Abstract
Introduction
Stem cell therapy is an experimental treatment for brain disorders. Although a cellular product, stem cells can be classified as biologics based on the cells’ secretion of therapeutic substances. Treatment with stem cell biologics may appeal to stroke because of the secondary cell death mechanisms, especially neuroinflammation, that are rampant from the onset and remain elevated during the progressive phase of the disease requiring multi-pronged biological targets to effectively abrogate the neurodegenerative pathology. However, the optimal delivery methods, among other logistical approaches (i.e., cell doses and timing of intervention), for stem cell therapy will need to be refined before stem cell biologics can be successfully utilized for stroke in large scale clinical trials.
Areas covered
In this review, we discuss how the innate qualities of stem cells characterize them as biologics, how stem cell transplantation may be an ideal treatment for stroke, and the various routes of stem cell administration that have been employed in various preclinical and clinical investigations.
Expert opinion
There is a need to optimize the delivery of stem cell biologics for stroke in order to guide the safe and effective translation of this therapy from the laboratory to the clinic.
Keywords: stem cells, cerebral ischemia, drug, inflammation, translational research
1. The dirty drug: Stem cells as “biologics”
Stroke is a neurodegenerative and neurovascular disorder with 795,000 cases occurring annually in the United States [1]. The disease is characterized by an occlusion of a blood vessel within the skull or neck that supplies blood to the brain, which consequentially reduces cerebral blood flow and induces cell death in the affected areas [2]. Additionally, ischemic stroke is accompanied by an increase in inflammatory events in the brain and periphery that contribute to further apoptosis and subsequent neurological damage [3]. Although tissue plasminogen activator (tPA) is available as a treatment for stroke, its narrow 4.5 hour window of administration following ischemic stroke limits its effectiveness in the clinic [4]. Thus, alternative treatment regimens are warranted. Stem cell therapy may be a viable treatment for stroke given stem cells’ neuroprotective properties and ability to mitigate inflammation in the brain, as well as in the periphery via the spleen [5,6]. For instance, stem cells transplanted systemically after stroke demonstrate preferential migration to the spleen [5]. Indeed, stem cells such as mesenchymal stem cells (MSCs) are “biologics” that exhibit the ability to home to sites of inflammation [5–7], which would enable them to effectively render their anti-inflammatory effects and hinder further pathological development of a stroke. As recent evidence points to the systemic inflammatory response as a key pathological component in exacerbating secondary cell death post-stroke [8], stem cell transplantation appears to be a promising therapy to attenuate this system-wide immune response and ameliorate stroke outcomes.
Generating a dependable source of transplantable cells with consistent ethical and quality control will be important to create effective, reproducible, and reliable cell therapies for ischemic stroke. Additionally, further research is necessary to identify the optimal cell types that are both safe and effective for transplantation. However, stem cells remain promising therapeutic biologics as they demonstrate multiple avenues for combating neurodegeneration such as by mitigating neuroinflammation and increasing angiogenesis, neurogenesis, and vasculogenesis [9–14]. Of note, the ischemic site in the stroke brain comprises a toxic microenvironment that is detrimental to stem cell survival and maintains a heightened state of inflammation [15,16]. Thus, mitigating this toxic microenvironment by lowering cytotoxicity and inflammation may prove beneficial for increasing the quantity of surviving grafted cells and enhancing their therapeutic effects.
Initially, the regenerative mechanism exerted by stem cell therapy in the nervous system was believed to be the replacement of dead or dying neurons. However, further stem cell transplantation investigations have demonstrated poor engraftment rates yet robust increases in functional recovery and neuronal survival [17]. One of the main mechanisms to explain these observations is the secretion of neurotrophic factors by the engrafted cells. Several growth factors are involved in cell survival pathways and limiting their decline represents a valid strategy to ameliorate stroke outcomes. Administering BDNF, VEGF, or other growth factors prevents apoptosis [18–20] and improves neurological outcomes in experimental disease models, but is unlikely to be effective in clinical settings. In contrast, stem cells are able to secrete a variety of neuroprotective factors, inducing anti-apoptotic and anti-inflammatory responses. Moreover, stem cells are sensitive and responsive, capable of regulating their growth factor secretion to proper levels and avoiding any dosage issues. This point is crucial because drug-induced overproduction can exert counter-productive neurological activities, such as how BDNF overexpression can induce epileptic seizures [21]. Another main mechanism of action is represented by the mobilization of endogenous stem cells. The discovery of brain regions which harbor endogenous stem cells (neurogenic niches), has challenged the dogma that mammalian adult brains are incapable of generating new neurons [22,23]. The endogenous mobilization of stem cells in the brain is only slightly effective in reverting or impeding the activation of cell death pathways. This is a result of the limited ability of endogenous stem cells to commit to the neuronal lineage, migrate, and differentiate from the neurogenic niches to the injured area [24]. To exert therapeutic activities, the endogenous stem cells need to migrate from these distal, neurogenic areas. Of note, transplanted stem cells mediate the mobilization of host stem cells [25]. This mechanism called a “biobridge” helps stem cells provide neuroprotective benefits and can facilitate stem cell migration to the injured area, enhancing the host’s regenerative activity [25]. Moreover, an additional mechanism of action has been reported involving secretome (microvesicles and exosomes) activity, which exerts therapeutic effects in neurovascular diseases [26].
While stem cell transplantation has promise as a potential treatment for ameliorating ischemic stroke, these grafted cells also carry possible risks including a host immune response to reject the transplanted cells and formation of detrimental teratomas. Immunosuppression regimens are an available solution for host rejection of stem cell grafts, though the risk of an undesirable immune response is dependent on the type of host [27]. Of note, stem cells with the capacity to differentiate into multiple cell lineages such as induced-pluripotent stem cells and embryonic-derived stem cells possess a higher chance of developing teratomas than adult stem cells [28]. Additionally, a reduction in the stemness of transplanted stem cells over time is actually favorable, as there is less risk for undesirable, excessive proliferation. This limitation is particularly dependent on specific cells’ ability to differentiate, though all stem cells possess this potential hazard.
To circumvent the host rejection response and teratoma development while preserving the regenerative benefits of stem cells, it has been proposed to use derivatives of stem cells such as vesicles, rather than the cells themselves. It is widely accepted that MSCs exert their action via paracrine effects via secretome or extracellular vesicles, rather than through transdifferentiation to replace injured neurons. Specifically, MSC-secreted extracellular vesicles are able to promote neural repair and to improve the functional outcome in stroke animal model. In this case, cell-derived therapies, such as components derived from secretomes possessing beneficial factors, can be utilized to increase neurogenesis and angiogenesis and decrease inflammatory processes [28–32]. Therefore, extracellular vesicles may be developed as a novel cell-free therapy for neurological disorders. Indeed, intravenous delivery of exosome-enriched vesicles released by bone marrow-derived MSCs significantly improve the neurite remodeling as well as neural plasticity in MCAO rats [33]. Stroke triggers the mobilization of bone marrow MSC-derived secretome in patients with severe stroke, and these vesicles exert restorative activity [34]. Such cell-free therapeutic effect is recently seen in a porcine model of stroke, demonstrating for the first time that hNSC-derived vesicles preserve functional activity and neural tissue integrity post-MCAO, suggesting that it may represent a potential therapy for stroke patients [35]. To date, systematic comparative vis-à-vis studies between transplantable cells and cell-free therapeutic substances are missing, which will be a key step in identifying the optimal regenerative medicine product for stroke therapy.
