Significance
The mammalian placenta is a target for endocrine-disrupting chemicals, such as the widely prevalent plasticizer bisphenol A (BPA). Consumer unease with BPA has led to manufacture of substitutes, such as bisphenol S (BPS). Here, we used a multiomics approach to study effects of developmental exposure to BPA and BPS on midgestational mouse placenta. BPA and BPS caused almost identical changes in gene expression; similar morphological defects in the junctional zone, which forms the interface of the placenta with maternal endometrium; and marked alterations in placental content of neurotransmitters serotonin and dopamine. The results imply that in rodents there could be associated effects on the placental–fetal brain axis. We also conclude that BPS should be regarded as hazardous as BPA.
Keywords: trophoblast, endocrine disruptor, transcriptomics, neurotransmitters, labyrinth
Abstract
Placental trophoblast cells are potentially at risk from circulating endocrine-disrupting chemicals, such as bisphenol A (BPA). To understand how BPA and the reputedly more inert bisphenol S (BPS) affect the placenta, C57BL6J mouse dams were fed 200 μg/kg body weight BPA or BPS daily for 2 wk and then bred. They continued to receive these chemicals until embryonic day 12.5, whereupon placental samples were collected and compared with unexposed controls. BPA and BPS altered the expression of an identical set of 13 genes. Both exposures led to a decrease in the area occupied by spongiotrophoblast relative to trophoblast giant cells (GCs) within the junctional zone, markedly reduced placental serotonin (5-HT) concentrations, and lowered 5-HT GC immunoreactivity. Concentrations of dopamine and 5-hydroxyindoleacetic acid, the main metabolite of serotonin, were increased. GC dopamine immunoreactivity was increased in BPA- and BPS-exposed placentas. A strong positive correlation between 5-HT+ GCs and reductions in spongiotrophoblast to GC area suggests that this neurotransmitter is essential for maintaining cells within the junctional zone. In contrast, a negative correlation existed between dopamine+ GCs and reductions in spongiotrophoblast to GC area ratio. These outcomes lead to the following conclusions. First, BPS exposure causes almost identical placental effects as BPA. Second, a major target of BPA/BPS is either spongiotrophoblast or GCs within the junctional zone. Third, imbalances in neurotransmitter-positive GCs and an observed decrease in docosahexaenoic acid and estradiol, also occurring in response to BPA/BPS exposure, likely affect the placental–brain axis of the developing mouse fetus.
The placenta is a transient organ that anchors the developing fetus to the wall of the reproductive tract and plays multifaceted roles in nutrient, gas, and waste exchange. In eutherian mammals, it produces a range of hormones and cytokine factors that couple the needs of the fetus to responses in the mother. Additionally, in many species, including rodents and humans, that have a hemochorial type of placentation, the syncytiotrophoblast cells involved in nutrient and gas exchange become bathed in maternal blood (1), rendering them potentially vulnerable to toxicants circulating within the maternal bloodstream. While the placenta detoxifies certain xenobiotic chemicals, as an endocrine organ, it can also be affected by those compounds that interfere with its endocrine functions. One such endocrine-disrupting chemical (EDC) that is widely prevalent in the environment and present in various household items is bisphenol A (BPA) (2). In 2013, it was estimated that 15 billion lbs. of this chemical were manufactured (3). Approximately 93% of the US population has detectable amounts of BPA in their urine, suggestive of widespread exposure (4). Exposure to BPA and bisphenol S (BPS) is primarily dietary (5, 6), although there are additional routes of contact (7, 8). Additionally, BPA can be readily transmitted from mother to offspring via the placenta (9, 10) and is, therefore, a major environmental concern during pregnancy. Because of consumer unease with BPA, more manufacturers are beginning to use BPA substitutes, such as BPS, as plasticizers in household products. However, some reports indicate that such analogs induce similar outcomes as BPA (11, 12).
In the recent decade, there have been several studies examining the effects of BPA on the mouse placenta (13–22). Other studies have focused on the effects of BPA in isolated human placenta lines and in term human placenta (23–33). A concern with using cells derived from human term tissues is that they fail to replicate the full range of placental responses associated with the critical early and midpregnancy periods (34). In addition, cell lines derived from neoplasms (e.g., BeWo, JEG-3, and JAR) or transformed in some manner may not have physiological equivalence to their normal trophoblast counterparts. Previous mouse studies have generally examined either transcriptomic responses or effects on placental morphology (13–22), but there are no definitive reports on the effects of the BPA analog, BPS (structures are shown in SI Appendix, Fig. S1). Additionally, sex differences have not been examined toward either chemical, even though the placenta is sexually dimorphic (1, 35).
Here, we have performed a comprehensive examination on how maternal exposure to BPA and BPS influence mouse placental development by midpregnancy. In doing so, we have considered whether the two chemicals provoke closely similar or distinctly different placental responses and hence, whether one chemical is potentially more benign than the other. Additionally, we examined the metabolomes of exposed and control placentas, especially for the presence of neurotransmitters, because it is now becoming evident that the placenta response to environmental insults likely influences development and function of other major fetal organs, especially the brain (1, 36).
Results
General Maternal and Fetal Measures.
Several outcomes in relation to pregnancy were compared between the BPA/BPS treatment groups and controls (SI Appendix, Tables S1 and S2). No differences were observed between the BPA/BPS and control groups in pregnancy success, maternal gestational weight, number of implantation sites, and number of fetuses. Nor were there differences in sex ratio when values were assessed according to deviation from the expected 1:1 ratio of males vs. females. However, when conceptus sex ratios in BPA and BPS groups were analyzed relative to each other and to controls, there was a trend for BPA exposure to show increased percentage of male conceptuses (56.9 ± 4.7 vs. 42.9 ± 7.9, P = 0.06). Additional experiments with many more animals would be needed to explore further whether sex ratio is distorted by BPA treatment.
Transcriptomic Analyses of Mouse Placental Tissues Exposed to BPA or BPS.
RNA sequencing (RNAseq) analysis was performed on placentas recovered from BPA- and BPS-treated and control dams at embryonic day 12.5 (e12.5), and the raw data have been deposited (37). An average of 97.06% of reads (∼124 million) was mapped. The average mapped read count after filtering the reads that mapped to random chromosomes and to mitochondrial DNA was ∼121 million (SI Appendix, Table S3).
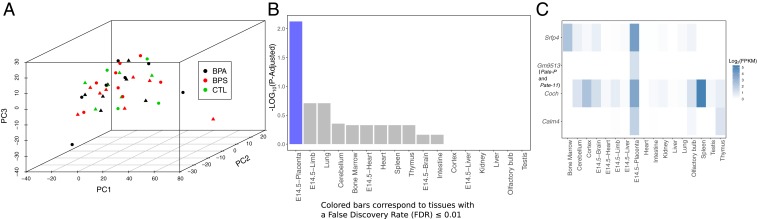
Principal component analyses (PCA) of the RNAseq data did not show any clear separation based on either treatment or the interaction of treatment with sex (Fig. 1A). Thus, we combined male and female placental results within the same group. Based on false discovery rate (FDR), only 11 genes were differentially expressed (FDR ≤ 0.05; fold change ≥ 1.5; transcripts per million ≥ 1) relative to controls in the BPS-exposed placentas, and 3 genes were differentially expressed in the BPA-exposed placentas (Table 1). On closer investigation of the differentially expressed genes (DEGs), both treatments influenced expression of a common set of 13 genes, of which 11 were down-regulated (P ≤ 0.05) and 2 (Actn2 and Efcab2) were modestly up-regulated (P ≤ 0.05). The gene set was enriched for at least four genes with transcripts that are typically augmented in placenta (Sfrp4, Coch, Gm9513, and Calm4) as determined by using the program TissueEnrich (38) (Fig. 1 B and C). One of them, Gm9513, has a sequence similar to that encoded by the Pate11 (prostate and testis expressed 11) locus on chromosome 9 of the mouse (http://www.informatics.jax.org/marker/MGI:3779923) and is a paralog of human PATE gene family members. In general, the data shown in Table 1 suggest that BPA and BPS have almost indistinguishable effects on gene expression in the e12.5 mouse placenta but influence the expression of only a handful of genes. There was no significant influence, as determined by FDR, of sex on expression of these 13 genes based on an analysis of treatment × sex interactions (SI Appendix, Table S4).
Fig. 1.
