Significance
Dietary habits, especially meat consumption, represent a key aspect in the behavior and evolution of fossil hominin species. Here, we explore zinc (Zn) isotope ratios in tooth enamel of fossil mammals. We show discrimination between different trophic levels and demonstrate that Zn isotopes could prove useful in paleodietary studies of fossil hominin, or other mammalian species, to assess their consumption of animal versus plant resources. We also demonstrate the high preservation potential of pristine diet-related Zn isotope ratios, even under tropical conditions with poor collagen preservation, such as the studied depositional context in Southeast Asia. However, assessing the preservation of original δ66Zn values is required for each fossil site as diagenesis may vary across and even within taphonomic settings.
Keywords: zinc, stable isotopes, diagenesis, trophic ecology, diet
Abstract
Stable carbon and nitrogen isotope ratios of collagen from bone and dentin have frequently been used for dietary reconstruction, but this method is limited by protein preservation. Isotopes of the trace element zinc (Zn) in bioapatite constitute a promising proxy to infer dietary information from extant and extinct vertebrates. The 66Zn/64Zn ratio (expressed as δ66Zn value) shows an enrichment of the heavy isotope in mammals along each trophic step. However, preservation of diet-related δ66Zn values in fossil teeth has not been assessed yet. Here, we analyzed enamel of fossil teeth from the Late Pleistocene (38.4–13.5 ka) mammalian assemblage of the Tam Hay Marklot (THM) cave in northeastern Laos, to reconstruct the food web and assess the preservation of original δ66Zn values. Distinct enamel δ66Zn values of the fossil taxa (δ66Zncarnivore < δ66Znomnivore < δ66Znherbivore) according to their expected feeding habits were observed, with a trophic carnivore-herbivore spacing of +0.60‰ and omnivores having intermediate values. Zn and trace element concentration profiles similar to those of modern teeth also indicate minimal impact of diagenesis on the enamel. While further work is needed to explore preservation for settings with different taphonomic conditions, the diet-related δ66Zn values in fossil enamel from THM cave suggest an excellent long-term preservation potential, even under tropical conditions that are well known to be adverse for collagen preservation. Zinc isotopes could thus provide a new tool to assess the diet of fossil hominins and associated fauna, as well as trophic relationships in past food webs.
Stable isotope analyses in archeology and paleontology have been frequently used to explore the diet of past human populations. Nitrogen stable isotope (δ15N) analysis of bone or dentin collagen is an established method for the trophic level assessment (1, 2). However, these analyses are confronted with the limitations that arise from the degree of protein preservation (3). Trophic level assessment of ancient mammals and hominins older than ∼100 kyr are, due to the lack of collagen preservation, currently out of reach. This timeframe is even shorter (∼15 kyr) in arid and wet tropical settings that nonetheless often represent key regions in human evolution, such as Africa (4, 5) and Asia (6, 7). However, beyond the classical collagen-bound nitrogen isotopes, trophic level reconstructions from enamel with different isotope systems have become feasible (8–11) and were recently applied to fossil and archeological specimens (9, 12–15). Using multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS) allows for the measurement of “nontraditional” stable isotopes from various elements (calcium, magnesium, zinc [Zn], and strontium [Sr]). Among these, Zn isotope ratios (66Zn/64Zn, expressed as δ66Zn value) constitute a promising dietary indicator (11, 14, 16–18). Indeed, Zn is incorporated as trace element in the enamel bioapatite and, thus, has a better long-term preservation potential compared to collagen-bound nitrogen, showing promise for dietary reconstructions in archeology and paleontology (19). It was not until 2012 that Van Heghe et al. (20) began investigating the causes of the variability of δ66Zn values in a pilot study. Since then, work on mammals from modern food webs, first in Africa (16, 17) and then in the Canadian Arctic (18), have established the relationship between δ66Zn of bioapatite and diet.
As currently understood, two factors influence the variability of δ66Zn values in a food web: the initial Zn isotope composition from the source of intake and biological Zn isotope fractionation occurring within the organism itself. In plants, the initial bioavailable Zn isotope composition is derived from the soil, which is in turn controlled by the nature of the underlying bedrock. Igneous rocks exhibit relatively similar δ66Zn values (+0.3 ± 0.14‰ [2σ]) (21, 22). Sedimentary rocks show much more variable δ66Zn values (21–23), with the highest values found in marine carbonates (+0.3 to +1.4‰) (24, 25). An initial biological fractionation in plants then occurs between the roots and the soil, which favors the absorption of heavy Zn isotopes relative to the litter layer in which they grow (26–29). An active uptake of heavy Zn isotopes then enhances the intraplant mobility of light Zn isotopes to the most aerial parts of the plants (26–28), leading to a general trend of progressively lower δ66Zn values from root to leaves, i.e., within different parts of a single plant, but also leading to variable δ66Zn values between different plant species (26–28). In animals, the body tissues’ δ66Zn values also depend on the Zn isotopic composition of the foods consumed. Different plants and parts of plants consumed will thus induce varying δ66Zn values in herbivores. Similarly, the δ66Zn values of body tissues in carnivores depend on the prey and parts of the prey consumed, with muscles usually exhibiting low δ66Zn values compared to the average Zn isotopic composition of the body (11, 16, 17, 30). Since plants usually have the most elevated δ66Zn values (11, 17) and muscles low values (11, 16, 17, 30), the resulting δ66Zn values of a trophic chain follow an opposite trend as to the classic trophic level tracer δ15Ncollagen values, that increase about 3–4‰ per trophic level (2). The higher the trophic level of an animal is, the lower the δ66Zn values of its body tissues are (11, 14, 16–18, 30).
