Significance
G protein-coupled receptors (GPCRs) are the largest family of cell surface receptors and respond to diverse extracellular stimuli to promote various cellular responses. However, different types of stimuli can activate the same GPCR and promote distinct signaling cascades that result in opposing cellular responses, a phenomenon termed biased signaling. The present study illustrates the utility of global phosphoproteomic profiling of signaling by a GPCR, protease-activated receptor-1, activated by two different proteases to identify pathways and a rich array of proteins that confer biased signaling and opposing functions on endothelial barrier integrity.
Keywords: actin, arrestin, GPCR, inflammation, thrombin
Abstract
Thrombin, a procoagulant protease, cleaves and activates protease-activated receptor-1 (PAR1) to promote inflammatory responses and endothelial dysfunction. In contrast, activated protein C (APC), an anticoagulant protease, activates PAR1 through a distinct cleavage site and promotes anti-inflammatory responses, prosurvival, and endothelial barrier stabilization. The distinct tethered ligands formed through cleavage of PAR1 by thrombin versus APC result in unique active receptor conformations that bias PAR1 signaling. Despite progress in understanding PAR1 biased signaling, the proteins and pathways utilized by thrombin versus APC signaling to induce opposing cellular functions are largely unknown. Here, we report the global phosphoproteome induced by thrombin and APC signaling in endothelial cells with the quantification of 11,266 unique phosphopeptides using multiplexed quantitative mass spectrometry. Our results reveal unique dynamic phosphoproteome profiles of thrombin and APC signaling, an enrichment of associated biological functions, including key modulators of endothelial barrier function, regulators of gene transcription, and specific kinases predicted to mediate PAR1 biased signaling. Using small interfering RNA to deplete a subset of phosphorylated proteins not previously linked to thrombin or APC signaling, a function for afadin and adducin-1 actin binding proteins in thrombin-induced endothelial barrier disruption is unveiled. Afadin depletion resulted in enhanced thrombin-promoted barrier permeability, whereas adducin-1 depletion completely ablated thrombin-induced barrier disruption without compromising p38 signaling. However, loss of adducin-1 blocked APC-induced Akt signaling. These studies define distinct thrombin and APC dynamic signaling profiles and a rich array of proteins and biological pathways that engender PAR1 biased signaling in endothelial cells.
Dysfunction of the vascular endothelium is a hallmark of inflammation and results in barrier permeability, vascular leakage, tissue edema, and organ failure in sepsis and other diseases (1, 2). Endothelial dysfunction is triggered by inflammatory mediators, many of which signal through G protein-coupled receptors (GPCRs) (3, 4). A key GPCR inflammatory mediator is thrombin, a procoagulant protease generated in response to vascular injury and inflammation (5). Thrombin stimulates numerous inflammatory responses, including endothelial barrier disruption (6) through activation of protease-activated receptor-1 (PAR1) (7, 8). Thrombin cleaves the N terminus of PAR1, unveiling a new N terminus that functions as a tethered ligand and binds intramolecularly to initiate receptor signaling (9). Activation of PAR1 by thrombin induces rapid heterotrimeric G protein signaling and disruption of the endothelial barrier through RhoA activation of Rho-associated kinase (ROCK) and phosphorylation of the myosin light chain (MLC) by MLC kinase (10). Thrombin-stimulated endothelial barrier permeability is also promoted by p38 mitogen-activated protein kinase (MAPK) signaling and occurs independent of MLC phosphorylation and RhoA signaling (11–13). Thus, multiple signaling pathways induced by thrombin mediate endothelial barrier disruption.
In addition to thrombin, other proteases can cleave and activate PAR1 through noncanonical sites and promote biased signaling. The distinct tethered ligands appear to preferentially stabilize unique active conformational states of PAR1, resulting in biased signaling. Unlike thrombin, activated protein C (APC), a native anticoagulant protease, activates PAR1 through cleavage at a distinct site in the N terminus (14) and induces protracted cytoprotective responses in endothelial cells (15). APC enhances endothelial barrier stabilization, prosurvival, and anti-inflammatory activities. APC-induced PAR1 endothelial barrier stabilization is specified by localization of both PAR1 and the APC cofactor endothelial protein C receptor (EPCR) in caveolae, plasma membrane microdomains enriched in caveolin-1 (16, 17). In addition, endothelial barrier stabilization induced by APC/PAR1 is mediated by β-arrestin-2 and dishevelled (Dvl-2) scaffolds and prolonged Rac1 signaling, rather than rapid heterotrimeric G protein signaling (18). However, the mechanisms by which thrombin and APC promote biased signaling to differentially regulate inflammation and endothelial barrier integrity remain poorly understood.
To elucidate the pathways and proteins that engender PAR1 biased signaling induced by thrombin and APC in human endothelial cells, we used a quantitative phosphoproteomic approach. Here, we report the global phosphoproteome of thrombin and APC signaling using tandem mass tag (TMT) mass spectrometry (MS). Our results reveal unique dynamic phosphoproteome profiles of thrombin and APC signaling and the identification of multiple enriched biological functions associated with microtubules, adherens junctions, and actin, all key modulators of endothelial barrier function as well as important mediators of gene expression. Distinct sets of kinases and subsets of proteins predicted to mediate thrombin and APC biased signaling were also identified. The function of a subset of phosphorylated proteins not previously linked to thrombin and APC signaling was examined and led to the identification of afadin and adducin-1 as modulators of endothelial barrier function. These studies identify a rich array of proteins and pathways that engender PAR1 biased signaling in human endothelial cells.
Results
Quantitative Phosphoproteomic Workflow for Thrombin and APC Signaling.