Effects of BPA/BPS on placental gene expression. (A) PCA plot using the top quartile of the most variable genes across the samples. The color in the plot represents the different treatments, and sex is represented by the shape (females are triangles, and males are dots; each symbol represents a single biological replicate for a given group). TissueEnrich analyses of differentially expressed (DE) genes in BPA/BPA vs. control placenta. (B) Tissue-specific gene enrichment analysis of genes down-regulated in BPA or BPS relative to controls shows enrichment for genes with placenta-specific expression. (C) Heatmap showing expression levels for placenta-specific genes that were down-regulated in BPA or BPS relative to controls across tissues assayed in the mouse ENCODE Project. Placenta-specific genes were determined by TissueEnrich. Calm4, Coch, and Sfrp4 are highly expressed in placenta and a small subset of other tissues (group enriched), whereas Gm9513 is only expressed in the placenta. FPKM, fragments per kilobase of transcript per million mapped reads.
Table 1.
DEGs in BPA or BPS vs. control placenta
| Gene symbol | Gene name | BPA | BPS | ||||||
| P value | FDR | Log2 fold change | Fold change | P value | FDR | Log2 fold change | Fold change | ||
| Actn2 | Actinin α2 | 1.20E-02 | 6.46E-01 | 0.69 | 1.62 | 3.04E-05 | 4.45E-02 | 1.11 | 2.17 |
| Calm4 | Calmodulin 2 | 1.13E-03 | 3.17E-01 | −3.26 | −9.55 | 5.54E-06 | 1.78E-02 | −4.49 | −22.52 |
| Coch | Cochlin | 5.02E-03 | 5.18E-01 | −2.47 | −5.55 | 1.27E-05 | 2.92E-02 | −3.80 | −13.91 |
| Cxcl14 | C-X-C Motif Chemokine Ligand 14 | 2.25E-06 | 1.81E-02 | −3.85 | −14.41 | 1.12E-04 | 1.05E-01 | −3.10 | −8.59 |
| Ear2/NR2F6 | Nuclear Receptor Subfamily 2 Group F Member 6 | 5.41E-05 | 1.09E-01 | −4.04 | −16.46 | 5.47E-06 | 1.78E-02 | −4.53 | −23.08 |
| Efcab2 | EF-Hand Calcium Binding Domain 2 | 5.49E-06 | 2.95E-02 | 0.59 | 1.51 | 8.20E-02 | 8.17E-01 | 0.23 | 1.17 |
| Epdr1 | Ependymin Related 1 | 1.36E-05 | 5.46E-02 | −2.05 | −4.14 | 2.33E-05 | 3.75E-02 | −1.96 | −3.91 |
| Gdf10 | Growth Differentiation Factor 10 | 2.36E-05 | 7.60E-02 | −2.36 | −5.15 | 2.45E-06 | 1.32E-02 | −2.61 | −6.09 |
| Gm9513/PATE1 | Prostate and Testis Expressed 1 | 5.28E-03 | 1.00E+00 | −2.16 | −4.48 | 5.18E-06 | 2.37E-02 | −3.54 | −11.6 |
| Guca2a | Guanylate cyclase activator 2a (guanylin) | 7.21E-04 | 2.76E-01 | −3.79 | −13.84 | 2.05E-05 | 3.66E-02 | −4.74 | −26.72 |
| Mmp3 | Matrix Metallopeptidase 3 | 5.53E-04 | 2.62E-01 | −2.67 | −6.38 | 8.32E-06 | 2.23E-02 | −3.46 | −11.04 |
| Rimklb | Ribosomal Modification Protein RimK Like Family Member B | 1.42E-03 | 3.41E-01 | −1.87 | −3.67 | 1.50E-05 | 3.02E-02 | −2.51 | −5.7 |
| Sfrp4 | Secreted Frizzled Related Protein 4 | 3.67E-08 | 5.91E-04 | −5.67 | −51.05 | 1.83E-07 | 2.95E-03 | −5.30 | −39.49 |
Protein–protein interactions involving RIMKIb to CALM4 and EPDR to SFRP4 were predicted by using the STRING Database (SI Appendix, Fig. S2). Functional enrichment analyses with the WEB-based Gene Set Analysis Toolkit (39) revealed that the Wingless Int-1 (Wnt) and chemokine signaling pathways, amino acid metabolism, and possibly, neurotransmission were likely affected by BPA/BPS exposures (SI Appendix, Fig. S3).
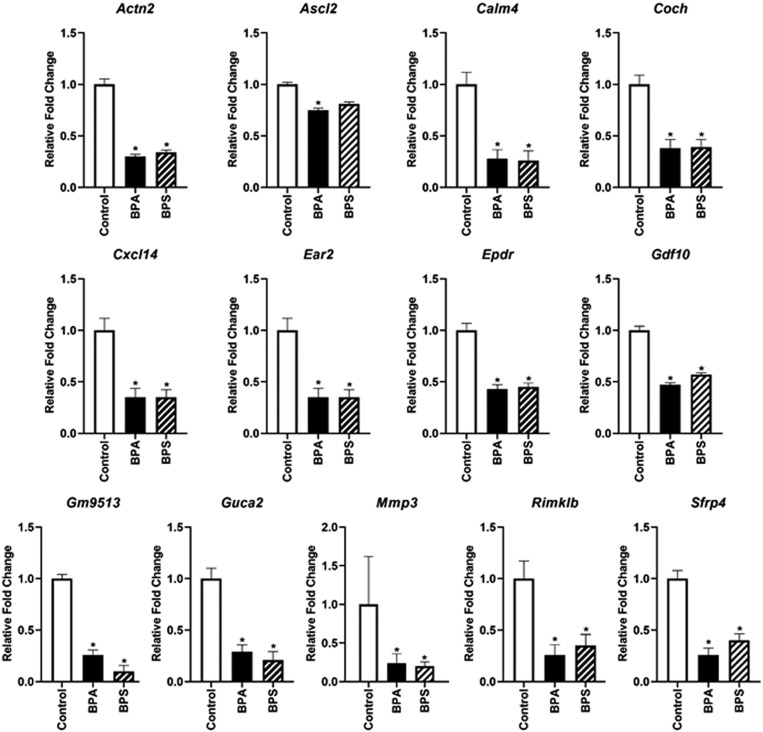
qPCR, in general, confirmed the RNAseq data shown in Table 1. The one exception was Actn2, which appeared to be slightly up-regulated as determined by RNAseq but down-regulated by the criterion of qPCR. Based on a previous report that BPA exposure altered the expression of Ascl2 (Achaete-Scute Family Basic Helix–loop–helix Transcription Factor 2) (18) and the contributory role of this imprinted gene in differentiation of the mouse placenta layers (40), we also screened the expression of this gene by qPCR analysis. Both BPA and BPS exposure appeared to reduce placental expression of Ascl2 modestly but with only BPA having a significant (P < 0.05) effect (Fig. 2). RNAseq data indicated no differences for Ascl2 expression after exposure to either BPA or BPS. The fold change data for these genes based on treatment × sex interactions are shown in SI Appendix, Table S5. Again, no significant effect of fetal sex was observed.
Fig. 2.
qPCR analyses of placental genes shown to be differentially expressed (DE) based on RNAseq analyses. qPCR analysis of the imprinted gene, Ascl2, was also analyzed as its expression may be affected by BPA (18). In the current results, it is also down-regulated in e12.5 placenta of BPA- but not BPS-exposed mice relative to controls. N = 10 to 16 biological replicates tested per group. *P ≤ 0.05.
Nontargeted Metabolomics in Fetal Placental Tissue.
A broad, nontargeted analysis of polar metabolites extracted from e12.5 placenta was performed, and the raw data have been deposited (41). These data revealed that BPA and BPS exposure resulted in a number of apparent changes based on P values ≤ 0.05 (Dataset S1 and SI Appendix, Fig. S4). As no differences were detected based on the interaction of treatment and sex, results from male and female placentas were considered together. BPA exposure lowered concentrations of d-fructose and the minor metabolites docosahexaenoic acid (DHA), sophorose (2-O-β-d-glucopyranosyl-d-glucose), and glycolic acid relative to controls (SI Appendix, Fig. S5). In BPS vs. control placentas, fatty acids were most affected, with significant decreases in stearic acid, palmitic acid, DHA, octadecenoic acid, and hexadecanoic (palmitic). d-ribose increased in concentration, while glycolic acid showed a decrease (as it did in BPA-exposed placentas).
Targeted Metabolomics of Neurotransmitters in Fetal Placental Tissue.