2. Stroke as a candidate target for stem cell biologics
Stroke can be classified as either ischemic or hemorrhagic. Of recent, ischemic stroke appears to be an ideal candidate for stem cell therapy [36, 37]. As mentioned above, at present, only a few treatment options exist for ischemic stroke and these regimens possess deficiencies that compromise their safety and efficacy in clinical settings. Thus, stem cell therapy emerges as an alluring potential treatment for the chronic symptoms of cerebral ischemia, such as the cell death and detrimental neuroinflammation associated with the subacute and chronic stages of ischemic stroke [38].
Three main phases characterize the ischemic events that occur after a stroke: the acute, subacute, and chronic phases. First, the initial few hours after the occlusion mark the acute phase of stroke and are accompanied by excitotoxicity and oxidative damage at the infarct area due to insufficient blood supply [39–41]. Cell death is exacerbated by the production of reactive oxygen species (ROS) and increasing concentrations of Na+ and Ca2+ ions in the ischemic region. The compromised homeostasis of ions enables water to enter the cells, resulting in the development of vasogenic edema in the infarct zone. Moreover, the neurons in the ischemic penumbra that initially survive the ischemic event begin to secrete signals that promote further cellular damage [39–41]. Next, the subacute phase involves elevated neuroinflammation and the secretion of matrix metalloproteases (MMPs), cellular adhesion molecules (CAMs), chemokines, and cytokines from damaged astrocytes, neurons, and microglia; and occurs during the early days after the onset of ischemia, following the acute phase [5,39–41]. Additional leukocytes are drawn to the infarct site due to elevated amounts of CAMs enabling increased leukocyte adhesion to cerebral vessels. Furthermore, as a result of rising MMP levels, blood-brain barrier (BBB) permeability increases, facilitating the infiltration of peripheral leukocytes into damaged regions and further escalating inflammation [39–41]. Finally, astrocytes and activated microglia perpetuate the state of heightened inflammation into the chronic stages of stroke that follow the subacute phase [39–41]. Additional macrophages and neutrophils from the periphery are recruited via the BBB due to the release of CAMs, chemokines, and cytokines from diseased brain cells. Consequently, the brain infrastructure is at risk to damage from the cell death and cerebral edema generated by this chronic inflammation [39–41].
Stem cell transplantation is capable of mitigating the heightened inflammation present in both the subacute and chronic phases of stroke. As the ischemic injury is aggravated by ongoing inflammatory processes during the subacute stage, it is critical to promote neuroprotection and preclude further damage during this period [36]. Additionally, facilitating anti-inflammatory and neuroregenerative events in the subacute and chronic phases are paramount for successful therapeutic outcomes [5,39–41]. Indeed, administering stem cells in the chronic phase of stroke can revitalize the brain, restore normal blood flow, repair the disruptions in the BBB, and reduce inflammation via regenerative processes such as angiogenesis, neurogenesis, synaptogenesis, and vasculogenesis. Moreover, the apoptosis, inflammation, mitochondrial dysfunction, and oxidative stress that lead to ischemia-mediated early secondary cell death can be inhibited by stem cell injections during the subacute stage [4,5,42,43]. Thus, stem cell therapy can fulfill the unmet need for an effective treatment targeting the subacute and chronic phases of ischemic stroke through its ability to promote recovery after stroke-induced damage by increasing reinnervation and diminishing inflammation.
The disease pathology of ischemic stroke is now thought to involve a mounting peripheral immune response in addition to the central neuroinflammation [44], and this connection between the peripheral systems and the brain is likely mediated through inflammatory signals in the circulatory system. Indeed, neuroinflammation is facilitated by both central and peripheral inflammation in which the initial ischemic insult creates an inflammatory response that is further enhanced by systemic inflammation [45]. Furthermore, lymphocytes, Tcells, macrophages, and monocytes pass through the BBB which has been compromised by the ischemic injury, and further elevate inflammation levels [5,45].
The systemic inflammation that accompanies ischemia in the brain may be mediated by the spleen. In fact, following ischemic stroke, spleen sizes decrease and splenocytes are released into the circulatory system, leading to increased neurodegeneration [46]. Additionally, the spleen has demonstrated a role in exacerbating other neurodegenerative diseases such as traumatic brain injury, as cognitive function and injury sizes improve when the spleen is removed post-injury [47].
The spleen appears to play a crucial role as a “seducer” in the body’s physiological response to transplanted stem cells [5,25]. Human bone marrow MSCs injected into the circulatory system preferentially migrate to the spleen after stroke [5]. Hence, stem cells may mitigate the spleen’s inflammatory processes post-stroke, given that these cells possess innate anti-inflammatory qualities. With the knowledge that the spleen contributes to inflammation and neurodegeneration during the chronic stage of stroke, anti-inflammatory treatments for ischemia may also benefit from approaches that target and abate the peripheral immune response, such as stem cell therapy.
The stem cells’ ability to modulate splenic activity in the chronic phase of stroke may prove to be beneficial for developing effective stroke treatments. These cells may not need to enter the brain and may possibly only need to interact with the spleen to exert their neuroprotective effects [5]. Indeed, if stem cells can reduce neuroinflammation via indirect avenues, their ability to ameliorate ischemic stroke will not be compromised by their limited potential to cross the BBB. This solves the dilemma in which systemic administration of stem cells is rendered less effective following repair of the damaged BBB. Furthermore, stem cell treatments that focus on the spleen in the chronic phase of stroke will remain potent even after the BBB is restored and impedes the bioavailability of therapeutics in the brain. Stem cell therapies at later post-stroke time points will benefit from additional studies that further investigate how stem cells interact with the spleen to reduce inflammation.
While several types of cells such as induced pluripotent stem cells, embryonic stem cells, amnion, umbilical cord blood, adipose, CTX0E3 cells, and NT2N cells have been employed in bench and clinical cell transplantation investigations for ischemic stroke [4,48–55], recent studies have focused on cells harvested from bone marrow. Multi-potent adult progenitor cells (MAPCs), SB623 cells, endothelial progenitor cells (EPCs), multilineage-differentiating stress enduring (Muse) cells, MSCs, and other stem cells derived from bone marrow demonstrate a favorable safety profile in several diseases [56,57] and have been comprehensively examined in various animal models [5,58], making them attractive for stem cell transplantation in stroke.
Indeed, stem cell transplantation will likely be beneficial for ischemic stroke given the stem cells’ ability to bolster neuroprotection and neuroregeneration in diseased animal models [58–63]. As the onset of stroke is uncertain, stem cell regimens will likely be used as a preventative or regenerative treatment in the clinic. The idea of stem cells as prophylactic biologics will be reinforced by advancements in diagnostic methods that incorporate genetics, co-morbidity factors, and family history to indicate patients that are prone to stroke and may benefit from cell transplantation. Clinical utilization of stem cells will benefit from future studies that probe these cells’ capabilities to impede the occurrence of an ischemic stroke and promote post-stroke recovery. Particular interest is provided in optimizing the route of stem cell delivery because of the need to distribute the biologics relased by the stem cells to the stroke brain. The rescue of the injured brain and resulting functional outcomes are likely to be dictated by the appropriate bioavailabity of the stem cells and their secereted factors within or close to the site of injury.
