Significance
This unique randomized, double-blind, placebo-controlled trial examined the susceptibility to false memories under the influence of cannabis, using a basic (DRM) and two applied (misinformation) paradigms. We used a highly powered experimental design, allowing us to test acute and residual drug effects. To achieve high reproducibility and ecological validity, the misinformation paradigms included an eyewitness and a perpetrator scenario, presented in a virtual-reality environment. We show across different paradigms that cannabis consistently increases susceptibility to false memories. The results have implications for police, legal professionals, and policymakers with regard to the treatment of cannabis-intoxicated witnesses and suspects and the validity of their statements.
Keywords: cannabis, THC, false memory, misinformation, DRM
Abstract
With the growing global acceptance of cannabis and its widespread use by eyewitnesses and suspects in legal cases, understanding the popular drug’s ramifications for memory is a pressing need. In a double-blind, randomized, placebo-controlled trial, we examined the acute and delayed effects of Δ9-tetrahydrocannabinol (THC) intoxication on susceptibility to false memory in 64 healthy volunteers. Memory was tested immediately (encoding and retrieval under drug influence) and 1 wk later (retrieval sober). We used three different methods (associative word lists and two misinformation tasks using virtual reality). Across all methods, we found evidence for enhanced false-memory effects in intoxicated participants. Specifically, intoxicated participants showed higher false recognition in the associative word-list task both at immediate and delayed test than controls. This yes bias became increasingly strong with decreasing levels of association between studied and test items. In a misinformation task, intoxicated participants were more susceptible to false-memory creation using a virtual-reality eyewitness scenario and virtual-reality perpetrator scenario. False-memory effects were mostly restricted to the acute-intoxication phase. Cannabis seems to increase false-memory proneness, with decreasing strength of association between an event and a test item, as assessed by different false-memory paradigms. Our findings have implications for how and when the police should interview suspects and eyewitnesses.
Cannabis is the most widely used illicit substance across the world, and its main psychoactive ingredient, Δ9-tetrahydrocannabinol (THC), has been associated with memory impairments (e.g., ref. 1). As a potential factor impacting memory, cannabis intoxication is an issue of particular interest from a legal perspective. That is, testimonies by eyewitnesses or suspects are oftentimes the only piece of evidence that triers of fact can use for legal decision making, and, thus, gathering reliable testimony is crucial. However, memory performance is imperfect, resulting sometimes in false memories (i.e., memories of nonexperienced events/details; refs. 2 and 3), and such false memories can have disastrous consequences in legal cases (e.g., wrongful convictions or false accusations). This phenomenon of false memory, combined with the fact that cannabis-intoxicated eyewitnesses and suspects are common (4), stresses the need to examine whether cannabis might facilitate false-memory production. Empirical work in this area is rather limited (see ref. 5 for a recent review). In the current experiment, we conducted a randomized, placebo-controlled study to test the impact of cannabis on false-memory formation using three prominent false-memory paradigms.
Core to many false-memory paradigms is the presentation of words or events to which one has been exposed before (“old”) or not (“new”). Old–new recognition decisions can be affected by response bias, a general tendency to respond to items in a systematic but potentially false direction (e.g., yes bias). Some people adopt a stricter decision criterion, requiring a higher level of memory strength to call an item old, while others may respond more liberally (6). We investigated whether individuals who are under the influence of cannabis express a different bias, and, if so, if it would be influenced by levels of association between old and new events. Single doses of cannabis have been found to cause deficits in decision making and working memory (7–9) that have been associated to increased cannabinoid receptor type 1 (CB1 receptor) activation in the hippocampus (10, 11). It is not clear, however, whether cannabis also affects the tendency of how individuals respond to events that may or may not have happened. It has been suggested that hippocampal CB1 receptor activation might underlie the formation of incidental associations (12), which would predict an increase in false memories.
The false-memory literature broadly distinguishes between two types: “Spontaneous” false memories arise due to internal cognitive processes, whereas “suggestion-based” false memories occur because of external suggestion (13, 14). A highly reliable and common method to evoke spontaneous false memories is the Deese/Roediger–McDermott (DRM) paradigm (15–17), in which people falsely remember words not actually presented in an associatively related list of words. Research on cannabis and DRM false-memory formation is sparse, but in a recent field study, we compared intoxicated vs. nonintoxicated cannabis users vs. a nonusing control group (18). No statistical difference between groups was found for the acceptance of “critical lures” (associated but nonpresented theme words). However, false alarms to nonpresented irrelevant stimuli (unrelated to theme) were increased in both sober and intoxicated cannabis users. These findings might be interpreted as reminiscent of a cannabis-induced response bias (“yes-saying” bias) that might vary depending on the strength of association between studied and test items (see also refs. 19 and 20 for related findings).
