Significance
Follicular B cells generally require T cell help to contribute to adaptive immunity, producing several isotypes of immunoglobulins that carry out distinct effector functions. Two specialized B cell subsets, marginal zone (MZ) and B-1 B cells, arise from different developmental steps and have different functions. They become activated to produce antibodies without T cell help and are the major sources of plasma IgM. We identified a viable missense allele of Ncstn in a forward genetic screen. We discovered that the development of MZ and B-1 B cells, TI antibody response, fur pigmentation, and intestinal homeostasis critically depend on the glycosylation status of NCSTN and the catalytic activity of γ-secretase toward its substrate Notch, a critical receptor in numerous developmental decisions.
Keywords: nicastrin, marginal zone B cells, B-1 B cells, T cell-independent antibody response
Abstract
γ-secretase is an intramembrane protease complex that catalyzes the proteolytic cleavage of amyloid precursor protein and Notch. Impaired γ-secretase function is associated with the development of Alzheimer’s disease and familial acne inversa in humans. In a forward genetic screen of mice with N-ethyl-N-nitrosourea-induced mutations for defects in adaptive immunity, we identified animals within a single pedigree exhibiting both hypopigmentation of the fur and diminished T cell-independent (TI) antibody responses. The causative mutation was in Ncstn, an essential gene encoding the protein nicastrin (NCSTN), a member of the γ-secretase complex that functions to recruit substrates for proteolysis. The missense mutation severely limits the glycosylation of NCSTN to its mature form and impairs the integrity of the γ-secretase complex as well as its catalytic activity toward its substrate Notch, a critical regulator of B cell and T cell development. Strikingly, however, this missense mutation affects B cell development but not thymocyte or T cell development. The Ncstn allele uncovered in these studies reveals an essential requirement for NCSTN during the type 2 transitional-marginal zone precursor stage and peritoneal B-1 B cell development, the TI antibody response, fur pigmentation, and intestinal homeostasis in mice.
B cell responses to antigens are classified as T cell-dependent (TD) or T cell-independent (TI) based on their need for T cell help in antibody production. Antigens eliciting a TD antibody response are proteins that are processed and presented to helper T cells in the context of MHC II molecules. The TD antibody responses are mediated by follicular B cells (also known as B-2 cells, the major B cell subset in the body) and are long-lasting to deploy high-affinity antibodies of multiple isotypes. In contrast, TI antigens, such as bacterial capsular polysaccharides and viral capsids, stimulate antibody responses that do not require MHC II-restricted T cell help (1). The TI antibody response is mediated by the marginal zone (MZ) and B-1 B cell populations, which expand on immunization in extrafollicular sites (2–4) and confer protective immunity by producing antigen-specific IgM without somatic hypermutation (4–7). Thus, TI responses give rise to less specific but more immediate protection compared with TD antibody responses.
B-2 cells are continuously replenished from precursors in bone marrow, where they undergo both positive and negative selection. Immature B cells in bone marrow migrate to the spleen, where they differentiate through two transitional stages and become mature naïve B-2 cells (8) or, alternatively, MZ B cells. Their fate is determined during the transitional stages and depends on signals from the B cell receptor, B cell activating factor, nuclear factor κ light chain enhancer of activated B cells, and Notch2, as well as signals involved in anatomical retention of MZ B cells in the spleen (9). In contrast, B-1 cells are generated mainly from fetal liver progenitors rather than bone marrow precursors, reside in the peritoneal cavity, and are maintained by self-renewal throughout the life of the organism (10). It is well established that the spleen is also required for B-1 (especially B-1a) cell development (11); however, the underlying mechanism(s) that mediate B-1 cell differentiation remain largely unknown.
The γ-secretase protease complex cleaves multiple type I membrane proteins, including amyloid precursor protein (APP) and Notch. APP undergoes proteolytic processing by either α- or β-secretase to release soluble APP ectodomains into the extracellular space. Then γ-secretase cleaves the remaining membrane-anchored APP C-terminal fragments (APP-CTFs) and generates p3 (the byproduct of α- and γ-secretase cleavages) or amyloid β peptides (the byproduct of β- and/or γ-secretase cleavage) together with the APP intracellular domain (12). Notch plays essential roles in thymic T cell lineage commitment (13), as well as in specification of MZ B cell versus B-2 cell fate (14), and it undergoes a series of proteolytic cleavages by ADAM family metalloproteases and γ-secretase to generate the Notch intracellular domain (NICD) (15). The γ-secretase complex consists of four core subunits: presenilin (PS), PS enhancer 2 (PEN-2), anterior pharynx-defective 1 (APH-1), and nicastrin (16). Nicastrin is a type I membrane protein with a large extracellular domain (17) that functions as a γ-secretase substrate receptor (18). Activation of the γ-secretase complex requires extensive N-linked glycosylation of nicastrin, which helps stabilize the protein (19). Mutations in γ-secretase complex proteins and impaired catalytic activity of the complex have been implicated in Alzheimer’s disease (AD) (20), familial type acne inversa (21), hypopigmentation (22, 23), and thymic hypoplasia (24); however, little is known about the role and function of the γ-secretase complex in B cell-mediated immunity. Here we describe the effect of a severely hypomorphic but viable missense mutation of Ncstn on MZ B cell and B-1 B cell development.
Results
Identification of a Viable Ncstn Missense Mutation.
