Significance
Despite being the first tight junction (TJ) component discovered and numerous in vitro studies showing its TJ-related functions, Ocln has remained elusive regarding its physiological functions because Ocln null mice develop normally. Here we demonstrate that Ocln null mammary gland epithelium has intact TJ integrity, which argues against it being an essential TJ component as previously expected; rather, Ocln plays a role in regulating protein secretion by binding to SNARE components. Ocln mutant milk-producing cells exhibit defective protein secretion and augmented endoplasmic reticulum stress and unfolded protein response, ultimately leading to protein expression shutdown and lactation failure in mutant mice. Our results thus shed important light on the essential physiological functions of Ocln in the vertebrate epithelium.
Keywords: alveolar differentiation, ER stress, unfolded protein response, vesicular trafficking
Abstract
Tight junctions (TJs) are fundamental features of both epithelium and endothelium and are indispensable for vertebrate organ formation and homeostasis. However, mice lacking Occludin (Ocln) develop relatively normally to term. Here we show that Ocln is essential for mammary gland physiology, as mutant mice fail to produce milk. Surprisingly, Ocln null mammary glands showed intact TJ function and normal epithelial morphogenesis, cell differentiation, and tissue polarity, suggesting that Ocln is not required for these processes. Using single-cell transcriptomics, we identified milk-producing cells (MPCs) and found they were progressively more prone to endoplasmic reticulum (ER) stress as protein production increased exponentially during late pregnancy and lactation. Importantly, Ocln loss in MPCs resulted in greatly heightened ER stress; this in turn led to increased apoptosis and acute shutdown of protein expression, ultimately leading to lactation failure in the mutant mice. We show that the increased ER stress was caused by a secretory failure of milk proteins in Ocln null cells. Consistent with an essential role in protein secretion, Occludin was seen to reside on secretory vesicles and to be bound to SNARE proteins. Taken together, our results demonstrate that Ocln protects MPCs from ER stress by facilitating SNARE-dependent protein secretion and raise the possibility that other TJ components may participate in functions similar to Ocln.
Tight junctions (TJs) are fundamental features of both epithelia and endothelia, where their “barrier” function prevents free exchange of nutrients, wastes, and metabolites between tissues and their environment (1). Located at the apical-basolateral juncture, TJs are also thought to form a structural platform on which multiple signaling cascades converge and integrate and are potentially essential for the emergence of various tissues and organs during metazoan evolution. As such, much effort has been devoted to understanding TJ functions in vertebrate organ development, homeostasis, and pathogenesis (2, 3).
At the protein level, TJs are composed of multiple components, including members of the four-pass transmembrane proteins that are part of the Occludin (OCLN) and Claudin families. TJs are also composed of “nonresident” cytoplasmic components, such as members of the ZO family, which connect TJs to the cytoskeleton by binding to actin filaments (4–6). Although OCLN was the first transmembrane TJ component discovered almost three decades ago, the full scope of its physiological functions has remained controversial (7). While initial in vitro studies showed that Ocln is indispensable for TJ function, they contradicted studies from in vivo experiments showing that mouse embryonic stem cells lacking Ocln form intact TJs and that mutant embryos develop normally to term (4, 8). However, Ocln null mice do exhibit defects in certain organs later in life, including the salivary gland, stomach, brain, and inner ear (8, 9), suggesting an unknown role of Ocln in homeostasis and maintenance of these organs.
As a secretory organ readily amenable to experimental manipulations, the mouse mammary gland has recently emerged as an invaluable system for dissecting TJ functions (10, 11). Unlike most other epithelial organs, the morphogenetic and cell differentiation processes and physiological functions of the mammary gland mostly occur during postnatal life. For example, at birth, the mammary gland is a simple ductal tree composed of only a few epithelial branches, with much of its stromal fat pad free of epithelium (12–14). It is only during puberty (which starts at 3 wk of age in mice), when active epithelial invasion initiates via a process known as branching morphogenesis until the fad-pad is completely filled with mammary epithelium (15, 16). During pregnancy, the epithelial tree undergoes alveologenesis where alveoli form on both sides of the epithelial branches and luminal cells undergo a concomitant maturation process (17, 18). These morphogenetic and differentiation processes are finely regulated by systemic hormones, including estrogen, progesterone, and prolactin, and their corresponding downstream signaling pathways (19–21).
The synthesis of milk components starts some time during pregnancy as part of the luminal maturation process (22). At this stage, these components are packaged mainly into cytoplasmic lipid droplets (CLDs) or secretory vesicles (SVs) containing aggregates of milk proteins, also known as casein micelles (CMs) (23–25). Secretory activation does not occur until parturition, due to a combination of progesterone withdrawal and a sharp prolactin increase, along with other possible changes in systemic or local factors (10, 11). As such, SVs fuse with the plasma membrane via a poorly understood exocytotic process and release their protein contents into the enlarging lumen (26). Once milk proteins have been secreted into the alveolar lumen, myoepithelial cells, under the stimulation of oxytocin, contract and squeeze the milk along the ductal network and eventually deliver nutrients to the nipple (27, 28).
Thus, the unique postnatal development and maturation processes make the mammary gland an ideal model for studying the role of Ocln and other TJ components in organ homeostasis and maintenance. Indeed, increasing evidence now shows that TJ integrity is essential for the secretory function of the mammary gland (22, 29, 30). As a defining feature of epithelial organs, for example, TJs are essential for tissue polarity (31) and thus are indispensable for mammary gland physiology. In addition, TJs of the mammary epithelium, which are initially very leaky and permeable to large metabolites during pregnancy, undergo a rapid “closure” process whereby they become much more impermeable on parturition (10, 11). Compromise to this closure process from a chemical insult (e.g., EGTA treatment), a pathological insult (e.g., inflammation due to mastitis), or increased intraluminal pressure due to milk stasis when suckling is stopped often leads to a reduction or cessation of milk secretion (32, 33).
Studies have shown that members of the Claudin and ZO families are expressed during mammary gland development (29). However, the precise role of these TJ components has remained largely unclear. A previous study found that mice lacking Ocln fail to nurse their young due to unknown reasons (8). In the present study, we hypothesized that Ocln is essential for milk secretion by regulating TJ function. Here we report data from our test of this hypothesis using a combination of genetics, biochemistry, confocal and electron microscopy, single-cell transcriptomics, and various other assays.
Results
Pups of Female Mice Lacking Ocln Died Shortly after Birth due to Severe Starvation because of Defective Mammary Gland Function.
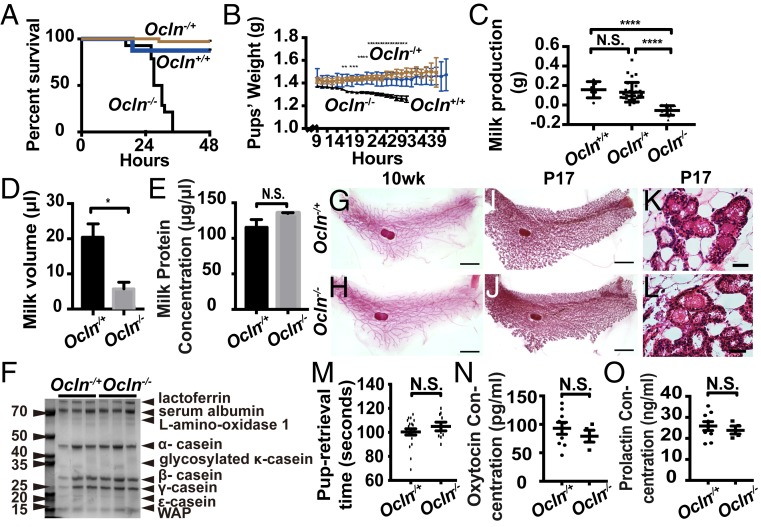
Consistent with a previous report (8), we found that pups born to Ocln−/− mice, which included all three genotypes Ocln+/+, Ocln −/+, and Ocln−/−, died within 48 h after birth (Fig. 1A). Moreover, their weight gain, as measured every 2 h during the first 48 h, was slower than that seen in pups born to Ocln+/+ or −/+ female mice (Fig. 1B). Using both an indirect (Fig. 1C) and direct (Fig. 1D) measurements of milk production (34), we found that Ocln null mice produced nearly 75% less milk compared with Ocln+/+ and Ocln−/+ mice. Because we observed no obvious differences between Ocln+/+ and Ocln−/+ control mice in these assays, we decided to use Ocln−/+ mice as the control mice for all subsequent analyses.
Fig. 1.