Thrombin and APC have been shown to activate PAR1 via unique cleavage sites within the N terminus at arginine (R)41 and R46, respectively. Thrombin disrupts the endothelial barrier through rapid heterotrimeric G protein signaling, whereas APC triggers slower β-arrestin-2-mediated endothelial barrier stabilization in human umbilical vein endothelial cell–derived EA.hy926 cells (Fig. 1A) (14, 16–18). To enable identification of phosphorylation events induced by these PAR1 biased agonists, EA.hy926 cells were stimulated with either thrombin for 0, 2.5, and 5 min or APC for 0, 15, or 30 min in three biological replicates to capture changes in phosphorylation closely linked to their respective biological responses. Cell lysates were collected, and phosphopeptides were enriched by TiO2, processed for TMT 10-plex labeling, and analyzed by liquid chromatography-MS2/MS3 to quantify the proteome and phosphoproteome (Fig. 1B) (19) (SI Appendix, Materials and Methods) We quantified 11,266 phosphopeptides representing 6,698 proteins with a false discovery rate of <1% (Dataset S1) (20, 21). The phosphosite distribution was 82% phosphoserine (pS), 15% phosphothreonine (pT), and 2.5% phosphotyrosine (pY) (Fig. 1C), with most of the peptides phosphorylated on single (61%) and double (30%) sites and fewer on triple (7%) and quadruple (1.1%) sites (Fig. 1D). To validate our studies, lysates from mass spectrometry samples stimulated with thrombin or APC were analyzed by immunoblotting and showed rapid thrombin-induced p38 phosphorylation at T180/Y182 (Fig. 1E) and APC-stimulated Akt S473 phosphorylation at later times (Fig. 1E) (12, 14). These results confirm that the expected signaling pathways in endothelial cells were activated by the appropriate PAR1 biased ligands.
Fig. 1.
(A) Thrombin (Th) activates PAR1 by cleavage at R41, which couples to heterotrimeric G proteins and disrupts the endothelial barrier. APC, bound to EPCR, cleaves PAR1 at R46, which signals via β-arrestin-2 (β-arr2) to promote endothelial barrier stabilization. (B) Endothelial cells treated with Th or APC were processed for quantitative mass spectrometry. Pie charts of phosphosite distribution (C) and the number of phosphosites per peptide distribution (D). (E) Cell lysates from mass spectrometry nontreated 0 min and Th and APC treatments immunoblotted for p38 or Akt phosphorylation.
Quantitative Phosphoproteomic Temporal Profiling of Thrombin and APC Signaling.
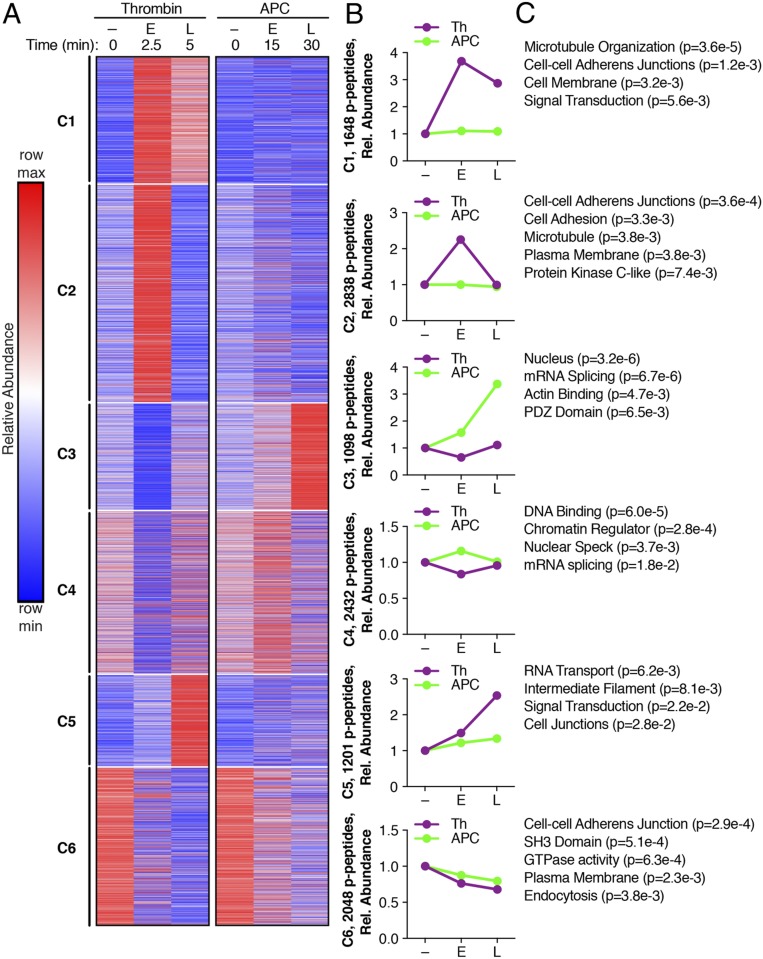
We next evaluated the temporal changes of the phosphoproteome induced by thrombin and APC using k-means clustering of the 11,266 phosphopeptides (Fig. 2 A and B) (Datasets S1 and S2) (22, 23). Each of the untreated 0 min replicates from the thrombin and APC subsets were compared by similarity matrices using Spearman’s correlation coefficients and were positively correlated (SI Appendix, Fig. S1), indicating minimal variances. The thrombin-induced phosphoproteome separated into three temporally distinct clusters (C), C1, C2, and C5, which displayed increases in phosphorylation at the early 2.5 min and late 5 min time points in C1, early time only in C2, and late time only in C5 compared to the untreated 0 min control conditions (Fig. 2 A and B). The subsets of phosphopeptides in C1, C2, and C5 were minimally altered by APC stimulation (Fig. 2 A and B). The APC-generated phosphoproteome clustered into two temporally distinct groups, C3 and C4. C3 showed a modest early increase in phosphorylation at 15 min and a pronounced late increase in phosphorylation at 30 min, whereas C4 showed only an early minimal increase in phosphorylation (Fig. 2 A and B). Thrombin caused a modest decrease in phosphorylation only at the early time point in both the C3 and C4 clusters of the APC phosphoproteome (Fig. 2 A and B). In the C6 cluster, both thrombin and APC caused a decrease in phosphorylation at both the early and late time points (Fig. 2 A and B). Thus, these data indicate that thrombin and APC induce distinct temporal dynamic changes in protein phosphorylation.
Fig. 2.
A k-means clustered heat map of the 11,266 quantified phosphopeptides (p-peptides) from Th and APC treatments (A) separated into six clusters. Increases and decreases in phosphorylation are represented by the red and blue colors, respectively. Color intensities depict phosphopeptide levels in each sample induced by Th or APC normalized to their respective 0 min control and relative (rel.) to the maximum and minimum abundances per row. (B) Th- and APC-induced changes in phosphopeptide abundance plotted against early (E) and late (L) times; – indicates 0 min non-treated. (C) Gene ontology enrichment analysis and rank based on statistical significance; P values were determined by Student’s t test.
