Significance
Understanding how infectious diseases spread is critical for preventing and containing outbreaks. While advances have been made in forecasting epidemics, much is still unknown. Here we show that the incubation period, the time between exposure to a pathogen and onset of symptoms, is an important factor in predicting spatiotemporal spread of disease and provides one explanation for the different trajectories of recent Ebola and cholera outbreaks in Sierra Leone. We find that outbreaks of pathogens with longer incubation periods, such as Ebola, tend to have less predictable spread, whereas pathogens with shorter incubation periods, such as cholera, spread in a more predictable, wavelike pattern. These findings have implications for the scale and timing of reactive interventions, such as vaccination campaigns.
Keywords: cholera, Ebola, epidemics, modeling, predictability
Abstract
Forecasting the spatiotemporal spread of infectious diseases during an outbreak is an important component of epidemic response. However, it remains challenging both methodologically and with respect to data requirements, as disease spread is influenced by numerous factors, including the pathogen’s underlying transmission parameters and epidemiological dynamics, social networks and population connectivity, and environmental conditions. Here, using data from Sierra Leone, we analyze the spatiotemporal dynamics of recent cholera and Ebola outbreaks and compare and contrast the spread of these two pathogens in the same population. We develop a simulation model of the spatial spread of an epidemic in order to examine the impact of a pathogen’s incubation period on the dynamics of spread and the predictability of outbreaks. We find that differences in the incubation period alone can determine the limits of predictability for diseases with different natural history, both empirically and in our simulations. Our results show that diseases with longer incubation periods, such as Ebola, where infected individuals can travel farther before becoming infectious, result in more long-distance sparking events and less predictable disease trajectories, as compared to the more predictable wave-like spread of diseases with shorter incubation periods, such as cholera.
Epidemics of emerging infectious diseases such as Ebola and Zika underscore the need to improve global capacity for surveillance and response (1–3). Forecasting the spatiotemporal spread of infectious diseases during an outbreak can enable responders to stay ahead of an epidemic. However, it remains challenging both methodologically and with respect to data requirements (4, 5), as disease spread is influenced by multiple factors, including the pathogen’s underlying transmission parameters and epidemiological dynamics, social networks and population connectivity, and environmental conditions (6–9). Previous forecasting efforts have had varying levels of success in predicting the total number of cases and spatiotemporal spread of outbreaks like Ebola, and few have actually been used in real time in the midst of an epidemic (7). Efforts to understand the likely performance of forecasts have shown that heterogeneity in contact structure and number of secondary infections can pose challenges, but reasonable predictions can be made in some cases, depending on disease-specific parameters (6). However, the epidemiological attributes that determine predictability remain uncertain in real-world settings (10–12).
The time from when individuals are infected to when they become infectious (the latent period) and to when they become symptomatic (the incubation period), and the relationship between the two, have been shown to play a large role in the epidemic potential of diseases (9, 13, 14). In particular, transmission that occurs during the incubation period before an individual develops symptoms can contribute to rapid disease spread. When the latent period is shorter than the incubation period for an infectious individual, presymptomatic transmission can be a strong driver of the total number of secondary infections by an infectious individual in a completely susceptible population (i.e., R0) (13, 14). Indeed, the basis of contact tracing protocols during an outbreak reflects the need to identify and contain individuals during the incubation period, and the relative effectiveness of interventions such as symptom monitoring or quarantine significantly depends on the relationship between infectiousness and symptoms (14). Additional related metrics, the generation interval (i.e., the time between infection of an infector−infectee pair) and the serial interval (i.e., the time between symptom onset of an infector−infectee pair), as well as their variances, can further impact the growth rate and total number of infections during an epidemic (15). The incubation period is also likely to play a particularly important role in determining the spatial spread of an epidemic, because one’s typical travel may continue prior to symptom onset, whereas travel behavior may change or stop altogether during illness (16), particularly when symptoms are severe or immobilizing; even if symptoms are mild, if one knows they are infected, behavior may also change, impacting transmission.
Back-to-back epidemics of cholera (2012−2013) and Ebola (2014−2015) in Sierra Leone present a unique opportunity to compare the spatial dynamics of two epidemics in the same population caused by pathogens with notable similarities in both the drivers of outbreaks and the interventions used to curtail them, including oral rehydration (17, 18). Both are transmitted through contact with contaminated diarrhea or vomitus (plus other bodily fluids for Ebola), and the basic reproductive number (R0) for both diseases is thought to be between 1 and 3 (19, 20). Both diseases can cause immobilizing gastrointestinal symptoms of diarrhea and vomiting, and, untreated, their case fatality rates can exceed 50% (21, 22). Cultural factors and rituals, such as traditional funeral practices, are known to influence the spread of both cholera (23) and Ebola (24), while water, sanitation, and hygiene programs are often used to slow the spread of each (25). Both epidemics occurred against a backdrop of an immunologically naïve population. Although it seems likely that travel patterns and the density and distribution of people were broadly similar over the time period in question, regular movements may have been more impacted during the Ebola epidemic than during the cholera epidemic due to travel restrictions, particularly during the multiday lockdowns (26). One critical difference between the dynamics of these diseases, however, is the incubation period, which is estimated at a median of 8 d to 12 d between infection and onset of symptoms for Ebola (1) and only 1 d to 2 d for cholera (27).