Pups of female mice lacking Ocln died shortly after birth due to severe starvation because of defective mammary gland function. (A) Kaplan–Meier survival analysis of the pups born to Ocln null homozygotes (−/−; n = 22), heterozygotes (−/+; n = 41), and wild-type (+/+; n = 18) female littermates. (B) Time course of weight gains of pups nursed by Ocln−/− (n = 3), Ocln−/+ (n = 6), and Ocln+/+ (n = 3) female mice. (C) Milk production as measured by the weight gain of pups during 30 min of feeding time after a 2-h separation from nursing Ocln female mice. (D) Milk volume as measured by direct harvests from the nipple. (E) Milk protein concentrations. (F) Milk protein composition as measured by Western blot analysis and Coomassie blue staining. (G–L) Epithelial morphology as indicated by whole-mount mammary gland staining with carmine red (G–J) and H&E staining (K and L) in Ocln null and control mice. (Scale bars: 4 mm in G–J; 50 µm in K and L.) (M) Time measured in seconds that took a nursing dam to retrieve pups separated from her. There were no significant differences in pup retrieval time between Ocln null and control mice. (N and O) Serum oxytocin (N) and prolactin (O) levels in Ocln null and control mice, as detected by enzyme-linked immunosorbent assay. N.S., not significant. N.S. ≥ 0.05; *P < 0.05; ****P < 0.0001.
Interestingly, despite reduced milk production, both the concentration and composition of milk proteins of Ocln null mice were similar to those of the control mice (Fig. 1 E and F). Indeed, an extensive analysis of milk protein composition using mass spectrometry showed that most of the major milk proteins, including casein proteins, were expressed at similar levels in Ocln null and control mice (SI Appendix, Fig. S1A). Taken together, these data indicate that pups born to Ocln null mice died because of severely reduced milk production in the mutant mice.
Whole-mount staining with carmine aluminum dye revealed the presence of a mature epithelial tree (Fig. 1 G and H) in both 10-wk-old adult Ocln null and control mice, and showed that their ductal networks were elaborated to a similar extent with alveolar structures during late pregnancy (Fig. 1 I–L). These data suggest that alveolar morphogenesis is not defective in Ocln null mice.
Pups of Ocln null mice may die for various reasons (SI Appendix, Fig. S1B); for example, pups may die because Ocln null dams had defective nursing behavior and failed to feed their pups. Therefore, we separated female mice from their pups and measured the amount of time that it took for the dams to retrieve pups to their nests for feeding. We observed a similar retrieval time in the Ocln null and control mice (Fig. 1M), suggesting that Ocln null mice did not suffer from defective nursing behavior. Alternatively, milk production may be reduced in Ocln null dams as a result of a systemic defect due to, for example, reduced levels of oxytocin or prolactin, both of which are produced by the pituitary gland and promote milk release or production through the myoepithelium or luminal epithelium, respectively. However, serum oxytocin and prolactin levels were similar in the Ocln null and Ocln−/+ control mice (Fig. 1 N and O).
Based on the foregoing findings, we concluded that pups of Ocln null females died from severe starvation due to reduced milk production by mutant dams. Furthermore, we concluded that the defects were most likely intrinsic to the mammary gland rather than extrinsic due to neurologic or systemic anomalies in mutant mice.
Luminal Epithelium Expressed OCLN and Was Defective in Mutant Mammary Glands.
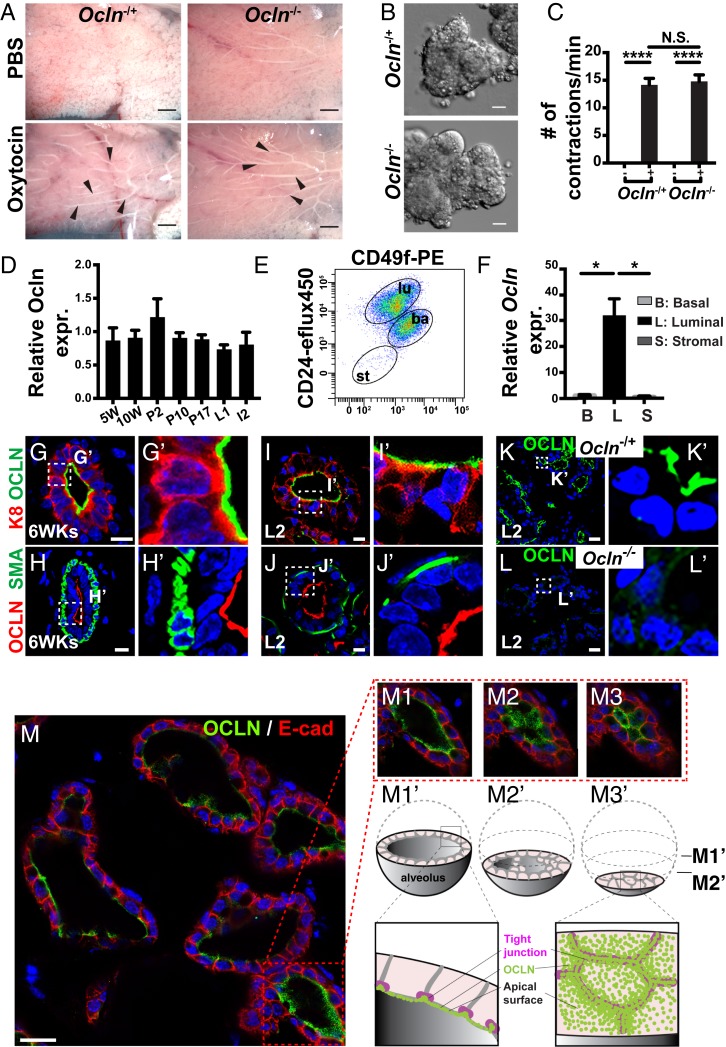
To determine whether mutant mammary glands were able to release milk on stimulation, we treated fresh whole-mount mammary glands with either oxytocin or PBS as a vehicle control. While PBS elicited no response from the mammary glands, we found that milk was released into the mammary ducts when both Ocln null and Ocln−/+ control mammary glands were stimulated by oxytocin (Fig. 2A). Indeed, we found that organoid, or a piece of mammary epithelium, preparations from both Ocln null and Ocln−/+ control mice responded to oxytocin treatment in vitro (Fig. 2B) and contracted at a similar frequency on stimulation (Fig. 2C and Movies S1 and S2).
Fig. 2.
Luminal epithelium expressed OCLN and was defective in mutant mammary glands. (A) Whole mammary glands from Ocln−/+ and Ocln−/− littermates were treated with PBS or PBS containing 20-mg/mL oxytocin, and the expulsion of milk into the ducts was evaluated. Arrowheads indicate milk release into the ducts after oxytocin exposure. (Scale bars: 2 mm.) (B and C) Mammary gland organoids were stimulated by oxytocin (B; 20 mg/mL) and the number of contractions was quantified in the presence (+) or absence (−) of oxytocin when observed under a differential interference contrast microscope. (Scale bars: 20 µm.) (D–F) Ocln mRNA expression levels as detected by qPCR. (D) RNA was harvested from mammary glands from female mice at 5 wk and 10 wk of age as virgins and at the P2, P10, P17, L1, and I2 stages. Values were normalized against actin expression, and Ocln expression at 5 wk was set as the base value against which other stages were compared. Data in the graph are mean ± SD. (E and F) MECs were sorted based on their expression of CD24 and integrin-α6 (CD49f). CD24medCD49fhi cells were basal cells, CD24hiCD49flow cells were luminal cells, and CD24negCD49fneg were stromal cells. RNA was harvested from the three cell partitions to generate DNA templates for qPCR reactions (F). (G–M3′) OCLN protein expression as detected by immunofluorescence at 6 wk after birth (G–H′) and on L2 (I–L′). (G–J′) OCLN expression, indicated by green (G, G′, I, and I′) or red (H, H′, J, and J′), was restricted to the luminal layer, which expresses K8 (green in G, G′, I, and I′), and was not found in the basal layer, which expressed smooth muscle actin (SMA; H, H′, J, and J′). (K–L′) OCLN protein expression was present in the Ocln −/+, but not in the Ocln−/−, mammary epithelium. Ocln−/+, n = 4; Ocln−/−, n = 4. The area in the dotted white box is shown in a close-up view on the right. (Scale bars: 10 µm.) (M–M3′) Serial scan along the z-axis showing close-up views (M1–M3) of an OCLN immunofluorescent-stained alveolus on L2 (M). (M1′–M3′) cartoon diagrams of M1–M3 to depict various areas of OCLN localization in the cell, from basal-lateral to apical membranes, which face the lumen, as lasers scanned progressively toward the bottom of the alveolus. Note that views in M2–M3′ accentuated OCLN staining at the cell boundaries, presumably because of its location to the TJs, as confirmed by immunoelectron microscopy (see Fig. 7A′). (Scale bar: 20 μm.) N.S. ≥ 0.05; *P < 0.05; ****P < 0.0001.