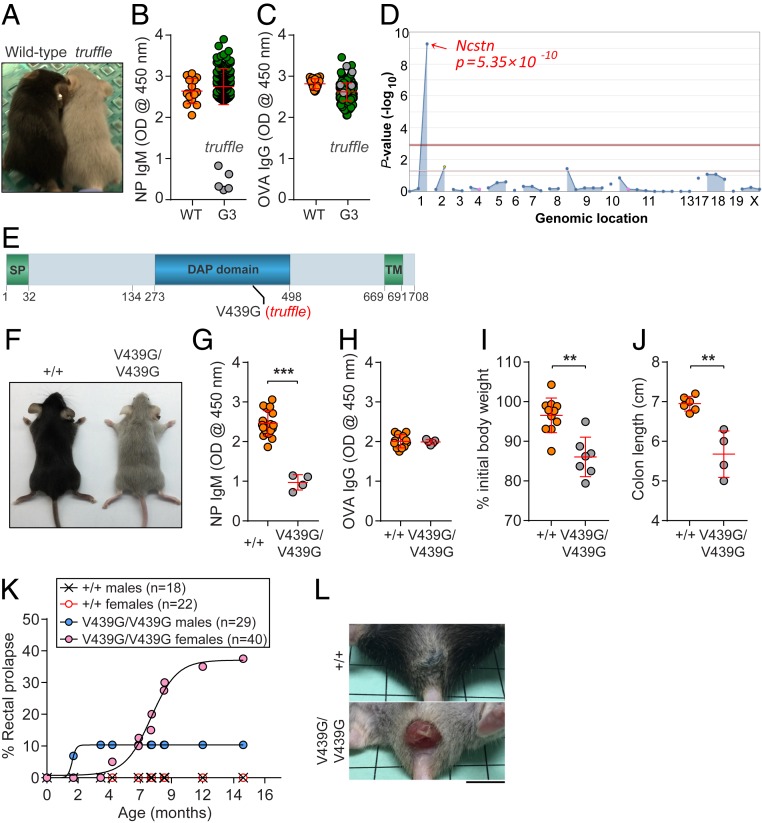
To identify genes required for the development and function of adaptive immunity, we carried out a forward genetic screen in third-generation (G3) C57BL/6J mice bred to carry homozygous and heterozygous mutations induced by N-ethyl-N-nitrosourea (ENU) in their great-grandsires (G0). During the course of adaptive immunity screens, we identified several mice from a single pedigree exhibiting hypopigmentation of the fur (Fig. 1A) and diminished TI antibody responses to (4-hydroxy-3-nitrophenyl) acetyl-Ficoll (NP-Ficoll) compared with wild-type C57BL/6J mice (Fig. 1B). The mice exhibited comparable TD antibody responses to aluminum hydroxide-precipitated ovalbumin (OVA/alum; Fig. 1C). The phenotype, named truffle, was transmitted as a recessive trait. By automated meiotic mapping (25), the truffle phenotype was correlated with a missense mutation in Ncstn (Fig. 1D), resulting in a valine (V)-to-glycine (G) substitution at position 439 (V439G) in the DYIGS and peptidase homologous (DAP) domain of the NCSTN protein (Fig. 1E) (18), which was predicted to be damaging by PolyPhen-2 (score = 1.000) (26).
Fig. 1.
The truffle phenotype. (A) Photograph of a male truffle mouse and wild-type littermate. (B) Decreased TI NP-specific antibodies in truffle mice (highlighted in gray dots) at 6 d after NP-Ficoll immunization. Data are from 247 G3 mice in a screen group that includes the truffle pedigree. Wild-type (C57BL/6J) mice immunized with the same antigen served as controls. Data are presented as absorbance at 450 nm. (C) Normal TD OVA-specific antibodies in truffle mice at 14 d after immunization with OVA/alum. (D) Manhattan plot. −Log10 P values (y-axis) plotted against the chromosomal positions of mutations (x-axis) identified in the affected pedigree. (E) Protein domains of mouse NCSTN (708-aa long). The location of the truffle mutation, which results in substitution of valine 439 to glycine (V439G) in Ncstn, is highlighted in red; SP, signal peptide; DAP, DYIGS and peptidase homologous; TM, transmembrane. (F) Photograph of a male mouse with CRISPR/Cas9-induced V439G substitution (NcstnV439G/V439G) and a wild-type littermate at age 8 wk. (G and H) TI (G) or TD (H) antibody responses in mice with the indicated genotypes for Ncstn following immunization with NP-Ficoll or OVA/alum, respectively. Data are presented as absorbance at 450 nm. (I and J) Weight loss (I) and colon length (J) analysis of mice with indicated genotypes on day 10 of treatment with 1.3% DSS. (K) Incidence of spontaneous rectal prolapse in wild-type and NcstnV439G/V439G mice. (L) Macroscopic view of rectal prolapse in NcstnV439G/V439G mice. Each symbol represents an individual mouse. P values were determined by Student’s t test. Results are representative of two (G and H) or three (I and J) independent experiments with 4 to 20 mice per genotype. Error bars indicate SD.
To verify causation, CRISPR/Cas9-mediated gene targeting was used to generate a single nucleotide replacement allele (A→C at chr1_172,070,009), causing the same amino acid change as that caused by the ENU-induced mutation (V439G). Consistent with truffle mice, NcstnV439G/V439G mice showed hypopigmentation of the fur (Fig. 1F) and diminished TI and normal TD antibody responses, respectively, to NP-Ficoll and OVA/alum immunization (Fig. 1 G and H) compared with wild-type littermates. These data conclusively established a causative relationship between the Ncstntruffle mutation and the observed phenotype in NcstnV439G/V439G mice.
Previous studies demonstrated the biological significance of γ-secretase protease-mediated Notch signaling in the maintenance of intestinal homeostasis and host defense functions in mice (27–29). To assess the effect of a viable Ncstn missense mutation on intestinal homeostasis, NcstnV439G/V439G mice and wild-type littermates were given 1.3% dextran sodium sulfate (DSS) in their drinking water for 7 d, and body weight was measured on days 0 and 10 after initiation of DSS treatment. The NcstnV439G/V439G mice developed severe colitis, losing 13.9% of their initial body weight by day 10 of treatment, compared with an average 3.5% of initial body weight lost by wild-type littermates (Fig. 1I). The NcstnV439G/V439G mice treated with DSS also displayed greater shortening of the colon compared with wild-type littermates (Fig. 1J). In the absence of DSS, NcstnV439G/V439G mice often developed rectal prolapse. A higher frequency of prolapse was observed among females compared with age-matched males (37.5% vs. 10.3%) (Fig. 1 K and L). Spontaneous rectal prolapse was very uncommon in wild-type mice (Fig. 1 K and L). These data demonstrate that hypopigmentation of fur, impaired TI antibody response, and diminished intestinal homeostasis all result from a viable Ncstn missense mutation in mice.
Impaired Immune Cell Development Caused by a Viable Ncstn Mutation in Mice.