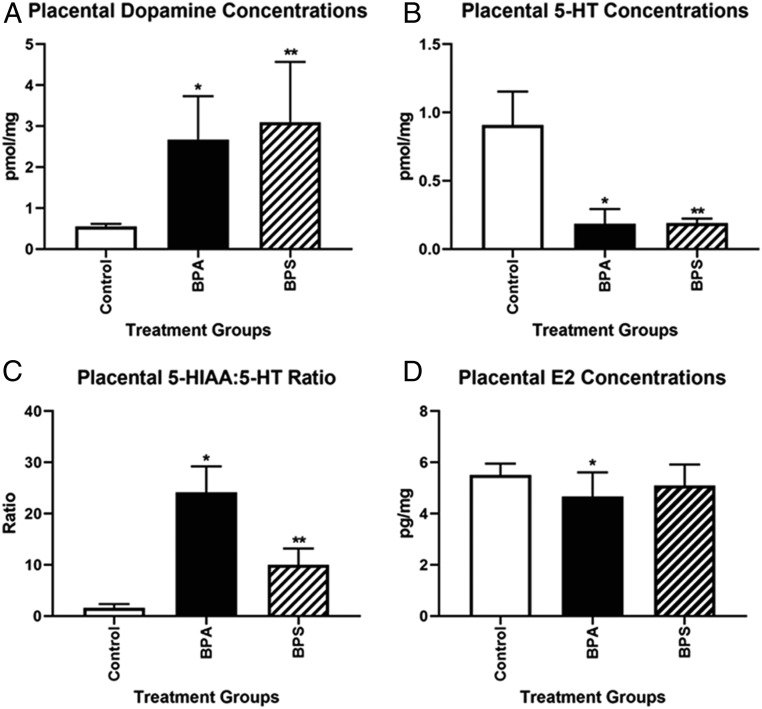
Maternal exposure to BPA and BPS significantly increased placental concentrations of dopamine (Fig. 3A). Conversely, there was a dramatic decrease in placental serotonin (5-HT) following BPA or BPS exposure (Fig. 3B). BPA and BPS increased the ratios of 5-hydroxyindoleacetic acid (5-HIAA; primary 5-HT metabolite) to 5-HT ∼25- and 10-fold, respectively (Fig. 3C). There was no effect of maternal treatment or maternal treatment × conceptus sex interaction for another neurotransmitter, γ-aminobutyric acid (GABA) (SI Appendix, Table S6).
Fig. 3.
Effects of BPA and BPS on the placental production of neurotransmitters and E2 at e12.5. (A) Both BPA and BPS increased placental dopamine concentrations. *P = 0.01; **P = 0.03. (B) Both EDCs reduced placental production of serotonin (5-HT). *P = 0.0001; **P = 0.0006. (C) The placental 5-HIAA:5-HT ratio was increased with both chemicals. *P = 0.0001; **P = 0.001. (D) Placental E2 concentrations were reduced in BPA vs. control group. N = 10 to 16 biological replicates per group. *P = 0.01.
Placental Estradiol, Estrone, Corticosterone, Testosterone, and Progesterone Concentrations.
Placental concentrations of estradiol (E2) were modestly but significantly reduced in BPA-exposed placentas compared with the control group (P = 0.01) (Fig. 3D). However, BPS exposure did not result in a significant effect. None of the other placental steroid hormones examined were affected by either maternal treatment or maternal treatment × conceptus sex interactions (SI Appendix, Table S7).
BPA/BPS-Induced Placental Histopathological Changes.
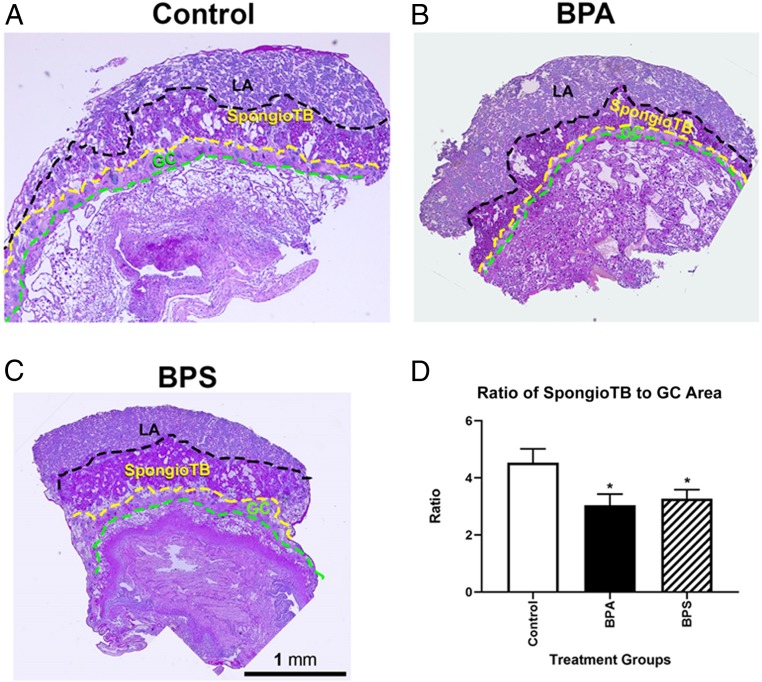
Within each placental section, the areas of labyrinth, spongiotrophoblast zone, and trophoblast giant cells (GCs) were measured. No interaction of maternal treatment and conceptus sex was observed, and thus, male and female placenta data within each group were combined to increase the power of the analyses. Exposure to either BPA or BPS reduced the ratio of the spongiotrophoblast zone to GC area (P ≤ 0.05) (Fig. 4), a result consistent with that reported by others for BPA (19, 21).
Fig. 4.
Histological images of placentas from control and BPA- and BPS-exposed mice. A–C depict the three main regions of the fetal placenta: labyrinth (LA), spongtiotrophoblast (spongioTB), and GCs. The areas of each these regions were determined as well as the ratio of the areas to each other. (D) BPA and BPS exposure reduces the spongioTB to GC ratio, suggestive that these trophoblast lineages are vulnerable to BPA/BPS exposure. N = 10 to 16 individuals (males and females combined) per group. *P ≤ 0.05.
5-HT and Dopamine Immunohistochemistry.
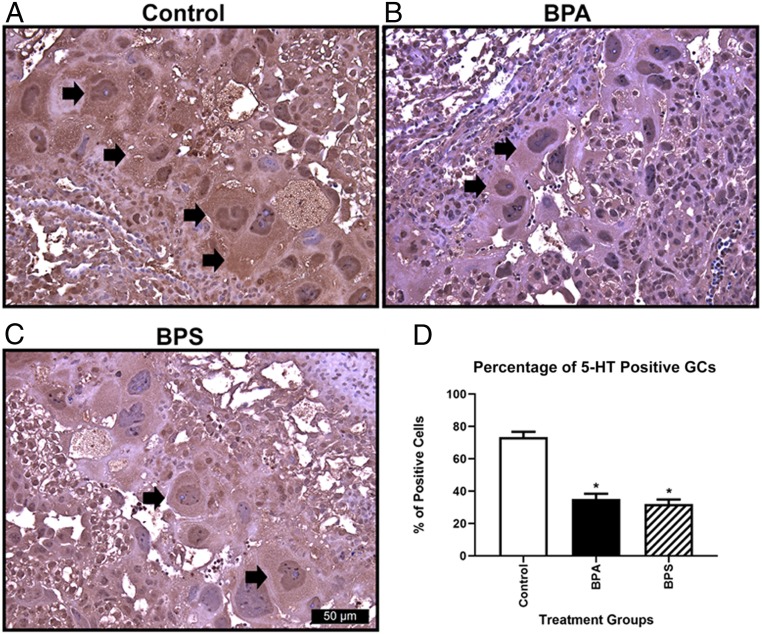
As a positive control and to establish optimal conditions for immunohistochemistry (IHC), we first confirmed that the antibody raised against 5-HT could be used to localize this neurotransmitter within neurons of the fetal brain (SI Appendix, Fig. S6 A and B). When these same conditions were applied to immunolocalize 5-HT in placental sections, it became clear that the highest expression was within trophoblast GCs of the junctional zone, although there was also some expression within the labyrinth region as well (Fig. 5 A–C). The percentage of serotonin-positive GCs was reduced in placentas that had been exposed to BPA and BPS relative to controls (P = 0.01) (Fig. 5D). Moreover, overall staining intensity in 5-HT–positive GCs also appeared to be depleted (Fig. 5A vs. Fig. 5 B and C). As there was no difference in the area occupied by GCs, we infer that there was less 5-HT in the GC compartment of BPA/BPS-exposed mice than in controls, a result consistent with whole-placental data shown in Fig. 3B.
Fig. 5.
Serotonin (5-HT) immunoreactivity in GCs. The 5-HT immunoreactivity in control (A), BPA (B), and BPS (C) GCs. (D) Determination of the percentage of 5-HT immunoreactivity GCs reveals that both BPA and BPS have decreased percentages of positively stained cells relative to control. Arrows indicate 5-HT positive immunoreactive GCs. N = 6 individuals per treatment group. *P = 0.01.