However, while enamel has been shown to be less prone to alteration than bone and dentin (31–36), it is nevertheless not immune from diagenetic processes (34–40). One key predicament to investigating paleoecology through the analysis of trace elements (such as Zn) is thus the absence of diagenetic alteration. Additionally, generalized diagenetic effects on Zn from enamel still remain mostly uncertain, as they seem to vary considerably from site to site (40–42). Therefore, careful investigations of potential postmortem alteration on trace elements in fossil teeth is crucial for each taphonomic setting to separate genuine ecological information from diagenetic alteration such as trace element incorporation, leaching, or replacement (40, 42, 43).
Tam Hay Marklot (THM) cave (filling of the cave, and its associated fauna, dated to 38.4–13.5 ka by U-Th analysis on teeth, SI Appendix, Supporting Information 1.3, Tables S8–S13, and Figs. S43–S46), in the northeastern part of Laos, Hua Pan Province, is situated in a subtropical latitudinal setting where preservation of organic material (i.e., collagen) is generally poor (44). This cave offers ideal conditions to rigorously assess the preservation potential of diet-related Zn isotopic composition in fossils, when compared to organic matter-bound dietary proxies such as N isotopes. Indeed, the complex and diverse sedimentary processes encountered in mainland Southeast Asia often lead to atypical preservation of the vertebrate assemblages, almost always originating from karst breccias (45–47). Subject to a highly variable climate- and water-dependent environment, these karst systems produce fossil assemblages that are often characteristic of long transportation processes through subterraneous cave networks, often with multiple reworking episodes (45–47). Furthermore, the surroundings of THM cave offer, at present-day and presumably also in the past, two types of photosynthetic pathways used by local plants, C3 and C4, thus allowing an additional and already well-established dietary tracer (δ13C) of the same specimens to be compared with the δ66Zn results. A detailed description of the regional geology and sedimentary deposits are presented in SI Appendix, Supporting Information 1.1.
Here, a multiisotope investigation was carried out on tooth enamel (δ66Zn, 87Sr/86Sr, δ13C, δ18O) and dentin collagen (δ15N), if still preserved, from the Late Pleistocene (38.4–13.5 ka) fossiliferous assemblage newly recovered in the THM cave in 2015. The preservation of diet-related Zn isotopic composition in fossil enamel was systematically investigated to assess the potential application of Zn isotope analysis for dietary reconstruction in deep time. In order to cover a broad range of distinct trophic levels and dietary habits, tooth enamel from 72 specimens belonging to 22 mammalian taxa was analyzed (SI Appendix, Table S1). A variety of small-, medium- and large-sized species were selected, covering a wide range of feeding categories including carnivores, omnivores, and herbivores (where a species’ specific trophic ecology was assigned based on analogous modern-day fauna’s dietary behaviors; SI Appendix, Table S1). Enamel from each specimen was sampled for δ66Zn, 87Sr/86Sr, δ13C, and δ18O isotope analyses (>5 mg per sample for Zn analysis; see SI Appendix, Supporting Information 3.1). Because kinetic and equilibrium biological fractionations of 87Sr/86Sr are negligible (48–51) and overwritten during normalization for instrumental mass bias (48), radiogenic 87Sr/86Sr ratios in animal bones and teeth reflect those of local bioavailable Sr sources (9, 48, 52, 53). Differences in 87Sr/86Sr would thus imply provenance from a distinct locality with a different geological bedrock type. The granitic bedrock found at THM locality is likely to exhibit higher 87Sr/86Sr associated with concomitant lower δ66Zn values, while the limestone bedrock would show the opposite trend. This could thus explain some of the variability observed in enamel δ66Zn values among the fossil teeth. The carbon isotopic composition (δ13C) of foods are incorporated into the tissues (i.e., bone and enamel) of the animals that eat them (54, 55). In terrestrial animals, the carbon of food webs is derived from plants that undergo either C3 or C4 photosynthesis (56), providing a complementary dietary tracer to δ66Zn values. While an array of complex variables are likely to induce variations in the oxygen isotopic composition (δ18O) of tooth enamel in homeothermic vertebrates (57–59), the present study seeks to explore possible relation between δ18O values and Zn isotopic composition, mostly relative to diet and physiology. A subsample of 23 specimens was also analyzed for dentin collagen δ13C and δ15N values in order to assess the preservation of organic material. When collagen preservation was sufficient (SI Appendix, Supporting Information 3.5), the δ15N values (n = 4) were compared with δ66Zn of the same specimen since the collagen-bound δ15N values reflect the amount of animal protein in the diet and can thus be used to assess trophic level (2). The impact of postmortem taphonomic alteration processes was assessed in situ with spatially resolved element concentration profiles on six fossil mammalian teeth as well as three modern ones for comparison, with distinct feeding behaviors (carnivorous, omnivorous, and herbivorous), digestive physiologies (foregut, hindgut, and carnivore) and phylogenetic histories (Artiodactyla, Perissodactyla, Carnivora, Rodentia, and Primates). Finally, in order to enhance the interpretative framework of Zn isotopic composition, we explored the relation between individual factors (diet, 87Sr/86Sr, δ13Capatite, δ18Oapatite, zinc concentration, and body mass) with δ66Zn values, by fitting a linear mixed model (LMMs; ref. 60) with a Gaussian error structure and identity link (61). A detailed description of the variation of the different stable isotope systems and the methods used in this study are presented in SI Appendix, Supporting Informations 2 and 3.
Results
Measured δ66Zn, 87Sr/86Sr, δ13C, δ18O, and δ15N values for all specimens and reference materials are summarized in SI Appendix, Supporting Information 4, Tables S3–S5, and Figs. S8–S10.