We hypothesize that the disease incubation period may be a particularly influential driver of different patterns of disease spread through space and time. We analyze the spatiotemporal dynamics of a cholera outbreak and an Ebola outbreak in Sierra Leone, both of which occurred over a similar time period. We develop a simulation model of the spatial spread of an epidemic and examine the impact of the incubation period on the dynamics of spread and the predictability of outbreaks. We find that differences in the incubation period alone can determine the limits of predictability for these diseases with different natural history, both empirically and in our simulations. Our results show that diseases with longer incubation periods, such as Ebola, where infected individuals can travel farther before becoming infectious, result in more long-distance sparking events and less predictable disease trajectories, as compared to the more predictable wave-like spread of diseases with shorter incubation periods, such as cholera.
Results
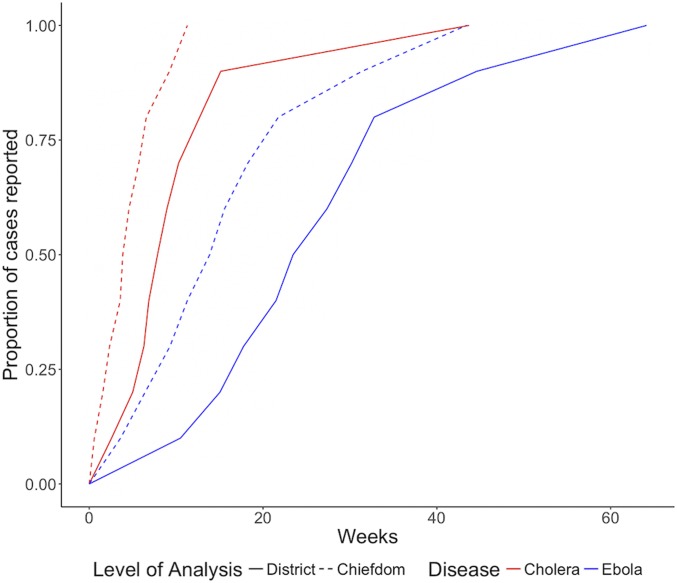
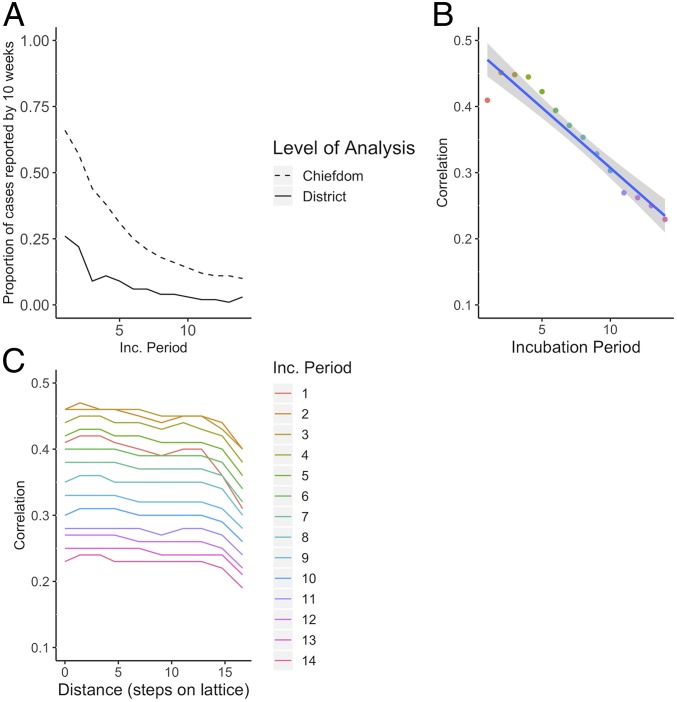
We first summarize the cholera and Ebola epidemics in terms of their dynamics in time and space. More cases were reported during the cholera epidemic (22,691) than during the Ebola epidemic (11,903); however, far fewer cholera cases were fatal (324 vs. 3,956). Both epidemics lasted for similar periods of time, with cholera (January 7, 2012 to May 14, 2013) occurring 2 y prior to Ebola (May 18, 2014 to September 12, 2015). Data for both outbreaks were reported at the chiefdom level, the third-level administrative units. The times between the onset of an outbreak and when half or all of its cases were reported were longer when outbreaks were aggregated by district (second-level administrative units, comprised of chiefdoms) instead of chiefdom (Fig. 1), which has implications for the optimal scale for surveillance and response measures. The median time for a chiefdom cholera outbreak to report half of its case total was 3.9 wk, and median outbreak duration was 11.3 wk. The median time for district outbreaks to report half of their cholera cases was 7.9 wk, and the median outbreak duration was 43.7 wk. Analysis of Ebola revealed similar trends, with chiefdoms reporting half of their cases at a median of 13.9 wk and median outbreak duration of 43.3 wk, and districts reporting half of their cases at a median of 23.5 wk and median outbreak duration of 64.1 wk.
Fig. 1.
The proportion of cholera and Ebola cases reported over time differed between district and chiefdom levels. The times between the onset of an outbreak and when half or all of its cases were reported were longer when outbreaks were aggregated by district instead of chiefdom, which has implications for the optimal scale for surveillance and response measures. The median time for a chiefdom cholera outbreak to report half of its case total was 3.9 wk, and a median of 7.9 wk for district cholera outbreaks. For Ebola, chiefdoms reported half of their cases at a median of 13.9 wk, and districts reported at a median of 23.5 wk.