To further characterize the immunologic defect caused by the Ncstn mutation, we immunophenotyped mice by complete blood count (CBC) testing and flow cytometry analysis of lymphoid and myeloid cells in blood, spleen, thymus, bone marrow, and peritoneum. Previous studies demonstrated that an inducible (Mx1-Cre) or a hematopoietic-specific (Vav-Cre) deletion of Ncstn caused thymic hypoplasia, an enlarged spleen, and aberrant accumulation of granulocyte-monocyte progenitors (GMPs), leukocytosis, and monocytosis (24). Unlike the phenotype observed in hematopoietic-specific NCSTN-deficient mice, gross examination of lymphoid organs showed that the NcstnV439G/V439G mice had no sign of thymic hypoplasia (Fig. 2A) or an enlarged splenic enlargement (Fig. 2B). We also examined the hematopoietic stem and progenitor cell (HSPC) populations in the bone marrow. The missense mutation in Ncstn did not appreciably affect the total HSPC population, including long-term-hematopoietic stem cells (HSCs), short-term HSCs, multipotent progenitors, common lymphoid progenitors, common myeloid progenitors, megakaryocyte–erythrocyte progenitors, or GMPs (Fig. 2C). Furthermore, CBC testing (Fig. 2D) and cell counts (Fig. 2E) showed that the NcstnV439G/V439G mice had comparable numbers of white blood cells, monocytes, neutrophils, and CD11b+ or CD11c+ myeloid cells compared with wild-type littermates. In addition, the development of thymocytes was not impaired in NcstnV439G/V439G mice compared with wild-type littermates (Fig. 2 F and G). No significant difference was found in CD3+ T cell number; however, NcstnV439G/V439G mice had slightly fewer CD8+ T cells in the spleen compared with wild-type littermates (Fig. 2H). Therefore, the CD4+-to-CD8+ T cell ratio was increased in NcstnV439G/V439G mice (Fig. 2I). Flow cytometry analysis of bone marrow showed that NcstnV439G/V439G mice had comparable numbers of B cell progenitors in the prepro-B stage, pro-B to pre-B transition, immature stage, and transitional B stage (Fig. 2 J–L). The frequency and numbers of mature recirculating B cells in the bone marrow appeared normal in NcstnV439G/V439G mice compared with wild-type littermates (Fig. 2 J and L); however, the surface IgD and IgM expression on mature recirculating B cells of NcstnV439G/V439G mice was decreased (Fig. 2 M and N). As a result, the mature recirculating B cell populations in the bone marrow of NcstnV439G/V439G mice has shifted to the lower left corner of the scatterplot compared with that of wild-type littermates (Fig. 2L). In the spleen, newly formed B cells that have migrated from the bone marrow undergo distinct transitional B cell stages (type 1 to 3 transitional B; T1 to T3) (9, 30). T2 B cells are direct precursors of follicular B cells, but a subset of these cells, termed T2 to MZ precursors (T2-MZP), are known as precursors of MZ B cells (31). MZ B cells and B-1 B cells, which predominate in the splenic MZ and peritoneal cavities, respectively, each contribute a massive wave of serum IgM production in response to immunization with TI antigens (4).
Fig. 2.
Impaired lymphocyte development caused by a Ncstn missense mutation in mice. (A and B) Representative photographs of thymus (A) and spleen (B). (C) Total numbers of stem and progenitor cell subsets per femur in the bone marrow of NcstnV439G/V439G mice and wild-type littermates as determined by flow cytometry. The FACS gating strategy for HSC subsets is described in detail in SI Appendix, Fig. S1. LSK+, Lineage-Sca-1+c-Kit+; LK+, lineage-Sca-1-c-Kit+. (D) Whole blood cell counts in 12-wk-old NcstnV439G/V439G mice and wild-type littermates. (E) Numbers of CD11b+ and CD11c+ myeloid cells in the spleen of 12-wk-old NcstnV439G/V439G mice and wild-type littermates. (F and G) Flow cytometry scatterplots of thymocytes (F) and total numbers of thymocyte subsets per thymus (G) isolated from 12-wk-old NcstnV439G/V439G mice and wild-type littermates. Numbers adjacent to outlined areas or in quadrants indicate the percentage of cells in each. Thymocytes were analyzed by flow cytometry for CD4, CD8, CD25, and CD44 surface markers. The FACS gating strategy for thymic T cell development is described in detail in SI Appendix, Fig. S2. (H and I) Numbers (H) and ratio (I) of splenic T cells in 12-wk-old NcstnV439G/V439G mice and wild-type littermates. (J) Numbers of B cell subsets in bone marrow, spleen, and peritoneal cavity of 12-wk-old NcstnV439G/V439G mice and wild-type littermates. (K, L, and O–T) Representative FACS plots showing B cell development in the bone marrow (K and L), spleen (O–R), and peritoneal cavity (S and T) from 12-wk-old NcstnV439G/V439G mice and wild-type littermates. The FACS gating strategy for B cell development is described in detail in SI Appendix, Fig. S3. (M and N) Surface IgD (M) and IgM (N) expression on peripheral blood B cells in 12-wk-old NcstnV439G/V439G mice and wild-type littermates. (U and V) Serum IgM (U) and IgG1 (V) concentration in unimmunized mice at 12 wk of age with indicated genotypes for Ncstn. Each symbol represents an individual mouse. P values were determined by Student’s t test. Data are representative of three independent experiments with 4 to 11 mice per genotype. Error bars indicate SD.
Importantly, a previous study demonstrated that conditional deletion of Notch2, which encodes one of the substrate proteins for the γ-secretase complex, results in complete arrest of MZ B cell development in mice (14). In contrast to Notch2, Notch1 has an indispensable function in early T cell development in mice (13). We observed a normal number of T1 and T2 transitional B cells in the spleens of NcstnV439G/V439G mice compared with wild-type littermates (Fig. 2J), but the development of T2-MZP cells (Fig. 2 J and P) and MZP-MZ B cells (Fig. 2 J, Q, and R) was significantly impaired. Furthermore, the frequency (Fig. 2S) and numbers (Fig. 2J) of peritoneal B-1 B cells, including the B-1a subpopulation (Fig. 2T), were significantly reduced in NcstnV439G/V439G mice compared with wild-type littermates. The proportion of the B-1b subpopulation in peritoneal B-1B cells was increased (Fig. 2T), but the total number was significantly reduced in NcstnV439G/V439G mice compared with wild-type littermates (Fig. 2J). Consequently, significantly decreased serum IgM levels (Fig. 2U), but normal IgG1 levels (Fig. 2V) were detected in NcstnV439G/V439G mice compared with wild-type littermates. Collectively, these data suggest that decreased peritoneal B-1 B cells and near-complete arrest of splenic MZ B cell development caused by a viable Ncstn mutation result in impaired TI antibody responses in NcstnV439G/V439G mice.
A Cell-Intrinsic Failure of Marginal Zone and B-1 B Cell Development.