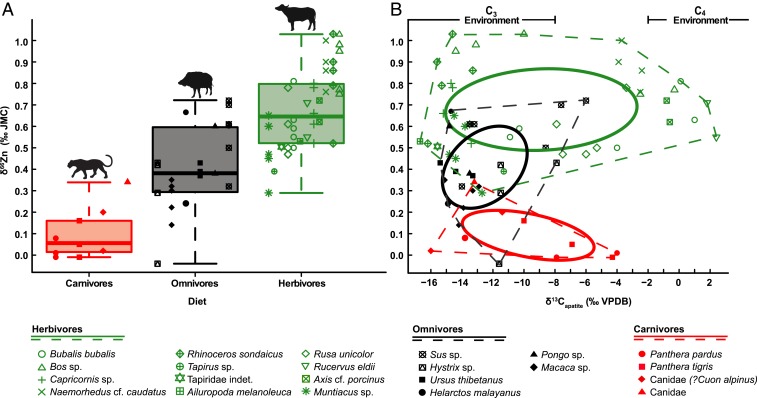
The total range of tooth enamel δ66Zn values from THM cave is 1.07‰, ranging from −0.04‰ to +1.03‰ (Fig. 1). Herbivores exhibit the highest δ66Zn values (δ66Zn = +0.68 ± 0.38‰ [2σ], n = 41), carnivores the lowest (δ66Zn = 0.09 ± 0.24‰ [2σ], n = 9) and the δ66Zn values of omnivores fall in between (δ66Zn = +0.41 ± 0.38‰ [2σ], n = 22) (Fig. 1 and SI Appendix, Table S3). Omnivorous taxa, on average, show the highest variability in the intrataxon ranges of their δ66Zn values (average δ66Zn range = 0.30 ± 0.34‰ [2σ]), compared to herbivores (average δ66Zn range = 0.28 ± 0.24‰ [2σ]) and carnivores (average δ66Zn range = 0.15 ± 0.10‰ [2σ]).
Fig. 1.
(A) Range of δ66Zn values (relative to the JMC-Lyon Zn isotope standard; ref. 74) in tooth enamel for carnivores (red), omnivores (black), and herbivores (green) of the THM cave assemblage. The boxes from the box and whisker plots represent the 25th–75th percentiles, with the median as a bold horizontal line. (B) Distribution of enamel δ66Zn versus δ13Capatite values of the THM cave assemblage (SI Appendix, Table S3), where “C3 environment” and “C4 environment” are, respectively, defined by δ13Capatite < −8‰ and > −2‰. Dashed lines represent the full range of variation and full lines represent 40% predictive ellipses (using R statistical software and package “SIBER”; refs. 72 and 75).
Sixty-nine of 72 specimens were analyzed for 87Sr/86Sr. Enamel 87Sr/86Sr display a broad range, from 0.7097 to 0.7243 (Δ = 0.0146); however, the majority of specimens clusters between 0.7135 and 0.7173 (52%, n = 36; SI Appendix, Table S3).
Enamel δ13C values range from −16.70‰ to 2.40‰ (n = 72), covering the full spectrum of values typical for pure subcanopy to open woodland C3 and C4 plant feeders (Fig. 1B and SI Appendix, Table S3). Fossil enamel from THM cave indicates that a predominant C3 environment existed in the cave surroundings but with a definite C4 grass component (56). The enamel δ18O values range from −5.85‰ to 0.2‰.
Collagen preservation of the teeth was poor, as only 4 (Muntiacus sp., Bos sp., Sus sp., and Rhinoceros sondaicus) of the 23 dentin samples yielded any collagen and even these fell below the 1% limit (∼0.46 ± 0.48% [2σ]; modern bones = ∼22%; ref. 3). Nonetheless, collagen extracts have C:N ratios characteristic of well-preserved collagen (3.26 ± 0.08 [2σ]) (3) (SI Appendix, Table S4 and Fig. S9). The δ15Ncollagen values associated with these specimens range from +3.15‰ to +10.56‰ and the δ13Ccollagen values from −24.0‰ to −9.1‰. The higher δ15Ncollagen values are in agreement with associated lower δ66Zn values, for taxa assigned an omnivorous diet (Sus sp. and Muntiacus sp.), and conversely the lower δ15Ncollagen values with higher δ66Zn values, representative of an herbivorous diet (Bos sp. and Rhinoceros sondaicus). The δ13Ccollagen and δ13Capatite values are also consistent for each specimen (SI Appendix, Tables S3 and S4).
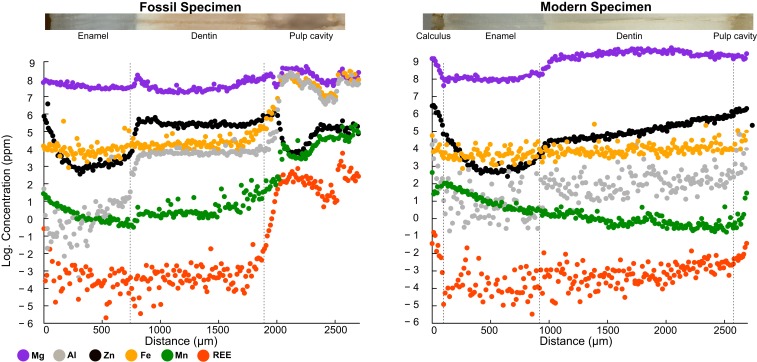
Zinc concentration distribution was investigated in 15 cross-sections from 6 fossil mammal teeth and compared to that of 10 cross sections from 3 modern specimens, to assess the impact of postmortem taphonomic processes on the enamel. Additionally, Fe, Mn, Al, Mg, and rare earth elements (REE, calculated as the sum of all measured REE concentrations), which are sensitive to diagenetic alteration, provided complementary tracers to discern the degree of diagenetic alteration of the enamel. Concentrations and distribution profiles of these elements were similar to those of modern teeth and were observed almost systematically across modern and fossil enamel samples, suggesting a lack of any significant diagenetic alteration (uptake or leaching) of trace elements in the latter. Similarly, an absence of relationship between δ66Zn values and average enamel concentration in various other trace elements, with potentially different susceptibilities for alteration (43), can be observed (SI Appendix, Figs. S35–S39). In contrast, the dentin and pulp cavity of the fossil teeth had higher concentrations of these elements indicating diagenetic alteration (Fig. 2). On 15 fossil tooth cross-sections, a total of 23 enamel segments were analyzed. Of these, only one enamel cross-section segment showed Zn concentration distribution that did not follow the characteristic pattern observed for modern enamel (n = 10) of higher concentration in the outermost layer that decreases toward a constant level inwards (SI Appendix, Fig. S30) (19, 62–64). While the distribution of this one segment may indicate some postmortem alteration, none of the other three enamel cross-section segments analyzed from that same specimen (Panthera pardus, 34505) displayed an atypical pattern. The absence of any significant postmortem Zn uptake is nonetheless further corroborated by the absence of a mixing line between Zn concentration and δ66Zn values (SI Appendix, Fig. S32). Altogether, a good preservation of the enamel seems to prevail in the fossil specimens, suggesting no alteration of original Zn contents and, hence, preservation of pristine biogenic δ66Zn values. Complete sets of spatial element concentration profiles are provided in SI Appendix, Figs. S12–S29.