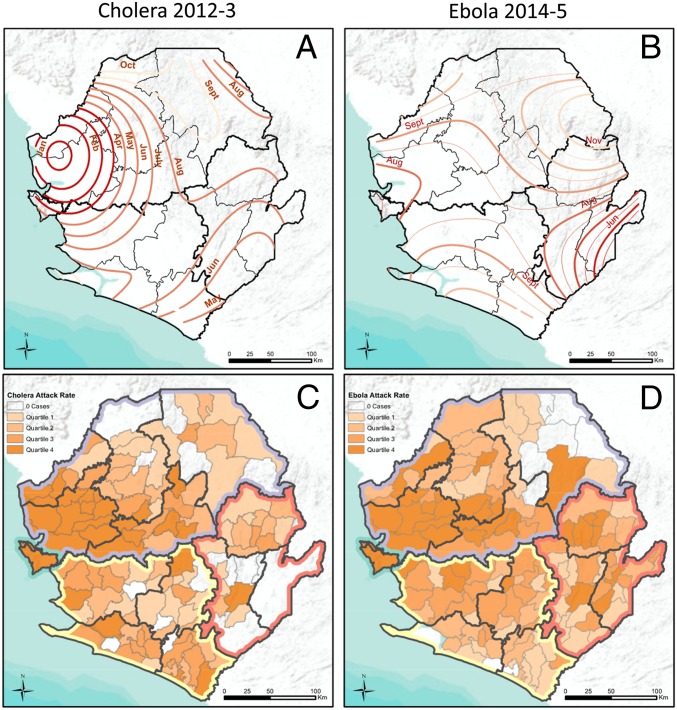
Both the cholera and Ebola epidemics were widespread, each reaching more than 75% of the country’s chiefdoms. However, their trajectories differed. The spread of cholera from the northwest followed a radial spatial dispersion gradually in all directions for the first 6 mo, while Ebola spread from the southeast for 2 mo before rapid expansion to the northwest which sparked the national epidemic (Fig. 2 A and B and Movie S1). These findings were statistically supported by space−time analysis of each epidemic, which revealed clusters of high case reporting of both diseases in western Sierra Leone and unique clusters of cholera in the south and Ebola in the east (SI Appendix, Fig. S1). The wave front of chiefdom cholera outbreak onset progressed more slowly and gradually than for Ebola, which exhibited faster and more discontinuous expansion, as shown by the larger spacing between monthly contour lines (Fig. 2 A and B). Despite their different trajectories, the geography of the epidemics largely overlapped, with clusters of high cumulative attack rates of cholera and Ebola observed in the north and west regions of Sierra Leone (Fig. 2 C and D) and confirmed through Local Moran’s I methods (SI Appendix, Fig. S2).
Fig. 2.
Spatial trend contours of disease spread and chiefdom attack rates highlight similarities and differences between the two epidemics. Spatial trend contours of cholera (A) and Ebola (B) chiefdom case onset dates spread from areas in dark red to light orange; thicker lines (A and B) show monthly increments, and thinner lines (B) show 2-wk increments. Each line of the same color represents the same timescale. Larger spacing between lines represents faster spread. Thick black lines denote regions, and thin black lines denote districts. Chiefdom attack rate quartiles for cholera (C) and Ebola (D) vary over space and regions. Colored boundaries denote regions, followed by bold black borders for districts and thin borders for chiefdoms.
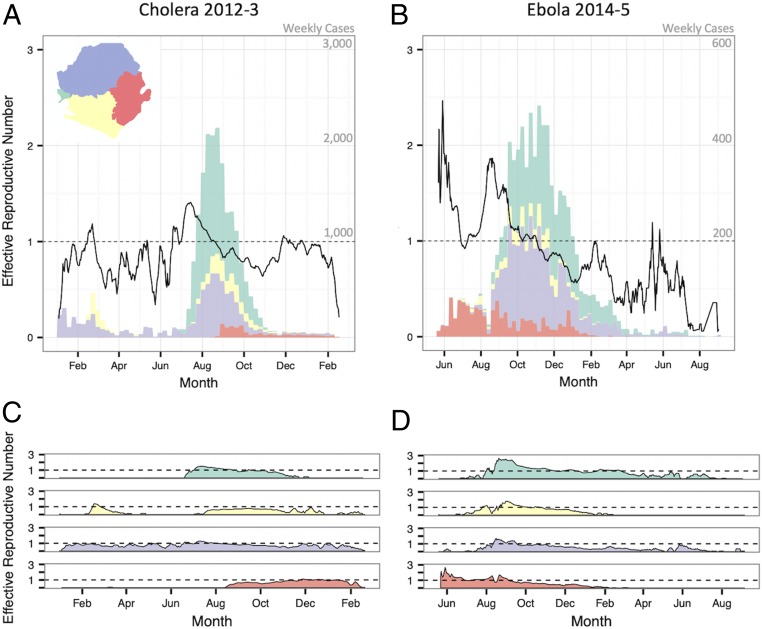
As a daily estimate of transmission intensity, we recorded the effective reproductive number on day t (Rt) and its variation over time nationally and by region (Fig. 3). While some areas sustained transmission (i.e., Rt > 1) of both cholera and Ebola for many days (e.g., Freetown in the west and Kenema Town in the east), 75% of chiefdoms during the cholera outbreak and 44% of chiefdoms during the Ebola outbreak recorded either zero cases or zero days with Rt > 1 (SI Appendix, Fig. S3). As expected, transmission intensity of both diseases was positively correlated in chiefdoms near each other (SI Appendix, Fig. S4). Correlation decayed with distance, consistent with local disease spread, and interchiefdom distances of over 100 km eliminated any evidence of positive correlation of disease presence, chiefdom outbreak time, case count, and cumulative attack rate (SI Appendix, Fig. S4). These metrics appear more highly correlated in space for cholera than for Ebola, although the confidence intervals overlap (SI Appendix, Fig. S4).
Fig. 3.
Weekly case counts show outbreak trajectory in the four regions of the country. The bars in A and B indicate the weekly case count on independent y axes of cholera and Ebola, respectively. Black lines show maximum likelihood estimates of Rt of cholera and Ebola epidemics nationally (A and B, respectively) and in each region (C and D, respectively).
