Abstract
Background
A comprehensive understanding of the pre-existing genetic variation in genes associated with antibiotic resistance in the Mycobacterium tuberculosis complex (MTBC) is needed to accurately interpret whole-genome sequencing data for genotypic drug susceptibility testing (DST).
Methods
We investigated mutations in 92 genes implicated in resistance to 21 anti-tuberculosis drugs using the genomes of 405 phylogenetically diverse MTBC strains. The role of phylogenetically informative mutations was assessed by routine phenotypic DST data for the first-line drugs isoniazid, rifampicin, ethambutol, and pyrazinamide from a separate collection of over 7000 clinical strains. Selected mutations/strains were further investigated by minimum inhibitory concentration (MIC) testing.
Results
Out of 547 phylogenetically informative mutations identified, 138 were classified as not correlating with resistance to first-line drugs. MIC testing did not reveal a discernible impact of a Rv1979c deletion shared by M. africanum lineage 5 strains on resistance to clofazimine. Finally, we found molecular evidence that some MTBC subgroups may be hyper-susceptible to bedaquiline and clofazimine by different loss-of-function mutations affecting a drug efflux pump subunit (MmpL5).
Conclusions
Our findings underline that the genetic diversity in MTBC has to be studied more systematically to inform the design of clinical trials and to define sound epidemiologic cut-off values (ECOFFs) for new and repurposed anti-tuberculosis drugs. In that regard, our comprehensive variant catalogue provides a solid basis for the interpretation of mutations in genotypic as well as in phenotypic DST assays.
Keywords: Mycobacterium tuberculosis, Drug resistance, Benign mutations, Intrinsic resistance
Background
Drug-resistant Mycobacterium tuberculosis complex (MTBC) strains are estimated to account for one third of all deaths due to antimicrobial resistance globally [1]. Owing to the inherently slow growth rate of MTBC, the only realistic way to diagnose the majority of drug-resistant cases is to use rapid genotypic drug-susceptibility testing (gDST), which ranges from targeted assays to whole-genome sequencing (WGS) [2]. In fact, it is becoming increasingly clear that gDST assays are better suited than phenotypic DST (pDST) to rule-in resistance caused by known mechanisms that only confer modest minimum inhibitory concentration (MIC) increases, such as for ethambutol (EMB) [3–5].
The accuracy of gDST depends on the ability to distinguish valid markers for resistance (i.e. mutations that directly confer resistance or, alternatively, play a compensatory role in resistance) from neutral mutations that do not alter the susceptibility to an antibiotic [6, 7]. In this context, one of the major confounders is the pre-existing variation in genes associated with resistance, which comprises neutral mutations and, more rarely, changes that confer intrinsic/natural resistance (i.e. resistance that arose by chance/genetic drift prior to the clinical use of a drug or a related agent with a shared resistance mechanism) [8]. Because MTBC displays a strictly clonal population structure without any lateral gene transfer, these mutations are typically phylogenetically informative and unique (i.e. they are markers for a particular subgroup of the global MTBC diversity). Consequently, they form a barcode that is exploited by some targeted gDST assays to provide an epidemiological typing result at no additional cost, albeit at a limited resolution compared with WGS [9–11]. By contrast, homoplastic mutations have arisen multiple times independently in the MTBC phylogeny and are, consequently, not markers for a single subgroup. If this diversity is not considered at the design stage of a gDST assay, they can result in systematic false-resistant results. Indeed, the World Health Organization (WHO) has just revised the reporting language for line probe assays to reflect this possibility (e.g. gyrA A90G causes false-resistance reports for fluoroquinolones with the Hain GenoType MTBDRsl assay) [12, 13].
The purpose of this study was, therefore, to catalogue phylogenetically informative mutations in 92 genes implicated in the resistance to a total of 21 antibiotics and, where possible, to identify neutral mutations amongst these changes by taking evolutionary information and pDST data into consideration. Moreover, we searched for evidence of previously unknown intrinsic resistance.
Methods
Strain collection
We analysed 405 phylogenetically diverse MTBC genomes, of which 214 were drawn from Comas et al. who studied the evolutionary history of MTBC using isolates from 46 countries [14]. This collection was supplemented with mostly pan-susceptible strains from the Research Center Borstel (n = 69) [10] and the Karolinska University Hospital in Sweden (n = 122) [4].
WGS
WGS at the Research Center Borstel was performed with Illumina Technology (MiSeq, NextSeq 500, HiSeq 2500) using Nextera XT library preparation kits as instructed by the manufacturer (Illumina, San Diego, CA, USA). Fastq files (raw sequencing data) for all strains analysed in this study are available from the European Nucleotide Archive, and details can be found within Additional file 1: Table S1. All genomes were analysed with the MTBseq pipeline [15]. First, reads were mapped to the M. tuberculosis H37Rv genome (GenBank ID: NC_000962.3) with BWA [16]. Alignments were then refined with the GATK [17] and Samtools [18] toolkits for base quality recalibration and alignment corrections for possible PCR and InDel artefact. Variants (SNPs and InDels) were called if the following criteria were met: a minimum coverage of four reads in both forward and reverse orientation, four reads calling the allele with at least a phred score of 20, and an allele frequency of 75%. Deletions in Rv1979c were identified manually as the above algorithms are not optimised to call large InDels.