Fig. 2.
Spatial element concentration profiles of Zn, Fe, Mn, Al, Mg, and REE in caprine teeth for a fossil (Capricornis sp., Left) and a modern (H. jemlahicus, Right) specimen. The Fe, Mn, Al, and REE (calculated as the sum of all measured REE concentrations) were selected as tracers for diagenetic alteration because of their relative abundance in soil matter, as well as their tendency to be enriched postmortem in fossil bioapatite. Thus, they most likely trace postmortem taphonomic alterations and element uptake from soil pore water. Note that in both photomicrographs the tracks of laser ablation line scans are visible.
Overall, the full-null LMM comparison was clearly significant (likelihood ratio test: X2 = 21.29, df = 2, P < 0.001) and allowed to assess which of the tested predictors were associated with variations in δ66Zn values. The δ13C and δ18O values, as well as the zinc concentration of each sample, appeared to have no significant relation with the variability of the δ66Zn values response (δ13Capatite values likelihood ratio test: X2 = 0.230, df = 1, P = 0.632; δ18Oapatite values likelihood ratio test: X2 = 0.135, df = 1, P = 0.713). Diet had a significant relation with δ66Zn values whereby omnivores and herbivores had clearly elevated values as compared to carnivores (LMM diet P < 0.05; SI Appendix, Table S6). Finally, 87Sr/86Sr and body mass both displayed a significant relation with the variability of the δ66Zn values (87Sr/86Sr likelihood ratio test: X2 = 12.101, df = 1, P = 0.001; body mass likelihood ratio test: X2 = 9.892, df = 1, P = 0.002). While (G)LMMs do not allow to get an estimated effect size for individual predictors, the effect size for the entirety of the fixed effects (“marginal R2”) is 0.63 and the one for the entirety of the fixed and random effects (“conditional R2”) is 0.85 (65).
Discussion
Preservation of Diet-Related Zn Isotope Compositions in Fossil Teeth.
The ordering of fossil taxa from THM cave according to their enamel δ66Zn values (δ66Zncarnivores < δ66Znomnivores < δ66Znherbivores) reflects trophic level differences that are in good agreement with their expected dietary habits (Fig. 1), as well as δ66Zn values observed for modern mammals from similar feeding categories (14, 16–18). This strongly suggests that the enamel of the 38.4–13.5 thousand-year-old fossil teeth from THM cave retained their original, diet-related Zn isotopic composition expected for each feeding category and, hence, was not altered by taphonomic processes. This is further supported by spatial distribution profiles of Zn across the fossil enamel with higher concentrations in the outermost enamel layer decreasing toward a constant level inwards, which is a characteristic pattern for modern teeth (Fig. 2 and SI Appendix, Figs. S18–S20 and S27–S30) (19, 62–64). The higher concentration in Zn in the first few tenths of microns of the outermost enamel layer is believed to be a biochemical signal that could be associated with the termination of the enamel maturation (63). While this pattern is systematically observed for all teeth (i.e., both fossil and modern ones), thus supporting the preservation of pristine biogenic Zn concentrations (and thus δ66Zn signatures), it also poses a challenge for distinguishing between original biogenic signature and postmortem diagenetic uptake. However, this layer of higher Zn concentration is systematically only <200 μm thick (Fig. 1 and SI Appendix, Figs. S12–S29) (19, 62–64) and, thus, lends further support to the preservation of a biogenic pattern. Furthermore, this outer Zn-rich layer is routinely removed mechanically during the enamel cleaning process for stable isotope analysis.
Due to the tropical setting of THM cave, collagen preservation is limited, as reflected by low collagen extraction success rate (i.e., n = 4) and low collagen yield (<1%). Nevertheless, the few δ15Ncollagen values that were obtained follow the expected trend in δ66Zn values, where relatively high δ15Ncollagen values are associated with relatively low δ66Zn values (18) (SI Appendix, Fig. S31). Finally, the low in vivo-like Mn, Fe, Al, Mg, and bulk REE contents, typical for enamel of modern mammal teeth (Fig. 2), demonstrate the lack of any significant diagenetic uptake of trace elements from the soil environment rich in these elements. Although postmortem taphonomic processes can vary significantly from one location to another due to site formations processes, age, environmental conditions, and soil composition (35, 66, 67), multiple lines of evidence presented in this study support the effective preservation of diet-related Zn isotopic composition in enamel of the investigated fossil teeth despite the adverse tropical setting of THM cave. This is encouraging for future applications of Zn isotopes in enamel of fossil teeth for dietary reconstructions.