To explore the biological functions associated with the distinct thrombin and APC phosphoproteomes, we analyzed and compared biological processes, cellular compartments, and molecular functions that were overrepresented in each cluster using gene ontology enrichment analysis (Dataset S2) (24, 25). Microtubule and adherens junctions were significantly enriched in the C1 and C2 clusters of the thrombin-induced phosphoproteome (Fig. 2C). C5 also showed thrombin-dependent enrichment of intermediate filaments, signal transduction, and cell junctions (Fig. 2C). Surprisingly, the C3 and C4 clusters in the APC phosphoproteome showed highly significant enrichment in the nucleus, messenger RNA (mRNA) splicing, and DNA binding and chromatin regulators (Fig. 2C). In addition, actin binding was enriched, although to a lesser extent, in C3, and decreases in phosphopeptides associated with adherens junctions were highly enriched in C6 and may be related to APC’s role in enhancing endothelial barrier stabilization (16, 18).
Thrombin and APC Significantly Altered Changes in Subsets of Phosphoproteins.
The k-means clustering analysis assumes that phosphopeptides targeted by the same kinase will yield similar temporal profiles (26). Thus, to identify the kinases known or predicted to target proteins in the specific clusters, we applied the motif-X algorithm (27) to define the most overrepresented kinase consensus motifs in each cluster and used NetworKIN to match the consensus sites with the kinases predicted to phosphorylate the site (Table 1) (Dataset S3) (28). Cyclin-dependent kinase 1 (CDK1) and protein kinase C-β (PKC-β) were among the most overrepresented motifs in the thrombin phosphoproteome. In contrast, the most overrepresented kinase consensus motif identified for the APC phosphoproteome in all three clusters was casein kinase 2-α (CK2), also known as protein kinase CK2 (Table 1). While CDK1 and PKC-β are known to mediate thrombin functions (5, 29), the role of CK2 in APC-triggered cellular responses is not known.
Table 1.
The top ranked identified protein kinase consensus motifs for phosphopeptides grouped into the distinct clusters (C) with a preference for serine or threonine and the kinases predicted to target these consensus sites
| Th or APC | Cluster | Motif | Kinases | Motif score | Matches | Fold increase |
| Th | C1, Ser | .....P.SP...K... | PKC-β | 622 | 25 | 29.9 |
| C1, Thr | .....SPT....... | CDK1 | 615 | 47 | 13.9 | |
| CK2-α | ||||||
| Th | C2, Ser | .......SP..SP.. | 625 | 32 | 18.1 | |
| .....P.SP...K.. | CDK1 | 623 | 35 | 26.8 | ||
| ....R..SP...... | PKC-β | 615 | 169 | 10.1 | ||
| C2, Thr | .......TPSP.... | 622 | 21 | 37.2 | ||
| .....SPT....... | 615 | 50 | 12.9 | |||
| .......TSP..... | 615 | 43 | 13.7 | |||
| APC | C3, Ser | ......RS.SP.... | CK2-α | 922 | 44 | 36.5 |
| ....RS.SP...... | CLK1 | 922 | 67 | 42.3 | ||
| .R.....SD.E.... | CDK1 | 629 | 30 | 96.8 | ||
| .K.....SD.E.... | 628 | 29 | 77.3 | |||
| .......S.EE...S | 623 | 20 | 39.4 | |||
| .......S.DE.... | 615 | 57 | 12.6 | |||
| C3, Thr | — | — | — | — | ||
| APC | C4, Ser | .......SP..SP.. | CK2-α | 923 | 90 | 18.8 |
| ......RS.SP.... | 923 | 49 | 23.7 | |||
| .....SDS..E.... | 923 | 47 | 31.4 | |||
| .......SDS..E.. | 923 | 45 | 32.6 | |||
| .......SSD.E... | 923 | 43 | 57.7 | |||
| .......SD.EE... | 631 | 81 | 50.0 | |||
| .......SDEE.... | 630 | 38 | 33.6 | |||
| ....RS.SP...... | 629 | 62 | 25.1 | |||
| .......S.SDE... | 627 | 23 | 38.1 | |||
| .......S.EDE... | 624 | 23 | 34.5 | |||
| .......S.P.SP.. | 623 | 33 | 17.0 | |||
| C4, Thr | .....SPT....... | 615 | 49 | 20.5 | ||
| Th | C5, Ser | ..R.R..S...E... | CDK1 | 623 | 23 | 39.5 |
| .....P.SP....P. | PKC-β | 623 | 55 | 26.4 | ||
| .....P.SP.....K | CK2-α | 622 | 28 | 43.6 | ||
| .....P.SP...... | 615 | 137 | 10.4 | |||
| C5, Thr | — | — | — | — | ||
| Th and APC | C6, Ser | ..K.S..SL...... | CK2-α | 631 | 34 | 36.5 |
| K....S.S..D.... | CDK1 | 627 | 24 | 38.6 | ||
| ....S..SSP..... | 626 | 42 | 14.3 | |||
| .......S..SSP.. | 625 | 36 | 15.2 | |||
| ....R.PSP...... | 623 | 44 | 25.8 | |||
| C6, Thr | ..F....TP.G.... | 630 | 21 | 281 | ||
| .....SPT....... | 615 | 41 | 16 |
The motif score (>500), number of matches in the query set, and the fold increase over the normal occurrence of the consensus site (>10-fold) are also provided. —, no motif detected.