Suggestion-based false memories are frequently studied by using the misinformation paradigm. Here, participants first view or are involved in an event (e.g., mock crime), then are exposed to misinformation (e.g., suggestive questions or misleading narrative containing false details), and, finally, receive a memory test. Exposure to postevent misinformation often results in people incorporating the suggested details into their memory reports, a phenomenon that is also known as the “misinformation effect” (for a review, see ref. 21). To our knowledge, no study thus far has implemented this method to study the effects of cannabis on suggestion-based false memory.
The current experiment aimed to assess the impact of cannabis intoxication on both spontaneous (DRM) and suggestion-based (misinformation) false-memory production in healthy, occasional cannabis users. The DRM method allowed us to specifically test recognition rates at different levels of association between old and new items. Thus, the level of association is highest for old (i.e., studied) words. Compared to this, the association for new words is lower, but highest for critical lures, less for related lures, and lowest for unrelated words. In general, the misinformation method is not constructed by using similar associative mechanisms as in the DRM method, but does contain questions about presented or nonpresented details that also differ in their level of association. That is, truly presented details were present at encoding and, thus, were highly linked with the experience, while suggested details were linked through the suggestion of being present in the scenario. Nonsuggested, nonpresented details were weakly linked to the experience. Since CB1 activation facilitates formation of incidental associations (12), we tested how cannabis affected the response bias for items with different associative strengths.
Another element of the current experiment was that we used virtual reality (VR) as a way to test the misinformation effect in subjects acting as eyewitnesses and perpetrators. Studies have traditionally employed methods such as case vignettes or videos (22), but also staged events (23), to expose participants to a mock crime event, presenting a trade-off of either maximizing internal or external validity. The scenarios in this study were administered in VR, a fully immersive technology that can overcome this trade-off by combining high experimental control and reusability with high degrees of realism, ecological validity, and feelings of presence (24). Misinformation was introduced through a combination of suggestive questions in a later interview and a virtual cowitness. The interview contained questions about truly presented details (“presented”), leading questions about nonpresented details (“suggested”), and neutral questions about nonpresented details (“nonsuggested”).
The study was conducted according to a double-blind, mixed-model, placebo-controlled design. Suggestion-induced false memory in VR scenarios was tested in a between-subjects design, whereas spontaneous false memory using the DRM paradigm was tested in a within-subjects design. In order to differentiate between acute and long-term drug effects, spontaneous and suggestion-based false memory were assessed at two time points: shortly after encoding (“immediate”) and 1 wk later (“delayed”). Both assessments are of practical relevance. Intoxication during encoding and retrieval phases often occurs in eyewitness situations, which may affect immediate memory of the witnessed event. However, people are not always interviewed immediately following a crime, so it is also imperative to include a retrieval condition in which participants are sober. Given the described findings of cannabis-induced memory impairment (1, 18, 20), cannabis intoxication was generally expected to result in higher false-memory rates, compared to a placebo condition.
Results
Means and SEs for all DRM and misinformation parameters can be viewed in Table 1. Demographic information, intoxication parameters, and additional analyses are displayed in SI Appendix.
Table 1.