Variation in Zn Isotopic Compositions in Tooth Enamel.
The overall mean value and range of δ66Zn values for each diet category, as well as the intraspecific δ66Zn variability of each taxon (SI Appendix, Supporting Information 4.1), are in agreement with their dietary habits and display nearly no overlap between carnivores and herbivores. Additionally, the LMM further confirmed that δ66Zn values differ between each of the three dietary categories. Carnivores exhibit the lowest δ66Zn values, in agreement with a strict carnivorous diet, and the smallest range of variation. In contrast, herbivores have significantly higher δ66Zn values than carnivores and many omnivores (Fig. 1). Herbivores also have a broad range of δ66Zn values being consistent with the consumption of a variety of different plants, plant parts, and their specific digestive strategies (foregut and hindgut fermentation). Omnivores display mostly intermediate δ66Zn values but occasionally exhibit values characteristic of carnivorous taxa or strictly herbivorous taxa (Fig. 1). Thus, omnivores exhibit the largest range of δ66Zn values covering all three dietary categories, most likely resulting from a varying proportion of meat (but also of plant and animal matter from vertebrates and invertebrates) in their diet. While not enough data are yet available to draw any definitive conclusions, it is likely that the lower end of their δ66Zn range reflect diets that are mostly composed of animal matter, whereas the upper range would be predominantly, if not entirely, comprised of plants. A single herbivore specimen (Muntiacus sp.) falls within the range of carnivores. However, this taxon is known at times to exhibit omnivorous dietary habits, feeding on bird’s eggs and small animals (68, 69). Furthermore, its associated high δ15N value attests to a diet that is, at the very least, not strictly limited to plant matter. Finally, both its δ66Zn and δ15N values are similar to that of a Sus sp., further supporting an omnivorous diet for this Muntiacus specimen.
Overall, the range of δ66Zn values for THM is smaller (1.07‰) than seen in a modern terrestrial food web of the Koobi Fora region of Turkana Basin in Kenya (1.24‰) (17), and the absolute δ66Zn values of the whole food web are also lower. This is likely the result of different faunal assemblages and environments between the two localities: THM cave was situated in a mostly forested setting, whereas Koobi Fora is mainly an open grassland landscape. Because trees are likely to exhibit lower δ66Zn values in their leaves compared to low growing herbaceous vegetation (26–28), this could explain why herbivores, and consequently carnivores, have lower enamel δ66Zn values at THM cave. The Zn isotopic composition of the local geology, seen as having a significant relation with δ66Zn values of THM cave, could also in part explain differences observed between these sites. The trophic spacing observed between mammalian carnivore-herbivore is also larger at THM (+0.60‰) than at Koobi Fora (+0.40‰). This is likely the result of the faunal assemblage from Koobi Fora, as it contains less species and specimens (n = 10 and n = 26, respectively), carnivores that do not prey on most or any of the herbivores listed, and hyenas’ higher δ66Zn values probably caused by bone consumption (17). As opposed to the Koobi Fora region, no clear distinction in δ66Zn values can be drawn between grazers and browsers at THM. However, two groups can be discerned in the δ66Zn values of browsers (established by δ13Capatite < −8‰ characteristic for C3 plant feeders), one with low (+0.52 ± 0.20‰ [2σ], n = 14) and the other with high (+0.90 ± 0.20‰ [2σ], n = 9) δ66Zn values (Fig. 1). In the upper range of the sampled browsers’ δ66Zn values (Fig. 1), a mixture of both foregut and hindgut fermenters as well as large and intermediate body-sized taxa are present. Consequently, we conclude that digestive physiology and body mass can be ruled out as factors explaining this variability. Maternal effects linked to breastfeeding or in utero tooth formation were also ruled out as causes to intragroup δ66Zn values variability, as the formation and emergence sequence of the sampled teeth (i.e., only teeth of adult individuals formed postweaning; SI Appendix, Table S1) goes against such interpretation. Therefore, the most likely explanation would be diet, most probably linked to the vertical layering of the vegetation in a given habitat. Because of progressively lower δ66Zn values observed within plants from root to leaves, browsing in lower vegetation layers on herbaceous understory plants should lead to higher, grazer-like δ66Zn values, while browsing in upper vegetation layers like the canopy should lead to lower δ66Zn values. This might be the reason for similar δ66Zn values between some browsers (e.g., Rhinoceros sondaicus and Bos sp.) and grazers (e.g., Rucervus eldii and Axis cf. porcinus, with δ13Capatite > −2‰ characteristic for C4 plant feeders) (Fig. 1).
Finally, the estimates obtained for 87Sr/86Sr and body mass from the LMM were in agreement with their respective expectation toward δ66Zn values in a food web (SI Appendix, Table S6). Based on the Sr and Zn isotope composition of crustal rocks (21–25, 70), an increase in 87Sr/86Sr ratios associated with a decrease in δ66Zn values was expected (SI Appendix, Table S6): Granitic bedrock usually exhibits higher 87Sr/86Sr associated with concomitant lower δ66Zn values while limestone bedrock show the opposite trend, both present at THM locality. Likewise, a positive relationship between δ13C and body mass due to 13C enrichment with increasing body mass was reported elsewhere (71) and seems to also apply for δ66Zn values (SI Appendix, Table S6). Conversely, no significant relation could be drawn between δ13C and δ18O values and δ66Zn values relative to diet and physiology. The LMM thus allowed us to successfully identify which of the tested predictors showed a significant relation with δ66Zn values, otherwise not always identified as such (SI Appendix, Fig. S10). However, their respective impact on δ66Zn values cannot be estimated, although it seems likely that its effect is limited since dietary habits are preserved. Further work (e.g., controlled feeding experiments) will be necessary to ascertain and quantify the impact of these factors on δ66Zn values in a broader and more general context, especially compared to diet.