3. Delivery routes of stem cell biologics in stroke
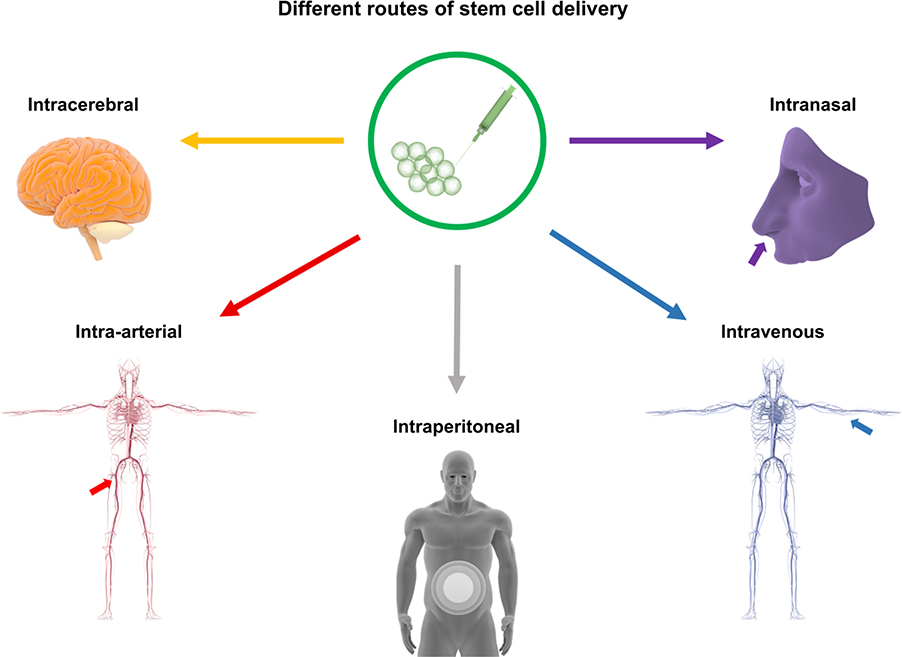
Stem cell therapy after stroke improves functional outcomes in preclinical studies. There are different routes for treatments, including intracerebral, intra-arterial, intraperitoneal, intraventricular, intravenous, and intranasal (Figure 1), but the most suitable route is still uncertain. After stroke, the original concept of transplanting exogenous stem cells is to reestablish the cytoarchitecture of injured tissue. This treatment involves the grafted cells’ survival in an inhospitable environment with inflammation, oxidative stress, glial scars, and cell death [15,16]. Initially, it was believed that intracerebral administration was the best route to enable exogenous neural stem cells (NSCs) to reach brain tissues. These cells are multipotent, able to self-renew and generate neural cells, and can replace lost neurons [64,65]. Subsequently, the mechanistic view of stem cell therapy has evolved into a by-stander effects, with stem cells such as NSCs exerting their functional effects through paracrine pathways, secreting different growth factors, and expressing mRNA. Besides NSCs, numerous other cell types can perform these paracrine functions and differentiate into multiple lineages, which is necessary for replacing lost neurons in the injured brain [66,67]. In addition to NSCs displaying cell replacement and by-stander effects in animal models [68–70], embryonic stem cells [71,72], induced pluripotent stem cells (iPSCs) [73,74], and MSCs have also been demonstrated to achieve such mechanisms of neurovascular repair.
Figure 1.
Different routes of stem cell delivery. Stem cell transplantation for stroke can be administered via several routes, including intracerebral, intra-arterial, intraperitoneal, intraventricular, intravenous, and intranasal treatment.
Intracerebral delivery indicates that more implanted cells exist in the injured regions compared to other administration routes, in which millions of stem cells are delivered into the brain and approximately one third are able to migrate to the injured areas [75–77], as well as to the intact hemisphere [78,79]. Intra-striatal injection of human iPSCs induces neural recovery as these cells are able to differentiate into immature and mature neurons, indicating that this administration route is feasible in subcortical stroke [80]. However, less invasive methods than stereotaxic injections have been used and the intracerebral method is not the only route capable of replacing lost neurons and neuronal connections. Using subarachnoid [81,82] and intraventricular [16] routes for therapeutic delivery in stroke rats improves cell survival and enriches the microenvironment. Additionally, exogenous stem cells administered via intraventricular techniques can reach ischemic areas after stroke.
Clinical trials have reported the feasibility and safety of NSCs. Although some patients show improvement, the trial results demonstrate no significant benefits in motor function [83]. The use of NSCs in patients, particularly the CTX0E03 (CTX) line, has been evaluated in the PISCES trial in order to test their safety after injection ( NCT01151124), since few in vitro and in vivo studies have been performed and are not enough to render significant conclusions. Interestingly, a single intracerebral dose of CTX (up to 20 million cells) induces no adverse events and is associated with improved neurological function [84].
Bone marrow stem cells have demonstrated effectiveness concomitant with some improvements in the neurological condition of patients [85,86]. Clinical trials performing intracerebroventricular administration of autologous bone marrow stem cells [87,88] and fetal cells [89] report ameliorations in functional activity compared with the control groups. However, numerous issues hinder intraventricular and intracerebral routes of stem cell administration for brain repair, such as poor cell availability, invasiveness, immune rejection, and uncertain cell “fate” within the brain, which represent important limits to translational applications [90]. To overcome these obstacles, less invasive routes of administration represent promising candidates for stem cell therapy after stroke.
Stem cell therapy through intra-arterial administration in stroke animal models has demonstrated positive results. The most common intra-arterial method requires the use of catheterization as a guide for the cells through the carotid artery, enabling the delivery of a large number of cells to the brain injury [91]. However, when using this delivery method, around 10% of exogenous cells reach the injured region [92]. Transplanting stem cells intra-arterially leads to the replacement of lost neural connections and induces the release of trophic factors that enhance brain repair [91]. Moreover, intra-arterial administration of NSCs confers successful recovery after stroke, implying that grafted cells do not need to be administered near the injured area to be effective. For stroke treatments in animal models, several different types of stem cells apart from NSCs have been delivered intra-arterially, including umbilical cord mesenchymal stromal cells, human umbilical cord blood mononuclear cells, and MSCs [91–93]. Intra-arterial delivery has the advantage of being less invasive compared to the intralesional routes and is a promising route for stem cell therapy in stroke patients. Exogenous stem cells administered intra-arterially can reach the brain, showing efficacy via improved recovery. However, the risk of vessel blockage has been noted due to the large size of the cells [94] or microemboli [95]. In contrast, no adverse effects from microemboli after intra-arterial administration have also been observed [91]. Systemic delivery of stem cells could also lead to vasculature blockage. Increasing extra-vascular activity from the lumen to parenchymal brain [96] or targeting cells through overexpression of molecules [97] represent strategies to increase engraftment of these cells to the brain and minimize microemboli formation. The current therapies for stroke, such as thrombectomy, include an intra-arterial intervention that is effective until 8 h after stroke [98]. Treatments featuring an intra-arterial thrombectomy procedure may be well worth combining with intra-arterial administration of stem cells, possibly offering an advantage in clinical translation.