Phylogenetic analysis
Regions annotated as repetitive elements (e.g. PPE and PE-PGRS gene families), InDels, multiple consecutive SNPs in a 12-bp window (possible InDel artefacts or rare recombination scars), and 92 genes implicated in antibiotic resistance (Additional file 2: Table S2) were excluded for the phylogenetic reconstruction. In the combined analysis, we considered all genome positions that fulfilled the aforementioned criteria for coverage and variant frequency in 95% of all samples in the datasets as valid and used the concatenated sequence alignment to calculate a maximum likelihood tree with Fast Tree [19], employing a GTR substitution model, 1000 resamples, and Gamma20 likelihood optimisation to account for rate heterogeneity amongst sites. The consensus tree was rooted with the “midpoint root” option in FigTree [20], and nodes were arranged in increasing order. MTBC strains were stratified into lineages and subgroups using the classification schemes by Coll et al. and/or Merker et al. [9, 21].
In the ML tree, we identified internal nodes/branches with very good statistical support (bootstrap values ≥ 0.9). Strains derived from one shared internal branch (i.e. most common recent ancestor) were assigned to groups, and group-specific mutations were extracted considering sequences of 92 genes implicated in antibiotic resistance (Additional file 2: Table S2).
Phylogenetic (branch-specific) mutations were further classified using pDST data (mainly MGIT 960) for the first-line drugs rifampicin (RIF), isoniazid (INH), EMB, and pyrazimanide (PZA) from the CRyPTIC consortium [7]. A mutation was regarded as likely neutral if > 90% of strains that did not harbour known resistance mutations were phenotypically susceptible, provided that pDST results were available for at least 10 strains.
MIC measurements
MIC values for INH (Sigma-Aldrich, Germany), prothionamide (PTO; Riemser, Germany), bedaquiline (BDQ; Janssen, USA), and clofazimine (CFZ; Sigma-Aldrich, Germany) were determined in the BACTEC MGIT 960 system (Becton Dickinson) in conjunction with the EpiCenter TBeXiST software. The following drug concentrations were tested: 0.0125, 0.025, 0.05, 0.1, and 0.4 μg/ml for INH; 0.3125, 0.625, 1.25, 2.5, and 5 μg/ml for PTO; and 0.0625, 0.125, 0.25, 0.5, 1, and 2 μg/ml for BDQ and CFZ. A strain was interpreted as resistant to a drug at a particular concentration if the drug-containing tube reached ≥ 100 growth units before the drug-free control tube with the 1:100 diluted suspension of the strain reached 400 growth units.
Results
MTBC strain collection and phylogeny
Our collection (n = 405) featured 296 evolutionary “modern”, i.e. TbD1 region deleted [22], M. tuberculosis strains (i.e. lineages 2–4), and 109 evolutionary “ancestral” (TbD1 region intact) MTBC lineages, ranging from lineages 1 and 7 (M. tuberculosis, n = 60) and lineages 5 and 6 (M. africanum, n = 35) to 14 animal-adapted species (i.e. M. pinnipedii, M. microti, M. orygis, M. caprae, M. bovis, including one M. bovis BCG vaccine strain (Additional file 1: Table S1). Of these isolates, only 40 (9.9%) had a mutation in the RIF resistance determining mutation in rpoB and were, consequently, RIF resistant.
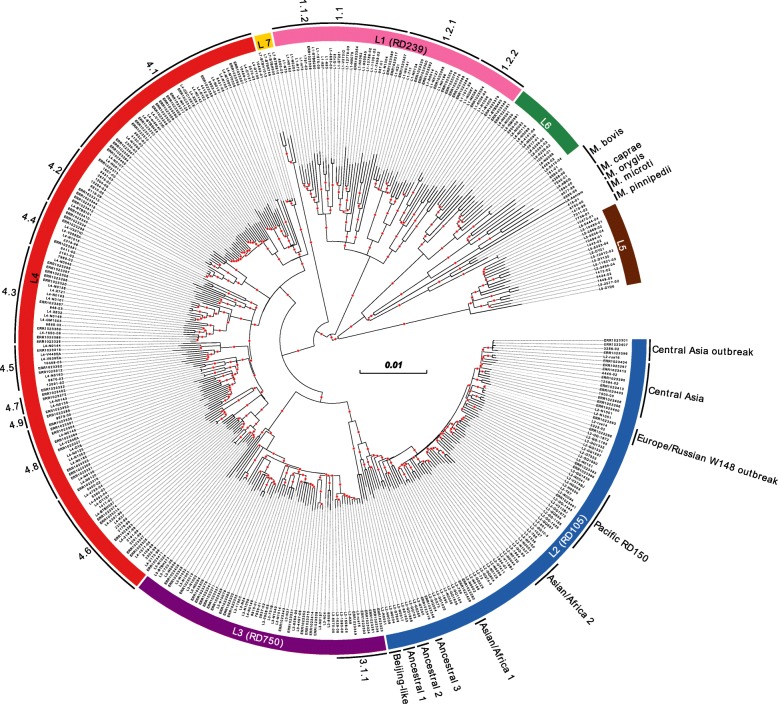
A maximum likelihood (ML) tree with a general time-reversible (GTR) substitution model and 1000 resamples was calculated based on 42,760 single nucleotide polymorphisms (SNPs), excluding mutations in repetitive regions and 92 genes implicated in antibiotic resistance (Additional file 2: Table S2). Next, we identified all internal nodes/branches with a bootstrap support of 0.9 and higher (n = 334). All isolates that shared a common node/branch with sufficient bootstrap support were grouped together, and group-specific changes, i.e. SNPs and insertions and deletions (InDels), in the aforementioned 92 genes were identified (Fig. 1).