The results so far showed that the myoepithelium was functionally normal and implied that the cause of lactation failure likely resided in the luminal epithelium, which is responsible for making milk in the mammary glands. To test this possibility, we first determined the spatiotemporal expression of Ocln during mammary gland development. Using quantitative real-time PCR (qPCR), we found that Ocln mRNA was expressed at all stages examined, including epithelial branching, pregnancy, lactation, and involution stages (Fig. 2D). Furthermore, using cDNA template preparations from different subpopulations of mammary gland cells, we found that Ocln was expressed in luminal epithelial cells and was absent from both basal cells and stromal fibroblasts (Fig. 2 E and F).
Indeed, using immunofluorescence microscopy, we found that OCLN protein was present in apical domains of the K8+ luminal epithelium and was absent from basal/myoepithelium, which was marked by smooth muscle actin expression at both 6 wk of age and during the L2 stage (Fig. 2 G–J′). We did not observe OCLN expression in Ocln null mice (Fig. 2 K–L′). Furthermore, serial scanning of the mammary gland alveoli along the z-axis (Fig. 2 M–M3′) revealed that in addition to punctate staining on the apical surfaces facing the lumen, OCLN was enriched at apical cell–cell boundaries (Fig. 2 M2–M3′). This is consistent with OCLN being a TJ component, as indicated by the data from our immunoelectron microscopy studies (see Fig. 7A′).
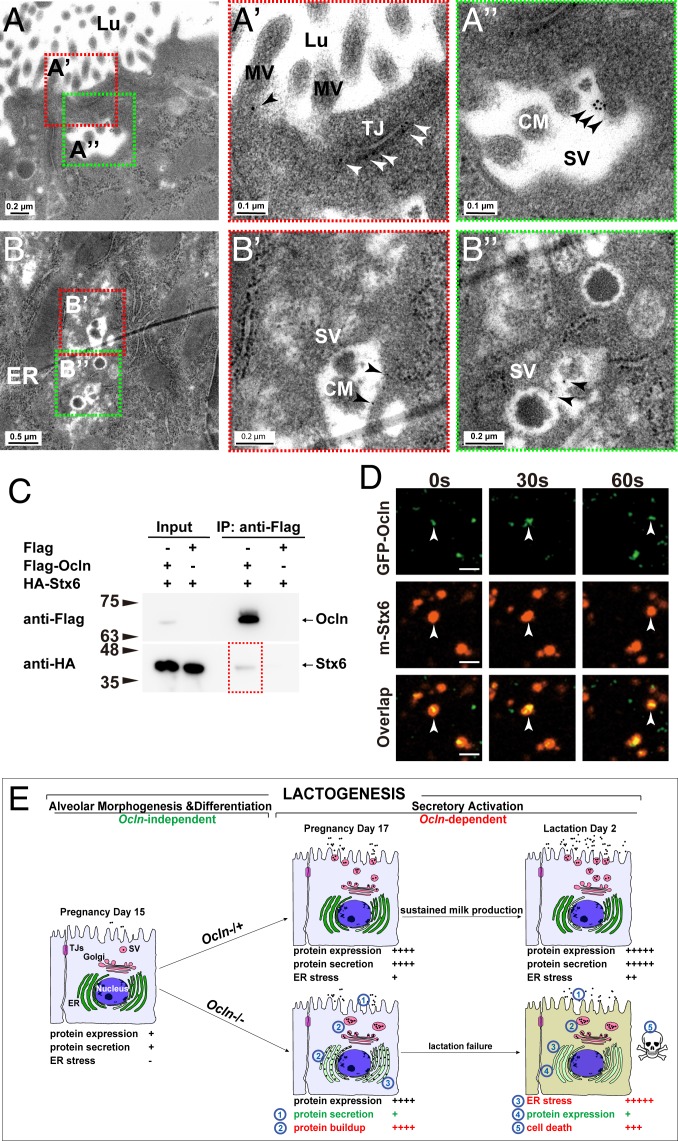
Fig. 7.
OCLN localized on SVs and bound to SNARE proteins. (A–B″) Localization of OCLN protein in luminal epithelial cells as detected by immunoelectron microscopy at the L2 stage. Primary antibodies against OCLN were visualized by a secondary antibody coupled to gold particles (black arrowheads). Overall, 19% of microvilli (out of a total of 304), 67% of TJs (out of a total of 12), and 40% of SVs (out of a total of 50) showed OCLN-positive staining. Areas in red or green dotted-boxes indicate close-up views on the right (A′, A″, B′, and B″). MV, microvillus; Lu, lumen; Nu, nucleus. Scale bars are as indicated. (C) Protein binding between OCLN and STX6 as detected by co-IP assays. OCLN was tagged by Flag protein, whereas STX6 was tagged by HA. Antibody against Flag was used for immunoprecipitation, and antibody against HA was used for subsequent Western blot analysis. Numbers indicate molecular weights of the markers (in kilodaltons). (D) Time course of localization of OCLN and STX6 as detected by fluorescent microscopy. GFP was fused in-frame with OCLN at the N terminus, whereas mCherry was fused in-frame with STX6. White arrowheads denote OCLN and STX6 particles over the time course of observation. Note that 20% of the OCLN particles colocalized with STX6 particles. (E) Schematic diagram of the lactogenic process, consisting of alveolar epithelial morphogenesis and differentiation, and secretory activation, which depends on Ocln function, in the mammary gland. In the Ocln−/+ control gland, there are progressive increases in milk protein expression and secretion, along with the accompanying stress buildup along the ER-Golgi secretory pathway during pregnancy and lactation. Ocln is essential for efficient protein secretion to meet the high demand of the greatly increased protein production and to relieve the resultant stress endured by the secretory pathway. As a result, copious amounts of milk are produced by Ocln control mammary glands to sustain pup growth. In mutant MPCs, loss of Ocln function results in defective protein secretion, which in turn causes a protein buildup along the ER-Golgi secretory pathway, including the ER and SVs. Consequently, ER stress and UPR are greatly increased, with an immediate response of shutdown of milk protein expression and, to a lesser extent, apoptosis of the MPCs that show long-term ER stress. As a result, milk production is greatly reduced, and Ocln mutant mice are unable to sustain pup survival.
Taken together, the foregoing data show that luminal epithelium is the tissue layer in which OCLN is expressed and is most likely the defective tissue responsible for the lactation failure phenotype observed in Ocln null mice. The evidence presented thus far is consistent with our hypothesis that Ocln regulates TJ functions essential for milk secretion.
Tissue Polarity and TJ Integrity Were Not Compromised by the Absence of Ocln in Mutant Mammary Glands.
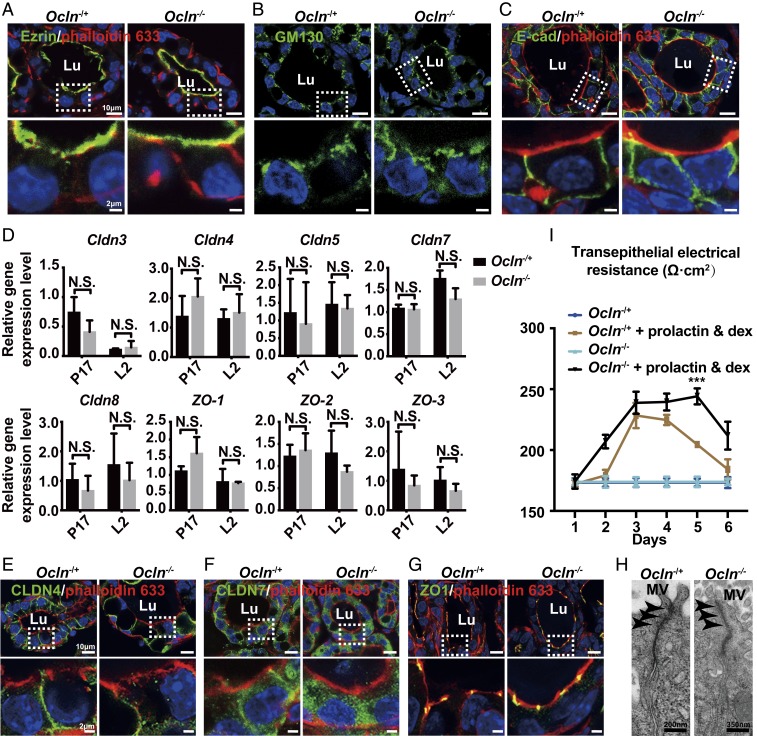
As a TJ component, Ocln may be essential for milk secretion by regulating one or both aspects of TJ function in the mammary gland, including epithelial polarity and/or barrier function. Thus, we first sought to determine whether tissue polarity was defective in Ocln null mammary glands. Using immunofluorescence against several polarity markers, including Ezrin, which denotes the apical surface; GM130, which marks Golgi orientations; and E-cadherin, which is expressed in the basolateral domains, we found that epithelial polarity was overall normal in Ocln null glands (Fig. 3 A–C).