Despite several studies exploring the utility of intra-arterial transplantation, its safety and efficacy remain inconclusive requiring further investigations. The mortality of stroke rodents after intra-arterial administration of NSCs is reduced using a microneedle instead of a catheter [99]. Moreover, microemboli occur in some cases, while other studies report no adverse effects from microemboli [91]. The infusion time, dose, and cell size can all contribute to an increase in the complications after intra-arterial graft transplantation. In fact, low infusion is linked to increased complications, while transplanting a high dose of cells triggers augmented embolic events [100]. Intriguingly, fast infusion has also induced an increase in embolic events [101]. In light of this, infusion velocity requires further investigations as the results remain contradictory. Additionally, reports regarding efficacy are equally as conflicting. While the intra-arterial route of administration in stroke animal models has demonstrated positive outcomes [91,92,99], intra-arterial delivered exogenous bone marrow MSCs exhibit limited ability to improve middle cerebral artery occlusion recovery in rats, even after indicating effective homing to the infarcted hemisphere [97–102]. These preclinical results indicate that further studies are necessary to identify clinically effective therapies for stroke [103]. Of note, clinical trials of cell therapies for stroke have also evaluated intra-arterial administration, showing that intra-arterial delivery of bone marrow cell grafts [104] or autologous bone marrow mononuclear cells [105] is safe and feasible. Moreover, 30% of patients with moderate to severe strokes show clinical improvement, and 40% of patients have a positive clinical outcome at 90 days [106].
Compared with intra-arterial administration, the intravenous route of cell delivery is more attractive because it is less invasive for stroke patients and equally effective compared to other delivery methods. In fact, most ongoing clinical trials use this route of administration [107]. Using intravenous delivery, the administered cells are confined to the peripheral organs, leading to low cell concentrations in the infarct zone [108]. Additionally, no cases of tumor formation or adverse effects have been reported. While intravenous and intra-arterial routes of administration show comparable protective properties and feasibility, intravenous delivery is considered preferable [91]. Different preclinical studies have reported promising results after intravenous cell therapy administration in stroke using several types of cells. Exogenous bone marrow stromal cells administered intravenously are able to migrate into the brain, survive, and improve recovery [109]. In addition, bone marrow mononuclear cells reduce lesion sizes and ameliorate functional outcomes in rats [110]. Moreover, adipose-derived MSCs improve brain plasticity and attenuate inflammation and apoptosis [111,112]. However, stem cell administration post-stroke may not be sufficient to enhance recovery in an aged brain environment [113]. iPSCs transplanted into the stroke-afflicted cortex are able to survive, differentiate into neurons, and improve functional recovery [114]. After intravenous NSC administration in rodents with intracerebral hemorrhage, these cells migrate and differentiate into astrocytes and neurons, and enhance post-stroke functionality in rodents [115]. Moreover, a small percentage of injected NSCs accumulate in the stroke injury area and most of these exogenous cells remain undifferentiated [116]. Interestingly, it has been demonstrated that NSCs migrate preferentially to the spleen compared to the brain and reduce apoptosis, inflammation, and edema formation after an ischemic insult. These effects are not observed in splenectomized rats, implicating that the stem cells provided this neuroprotection by interrupting splenic inflammatory responses [117]. Additionally, human bone marrow stem cells administered intravenously also migrate more to the spleen than to the brain, attenuating inflammation and reducing the infarct area in the striatum. Indeed, these results demonstrate that stem cells injected intravenously represent a potential therapy for post-acute stroke capable of abrogating the inflammation-plagued secondary cell death [5].
Many clinical studies have employed cell-based therapies with intravenous administration. For instance, MSCs injected intravenously have improved neurological deficits in five patients with severe stroke [118]. A clinical evaluation of the efficacy and safety of intravenously injected autologous MSCs in a larger cohort has deemed this treatment as safe for 5-years follow-up [119]. Additionally, autologous MSCs expanded using autologous human serum are safe and capable of reducing the ischemic lesion by more than 20% after one week of treatment [120]. Other types of cells such as bone marrow mononuclear cells have been administered intravenously and have demonstrated feasibility and safety in patients with stroke [121,122]. After autologous cell transplants in the chronic phase of stroke, patients have shown improvements in the Barthel Index and augmented brain plasticity without adverse effects [123]. Recently, intravenous transplantations of autologous bone marrow mononuclear cells have also demonstrated enhanced neurological recovery and cerebral blood flow in stroke patients [124]. Overall, autologous mononuclear stem cell treatment via intravenous administration has been demonstrated to be feasible and safe for stroke patients, and there are several ongoing clinical trials testing the feasibility, safety, and efficacy of intravenous administration of other cell types in acute stroke.
In tandem with intra-arterial and intraperitoneal routes, the intraperitoneal route of administration stands as another potent delivery method but it remains underexplored that necessitates the need for using in vivo stroke models to assess safety, efficacy, and feasibility as a prior step to clinical studies. As noted above, the most appealing feature of intraperitoneal route is its minimally invasive procedure thus lessening the trauma associated with stem cell delivery associated with the other cell delivery approaches. However, the minimal migration of the intraperitoneally transplanted cells may limit the successful deposition of the cells and their biologics into the ischemic brain and inflammatory sites, which would necessitate increasing the cell dose to achieve an efficacious outcome. In the end, laboratory studies are needed to enhance cell migration with the intraperineal route. When contemplating with the intraperitoneal route of stem cell injections for stroke, it has been demonstrated that the grafted cell distribution may affect the therapeutic outcomes. For instance, higher quantities of MSCs reach the spleen, lungs, and brain when injected intravenously, relative to the intraperitoneal route [125]. That relatively few stem cells reach the ischemic brain or inflammatory peripheral organs (i.e., spleen) may warrant an ample amount of cell dose for intraperitoneal route of delivery. Such logistical requirements may limit the use of intraperitoneal route, despite its minimally invasive approach that appears practical in the clinical setting of stroke.
Another minimally invasive procedure but allows robust cell migration potential is the intranasal administration which has gained traction for stem cell delivery primarily because of its safe, effective, and feasible route of delivery. Intranasal delivery is the most recent route used for cell-based treatments for stroke and currently, only preclinical studies utilizing this method have been performed. Of interest, intranasally administered cells are able to bypass the BBB and reach the brain [126]. These intranasally delivered cells migrate from the nose through the olfactory bulb or cerebrospinal fluid [127]. Additional experimental investigations probing proper dosages and techniques to reduce cell clumping or other adverse effects are necessary to advance this route of delivery. Compared with the other peripheral routes of delivery, the intranasal route appears to circumvent the problem of directing cells to the ischemic brain. Mouse models of ischemia treated with intranasally administered bone marrow MSCs have shown enhanced cell homing to the ischemic area and optimized therapeutic efficacy [128]. Additionally, intranasal delivery of bone marrow MSCs in neonatal stroke rats reduces infarct sizes and BBB disruption. Moreover, these animals show improved brain plasticity, enhanced cerebral blood flow, and increased functional recovery [129]. In a comparison between an intranasal delivery of MSCs and BDNF-secreting MSCs in neonatal hypoxic-ischemic brain injury rats, it has been demonstrated that both treatments reduce brain injury, ameliorate behavioral performance, and promote cell proliferation after stroke [130]. To reduce possible tumorigenic effects and increase the survival rate of grafted cells after intranasal administration, conditional medium can be used. Indeed, intranasal administration of conditional medium from human umbilical cord MSCs ameliorates functional outcomes, reduces BBB damage, and improves the vasculature post-stroke [131]. Additional experimental investigations probing proper dosages and techniques to reduce cell clumping or other adverse effects are necessary to advance this route of delivery. Compared with intraperitoneal route, the intranasal route appears to circumvent the problem of directing cells to the ischemic brain.