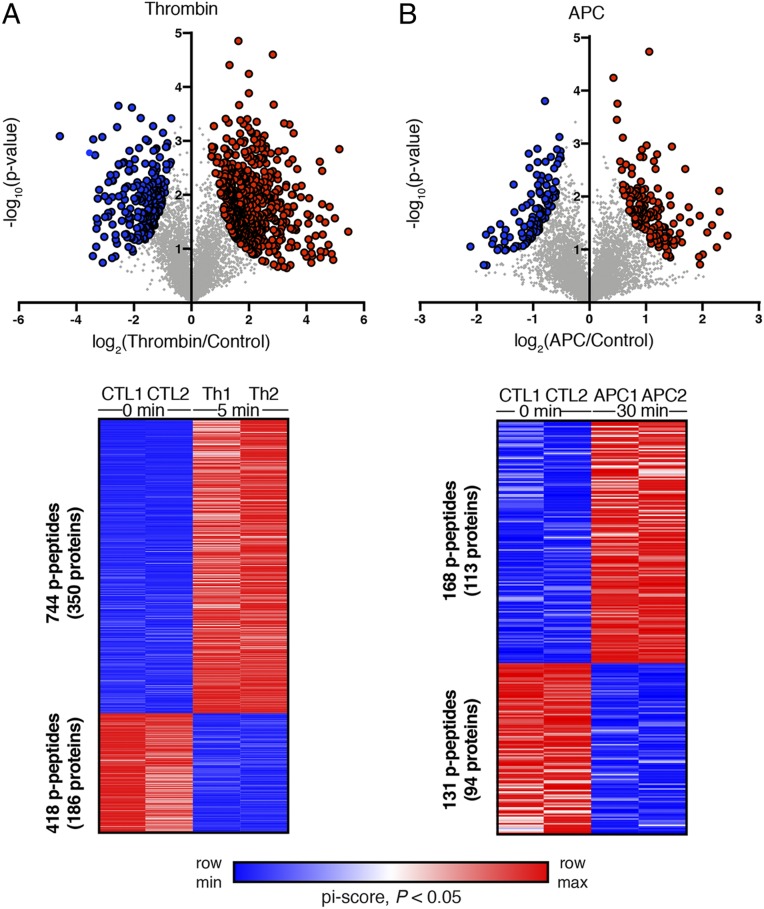
We next examined the effect of thrombin and APC on specific sets of phosphopeptides using pi-score analysis that combines statistical significance with agonist-induced fold change in phosphorylation into a single, comparable value (30). Thrombin induced significant changes in a total of 1,162 phosphopeptides, 744 peptides from 350 unique proteins with increases in phosphorylation and 418 peptides from 186 unique proteins with decreases in phosphorylation (Fig. 3A) (Dataset S4). Incubation with APC resulted in significant changes in a total of 299 phosphopeptides, of which 168 peptides from 113 unique proteins exhibited increases in phosphorylation and 131 peptides from 94 unique proteins showed decreases in phosphorylation (Fig. 3B) (Dataset S4). The corresponding heat maps of the individual TMT-MS replicates are highly similar (Fig. 3 A and B), verifying the reproducibility of the data. Together, these analyses indicate that thrombin and APC activate distinct sets of kinases and subsets of phosphorylated proteins in endothelial cells, consistent with the concept of PAR1 biased signaling.
Fig. 3.
Volcano plots and heat maps of significantly altered (pi-score, P < 0.05) p-peptide replicates of (A) Th- and (B) APC-treated endothelial cells. Log-transformed P values (Student’s t test) associated with individual phosphopeptides are plotted against the log-transformed fold change in abundance between 0 min control (Ctrl), Th, and APC treatments. Color intensities depict changes in phosphopeptide levels relative to the maximum (red) and minimum (blue) abundances per row.
Heat Maps and Diagrams of Candidate Proteins Phosphorylated by Thrombin or APC.
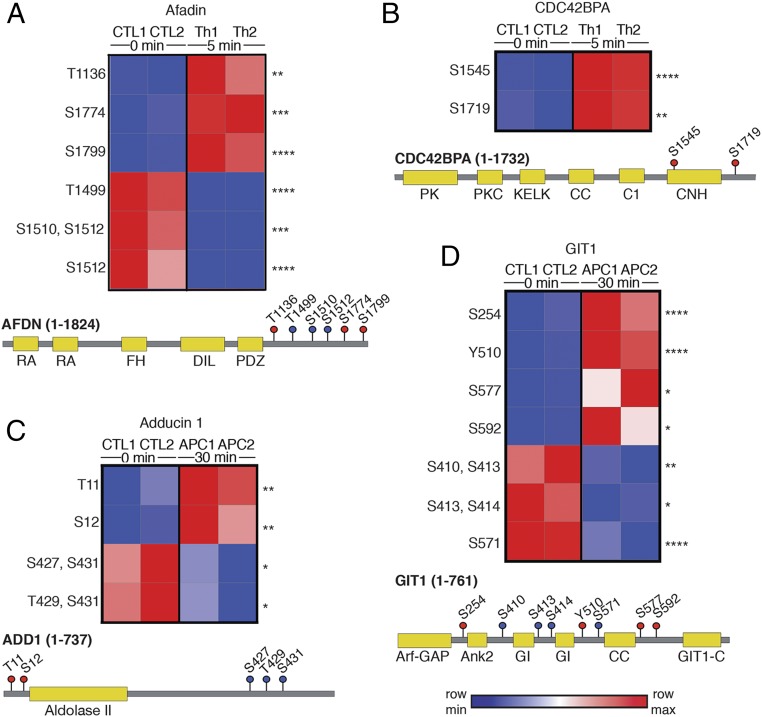
Given the abundance of phosphorylated proteins associated with adherens junctions, microtubules, and actin binding, all of which regulate endothelial barrier disruption, we hypothesize that a subset of phosphorylated proteins not previously linked to thrombin or APC function might regulate endothelial barrier integrity. Afadin (AFDN) is an actin filament-binding protein that associates with adherens junctions (31, 32) and showed significant increases in phosphorylation at three sites and decreases in phosphorylation at three other sites, all within the C-terminal domain, following thrombin stimulation (Fig. 4A). The CDC42 binding protein kinase alpha (CDC42BPA) protein, also known as MRCKA, is an effector of CDC42-induced peripheral actin formation and cytoskeletal reorganization (33) and also displayed significant increases in phosphorylation at two serine sites within the C-terminal region in thrombin-treated cells (Fig. 4B). Neither afadin nor CDC42BPA showed significant changes in phosphorylation in APC-stimulated cells (Dataset S4). We also identified two proteins, adducin-1 (ADD1) and GPCR interacting protein 1 (GIT1), that showed significant changes in phosphorylation after APC but not thrombin stimulation (Fig. 4 C and D) (Dataset S4). Adducin-1 is a key regulator of actin dynamics (34), and GIT1 is a scaffold protein that regulates focal contacts and cytoskeletal dynamics (35). APC induced significant increases in adducin-1 phosphorylation at two N-terminal sites and decreases in phosphorylation at three C-terminal sites (Fig. 4C). GIT1 was extensively phosphorylated throughout the protein, with four sites displaying increased phosphorylation and a distinct set of four other sites showing a decrease in phosphorylation after APC stimulation (Fig. 4D).
Fig. 4.