Fig. 3.
Tissue polarity and TJ integrity were not compromised by the absence of Ocln in mutant mammary glands. (A–C) Protein expression (green) of several tissue polarity markers, including Ezrin (A), GM130 (B), and E-Cad (C), as detected by immunofluorescent staining at the L2 stage. Actin filament (red) was detected using phalloidin-Alexa 633 (A and C). Samples were counterstained with the nuclear dye DAPI (blue). (Insets) Close-up views of the alveolar epithelia. Note that GM130 and E-Cad staining was slightly more diffuse in the mutant than in the control, but their overall expression patterns were relatively normal. Ocln−/+, n = 3; Ocln−/−, n = 3. (Scale bars, A–C: Top panels, 10 μm; Bottom panels, 2 μm.) (D) mRNA expression of members of the Claudin (A) and ZO (B) families as detected by qPCR at the P17 and L2 stages. Values were normalized against actin expression, and the expression of each gene in Ocln−/+ at P17 was set as base value against which other stages were compared. The multiple t test was performed for statistical analysis, and SEM was used. (E–G) Protein expression (green) of CLDN4 (E), CLDN7 (F), and ZO1 (G), as detected by immunofluorescent staining at the L2 stage. Actin filament (red) was detected using phalloidin-Alexa 633. Samples were counterstained with nuclear dye DAPI (blue). (Insets) Close-up views of the epithelia. Ocln−/+, n = 3; Ocln−/−, n = 3. (H) TJs of the alveolar epithelial as revealed by TEM in Ocln−/+ and Ocln−/− mice at the L2 stage. MV, microvilli. Black arrowheads denote TJs. (Scale bar: Left, 200 nm; Right, 350 nm.) (I) Time course of transepithelial electrical resistance of Ocln−/+ and Ocln−/− mammary epithelia cultured in Transwell inserts under the conditions indicated. N.S. ≥ 0.05; ***P < 0.001.
We next evaluated whether TJs were normal in Ocln mutant glands. Using qPCR, we found that mRNA expression levels of members of the Claudin and ZO families, including Claudin3, 4, 5, 7, 8, and 11 and ZO1, 2, and 3, were all normal when Ocln null and control mammary glands were compared at pregnancy day 17 (P17) and at lactation day 2 (L2) (Fig. 3D and SI Appendix, Fig. S2A). Using immunofluorescence against some of these TJ components, including CLDN4, CLDN7, and ZO1, we confirmed that their protein expression indeed was not significantly changed in Ocln null mice compared with control mice at the L2 stage (Fig. 3 E–G). Furthermore, using transmission electron microscopy (TEM), we observed morphologically normal TJs in both mutant and control mammary glands at the L2 stage (Fig. 3H).
Next, we directly tested whether the best-characterized “barrier” function of TJs was compromised in Ocln null glands. To this end, we used a biotin-labeled fluorescent exclusion dye and examined the “leakiness” of the alveolar epithelium under different treatment conditions. As expected, EGTA treatment made the alveoli leakier, as evident from dye influx into the lumen, presumably due to the disruption of the TJ barrier and other cell–cell adhesions (SI Appendix, Fig. S2B). No dye was observed in the lumen in medium lacking EGTA in either Ocln null or control alveoli, suggesting that barrier function was normal in both null and control epithelium (SI Appendix, Fig. S2B). Moreover, we measured transepithelial electrical resistance (TEER) to directly evaluate paracellular ion transportation in both Ocln null and control alveolar epithelia. We detected an increase in TEER when epithelial cells were treated with prolactin and dexamethasone but otherwise observed generally similar results in Ocln null and control alveolar epithelia (Fig. 3I).
Taken together, these results suggest that Ocln is not required for either tissue polarity or TJ integrity in the luminal epithelium. Thus, contrary to our aforementioned hypothesis, the data indicate that a defect in tissue polarity or TJ function is not the likely cause of lactation failure in Ocln mutant mice.
Ocln Was Not Required for Alveolar Morphogenesis or Cell Differentiation.
It is possible that Ocln may be required for alveolar differentiation and thus for mammary gland milk production. Thus, we next examined mRNA expression of several essential regulators of this process, including Elf5, C/EBPb, RankL, Esr1, and others. We observed no significant differences in the expression of these regulators between Ocln null and control mice (SI Appendix, Fig. S3A). Moreover, activation of STAT5a, an essential downstream effector of prolactin signaling, was also normal in Ocln mutant mammary glands, based on its phosphorylation status as measured by Western blot analysis (SI Appendix, Fig. S3 D and C). Finally, we examined mRNA expression of an array of differentiation markers of the alveolar epithelium, including α-lactalbumin and Wap, and found that they were expressed at similar levels in Ocln null and control mammary epithelium (SI Appendix, Fig. S3D).
Taken together, the forgoing data show that luminal epithelial differentiation was largely normal in the Ocln null mice. Thus, Ocln likely is not required for alveologenesis in the mammary gland.
Ocln-Deficient Mammary Glands had a Lower than Normal Percentage of Milk-Producing Cells due in Part to an Increase in Apoptosis.
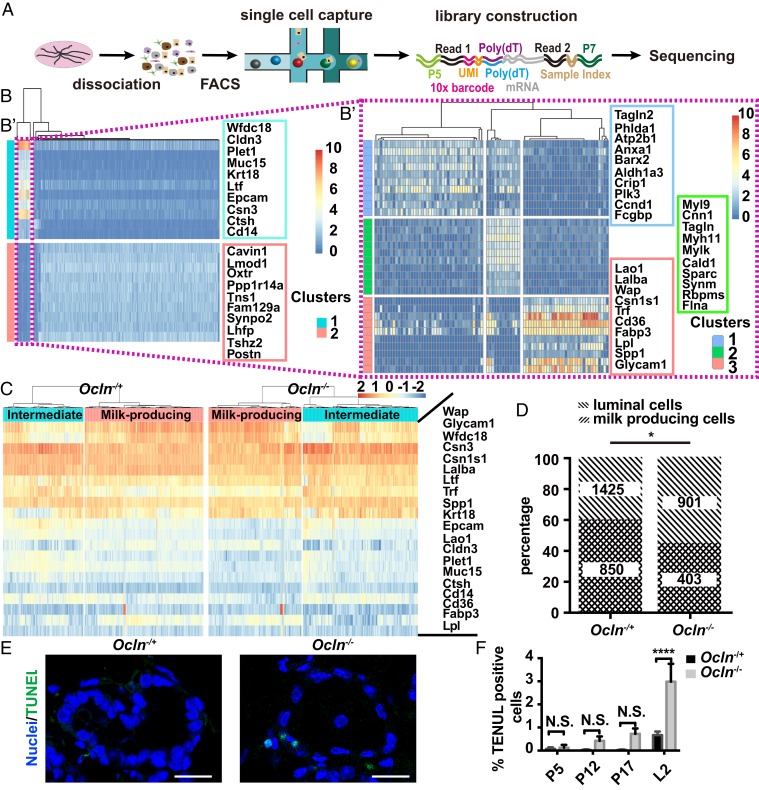
Although alveologenesis was overall normal in Ocln null mice, it is possible that differentiated cells, such as milk-producing cells (MPCs), may require Ocln for subsequent clonal expansion, survival, or other aspects of normal homeostasis and function. Because a reliable method for identifying MPCs is not yet available, we used the recently developed single-cell RNA sequencing (scRNA-seq) technology to isolate MPCs from Ocln null and control mammary glands in an unbiased manner. To this end, we prepared cDNA libraries from 1,463 mammary epithelial cells (MECs) from Ocln null mice and 1,960 MECs from Ocln−/+ mice at the L2 stage and then subjected them to sequencing (Fig. 4A).
Fig. 4.
Ocln-deficient mammary glands had a below-normal percentage of MPCs, due in part to an increase in apoptosis. (A) Diagram depicting the experimental procedures of single-cell analyses of Ocln−/+ and Ocln−/− mammary glands to determine the percentage of MPCs in these glands. (B and B′) Identification of marker genes of MPCs using published single-cell transcriptomics (35). (B) Heat map of epithelial subtypes and their gene expression levels during lactation. (B′) Luminal cells during lactation were further analyzed and divided into subtypes. The milk-producing subtype was identified along with their 20 most highly expressed marker genes. (C and D) Subtyping of luminal epithelial cells using these 20 MPC marker genes to identify the percentages of MPCs in Ocln−/+ and Ocln−/− mice (C). Percentages of MPCs among all luminal cells in Ocln−/+ and Ocln−/− mice (D). The two-sided χ2 test was performed for statistical analysis. (E and F) Cell death as detected by TUNEL staining (green) at the P5, P10, P17, and L2 stages (E) and as quantified (F). No statistically significant differences were detected at the times indicated (unpaired two-tailed Student’s t test). (Scale bars: 20 µm.) N.S. ≥ 0.05; *P < 0.05; ****P < 0.0001.