Finally, an invasive route of delivery has also been explored for stem cell administration. Intralesional (intracerebral, intraventricular, or subarachnoid) route of administration is that the transplanted cells participate in reestablishing and reconstructing the cytoarchitecture of damaged tissue after stroke. In fact, it has been demonstrated that these cells can replace most of the lost neurons after stroke [76,132]. However, this type of delivery has the disadvantage of a limited survival rate in an inhospitable milieu [15,16]. To overcome this obstacle and improve grafted cell survival, migration, differentiation, and proliferation, different hydrogels have been used. For instance, encouraging results for stroke therapies has been reported using Matrigel or hyaluronic acid (HA), which are promising material for delivering cells to neural tissues [133].
In summary, several delivery methods for stem cells exist in the laboratory which warrant closer examination to reveal each particular route’s safety and efficacy. Clinical studies have tested different routes of administration as well, but since the transplant protocols vary between the clinical trials, assessment of superiority of a particular route of cell delivery compared to others remains elusive.
4. Expert opinion
Stem cell therapy is an experimental treatment for neurological disorders, including stroke. Although stem cells are a cellular product, they may be considered as “biologics” mainly because of their robust secretion of therapeutic substances, such as neurotrophic, anti-inflammatory, anti-oxidative, and anti-apoptotic factors among others, which have been shown to promote neurovascular recovery after cerebral ischemia. Such stem cell-based biologics approach has targeted stroke primarily due to the secondary cell death pathways accompanying the disease, characterized by downregulated levels of therapeutic substances which the stem cells are known to secrete as noted above, specifically neuroinflammation. That stem cells may represent the dirty drug designation appeals to stroke with its progressive phase associated with multiple cell death pathways requiring a multi-pronged approach to effectively abrogate the neurodegenerative pathology. Preclinical studies have shown the safety and efficacy of stem cell therapy in many stroke models. Limited clinical trials have been underway, and largely show the safety of transplanted stem cells in stroke patients. Efficacy of stem cells in the clinic remains elusive. Clearly, there is a need to improve the transplant regimen in order to realize not just safety, but also efficacy outcomes in the clinic. A key translational research gap may be related to finding the optimal route of delivery. Recognizing the stem cells stand as potent biologics may facilitate finding this optimal stem cell administration approach. To this end, determining the different therapeutic substances, such as anti-inflammatory factors, secreted by stem cells and amplifying their levels, as well as their biological function, may enhance the clinical outcomes of stem cell therapy. Moreover, equating stem cells as biologics with pharmacological properties may allow modification in their delivery method, such that solid and stable functional benefits are achieved. We summarize the different routes of stem delivery in Table 1, which show that each stem cell delivery route necessitates the need for adjusting the dose and timing of stem cells [118, 119, 125–131]. Increasing evidence shows that several types of stem cells reside in the CNS and non-CNS. As mentioned, some of them are not yet transplanted into post-stroke animals or translated in clinic, but they showed the potential to contribute to the brain repair process following stroke [134]. Since the research in this field is of high interest, this potential therapeutic activity will exploit to treat stroke patients in the near future. Because stroke is associated with distinct phases, namely acute, subacute and chronic, as discussed above, the optimal route will need to cater to the timing of intervention based on the stroke stage. Similarly, the cell dose associated with the route and timing of stroke will need to be modified accordingly. In the end, there is likely not a universal route and dose across the phases of stroke, but a range of routes and doses that will be stroke timing-dependent. As a rule of thumb, acute and subacute phases of stroke associated with elevated inflammatory signals acting as cell migratory “help me” signals will likely allow effective outcomes with minimally invasive procedures such as intravenous, intra-arterial, intranasal, and intraperitoneal routes with higher doses. On the other hand, the chronic state of stroke characterized by waning inflammatory signals will necessitate intracerebral or intraventricular administration to deliver the lower doses of cells within or near the ischemic brain region. Integrating our basic science knowledge on the onset and progressive nature of stroke pathology with the translational optimization of stem cell routes and doses will improve the successful clinical application of stem cell biologics for stroke.
Table 1.
Routes, doses, and timing of stem cell administration in stroke.
| Type of Study | Route | Type of Stem Cells | Physiological Effect | Dose | Timing | Ref. |
|---|---|---|---|---|---|---|
| Preclinical | IP | MSCs MNCs |
Improved peripheral distribution compared to IV | 1 million | 1 day | [118] |
| Preclinical | IC | CTX0E03 | Promotion of behavioural recovery and endogenous neurogenesis | 4500, 45000 or 450000 | 4 weeks | [133] |
| Clinical | IC | SB623 | Improvement in clinical outcome end points | 2.5, 5, 10 million | 6 – 60 months | [134] |
| Preclinical | IA | hESCs | Decreased brain injury | 1 million | 1 day | [135] |
| Clinical | IA | Autologous CD34+ selected stem/progenitor | Improvement in functional scores and reduction in lesion volume | 100 million | 7 days | [136] |
| Preclinical | IV | hNSCs | Improvement in functional recovery | 5 million | 1 – 3 days | [137] |
| Clinical | IV | MAPC | Safety and efficacy No significant improvement |
400, 1200 million | 1 – 2 days | [138] |
| Preclinical | IN | BMSCs | Reduction in brain infarct volume and cell death | 1 million | 1 day | [119] |
| Clinical | IN | BMSCs | Improvement in clinical outcome | 50 million | 7 days | [139] |
In summary, stem cells are cellular products that can be considered as biologics based on their secretion of therapeutic substances, which have been shown to harness regenerative mechanisms in stroke models. Recognizing stem cells as biologics allows optimization of stem cell delivery routes that take advantage of their drug-like properties, such as absorption and metabolism of stem cell-secreted factors. Such optimized stem cell delivery route, together with appropriate dose and timing of administration, will improve functional outcomes of cell therapy in stroke.
Article Highlights.
Although long considered as a cellular product, stem cells can be classified as biologics based on the cells’ secretion of therapeutic substances
Stem cell biologics stand as potent stroke therapeutics for sequestering secondary cell death processes
Cell death cascades, especially aberrant neuroinflammation, plague stroke during the neurodegenerative progression of the disease
The multifactorial progressive phase of the disease requires multi-pronged biological targets to effectively abrogate the neurodegenerative pathology
Optimizing the transplant regimen, in particular the route of delivery, will advance the use of stem cell biologics for stroke therapy
Acknowledgments
Funding
This paper was funded by the National Institute of Health.
Footnotes
Declaration of interest
C Borlongan was funded and received royalties and stock options from Astellas, As-terias, Sanbio, Athersys, KMPHC, and International Stem Cell Corporation; and also received consultant compensation for Chiesi Farmaceutici. He also holds patents and patent applications related to stem cell biology and therapy. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catanese L, Tarsia J, Fisher M. Acute Ischemic Stroke Therapy Overview. Circ Res. 2017;120(3):541–558.*, of interest: This manuscript presents novel treatment paradigms based on intraarterial device treatment to potentially increase the number of patients who can be treated despite long transport times and to ameliorate the consequences of reperfusion injury.
- 3.Stonesifer C, Corey S, Ghanekar S, et al. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158:94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dailey T, Metcalf C, Mosley YI, et al. An Update on Translating Stem Cell Therapy for Stroke from Bench to Bedside. J Clin Med. 2013;2(4):220–241.**, of considerable interest: This review paper discusses the current knowledge of stem cell research in neurological disease, mainly stroke, with a focus on its benefits, limitations, and clinical potential.