Means from DRM and misinformation parameters (rates in proportions)
| Cannabis condition | Placebo condition | |||
| Immediate | Delayed | Immediate | Delayed | |
| DRM | ||||
| n | 63 | 63 | 64 | 63 |
| True recognition (old) | 0.68 (0.02) | 0.46 (0.02) | 0.68 (0.02) | 0.51 (0.02) |
| False alarms (critical) | 0.62 (0.02) | 0.59 (0.03) | 0.56 (0.03) | 0.64 (0.02) |
| False alarms (related) | 0.42 (0.03) | 0.44 (0.03) | 0.27 (0.03) | 0.44 (0.03) |
| False alarms (unrelated) | 0.40 (0.03) | 0.39 (0.03) | 0.16 (0.02) | 0.31 (0.03) |
| Net accuracy | 0.68 (0.08) | 0.54 (0.05) | 0.75 (0.13) | 0.57 (0.08) |
| Misinformation eyewitness | ||||
| n | 32 | 31 | 32 | 31 |
| Presented | 0.78 (0.02) | 0.71 (0.02) | 0.78 (0.02) | 0.74 (0.02) |
| Suggested | 0.19 (0.04) | 0.19 (0.03) | 0.08 (0.02) | 0.17 (0.03) |
| Nonsuggested | 0.06 (0.02) | 0.17 (0.03) | 0.01 (0.01) | 0.10 (0.02) |
| Misinformation perpetrator | ||||
| n | 31 | 31 | 32 | 31 |
| Presented | 0.68 (0.03) | 0.62 (0.03) | 0.65 (0.03) | 0.67 (0.03) |
| Suggested | 0.31 (0.05) | 0.28 (0.04) | 0.23 (0.03) | 0.27 (0.03) |
| Nonsuggested | 0.08 (0.02) | 0.07 (0.02) | 0.01 (0.01) | 0.03 (0.01) |
Values shown are mean (SE).
DRM.
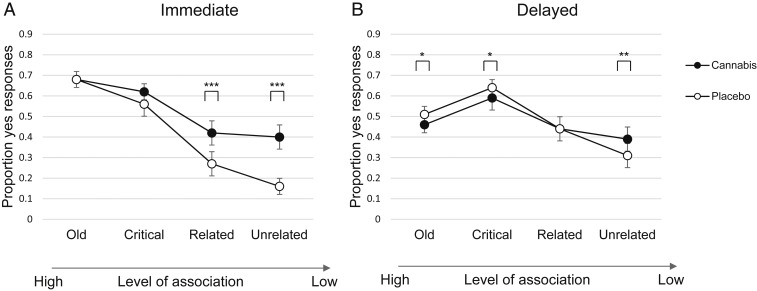
Fig. 1 depicts the mean DRM true and false memory rates for the two drug conditions at immediate (Fig. 1A) and delayed (Fig. 1B) test. As can be seen in Fig. 1A, cannabis-intoxicated individuals had higher false-memory rates compared to the placebo condition at immediate test. This effect depended on the level of association between studied and tested words. Statistically, this was reflected in an interaction effect between drug and level of association [F(2.67, 167.52) = 14.83, P < 0.001, ω2 = 0.08]. Cannabis increased false memories of related lures [F(1, 62) = 21.50, P < 0.001, Cohen’s d = 0.58] and unrelated words [F(1, 62) = 62.53, P < 0.001, Cohen’s d = 1.0], where levels of association were low. However, cannabis did not affect the response to old words (true memory) and critical lures, where levels of association were high.
Fig. 1.
DRM mean scores in proportions from immediate test (A) and delayed test (B) by drug condition. *P < 0.05; **P < 0.01; ***P < 0.001 (pairwise comparisons). Error bars represent 95% CIs.
The delayed test also revealed a significant interaction between drug and level of association [F(2.59, 155.46) = 7.60, P < 0.001, ω2 = 0.02]. Cannabis increased false memories for unrelated words [F(1, 60) = 8.85, P = 0.004, Cohen’s d = 0.38;], but decreased false memories of critical lures [F(1, 60) = 4.37, P = 0.041, Cohen’s d = 0.27] and true memory [F(1, 60) = 6.20, P = 0.016, Cohen’s d = 0.32].
Overall, cannabis-intoxicated participants had lower memory accuracy (net accuracy = ratio of true memory to total memory), both in the immediate test [t(62) = 3.67, P < 0.001, Cohen’s d = 0.46] and the delayed test [t(60) = 2.49, P = 0.015, Cohen’s d = 0.32], as compared to placebo.
Misinformation Paradigm.
Eyewitness scenario.