Heat maps and diagrams of proteins phosphorylated by Th (A) afadin and (B) CDC42BPA or by APC (C) adducin-1 and (D) GIT1. Increases and decreases in phosphorylation sites are indicated by red and blue color intensities, respectively. Statistical significance was determined by two-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001). Cartoon diagrams show the protein domain structure and phosphorylation site location; red and blue dots with residue numbers indicate increases and decreases in phosphorylation, respectively. Color intensity is indicative of the extent of phosphorylation. All sites are conserved across human, mouse, and rat species.
Afadin and Adducin-1 Regulate Thrombin-Induced Endothelial Barrier Disruption.
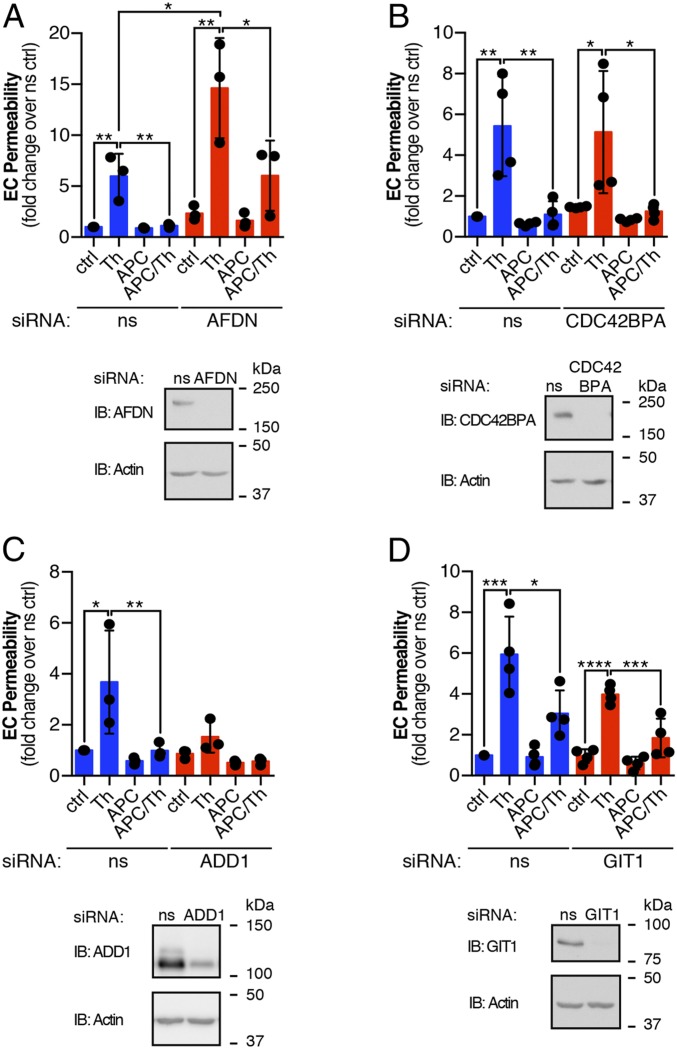
To assess the function of these proteins in endothelial barrier regulation, we used small interfering RNA (siRNA)-targeted depletion and examined thrombin-induced endothelial barrier disruption and APC-promoted endothelial barrier protection. Confluent monolayers of EA.hy926 cells were transfected with either control nonspecific siRNA or target-specific siRNAs that were optimized for efficient knockdown of protein expression (SI Appendix, Fig. S2). In control siRNA-transfected cells, thrombin caused an approximately fivefold increase in endothelial barrier permeability that was reduced to basal levels in APC pretreated cells (Fig. 5A), consistent with a role for APC in endothelial barrier protection (18). However, the magnitude of thrombin-induced barrier permeability was significantly greater, ∼15-fold, in afadin-depleted cells compared to control siRNA cells (Fig. 5A), suggesting afadin functions in regulation of endothelial barrier stability. Although APC-induced barrier protection was more variable in the afadin-depleted cells and reduced to approximately fivefold compared to control siRNA cells, it was significantly decreased relative to thrombin (Fig. 5A). In contrast, depletion of CDC42BPA had no effect on the magnitude of thrombin-induced endothelial barrier disruption or on the extent of barrier protection promoted by APC (Fig. 5B). Next, we evaluated adducin-1 and GIT1, which are phosphorylated by APC and not thrombin, in endothelial barrier function. Thrombin induced an approximately fourfold increase in endothelial barrier permeability in control siRNA cells, whereas depletion of adducin-1 virtually ablated thrombin-induced endothelial barrier permeability (Fig. 5C). The significant loss of thrombin-induced endothelial barrier disruption precluded the assessment of APC on barrier protection. In contrast, knockdown of GIT1 failed to alter either thrombin or APC regulation of endothelial barrier permeability (Fig. 5D), suggesting that afadin and adducin-1 actin binding proteins are important for regulation of barrier function.
Fig. 5.
EA.hy926 endothelial cells (EC) transfected with nonspecific (ns) and either (A) afadin, (B) CDC42BPA, (C) adducin-1, or (D) GIT1 siRNAs were pretreated with APC (180 min) prior to Th stimulation (10 min) and barrier permeability monitored at 30 min. Cell lysates were immunoblotted (IB) as indicated. The data (mean ± SD, n = 3 or 4) are expressed as the fold over the ns siRNA control and are analyzed by ANOVA for comparisons between or within groups (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Intercellular Gap Formation and Actin Polymerization Induced by Thrombin and APC.