- 5.Acosta SA, Tajiri N, Hoover J, et al. Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke. Stroke. 2015;46(9):2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu K, Lee JY, Kaneko Y, et al. Human stem cells transplanted into the rat stroke brain migrate to spleen via lymphatic and inflammation pathways. Haematologica. 2018.**, of considerable interest: This study for the first time demonstrates brain-to-periphery migration of stem cells, advancing the novel concept of harnessing the lymphatic system in mobilizing stem cells to sequester peripheral inflammation as a brain repair strategy.
- 7.Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419–427. [DOI] [PubMed] [Google Scholar]
- 8.Marcet P, Santos N, Borlongan CV. When friend turns foe: central and peripheral neuroinflammation in central nervous system injury. Neuroimmunol Neuroinflamm. 2017;4:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boltze J, Reich DM, Hau S, et al. Assessment of neuroprotective effects of human umbilical cord blood mononuclear cell subpopulations in vitro and in vivo. Cell Transplant. 2012;21(4):723–737. [DOI] [PubMed] [Google Scholar]
- 10.Henning RJ, Shariff M, Eadula U, et al. Human cord blood mononuclear cells decrease cytokines and inflammatory cells in acute myocardial infarction. Stem Cells Dev. 2008;17(6):1207–1219. [DOI] [PubMed] [Google Scholar]
- 11.Pimentel-Coelho PM, Rosado-de-Castro PH, da Fonseca LM, et al. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2012;71(4 Pt 2):464–473. [DOI] [PubMed] [Google Scholar]
- 12.Acosta SA, Franzese N, Staples M, et al. Human Umbilical Cord Blood for Transplantation Therapy in Myocardial Infarction. J Stem Cell Res Ther. 2013(Suppl 4). [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EJ, Choi EK, Kang SK, et al. N-cadherin determines individual variations in the therapeutic efficacy of human umbilical cord blood-derived mesenchymal stem cells in a rat model of myocardial infarction. Mol Ther. 2012;20(1):155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li T, Ma Q, Ning M, et al. Cotransplantation of human umbilical cord-derived mesenchymal stem cells and umbilical cord blood-derived CD34(+) cells in a rabbit model of myocardial infarction. Mol Cell Biochem. 2014;387(1–2):91–100. [DOI] [PubMed] [Google Scholar]
- 15.Buhnemann C, Scholz A, Bernreuther C, et al. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129(Pt 12):3238–3248. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Ye R, Yan T, et al. Cell based therapies for ischemic stroke: from basic science to bedside. Prog Neurobiol. 2014;115:92–115.**, of considerable interest: This review paper provides a comprehensive synopsis of preclinical evidence and clinical experience of various donor cell types, their restorative mechanisms, delivery routes, imaging strategies, future perspectives and challenges for translating cell therapies as a neurorestorative regimen in clinical applications.
- 17.Dela Pena I, Sanberg PR, Acosta S, et al. Stem cells and G-CSF for treating neuroinflammation in traumatic brain injury: aging as a comorbidity factor. J Neurosurg Sci. 2014;58(3):145–149. [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiq I, Park E, Liu E, et al. Treatment of traumatic brain injury using zinc-finger protein gene therapy targeting VEGF-A. J Neurotrauma. 2012;29(17):2647–2659. [DOI] [PubMed] [Google Scholar]
- 19.Lin D, Wu Q, Lin X, et al. Brain-derived Neurotrophic Factor Signaling Pathway: Modulation by Acupuncture in Telomerase Knockout Mice. Altern Ther Health Med. 2015;21(6):36–46. [PubMed] [Google Scholar]
- 20.Kim HJ, Lee JH, Kim SH. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010;27(1):131–138. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Yang M, Kim J, et al. Developmental and degenerative modulation of brain-derived neurotrophic factor transcript variants in the mouse hippocampus. Int J Dev Neurosci. 2014;38:68–73. [DOI] [PubMed] [Google Scholar]
- 22.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. [DOI] [PubMed] [Google Scholar]
- 23.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liska MG, Crowley MG, Nguyen H, et al. Biobridge concept in stem cell therapy for ischemic stroke. J Neurosurg Sci. 2017;61(2):173–179. [DOI] [PubMed] [Google Scholar]
- 25.Tajiri N, Kaneko Y, Shinozuka K, et al. Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One. 2013;8(9):e74857.*, of interest: This study discusses novel observations of a stem cell mechanism that involves attracting a host cell to engage in brain repair, harnessing a stem cell-paved bio-bridge beyond cell replacement and trophic factor secretion for the treatment of traumatic brain injury and other neurological disorders.
- 26.Zhang Y, Chopp M, Zhang ZG, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imberti B, Monti M, Casiraghi F. Pluripotent stem cells and tolerance induction in organ transplantation. Curr Opin Organ Transplant. 2015;20(1):86–93. [DOI] [PubMed] [Google Scholar]
- 28.Stone LL, Grande A, Low WC. Neural repair and neuroprotection with stem cells in ischemic stroke. Brain Sci. 2013;3(2):599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xin H, Li Y, Buller B, et al. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30(7):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xin H, Li Y, Chopp M. Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 2014;8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chopp M, Zhang ZG. Emerging potential of exosomes and noncoding microRNAs for the treatment of neurological injury/diseases. Expert Opin Emerg Drugs. 2015;20(4):523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enrichedextracellular particles. Stem Cells. 2013; 31(12):2737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moon GJ, Sung JH, Kim DH, et al. Application of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Stroke:Biodistribution and MicroRNA Study. Transl Stroke Res. 2018.*, of interest: This paper shows detection of different miRNAs that are essential for promoting neurogenesis and angiogenesis for stroke treatment, with similar medicinal capacity as stem cells but an improved safety profile that overcomes cell-associated limitations in stem cell therapy.