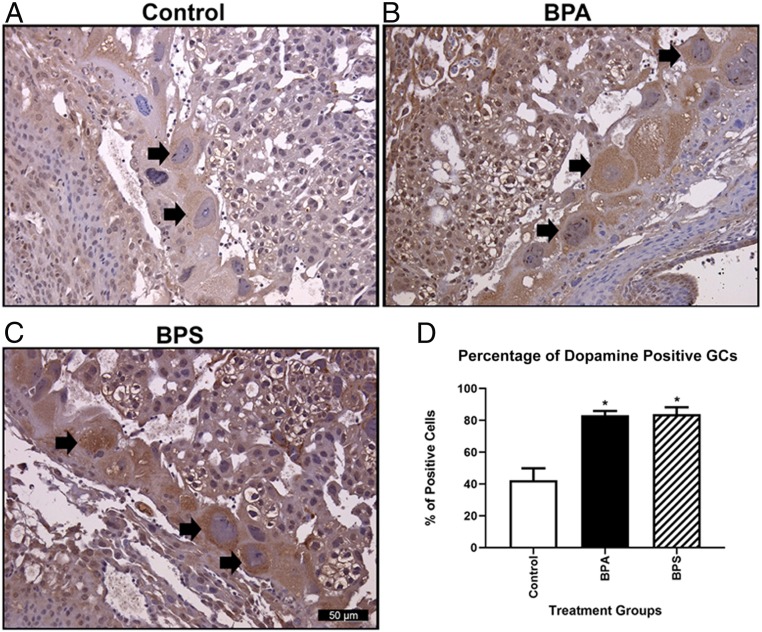
We established conditions for dopamine IHC on fetal brain sections similarly to those developed for 5-HT (SI Appendix, Fig. S6 C and D). As with 5-HT, the primary cells in the placenta staining positively for dopamine were the GCs (Fig. 6 A–C). In contrast to 5-HT, however, BPA/BPS exposure increased the percentage of dopamine-positive GCs (P = 0.001) (Fig. 6D) and increased the intensity of dopamine staining in those cells. These results are consistent with the overall two- to threefold increase in placental dopamine levels brought about by BPA/BPS exposure (Fig. 3C).
Fig. 6.
Dopamine immunoreactivity in GCs. Dopamine immunoreactivity in control (A), BPA (B), and BPS (C) GCs. (D) Determination of the percentage of dopamine immunoreactivity GCs reveals that both BPA and BPS have increased percentages of positively stained cells relative to control. Arrows indicate dopamine positive immunoreactive GCs. N = 6 individuals per treatment group. *P = 0.001.
Correlations among Analytical Data.
To examine associations between gene expression; changes in concentrations of metabolites, neurotransmitters, and steroid hormones; placental histology; and 5-HT and dopamine immunoreactivity in GCs, we used the mixOmics program (42) (SI Appendix, Fig. S7). This R-plot analysis, which does not take into account directional changes, revealed a significant correlation (r = 0.61) between the observed levels of polar metabolites and steroid hormone concentrations, suggesting a possible link between the shifts in metabolite levels brought about by BPA/BPS exposures and reduced estrogen concentrations. As inferred above, concentrations of the neurotransmitters, 5-HT and dopamine, correlated with the IHC data (r = 0.55 for 5-HT; r = 0.8 for dopamine). There was also a strong indication that the reduced area of spongiotrophoblast to GCs noted in BPA/BPS-exposed placentas was linked to the changes in neurotransmitter levels (r = 0.52).
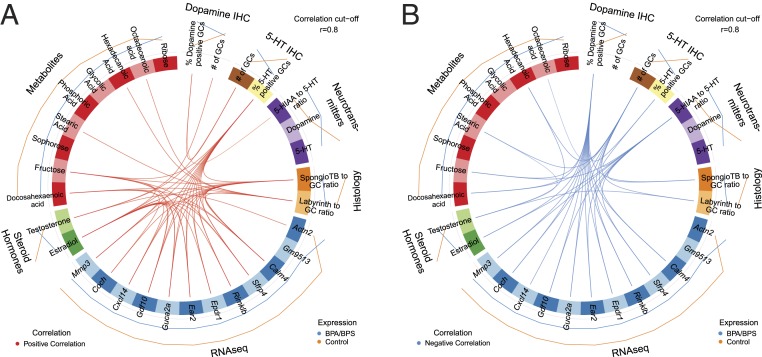
We then generated circos plots by using the combined BPA/BPS data in order to provide greater power to the analyses (Fig. 7A), and this approach revealed more detailed positive and negative correlations across datasets (Fig. 7B). The percentage of GCs positive for 5-HT, but not the concentration of 5-HT itself, correlated positively (r ≥ 0.8) with both placental fructose and placental E2 concentrations and most intriguingly, expression of Calm4, Sfrp4, Rimk1b, Epdr1, Ear2, Guca2a, Gdf10, Cxc14, Coch, and Mmp3 (i.e., 10 of the 11 genes down-regulated by exposure to BPA/BPS). The one exception, Gm9513, was not positively correlated with the number of 5-HT–positive GCs when a cutoff of r ≥ 0.8 was used. E2 concentrations correlated strongly and positively with many of the same aspects of phenotype as 5-HT–positive GCs. Examples include fructose concentration and expression of many of the same DEGs listed above.
Fig. 7.
Circos plot correlations for BPA/BPS-exposed vs. control placenta that includes DEGs, 5-HT IHC, dopamine IHC, neurotransmitter concentrations, histological proportions of the placenta, steroid hormones, and significant metabolite differences. Correlation value was set to 0.8. Red lines in the center indicate a positive correlation (A), whereas blue lines indicate a negative correlation (B). Lines on the outside of the circle indicate whether the value was greater in BPA/BPS (blue) or controls (orange). For this analysis to work, each category has to include more than one end point. Thus, number of GCs, labyrinth to GC ratio, and placenta testosterone concentrations were included even though they did not show treatment differences.
By contrast, as the percentage of GCs positive for dopamine increased, there was a corresponding loss of 5-HT–positive GCs and a negative correlation with expression of Calm4, Sfrp4, Rimk1b, Epdr1, Ear2, Guca2a, Gdf10, Cxc14, Coch, and Mmp3 and fructose and E2 concentrations (Fig. 7B). Again, it was the number of dopamine-positive cells rather than dopamine concentration per se that underpinned the correlations at r ≥ 0.8. Finally, as the 5-HIAA:5-HT ratio increased, the area of spongiotrophoblast relative to GCs in the junctional zone shrank.
Discussion
In humans, abnormal placental responses to EDCs, especially BPA, have been proposed to lead to early pregnancy loss; placental diseases, such as preeclampsia and preterm birth; and adverse health effects on offspring after birth and into adulthood, although none of these postulates have been definitively proven (43). However, it is becoming apparent in rodents that placental responses to in utero environmental insults can shape fetal brain development and postnatal behaviors of offspring through the placental–brain axis (1, 36). Although the Food and Drug Administration has not banned BPA outright in response to concerns, especially in relation to human infant exposures, manufacturers have sought alternatives, one of which is the structurally related chemical, BPS (SI Appendix, Fig. S1). Here, we have sought to compare the effects of BPA and BPS fed to pregnant mice on the phenotype of the e12.5 placenta, a stage when all of the essential features of the functionally mature placenta are evident. Our general conclusion is that BPA and BPS exert remarkably similar effects on placental gene expression, metabolome, and placental organization as well as on the content of the neurotransmitters, dopamine and 5-HT, raising the likelihood that the substitute chemical is potentially as harmful as its predecessor.
Past studies in rodents, mainly in mice, suggest that BPA can affect expression of several genes in the placenta (13–16, 18, 20, 44, 45). Some of these reports focused on a few select candidates (14–16, 18, 20, 44, 45), especially ones known to be imprinted (14, 18), while others used a global approach based on microarray analyses (13, 20) but were often underpowered. In addition, doses of BPA fed to the mother varied as did the time in pregnancy when the placentas were analyzed. Whatever the reasons, none of these earlier studies on BPA placental effects aligned with ours in terms of DEGs. One similarity with two earlier studies, however, is that the WNT signaling pathway, which is important in the functional development of trophoblast in both the human (46) and the mouse (47), appeared affected by BPA and BPS (21, 22). We have confidence in our study because it was robust both with regard to the number of samples analyzed (N = 10 to 16 biological replicates per treatment) and in the statistical treatment of data. Perhaps most reassuring was that BPA and BPS exposures both regulated a small set of 13 genes, leaving the vast majority of other expressed genes unaffected. This outcome strongly suggests that the chemicals targeted the placental transcriptome narrowly and selectively.