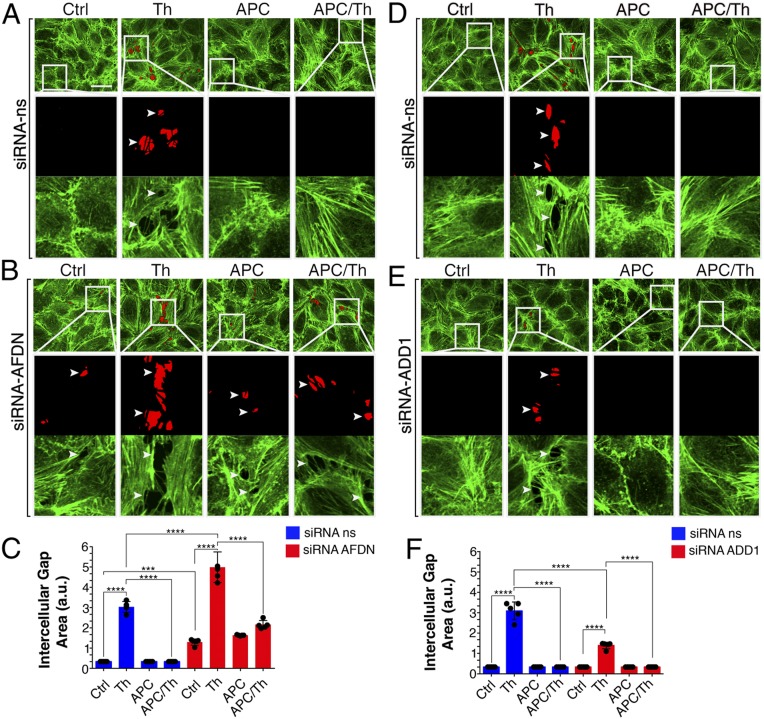
To gain insight into how afadin and adducin-1 modulate endothelial barrier function, we quantitatively determined the intercellular gap formation induced by thrombin and the extent of protection promoted by APC. The actin cytoskeleton is important for endothelial junction assembly, stabilization, and maintenance and was visualized using phalloidin (36, 37). An interconnected monolayer of endothelial cells with prominent actin bundles with negligible gap formation was observed in untreated control siRNA cells (Fig. 6 A–C), whereas incubation with thrombin caused a significant threefold increase in gap formation (Fig. 6 A–C). As expected, APC pretreatment significantly inhibited thrombin-induced gap formation in control siRNA cells (Fig. 6 A and C), consistent with a role for APC in barrier protection (Fig. 5) (18, 38). In cells depleted of afadin, gap formation was already evident in untreated control siRNA cells; however, thrombin caused an even greater fivefold increase in gap formation compared to thrombin-treated control siRNA cells (Fig. 6 B and C). Despite enhanced thrombin-induced barrier permeability, APC retained its ability to significantly reduce thrombin-promoted barrier disruption in afadin-depleted cells (Fig. 6 B and C). In contrast to afadin, thrombin-stimulated barrier permeability was significantly reduced in adducin-1 deficient cells compared to control siRNA cells (Fig. 6 D–F). Although thrombin-induced barrier permeability was substantially attenuated, APC further diminished thrombin-stimulated permeability in adducin-1 knockdown cells (Fig. 6 D–F). These findings indicate that adducin-1 and afadin, key regulators of actin dynamics, mediate opposing effects on thrombin-stimulated endothelial barrier disruption.
Fig. 6.
EA.hy926 cells transfected with (A) nonspecific (ns) or (B) afadin siRNAs or (D) nonspecific or (E) adducin-1 siRNAs. Cells were left untreated (Ctrl) or treated with Th (30 min) or APC (180 min) prior to Th (APC/Th). Cells were imaged by confocal microscopy. Intercellular gap formation (red areas and white arrowheads) were visualized. Boxed areas are 5× magnifications. (C and F) Quantification of the intercellular gap is the average area of six random fields per condition from five independent experiments. The data (mean ± SD, n = 5) are expressed in arbitrary units (a.u.) and analyzed by ANOVA for comparisons between and within groups (***P < 0.001; ****P < 0.0001). (Scale bar, 10 μm.)
Thrombin and APC Signaling in Endothelial Cells.
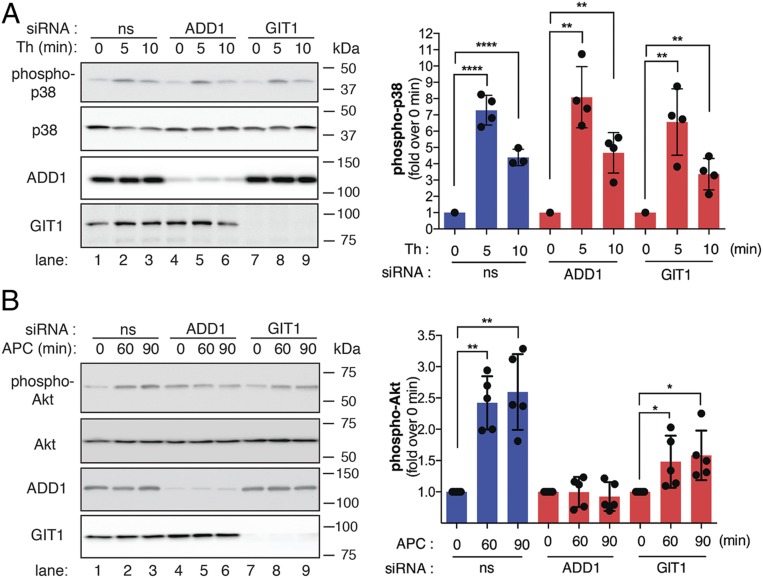
To ensure that thrombin activation of PAR1 signaling remained intact in adducin-1 siRNA-depleted cells, we examined p38 phosphorylation. In control siRNA-transfected cells, thrombin induced an approximately sevenfold peak increase in p38 activation at 5 min that remained elevated, approximately fivefold at 10 min (Fig. 7A, lanes 1 to 3), similar to that previously reported (12). A similar trend in thrombin-induced p38 activation was observed in both adducin-1 and GIT1 siRNA-depleted cells (Fig. 7A, lanes 4 to 9), indicating that thrombin signaling remains intact. These findings suggest that the effect of adducin-1 on thrombin-induced endothelial barrier permeability occurs either downstream or independent of p38 signaling. Interestingly, APC-induced activation of Akt was virtually abolished in adducin-1 knockdown cells (Fig. 7B, lanes 4 to 6) but remained intact in nonspecific siRNA- and GIT1-transfected cells (Fig. 7B, lanes 1 to 3 and 7 to 9), suggesting that the actin cytoskeleton may function in APC/PAR1-induced cytoprotective signaling in endothelial cells.
Fig. 7.
EA.hy926 cells transfected with nonspecific (ns), adducin-1, or GIT1 siRNAs were stimulated with (A) Th or (B) APC, lysed, and immunoblotted. Immunoblots representative of three independent experiments are shown. The data (mean ± SD, n = 3) are expressed as the fold over 0 min and analyzed by Student’s t test (*P < 0.05, **P < 0.01, ****P < 0.0001)
Discussion
PAR1 is activated by both thrombin and APC through distinct N-terminal cleavage sites, which causes opposite responses in endothelial cells through poorly characterized pathways (18, 39). Phosphorylation is a key regulator of protein function and many important biological responses. Thus, to understand how thrombin and APC induce PAR1 biased signaling to promote opposing cellular functions, we performed a comprehensive quantitative phosphoproteomic analysis using human cultured endothelial cells. We found that thrombin and APC induced distinct dynamic changes in the phosphoproteome that are targeted by specific sets of kinases. Our study further indicated that the majority of phosphorylated proteins identified in the thrombin phosphoproteome are associated with adherens junctions and the endothelial barrier function, whereas the APC phosphoproteome is closely affiliated with gene expression and actin. We interrogated the function of a subset of phosphoproteins not previously linked to thrombin or APC signaling and discovered that afadin and adducin-1, actin binding proteins, are important for thrombin-induced endothelial barrier disruption. These studies provide a comprehensive global analysis of thrombin and APC signaling and reveal a rich array of proteins and pathways that engender PAR1 biased signaling in endothelial cells.