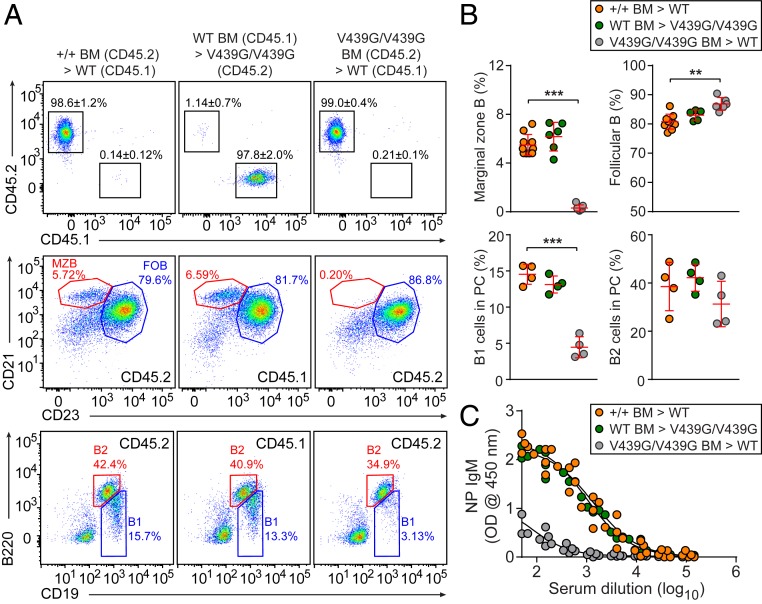
To determine the cellular origins of the Ncstn-associated defects in MZ B cell and B-1 B cell development, we reconstituted irradiated wild-type (CD45.1) or NcstnV439G/V439G mice (CD45.2) with unmixed Ncstn+/+ (CD45.2), NcstnV439G/V439G (CD45.2), or wild-type (CD45.1) bone marrow cells. The bone marrow cells from NcstnV439G/V439G donors were unable to repopulate MZ B cells and B-1 B cells in wild-type recipients (CD45.1) as efficiently as cells derived from wild-type donors (Fig. 3 A and B). In contrast, bone marrow cells from wild-type donors (CD45.1) were able to fully repopulate MZ B cells and B-1 B cells in NcstnV439G/V439G recipients (CD45.2) (Fig. 3 A and B). In addition, irradiated NcstnV439G/V439G recipients reconstituted with wild-type bone marrow mounted TI antibody responses comparable to those of irradiated wild-type recipients engrafted with wild-type bone marrow (Fig. 3C). However, irradiated wild-type recipients engrafted with NcstnV439G/V439G bone marrow showed significantly decreased TI antibody responses (Fig. 3C). This suggests a specific cell-autonomous effect of a viable Ncstn mutation on MZ B cell and B-1 B cell development and TI antibody responses. We note that the B-1 B cells examined here originated in the bone marrow (32) and might not necessarily reflect the phenotype of those originating from fetal liver.
Fig. 3.
A cell-intrinsic failure of MZ and B1 B cell development. (A) Repopulation of donor-derived lymphocytes in the peripheral blood (Top), MZ and follicular B cells in the spleen (Middle), and B1 cells in the peritoneal cavity (Bottom) of recipients at 10 wk after reconstitution of bone marrow isolated from mice with indicated genotypes. Numbers adjacent to outlined areas or in quadrants indicate percent cells in each population. (B) The frequencies of B cell subsets in the bone marrow chimeras as determined by flow cytometry. (C) TI antibody responses after immunization with NP-Ficoll in bone marrow chimeras at 10 wk after reconstitution. Data are presented as absorbance at 450 nm. Each symbol represents an individual mouse. P values were determined by one-way analysis of variance with Dunnett’s test for multiple comparisons. Data are representative of two independent experiments with four to nine mice per genotype. Error bars indicate SD.
Effect of a Viable Ncstn Mutation on Mature and Functional γ-Secretase Complex Formation.
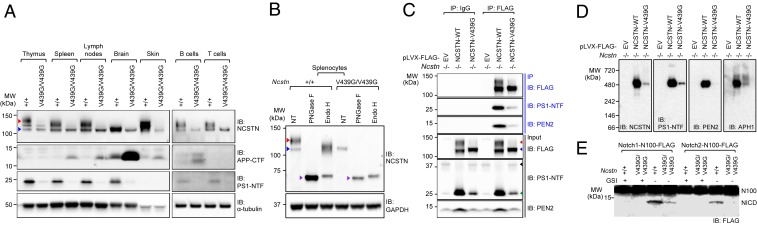
To investigate the effect of the NcstnV439G/V439G mutation at a molecular level, we isolated total cell lysates (TCLs) from thymus, spleen, lymph nodes, brain, skin, splenic pan T cells, and splenic pan B cells from NcstnV439G/V439G mice and wild-type littermates. TCLs were subjected to Western blot analysis to check expression levels of γ-secretase complex proteins. The predicted molecular mass of NCSTN is ∼80 kDa (17). In cells, NCSTN exists in higher (150 kDa) and intermediate (110 kDa) molecular weight forms due to glycosylation events (33). It has been shown that a functional γ-secretase complex has the 150-kDa glycosylated form of NCSTN (34). Immunoblot analysis revealed significant reduction in the quantity of the 150-kDa form of NCSTN in TCLs isolated from NcstnV439G/V439G mice compared with those from wild-type littermates (highlighted by the red arrowhead in Fig. 4A).
Fig. 4.
Effect of a Ncstn mutation in mature and functional γ-secretase complex formation. (A) Immunoblot analysis of NCSTN, APP-CTF, and PS1-NTF in TCLs of thymus, spleen, lymph nodes, brain, skin, splenic B220+, and CD3+ T cells from NcstnV439G/V439G mice and wild-type littermates. The red arrowhead indicates the mature form of NCSTN; blue arrowhead, the intermediate form of NCSTN. (B) Glycosidase treatments of NCSTN in splenocytes isolated from NcstnV439G/V439G mice or wild-type littermates. Endo H and PNGase F treatments show the sensitivity of the various bands to deglycosylation. The purple arrowhead indicates nonglycosylated NCSTN. (C) TCLs isolated from Ncstn−/− MEFs stably expressing FLAG-tagged wild-type, mutant (V439G) NCSTN, or empty vector (EV) were immunoprecipitated using anti-FLAG M2 agarose beads and immunoblotted with antibodies against FLAG, PS1, and PEN2. The red arrowhead indicates the mature form of NCSTN; blue arrowhead, the intermediate form of NCSTN; black arrowhead, full-length PS1; green arrowhead, PS1-NTF. (D) BN-PAGE analysis of γ-secretase subunits (nicastrin, PS1, PEN2, and APH1) from an equivalent quantity of total membrane proteins from Ncstn−/− MEFs stably expressing FLAG-tagged wild-type, mutant (V439G) NCSTN, or EV control. (E) γ-secretase activity was measured by mixing a FLAG-tagged mouse Notch1 or 2 substrate (N1- or N2-N100) with microsomal proteins isolated from NcstnV439G/V439G mice and wild-type littermates in the presence or absence of the γ-secretase inhibitor DAPT in vitro. N1-N100 and N2-N100 are membrane-tethered Notch fragments resulting from ectodomain shedding of Notch1 and 2, respectively. Both N1- and N2-N100 and their γ-secretase-cleaved product, NICD, were visualized by immunoblotting using an anti-FLAG antibody. Data are representative of three independent experiments.