Simulations.
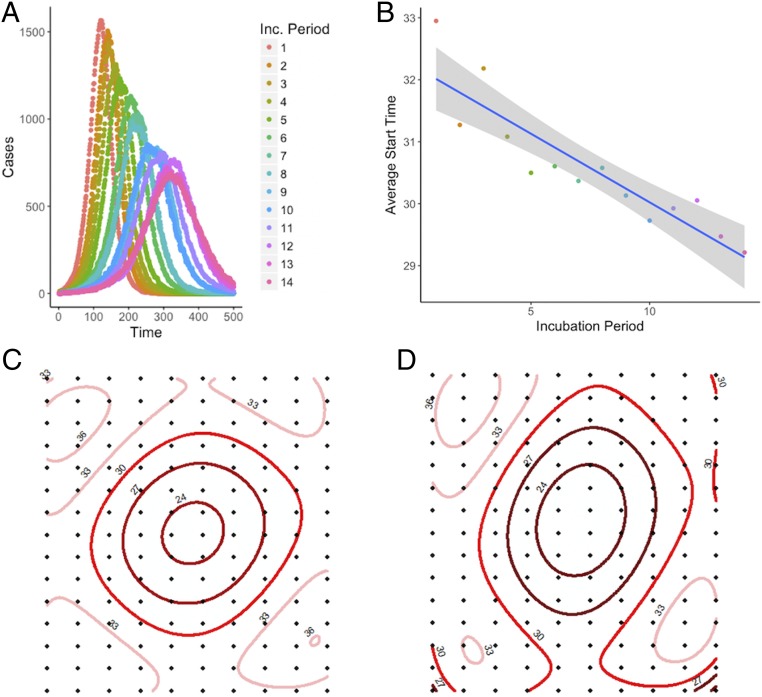
To examine the role of the incubation period in the spread of disease, we simulated outbreaks characterized by varying incubation periods among agents distributed evenly on a spatial lattice, with movement between populations in the lattice based on a gravity model. These simulations show a systematic relationship between the incubation period and spatiotemporal patterns of disease spread. As expected, simulated epidemic curves of diseases with shorter incubation periods were more acute, while diseases with longer incubation periods peaked later (Fig. 4A). Although epidemics tend to last longer for diseases with longer incubation periods, the spread of the disease to more distant locations can progress more quickly, causing a discontinuous and more rapidly spreading wave front (Fig. 4 C and D). In the first 50 d of our simulations, locations farther from the origin of the epidemic experienced cases earlier, on average, in simulations with longer incubation periods compared to those with shorter incubation periods, likely due to long-distance sparking events from infected agents traveling during the incubation period (Fig. 4B). The dispersion kernel Kx(d), the probability that an agent will end up at a position separated a distance d from the initial position after x days, is more homogeneously spread and has nonvanishing probabilities at greater distances the higher the incubation period (x), explaining the enhancement in sparking events (SI Appendix, Fig. S5).
Fig. 4.
Results of 700 simulations of 14 different incubation periods show the impact of incubation period on disease spread. Epidemics with shorter incubation periods are more acute than epidemics with longer incubation periods (A). The average start time of epidemics at all locations over the first 50 d of outbreak is later for shorter incubation periods than for longer ones (B). Spatial trend contours of the first 50 d of simulated outbreaks with shorter incubation period (2 d) (C) and longer incubation period (10 d) (D), spreading from areas in dark red to light red, show that shorter incubation periods result in a more wave-like spread, and longer incubation periods result in more long-distance sparking events; numbers show average start day relative to start of the outbreak.
Simulations on a lattice with relative population size based on Sierra Leone’s chiefdom census data (as opposed to the evenly distributed populations in the original lattice simulations) support the finding that the duration of epidemics is longer on a district (i.e., group of lattice points) rather than chiefdom (i.e., individual lattice point) scale, with duration lengthening with increasing incubation periods (Fig. 5A).
Fig. 5.
The incubation period impacts the timing of outbreaks and, as a result, the correlation. As the incubation period increases, the proportion of cases reported by 10 wk, when a reactive vaccination campaign might begin, decreases in simulated epidemics (A). As the incubation period increases, the average correlation overall (B) and by distance from origin of simulated outbreaks (C) decreases.
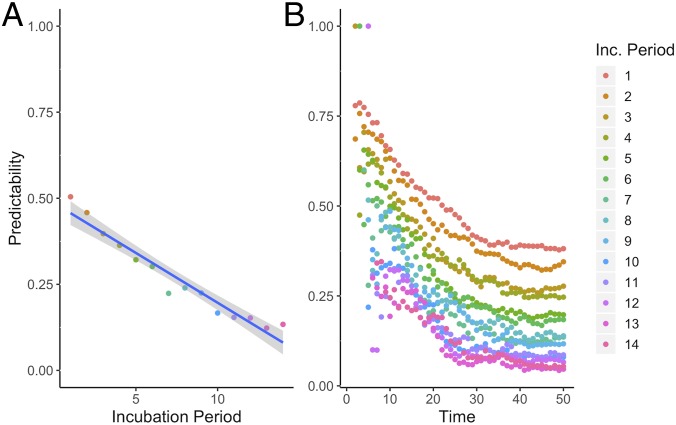
Consistent with the correlation analysis comparing Sierra Leone’s cholera and Ebola outbreaks, time series from simulated outbreaks with shorter incubation periods were more highly correlated than those from simulations with longer incubation periods, with correlation decaying as distance between locations on the lattice increased (Fig. 5 B and C). Higher correlation suggests increased predictability, which the results of the overlap function support (Fig. 6). As the incubation period lengthened, the average predictability during the beginning of the outbreak decreased as the epidemics spread via unpredictable sparking patterns. Predictability plateaued as the outbreaks became widespread.
