The cells of all organisms are sugarcoated with polysaccharides, glycoproteins, and glycolipids. In multicellular organisms these polymers not only have a structural role in the organization of tissues and organs, but also have signaling activities. In the animal extracellular matrix for instance, the negatively charged hyaluronic acid (HA) polymers contribute to morphogenesis through mechanosensing, whereas HA fragments that are released from the polymer under certain conditions trigger inflammatory responses and angiogenesis (1). In plants, pectins are the major charged polysaccharides in the cell wall and thus can be considered the equivalent of HA. In addition, pectin-derived oligosaccharides released from the host cell wall during pathogen infection have been shown to trigger immune responses (2–4). Gallego-Giraldo et al. (5) in PNAS now provide evidence for a role of pectin oligosaccharide signaling also in the response of plant cells to cell wall perturbation.
Immune Responses Induced by Genetic Modification of Lignins
The study is part of a larger effort of Gallego-Giraldo et al. (5) to tailor plant biomass for improved digestibility (for animal feed) or conversion into fermentable sugars (saccharification, to produce second-generation biofuels and chemicals) (6). Plants, in contrast to animals, contain lignins in their extracellular matrix, besides polysaccharides and glycoproteins. Lignins consist of oxidatively cross-linked phenolic molecules, which form a hydrophobic network attached to the polysaccharides. Previous studies by Gallego-Giraldo et al. and others have shown that lignins are a major obstacle for biomass digestibility/saccharification, mainly because they limit the access of cell wall degrading enzymes to their substrates (6). A commonly used strategy to overcome this obstacle is to reduce the lignin content or to change lignin composition through genetic modification of lignin biosynthetic enzymes (7). A major problem with this strategy is that lignin reduction frequently interferes with plant growth. This reflects not only structural defects due to the lack of lignins, for instance in water-transporting xylem vessels, but also immune responses induced by the cell wall modifications (8, 9). With the aim to overcome this bottleneck for the genetic improvement of biomass feedstocks, Gallego-Giraldo et al. (5) used the model plant Arabidopsis to study the molecular mechanisms underlying the induction of immune responses upon lignin reduction.
A Role for Pectin Metabolism in These Immune Responses
Gallego-Giraldo et al. (5) chose two semidwarf lines with reduced lignin content, obtained by the genetic modification of distinct lignin biosynthesis genes. Comparison of the transcriptomes with that of wild-type (WT) plants showed that both lignin-deficient lines overexpressed a suite of pathogenesis-related (PR) genes, confirming the activation of immune responses in these lines. The most strongly up-regulated transcript in both lignin-deficient lines, however, encoded the pectin-hydrolyzing enzyme polygalacturonase ADPG1. Interestingly, ADPG1 expression was essential for the induction of some of the PR genes, since no such induction was observed in the lignin-deficient lines in which ADPG1 was inactivated. This suggested that ADPG1 itself or cell wall fragments released by ADPG1 might act as an elicitor of PR gene expression. The latter possibility was confirmed by showing that PR gene expression could be elicited with water extracts from lignin-deficient walls but not from walls from WT or lignin-deficient lines lacking ADPG1. In addition, treatment of the latter extracts with recombinant ADPG1 restored the elicitor activity and this was not seen with ADPG1 itself or ADPG1-treated extracts from WT walls.
The emerging view is that the induction of the expression of these PR genes upon lignin modification involves a two-step process: First, the reduced lignin content facilitates the release of material harboring a latent elicitor but also causes the up-regulation of the enzyme (ADPG1) required for the activation of the latent elicitor. Next, the active species triggers the immune response at least in part through the up-regulation of defense genes (Fig. 1). The pectin-derived elicitor therefore integrates two pieces of information: the presence of latent elicitor (which may simply reflect physical changes in the cell wall or the activity of cell wall enzymes) and the induction of the activating enzyme (depending on signaling). It will be interesting to see whether this dual recognition is part of a failsafe system in the critical choice between growth and immunity.
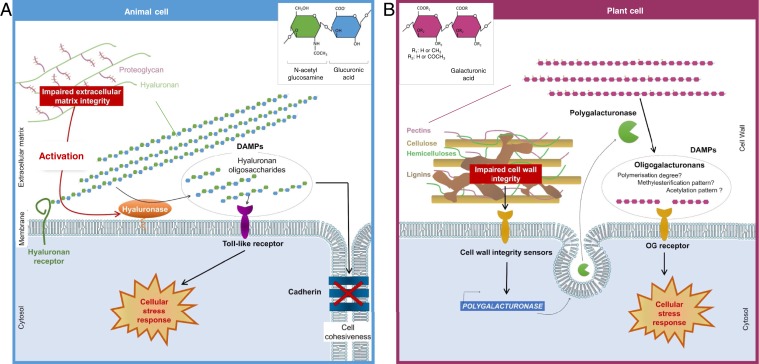
Fig. 1.
Stress and immune signaling through extracellular matrix fragments in animals or plants. (A) In disease states of animal cells, hyaluronans (HA) can be converted into HA fragments due to increased expression and activity of plasma membrane-associated extracellular hyaluronidases. HA fragments serve in turn as damage-associated molecular patterns (DAMPs) and are capable of initiating receptor-mediated signaling and altering cell cohesiveness. (B) In plant cells, changes in lignin composition initiate a signaling cascade, presumably through activation of cell wall integrity receptors, which induce the expression of a polygalacturonase. This pectin-hydrolyzing enzyme releases, from soluble cell wall fractions, signaling molecules, which elicit the expression of immune response genes.