To determine marker genes specific to MPCs, we used recently published databases of single-cell transcriptomics at various stages of mammary gland development (GSE106273) (35). We first identified the luminal subpopulation of MECs and their corresponding markers, then isolated MPCs from this luminal population and identified their corresponding top-20 marker genes (Fig. 4B′). After filtering out low-quality cells and basal cells, we were left with 910 Ocln null and 1,425 control luminal cells, which were further subdivided into MPCs and intermediate cells using the aforementioned 20 MPC-specific marker genes (Fig. 4C). We found that MPCs accounted for 60% of the total luminal cells in Ocln−/+ glands, compared with only 45% in Ocln null glands (Fig. 4D). Thus, the data indicate that the percentage of MPCs in the luminal epithelium was 15% lower in Ocln null glands compared with control glands.
To determine the cause of the reduced MPC percentage in the luminal epithelium, we assayed cell proliferation and death using 5-ethynyl-2´-deoxyuridine incorporation and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analyses, respectively, during various stages of pregnancy and lactation. We found similar percentages of proliferating cells in Ocln null glands and control glands at all stages examined (SI Appendix, Fig. S4 A and B). In contrast, the percentage of apoptotic cells in Ocln null glands was similar to that in control glands at most stages except the L2 stage, when it was threefold higher in Ocln null glands (Fig. 4 E and F).
Taken together, these data reveal a 15% decrease of MPCs in Ocln null mammary glands compared with control glands, presumably due, at least in part, to increased cell death in the mutant glands during lactation. However, this 15% reduction of MPCs in Ocln null luminal epithelium is difficult to fully account for the lactation failure in the mutant gland, which had 75% less milk production than control glands (Fig. 1 C and D).
Alveolar Cells Lacking Ocln Showed Heightened Endoplasmic Reticulum Stress and Unfolded Protein Response.
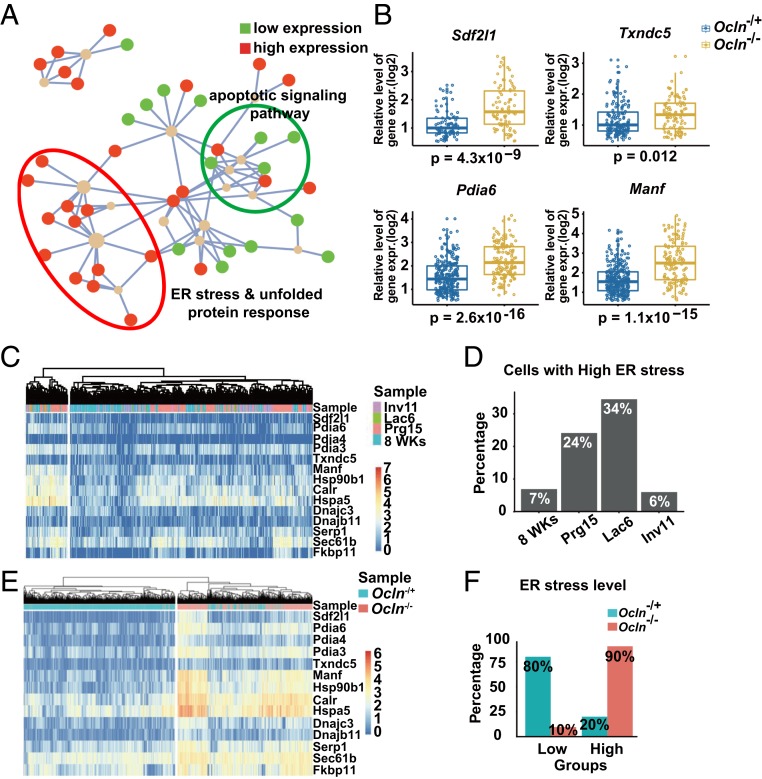
To determine the cause of increased cell death in Ocln null cells, we directly compared luminal Ocln-expressing cells from both null and control glands and examined changes in gene expression as a result of Ocln loss. We identified the 82 most up-regulated and down-regulated genes (SI Appendix, Fig. S5A) and performed Gene Ontology analysis to identify their functional relationships. Remarkably, 14 of these 82 genes, all of which were up-regulated, belonged to the endoplasmic reticulum (ER) stress and unfolded protein response (UPR) pathways, while 10 belonged to the apoptotic signaling pathway (Fig. 5 A and B and SI Appendix, Figs. S5B and S6A).
Fig. 5.
Alveolar cells lacking Ocln showed heightened ER stress and UPR. (A) Gene Ontology analysis of the differentially (P < 0.05) expressed genes (detailed list in SI Appendix, Fig. 5A) in luminal cells of Ocln control and null mice to determine the biological processes with which these genes might be involved. Red dots illustrate up-regulated genes; green dots, down-regulated genes. (B) Some of the highly expressed ER stress genes from Ocln control and null single luminal cells. Each dot indicates the expression level based on log2 of the genes in a single cell. The t test was performed for statistical analysis. (C and D) Determination of the percentage of highly ER-stressed luminal cells from published databases of single-cell transcriptomics (35). (C) The different stages of mammary gland development examined were 8 wk, on pregnancy day (Prg) 15, on lactation day (Lac) 6, and on involution day (Inv) 11. Red indicates enrichment of expression of the gene set; blue, suppression of expression. (D) Percentages of luminal epithelial cells that had either high or low expression levels of ER stress genes at these stages. (E and F) Identification of subgroups of luminal cells showing high or low levels of ER stress in Ocln−/+ and Ocln−/− mice. (E) Heatmap of expression of ER stress genes in single cells. (F) Percentages of highly or lowly ER stress cells in Ocln−/+ and Ocln−/− mice.
Previous studies have shown that ER stress and UPR are up-regulated during milk production (36, 37). To determine whether the up-regulation of ER stress and UPR in Ocln null glands is normal, we first sought to mine the published scRNA-seq datasets during virgin, pregnancy, lactation, and involution stages (35). We identified a progressive increase in the percentage of cells with high ER stress levels from the virgin to pregnancy and lactation stages. The percentage of cells with high ER stress during involution, when the demand for protein translation and secretion is no longer high, similarly returned to a state indistinguishable from that of virgin females (Fig. 5 C and D). These data confirm that increased ER stress is a normal aspect of lactation physiology, presumably due to a heightened load of protein production in the ER-Golgi secretory pathway.
We next examined expression levels of the 14 genes in the ER stress and UPR pathway from Ocln null and Ocln−/+ glands. We found that in both types of glands, MPCs could be subdivided into two groups: one with low ER stress levels and another with high ER stress levels (Fig. 5C). Indeed, in Ocln null glands, 90% of the MPCs were in the high ER stress group, and only 10% were in the low ER stress group. This is in contrast to the control glands, in which the majority of the MPCs (80%) were in the low ER stress group.
Taken together, our data suggest that Ocln is required to relieve stress normally imposed by the high demand of protein translation and secretion during pregnancy and lactation; in its absence, ER stress is not relieved in luminal epithelial cells, leading to an increase in the percentage of MPCs expressing high ER stress and UPR.
Milk Protein Secretion Was Defective in Ocln Mutant Mammary Glands.