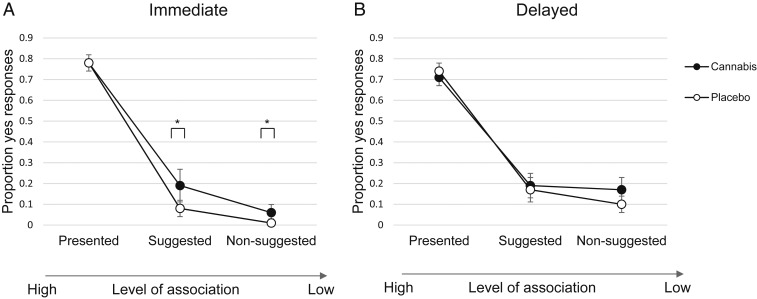
Fig. 2 shows the cannabis and placebo groups’ true- and false-memory rates for the eyewitness VR scenario at immediate (Fig. 2A) and delayed (Fig. 2B) test. As can be seen in the figure, the cannabis group had higher false-memory rates when still intoxicated (Fig. 2A), but this effect disappeared after 1 wk when sober again (Fig. 2B). True memory was not affected by cannabis at immediate test [presented details; F(1, 62) = 2.76−30, P = 1.0]. However, cannabis-intoxicated participants showed higher false memories of suggested and nonsuggested details than the placebo group [F(1, 62) = 6.19, P = 0.016, Cohen’s d = 0.62; and F(1, 62) = 4.59, P = 0.036, Cohen’s d = 0.54, respectively]. This was reflected by a group by level of association interaction [F(1.62, 100.19) = 3.43, P = 0.046, ω2 = 0.02].
Fig. 2.
Eyewitness mean scores in proportions from immediate test (A) and delayed test (B) by drug condition. *P < 0.05 (pairwise comparisons). Error bars represent 95% CIs.
Regarding the analyses of the delayed condition, no statistically significant interaction was detected anymore.
Perpetrator scenario.
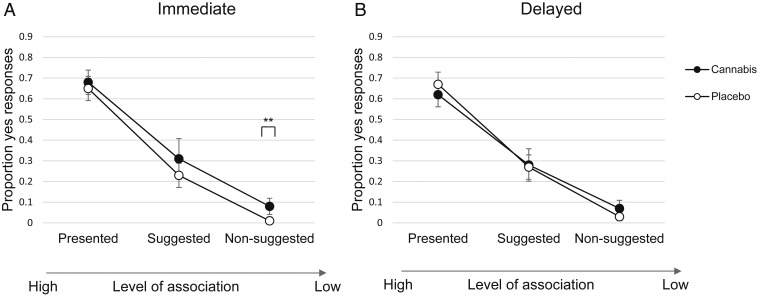
Fig. 3 depicts the two groups’ true- and false-memory rates for the perpetrator VR scenario in the immediate (Fig. 3A) and delayed (Fig. 3B) conditions. In Fig. 3A, it is visible that the cannabis group had higher false-memory rates while under the influence, compared to placebo. At 1-wk follow-up, no group differences were detectable anymore (Fig. 3B). Analysis of the immediate condition revealed a main effect of group [F(1, 61) = 5.79, P = 0.019, ω2 = 0.07], with cannabis-intoxicated participants showing the highest false-memory rates of nonsuggested details [F(1, 61) = 11.56, P = 0.001, Cohen’s d = 0.86]. No statistically significant differences between cannabis and placebo were detected for true memory [F(1, 61) = 0.40, P = 0.53] or false memory for suggested details [F(1, 61) = 2.23, P = 0.14].
Fig. 3.
Perpetrator mean scores in proportions from immediate test (A) and delayed test (B) by drug condition. **P < 0.01 (pairwise comparisons). Error bars represent 95% CIs.
At delayed test, no statistically significant interaction effect or group main effect emerged.
Discussion
We exampled the effects of cannabis on the susceptibility to form spontaneous and suggestion-based false memories and generally found more false memories in intoxicated participants. These elevated false-memory rates were observed in all paradigms and were most pronounced in the immediate condition, when memory tests took place while people were still acutely intoxicated. This fits well with research suggesting that cannabis robustly increases false memory at the retrieval stage (i.e., the memory test; ref. 20). However, some effects in the DRM persisted after 1 wk when people were sober again, indicating some THC-induced encoding (i.e., intake of information) impairments as well.