Fig. 6.
The incubation period impacts the predictability of disease spread. Longer incubation periods have lower overlap (predictability) in the first 50 d (A). Over time, the predictability decreases (B), with longer incubation periods consistently having lower predictability.
Discussion
Analysis of the cholera and Ebola epidemics revealed commonalities and differences in the way these pathogens spread throughout Sierra Leone, and our simulations suggest the differences in the incubation period reproduce these differences. Spatial diffusion of Ebola occurred more quickly than cholera, as evidenced by the wave front contour lines and further supported by statistical tests considering a subset excluding cholera cases before the brief respite in June (SI Appendix, Fig. S6). Additionally, cholera metrics were more correlated in space than Ebola metrics. Our model simulations suggest that these findings are potentially due to the counterintuitive role of the longer incubation period for Ebola as compared to cholera. Travel during the incubation period will be a key driver of geographic disease dispersion and predictability, especially in a population of individuals who decrease mobility when ill. Consequently, diseases with longer incubation periods will tend to have more long-distance sparking events caused by infected, but healthy, individuals traveling during the incubation period. This will result in faster epidemic dispersion to distant, unpredictable locations. These findings are in line with Marvel et al.’s (28) results, which found epidemic wave fronts are less likely to occur for mobility kernels that decay more slowly; when the incubation period is longer, the effective mobility kernel can span to more distant places, making sparking events more probable given the same number of transmission events.
Similar results were also obtained when infectious agents did not decrease mobility when ill, suggesting that travel during the incubation period has more influence on correlation and predictability than travel during the infectious period. While many other factors will influence wave speed, continuity, and epidemic synchrony, our simulations showed that small changes in the incubation period can powerfully influence epidemic dynamics. For example, environmental persistence of Vibrio cholerae in a local water source can potentially lead to a longer serial interval for local transmission (29) and a fatter right tail in offspring distribution via superspreading. Following the dynamics of cholera and Ebola, our models assumed that the incubation and latent periods were equal; however, presymptomatic infectiousness may be an important factor increasing spatial heterogeneity of onward transmission, especially in the context of decreasing mobility when ill. For a disease with presymptomatic infectiousness, we would expect to continue to see a positive correlation between long-range sparking events and the incubation period, as well as a greater likelihood of intermediate-range sparking events as infectiousness increasingly precedes symptom onset and travelers transmit en route.
The incubation period has already been recognized as an important component for understanding epidemics and control (13), with the conventional knowledge that long incubation periods allow more time for responders to scale up interventions against the overall epidemic and are therefore advantageous for disease control efforts. Here we demonstrated a counterintuitive mechanism whereby a longer incubation period may, in fact, hinder a response by decreasing the predictability of outbreaks and increasing their geographic scope as well as of the needs of surveillance and response. We use simulations to reproduce the double-edged sword of the influence of the disease incubation period on reactive interventions.
Reactive vaccination strategies exist for both cholera and Ebola outbreaks, and a better understanding of spatiotemporal spread can facilitate locally preemptive vaccination to target locations at high risk of introduction (30–32). Reactive vaccination campaigns must consider both the expected duration of an outbreak at a given spatial scale and the predictability of its spread. We found that both epidemics lasted longer at the district level than chiefdom level, likely due to the larger spatial scale of the districts. For cholera, we showed that chiefdom outbreaks tended to report half of their cases within ∼4 wk, suggesting that reactive vaccination of a chiefdom triggered by detection of a case may not be early enough to avert an outbreak, and, instead, intervening at a wider scale, such as districts, might provide more favorable timing for intervention targeting. We posit, for future study, that regional ring vaccination strategies may be better suited to diseases with short incubation periods, while contact ring vaccination strategies may be better suited to diseases with longer incubation periods, due to their regional unpredictability and the longer intervals between generations in infection.
There are limitations to our work with regard to data as well as methods. Few cholera cases were confirmed during the epidemic, and therefore we depend on the clinical definition as well as the cases that were detected and recorded by the surveillance system. Ebola surveillance data are similarly prone to differences in reporting rates, but the use of only confirmed cases yielded similar results to those reported above using both confirmed and suspected cases. Our estimates for the effective reproductive number depend on, and absorb the limitations of, case data, serial interval estimates, and the chiefdom connectivity matrix. Specifically, we assume all cases in our dataset acquired infection from others in the dataset, thereby excluding missing cases and asymptomatic transmitters. However, this method has been shown to be robust to cases missing at random, and we furthermore expect the role of asymptomatic transmission to be limited for both diseases, due to the strong correlation between pathogen load, symptoms, and infectiousness (33, 34).
Further, we assume no changes to the serial interval for either cholera or Ebola during the course of the epidemics. For cholera, specifically, waterborne transmission could potentially lead to a heavy right tail in serial intervals or change the distribution as pathogen accumulates or clears from a drinking source. Household data in Bangladesh, where the role of water contamination is expected to be large, suggest few serial intervals beyond 7 d (35). The geographic spread of cholera in Sierra Leone from the northwest and south toward the center of the country was not consistent with the direction of key waterways in the country, which primarily run from the eastern highlands to the western shores, suggesting population density and human-to-human contact likely played a larger role than water sources in this outbreak.
Finally, our simulation model provides a proof-of-concept test of the hypothesis of the impact of the incubation period on disease spread and makes several simplifying assumptions. These assumptions could be relaxed in future work, including the complete overlap of symptoms and infectiousness and constant or structured diffusion of agents, for example, without increased probability of returning “home,” which could decrease the overall distance exposed agents travel and therefore lower the probability of longer-range sparking events. One could use other models for the mobility of the agents, such as the one by Song et al. (36) which includes probabilities for returning to already visited places, as well as for exploration of locations not previously visited. In general, complex travel patterns are difficult to measure in real populations and are highly context-specific, interacting in critical ways with the epidemiological drivers of epidemics examined here.