To further characterize mutant NCSTN, we subjected TCLs to glycosidase analysis. Peptide:N-glycosidase F (PNGase F) treatment, which removes all N-linked oligosaccharides regardless of complexity, fully converted wild-type and V439G NCSTN into an ∼70-kDa nonglycosylated form (highlighted by purple arrowheads in Fig. 4B). This suggests that both wild-type and mutant NCSTN have N-linked glycosylation. While most N-linked glycans in mature NCSTN molecules are complex oligosaccharides, some are high-mannose residues. When TCLs were treated with endoglycosidase H (Endo H), which cleaves only high mannose residues, the 150- and 110-kDa forms of wild-type NCSTN downshifted to the 110-kDa and 70-kDa forms, respectively. However, Endo H treatment of mutant NCSTN downshifted the 110-kDa form to the 70-kDa form (Fig. 4B). This result suggests that unlike in wild-type NCSTN, most of the N-linked sugars in mutant NCSTN are high-mannose residues. Furthermore, TCLs from NcstnV439G/V439G mice contained an increased quantity of α- or β-secretase–cleaved APP CTF and a decreased quantity of the active PS1 species N-terminal fragment (PS1-NTF) compared with that in wild-type littermates (Fig. 4A).
Assembly of NCSTN with other γ-secretase components occurs in the ER and glycosylation occurs during transit through the Golgi apparatus (12). To assess the effect of the V439G substitution in these cellular processes, we generated Ncstn−/− cell lines stably expressing FLAG-tagged wild-type or mutant NCSTN. As expected, a significant reduction in the higher molecular weight form (150 kDa) of NCSTN was observed in TCLs extracted from Ncstn−/− cells expressing mutant NCSTN compared with cells expressing wild-type NCSTN (indicated by the red arrowhead in Fig. 4C). In addition, decreased levels of PEN2 and PS1 endoproteolytic fragments (PS1-NTF; indicated by the green arrowhead in Fig. 4C) were also observed in Ncstn−/− cells expressing mutant NCSTN compared with cells expressing wild-type NCSTN. Since the glycosylation status of NCSTN influences mature γ-secretase complex assembly (35), we determined the effect of the mutation in binding with other γ-secretase components. Coimmunoprecipitation assays revealed that mutant NCSTN, which exists predominantly in an intermediate molecular weight form, is able to interact with other γ-secretase components, including PS1 and PEN2 (Fig. 4C).
We next determined whether the mutation affects mature γ-secretase complex integrity and function. Blue native-PAGE (BN-PAGE) analysis of the membrane fraction isolated from Ncstn−/− cells expressing wild-type or mutant NCSTN indicated that the mutant NCSTN can form the mature γ-secretase complex (∼480 kDa). However, the level of the mature complex is significantly decreased in cells expressing mutant NCSTN, suggesting that the mutation impairs the integrity of the mature γ-secretase complex (Fig. 4D). Furthermore, the mutation resulted in clear inhibition of γ-secretase activity toward Notch2 but had a milder effect on its activity toward Notch1 (Fig. 4E). These results suggest that the glycosylation status of NCSTN influences the integrity and catalytic activity of the γ-secretase complex.
Discussion
The role of the γ-secretase complex in regulating intramembrane proteolysis has been extensively studied and is well recognized. However, the significance of NCSTN and other γ-secretase proteins in immunity remains unclear due to the lack of viable animal models. Taking advantage of our forward genetic screen of mice with mutations induced by ENU, we have identified a previously undescribed γ-secretase–inactivating mutation and have demonstrated that the γ-secretase complex/Notch signaling axis controls pigmentation, intestinal homeostasis, MZ B cell and B-1 B cell development, and TI B cell responses in mice. These phenotypes observed in mice carrying a viable hypomorphic missense mutation of Ncstn may stem from reduced glycosylation of NCSTN. Our data indicate that the glycosylation status of NCSTN critically influences the integrity of the γ-secretase complex, as well as the catalytic activity toward its substrate Notch.
The γ-secretase complex offers a valuable system for studies in multiple fields, because the protein complex cleaves several type I membrane proteins, including Notch, APP, and others. Notch signaling is essential for cell-cell communication during development, and it controls melanocyte differentiation in hair follicles (22, 36), controls hematopoiesis (13, 14), and promotes proliferation of intestinal crypts (27–29). Through its signaling cascade, γ-secretase—mediated cleavage of Notch releases the NICD, which moves to the nucleus to regulate gene expression. Consistent with several phenotypes observed in Notch-deficient mice, we found that the V439G substitution leads to hypopigmentation of fur and spontaneous colitis in mice, manifested by rectal prolapse. Given the widespread effect of altering NCSTN glycosylation in multiple tissues, we consider it likely that impaired proteolytic cleavage of Notch also occurs in the skin and intestinal epithelium of NcstnV439G/V439G mice. Our data suggest that the NCSTN V439G-containing γ-secretase complex retains some proteolytic function toward Notch substrates.
It has been reported that there is no heterozygous effect of Notch1 deletion (Notch1f/−;Mx1-Cre) in T cell development (13). In contrast, haploinsufficiency of Notch2 in B cells (Notch2f/+;Mx1 or CD19-Cre) significantly impairs MZ B cell development, with mutants displaying between one-sixth and one-fourth the number of MZ B cells observed in the control mice (Notch2+/+;Mx1-Cre) (14). The phenotypic effects of homozygous NcstnV439G suggest that these mice have less Notch1/2 activity than has been observed in heterozygous Notch1 or Notch2 mutants.
Comparing the activity of wild-type and mutant NCSTN in the γ-secretase complex revealed that Notch1 processing is more resistant to the effect of mutant NCSTN than Notch2 processing. Therefore, Notch2 processing appears to be reduced to a point at which it causes a defect in MZ B cell and B-1 B cell development, while there is sufficient processing of Notch1 to permit normal T cell differentiation in NcstnV439G/V439G mice. Complete ablation of Ncstn causes prenatal lethality in mice due to deficient Notch signaling (24). In contrast, offspring with the NcstnV439G/V439G genotype were born at expected Mendelian frequencies (P = 0.2698, χ2 test; n = 100 mice: 27 +/+, 55 V439G/+, and 18 V439G/V439G). We cannot yet explain why the missense Ncstn allele is viable in mice, but we postulate that residual γ-secretase complex activity in these mice is sufficient for critical developmental processes.