While activation of PAR1 signaling by thrombin is rapid and catalyzed through coupling to heterotrimeric G proteins, APC-triggered PAR1 cytoprotective signaling is slower and mediated by β-arrestin-2-facilitated protein-protein interactions (18, 40, 41). Our phosphoproteomic analysis demonstrates that thrombin causes rapid increases in phosphorylation of subsets of proteins at the plasma membrane that are associated with modulation of adherens junctions. Adherens junctions are critical for maintaining endothelial cell–cell contacts and control barrier integrity (42, 43). The cytoskeleton is also a key regulator of barrier function and is controlled by a dynamic network of actin fibers, microtubules, and intermediate filaments (43, 44). The phosphorylation status of these same sets of proteins was minimally altered by APC stimulation. In contrast, the majority of APC-induced phosphorylated proteins were associated with the nucleus and affiliated with gene transcription and expression, consistent with previous studies showing APC modulates transcription of genes affiliated with proinflammatory and proapoptotic pathways in cytokine-stimulated endothelial cells (45, 46). A subset of APC-induced phosphoproteins was also associated with actin binding, which likely has a role in endothelial barrier stabilization through modulation of the actin cytoskeleton.
Thrombin causes barrier disruption through RhoA, MLCK, and p38 signaling and modulation of adherens junction proteins through protein phosphorylation and dephosphorylation (10, 12). However, the role of actin binding proteins and other modulators of the cytoskeleton in regulation of endothelial barrier stability by thrombin or APC signaling remains poorly understood. We found that afadin and adducin-1, two actin binding proteins, are extensively phosphorylated by thrombin and APC, respectively, and have significant but opposite effects on endothelial barrier permeability induced by thrombin. Depletion of adducin-1 virtually ablated thrombin-induced barrier permeability, without compromising p38 signaling, suggesting that adducin-1 is a key regulator of barrier function downstream of receptor signaling. However, loss of adducin-1 also caused significant disruption of APC-mediated Akt activation, indicating that APC activation of PAR1 signaling may also be modulated by the actin cytoskeleton. The precise mechanism by which perturbation of Akt signaling modulates APC/PAR1-induced endothelial barrier stabilization is not clear.
We also show that afadin and adducin-1 are phosphorylated on multiple conserved sites following agonist stimulation. However, with the exception of the adducin-1 S12 residue, which has been implicated in protein–protein interactions (47), all of the other identified phosphosites have no known function. Using the motif-X and NetworKIN algorithms, we identified CDK1 and PKC-β as the predominant kinases that mediate thrombin-induced protein phosphorylation, consistent their reported roles in promoting thrombin-mediated cellular proliferation and endothelial barrier dysfunction (5, 29). In contrast, CK2 appears to be the key regulator of APC-induced protein phosphorylation; however, the function of CK2 in the regulation of APC-induced cytoprotection is not known. Although phosphorylation is an important posttranslational modification with widespread functions, most phosphorylation sites are assigned to only 20% of known kinases, and the vast majority phosphorylation sites have not been studied and lack any known function (48). All of the thrombin and APC-induced phosphorylated sites identified in afadin and adducin-1 are conserved across species, suggesting they serve similar biological functions. Nonetheless, further analysis is necessary to examine the role of predicted kinases in mediating afadin and adducin-1 phosphorylation and their contribution to thrombin-induced endothelial barrier disruption. The current study examines the dynamics of phosphorylation at different times critical for either thrombin-induced barrier disruption or APC-induced barrier stabilization and therefore does not preclude the possibility that the biased agonists might share certain phosphorylation events at overlapping time points. In summary, our global analysis of the thrombin and APC phosphoproteome provides critical important information regarding the proteins and pathways that engender endogenous PAR1 biased signaling in human endothelial cells.
Materials and Methods
Reagents and antibodies, endothelial cell culture, cell transfections, immunoblotting, endothelial barrier permeability, cell imaging, mass spectrometry, and statistical analysis are described in SI Appendix, Materials and Methods.
Data Availability.
The mass spectrometry data have been deposited on MassIVE (MSV000084604; https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=562332e622c74f369103cb90917b4971) and on the ProteomeXchange (PXD016368; http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD016368).
Supplementary Material
Acknowledgments
This work was supported by NIH/National Institute of General Medical Sciences (NIGMS) Grants R35 GM127121 (to J.T.); NIH/NIGMS Grant K12 GM068524 (to O.M.-I. and J.D.L.); the University of California President’s Postdoctoral Fellowship Program (O.M.-I); and NIH/NIGMS Grants T32 GM007752 and NIH/NIAMS T32 AR064194 (to J.M.W.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The mass spectrometry data are deposited on MassIVE MSV000084604 and the ProteomeXchange PXD016368.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1917295117/-/DCSupplemental.