Fig. 1.
MTBC phylogeny. ML phylogeny based on 42,760 SNPs from 405 genomes using a general time-reversible substitution model and 1000 resamples. Seven major MTBC lineages and animal-adapted species are highlighted. Where warranted, these were differentiated further into subgroups (as shown on the circumference of the figure). Red dots indicate branches (n = 334) with a resampling support of > 0.9 and which were investigated for branch-specific mutations in 92 genes implicated in antibiotic resistance
Phylogenetically informative and unique mutations: an overview
We found a total of 557 group-specific mutations in the 92 genes that had either been implicated in antibiotic resistance, are confirmed resistance mechanisms, or are involved in compensating for the reduced fitness of resistance mutations in other genes. Out of 557 mutations, we excluded 26 mutations as they were found in groups that only comprised strains with a pairwise distance of up to five SNPs, suggesting recent transmission. However, three H37Rv-specific mutations (4.9 subgroup) and the aforementioned gyrA A90G change (4.6.1.2 subgroup) were exempt from this rule given their phylogenetic importance [12]. This list of mutations was further supplemented with three known phylogenetic mutations (i.e. tlyA N236K in the 4.6.2 subgroup [23], cycA G122S in BCG, and mmaA3 G98D in a subgroup of BCG [8]) that confer intrinsic antibiotic resistance (Additional file 3: Table S3). These three mutations were not highlighted by our algorithm because they featured only in single strains in our collection. Where possible, we compared the resulting 539 mutations with the typing scheme by Coll et al., the most widely used method to stratify WGS data for MTBC [9]. Of the 89 SNPs that were in common, 82 results were in agreement. Subsequent personal communications with Francesc Coll revealed that the seven discrepancies were due to errors in his study and were eventually resolved (Additional file 3: Table S3).
Phylogenetically informative and unique mutations: impact on resistance
We then proceeded to identify mutations that were likely neutral (i.e. do not correlate with resistance) using previously published pDST data for RIF, INH, EMB, and PZA for over 7000 isolates [7], yielding 138 neutral mutations (Additional file 3: Table S3).
Notably, we identified five group-specific mutations that had been linked with drug resistance in the literature. This included the ndh R268H mutation, which had been proposed as an isoniazid resistance marker, and we classified it as a marker for a subgroup of lineage 1.1.2 [24]. Yet, our analysis of routine pDST data and additional MIC testing demonstrated that ndh R268H was likely neutral (Additional file 4: Tables S4). Moreover, we undertook MIC testing to investigate the roles of the ethA M1R, S266R, and G413D mutations, which had been previously associated with PTO and ethionamide (ETO) resistance (Additional file 4: Table S4) [25–27]. Of these mutations, only isolates with the ethA M1R mutation tested resistant to PTO, which was expected as this mutation should abolish the start codon of ethA. This particular mutation was shared by four isolates that formed a subgroup within lineage 4.2.2 (TUR genotype) with a median pairwise genetic distance of 22 SNPs and were at least resistant to INH. Consequently, it was unclear whether this mutation arose in response to the exposure to PTO/ETO or whether this represented an example of intrinsic resistance.
In addition, we measured the CFZ MICs for the Rv1979c V52G mutation, which yielded MICs in the susceptible range (Additional file 4: Table S4). This result, therefore, supported a recent study that found that this alteration, which was shared by a group of two Beijing isolates, probably does not confer CFZ resistance [28].
Our analysis supported the hypothesis that the entire MTBC branch that comprises both M. africanum lineages and the animal-adapted strains (M. africanum/animal branch) likely has intrinsically elevated MICs to cycloserine (DCS), owing to a 1-bp frameshift deletion in ald, which encodes alanine dehydrogenase (Additional file 3: Table S3) [5, 29]. In addition, we expect the DCS MIC to be raised further in M. microti and M. pinnipedii given that both species harbour a frameshift in cycA, which should impede DCS uptake, as is the case in all BCG variants due to a G122S mutation in the same gene [5, 29, 30].