How the initial cell wall perturbation is perceived is not known. It may involve the receptor-mediated detection of wall components, different or not from the latent elicitor, or some type of mechanosensing (10). Receptors for the ADPG1-induced elicitors also remain to be identified.
Insights into the Molecular Structure of the Elicitor
The exact nature of the elicitor activity released by the polygalacturonase ADPG1 also remains elusive. Polygalacturonases hydrolyze the α-1,4 linkages of low methyl-esterified homogalacturonans and have a substrate preference for linear polygalacturonic acids. The recombinant ADPG1 instead appears to prefer more complex substrates. This is suggested by its preference for the heterogeneous and highly methylated apple pectins over polygalacturonic acid and the observation that the elicitor activity in water extracts from lignin-deficient walls was abolished by treatment with a generic polygalacturonase. In addition, this elicitor activity disappeared upon treatment with enzymes that cleave arabinan and, to a lesser extent, fucose or xyloglucan, respectively. Therefore, the elicitor structures in these extracts appear to be more complex than the previously reported linear oligogalacturonides (3).
Interestingly, ADPG1 was previously shown to have also a role in normal plant development, where it is exclusively expressed during, and required for, anther dehiscence (11). It is tempting to speculate that ADPG1-released oligosaccharide elicitors are also involved in this process.
More than 40 y after the formulation of the “oligosaccharin hypothesis” to explain the elicitation of plant defenses by specific fungal cell wall fragments (12), interest is growing again for the signaling role of oligosaccharides in plant development and plant–microbe interactions. Carbohydrates, with their extreme structural diversity, are in principle efficient information carriers in addition to nucleic acids and proteins. The structural elucidation of these signaling molecules is now facilitated by recently improved analytical methods, which for instance revealed the unsuspected complexity of the population of oligogalacturonides that accumulate during infection of plant tissues with the necrotrophic fungus Botrytis cinerea (13). A major challenge for the coming years will be to investigate to what extent animals and plants use signaling oligosaccharides during development and for immunity and to decode the underlying grammar.
Acknowledgments
A.V. and H.H.’s research was supported by the “Agence Nationale de Recherche” project ANR-14-CE34-0010-03 “Pectosign” (to H.H.) and a National Research Institute for Agriculture, Food and Environment tenure track grant (to A.V.). The Institut Jean Pierre Bourgin benefits from the support of Saclay Plant Sciences-SPS (ANR-17-EUR-0007).
Footnotes
The authors declare no competing interest.
See companion article on page 3281 in issue 6 of volume 117.
References
- 1.Joy R. A., Vikkath N., Ariyannur P. S., Metabolism and mechanisms of action of hyaluronan in human biology. Drug Metab. Pers. Ther. 33, 15–32 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Nothnagel E. A., McNeil M., Albersheim P., Dell A., Host–pathogen interactions. XXII. A galacturonic acid oligosaccharide from plant cell walls elicits phytoalexins. Plant Physiol. 71, 916–926 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spiro M. D., et al. , Purification and characterization of biologically active 1,4-linked α-D-oligogalacturonides after partial digestion of polygalacturonic acid with endopolygalacturonase. Carbohydr. Res. 247, 9–20 (1993). [Google Scholar]
- 4.Hahn M. G., Darvill A. G., Albersheim P., Host-pathogen interactions: XIX. The endogenous elicitor, a fragment of a plant cell wall polysaccharide that elicits phytoalexin accumulation in soybeans. Plant Physiol. 68, 1161–1169 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallego-Giraldo L., et al. , ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE 1 (ADPG1) releases latent defense signals in stems with reduced lignin content. Proc. Natl. Acad. Sci. U.S.A. 117, 3281–3290 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang P., Dudareva N., Morgan J. A., Chapple C., Genetic manipulation of lignocellulosic biomass for bioenergy. Curr. Opin. Chem. Biol. 29, 32–39 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Dos Santos A. C., Ximenes E., Kim Y., Ladisch M. R., Lignin-enzyme interactions in the hydrolysis of lignocellulosic biomass. Trends Biotechnol. 37, 518–531 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Miedes E., Vanholme R., Boerjan W., Molina A., The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 5, 358 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Man Ha C., et al. , Ectopic defense gene expression is associated with growth defects in Medicago truncatula lignin pathway mutants. Plant Physiol. 181, 63–84 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamant O., Haswell E. S., Life behind the wall: Sensing mechanical cues in plants. BMC Biol. 15, 59 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa M., Kay P., Wilson S., Swain S. M., ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are Polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21, 216–233 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albersheim P., et al. , “Oligosaccharins: Naturally occurring carbohydrates with biological regulatory functions” in Structure and Function of Plant Genomes, Ciferri O., Dure L., Eds. (Plenum Publishing Corp., New York, NY, 1983), pp. 293–312. [Google Scholar]
- 13.Voxeur A., et al. , Oligogalacturonide production upon Arabidopsis thaliana-Botrytis cinerea interaction. Proc. Natl. Acad. Sci. U.S.A. 116, 19743–19752 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]