- 35.Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, Swetenburg RL, Hess DC, West FD, Stice SL. Human Neural Stem Cell Extracellular Vesicles Improve Recovery in a Porcine Model of Ischemic Stroke. Stroke. 2018;49(5):1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borlongan CV, Glover LE, Sanberg PR, et al. Permeating the blood brain barrier and abrogating the inflammation in stroke: implications for stroke therapy. Curr Pharm Des. 2012;18(25):3670–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Truelsen T, Krarup LH, Iversen HK, et al. Causes of Death Data in the Global Burden of Disease Estimates for Ischemic and Hemorrhagic Stroke. Neuroepidemiology. 2015;45(3):152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin R, Liu L, Zhang S, et al. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. 2013;6(5):834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ceulemans AG, Zgavc T, Kooijman R, et al. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. J Neuroinflammation. 2010;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park DH, Borlongan CV, Willing AE, et al. Human umbilical cord blood cell grafts for brain ischemia. Cell Transplant. 2009;18(9):985–998. [DOI] [PubMed] [Google Scholar]
- 43.Park DH, Eve DJ, Musso J 3rd, et al. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev. 2009;18(5):693–702. [DOI] [PubMed] [Google Scholar]
- 44.dela Pena IC, Yoo A, Tajiri N, et al. Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis. J Cereb Blood Flow Metab. 2015;35(2):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dziedzic T Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;15(5):523–531. [DOI] [PubMed] [Google Scholar]
- 46.Seifert HA, Hall AA, Chapman CB, et al. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7(4):1017–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang BJ, Men XJ, Lu ZQ, et al. Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin Med J (Engl). 2013;126(12):2354–2360. [PubMed] [Google Scholar]
- 48.Borlongan CV, Sanberg PR, Freeman TB. Neural transplantation for neurodegenerative disorders. Lancet. 1999;353 Suppl 1:SI29–30. [DOI] [PubMed] [Google Scholar]
- 49.Borlongan CV, Fournier C, Stahl CE, et al. Gene therapy, cell transplantation and stroke. Front Biosci. 2006;11:1090–1101. [DOI] [PubMed] [Google Scholar]
- 50.Borlongan CV, Kaneko Y, Maki M, et al. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19(4):439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borlongan CV. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 2009;40(3 Suppl):S146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonucci I, Stuppia L, Kaneko Y, et al. Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant. 2011;20(6):789–795. [DOI] [PubMed] [Google Scholar]
- 53.Tajiri N, Acosta S, Glover LE, et al. Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats. PLoS One. 2012;7(8):e43779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maya-Espinosa G, Collazo-Navarrete O, Millan-Aldaco D, et al. Mouse embryonic stem cell-derived cells reveal niches that support neuronal differentiation in the adult rat brain. Stem Cells. 2015;33(2):491–502. [DOI] [PubMed] [Google Scholar]
- 55.Stevanato L, Thanabalasundaram L, Vysokov N, et al. Investigation of Content, Stoichiometry and Transfer of miRNA from Human Neural Stem Cell Line Derived Exosomes. PLoS One. 2016;11(1):e0146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104(34):13798–13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Y, Yasuhara T, Hara K, et al. Transplantation of bone marrow-derived stem cells: a promising therapy for stroke. Cell Transplant. 2007;16(2):159–169. [PubMed] [Google Scholar]
- 58.Tajiri N, Duncan K, Antoine A, et al. Stem cell-paved biobridge facilitates neural repair in traumatic brain injury. Front Syst Neurosci. 2014;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borlongan CV, Tajima Y, Trojanowski JQ, et al. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149(2):310–321. [DOI] [PubMed] [Google Scholar]
- 60.Borlongan CV, Saporta S, Poulos SG, et al. Viability and survival of hNT neurons determine degree of functional recovery in grafted ischemic rats. Neuroreport. 1998;9(12):2837–2842. [DOI] [PubMed] [Google Scholar]
- 61.Borlongan CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998;9(16):3615–3621. [DOI] [PubMed] [Google Scholar]
- 62.Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3(4):466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–256. [DOI] [PubMed] [Google Scholar]
- 64.Shi X, Yan C, Liu B, et al. miR-381 Regulates Neural Stem Cell Proliferation and Differentiation via Regulating Hes1 Expression. PLoS One. 2015;10(10):e0138973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Hoornaert CJ, Le Blon D, Quarta A, et al. Concise Review: Innate and Adaptive Immune Recognition of Allogeneic and Xenogeneic Cell Transplants in the Central Nervous System. Stem Cells Transl Med. 2017;6(5):1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5(1):121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu W, Zheng J, Gao L, et al. Neuroprotective Effects of Stem Cells in Ischemic Stroke. Stem Cells Int. 2017;2017:4653936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hou B, Ma J, Guo X, et al. Exogenous Neural Stem Cells Transplantation as a Potential Therapy for Photothrombotic Ischemia Stroke in Kunming Mice Model. Mol Neurobiol. 2017;54(2):1254–1262. [DOI] [PubMed] [Google Scholar]
- 69.Nishino H, Borlongan CV. Restoration of function by neural transplantation in the ischemic brain. Prog Brain Res. 2000;127:461–476. [DOI] [PubMed] [Google Scholar]
- 70.Savitz SI, Rosenbaum DM, Dinsmore JH, et al. Cell transplantation for stroke. Ann Neurol. 2002;52(3):266–275. [DOI] [PubMed] [Google Scholar]
- 71.Hicks AU, Lappalainen RS, Narkilahti S, et al. Transplantation of human embryonic stem cell-derived neural precursor cells and enriched environment after cortical stroke in rats: cell survival and functional recovery. Eur J Neurosci. 2009;29(3):562–574. [DOI] [PubMed] [Google Scholar]
- 72.Hayashi J, Takagi Y, Fukuda H, et al. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26(7):906–914. [DOI] [PubMed] [Google Scholar]
- 73.Kawai H, Yamashita T, Ohta Y, et al. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab. 2010;30(8):1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang M, Lv L, Ji H, et al. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem. 2011;354(1–2):67–75. [DOI] [PubMed] [Google Scholar]
- 75.De Feo D, Merlini A, Laterza C, et al. Neural stem cell transplantation in central nervous system disorders: from cell replacement to neuroprotection. Curr Opin Neurol. 2012;25(3):322–333. [DOI] [PubMed] [Google Scholar]
- 76.Kelly S, Bliss TM, Shah AK, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci U S A. 2004;101(32):11839–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Darsalia V, Kallur T, Kokaia Z. Survival, migration and neuronal differentiation of human fetal striatal and cortical neural stem cells grafted in stroke-damaged rat striatum. Eur J Neurosci. 2007;26(3):605–614. [DOI] [PubMed] [Google Scholar]
- 78.Modo M, Stroemer RP, Tang E, et al. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002;33(9):2270–2278. [DOI] [PubMed] [Google Scholar]
- 79.Veizovic T, Beech JS, Stroemer RP, et al. Resolution of stroke deficits following contralateral grafts of conditionally immortal neuroepithelial stem cells. Stroke. 2001;32(4):1012–1019. [DOI] [PubMed] [Google Scholar]
- 80.Popa-Wagner A, Buga AM, Doeppner TR, et al. Stem cell therapies in preclinical models of stroke associated with aging. Front Cell Neurosci. 2014;8:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim JY, Jeong CH, Jun JA, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2(5):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen SJ, Chang CM, Tsai SK, et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010;19(11):1757–1767. [DOI] [PubMed] [Google Scholar]
- 83.Kondziolka D, Steinberg GK, Wechsler L, et al. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg. 2005;103(1):38–45. [DOI] [PubMed] [Google Scholar]
- 84.Kalladka D, Sinden J, Pollock K, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. Lancet. 2016;388(10046):787–796. [DOI] [PubMed] [Google Scholar]
- 85.Suarez-Monteagudo C, Hernandez-Ramirez P, Alvarez-Gonzalez L, et al. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27(3):151–161. [DOI] [PubMed] [Google Scholar]
- 86.Li ZM, Zhang ZT, Guo CJ, et al. Autologous bone marrow mononuclear cell implantation for intracerebral hemorrhage-a prospective clinical observation. Clin Neurol Neurosurg. 2013;115(1):72–76. [DOI] [PubMed] [Google Scholar]