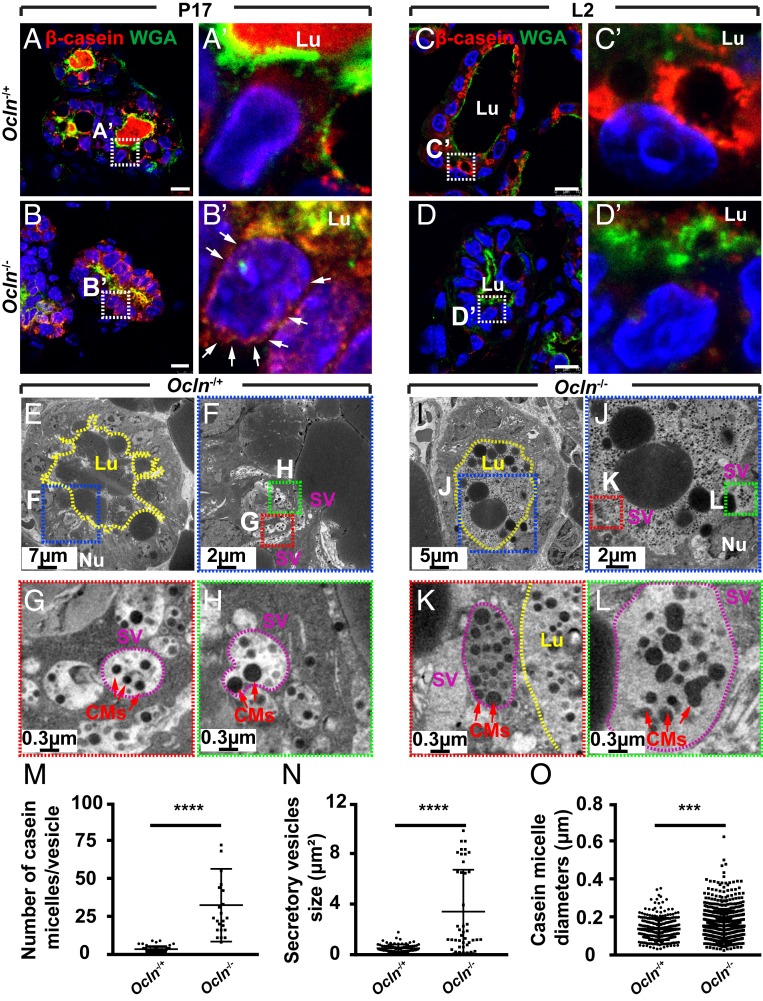
While prolonged or long-term ER stress can lead to apoptosis, an immediate or short-term response to ER stress is often the shutdown of the protein translation machinery (38). Therefore, we predicted that milk protein expression would be reduced in Ocln null mammary glands. To test this possibility, we used immunofluorescence microscopy to examine β-casein expression during pregnancy and lactation. We found β-casein expression by alveolar epithelial cells at the P17 stage, when it was also found in the lumen (Fig. 6 A and A′). By the L2 stage, β-casein expression was greatly increased, as evidenced by a huge buildup close to the apical surface (Fig. 6 C and C′). Surprisingly, we found that although β-casein was expressed by Ocln null alveolar cells at P17, it was mostly perinuclear rather than being secreted into the lumen as in the control alveolus (Fig. 6 B and B′). By the L2 stage, β-casein expression was indeed greatly diminished in Ocln null alveoli, as predicted (Fig. 6 D and D′).
Fig. 6.
Milk protein secretion was defective in Ocln null mammary glands. (A–D′) β-casein protein expression (red) as detected by immunofluorescence on mammary epithelium of Ocln−/+ and Ocln−/− mice at the P17 stage (Ocln−/+, n = 3; Ocln−/−, n = 5) and the L2 stage (Ocln−/+, n = 7; Ocln−/−, n = 7). WGA staining (green) marked the apical surface of the alveoli and was a demarcation of the lumen. Note that β-casein protein was perinucleus (white arrows), presumably in the ER in null mice at P17 (B and B′), and was greatly diminished at L2 (D and D′) compared with Ocln−/+ mice at these stages (A, A′, C, and C′). Samples were counterstained with nuclear dye DAPI (blue). (Insets) Close-up views of the alveolar epithelia. (Scale bars: 10 µm.) (E–O) TEM micrographs of mammary gland alveoli from Ocln−/+ and Ocln−/− mice at the L2 stage (E–L). Note that while SVs (purple circles) usually contained a few CMs (red arrows) in Ocln−/+ alveoli, they often contained far more CMs in Ocln−/− SVs, which were often larger than normal in mutant alveoli. Lu, lumen; Nu, nucleus. Scale bars are as indicated. (M–O) Quantitative comparisons of SVs and CMs between Ocln−/+ and Ocln−/− mice. Statistical analysis was performed using t test; ***P < 0.001; ****P < 0.0001.
The perinuclear presence of β-casein, presumably an indication of its accumulation in the ER-Golgi secretory pathway, may result from defective secretion of milk proteins. To test this possibility, we examined tissue preparations from Ocln null and control mammary glands by TEM at the L2 stage. We observed a large number of SVs, each containing a few CMs, close to the apical membrane in Ocln control alveolar cells (Fig. 6 E–H). Interestingly, the SVs in Ocln null alveolar cells were often larger and contained a greater number of CMs that were often larger and more irregular than those in Ocln−/+ glands (Fig. 6 I–O and SI Appendix, Fig. S7 A–D).
Together, these data support the function of Ocln in regulating protein secretion. Thus, in the absence of Ocln function, milk protein secretion becomes defective. This then leads to protein buildup in the secretory pathway and subsequent increases in ER stress and UPR in the mutant alveolar cells. These findings suggest that the lactation phenotype observed in Ocln null mammary glands is an indirect consequence of down-regulation of milk protein expression due to increased ER stress in the absence of Ocln function.
OCLN Localized to Secretory Vesicles and Bound to SNARE Proteins.
Ocln may regulate protein secretion directly or indirectly. To differentiate these two mechanisms, we examined whether OCLN protein localized to the protein secretory machinery. Using immunoelectron microscopy on ultrathin mammary gland tissue sections at the L2 stage, we identified the presence of OCLN in TJs, as expected (Fig. 7 A and A′). Consistent with our immunofluorescence data showing punctate staining of OCLN (SI Appendix, Fig. S3 D–E′), we found that OCLN was present on microvilli on the apical surfaces of luminal epithelial cells (Fig. 7 A and A′). Importantly, OCLN was also detected on the SVs (Fig. 7 A, A″, and B–B), whose characteristic CMs distinguish them from endocytic vesicles. These findings suggest that OCLN is a part of the protein secretory machinery on the SVs.
SNARE proteins are essential regulators of protein secretion that directly participate in the fusion process between SVs and the plasma membrane (39). OCLN may regulate protein secretion and may bind to SNARE protein and directly participate in the secretory process. Thus, we used two independent methods to examine whether OCLN binds to SNARE proteins, including VAMP4, SNAP23, and Syntax6 (STX6), which are expressed in the lactating mammary gland (40). We first “tagged” OCLN and SNARE proteins at their N termini with FLAG and hemagglutinin (HA) peptides, respectively, then subjected these fusion proteins to coimmunoprecipitation (co-IP) to test their potential binding. We found that STX6 bound to OCLN (Fig. 7C), as did VAMP4 and SNAP23 (SI Appendix, Fig. S8 A and B). These in vitro data demonstrate that OCLN could bind to several SNARE proteins.
If OCLN can bind to SNARE proteins inside living cells, they should be able to colocalize at the same places under live imaging microscopy. To test this possibility, we fused OCLN and SNARE proteins at their N-termini with green fluorescent protein (GFP) and mCherry, respectively. Lentiviral constructs expressing these fusion proteins were then used to transfect primary MECs, which were observed under fluorescence confocal microscopy. We found that OCLN frequently colocalized with the SNARE proteins, and they often migrated together inside the cells (Fig. 7E, SI Appendix, Fig. S6 C and D and Table S1, and Movies S3–S5).
Taken together, the foregoing data demonstrate that OCLN localizes to SVs and could directly participate in secretion by binding to SNARE protein in MECs.
Discussion
Previous studies have shown that mice lacking Ocln fail to nurse their young for unknown reasons. Here we confirmed the lactation phenotype and tested our hypothesis that Ocln is essential for milk secretion by regulating TJ function. Consistent with this hypothesis, we found that lactation failure was intrinsic to defects in the mutant mammary gland rather than systemic in the Ocln null mice. Furthermore, OCLN was expressed throughout mammary development and was restricted to the luminal epithelium, where TJs reside. Contrary to our hypothesis, however, Ocln null epithelium showed normal morphogenesis, cell differentiation, tissue polarity, and TJ integrity and function. Remarkably, we found that MPCs lacking Ocln exhibited high levels of ER stress and UPR owing to defective protein secretion. This in turn caused a shutdown of milk protein expression in Ocln null MPCs and ultimately led to lactation failure in the mutant mice. Consistent with a role in protein secretion, OCLN resided on SVs and bound to SNARE proteins, including VAMP4. Taken together, our results demonstrate that Ocln directly regulates apical protein secretion.
Ocln Is Not Required for the Conventional Functions of the TJs in the Epithelium.
Our hypothesis that Ocln is essential for milk secretion by regulating TJ function was based on the well-accepted notion that TJ functions are essential for epithelial polarity and lactation in the mammary gland (22, 29, 30). This hypothesis was supported by our initial results showing that the defective tissue responsible for the lactation phenotype is the luminal epithelium, which expresses Ocln and contains MPCs. However, our subsequent results showed that the expression of TJ components was normal, and that both the morphology and functions of TJs were unaffected by Ocln loss in the mutant mammary epithelium. The data thus disapprove our hypothesis and conclusively demonstrate that Ocln is not required for TJ integrity in the mammary gland.