Missense mutations in the PS1/2 subunit of the γ-secretase complex cause early-onset familial AD (FAD). The mechanism by which these mutations lead to AD remains controversial (20). While a prevailing view is that they are gain-of-function mutations that cause γ-secretase–mediated overproduction of toxic β-amyloid peptides in AD brains, others believe that they are loss-of-function mutations during AD pathogenesis. In this regard, the viable Ncstn missense allele provides a novel tool for testing whether partial loss of γ-secretase activity would suppress AD phenotypes in mice—that is, if PS missense FAD mutations are bona fide gain-of-function mutations. Alternatively, it would be of interest to learn whether the Ncstn V439G mutation facilitates AD development—that is, if PS FAD mutations contribute to AD via a loss-of-function mechanism. Future research will explore these and other questions pertinent to the understanding of AD pathogenesis using this mouse model, including but not limited to the relationships among γ-secretase deficiency, impaired immunity, amyloid pathology, neuronal death, and loss of memory. In view of the strong phenotypic effect and importance of NCSTN in diverse cellular processes, this viable Ncstn missense allele will be useful to researchers in numerous fields.
Materials and Methods
Mice.
C57BL/6J male mice age 8- to 10-wk purchased from The Jackson Laboratory were mutagenized with ENU, as described previously (37). Mutagenized G0 males were bred to C57BL/6J females, and the resulting G1 males were crossed to C57BL/6J females to produce G2 mice. G2 females were backcrossed to their G1 sires to yield G3 mice, which were screened for phenotypes. Whole-exome sequencing and meiotic mapping were performed as described previously (25).
The NcstnV439G/V439G mice were generated using a CRISPR/Cas9 system as described previously (38, 39). Female C57BL/6J mice were superovulated by injection of 6.5 U of pregnant mare serum gonadotropin (Millipore Sigma), followed by injection of 6.5 U of human chorionic gonadotropin (Sigma-Aldrich) 48 h later. The superovulated mice were subsequently mated overnight with C57BL/6J male mice. The next day, fertilized eggs were collected from the oviducts, and in vitro-transcribed Cas9 mRNA (50 ng/μL) and Ncstn small base-pairing guide RNA (50 ng/μL; 5′- GGCTCGAAACATCTCTGGCG-3′) were injected into the cytoplasm or pronucleus of the embryos. The injected embryos were cultured in M16 medium (Sigma-Aldrich) at 37 °C in 5% CO2. For the production of mutant mice, two-cell stage embryos were transferred into the ampulla of the oviduct (10 to 20 embryos per oviduct) of pseudopregnant Hsd:ICR (CD-1) female mice (Harlan Laboratories). All experiments in this study were approved by the University of Texas Southwestern Medical Center’s Institutional Animal Care and Use Committee.
Immunization and Enzyme-Linked Immunosorbent Assay.
Eight- to 12-wk-old G3 mice or NcstnV439G/V439G and wild-type littermates were immunized with the TD antigen OVA/alum (200 μg, i.m.; Invivogen) on day 0 and with the TI antigen NP50-AECM-Ficoll (50 μg, i.p.; Biosearch Technologies) on day 8, as described previously (40). At 6 d after NP50-AECM-Ficoll immunization, blood was collected in MiniCollect tubes (Mercedes Medical) and centrifuged at 1,500 × g to separate the serum for enzyme-linked immunosorbent assay (ELISA) analysis.
ELISA analysis of antigen-specific IgG and IgM responses was performed as described previously (38, 39), Nunc MaxiSorp flat-bottom 96-well microplates (Thermo Fisher Scientific) were coated with 5 µg/mL soluble OVA (Invivogen) or NP8-BSA (Biosearch Technologies) and incubated at 4 °C overnight. Plates were washed four times with washing buffer (0.05% [vol/vol] Tween-20 in PBS) using a BioTek microplate washer, then blocked with 1% (vol/vol) BSA for 1 h at room temperature. Serum samples were serially diluted in 1% (vol/vol) BSA, after which the 1:50 and 1:150 dilutions were added to the prepared ELISA plates. After a 2-h incubation, the plates were washed eight times with washing buffer and then incubated with HRP-conjugated goat anti-mouse IgG or IgM for 1 h at room temperature. Plates were again washed eight times with washing buffer, then developed with KPL SureBlue TMB Microwell Peroxidase Substrate and TMB Stop Solution (SeraCare). Absorbance was measured at 450 nm on a Synergy Neo2 Plate Reader (BioTek). Basal levels of anti-OVA IgG and anti-NP IgM were determined using preimmune serum. All ELISA data shown represent the 1:150 serum dilution.
For determination of serum antibody isotype levels (IgG1 and IgM), freshly isolated serum was subjected to sandwich ELISA analysis according to the manufacturer’s instructions (Invitrogen).
DSS-Induced Colitis Screen.
For DSS-induced colitis induction (36,000 to 50,000 molecular weight; MP Biomedicals), 8- to 12-wk-old mice received 1.3% DSS in the drinking water for 7 d, followed by 3 d off DSS, as described previously (41). Body weight was recorded daily and reported as a percentage relative to the pretreatment body weight.
Flow Cytometry.
Bone marrow cells, thymocytes, splenocytes, peripheral blood, or peritoneal cells were isolated, and red blood cell (RBC) lysis buffer was added to remove the RBCs. Cells were stained at a 1:200 dilution with mouse fluorochrome-conjugated monoclonal antibodies specific for the following murine cell surface markers encompassing the major immune lineages: B220 (clone RA3-6B2; BD), CD19 (clone 1D3; BD), IgM (clone R6-60.2; BD), IgD (clone 11-26c.2a; Biolegend), CD3ε (clone 145-2C11; BD), CD4 (clone RM4-5; BD), CD5 (clone 53-7.3; BD), CD11c (clone HL3; BD), CD44 (clone IM7; BD), CD43 (clone S7; BD), CD25 (clone PC61; Biolegend), CD21/CD35 (clone 7E9; Biolegend), CD23 (clone B3B4; BD), Ly-51 (clone BP-1; BD), CD8α (clone 53-6.7; Biolegend), CD11b (clone M1/70; Biolegend), NK1.1 (clone PK136; BD), F4/80 (clone BM8.1; Tonbo), and CD62L (clone MEL-1; Tonbo), and in the presence of Fc shield (clone 2.4G2; Tonbo) for 1 h at 4 °C. After staining, cells were washed twice in PBS and analyzed by flow cytometry. Data were acquired on an LSRFortessa cell analyzer (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Bone Marrow Chimeras.