- 87.Sharma AK, Sane HM, Paranjape AA, et al. The effect of autologous bone marrow mononuclear cell transplantation on the survival duration in Amyotrophic Lateral Sclerosis - a retrospective controlled study. Am J Stem Cells. 2015;4(1):50–65. [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma A, Sane H, Gokulchandran N, et al. Autologous bone marrow mononuclear cells intrathecal transplantation in chronic stroke. Stroke Res Treat. 2014;2014:234095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rabinovich SS, Seledtsov VI, Banul NV, et al. Cell therapy of brain stroke. Bull Exp Biol Med. 2005;139(1):126–128. [DOI] [PubMed] [Google Scholar]
- 90.Wu Y, Wu J, Ju R, et al. Comparison of intracerebral transplantation effects of different stem cells on rodent stroke models. Cell Biochem Funct. 2015;33(4):174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gutierrez-Fernandez M, Rodriguez-Frutos B, Alvarez-Grech J, et al. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405. [DOI] [PubMed] [Google Scholar]
- 92.Li Y, Chen J, Wang L, et al. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56(12):1666–1672. [DOI] [PubMed] [Google Scholar]
- 93.Shen LH, Li Y, Chen J, et al. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137(2):393–399. [DOI] [PubMed] [Google Scholar]
- 94.Ge J, Guo L, Wang S, et al. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev. 2014;10(2):295–303. [DOI] [PubMed] [Google Scholar]
- 95.Walczak P, Zhang J, Gilad AA, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39(5):1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guzman R, De Los Angeles A, Cheshier S, et al. Intracarotid injection of fluorescence activated cell-sorted CD49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39(4):1300–1306. [DOI] [PubMed] [Google Scholar]
- 97.Gorelik M, Orukari I, Wang J, et al. Use of MR cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology. 2012;265(1):175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. [DOI] [PubMed] [Google Scholar]
- 99.Chua JY, Pendharkar AV, Wang N, et al. Intra-arterial injection of neural stem cells using a microneedle technique does not cause microembolic strokes. J Cereb Blood Flow Metab. 2011;31(5):1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cui LL, Kerkela E, Bakreen A, et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Janowski M, Lyczek A, Engels C, et al. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab. 2013;33(6):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mitkari B, Nitzsche F, Kerkela E, et al. Human bone marrow mesenchymal stem/stromal cells produce efficient localization in the brain and enhanced angiogenesis after intra-arterial delivery in rats with cerebral ischemia, but this is not translated to behavioral recovery. Behav Brain Res. 2014;259:50–59. [DOI] [PubMed] [Google Scholar]
- 103.Savitz SI, Chopp M, Deans R, et al. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke. 2011;42(3):825–829. [DOI] [PubMed] [Google Scholar]
- 104.Moniche F, Gonzalez A, Gonzalez-Marcos JR, et al. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43(8):2242–2244. [DOI] [PubMed] [Google Scholar]
- 105.Battistella V, de Freitas GR, da Fonseca LM, et al. Safety of autologous bone marrow mononuclear cell transplantation in patients with nonacute ischemic stroke. Regen Med. 2011;6(1):45–52. [DOI] [PubMed] [Google Scholar]
- 106.Friedrich MA, Martins MP, Araujo MD, et al. Intra-arterial infusion of autologous bone marrow mononuclear cells in patients with moderate to severe middle cerebral artery acute ischemic stroke. Cell Transplant. 2012;21 Suppl 1:S13–21. [DOI] [PubMed] [Google Scholar]
- 107.Detante O, Jaillard A, Moisan A, et al. Biotherapies in stroke. Rev Neurol (Paris). 2014;170(12):779–798. [DOI] [PubMed] [Google Scholar]
- 108.Boltze J, Arnold A, Walczak P, et al. The Dark Side of the Force - Constraints and Complications of Cell Therapies for Stroke. Front Neurol. 2015;6:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. [DOI] [PubMed] [Google Scholar]
- 110.Iihoshi S, Honmou O, Houkin K, et al. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 111.Leu S, Lin YC, Yuen CM, et al. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J Transl Med. 2010;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gutierrez-Fernandez M, Rodriguez-Frutos B, Ramos-Cejudo J, et al. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther. 2013;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balseanu AT, Buga AM, Catalin B, et al. Multimodal Approaches for Regenerative Stroke Therapies: Combination of Granulocyte Colony-Stimulating Factor with Bone Marrow Mesenchymal Stem Cells is Not Superior to G-CSF Alone. Front Aging Neurosci. 2014;6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tatarishvili J, Oki K, Monni E, et al. Human induced pluripotent stem cells improve recovery in stroke-injured aged rats. Restor Neurol Neurosci. 2014;32(4):547–558. [DOI] [PubMed] [Google Scholar]
- 115.Jeong SW, Chu K, Jung KH, et al. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34(9):2258–2263. [DOI] [PubMed] [Google Scholar]
- 116.Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132(Pt 8):2239–2251. [DOI] [PubMed] [Google Scholar]
- 117.Lee ST, Chu K, Jung KH, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131(Pt 3):616–629. [DOI] [PubMed] [Google Scholar]
- 118.Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57(6):874–882. [DOI] [PubMed] [Google Scholar]
- 119.Lee JS, Hong JM, Moon GJ, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–1106. [DOI] [PubMed] [Google Scholar]
- 120.Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134(Pt 6):1790–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Savitz SI, Misra V, Kasam M, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70(1):59–69. [DOI] [PubMed] [Google Scholar]
- 122.Prasad K, Mohanty S, Bhatia R, et al. Autologous intravenous bone marrow mononuclear cell therapy for patients with subacute ischaemic stroke: a pilot study. Indian J Med Res. 2012;136(2):221–228. [PMC free article] [PubMed] [Google Scholar]
- 123.Bhasin A, Srivastava M, Bhatia R, et al. Autologous intravenous mononuclear stem cell therapy in chronic ischemic stroke. J Stem Cells Regen Med. 2012;8(3):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taguchi A, Sakai C, Soma T, et al. Intravenous Autologous Bone Marrow Mononuclear Cell Transplantation for Stroke: Phase1/2a Clinical Trial in a Homogeneous Group of Stroke Patients. Stem Cells Dev. 2015;24(19):2207–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohshima M, Taguchi A, Tsuda H, et al. Intraperitoneal and intravenous deliveries are not comparable in terms of drug efficacy and cell distribution in neonatal mice with hypoxia-ischemia. Brain Dev. 2015;37(4):376–386.**, of considerable interest: This study demonstrates that the administration route influences the effects of drugs and cell distribution.
- 126.Lioutas VA, Alfaro-Martinez F, Bedoya F, et al. Intranasal Insulin and Insulin-Like Growth Factor 1 as Neuroprotectants in Acute Ischemic Stroke. Transl Stroke Res. 2015;6(4):264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Danielyan L, Schafer R, von Ameln-Mayerhofer A, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88(6):315–324. [DOI] [PubMed] [Google Scholar]
- 128.Wei N, Yu SP, Gu X, et al. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013;22(6):977–991. [DOI] [PubMed] [Google Scholar]
- 129.Wei ZZ, Gu X, Ferdinand A, et al. Intranasal delivery of bone marrow mesenchymal stem cells improved neurovascular regeneration and rescued neuropsychiatric deficits after neonatal stroke in rats. Cell Transplant. 2015;24(3):391–402. [DOI] [PubMed] [Google Scholar]
- 130.van Velthoven CT, Sheldon RA, Kavelaars A, et al. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke. 2013;44(5):1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao Q, Hu J, Xiang J, et al. Intranasal administration of human umbilical cord mesenchymal stem cells-conditioned medium enhances vascular remodeling after stroke. Brain Res. 2015;1624:489–496. [DOI] [PubMed] [Google Scholar]
- 132.Pollock K, Stroemer P, Patel S, et al. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol. 2006;199(1):143–155. [DOI] [PubMed] [Google Scholar]
- 133.Marquardt LM, Heilshorn SC. Design of Injectable Materials to Improve Stem Cell Transplantation. Curr Stem Cell Rep. 2016;2(3):207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takagi T, Yoshimura S, Sakuma R, et al. Novel Regenerative Therapies Based on Regionally Induced Multipotent Stem Cells in Post-Stroke Brains: Their Origin, Characterization, and Perspective. Transl Stroke Res. 2017;8(6):515–528. [DOI] [PubMed] [Google Scholar]