The findings obtained from the DRM paradigm extend and replicate previous findings in several ways. Acute cannabis use did not significantly affect immediate true-recognition performance, or false recognition of critical lures, which was similarly shown in the coffeeshop field study (18). However, cannabis elevated immediate false-memory rates for related and unrelated stimuli (medium and large effect sizes, respectively). Moreover, memory performance during cannabis and placebo was similar when presented with old words and critical lures, but false-recognition rates were much higher during cannabis intoxication when confronted with words that were poorly associated to the old words, suggesting that cannabis induced a response bias toward less-associated new items. Remarkably, this latter effect was still present at follow-up 1 wk later when participants were sober again. At follow-up, we also found that the cannabis condition had lower true memories and lower false memories of critical lures, compared to placebo (although not robustly; SI Appendix). This could be reflective of THC-induced encoding impairments, where impaired processing during the study phase could result in decreased memory for studied words, thereby also reducing memory for similar, easily confusable items. This is consistent with other research in this field, where THC at encoding reduced DRM false-memory formation (19), suggesting that at encoding, cannabis might reduce memory for the relatedness of presented words. This also seems in line with the current finding that the decrease in false-memory frequency for unrelated words as compared to related words was most pronounced in the placebo condition. Overall, these results fit well with previous studies that disentangled the effects of cannabis on different memory stages (19, 20), and an emerging picture seems to be that elevated false memories are the norm when THC affects retrieval.
Cannabis also amplified susceptibility to suggestion-based false memories in the misinformation paradigm. In the eyewitness scenario, intoxicated participants showed the highest false-memory rates in response to leading questions about suggested details, but also neutral questions about nonpresented details. In the perpetrator scenario, an overall higher tendency to respond “yes” to all questions among intoxicated participants was detected. This effect was primarily driven by false memories of nonsuggested, nonpresented details, a response pattern that might increase the risk of false reporting and might be more indicative of a general response bias. These differences between groups were restricted to the immediate condition and disappeared at follow-up 1 wk later, indicating that THC-induced impairments might be most detrimental to retrieval. However, by inspecting the mean scores, it becomes clear that the cannabis group did not necessarily improve over time, but, rather, the placebo group worsened at follow-up, thus performing more similarly to the cannabis group after 1 wk had passed. This is in line with research showing that memory decays over time and that people are more prone to be influenced by misinformation with increasing length between event and postevent misinformation because they are less likely to detect discrepancies (discrepancy-detection principle; refs. 21 and 25). Due to the placebo group deteriorating with time, no statistical differences in memory performance were detected at follow-up.
On a broader level, we detected that cannabis-intoxicated people seemed to show a tendency toward more liberal responding under conditions of uncertainty. Why might this be so? An increase in irrelevant associations might stem from increased incidental learning due to activation of hippocampal CB1 receptors (12). High densities of cannabinoid receptors in the hippocampus and cortex have been suggested as playing a role in the cognitive effects of cannabis (26). These effects include a loosening of associations, fragmentation of thought, and heightened distractibility. Such reductions in focus and increments in mental activity could well account for the increase in false recognitions of irrelevant or unrelated words or events on all memory tasks. Some responses might also be explained by impaired source monitoring—for example, by confusing information from external sources (e.g., cowitness) with internal ones (own memory; ref. 27). Increased irrelevant associations due to cannabis might contribute to source misattributions and, therefore, memory errors.
The current study has several practical implications. The most important message from this study is that cannabis exerted a general impact on memory by increasing various types of recollective errors. Although there is debate on whether different types of false memories are related to each other (3, 28), the current study shows that intoxicated individuals might be at high risk to form all kinds of memory errors, which can be perilous in investigative interviewing settings. In addition, intoxicated individuals were more vulnerable to suggestive questions while still under acute influence, but this effect disappeared at 1-wk follow-up. In terms of interviewing witnesses, victims, or suspects after the incidence of a crime, this means that interviewing while the individual is still intoxicated should be minimized due to elevated risk of false reporting. Questioning should ideally take place as soon as the person has sobered up to prevent memory decay due to time. However, a person under the influence of cannabis during an event might still show a yes bias toward some new information later. Thus, cannabis-intoxicated individuals might have to be treated as a vulnerable group, similar to child or elderly witnesses/suspects.
Future replication is needed to support this study’s findings and could include measures of free recall and metacognition on top of recognition memory. For example, it is important to examine how confident cannabis-intoxicated individuals are when making memory errors. Future studies might also explore whether memory errors introduced during an intoxicated interview would persist and appear in later interviews, adding to the potential costs of interviewing people while they are still intoxicated.
To recap, this study has provided evidence that using cannabis elevates the risk of creating different types of false memories. Cannabis-intoxicated witnesses and suspects pose a vulnerable group and might profitably be identified as such, and while drug testing is a routine procedure with suspects, this is not the case for witnesses or victims (29). Although cannabis is oftentimes connected with positive effects (e.g., pain reduction), it might also lead to hazy memories, which eventually opens the door for a negative effect: increases in false memories.