What particular roles any of the genes that are most influenced by BPA and BPS might play in the development of spongiotrophoblast and GCs (Fig. 4) are unclear. In our experiments, we detected a reduction in spongiotrophoblast to GC area with both treatments. BPA/BPS-induced differences in these placental cellular components may at least partially account for changes in gene expression in response to these treatments. Other studies agree that placentas from pregnant mice exposed to BPA show degenerative changes in spongiotrophoblast and/or GC layers (19, 21). A similar reduction of spongiotrophoblast area was evident in mice with a partial loss of Ascl2 (also called Mash2), a maternally imprinted gene in the IC2 cluster (40). Homozygous mutant mice that lack this gene exhibit a complete loss of spongiotrophoblast cells and die by e10 (48), while heterozygous Ascl2−/+ mice show extra layers of parietal (external to the junctional zone) GCs (48, 49). Exposure to a very high dose of BPA (10 mg/kg body weight per day) but not a lower dose (10 μg/kg body weight per day) has been reported to result in loss of imprinting and increased expression of Ascl2 from what should have been the repressed allele in e9.5 placenta (18). We failed to detect any changes in Ascl2 expression relative to controls by RNAseq analysis, although there was a hint of decreased expression in the qPCR measurements (Fig. 2). It remains unclear, therefore, whether the reduction in spongiotrophoblast area associated with BPA and BPS exposures involve Ascl2.
One gene with expression that was down-regulated in both BPA- and BPS-exposed placentas was Gm9513, which belongs to the Pate gene family, of which there are 14 members in the mouse and 4 in the human (50). Specifically, Gm9513 appears to correspond to Pate11 (originally Pate P) and is a possible ortholog of the human PATE 1 gene. Murine PATE11 seems to be relatively specific to the rodent placenta (rather than prostate and testis). PATE-like proteins have been implicated in having neuroregulatory roles and appear localized to cells with a neuroendocrine phenotype (51). At this point, however, the possible roles of Gm9513 and those of the other 10 genes down-regulated by BPA/BPS (Calm4, Sfrp4, Rimk1b, Epdr1, Ear2, Guca2a, Gdf10, Cxc14, Coch, and Mmp3) in regulating placental development are unclear. Notably, however, the reduction in spongiotrophoblast to GC area, the loss of 5-HT–positive GCs, and gain in dopamine-positive GCs do not seem to be strongly correlated with the down-regulation of Gm9513 but instead, with the other 10 genes (Fig. 7). Of these, Sfrp4 (Secreted Frizzled Protein 4) was most dramatically silenced by BPA/BPS exposure (Table 1) and is a soluble inhibitor of WNT signaling, a pathway that participates in trophoblast lineage development (52). Sfrp4−/− mutant female mice produce fewer pups per litters than controls (53), but the basis of this phenotype and whether it is associated with placentation are unknown. There is a similar lack of information with regard to a role for the other genes in mouse placentation.
The metabolomics studies on polar metabolites in the placenta indicated that BPA and BPS had minor effects, with only two metabolites, DHA and glycolic acid, significantly down-regulated by exposure to both chemicals. DHA, in particular, is essential for normal fetal brain development and regulation of levels of neurotrophin and nerve growth factor (54–57). BPS but not BPA caused reductions in stearic and palmitic acids, which are also important precursors of brain lipid components. Thus, exposure to either chemical could impact the placental–brain axis of the fetus by indirectly reducing the concentrations of a few key metabolites. Importantly, however, the variance in the metabolome data may have precluded significant shifts in other metabolites from being noted.
Perhaps the most dramatic effects of both BPA and BPS exposure were to reduce the concentration of 5-HT by over 80%, raise that of its immediate metabolite 5-HIAA, and increase the concentration of a second neurotransmitter, dopamine, three- to fivefold (Fig. 3), while the third neurotransmitter measured, GABA, was not affected (SI Appendix, Table S6). 5-HT has been previously reported to be present in the mouse placenta from e9 through e12 (58), with its presence largely limited to the GCs after e10.5 (59). One crucial role for placental 5-HT is thought to be in the development of the fetal forebrain (60) by providing a vital supplementary source of the neurotransmitter. Hence, a reduction in the number of 5-HT–positive GCs and in total 5-HT placental levels might starve the fetal brain at a critical time in its maturation, leading to long-term defects in behavior that have previously been associated with BPA exposure. By contrast to 5-HT, there is little information relating to the occurrence of dopamine in the placenta, although its presence in amniotic fluid has been inferred to have a placental origin (61). Our data demonstrate that dopamine is present in the mouse placenta in amounts comparable with those of 5-HT (Fig. 3) and that, like 5-HT, dopamine is highly concentrated in GCs within the junctional zone (Fig. 6). Whether there are classes of trophoblast GCs that can be distinguished by their 5-HT vs. dopamine content remains to be determined.
Two mouse models have been used to examine the role of 5-HT in mouse placentation. One is the tryptophan hydroxylase (Tph1) knockout mouse, which is unable to synthesize 5-HT (62), while the second lacks the Solute Carrier Family 6 Member 4 (SLC6A4), a transporter required for uptake of 5-HT (62). Both strains of mice had slightly smaller placentas than their wild-type counterparts with abnormalities mainly confined to spongiotrophoblast of the junctional zone. In other words, these knockouts provide similar phenotypes to BPA/BPS exposures. Together, these and other studies suggest that 5-HT is needed for trophoblast maintenance (63), especially within the junctional zone, and that the deleterious effects of BPA/BPS on spongiotrophoblast to GC area may be due, at least in part, to the lack of availability of 5-HT from the adjacent GCs. Interestingly, although dopamine itself has not been shown to accumulate in the rat placenta, the predominant trophoblast cells that express dopamine D1 and D2 receptors are spongiotrophoblast and GCs of the junctional zone (64), raising the possibility that the locally increased concentrations of dopamine, in addition to the reduction in 5-HT, might contribute to the observed pathology caused by BPA/BPS exposure in the mouse.
Whether the changes in concentrations of 5-HT or dopamine caused as a result of BPA/BPS exposures are on the biosynthetic or degradative pathways is unclear at present, although the accumulation of 5-HIAA could imply that 5-HT was being metabolized at a higher rate in the exposed placentas. However, there were no significant alterations in the transcript levels of the genes implicated in either the biosynthesis (e.g., tryptophan hydroxylase [TPH1 and -2], dihydroxyphenylalanine [DOPA] decarboxylase [DDC], tyrosine hydroxylase [TH]) or degradation (e.g., monoamine oxidases [MAO], catechol-O-methyl transferase [COMPT]) of 5-HT and dopamine.
Our multiomics integrative approach and the construction of circos plots revealed that surprisingly robust correlations could be made between diverse features of the BPA/BPS phenotype (Fig. 7), including, for example, the positive correlations observed between the majority of the DEGs, numbers of 5-HT–positive GCs, and placental estradiol and fructose concentrations. From these and other data discussed earlier, we infer, as others have done before (19, 21), that the target of BPA/BPS exposure is located in the junctional zone of the rodent placenta, most likely either the trophoblast GCs or spongiotrophoblast adjacent to them. As GCs are enriched for steroidogenic enzymes (65–68) and E2 may have a role in regulating expression of genes, such as tryptophan hydroxylase-2 (Tph2), Maoa, Maob, Sert, and 5-HT receptor 1a (Htr1a) (69), declining E2 concentrations in GCs could trigger reductions in 5-HT production and secondary changes in spongiotrophoblast that relies on GCs for neuroendocrine support. One previous study in rats has shown that BPA exposure reduces P450 aromatase activity in the ovary (70), but the gene encoding this protein was not among those regulated in the present placental study. It should also be noted that BPA and BPS effects on total placental E2 concentrations were modest and that only the data for BPA were significant. Importantly, however, the levels of E2 (as well as those of fructose and all other metabolites) were derived from whole-placental extracts. Local effects in the spongiotrophoblast and GCs might be considerably more dramatic, thereby explaining many of the strong correlations seen in Fig. 7.
While maternal or fetal serum BPA/BPS concentrations in the current studies were not assessed because of technical issues arising from storing the frozen tissues in polypropylene tubes, the dose of BPA used was within the presumptive “no observed adverse effect level” (NOAEL) (71–75) that yields serum concentrations comparable with those identified in human populations (5, 10, 76–79). The BPS dose was chosen based on previous reports suggesting that it caused changes in maternal and offspring behavioral patterns (80, 81). This BPS dose falls well below the toxicological NOAEL of 10 mg/kg per day (82) and can thus be considered to be low.