The threat of cholera and Ebola reemergence in Sierra Leone remains a concern (37). We have shown that differences in incubation period alone are a powerful driver of geographic dispersion and merit further study. Although this study only examines one epidemic from each disease, the size of these epidemics, combined with simulation results from our model, can lend information toward a better understanding of each disease and our ability to predict disease spread. This work can inform development of international preparedness and response strategies and ensure timely and effective interventions.
Methods
Data.
Cholera cases were reported to the Sierra Leone Ministry of Health and Sanitation by treatment facilities throughout Sierra Leone between January 1, 2012 and May 15, 2013 (38). Following standard World Health Organization (WHO) definitions (39), a suspected cholera case was defined as acute onset of watery diarrhea or severe dehydration in a person aged 5 y or older in a region without a known cholera outbreak; once the government of Sierra Leone declared an outbreak of cholera on February 27, 2012, any case of acute watery diarrhea could henceforth be included as a suspected cholera case. Data were compiled and anonymized by the WHO for analysis, with case reports temporally resolved by day and spatially resolved by chiefdom. For Ebola, we used a published dataset of 8,358 confirmed and 3,545 suspected Ebola cases reported to the Sierra Leone Ministry of Health and Sanitation from May 2014 to September 2015 (40). Our analysis included both suspected and confirmed cases of Ebola according to standard WHO definitions (41). Population estimates for 2012 and 2014 were imputed by chiefdom using a linear fit between chiefdom population estimates from the 2004 and 2015 Population and Housing Censuses (42). Data and code are available on Github (43).
Sierra Leone has four administrative regions, which are divided into 14 districts. Freetown, the capital and largest city, comprises 2 districts; the remaining 12 districts are subdivided into 149 chiefdoms, with a median of 11.5 chiefdoms per district. Chiefdom, as the finest administrative unit available for cases of both cholera and Ebola, was considered the unit of observation and the unit of analysis (with the exception of cases in Freetown which were solely reported at district level), as it is the likely scale of intervention campaigns like vaccination. To understand what would have been observed at a coarser spatial scale that is more common for surveillance, we additionally aggregated cases by district.
Spatiotemporal Analysis.
We defined the first outbreak week for each chiefdom as the week of the first reported case in that chiefdom. We visualized outbreak spread using a contour map of outbreak wave front direction and speed (40). Contours of spatial spread were generated using ArcMap 10.3.1 Spatial Analyst extension by applying a fourth-degree polynomial trend interpolation of chiefdom onset dates and generating contour lines of this surface in 2- to 4-wk increments. With this method, more closely spaced contour lines indicate slower propagation, similar to the slope of a topographic map of elevation.
To identify space−time clusters, using the SaTScan software package (44), we ran a retrospective discrete Poisson-based Scan Statistic over the entirety of the outbreaks for which data were available, namely, 16 mo of cholera data and 17 mo of Ebola data. Disease case reports were assumed to be Poisson-distributed given chiefdom population size. The unit of time aggregation for the analysis was specified as the median serial interval for each disease [5 d for cholera (45, 46) and 13.3 d for Ebola (1)].
We calculated spline correlograms for four chiefdom outbreak metrics to measure spatial correlation of date of first case, case count, attack rate, and disease presence (yes/no). The maximum centroid-to-centroid distance was set to 150 km, approximately the radius of Sierra Leone. We used the spline.correlog function of the R package ncf for each disease and all chiefdom pairs (47).
We estimated the daily effective reproductive number (Rt), the average number of onward infections generated by cases with onset on day t, using methods described by Wallinga and Teunis (48) and extended to metapopulations by White et al. (49). This maximum likelihood method estimates the probability that an observed case was the infector for each subsequent case by leveraging information on the daily case count, the serial interval distribution, and a weights matrix that quantifies relative contact frequency within and between chiefdoms. The serial interval for cholera was assumed to follow a gamma distribution (rate = 0.1, shape = 0.5) with a median of 5 d, as has been used previously after consideration of both fast, person-to-person, and slow, environmental, transmission routes (45, 46). The serial interval for Ebola was assumed to follow a gamma distribution (rate = 0.17, shape = 2.59) with a median of 13.3 d derived from the estimates by the WHO Ebola Response Team (1). The contact frequency between two given chiefdoms was assumed to decrease with squared distance between the chiefdom centroids. Additional weights matrices with different functional forms for distance decay yielded qualitatively similar measurements of Rt.
Model.