Conclusion
In this study, the first Zn isotope dataset of fossil tooth enamel, from a Late Pleistocene Southeast Asian faunal assemblage (∼38.4–13.5 ka) from the THM cave in northeastern Laos, Hua Pan Province, is presented. We show multiple lines of evidence that support the lack of significant postmortem diagenetic trace element uptake from the soil environment into the enamel of fossil teeth. Enamel profiles along tooth cross sections do not display any identifiable postmortem alteration of biogenic Zn concentration gradients or diet-related δ66Zn by postmortem processes. The classic trophic-level tracer δ15Ncollagen (only obtained for four samples) displayed an expected inverse trophic relation with δ66Zn of the same teeth, further supporting the preservation of original δ66Zn values. The Late Pleistocene mammal teeth from THM thus retained pristine, diet-related δ66Zn values in their tooth enamel that are in good agreement with expected dietary habits of the concerned taxa. For this fossil food web, a trophic level spacing of −0.60‰ between herbivores and carnivores was found, while omnivores had intermediate δ66Zn values being 0.30‰ lower or higher to herbivores and carnivores, respectively. Thus, carnivores have the lowest, omnivores intermediate, and herbivores the highest δ66Zn values. Contrary to what was previously observed in an African grassland environment regarding the distinction of browsers and grazers (17), no obvious relation was found between δ13C and δ66Zn values. However, both the local geology and the body mass showed a significant relation with consumer’s δ66Zn values, as expected. Further studies from other sites and from controlled feeding experiments will be necessary to ascertain the factors at play and their impact on the variability of δ66Zn values in consumer (hard) tissues. While a systematic, site-specific assessment of the extent of diagenetic alterations of biogenic compositions in fossils is required, the results obtained from THM cave show promise for a high preservation potential of δ66Zn values in fossil enamel. Applying δ66Zn as dietary tracer could thus open new research avenues in paleontology and archeology, providing us with a powerful and much-needed isotopic trophic tracer for prehistoric and geological time periods (>100 kyr) or settings that lack collagen preservation, given pristine δ66Zn values are preserved.
Methods
Sample Collection.
The material used in this study consists of a selection of diverse taxa from the THM assemblage, covering a large range of distinct dietary habits. Within each taxon, the same teeth on the dental row (e.g., left p2), or different teeth but with various wear stages (e.g., left and right p2), were selected to ensure they belonged to different individuals. A total of 72 teeth, belonging to 22 distinct species and/or genera, were selected for the present isotopic analysis. One to six specimens per species were used (SI Appendix, Table S1).
Stable Isotope Analysis.
Zn and Sr isotopic ratios from teeth enamel were measured on a Thermo Scientific Neptune MC-ICP-MS and C and N isotopic ratios from teeth dentin were conducted using a Thermo Finnigan Flash EA coupled to a Delta V isotope ratio mass spectrometer, at the Max Planck Institute for Evolutionary Anthropology in Leipzig, and following the protocols in SI Appendix, Supporting Informations 3.2, 3.3, and 3.5. Stable C and O isotopic composition of every sample were analyzed using a Thermo Delta V Advantage isotopic mass spectrometer coupled to a Thermo Kiel IV Carbonate Device chemical preparer, at the “Service de Spectrométrie de Masse Isotopique du Muséum” in Paris, using the protocol described in SI Appendix, Supporting Information 3.4.
Spatial Element Concentration Profiles Analytical Technique.
Spatial element concentration profiles were conducted on six fossil teeth (Capricornis sp., Ursus thibetanus, P. pardus, Sus sp. Bubalus bubalis, and Macaca sp.) and three modern teeth (Bison bison, Hemitragus jemlahicus, and Pteronura brasiliensis) of various feeding behaviors (carnivorous, omnivorous, and herbivorous), digestive physiologies (foregut, hindgut, and carnivore) and mammalian order (Artiodactyla, Perissodactyla, Carnivora, Rodentia, and Primates). The measurement routines were performed with a Thermo Scientific Element 2 single collector sector-field ICP-MS coupled with a New Wave UP213 Nd:YAG laser ablation system, at the Max Planck Institute for Chemistry (Mainz), as described in SI Appendix, Supporting Information 3.6.
Statistical Analysis.
All statistical analyses were performed using the statistical program R (version 3.6.1) (72). To test our hypotheses of predictors associated with variability in δ66Zn values, we fitted a LMM (60) with a Gaussian error structure and identity link (61) using the R-package “lme4” (version 1.1–17) (73). The full method is reported in SI Appendix, Supporting Information 3.7.
A complete description of the material and methods used in this study is presented in SI Appendix, Supporting Information 3. All data discussed in the paper is available to readers in SI Appendix.