Bone marrow chimeras were prepared as described previously (38, 39). Recipient mice were lethally irradiated with 13 Gy via gamma radiation (X-RAD 320 Precision X-Ray; Accela). Each mouse received an i.v. injection of 5 × 106 bone marrow cells derived from donor tibias and femurs. The mice were maintained on antibiotics for 4 wk after engraftment. At 12 wk after bone marrow engraftment, the chimeras were euthanized to assess immune cell development in spleen, peripheral blood, and peritoneal cavity, which were analyzed by flow cytometry. Chimerism was assessed using congenic CD45 markers.
Cell Culture, Isolation of Primary Lymphocytes, Immunoprecipitation, and Immunoblot Analysis.
FLAG-tagged wild-type or mutant (V439G) mouse NCSTN-expressing cell lines were generated in Ncstn−/− mouse embryonic fibroblasts (MEFs) (18). The Ncstn−/− MEFs were infected with lentivirus encoding either C-terminal FLAG-tagged wild-type or mutant (V439G) mouse Ncstn. At 48 h after infection, puromycin at a final concentration of 2 µg/mL was added to create stable cell lines.
Splenic pan B and T cells were purified using the EasySep Mouse Pan B and T Cell Isolation Kits (StemCell Technologies), respectively, according to the manufacturer’s instructions. Purity exceeded 95% in all experiments as tested by flow cytometry.
Immunoprecipitation was performed using anti-FLAG M2 agarose beads (Sigma-Aldrich) in wild-type or mutant (V439G) mouse NCSTN-expressing Ncstn−/− MEFs. Cells were harvested in CHAPSO lysis buffer (50 mM Tris⋅HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% [vol/vol] CHAPSO, and protease inhibitors) for 45 min at 4 °C. TCLs and immunoprecipitates were analyzed using anti-FLAG M2 (Sigma-Aldrich), anti-HA (Cell Signaling Technology), anti-PEN2 (Cell Signaling Technology), and anti-PS1 (A14; ref. 42) antibodies using standard procedures for immunoblot analysis as described below.
For Western blot analysis, cells were lysed in lysis buffer composed of 1% (wt/vol) SDS (Thermo Fisher Scientific), 0.01% (wt/vol) Benzonase (Sigma-Aldrich), and protease inhibitor mixture (Cell Signaling Technology) for 30 min at 4 °C. Protein concentration was measured using a BCA assay (Pierce). Here 10 μg of protein was separated on 4% to 12% Bris-Tris protein gels (Life Technologies), and proteins were transferred to nitrocellulose membranes (Bio-Rad) for 45 min at 13 V. After blocking in Tris-buffered saline containing 0.05% (vol/vol) Tween-20 (TBS-T) with 5% (wt/vol) nonfat dry milk (NFDM) at room temperature for 1 h, the membrane was incubated overnight with primary antibodies anti-NCSTN (Cell Signaling Technology), anti-APP (Sigma-Aldrich), anti-APH1 (H2D2; ref. 43), anti-PS1 (A14; ref. 42), anti–α-tubulin (Cell Signaling Technology), and anti-GAPDH (Cell Signaling Technology) at 4 °C in 5% (wt/vol) NFDM in TBS-T with gentle rocking. The membrane was then incubated with secondary goat anti-rabbit or mouse IgG-HRP antibody (Thermo Fisher Scientific) for 1 h at room temperature with gentle rocking. The chemiluminescence signal was developed using the SuperSignal West Dura Extended-Duration Substrate Kit (Thermo Fisher Scientific) and detected using the G:Box Chemi XX6 gel doc system (Syngene).
Isolation of Plasma Membrane and De-Glycosylation Assay.
To analyze glycosylation patterns of wild-type and mutant (V439G) NCSTN, freshly isolated splenocytes were lysed in SDS lysis buffer, as described previously (35). TCLs were treated with Endo H (500 mU/mL; New England BioLabs) or PNGase (200 U/mL; New England BioLabs) according to the manufacturer’s instructions and analyzed by immunoblotting using anti-FLAG M2 (Sigma-Aldrich) and anti-GAPDH (Cell Signaling Technology) antibodies.
Blue Native-PAGE Analysis.
Collected cells were washed with ice-cold PBS and suspended in ice-cold Pipes buffer (50 mM Pipes pH 7.0, 250 mM sucrose, 1 mM EGTA, and protease inhibitors). Cells were lysed by bioruptor sonication in an ice water bath, and the nuclear fraction was removed by centrifugation at 600 × g for 10 min at 4 °C. The membrane fraction was isolated by ultra-centrifugation at 100,000 × g for 1 h at 4 °C, and membrane proteins were extracted with a solubilization buffer (50 mM Pipes pH 7.0, 250 mM sucrose, 1 mM EGTA, 1% digitonin, and protease inhibitors). The same amount of soluble proteins was subjected to Native-PAGE using the NativePAGE Novex Bis-Tris Gel System (Life Technologies). The gel was transferred to a PVDF membrane and destained with water/methanol/acetic acid (5/4/1) for 30 min. The membrane was further used for Western blot analysis.
In Vitro γ-Secretase Activity Assay.
To measure the effect of the V439G substitution in γ-secretase function, γ-secretase activity was assessed in vitro as described previously (18). In brief, brains isolated from wild-type and NcstnV439G/V439G mice were suspended in ice-cold Pipes buffer (50 mM Pipes pH 7.0, 250 mM sucrose, 1 mM EGTA, and protease inhibitors) and gently sonicated. Pellets containing the nuclear fraction were removed by centrifugation at 600 × g for 10 min at 4 °C. Cleared supernatants containing the microsome fraction were isolated by ultra-centrifugation at 100,000 × g for 1 h at 4 °C and solubilized in 1% CHAPSO buffer (50 mM Pipes pH 7.0, 250 mM sucrose, 1 mM EGTA, and protease inhibitors). An equal wt/vol of microsomal protein was incubated with FLAG-tagged mouse-N1-N100 and N2-N100 in the presence or absence of the γ-secretase inhibitor DAPT (100 µM) for 24 h at 37 °C. N1-N100 contains Val1711-Glu1809 of mouse Notch-1 and is tagged with a C-terminal Flag/His (18). N2-N100 contains Val1668-Pro1766 of mouse Notch-2 with a C-terminal Flag/His tag (this study). The products were analyzed by immunoblotting using an anti-FLAG antibody (Sigma-Aldrich).
Statistical Analysis.