Materials and Methods
The study was approved by the standing Medical Ethics Committee of Maastricht University and the South East Sydney Local Health District Human Research Ethics Committee and was conducted according to the current revision of the Declaration of Helsinki (amended in 2013, Fortaleza) and the International Conference on Harmonization Guidelines for Good Clinical Practice. All subjects were fully informed of study procedures, adverse reactions to drug treatments, legal rights and responsibilities, expected benefits of a general scientific nature, and their right for voluntary termination without penalty or censure. All subjects gave written informed consent and received financial compensation (€150/AUS$200) for their participation. A permit for obtaining, storing, and administering medicinal cannabis was obtained from the Dutch drug-enforcement administration (Sydney: New South Wales Ministry of Health). The study was registered at the Netherlands National Trial Register (Nederlandse Trial Register, NL6494).
The study was set up as an international, multicenter, clinical drug trial between two sites: Maastricht University and the University of Sydney. A total of 64 healthy, occasional cannabis users (32 female, 32 male, mean age and SD: 22.7, 2.6) completed the present study, i.e., underwent a medical screening, a training, and both treatment conditions including follow-up (for demographic and drug history information, see SI Appendix, Table S1). On separate test days, each subject inhaled the vapor of a single dose of cannabis (300 μg of THC per kg of body weight) or a placebo. A single dose of vaporized THC has a rapid onset and reaches peak concentration in blood plasma within 10 min. The psychoactive effects of cannabis are experienced immediately after smoking, with peak levels of intoxication occurring after 15 to 30 min. Cognitive impairment is most prominent during 2 h after smoking, but may be detectable up to 4 to 6 h after smoking (8, 30).
Fifteen DRM associative word lists were presented on each test day, followed by a recognition test. Misinformation paradigm materials were presented in the form of a VR scenario (eyewitness vs. perpetrator), followed by a forced-choice memory interview. The eyewitness scenario showed a fight at a train station (5 min total duration), whereas the perpetrator scenario involved theft of a handbag at a bar (2 min duration; see SI Appendix for more details). Each participant was exposed once to each scenario on separate test days, counterbalanced with treatment condition (see SI Appendix, Fig. S1 for a detailed schematic representation of randomization sequences). Follow-up memory tests were conducted 7 (±1) d following each test day. All materials and data can be found on the Open Science Framework (https://osf.io/k5v8c/). Detailed explanations of the materials used, procedure, study design, and administration can be found in SI Appendix.
Supplementary Material
Acknowledgments
We thank Richard Benning (Maastricht University) for creating the VR environments used in this research; Consuelo Rivas, Sarah Hutchinson, Nicholas Lintzeris, Therese Chan, Man Cho Leung, Kelvin Cao, Vicky Hayes, and Apo Demirkol at the Langton Center, Sydney NSW Australia; Elodie Chiarovano and Hamish MacDougall from the University of Sydney; and students Marius Cordt, Julia Gros, Kayley van Uden, Rosalie Mourmans, Floor van der Steur, Didi Delsing, Rachel Heutz, Eva van Rosmalen, Elsa Roudot, Beatrice da Rios, and Naemi Welter for collecting data. This research was supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek Research Talent Grant 406.16.529 (to L.K., H.O., and J.G.R. in 2016).
Footnotes
The authors declare no competing interest.
Data deposition: All materials and data can be found on the Open Science Framework (https://osf.io/k5v8c/).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920162117/-/DCSupplemental.