The present studies were solely designed to examine how BPA/BPS exposure affects the midgestational placenta (e12.5). Although we cannot be sure, it seems likely that the effects noted (e.g., on neurotransmitter levels) persist until parturition, and it does not seem unreasonable to infer that these changes directly influence fetal brain development, possibly leading to permanent neurobehavioral effects. We and others have shown that developmental exposure of fetuses in various rodent models to BPA/BPS can alter later neurobehavioral responses (76, 80, 81, 83–87). To tease apart potential BPA/BPS-induced effects through the placental–brain axis vs. direct effects on the fetal brain, it may be necessary to create conditional knockout mice that lack BPA/BPS-responsive genes in the GCs and spongiotrophoblast. Such mice are presently though unavailable.
Our study reinforces the concept that BPA or BPS exposure alter the allocation of the placental cells within the junctional zone and suggests that this may be achieved by altering the balance of 5-HT and dopamine in GCs. These observations imply that there are likely to be associated effects on the fetal brain via the placental–brain axis and raise further concerns about possible neurodevelopmental and other disorders arising in exposed offspring, at least in rodents. Caution is suggested, however, in relating these inferences to the human whose placental development differs from that of the mouse and where there is no obvious homolog for the junctional zone and its component spongiotrophoblast and GCs (88, 89).
Experimental Procedures
Sections on chemicals and reagents, fetal PCR sexing, placental RNA isolation, Illumina TruSeq RNA library preparation, sequencing and analyses, qPCR, and metabolomics, including analysis of neurotransmitters, steroid hormones, and integrative correlation analyses, are described in SI Appendix, Supplemental Methods.
Animals and Treatments.
All animal experiments were approved by the University of Missouri Animal Care and Use Committee (Protocol #8693) and conformed to the NIH Guide for the Care and Use of Laboratory Animals (90). Five- to six-week-old C57BL6J female and male mice (Jackson Labs) were placed in polypropylene cages and fed a phytoestrogen-free diet (AIN93G; Envingo). Females were provided a week habituation period before treatments were initiated. They were then randomly selected to be in one of three treatments groups: 1) provided a daily Nabisco Nilla wafer (Nabisco) containing 70% ethanol (8- to 24-μL volume as adjusted for body weight that was air dried beforehand onto the wafer); 2) provided a daily wafer containing BPA (200 μg/kg body weight reconstituted in ethanol with a similar volume as vehicle control and air dried onto the wafer; and 3) provided a daily wafer containing BPS (200 μg/kg body weight reconstituted in ethanol with a similar volume as vehicle control and air dried onto the wafer). The BPA dose falls below the diet-administered maximum nontoxic dose for rodents (200 mg/kg body weight per day), is within the presumptive NOAEL (71–75), and yields serum concentrations comparable with those identified in human populations unknowingly exposed to this chemical (5, 10, 76–79). The BPS dose was chosen based on previous reports suggesting that it disrupted maternal and offspring behavioral patterns (80, 81). This BPS dose falls well below the toxicological NOAEL of 10 mg/kg per day (82) and can thus be considered a low dosage. Females were weighed weekly, and volume of ethanol, BPA, or BPS pipetted on the wafer was accordingly adjusted. To minimize background BPA/BPS contamination, animals were housed in polypropylene cages with aspen shavings rather than corn cob and provided glass water bottles. BPA/BPS was stored in amber glass bottles with aluminum foil around them to minimize exposure to light. Both chemicals were reconstituted in polypropylene tubes. After 2 wk of receiving one of these treatments, females were paired with potential breeder males and checked the next morning for a vaginal plug. When a vaginal plug was observed, this was considered e0.5 postcoitus. If no vaginal plug was observed in the morning, the males were placed in separate cages and then repaired that evening. Females were continued on the respective treatments until they were humanely euthanized at e12.5. At this time, each uterine horn was incised, and the uterine position of each conceptus was delineated. Fetal tissue was collected for PCR sexing. Half of the fetal placental tissue (as determined by the discoid morphology) where the underlying uterine tissue was dissected away was frozen in liquid nitrogen, and the other half of the fetal placenta with underlying uterine tissue was fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline solution (PBS) for histological analyses.
Placental Histology.
Before embedding, placenta tissue for histology was marked with tattoo ink to aid in orientation. All attempts were made to obtain a cross-section through all fetal placental layers. In those sections where such layers were only partially included or missing, the tissue block was deparaffinized and reoriented until such sections could be obtained. In those placentas where adequate orientation was not achieved, they were replaced with same-sex placental samples from the same dam. Sections of placenta for histology were fixed overnight in a 4% PFA solution at 4 °C. They were then subjected to three washes in 1× PBS before being placed in histology cassettes (Fisher Scientific) and stored in 70% ethanol until being processed. Histological sections (4- to 5-μm thick) were cut at the Idexx Co. Histology Laboratory and stained with periodic acid-Schiff (PAS)-Hematoxylin. Histological slides were viewed, and images were photographed under a Leica DM 5500B upright microscope with a Leica DFC290 color digital camera at the University of Missouri Cytology Core Facility. Morphometric analyses on the obtained images were performed with ImageJ, NIH (https://imagej.nih.gov/ij/). The labyrinth, spongiotrophoblast, and GC areas were measured within each placental section.
5-HT and Dopamine Immunohistochemistry and Morphometric Analyses.
Paraffin-embedded sections (5 μm) of placental tissue were prepared on glass slides, deparaffinized in xylene, and rehydrated in a graded series of ethanol. Sections were subsequently incubated at room temperature (20 to 21 °C) with blocking buffer containing 10% (vol/vol) normal serum with 1% (wt/vol) bovine serum albumin (BSA) for 2 h and with primary anti–5-HT antibody (ab6336; Abcam) diluted 1:80 (vol/vol) at 4 °C overnight. For negative controls, adjacent tissue sections were incubated with 1% (wt/vol) BSA in PBS. The slides were washed twice in 0.025% Triton X-100 in PBS (5 min each), incubated in 3% (vol/vol) H2O2 in PBS for 30 min, and incubated for 60 min at room temperature (20 to 21 °C) with horseradish peroxidase (HRP)–polymer secondary antibody (PI-9400; Vector Laboratories) diluted to 1:400 (vol/vol). Staining was visualized by using the 3,3'-diaminobenzidine (DAB) peroxidase substrate kit (SK-4100; Vector Laboratories) and DAB Enhancer (S1961; Dako). Sections were counterstained with Mayer's hematoxylin and mounted with Permount mounting medium (SP15-500; Thermo Fisher Scientific).
For dopamine immunohistochemistry, a similar procedure was followed except for the following. The primary antibody was a rabbit polyclonal against dopamine (ab8888; Abcam) diluted 1:100 (vol/vol). The secondary antibody was HRP anti-rabbit immunoglobulin G (IgG) (PI-1000; Vector Laboratories) diluted 1:400 (vol/vol).
Sections were photographed with a Leica DFC290 Color Digital Camera mounted on a Leica DM 5500B Compound Microscope (Leica Camera Inc.). Images taken from same tissue sections were stitched together by using Leica Application Suite software (V4.12; Leica). The GC region was delineated with Adobe Photoshop (CC 2018; Adobe). Percentage of serotonin 5-HT–immunoreactive and dopamine-immunoreactive GCs vs. total number of GCs was used for statistical analyses and graphing purposes.
Statistical Analyses.
All dependent variables were analyzed for normality by using the Wilk–Shapiro test (Statistical Analysis Systems [SAS] v9.4). Fetal sex ratio data were analyzed by using the generalized linear model of the SAS version 9.4 (PROC GENMOD procedure). The data were distributed as a binomial and transformed by means of a logit-link function. The χ2 test was used to test deviation from a 1:1 ratio (i.e., a value of 0.5 for the fraction of male fetuses) as well as for differences in sex ratio between groups. The antilog of the logit and the antilog of the differences between logit estimates produced the odds and odds ratio, respectively. The χ2 test was also used to test differences in pregnancy rates between groups. Number of implantation sites and fetuses and gestational body weight gain were analyzed by using the PROC MIXED procedure in SAS version 9.4. Differences between groups were determined by the Fisher least significant difference. Individual dam was considered the experimental unit and used as error term to control for potential litter effects.
Placental estradiol, estrone, corticosterone, testosterone, progesterone, GABA, dopamine, 5-HT, and 5-HIAA concentrations were logarithmically transformed to approach a normal distribution for data analysis. Data for the dependent variables of placental hormone and neurotransmitter concentrations; ratios between labyrinth, junctional zone, and GC areas in placenta; blood vessel size within the labyrinth region data; and percentage of 5-HT immunoreactive-positive GCs relative to total number of GCs were analyzed by the PROC MIXED procedure of SAS v9.4. Sources of variation considered were treatment, sex, and treatment × sex interaction with an individual mouse serving as the experimental unit to determine treatment effects. Gene expression data determined by qPCR analyses were normalized by using combined average delta cycle threshold (dCt) values of three housekeeping genes Rpl7, Porl2a, Ubc and analyzed by the PROC GLM procedure of SAS. Graphs are based, however, on 2−∆∆Ct values relative to the control values with group (males and females combined) mean value set to one for graphing purposes. To examine treatment × sex interactions, the group mean for control males or females was then determined based on the mean value of these sexes relative to the group mean value of one. For all data, a P value of ≤0.05 was considered significant. All data are presented as means ± SEM.