We simulated an agent-based model with 45,000 agents distributed equally in 150 locations, evenly spaced on a 15 × 10 lattice. Infected agents progressed through a traditional susceptible−exposed−infectious−recovered compartmental transmission framework. We assumed the incubation period (i.e., the time from exposure to symptom onset) overlapped completely with the latent period (i.e., the time from exposure to onset of infectiousness). Similarly, the duration of illnesses (5 d) aligned with the duration of infectiousness. The serial interval, which comprises both the incubation period and duration of infectiousness, can strongly influence epidemic dynamics. However, to isolate the impact of presymptomatic travel on spatiotemporal patterns of disease spread, in our simulations, we held the duration of infectiousness constant. The attack rate also remained constant throughout the epidemics, with an R0 of 1.5; simulations with larger R0s (e.g., 3) returned similar results. Movement of agents between two locations was simulated through a daily travel connectivity matrix A based on a gravity model, whereby connectivity was proportional to the population sizes of each location and the inverse squared distance between them (50). Different parametrizations of the gravity model, as well as simulations with relative population size based on Sierra Leone’s chiefdom census data (51), yielded similar results. Note that the effective dispersion kernel after x days, Kx(d), mentioned in the simulation results is different from the daily mobility matrix A. In our simulations, the mobility matrix is fixed independently of the disease, but the dispersion kernel that is relevant for each disease depends on the incubation period. The element Aij of the mobility matrix A describes the probability that an agent will travel from location i to location j in 1 d. These elements depend on the populations of those locations and the distance between them as in a gravity model for mobility. The dispersion kernel Kx(d) measures the probability of finding an agent at a distance d from the place where she was x days before. Therefore, the dispersion kernel is a direct consequence of the daily mobility matrix. It will tell us, for infected agents, the probability of being at a distance d from where they became infected after x days. As travel is stopped once they become infectious, the relevant dispersion kernel for each disease will be the one for which x equals the incubation period.
Susceptible, exposed, and recovered individuals had a daily probability of movement. To simulate the impact of a reduction in mobility during illness, agents in the model had their movement reduced as far as zero throughout the course of their period of infectiousness (and, equivalently, illness). Holding all other parameters constant, we conducted 700 simulations of epidemics for incubation periods ranging from 1 d to 14 d. We seeded the epidemic at the same location near the center of the lattice for all simulations.
Synchrony was assessed with the R package ncf functions mSynch and Correlog.Nc (47), which both estimate the correlation between the time series in each of the 150 locations across the 500 d of the simulations, with the latter incorporating distance (47). To assess the impact of the incubation period on the initial speed of spread, we calculated the average start time across all locations in the first 50 d of the outbreaks as well as at increasing distances from the location on the lattice where the outbreaks began.
To estimate the predictability of outbreak spread in space and time, we adapted an overlap function used to measure predictability of a severe acute respiratory syndrome (SARS) outbreak (9). In each simulation, a vector represents the proportion of all infected individuals at time t who are at location j. In a system with high predictability, will be similar across simulations. The overlap between simulations I and II can be estimated by . Θ(t) ranges from 0 to 1, with a higher value indicating more overlap and thus more predictability. We estimated predictability at each time point by calculating the average of the overlap functions for each pair of simulations for each incubation period. We calculated the average overlap across time points to provide a summary metric for predictability of each incubation period.
Data Availability.
Code and data are available on Github (43): https://github.com/rek160/Sierra-Leone-Cholera-Ebola.
Supplementary Material
Acknowledgments
We thank Dr. Foday Dafae for early support of this work. This work was supported by Award U54GM088558 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Code and data are available on Github, https://github.com/rek160/Sierra-Leone-Cholera-Ebola.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913052117/-/DCSupplemental.
References
- 1.Agua-Agum J., et al. ; WHO Ebola Response Team , West African Ebola epidemic after one year–Slowing but not yet under control. N. Engl. J. Med. 372, 584–587 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention , Zika virus–Transmission and risks. https://www.cdc.gov/zika/index.html. Accessed 22 November 2018.
- 3.Gates B., The next epidemic–Lessons from Ebola. N. Engl. J. Med. 372, 1381–1384 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Desai A. N., et al. , Real-time epidemic forecasting: Challenges and opportunities. Health Secur. 17, 268–275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahn R., et al. , Rapid forecasting of cholera risk in mozambique: Translational challenges and opportunities. Prehosp. Disaster Med. 34, 557–562 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Scarpino S. V., Petri G., On the predictability of infectious disease outbreaks. Nat. Commun. 10, 898 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraemer M. U. G., et al. , Reconstruction and prediction of viral disease epidemics. Epidemiol. Infect. 147, e34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyers L. A., Pourbohloul B., Newman M. E. J., Skowronski D. M., Brunham R. C., Network theory and SARS: Predicting outbreak diversity. J. Theor. Biol. 232, 71–81 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colizza V., Barrat A., Barthélemy M., Vespignani A., Predictability and epidemic pathways in global outbreaks of infectious diseases: The SARS case study. BMC Med. 5, 34 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk S., Camacho A., Kucharski A. J., Eggo R. M., Edmunds W. J., Real-time forecasting of infectious disease dynamics with a stochastic semi-mechanistic model. Epidemics 22, 56–61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chretien J.-P., Riley S., George D. B., Mathematical modeling of the West Africa Ebola epidemic. eLife 4, e09186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viboud C., et al. ; RAPIDD Ebola Forecasting Challenge group , The RAPIDD ebola forecasting challenge: Synthesis and lessons learnt. Epidemics 22, 13–21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser C., Riley S., Anderson R. M., Ferguson N. M., Factors that make an infectious disease outbreak controllable. Proc. Natl. Acad. Sci. U. S. A. 101, 6146–6151 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peak C. M., Childs L. M., Grad Y. H., Buckee C. O., Comparing nonpharmaceutical interventions for containing emerging epidemics. Proc. Natl. Acad. Sci. U. S. A. 114, 4023–4028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallinga J., Lipsitch M., How generation intervals shape the relationship between growth rates and reproductive numbers. Proc. Biol. Sci. 274, 599–604 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haw D. J., et al. , Differential mobility and local variation in infection attack rate. PLOS Comput. Biol. 15, e1006600 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalin D. R., Hirschhorn N., Ebola and cholera. Am. J. Trop. Med. Hyg. 92, 1081 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamontagne F., et al. , Evidence-based guidelines for supportive care of patients with Ebola virus disease. Lancet 391, 700–708 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Althaus C. L., Estimating the reproduction number of Ebola virus (EBOV) during the 2014 outbreak in West Africa. PLoS Currents, 10.1371/currents.outbreaks.91afb5e0f279e7f29e7056095255b288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukandavire Z., Morris J. G., Modeling the epidemiology of cholera to prevent disease transmission in developing countries. Microbiol. Spectr., 10.1128/microbiolspec.VE-0011-2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization , Ebola virus disease. https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease. Accessed 22 November 2018.