The statistical significance of differences between groups was analyzed using GraphPad Prism by performing the indicated statistical tests. Differences in the raw values among groups were considered statistically significant at P < 0.05. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant with P > 0.05.
Data Availability.
All data are contained in the main text and SI Appendix. The truffle mouse strain is available from the Mutant Mouse Resource and Research Center (C57BL/6J-MtgxR3969Btlr/Mmmh; stock no. 040937-MU). Strain information is available at https://mutagenetix.utsouthwestern.edu/incidental/incidental_rec.cfm?mid=310864&rn=20&rl=41&so=&ac=1&r0=0&nr=100&scd=r3969.
Supplementary Material
Acknowledgments
This work was supported by the NIH (Grants R01 AI125581, to B.B. and R01 NS079796 and RF1-AG064909, to G.Y.) and by the Lyda Hill Foundation (B.B.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916645117/-/DCSupplemental.
References
- 1.Mond J. J., Lees A., Snapper C. M., T cell-independent antigens type 2. Annu. Rev. Immunol. 13, 655–692 (1995). [DOI] [PubMed] [Google Scholar]
- 2.García de Vinuesa C., O’Leary P., Sze D. M., Toellner K. M., MacLennan I. C., T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur. J. Immunol. 29, 1314–1323 (1999). [DOI] [PubMed] [Google Scholar]
- 3.Martin F., Kearney J. F., Marginal-zone B cells. Nat. Rev. Immunol. 2, 323–335 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Martin F., Oliver A. M., Kearney J. F., Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14, 617–629 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Alugupalli K. R., et al. , The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J. Immunol. 170, 3819–3827 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Alugupalli K. R., et al. , B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 21, 379–390 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Haas K. M., Poe J. C., Steeber D. A., Tedder T. F., B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23, 7–18 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Allman D., et al. , Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 167, 6834–6840 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Pillai S., Cariappa A., The follicular versus marginal zone B lymphocyte cell fate decision. Nat. Rev. Immunol. 9, 767–777 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A., Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 161, 1554–1568 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wardemann H., Boehm T., Dear N., Carsetti R., B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J. Exp. Med. 195, 771–780 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dries D. R., Yu G., Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer’s disease. Curr. Alzheimer Res. 5, 132–146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radtke F., et al. , Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Saito T., et al. , Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity 18, 675–685 (2003). [DOI] [PubMed] [Google Scholar]
- 15.De Strooper B., et al. , A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Kopan R., Ilagan M. X., Gamma-secretase: Proteasome of the membrane? Nat. Rev. Mol. Cell Biol. 5, 499–504 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Yu G., et al. , Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature 407, 48–54 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Shah S., et al. , Nicastrin functions as a gamma-secretase-substrate receptor. Cell 122, 435–447 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Shirotani K., et al. , Gamma-secretase activity is associated with a conformational change of nicastrin. J. Biol. Chem. 278, 16474–16477 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Kelleher R. J. 3rd, Shen J., Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 114, 629–631 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang B., et al. , Gamma-secretase gene mutations in familial acne inversa. Science 330, 1065 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Aubin-Houzelstein G., et al. , Melanoblasts’ proper location and timed differentiation depend on Notch/RBP-J signaling in postnatal hair follicles. J. Invest. Dermatol. 128, 2686–2695 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Wang R., Tang P., Wang P., Boissy R. E., Zheng H., Regulation of tyrosinase trafficking and processing by presenilins: Partial loss of function by familial Alzheimer’s disease mutation. Proc. Natl. Acad. Sci. U.S.A. 103, 353–358 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinakis A., et al. , A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature 473, 230–233 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T., et al. , Real-time resolution of point mutations that cause phenovariance in mice. Proc. Natl. Acad. Sci. U.S.A. 112, E440–E449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adzhubei I. A., et al. , A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fre S., et al. , Notch signals control the fate of immature progenitor cells in the intestine. Nature 435, 964–968 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Obata Y., et al. , Epithelial cell-intrinsic Notch signaling plays an essential role in the maintenance of gut immune homeostasis. J. Immunol. 188, 2427–2436 (2012). [DOI] [PubMed] [Google Scholar]
- 29.van Es J. H., et al. , Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Lopes-Carvalho T., Kearney J. F., Development and selection of marginal zone B cells. Immunol. Rev. 197, 192–205 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Srivastava B., Quinn W. J. 3rd, Hazard K., Erikson J., Allman D., Characterization of marginal zone B cell precursors. J. Exp. Med. 202, 1225–1234 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montecino-Rodriguez E., Leathers H., Dorshkind K., Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7, 293–301 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Kimberly W. T., et al. , Complex N-linked glycosylated nicastrin associates with active gamma-secretase and undergoes tight cellular regulation. J. Biol. Chem. 277, 35113–35117 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Esler W. P., et al. , Activity-dependent isolation of the presenilin- gamma -secretase complex reveals nicastrin and a gamma substrate. Proc. Natl. Acad. Sci. U.S.A. 99, 2720–2725 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang D. S., et al. , Mature glycosylation and trafficking of nicastrin modulate its binding to presenilins. J. Biol. Chem. 277, 28135–28142 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Moriyama M., et al. , Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J. Cell Biol. 173, 333–339 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgel P., Du X., Hoebe K., Beutler B., ENU mutagenesis in mice. Methods Mol. Biol. 415, 1–16 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Choi J. H., et al. , IgD class switching is initiated by microbiota and limited to mucosa-associated lymphoid tissue in mice. Proc. Natl. Acad. Sci. U.S.A. 114, E1196–E1204 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi J. H., et al. , LMBR1L regulates lymphopoiesis through Wnt/β-catenin signaling. Science 364, eaau0812 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold C. N., et al. , A forward genetic screen reveals roles for Nfkbid, Zeb1, and Ruvbl2 in humoral immunity. Proc. Natl. Acad. Sci. U.S.A. 109, 12286–12293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang K. W., et al. , Enhanced susceptibility to chemically induced colitis caused by excessive endosomal TLR signaling in LRBA-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 116, 11380–11389 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thinakaran G., et al. , Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron 17, 181–190 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Lee S. F., et al. , Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-beta precursor protein and Notch. J. Biol. Chem. 277, 45013–45019 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained in the main text and SI Appendix. The truffle mouse strain is available from the Mutant Mouse Resource and Research Center (C57BL/6J-MtgxR3969Btlr/Mmmh; stock no. 040937-MU). Strain information is available at https://mutagenetix.utsouthwestern.edu/incidental/incidental_rec.cfm?mid=310864&rn=20&rl=41&so=&ac=1&r0=0&nr=100&scd=r3969.