References
- 1.Broyd S. J., van Hell H. H., Beale C., Yücel M., Solowij N., Acute and chronic effects of cannabinoids on human cognition—A systematic review. Biol. Psychiatry 79, 557–567 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Loftus E. F., Memories of things unseen. Curr. Dir. Psychol. Sci. 13, 145–147 (2004). [Google Scholar]
- 3.Otgaar H., Howe M. L., Brackmann N., Smeets T., The malleability of developmental trends in neutral and negative memory illusions. J. Exp. Psychol. Gen. 145, 31–55 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans J. R., Schreiber Compo N., Russano M. B., Intoxicated witnesses and suspects: Procedures and prevalence according to law enforcement. Psychol. Public Policy Law 15, 194–221 (2009). [Google Scholar]
- 5.Flowe H. D., Colloff M. F., Kloft L., Jores T., Stevens L. M., “Impact of alcohol and other drugs on eyewitness memory” in The Routledge International Handbook of Legal and Investigative Psychology, Bull R., Blandón-Gitlin I., Eds. (Routledge, London, UK, 2020), chap. 10, pp. 149–162. [Google Scholar]
- 6.Wright D. B., Gabbert F., Memon A., London K., Changing the criterion for memory conformity in free recall and recognition. Memory 16, 137–148 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Ranganathan M., D’Souza D. C., The acute effects of cannabinoids on memory in humans: A review. Psychopharmacology (Berl.) 188, 425–444 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Ramaekers J. G., et al. , High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology 31, 2296–2303 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Adam K. C. S., Doss M. K., Pabon E., Vogel E. K., de Wit H., Δ9-Tetrahydrocannabinol (THC) impairs visual working memory performance. bioRxiv:10.1101/778068 (23 September 2019). [DOI] [PMC free article] [PubMed]
- 10.Mizrahi R., Watts J. J., Tseng K. Y., Mechanisms contributing to cognitive deficits in cannabis users. Neuropharmacology 124, 84–88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloomfield M. A. P., et al. , The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol. Ther. 195, 132–161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busquets-Garcia A., et al. , Hippocampal CB1 receptors control incidental associations. Neuron 99, 1247–1259.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Mazzoni G., Naturally occurring and suggestion-dependent memory distortions; the convergence of disparate research traditions. Eur. Psychol. 7, 17–30 (2002). [Google Scholar]
- 14.Brainerd C. J., Developmental reversals in false memory: A new look at the reliability of children’s evidence. Curr. Dir. Psychol. Sci. 22, 335–341 (2013). [Google Scholar]
- 15.Deese J., On the prediction of occurrence of particular verbal intrusions in immediate recall. J. Exp. Psychol. 58, 17–22 (1959). [DOI] [PubMed] [Google Scholar]
- 16.Roediger H. L., McDermott K. B., Creating false memories: Remembering words not presented in lists. J. Exp. Psychol. Learn. Mem. Cogn. 21, 803–814 (1995). [Google Scholar]
- 17.Gallo D. A., False memories and fantastic beliefs: 15 years of the DRM illusion. Mem. Cognit. 38, 833–848 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Kloft L., et al. , False memory formation in cannabis users: A field study. Psychopharmacology (Berl.) 236, 3439–3450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballard M. E., Gallo D. A., de Wit H., Psychoactive drugs and false memory: Comparison of dextroamphetamine and δ-9-tetrahydrocannabinol on false recognition. Psychopharmacology (Berl.) 219, 15–24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doss M. K., Weafer J., Gallo D. A., de Wit H., Δ9-tetrahydrocannabinol at retrieval drives false recollection of neutral and emotional memories. Biol. Psychiatry 84, 743–750 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Loftus E. F., Planting misinformation in the human mind: A 30-year investigation of the malleability of memory. Learn. Mem. 12, 361–366 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Vredeveldt A., Charman S. D., den Blanken A., Hooydonk M., Effects of cannabis on eyewitness memory: A field study. Appl. Cogn. Psychol. 32, 420–428 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuille J. C., Tollestrup P. A., Marxsen D., Porter S., Herve H. F., An exploration on the effects of marijuana on eyewitness memory. Int. J. Law Psychiatry 21, 117–128 (1998). [DOI] [PubMed] [Google Scholar]
- 24.van Gelder J.-L., Otte M., Luciano E. C., Using virtual reality in criminological research. Crime Sci. 3, 10 (2014). [Google Scholar]
- 25.Loftus E. F., Miller D. G., Burns H. J., Semantic integration of verbal information into a visual memory. J. Exp. Psychol. Hum. Learn. 4, 19–31 (1978). [PubMed] [Google Scholar]
- 26.Solowij N., Cannabis and Cognitive Functioning (Cambridge University Press, Cambridge, UK, 2006). [Google Scholar]
- 27.Johnson M. K., Hashtroudi S., Lindsay D. S., Source monitoring. Psychol. Bull. 114, 3–28 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Ost J., et al. , False memory ≠ false memory: DRM errors are unrelated to the misinformation effect. PLoS One 8, e57939 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crossland D., Kneller W., Wilcock R., Intoxicated eyewitnesses: Prevalence and procedures according to England’s police officers. Psychol. Crime Law 24, 979–997 (2018). [Google Scholar]
- 30.Grotenhermen F., Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 42, 327–360 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.