In contrast to these cases, this study also raised the prospect that some subgroups may be more susceptible to particular antibiotics due to their specific genetic background. For example, we observed different loss-of-function (LOF) mutations in mmpL5 in two genetic backgrounds (in subgroups of lineages 1.1.1.1 and 4.6) that should render them hyper-susceptible to bedaquiline (BDQ) and CFZ [31]. Moreover, we observed LOF mutations in eis and its transcriptional activator whiB7, which might make the respective genotypes more susceptible to kanamycin (KAN) [4, 32]. Finally, we confirmed that most lineage 2 strains share a frameshift in the tap efflux pump, which means that mutations that result in the overexpression of whiB7 cannot confer streptomycin (STR) resistance in these strains [33].
Convergent evolution: impact on resistance
We observed 27 changes that had evolved independently in multiple genetic backgrounds and, consequently, were not markers for only one phylogenetic group (Additional file 5: Table S5), which is typically a sign of positive selection [34]. Indeed, several classical resistance mutations featured in this category, which were likely selected in response to antibiotic treatment (e.g. rpoB S450L and rpsL K43R). Another well-understood mutation affected codon 220 of pykA. A glutamic acid to aspartic acid change, which results in the inability to grow on glycerol as the sole carbon source, occurred at the base of the M. africanum/animal branch [35]. It has already been reported that some strains within this group (i.e. M. suricattae and all BCG variants) independently regained the ability to grow on glycerol by reverting back to glutamic acid [29]. Here, we found that this occurred on two more occasions, i.e. in one lineage 6 strain (L6-N0060) and in our variant of M. bovis ATCC 19210 (9564-00) [36].
As previously reported, Rv1979c was deleted independently in all M. africanum lineage 5 strains (del Rv1978-Rv1979c [37]), in our two M. pinnipedii strains (del Rv1964-Rv1979c [38]), and also in more recently derived BCG variants (Rv1964-Rv1988, i.e. RD2) [39, 40]. Given that mutations in Rv1979c are implicated in CFZ resistance, this raised the possibility that intrinsic resistance to CFZ might have arisen on at least three occasions in MTBC [5, 28, 41]. However, testing of five M. africanum lineage 5 strains did not reveal a discernible increase of the CFZ MIC (Additional file 4: Table S4).
There was at least one example where convergent evolution was misleading. The ancestral nucleotide at position -32 upstream of ald is adenine whereas glycine was acquired independently by M. africanum lineage 5 and a group of lineage 4 strains (i.e. 4.5, 4.6, 4.7, 4.8, and 4.9). Yet, because ald is inactive in lineage 5, as mentioned above, this mutation cannot have the same effect in both genetic backgrounds.
Discussion
Compared with most bacterial pathogens, MTBC is monomorphic [42]. Nevertheless, it has been known for more than 60 years that this limited diversity can result in intrinsic resistance [43]. Indeed, if resistance to an antibiotic can arise by LOF mutations that do not have major adverse consequences for bacterial fitness, it is not a question of whether intrinsic resistance exists but, rather, how widespread this phenotype is. This, in turn, is a function of how deeply rooted this phenotype is in the phylogenetic tree of MTBC and how well these strains have subsequently transmitted. For example, the pncA H57D mutation, which is estimated to have evolved approximately 900 years ago [44], is shared by the vast majority of M. bovis strains and consequently renders them intrinsically resistant to PZA [45]. Yet, owing to the control policies introduced in well-resourced countries over the past century, M. bovis is responsible for fewer than 3% of human tuberculosis (TB) cases globally [46].
The remaining experimentally confirmed cases of intrinsic resistance have arisen more recently in MTBC and, therefore, are less frequent. These include the intrinsic capreomycin resistance of some lineage 4.6.2 strains (Cameroon genotype) due to the tlyA N236K mutation [23], the high-level DCS resistance shared by all BCG variants [29], and the intrinsic resistance to INH and ETH/PRO of BCG variants derived after 1926 as a result of mmaA3 G98D [39]. Finally, M. canettii, which is not strictly speaking part of MTBC, is intrinsically resistant to PZA and pretomanid [45, 47, 48].
Yet, the molecular evidence from this study underscores that even more deeply rooted and thus older instances of intrinsic resistance have either not been studied sufficiently or may have been missed completely. The possibility that the entire M. africanum/animal branch likely has intrinsically elevated MICs to DCS is particularly concerning in light of the severe toxicity of DCS and terizidone [5, 29]. More MIC data are urgently needed to inform pharmacokinetic/pharmacodynamic (PK/PD) modelling to set a clinical breakpoint for DCS and to assess whether this increase is clinically relevant [49].
Conversely, genetic diversity may also confer hyper-susceptibility, which has not been studied systematically in the TB field as MICs are typically truncated at the lower end, i.e. sufficiently low concentrations are not typically tested to define the lower end of “susceptible” MIC distributions [5].
MIC testing of M. africanum lineage 5 strains did not confirm the role of the deletion of Rv1979c in CFZ resistance, as previously hypothesised [5]. This apparent contradiction might be explained if only specific gain-of-function mutations as opposed to LOF mutations in this gene, which includes a possible permease, confer resistance [28, 41]. Knockout and complementation experiments are currently ongoing to investigate this question further.