References
- 1.Goldenberg N. M., Steinberg B. E., Slutsky A. S., Lee W. L., Broken barriers: A new take on sepsis pathogenesis. Sci. Transl. Med. 3, 88ps25 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Weis S. M., Vascular permeability in cardiovascular disease and cancer. Curr. Opin. Hematol. 15, 243–249 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Sun L., Ye R. D., Role of G protein-coupled receptors in inflammation. Acta Pharmacol. Sin. 33, 342–350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goddard L. M., Iruela-Arispe M. L., Cellular and molecular regulation of vascular permeability. Thromb. Haemost. 109, 407–415 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coughlin S. R., Protease-activated receptors in hemostasis, thrombosis and vascular biology. J. Thromb. Haemost. 3, 1800–1814 (2005). [DOI] [PubMed] [Google Scholar]
- 6.Popović M., et al. , Thrombin and vascular inflammation. Mol. Cell. Biochem. 359, 301–313 (2012). [DOI] [PubMed] [Google Scholar]
- 7.O’Brien P. J., et al. , Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J. Biol. Chem. 275, 13502–13509 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Griffin C. T., Srinivasan Y., Zheng Y.-W., Huang W., Coughlin S. R., A role for thrombin receptor signaling in endothelial cells during embryonic development. Science 293, 1666–1670 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R., Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell 64, 1057–1068 (1991). [DOI] [PubMed] [Google Scholar]
- 10.Komarova Y., Malik A. B., Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu. Rev. Physiol. 72, 463–493 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Borbiev T., et al. , p38 MAP kinase-dependent regulation of endothelial cell permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L911–L918 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Grimsey N. J., et al. , Ubiquitin plays an atypical role in GPCR-induced p38 MAP kinase activation on endosomes. J. Cell Biol. 210, 1117–1131 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimsey N. J., et al. , A tyrosine switch on NEDD4-2 E3 ligase transmits GPCR inflammatory signaling. Cell Rep. 24, 3312–3323.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosnier L. O., Sinha R. K., Burnier L., Bouwens E. A., Griffin J. H., Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood 120, 5237–5246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin J. H., Zlokovic B. V., Mosnier L. O., Activated protein C, protease activated receptor 1, and neuroprotection. Blood 132, 159–169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo A., Soh U. J., Paing M. M., Arora P., Trejo J., Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc. Natl. Acad. Sci. U.S.A. 106, 6393–6397 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae J. S., Yang L., Rezaie A. R., Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 104, 2867–2872 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soh U. J., Trejo J., Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through β-arrestin and dishevelled-2 scaffolds. Proc. Natl. Acad. Sci. U.S.A. 108, E1372–E1380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapek J. D., Jr, et al. , Detection of dysregulated protein-association networks by high-throughput proteomics predicts cancer vulnerabilities. Nat. Biotechnol. 35, 983–989 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez D., Wozniak J., Thrombin/APC signaling through PAR1. MassIVE. https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=562332e622c74f369103cb90917b4971. Deposited 20 November 2019.
- 21.Gonzalez D., Wozniak J., Thrombin/APC signaling through PAR1. ProteomeXchange. http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD016368. Deposited 20 November 2019.
- 22.Wu J., Advances in K-Means Clustering (Springer, New York, 2012). [Google Scholar]
- 23.Ernst J., Bar-Joseph Z., STEM: A tool for the analysis of short time series gene expression data. BMC Bioinformatics 7, 191 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo H., et al. , Neuroprotective activities of activated protein C mutant with reduced anticoagulant activity. Eur. J. Neurosci. 29, 1119–1130 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Citro S., et al. , Phospholipase Cepsilon is a nexus for Rho and Rap-mediated G protein-coupled receptor-induced astrocyte proliferation. Proc. Natl. Acad. Sci. U.S.A. 104, 15543–15548 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Graaf E. L., Giansanti P., Altelaar A. F., Heck A. J., Single-step enrichment by Ti4+-IMAC and label-free quantitation enables in-depth monitoring of phosphorylation dynamics with high reproducibility and temporal resolution. Mol. Cell. Proteomics 13, 2426–2434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou M. F., Schwartz D., Using the scan-x Web site to predict protein post-translational modifications. Curr. Protoc. Bioinformatics 36, 13.16.1–13.16.8 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Linding R., et al. , NetworKIN: A resource for exploring cellular phosphorylation networks. Nucleic Acids Res. 36, D695–D699 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soh U. J., Dores M. R., Chen B., Trejo J., Signal transduction by protease-activated receptors. Br. J. Pharmacol. 160, 191–203 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Y., et al. , A novel significance score for gene selection and ranking. Bioinformatics 30, 801–807 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda W., et al. , Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J. Cell Biol. 146, 1117–1132 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato T., et al. , Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 281, 5288–5299 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Zhao Z., Manser E., Myotonic dystrophy kinase-related Cdc42-binding kinases (MRCK), the ROCK-like effectors of Cdc42 and Rac1. Small GTPases 6, 81–88 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuoka Y., Li X., Bennett V., Adducin: Structure, function and regulation. Cell. Mol. Life Sci. 57, 884–895 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoefen R. J., Berk B. C., The multifunctional GIT family of proteins. J. Cell Sci. 119, 1469–1475 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Hong S., Troyanovsky R. B., Troyanovsky S. M., Binding to F-actin guides cadherin cluster assembly, stability, and movement. J. Cell Biol. 201, 131–143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huveneers S., et al. , Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J. Cell Biol. 196, 641–652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finigan J. H., et al. , Activated protein C mediates novel lung endothelial barrier enhancement: Role of sphingosine 1-phosphate receptor transactivation. J. Biol. Chem. 280, 17286–17293 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Griffin J. H., Zlokovic B. V., Mosnier L. O., Activated protein C: Biased for translation. Blood 125, 2898–2907 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy R. V., Ardeshirylajimi A., Dinarvand P., Yang L., Rezaie A. R., Occupancy of human EPCR by protein C induces β-arrestin-2 biased PAR1 signaling by both APC and thrombin. Blood 128, 1884–1893 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanki H., et al. , β-arrestin-2 in PAR-1-biased signaling has a crucial role in endothelial function via PDGF-β in stroke. Cell Death Dis. 10, 100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dejana E., Tournier-Lasserve E., Weinstein B. M., The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev. Cell 16, 209–221 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Giannotta M., Trani M., Dejana E., VE-cadherin and endothelial adherens junctions: Active guardians of vascular integrity. Dev. Cell 26, 441–454 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Ingber D. E., Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ. Res. 91, 877–887 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Riewald M., Ruf W., Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J. Biol. Chem. 280, 19808–19814 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Riewald M., Petrovan R. J., Donner A., Mueller B. M., Ruf W., Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 296, 1880–1882 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Chan P. C., Hsu R. Y., Liu C. W., Lai C. C., Chen H. C., Adducin-1 is essential for mitotic spindle assembly through its interaction with myosin-X. J. Cell Biol. 204, 19–28 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Needham E. J., Parker B. L., Burykin T., James D. E., Humphrey S. J., Illuminating the dark phosphoproteome. Sci. Signal. 12, eaau8645 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry data have been deposited on MassIVE (MSV000084604; https://massive.ucsd.edu/ProteoSAFe/dataset.jsp?task=562332e622c74f369103cb90917b4971) and on the ProteomeXchange (PXD016368; http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD016368).