Supplementary Material
Acknowledgments
We thank K. Schilling and B. Brumme (Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology, Leipzig) as well as O. Tombret (UMR 7209 AASPE) for technical support; S. Steinbrenner (Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology, Leipzig) who performed the C and N analysis; R. Mundry (Department of Primatology, Max Planck Institute for Evolutionary Anthropology, Leipzig) for his valuable help and insight with the LMM; R. Barr, C. Zickert, L. Därr, A. Salzer, and L. Schymanski (Multimedia Department, Max Planck Institute for Evolutionary Anthropology, Leipzig) for their help with pictures and figure presentation; Christine Lefèvre, Joséphine Lesur, and Aurélie Verguin of the UMR 7209 (AASPE), Muséum National d’Histoire Naturelle, in Paris, for the agreement and access to the mammal collection; and M. Sponheimer and P. Telouk for their helpful discussions. We would like to acknowledge the support and thank the Max Planck Society and the Deutsche Forschungsgemeinschaft (“PALÄODIET” Project 378496604) for funding this study. T.T. and K.J. received funding by the European Research Council under the European Union’s Horizon 2020 research and innovation program Grant Agreements 681450 and 803676, respectively. Funding for the excavation of the Marklot cave in 2015 was provided by the University of Strasbourg (Unistra/EOST UMR 7516) and the Unité Propre de Recherche (UPR) 2147 of CNRS Dynamique de l'évolution humaine, France, and the University of Illinois at Urbana–Champaign. Finally, we would like also to thank V.S. and S. Luangaphay of the Department of National Heritage, Ministry of Information and Culture in Vientiane, Laos, for their authorization to study the Marklot fauna.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911744117/-/DCSupplemental.
References
- 1.Deniro M. J., Epstein S., Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Cosmochim. Acta 45, 341–351 (1981). [Google Scholar]
- 2.Schoeninger M. J., DeNiro M. J., Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim. Cosmochim. Acta 48, 625–639 (1984). [Google Scholar]
- 3.van Klinken G. J., Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 26, 687–695 (1999). [Google Scholar]
- 4.Ambrose S. H., Effects of diet, climate and physiology on nitrogen isotope abundances in terrestrial foodwebs. J. Archaeol. Sci. 18, 293–317 (1991). [Google Scholar]
- 5.van de Loosdrecht M., et al. , Pleistocene North African genomes link Near Eastern and sub-Saharan African human populations. Science 360, 548–552 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Clarkson C., et al. , The oldest and longest enduring microlithic sequence in India: 35 000 years of modern human occupation and change at the Jwalapuram Locality 9 rockshelter. Antiquity 83, 326–348 (2009). [Google Scholar]
- 7.Krigbaum J., Reconstructing human subsistence in the West Mouth (Niah Cave, Sarawak) burial series using stable isotopes of carbon. Asian Perspect. 44, 73–89 (2005). [Google Scholar]
- 8.Chu N.-C., Henderson G. M., Belshaw N. S., Hedges R. E. M., Establishing the potential of Ca isotopes as proxy for consumption of dairy products. Appl. Geochem. 21, 1656–1667 (2006). [Google Scholar]
- 9.Knudson K. J., et al. , Introducing δ88/86Sr analysis in archaeology: A demonstration of the utility of strontium isotope fractionation in paleodietary studies. J. Archaeol. Sci. 37, 2352–2364 (2010). [Google Scholar]
- 10.Martin J. E., Vance D., Balter V., Natural variation of magnesium isotopes in mammal bones and teeth from two South African trophic chains. Geochim. Cosmochim. Acta 130, 12–20 (2014). [Google Scholar]
- 11.Costas-Rodríguez M., Van Heghe L., Vanhaecke F., Evidence for a possible dietary effect on the isotopic composition of Zn in blood via isotopic analysis of food products by multi-collector ICP-mass spectrometry. Metallomics 6, 139–146 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Martin J. E., Tacail T., Cerling T. E., Balter V., Calcium isotopes in enamel of modern and Plio-Pleistocene East African mammals. Earth Planet. Sci. Lett. 503, 227–235 (2018). [Google Scholar]
- 13.Martin J. E., Vance D., Balter V., Magnesium stable isotope ecology using mammal tooth enamel. Proc. Natl. Acad. Sci. U.S.A. 112, 430–435 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaouen K., et al. , Tracing intensive fish and meat consumption using Zn isotope ratios: Evidence from a historical Breton population (Rennes, France). Sci. Rep. 8, 5077 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balter V., et al. , Calcium stable isotopes place Devonian conodonts as first level consumers. Geochem. Perspect. Lett. 10, 36–39 (2019). [Google Scholar]
- 16.Jaouen K., Pons M.-L., Balter V., Iron, copper and zinc isotopic fractionation up mammal trophic chains. Earth Planet. Sci. Lett. 374, 164–172 (2013). [Google Scholar]
- 17.Jaouen K., Beasley M., Schoeninger M., Hublin J.-J., Richards M. P., Zinc isotope ratios of bones and teeth as new dietary indicators: Results from a modern food web (Koobi Fora, Kenya). Sci. Rep. 6, 26281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaouen K., Szpak P., Richards M. P., Zinc isotope ratios as indicators of diet and trophic level in Arctic marine mammals. PLoS One 11, e0152299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean C., Le Cabec A., Spiers K., Zhang Y., Garrevoet J., Incremental distribution of strontium and zinc in great ape and fossil hominin cementum using synchrotron X-ray fluorescence mapping. J. R. Soc. Interface 15, 20170626 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heghe L. V., Engström E., Rodushkin I., Cloquet C., Vanhaecke F., Isotopic analysis of the metabolically relevant transition metals Cu, Fe and Zn in human blood from vegetarians and omnivores using multi-collector ICP-mass spectrometry. J. Anal. At. Spectrom. 27, 1327–1334 (2012). [Google Scholar]