Finally, epistatic interactions may affect the way in which mutations are interpreted, as illustrated by the effect of whiB7 promoter-up mutations, which confer cross-resistance to KAN and STR [32]. However, this is only the case in genetic backgrounds in which both eis and tap are functional, which is not always the case (e.g. in almost the entire lineage 2) [4, 33].
The number of open questions raised by our study is symptomatic of the lack of rigour used to define breakpoints to anti-TB drugs [3, 5, 50, 51]. The recent endorsement of an MIC reference method by the European Committee for Antimicrobial Susceptibility Testing (EUCAST) and associated guidelines to calibrate other methods, such as the widely used MGIT 960 system, are designed to address these shortcomings [52]. Indeed, these guidelines stipulate that representatives of lineages 1–7 must be tested to define sound epidemiologic cut-off values (ECOFFs). It would be in the interest of pharmaceutical companies to follow the EUCAST guidelines as early as possible during drug development to identify agents that may not be equally effective against major MTBC genotypes [53]. These antibiotics could either be abandoned or their development adjusted to gather evidence that genotypes with intrinsically elevated MICs are treatable at either standard or increased dosing (e.g. using nonclinical models or by choosing clinical trial sites in countries where these genotypes are sufficiently frequent to provide enough statistical power to study these questions comprehensively [54–56]).
Conclusion
We provide a comprehensive catalogue of phylogenetically informative mutations in genes implicated in drug resistance in MTBC. Our analysis underlines that despite being monomorphic, the genetic diversity in MTBC has to be studied systematically to inform the interpretation of gDST results for existing drugs as well as the development of urgently needed novel agents.
Supplementary information
Additional file 1: Table S1. Metadata for 405 MTBC isolates/datasets used in this study.
Additional file 2: Table S2. 92 genes (coding and upstream regions) implicated in antibiotic resistance.
Additional file 3: Table S3. Catalogue of 547 phylogenetically informative mutations in 92 genes implicated in drug resistance to 21 anti-TB drugs.
Additional file 4: Table S4. MIC results for isolates with ndh R268H, ethA M1R, ethA S266R, ethA G413D, or Rv1979c V52G mutations, or a Rv1978-Rv1979c deletion.
Additional file 5: Table S5. Overview of homoplasic mutations.
Acknowledgements
TMW was an NIHR Academic Clinical Lecturer and is now a Wellcome Trust Clinical Career Development Fellow (214560/Z/18/Z). TS is supported by the Swedish Heart and Lung Foundation (Oscar II Jubilee Foundation) and the Swedish Research Council. SJP is supported by the Health Innovation Challenge Fund (WT098600, HICF-T5-342), a parallel funding partnership between the Department of Health and Wellcome Trust. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health or Wellcome Trust. FPM was supported by a research grant by Joachim Herz Foundation (Biomedical Physics of Infection Consortium).
We thank Vanessa Mohr, Fenja Boysen, and Tanja Ubben (all Research Center Borstel) for their technical assistance.
Authors’ contributions
MM and SN conceived the project. MM, TAK, IB, and TW analysed the genomic data. TAK, PF, DM, JP, SJP, TS, and TW contributed to the construction of the data. SA, EC, KÄ, PJ, TS, and FM generated the phenotypic drug susceptibility test results. SN supervised the research. CK and TS assisted in the assessment of resistance-mediating mutations and contributed intellectual insights and guidance. MM, TW, CK, and SN wrote the paper. The authors read and approved the final manuscript.
Funding
Parts of the work were funded by the Leibniz Science Campus “Evolutionary Medicine of the Lung” (EvoLung) and the German Center for Infection Research (DZIF).
Availability of data and materials
Fastq files (raw sequencing data) for all strains analysed in this study are available from the European Nucleotide Archive, and details can be found within Additional file 1: Table S1.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
CUK is a consultant for the WHO Regional Office for Europe, Becton Dickinson, and QuantuMDx Group Ltd. CUK is an unpaid advisor to GenoScreen and consulted for the Foundation for Innovative New Diagnostics, which involved work for Cepheid Inc., Hain Lifescience, and WHO. The Bill & Melinda Gates Foundation and Hain Lifescience covered CUK’s travel and accommodation to present at meetings. The Global Alliance for TB Drug Development Inc. and Otsuka Novel Products GmbH have supplied CUK with antibiotics for in vitro research. YD Diagnostics has provided CUK with assays for an evaluation. JP is a paid consultant to Next Gen Diagnostics. All remaining authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13073-020-00726-5.
References
- 1.Stop TB Partnership | G20 Leaders Elevate TB challenge to Heads of State Level [Internet]. [cited 2017 Nov 24]. Available from: http://www.stoptb.org/news/stories/2017/ns17_044.asp.