Supplementary Material
Acknowledgments
R.M.R. is supported by National Institute of Child Health and Human Development (NICHD) Grant HD094937. G.T. is supported by NICHD Grant RHD096083A. C.S.R. is supported by National Institute of Environmental Health Sciences (NIEHS) Grant 1R01ES025547. We acknowledge NIH Shared Instrumentation Grant 1S10OD018141 (to N.D.D.) for hormone analyses. We thank the University of Missouri Office of Research and the University of Florida Office of Research for their financial support to the University of Missouri Metabolomics Center and the University of Florida Interdisciplinary Center for Biotechnology Research Proteomics and Mass Spectrometry Core, respectively. We are grateful for assistance provided by Michelle J. Farrington, Jessica A. Kinkade, and the undergraduate research assistants who assisted in taking care of the mice and with the tissue collections. We are also grateful to the staff at the University of Missouri Cytology Core Facility who assisted with obtaining the placenta histological images. We thank Dr. Kim-Anh Lê Cao and Abolfazl Jalal Abadi, University of Melbourne, for their assistance with the mixOmics program.
Footnotes
The authors declare no competing interest.
Data deposition: The RNA sequence data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database (accession no. 141322). The raw Gas Chromatography Mass Spectrometry (GC/MS) metabolomics data are available at Metabolomics Workbench (Project ID PR000891).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1919563117/-/DCSupplemental.
References
- 1.Rosenfeld C. S., Sex-specific placental responses in fetal development. Endocrinology 156, 3422–3434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schug T. T., Janesick A., Blumberg B., Heindel J. J., Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 127, 204–215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GrandViewResearch, Global bisphenol A (BPA) market by appliation (appliances, automotive, consumer, construction, electrical & electronics) expected to reach USD 20.03 billion by 2020. Digital J., 24 June, 2014. http://www.digitaljournal.com/pr/2009287. Accessed 20 January 2020.
- 4.Calafat A. M., Ye X., Wong L. Y., Reidy J. A., Needham L. L., Exposure of the U.S. Population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 116, 39–44 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sieli P. T., et al. , Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ. Health Perspect. 119, 1260–1265 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galloway T., et al. , Daily bisphenol A excretion and associations with sex hormone concentrations: Results from the InCHIANTI adult population study. Environ. Health Perspect. 118, 1603–1608 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue J., Wan Y., Kannan K., Occurrence of bisphenols, bisphenol A diglycidyl ethers (BADGEs), and novolac glycidyl ethers (NOGEs) in indoor air from Albany, New York, USA, and its implications for inhalation exposure. Chemosphere 151, 1–8 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Hines C. J., et al. , An evaluation of the relationship among urine, air, and hand measures of exposure to bisphenol A (BPA) in US manufacturing workers. Ann. Work Expo. Health 62, 840–851 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.vom Saal F. S., et al. , Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 24, 131–138 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandenberg L. N., Hauser R., Marcus M., Olea N., Welshons W. V., Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld C. S., Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues. Front. Neuroendocrinol. 47, 123–133 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L. H., et al. , Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Sci. Total Environ. 615, 87–98 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Imanishi S., et al. , Effects of oral exposure of bisphenol A on mRNA expression of nuclear receptors in murine placentae assessed by DNA microarray. J. Reprod. Dev. 49, 329–336 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Kang E. R., et al. , Effects of endocrine disruptors on imprinted gene expression in the mouse embryo. Epigenetics 6, 937–950 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lan X., et al. , Bisphenol A exposure promotes HTR-8/SVneo cell migration and impairs mouse placentation involving upregulation of integrin-β1 and MMP-9 and stimulation of MAPK and PI3K signaling pathways. Oncotarget 8, 51507–51521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J. H., et al. , Effects of octylphenol and bisphenol A on the metal cation transporter channels of mouse placentas. Int. J. Environ. Res. Public Health 13, E965 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller J. E., et al. , Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Sci. Rep. 8, 9196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Susiarjo M., Sasson I., Mesaros C., Bartolomei M. S., Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 9, e1003401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tachibana T., et al. , Effects of bisphenol A (BPA) on placentation and survival of the neonates in mice. J. Reprod. Dev. 53, 509–514 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Tait S., Tassinari R., Maranghi F., Mantovani A., Toxicogenomic analysis of placenta samples from mice exposed to different doses of BPA. Genom. Data 4, 109–111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tait S., Tassinari R., Maranghi F., Mantovani A., Bisphenol A affects placental layers morphology and angiogenesis during early pregnancy phase in mice. J. Appl. Toxicol. 35, 1278–1291 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Ye Y., Tang Y., Xiong Y., Feng L., Li X., Bisphenol A exposure alters placentation and causes preeclampsia-like features in pregnant mice involved in reprogramming of DNA methylation of WNT2. FASEB J. 33, 2732–2742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu P. W., et al. , Low-dose bisphenol A activates the ERK signaling pathway and attenuates steroidogenic gene expression in human placental cells. Biol. Reprod. 98, 250–258 (2018). [DOI] [PubMed] [Google Scholar]
- 24.De Felice B., et al. , Genome-wide microRNA expression profiling in placentas from pregnant women exposed to BPA. BMC Med. Genomics 8, 56 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q., et al. , Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children’s Study (NCS). Epigenetics 10, 793–802 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morice L., et al. , Antiproliferative and proapoptotic effects of bisphenol A on human trophoblastic JEG-3 cells. Reprod. Toxicol. 32, 69–76 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Albaladejo E., Fernandes D., Lacorte S., Porte C., Comparative toxicity, oxidative stress and endocrine disruption potential of plasticizers in JEG-3 human placental cells. Toxicol. In Vitro 38, 41–48 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Ponniah M., Billett E. E., De Girolamo L. A., Bisphenol A increases BeWo trophoblast survival in stress-induced paradigms through regulation of oxidative stress and apoptosis. Chem. Res. Toxicol. 28, 1693–1703 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Rajakumar C., Guan H., Langlois D., Cernea M., Yang K., Bisphenol A disrupts gene expression in human placental trophoblast cells. Reprod. Toxicol. 53, 39–44 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Sieppi E., et al. , The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures. Mol. Cell. Endocrinol. 429, 41–49 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Spagnoletti A., et al. , Low concentrations of Bisphenol A and para-Nonylphenol affect extravillous pathway of human trophoblast cells. Mol. Cell. Endocrinol. 412, 56–64 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Speidel J. T., Xu M., Abdel-Rahman S. Z., Bisphenol A (BPA) and bisphenol S (BPS) alter the promoter activity of the ABCB1 gene encoding P-glycoprotein in the human placenta in a haplotype-dependent manner. Toxicol. Appl. Pharmacol. 359, 47–54 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z. Y., et al. , Effect of Bisphenol A on invasion ability of human trophoblastic cell line BeWo. Int. J. Clin. Exp. Pathol. 8, 14355–14364 (2015). [PMC free article] [PubMed] [Google Scholar]
- 34.Jain A., Ezashi T., Roberts R. M., Tuteja G., Deciphering transcriptional regulation in human embryonic stem cells specified towards a trophoblast fate. Sci. Rep. 7, 17257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao J., et al. , Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc. Natl. Acad. Sci. U.S.A. 107, 5557–5562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behura S. K., Kelleher A. M., Spencer T. E., Evidence for functional interactions between the placenta and brain in pregnant mice. FASEB J. 33, 4261–4272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain A., et al. Placental transcriptome at midgestation from BPA-, BPS- exposed, and control mice. Gene Expression Omnibus (GEO). https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE141322. Deposited 2 December 2019.
- 38.Jain A., Tuteja G., TissueEnrich: Tissue-specific gene enrichment analysis. Bioinformatics 35, 1966–1967 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J., Duncan D., Shi Z., Zhang B., WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): Update 2013. Nucleic Acids Res. 41, W77–W83 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh-McGinnis R., Bogutz A. B., Lefebvre L., Partial loss of Ascl2 function affects all three layers of the mature placenta and causes intrauterine growth restriction. Dev. Biol. 351, 277–286 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumner B. W., et al. , Placental metabolome at midgestation from BPA-, BPS- exposed, and control mice. Metabolomics Workbench. https://www.metabolomicsworkbench.org/data/DRCCMetadata.php?Mode=Project&ProjectID=PR000891. Deposited 3 December 2019.