- 22.World Health Organization , Cholera: Mechanism for control and prevention. https://www.who.int/cholera/technical/secretariat_report/en/. Accessed 22 November 2018.
- 23.World Health Organization , Cholera fact sheet. https://www.who.int/news-room/fact-sheets/detail/cholera. Accessed 22 November 2018.
- 24.Victory K. R., Coronado F., Ifono S. O., Soropogui T., Dahl B. A.; Centers for Disease Control and Prevention , Ebola transmission linked to a single traditional funeral ceremony–Kissidougou, Guinea, December, 2014-January 2015. MMWR Morb. Mortal. Wkly. Rep. 64, 386–388 (2015). [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention , Global Water, Sanitation, & Hygiene (WASH). https://www.cdc.gov/healthywater/global/wash_statistics.html. Accessed 22 November 2018.
- 26.Peak C. M., et al. , Population mobility reductions associated with travel restrictions during the Ebola epidemic in Sierra Leone: Use of mobile phone data. Int. J. Epidemiol. 47, 1562–1570 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azman A. S., Rudolph K. E., Cummings D. A. T., Lessler J., The incubation period of cholera: A systematic review. J. Infect. 66, 432–438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marvel S. A., Martin T., Doering C. R., Lusseau D., Newman M. E. J., The small-world effect is a modern phenomenon. arXiv:13102636 (9 October 2013).
- 29.Azman A. S., et al. , The impact of a one-dose versus two-dose oral cholera vaccine regimen in outbreak settings: A modeling study. PLoS Med. 12, e1001867 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finger F., et al. , The potential impact of case-area targeted interventions in response to cholera outbreaks: A modeling study. PLoS Med. 15, e1002509 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azman A. S., et al. , Effectiveness of one dose of oral cholera vaccine in response to an outbreak: A case-cohort study. Lancet Glob. Health 4, e856–e863 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Henao-Restrepo A. M., et al. , Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 389, 505–518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glynn J. R., et al. , Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: A cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet Infect. Dis. 17, 645–653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson E. J., Harris J. B., Morris J. G. Jr, Calderwood S. B., Camilli A., Cholera transmission: The host, pathogen and bacteriophage dynamic. Nat. Rev. Microbiol. 7, 693–702 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weil A. A., et al. , Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin. Infect. Dis. 49, 1473–1479 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song C., Koren T., Wang P., Barabási A.-L., Modelling the scaling properties of human mobility. Nat. Phys. 6, 818–823 (2010). [Google Scholar]
- 37.World Health Organization , Sierra Leone to begin cholera vaccination drive in disaster-affected areas (2017). https://www.who.int/news-room/detail/05-09-2017-sierra-leone-to-begin-cholera-vaccination-drive-in-disaster-affected-areas. Accessed 6 February 2020.
- 38.Humanitarian Data Exchange (HDX) , Sierra Leone - Health. https://data.humdata.org/dataset/sierra-leone-health. Accessed 1 April 2017.
- 39.World Health Organization , “Global Task Force on Cholera Control Prevention and control of cholera outbreaks” (WHO policy and recommendations). https://www.who.int/cholera/technical/prevention/control/en/. Accessed 6 February 2020.
- 40.Fang L.-Q., et al. , Transmission dynamics of Ebola virus disease and intervention effectiveness in Sierra Leone. Proc. Natl. Acad. Sci. U. S. A. 113, 4488–4493 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization , “Case definition recommendations for Ebola or Marburg virus diseases” (2014). https://www.who.int/csr/resources/publications/ebola/ebola-case-definition-contact-en.pdf. Accessed 6 February 2020.
- 42.Statistics Sierra Leone , Census. https://www.statistics.sl/index.php/census.html. Accessed 18 November 2018.
- 43.Kahn R., Sierra-Leone-cholera-ebola. https://github.com/rek160/Sierra-Leone-Cholera-Ebola. Accessed 16 July 2019.
- 44.Kulldorff M., Inc I. M. S., SaTScan (TM) v7. 0: Software for the spatial and space-time scan statistics. https//satscan.org. Accessed 18 January 2018.
- 45.Azman A. S., et al. , Population-level effect of cholera vaccine on displaced populations, South Sudan, 2014. Emerg. Infect. Dis. 22, 1067–1070 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Azman A. S., et al. , Urban cholera transmission hotspots and their implications for reactive vaccination: Evidence from Bissau City, Guinea Bissau. PLoS Negl. Trop. Dis. 6, e1901 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjørnstad O. N., Ims R. A., Lambin X., Spatial population dynamics: Analyzing patterns and processes of population synchrony. Trends Ecol. Evol. 14, 427–432 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Wallinga J., Teunis P., Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am. J. Epidemiol. 160, 509–516 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White L. F., Archer B., Pagano M., Estimating the reproductive number in the presence of spatial heterogeneity of transmission patterns. Int. J. Health Geogr. 12, 35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dudas G., et al. , Virus genomes reveal factors that spread and sustained the Ebola epidemic. Nature 544, 309–315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Statistics Sierra Leone , 2015 population and housing census. https://www.statistics.sl/images/StatisticsSL/Documents/final-results_-2015_population_and_housing_census.pdf. Accessed 22 November 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Code and data are available on Github (43): https://github.com/rek160/Sierra-Leone-Cholera-Ebola.