- 2.Dheda K, Gumbo T, Maartens G, Dooley KE, Murray M, Furin J, et al. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med. 2019;7:820–826. doi: 10.1016/S2213-2600(19)30263-2. [DOI] [PubMed] [Google Scholar]
- 3.Schön T, Miotto P, Köser CU, Viveiros M, Böttger E, Cambau E. Mycobacterium tuberculosis drug-resistance testing: challenges, recent developments and perspectives. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2017;23:154–160. doi: 10.1016/j.cmi.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Heyckendorf J, Andres S, Köser CU, Olaru ID, Schön T, Sturegård E, et al. What is resistance? Impact of phenotypic versus molecular drug resistance testing on multi- and extensively drug-resistant tuberculosis therapy. Antimicrob Agents Chemother. 2018;62(2). [DOI] [PMC free article] [PubMed]
- 5.World Health Organization. (2018). Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. World Health Organization. https://apps.who.int/iris/handle/10665/260470. Lizenz: CC BY-NC-SA 3.0 IGO.
- 6.Miotto Paolo, Tessema Belay, Tagliani Elisa, Chindelevitch Leonid, Starks Angela M., Emerson Claudia, Hanna Debra, Kim Peter S., Liwski Richard, Zignol Matteo, Gilpin Christopher, Niemann Stefan, Denkinger Claudia M., Fleming Joy, Warren Robin M., Crook Derrick, Posey James, Gagneux Sebastien, Hoffner Sven, Rodrigues Camilla, Comas Iñaki, Engelthaler David M., Murray Megan, Alland David, Rigouts Leen, Lange Christoph, Dheda Keertan, Hasan Rumina, Ranganathan Uma Devi K., McNerney Ruth, Ezewudo Matthew, Cirillo Daniela M., Schito Marco, Köser Claudio U., Rodwell Timothy C. A standardised method for interpreting the association between mutations and phenotypic drug resistance inMycobacterium tuberculosis. European Respiratory Journal. 2017;50(6):1701354. doi: 10.1183/13993003.01354-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CRyPTIC Consortium and the 100,000 Genomes Project Prediction of susceptibility to first-line tuberculosis drugs by DNA sequencing. N Engl J Med. 2018;379:1403–1415. doi: 10.1056/NEJMoa1800474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Köser Claudio U., Feuerriegel Silke, Summers David K., Archer John A. C., Niemann Stefan. Importance of the Genetic Diversity within the Mycobacterium tuberculosis Complex for the Development of Novel Antibiotics and Diagnostic Tests of Drug Resistance. Antimicrobial Agents and Chemotherapy. 2012;56(12):6080–6087. doi: 10.1128/AAC.01641-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coll F, McNerney R, Guerra-Assunção JA, Glynn JR, Perdigão J, Viveiros M, et al. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat Commun. 2014;5:4812. doi: 10.1038/ncomms5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuerriegel S, Köser CU, Niemann S. Phylogenetic polymorphisms in antibiotic resistance genes of the Mycobacterium tuberculosis complex. J Antimicrob Chemother. 2014;69:1205–1210. doi: 10.1093/jac/dkt535. [DOI] [PubMed] [Google Scholar]
- 11.El Achkar S, Demanche C, Osman M, Rafei R, Ismail MB, Yaacoub H, et al. Drug-resistant tuberculosis, Lebanon, 2016–2017. Emerg Infect Dis 2019;25:564–568. [DOI] [PMC free article] [PubMed]
- 12.Ajileye A, Alvarez N, Merker M, Walker TM, Akter S, Brown K, et al. Some synonymous and nonsynonymous gyrA mutations in Mycobacterium tuberculosis lead to systematic false-positive fluoroquinolone resistance results with the Hain GenoType MTBDRsl assays. Antimicrob Agents Chemother. 2017;61(4). [DOI] [PMC free article] [PubMed]
- 13.Global Laboratory Initiative. Line probe assays for drug-resistant tuberculosis detection. Interpretation and reporting guide for laboratory staff and clinicians. http://www.stoptb.org/wg/gli/assets/documents/LPA_test_web_ready.pdf (Accessed 2.11.2018).
- 14.Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet. 2013;45:1176–1182. doi: 10.1038/ng.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohl TA, Utpatel C, Schleusener V, De Filippo MR, Beckert P, Cirillo DM, et al. MTBseq: a comprehensive pipeline for whole genome sequence analysis of Mycobacterium tuberculosis complex isolates. PeerJ. 2018;6:e5895. doi: 10.7717/peerj.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinforma Oxf Engl. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinforma Oxf Engl. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price MN, Dehal PS, Arkin AP. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FigTree [Internet]. [cited 2013 Oct 23]. Available from: http://tree.bio.ed.ac.uk/software/figtree/.
- 21.Merker M, Blin C, Mona S, Duforet-Frebourg N, Lecher S, Willery E, et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat Genet. 2015;47:242–249. doi: 10.1038/ng.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, Eiglmeier K, et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A. 2002;99:3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker TM, Merker M, Knoblauch AM, Helbling P, Schoch OD, van der Werf MJ, et al. A cluster of multidrug-resistant Mycobacterium tuberculosis among patients arriving in Europe from the Horn of Africa: a molecular epidemiological study. Lancet Infect Dis. 2018.