- 42.Rohart F., Gautier B., Singh A., Lê Cao K.-A., mixOmics: An R package for 'omics feature selection and multiple data integration. PLOS Comput. Biol. 13, e1005752 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pergialiotis V., et al. , Bisphenol A and adverse pregnancy outcomes: A systematic review of the literature. J. Matern. Fetal Neonatal Med. 31, 3320–3327 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Xu X., et al. , Associations of cadmium, bisphenol A and polychlorinated biphenyl co-exposure in utero with placental gene expression and neonatal outcomes. Reprod. Toxicol. 52, 62–70 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Tan W., et al. , Bisphenol A differentially activates protein kinase C isoforms in murine placental tissue. Toxicol. Appl. Pharmacol. 269, 163–168 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Knöfler M., et al. , Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell. Mol. Life Sci. 76, 3479–3496 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu D., Gong X., Miao L., Fang J., Zhang J., Efficient induction of syncytiotrophoblast layer II cells from trophoblast stem Cells by canonical Wnt signaling activation. Stem Cell Reports 9, 2034–2049 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guillemot F., Nagy A., Auerbach A., Rossant J., Joyner A. L., Essential role of Mash-2 in extraembryonic development. Nature 371, 333–336 (1994). [DOI] [PubMed] [Google Scholar]
- 49.Tanaka M., Gertsenstein M., Rossant J., Nagy A., Mash2 acts cell autonomously in mouse spongiotrophoblast development. Dev. Biol. 190, 55–65 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Loughner C. L., et al. , Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum. Genomics 10, 10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levitin F., et al. , PATE gene clusters code for multiple, secreted TFP/Ly-6/uPAR proteins that are expressed in reproductive and neuron-rich tissues and possess neuromodulatory activity. J. Biol. Chem. 283, 16928–16939 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knöfler M., Pollheimer J., Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling. Front. Genet. 4, 190 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christov M., Koren S., Yuan Q., Baron R., Lanske B., Genetic ablation of sfrp4 in mice does not affect serum phosphate homeostasis. Endocrinology 152, 2031–2036 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meher A. P., et al. , Placental DHA and mRNA levels of PPARγ and LXRα and their relationship to birth weight. J. Clin. Lipidol. 10, 767–774 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Dhobale M., Neurotrophins: Role in adverse pregnancy outcome. Int. J. Dev. Neurosci. 37, 8–14 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Larqué E., et al. , Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 94 (suppl.), 1908S–1913S (2011). [DOI] [PubMed] [Google Scholar]
- 57.Crawford M. A., Hassam A. G., Williams G., Essential fatty acids and fetal brain growth. Lancet 1, 452–453 (1976). [DOI] [PubMed] [Google Scholar]
- 58.Tuteja G., Chung T., Bejerano G., Changes in the enhancer landscape during early placental development uncover a trophoblast invasion gene-enhancer network. Placenta 37, 45–55 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yavarone M. S., Shuey D. L., Sadler T. W., Lauder J. M., Serotonin uptake in the ectoplacental cone and placenta of the mouse. Placenta 14, 149–161 (1993). [DOI] [PubMed] [Google Scholar]
- 60.Bonnin A., et al. , A transient placental source of serotonin for the fetal forebrain. Nature 472, 347–350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ben-Jonathan N., Munsick R. A., Dopamine and prolactin in human pregnancy. J. Clin. Endocrinol. Metab. 51, 1019–1025 (1980). [DOI] [PubMed] [Google Scholar]
- 62.Hadden C., et al. , Serotonin transporter protects the placental cells against apoptosis in caspase 3-independent pathway. J. Cell. Physiol. 232, 3520–3529 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenfeld C. S., Placental serotonin signaling, pregnancy outcomes, and regulation of fetal brain development. Biol. Reprod., ioz204 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim M. O., et al. , Colocalization of dopamine D1 and D2 receptor mRNAs in rat placenta. Mol. Cells 7, 710–714 (1997). [PubMed] [Google Scholar]
- 65.Schiff R., Arensburg J., Itin A., Keshet E., Orly J., Expression and cellular localization of uterine side-chain cleavage cytochrome P450 messenger ribonucleic acid during early pregnancy in mice. Endocrinology 133, 529–537 (1993). [DOI] [PubMed] [Google Scholar]
- 66.Arensburg J., Payne A. H., Orly J., Expression of steroidogenic genes in maternal and extraembryonic cells during early pregnancy in mice. Endocrinology 140, 5220–5232 (1999). [DOI] [PubMed] [Google Scholar]
- 67.Peng L., Arensburg J., Orly J., Payne A. H., The murine 3beta-hydroxysteroid dehydrogenase (3beta-HSD) gene family: A postulated role for 3beta-HSD VI during early pregnancy. Mol. Cell. Endocrinol. 187, 213–221 (2002). [DOI] [PubMed] [Google Scholar]
- 68.Peng L., Payne A. H., AP-2 gamma and the homeodomain protein distal-less 3 are required for placental-specific expression of the murine 3 beta-hydroxysteroid dehydrogenase VI gene, Hsd3b6. J. Biol. Chem. 277, 7945–7954 (2002). [DOI] [PubMed] [Google Scholar]
- 69.Hernández-Hernández O. T., Martínez-Mota L., Herrera-Pérez J. J., Jiménez-Rubio G., Role of estradiol in the expression of genes involved in serotonin neurotransmission: Implications for female depression. Curr. Neuropharmacol. 17, 459–471 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee S. G., et al. , Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17β-estradiol synthesis via downregulation of aromatase in rat ovary. Environ. Health Perspect. 121, 663–669 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson O. S., et al. , Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ. Mol. Mutagen. 53, 334–342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dolinoy D. C., Huang D., Jirtle R. L., Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. U.S.A. 104, 13056–13061 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dolinoy D. C., Weidman J. R., Waterland R. A., Jirtle R. L., Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 114, 567–572 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox K. H., Gatewood J. D., Howeth C., Rissman E. F., Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Horm. Behav. 58, 754–761 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vandenberg L. N., Chahoud I., Padmanabhan V., Paumgartten F. J., Schoenfelder G., Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol A. Environ. Health Perspect. 118, 1051–1054 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jašarević E., et al. , Sex and dose-dependent effects of developmental exposure to bisphenol A on anxiety and spatial learning in deer mice (Peromyscus maniculatus bairdii) offspring. Horm. Behav. 63, 180–189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Padmanabhan V., et al. , Maternal bisphenol-A levels at delivery: A looming problem? J. Perinatol. 28, 258–263 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teeguarden J. G., et al. , Twenty-four hour human urine and serum profiles of bisphenol a during high-dietary exposure. Toxicol. Sci. 123, 48–57 (2011). [DOI] [PubMed] [Google Scholar]
- 79.Vandenberg L. N., et al. , Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 118, 1055–1070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim B., Colon E., Chawla S., Vandenberg L. N., Suvorov A., Endocrine disruptors alter social behaviors and indirectly influence social hierarchies via changes in body weight. Environ. Health 14, 64 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.LaPlante C. D., Catanese M. C., Bansal R., Vandenberg L. N., Bisphenol S alters the lactating mammary gland and nursing behaviors in mice exposed during pregnancy and lactation. Endocrinology 158, 3448–3461 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.US Environmental Protection Agency, “Bisphenol A alternatives in thermal paper: Final report” (Rep. August 2015, US Environmental Protection Agency, 2015, https://www.epa.gov/sites/production/files/2015-08/documents/bpa_final.pdf).
- 83.Johnson S. A., et al. , Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: A CLARITY-BPA study. Horm. Behav. 80, 139–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson S. A., et al. , Disruption of parenting behaviors in California mice, a monogamous rodent species, by endocrine disrupting chemicals. PLoS One 10, e0126284 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams S. A., et al. , Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): A monogamous animal model. PLoS One 8, e55698 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jašarević E., et al. , Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc. Natl. Acad. Sci. U.S.A. 108, 11715–11720 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.da Silva B. S., et al. , Short and long-term effects of bisphenol S (BPS) exposure during pregnancy and lactation on plasma lipids, hormones, and behavior in rats. Environ. Pollut. 250, 312–322 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Georgiades P., Ferguson-Smith A. C., Burton G. J., Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23, 3–19 (2002). [DOI] [PubMed] [Google Scholar]
- 89.Malassiné A., Frendo J. L., Evain-Brion D., A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 9, 531–539 (2003). [DOI] [PubMed] [Google Scholar]
- 90.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.