While our data do not support the early in vitro studies suggesting a conventional barrier role of Ocln (4, 7), they are consistent with more recent in vivo studies showing normal TJ assembly and maintenance despite the absence of Ocln (9). Our findings are also consistent with the reports showing normal TJs in the intestinal (8), gastric, bladder (41), and cochlea epithelia lacking Ocln (42). Based on our findings, while we cannot exclude the possibility that Ocln may function in a traditional barrier role of TJs in certain developmental contexts, we conclude that in most physiological conditions examined thus far, including in the mammary gland, Ocln is not required for TJ integrity or function.
Lactation Failure of Ocln Null Mice Is an Indirect Consequence of Defective Milk Protein Secretion in MECs.
Several lines of evidence suggest that protein secretion is defective in Ocln null MPCs. First, our data show that OCLN directly resides on the membranous structures along the secretory pathway, including SVs and the plasma membrane. Second, both co-IP and live-imaging microscopy experiments show that OCLN can bind to SNARE proteins, which are essential regulators of protein secretion. Third, in the absence of Ocln, the secretory machinery shows abnormal morphology, including SVs bulging outside of the plasma membrane, abnormally large SVs, and distended ER. Finally, conspicuous protein buildup in both the SVs and ER was observed in Ocln mutant cells. We conclude that Ocln regulates protein secretion in the mammary gland luminal epithelium.
Although ER stress is frequently caused by unfolded or misfolded protein buildup (38), it can also be triggered by defects in ER-Golgi vesicular trafficking (43) and lysosomal transport (44). Our data further show that ER stress could be a normal physiological response to a high demand of protein expression and secretion during pregnancy and especially during lactation. Given the significant up-regulation of protein expression during lactation, it is conceivable all the steps along the protein secretory pathway, including folding, unfolding (of misfolded proteins), degradation, modification, transportation, and secretion, need to efficiently processed. Compromise of any of these steps (e.g., defective secretion resulting from Ocln loss) could cause protein buildup along the secretory pathway and thus exacerbate the ER stress already occurring as a physiological response to lactation.
Our data show a progressive increase in milk protein expression at the mRNA level throughout pregnancy. Thus, compared with their expression at P5, Wap and α-lactalbumin mRNA levels were tens of times higher at P12 and hundreds of times higher at P17 (Fig. 3P). Interestingly, by P17, pronounced milk protein secretion was already present in the alveolar lumen (Fig. 6A). Indeed, β-casein secretion defect in the Ocln null mammary glands was obvious as early as P17. These data argue against current models suggesting that parturition is a demarcation that separates alveolar differentiation from secretory activation (22, 25). Taken together, these data suggest that secretory activation occurs before parturition. A previous report suggested that milk secretion has both a constitutive and a regulative pathway (45); thus, it remains possible that constitutive secretion occurs early while it is the regulative pathway activated after parturition.
Taken together, our results show that the lactogenic process in the mammary gland is composed of two phases: alveolar epithelial morphogenesis and differentiation followed by secretory activation. Unlike the first phase, secretory activation depends on Ocln function. In the control gland, there are progressive increases in milk protein expression and secretion, along with accompanying stress buildup along the ER-Golgi secretory pathway during pregnancy and lactation. Ocln is essential for efficient protein secretion to meet the high demand of the greatly increased protein production and relieve the resultant stress endured by the secretory pathway. As a result, copious amounts of milk are produced by Ocln control mammary glands to sustain pup growth. In contrast, in the mutant glands, Ocln loss results in defective protein secretion, which in turn causes protein buildup along the ER-Golgi secretory pathway, including the ER and SVs. Consequently, ER stress and UPR are greatly increased, leading a shutdown of milk protein expression and, to a lesser extent, apoptosis of some MPCs. As a result, milk production is greatly diminished, and Ocln mutant mice fail to nurse their pups (Fig. 7E).
Mechanism of Ocln Regulation of Protein Secretion.
Although our data conclusively demonstrate that Ocln regulates protein secretion in the mammary gland, it is highly unlikely that Ocln is required for the baseline protein secretion essential for cell survival. Indeed, it is well known that defects in the basic protein secretion pathway are lethal to single eukaryotic cells and embryos (46, 47). In contrast, mammary gland formation was normal in Ocln null mice. Thus, Ocln appears to promote protein secretion efficiency and is essential for a phase of mammary gland development starting in mid-pregnancy, when a greatly up-regulated protein expression machinery demands effective secretion at the same time.
At present, the molecular detail of how Ocln may facilitate the secretion process has remained unclear. As a finely regulated multistep process, protein secretion is composed of docking, priming, and fusion of secretory vesicles (39). Together with the SNARE proteins, Ocln may regulate one or more of these steps. It is interesting that abnormal fusion of SVs with the plasma membrane was observed in Ocln null alveolar cells (SI Appendix, Fig. S6E). Thus, OCLN may regulate or facilitate the fusion process between the SVs and plasma membrane. However, we cannot exclude the possibility that OCLN may participate in other aspects of protein secretion as well, including size control of the SVs, which were much larger in Ocln null MPCs than in control MPCs (Fig. 6 and SI Appendix, Fig. S6).
Even though it is the only member of its family, OCLN shares with several other proteins a “MARVEL” domain in its four-pass transmembrane domains (48). The function of the MARVEL domain is currently unknown, although it may be important for protein–lipid interactions (49). However, MAL, the founding member of the MARVEL family, has been shown to play a role in apical protein and membrane trafficking (50–52). Thus, a functional dissection of the different domains of Ocln, especially the MARVEL domain, is important to learn how Ocln regulates protein secretion in the mammary gland.
Secretory Regulation: A General Function of Ocln?
An important question is whether Ocln regulates protein secretion in organs other than the mammary gland. Interestingly, previous reports have shown that disruption of Ocln in several contexts causes activation of the extrinsic pathway of apoptosis (53, 54). Furthermore, Ocln null mice are deaf because their outer hair cells are lost due to apoptosis (42). The mutant mice also suffer from a loss of chief and parietal cells in the stomach at around 6 wk of age (8, 41). In all these cases, why and how Ocln null cells are remain unclear.
As evident from TUNEL analysis and scRNA transcriptomics (Figs. 4 E and F and 5A), our results show that Ocln null cells undergo apoptosis as a result of heightened ER stress due to defective protein secretion. Consistent with a role in secretory regulation, our preliminary results show that OCLN is expressed in certain secretory cells of the stomach, salivary glands, and pancreas, some of which are lost during postnatal life of Ocln null mice (8). Thus, whether Ocln null cells are apoptotic in other developmental contexts and, if so, whether apoptosis is a result of defective secretion remain interesting questions for future studies.
Here we have described functions of Ocln, a prototypical TJ component whose physiological function has remained controversial. Future studies should examine whether Ocln has similar functions in professional secretory cells of other vertebrate organs as well. Moreover, it is tempting to speculate whether other TJ components participate in such functions as protein secretion, as we report here for Ocln. Future studies should provide insight into whether there is a new dimension of functions that can be ascribed to these “old” molecules.
Methods and Materials
Mouse Strain, Survival Rate, and Weight Gain Measurements.
Mice carrying the Ocln allele were genotyped as described previously (9). Mice were housed and maintained according to regulations of the ShanghaiTech University’s Institutional Animal Care and Use Committee (IACUC 2015SHT0006). Female mice were mated with males between age 10 and 12 wk. Pup survival was calculated as the percentage of surviving pups in each litter at 48 h postpartum. For cross-feeding experiments, seven heterozygote pups born to wild-type female mice were fostered to each wild-type or mutant mother at the time of parturition. The pups were weighed every 2 h for 2 d, and average pup weight was calculated. Survival of the pups was recorded to perform the Kaplan–Meier survival analysis.
Measurement of TEER.
Primary MECs were grown on collagen-coated polycarbonate membrane Transwell filter inserts in a 24-well plate at a density of 1 × 105 cells/cm2. Culture medium containing DMEM/F12 (Invitrogen) with 10% (vol/vol) FBS (Sigma-Aldrich), 5 μg/mL insulin, 1 μg/mL hydrocortisone, 10 ng/mL EGF, 100 U/mL penicillin, and 100 μg/mL streptomycin was used. The medium was changed every 2 d until a confluent monolayer formed. TEER was measured using a Millicell ERS-2 epithelial voltohmmeter (Merck Millipore).
Detailed methods are available in the SI Appendix.
Data Availability.
The data to perform the analyses have been deposited in the SRA database under accession code SRR10481973 and SRR10481974.