- 24.Lee AS, Teo AS, Wong SY. Novel mutations in ndh in isoniazid-resistant Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother. 2001;45:2157–2159. doi: 10.1128/AAC.45.7.2157-2159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brossier F, Veziris N, Truffot-Pernot C, Jarlier V, Sougakoff W. Molecular investigation of resistance to the antituberculous drug ethionamide in multidrug-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2011;55:355–360. doi: 10.1128/AAC.01030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado D, Perdigão J, Ramos J, Couto I, Portugal I, Ritter C, et al. High-level resistance to isoniazid and ethionamide in multidrug-resistant Mycobacterium tuberculosis of the Lisboa family is associated with inhA double mutations. J Antimicrob Chemother. 2013;68:1728–1732. doi: 10.1093/jac/dkt090. [DOI] [PubMed] [Google Scholar]
- 27.Morlock GP, Metchock B, Sikes D, Crawford JT, Cooksey RC. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother. 2003;47:3799–3805. doi: 10.1128/AAC.47.12.3799-3805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Wang B, Hu M, Huo F, Guo S, Jing W, et al. Primary clofazimine and bedaquiline resistance among isolates from patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2017;61(6). [DOI] [PMC free article] [PubMed]
- 29.Desjardins CA, Cohen KA, Munsamy V, Abeel T, Maharaj K, Walker BJ, et al. Genomic and functional analyses of Mycobacterium tuberculosis strains implicate ald in D-cycloserine resistance. Nat Genet. 2016;48:544–551. doi: 10.1038/ng.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JM, Uplekar S, Gordon SV, Cole ST. A point mutation in cycA partially contributes to the D-cycloserine resistance trait of Mycobacterium bovis BCG vaccine strains. PLoS One. 2012;7:e43467. doi: 10.1371/journal.pone.0043467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, et al. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One. 2014;9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeves AZ, Campbell PJ, Sultana R, Malik S, Murray M, Plikaytis BB, et al. Aminoglycoside cross-resistance in Mycobacterium tuberculosis due to mutations in the 5′ untranslated region of whiB7. Antimicrob Agents Chemother. 2013;57:1857–1865. doi: 10.1128/AAC.02191-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Köser CU, Bryant JM, Parkhill J, Peacock SJ. Consequences of whiB7 (Rv3197A) mutations in Beijing genotype isolates of the Mycobacterium tuberculosis complex. Antimicrob Agents Chemother. 2013;57:3461. doi: 10.1128/AAC.00626-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakatani Y, Opel-Reading HK, Merker M, Machado D, Andres S, Kumar SS, et al. Role of alanine racemase mutations in Mycobacterium tuberculosis D-cycloserine resistance. Antimicrob Agents Chemother. 2017;61(12). [DOI] [PMC free article] [PubMed]
- 35.Keating LA, Wheeler PR, Mansoor H, Inwald JK, Dale J, Hewinson RG, et al. The pyruvate requirement of some members of the Mycobacterium tuberculosis complex is due to an inactive pyruvate kinase: implications for in vivo growth. Mol Microbiol. 2005;56:163–174. doi: 10.1111/j.1365-2958.2005.04524.x. [DOI] [PubMed] [Google Scholar]
- 36.Riojas MA, McGough KJ, Rider-Riojas CJ, Rastogi N, Hazbón MH. Phylogenomic analysis of the species of the Mycobacterium tuberculosis complex demonstrates that Mycobacterium africanum, Mycobacterium bovis, Mycobacterium caprae, Mycobacterium microti and Mycobacterium pinnipedii are later heterotypic synonyms of Mycobacterium tuberculosis. Int J Syst Evol Microbiol. 2018;68:324–332. doi: 10.1099/ijsem.0.002507. [DOI] [PubMed] [Google Scholar]
- 37.Ates LS, Dippenaar A, Sayes F, Pawlik A, Bouchier C, Ma L, et al. Unexpected genomic and phenotypic diversity of Mycobacterium africanum lineage 5 affects drug resistance, protein secretion, and immunogenicity. Genome Biol Evol. 2018;10:1858–1874. doi: 10.1093/gbe/evy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marmiesse M, Brodin P, Buchrieser C, Gutierrez C, Simoes N, Vincent V, et al. Macro-array and bioinformatic analyses reveal mycobacterial “core” genes, variation in the ESAT-6 gene family and new phylogenetic markers for the Mycobacterium tuberculosis complex. Microbiol Read Engl. 2004;150:483–496. doi: 10.1099/mic.0.26662-0. [DOI] [PubMed] [Google Scholar]
- 39.Abdallah AM, Hill-Cawthorne GA, Otto TD, Coll F, Guerra-Assunção JA, Gao G, et al. Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations. Sci Rep. 2015;5:15443. doi: 10.1038/srep15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yew WW, Liang D, Chan DP, Shi W, Zhang Y. Molecular mechanisms of clofazimine resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2017;72:2943–2944. doi: 10.1093/jac/dkx227. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Chen J, Cui P, Shi W, Zhang W, Zhang Y. Identification of novel mutations associated with clofazimine resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2015;70:2507–2510. doi: 10.1093/jac/dkv150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Achtman M. Evolution, population structure, and phylogeography of genetically monomorphic bacterial pathogens. Annu Rev Microbiol. 2008;62:53–70. doi: 10.1146/annurev.micro.62.081307.162832. [DOI] [PubMed] [Google Scholar]
- 43.McDERMOTT W, Tompsett R. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am Rev Tuberc. 1954;70:748–754. doi: 10.1164/art.1954.70.4.748. [DOI] [PubMed] [Google Scholar]
- 44.Loiseau C, Menardo F, Aseffa A, Hailu E, Gumi B, Ameni G, et al. An African origin for Mycobacterium bovis. Evol Med Public Health. [cited 2020 Feb 19]; Available from: https://academic.oup.com/emph/advance-article/doi/10.1093/emph/eoaa005/5719036. [DOI] [PMC free article] [PubMed]
- 45.Loiseau C, Brites D, Moser I, Coll F, Pourcel C, Robbe-Austerman S, et al. Revised interpretation of the Hain Lifescience GenoType MTBC to differentiate Mycobacterium canettii and members of the Mycobacterium tuberculosis complex. Antimicrob Agents Chemother. 2019;63(6). [DOI] [PMC free article] [PubMed]
- 46.Müller B, Dürr S, Alonso S, Hattendorf J, Laisse CJM, Parsons SDC, et al. Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg Infect Dis. 2013;19:899–908. doi: 10.3201/eid1906.120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feuerriegel S, Köser CU, Baù D, Rüsch-Gerdes S, Summers DK, Archer JAC, et al. Impact of Fgd1 and ddn diversity in Mycobacterium tuberculosis complex on in vitro susceptibility to PA-824. Antimicrob Agents Chemother. 2011;55:5718–5722. doi: 10.1128/AAC.05500-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feuerriegel S, Köser CU, Richter E, Niemann S. Mycobacterium canettii is intrinsically resistant to both pyrazinamide and pyrazinoic acid. J Antimicrob Chemother. 2013;68:1439–1440. doi: 10.1093/jac/dkt042. [DOI] [PubMed] [Google Scholar]
- 49.Deshpande D, Alffenaar J-WC, Köser CU, Dheda K, Chapagain ML, Simbar N, et al. D-Cycloserine pharmacokinetics/pharmacodynamics, susceptibility, and dosing implications in multidrug-resistant tuberculosis: a Faustian deal. Clin Infect Dis Off Publ Infect Dis Soc Am. 2018;67:S308–S316. doi: 10.1093/cid/ciy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Köser CU, Maurer FP, Kranzer K. “Those who cannot remember the past are condemned to repeat it”: drug-susceptibility testing for bedaquiline and delamanid. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2019;80S:S32–S35. doi: 10.1016/j.ijid.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 51.Schön T, Matuschek E, Mohamed S, Utukuri M, Heysell S, Alffenaar J-W, et al. Standards for MIC testing that apply to the majority of bacterial pathogens should also be enforced for Mycobacterium tuberculosis complex. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2019;25:403–405. doi: 10.1016/j.cmi.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.EUCAST . SOP for calibrating surrogate MIC methods for M. tuberculosisagainsttheEUCAST reference MIC method. 2019. [Google Scholar]
- 53.Köser CU, Javid B, Liddell K, Ellington MJ, Feuerriegel S, Niemann S, et al. Drug-resistance mechanisms and tuberculosis drugs. Lancet Lond Engl. 2015;385:305–307. doi: 10.1016/S0140-6736(14)62450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kahlmeter G. The 2014 Garrod Lecture: EUCAST - are we heading towards international agreement? J Antimicrob Chemother. 2015;70:2427–2439. doi: 10.1093/jac/dkv145. [DOI] [PubMed] [Google Scholar]
- 55.Kahlmeter G, Giske CG, Kirn TJ, Sharp SE. Point-Counterpoint: differences between the European Committee on Antimicrobial Susceptibility Testing and the Clinical Laboratory Standards Institute Recommendations for Reporting Antimicrobial Susceptibility Results. J Clin Microbiol. 2019;57(9). [DOI] [PMC free article] [PubMed]
- 56.Gumbo T, Lenaerts AJ, Hanna D, Romero K, Nuermberger E. Nonclinical models for antituberculosis drug development: a landscape analysis. J Infect Dis. 2015;211(Suppl 3):S83–S95. doi: 10.1093/infdis/jiv183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Metadata for 405 MTBC isolates/datasets used in this study.
Additional file 2: Table S2. 92 genes (coding and upstream regions) implicated in antibiotic resistance.
Additional file 3: Table S3. Catalogue of 547 phylogenetically informative mutations in 92 genes implicated in drug resistance to 21 anti-TB drugs.
Additional file 4: Table S4. MIC results for isolates with ndh R268H, ethA M1R, ethA S266R, ethA G413D, or Rv1979c V52G mutations, or a Rv1978-Rv1979c deletion.
Additional file 5: Table S5. Overview of homoplasic mutations.
Data Availability Statement
Fastq files (raw sequencing data) for all strains analysed in this study are available from the European Nucleotide Archive, and details can be found within Additional file 1: Table S1.