Supplementary Material
Acknowledgments
We thank Dr. Winnie Shum for sharing materials and reagents and Drs. Christopher Antos and Jianlong Sun for critically reading the manuscript. We also thank the Mouse Core Facility at the National Institute for Protein Science Center at Shanghai, Jiabo Biotechnology; Drs. Yu Kong and Xu Wang (Electron Microscopy Facilities of Neuroscience, Chinese Academy of Science) for assistance with EM sample preparation and EM images analyzation work; and the Molecular Imaging Core Facility and the Bio-Electron Microscopy Facility at ShanghaiTech University. This work was supported by grants from the Ministry of Science and Technology of China (2017YFA0103502, to P.L.), National Science Foundation of China (31671494, to P.L. and 31871332, to L.Z.), and National Key Research and Development Program of China (2018YFC1004602, to L.Z.), and startup funds from ShanghaiTech University (to P.L. and L.Z.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Database deposition: The sequences reported in this paper have been deposited in the National Center for Biotechnology Information Sequence Read Archive (SRA) (www.ncbi.nlm.nih.gov/sra/) (accession nos. SRR10481973 and SRR10481974).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1909731117/-/DCSupplemental.
References
- 1.Furuse M., Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2, a002907 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zihni C., Balda M. S., Matter K., Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J. Cell Sci. 127, 3401–3413 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Zihni C., Mills C., Matter K., Balda M. S., Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 17, 564–580 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Feldman G. J., Mullin J. M., Ryan M. P., Occludin: Structure, function and regulation. Adv. Drug Deliv. Rev. 57, 883–917 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Cummins P. M., Occludin: One protein, many forms. Mol. Cell. Biol. 32, 242–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dörfel M. J., Huber O., Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J. Biomed. Biotechnol. 2012, 807356 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuse M., et al. , Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123, 1777–1788 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saitou M., et al. , Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11, 4131–4142 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saitou M., et al. , Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J. Cell Biol. 141, 397–408 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stelwagen K., Singh K., The role of tight junctions in mammary gland function. J. Mammary Gland Biol. Neoplasia 19, 131–138 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Nguyen D. A., Neville M. C., Tight junction regulation in the mammary gland. J. Mammary Gland Biol. Neoplasia 3, 233–246 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Howard B. A., Veltmaat J. M., Embryonic mammary gland development: a domain of fundamental research with high relevance for breast cancer research. Preface. J. Mammary Gland Biol. Neoplasia 18, 89–91 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Howard B. A., Lu P., Stromal regulation of embryonic and postnatal mammary epithelial development and differentiation. Semin. Cell Dev. Biol. 25–26, 43–51 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Lu P., Werb Z., Patterning mechanisms of branched organs. Science 322, 1506–1509 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu P., Sternlicht M. D., Werb Z., Comparative mechanisms of branching morphogenesis in diverse systems. J. Mammary Gland Biol. Neoplasia 11, 213–228 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sternlicht M. D., Kouros-Mehr H., Lu P., Werb Z., Hormonal and local control of mammary branching morphogenesis. Differentiation 74, 365–381 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brisken C., Rajaram R. D., Alveolar and lactogenic differentiation. J. Mammary Gland Biol. Neoplasia 11, 239–248 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Inman J. L., Robertson C., Mott J. D., Bissell M. J., Mammary gland development: Cell fate specification, stem cells and the microenvironment. Development 142, 1028–1042 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Oakes S. R., Hilton H. N., Ormandy C. J., The alveolar switch: Coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res. 8, 207 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visvader J. E., Stingl J., Mammary stem cells and the differentiation hierarchy: Current status and perspectives. Genes Dev. 28, 1143–1158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson C. J., Khaled W. T., Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development 135, 995–1003 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Truchet S., Honvo-Houéto E., Physiology of milk secretion. Best Pract. Res. Clin. Endocrinol. Metab. 31, 367–384 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Boulan E., Macara I. G., Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 15, 225–242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellman I., Nelson W. J., Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 9, 833–845 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McManaman J. L., Neville M. C., Mammary physiology and milk secretion. Adv. Drug Deliv. Rev. 55, 629–641 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Anderson S. M., Rudolph M. C., McManaman J. L., Neville M. C., Key stages in mammary gland development. Secretory activation in the mammary gland: It’s not just about milk protein synthesis! Breast Cancer Res. 9, 204 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart M. K., et al. , The severity of mammary gland developmental defects is linked to the overall functional status of Cx43 as revealed by genetically modified mice. Biochem. J. 449, 401–413 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mroue R., Inman J., Mott J., Budunova I., Bissell M. J., Asymmetric expression of connexins between luminal epithelial—and myoepithelial—cells is essential for contractile function of the mammary gland. Dev. Biol. 399, 15–26 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumgartner H. K., et al. , Developmental expression of claudins in the mammary gland. J. Mammary Gland Biol. Neoplasia 22, 141–157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Truchet S., Chat S., Ollivier-Bousquet M., Milk secretion: The role of SNARE proteins. J. Mammary Gland Biol. Neoplasia 19, 119–130 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Shin K., Fogg V. C., Margolis B., Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22, 207–235 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Nguyen D. A., Parlow A. F., Neville M. C., Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J. Endocrinol. 170, 347–356 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Fischer A., et al. , Impaired tight junction sealing and precocious involution in mammary glands of PKN1 transgenic mice. J. Cell Sci. 120, 2272–2283 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Zhang R., et al. , Th-POK regulates mammary gland lactation through mTOR-SREBP pathway. PLoS Genet. 14, e1007211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bach K., et al. , Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing. Nat. Commun. 8, 2128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Invernizzi G., Naeem A., Loor J. J., Short communication: Endoplasmic reticulum stress gene network expression in bovine mammary tissue during the lactation cycle. J. Dairy Sci. 95, 2562–2566 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Ren S., et al. , IRE1 phosphatase PP2Ce regulates adaptive ER stress response in the postpartum mammary gland. PLoS One 9, e111606 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter P., Ron D., The unfolded protein response: From stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Han J., Pluhackova K., Böckmann R. A., The multifaceted role of SNARE proteins in membrane fusion. Front. Physiol. 8, 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chat S., et al. , Characterisation of the potential SNARE proteins relevant to milk product release by mouse mammary epithelial cells. Eur. J. Cell Biol. 90, 401–413 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Schulzke J. D., et al. , Epithelial transport and barrier function in occludin-deficient mice. Biochim. Biophys. Acta 1669, 34–42 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Kitajiri S., et al. , Deafness in occludin-deficient mice with dislocation of tricellulin and progressive apoptosis of the hair cells. Biol. Open 3, 759–766 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper A. A., et al. , Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313, 324–328 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson J. L., et al. , Upregulation of the Rab27a-dependent trafficking and secretory mechanisms improves lysosomal transport, alleviates endoplasmic reticulum stress, and reduces lysosome overload in cystinosis. Mol. Cell. Biol. 33, 2950–2962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner M. D., Rennison M. E., Handel S. E., Wilde C. J., Burgoyne R. D., Proteins are secreted by both constitutive and regulated secretory pathways in lactating mouse mammary epithelial cells. J. Cell Biol. 117, 269–278 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suh Y. H., et al. , Deletion of SNAP-23 results in pre-implantation embryonic lethality in mice. PLoS One 6, e18444 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lodish H. F., Molecular Cell Biology (Macmillan Learning, ed. 8, 2016). [Google Scholar]
- 48.Sánchez-Pulido L., Martín-Belmonte F., Valencia A., Alonso M. A., MARVEL: A conserved domain involved in membrane apposition events. Trends Biochem. Sci. 27, 599–601 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Raleigh D. R., et al. , Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol. Biol. Cell 21, 1200–1213 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puertollano R., et al. , The MAL proteolipid is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin in Madin-Darby canine kidney cells. J. Cell Biol. 145, 141–151 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puertollano R., Alonso M. A., MAL, an integral element of the apical sorting machinery, is an itinerant protein that cycles between the trans-Golgi network and the plasma membrane. Mol. Biol. Cell 10, 3435–3447 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martín-Belmonte F., Arvan P., Alonso M. A., MAL mediates apical transport of secretory proteins in polarized epithelial Madin-Darby canine kidney cells. J. Biol. Chem. 276, 49337–49342 (2001). [DOI] [PubMed] [Google Scholar]
- 53.Beeman N., Webb P. G., Baumgartner H. K., Occludin is required for apoptosis when claudin-claudin interactions are disrupted. Cell Death Dis. 3, e273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beeman N. E., Baumgartner H. K., Webb P. G., Schaack J. B., Neville M. C., Disruption of occludin function in polarized epithelial cells activates the extrinsic pathway of apoptosis leading to cell extrusion without loss of transepithelial resistance. BMC Cell Biol. 10, 85 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data to perform the analyses have been deposited in the SRA database under accession code SRR10481973 and SRR10481974.