- 21.Cloquet C., Carignan J., Lehmann M. F., Vanhaecke F., Variation in the isotopic composition of zinc in the natural environment and the use of zinc isotopes in biogeosciences: A review. Anal. Bioanal. Chem. 390, 451–463 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Moynier F., Vance D., Fujii T., Savage P., The isotope geochemistry of zinc and copper. Rev. Mineral. Geochem. 82, 543–600 (2017). [Google Scholar]
- 23.Maréchal C. N., Nicolas E., Douchet C., Albarède F., Abundance of zinc isotopes as a marine biogeochemical tracer. Geochem. Geophys. Geosyst. 1, 1015 (2000). [Google Scholar]
- 24.Luck J.-M., Ben Othman D., Albarède F., Telouk P., “Pb, Zn and Cu isotopic variations and trace elements in rain” in Geochemistry of the Earth’s Surface, Armansson H., Ed. (CRC Press, 1999), pp. 199–203. [Google Scholar]
- 25.Pichat S., Douchet C., Albarède F., Zinc isotope variations in deep-sea carbonates from the eastern equatorial Pacific over the last 175 ka. Earth Planet. Sci. Lett. 210, 167–178 (2003). [Google Scholar]
- 26.Weiss D. J., et al. , Isotopic discrimination of zinc in higher plants. New Phytol. 165, 703–710 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Viers J., et al. , Evidence of Zn isotopic fractionation in a soil–plant system of a pristine tropical watershed (Nsimi, Cameroon). Chem. Geol. 239, 124–137 (2007). [Google Scholar]
- 28.Moynier F., et al. , Isotopic fractionation and transport mechanisms of Zn in plants. Chem. Geol. 267, 125–130 (2009). [Google Scholar]
- 29.Aucour A. M., Pichat S., Macnair M. R., Oger P., Fractionation of stable zinc isotopes in the zinc hyperaccumulator Arabidopsis halleri and nonaccumulator Arabidopsis petraea. Environ. Sci. Technol. 45, 9212–9217 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Balter V., et al. , Bodily variability of zinc natural isotope abundances in sheep. Rapid Commun. Mass Spectrom. 24, 605–612 (2010). [DOI] [PubMed] [Google Scholar]
- 31.Lee-Thorp J. A., van der Merwe N. J., Aspects of the chemistry of modern and fossil biological apatites. J. Archaeol. Sci. 18, 343–354 (1991). [Google Scholar]
- 32.Budd P., Montgomery J., Barreiro B., Thomas R. G., Differential diagenesis of strontium in archaeological human dental tissues. Appl. Geochem. 15, 687–694 (2000). [Google Scholar]
- 33.Dauphin Y., Williams C. T., Diagenetic trends of dental tissues. C. R. Palevol 3, 583–590 (2004). [Google Scholar]
- 34.Michel V., Ildefonse Ph., Morin G., Assessment of archaeological bone and dentine preservation from Lazaret Cave (Middle Pleistocene) in France. Palaeogeogr. Palaeoclimatol. Palaeoecol. 126, 109–119 (1996). [Google Scholar]
- 35.Kohn M. J., Schoeninger M. J., Barker W. W., Altered states: Effects of diagenesis on fossil tooth chemistry. Geochim. Cosmochim. Acta 63, 2737–2747 (1999). [Google Scholar]
- 36.Trueman C. N., Tuross N., Trace elements in recent and fossil bone apatite. Rev. Mineral. Geochem. 48, 489–521 (2002). [Google Scholar]
- 37.Sponheimer M., Lee-Thorp J. A., Oxygen isotopes in enamel carbonate and their ecological significance. J. Archaeol. Sci. 26, 723–728 (1999). [Google Scholar]
- 38.Schoeninger M. J., Hallin K., Reeser H., Valley J. W., Fournelle J., Isotopic alteration of mammalian tooth enamel. Int. J. Osteoarchaeol. 13, 11–19 (2003). [Google Scholar]
- 39.Grandjean P., Albarède F., Ion probe measurement of rare earth elements in biogenic phosphates. Geochim. Cosmochim. Acta 53, 3179–3183 (1989). [Google Scholar]
- 40.Sponheimer M., Lee-Thorp J. A., Enamel diagenesis at South African Australopith sites: Implications for paleoecological reconstruction with trace elements. Geochim. Cosmochim. Acta 70, 1644–1654 (2006). [Google Scholar]
- 41.Bocherens H., Brinkman D. B., Dauphin Y., Mariotti A., Microstructural and geochemical investigations on Late Cretaceous archosaur teeth from Alberta, Canada. Can. J. Earth Sci. 31, 783–792 (1994). [Google Scholar]
- 42.Hinz E. A., Kohn M. J., The effect of tissue structure and soil chemistry on trace element uptake in fossils. Geochim. Cosmochim. Acta 74, 3213–3231 (2010). [Google Scholar]
- 43.Reynard B., Balter V., Trace elements and their isotopes in bones and teeth: Diet, environments, diagenesis, and dating of archeological and paleontological samples. Palaeogeogr. Palaeoclimatol. Palaeoecol. 416, 4–16 (2014). [Google Scholar]
- 44.Pestle W. J., Colvard M., Bone collagen preservation in the tropics: A case study from ancient Puerto Rico. J. Archaeol. Sci. 39, 2079–2090 (2012). [Google Scholar]
- 45.Duringer P., Bacon A.-M., Sayavongkhamdy T., Nguyen T. K. T., Karst development, breccias history, and mammalian assemblages in Southeast Asia: A brief review. C. R. Palevol 11, 133–157 (2012). [Google Scholar]
- 46.Bacon A.-M., et al. , Late Pleistocene mammalian assemblages of Southeast Asia: New dating, mortality profiles and evolution of the predator–prey relationships in an environmental context. Palaeogeogr. Palaeoclimatol. Palaeoecol. 422, 101–127 (2015). [Google Scholar]
- 47.Bacon A.-M., et al. , Testing the savannah corridor hypothesis during MIS2: The Boh Dambang hyena site in southern Cambodia. Quat. Int. 464, 417–439 (2018). [Google Scholar]
- 48.Bentley R. A., Strontium isotopes from the earth to the archaeological skeleton: A review. J. Archaeol. Method Theory 13, 135–187 (2006). [Google Scholar]
- 49.Price T. D., et al. , Isotopic studies of human skeletal remains from a sixteenth to seventeenth century AD churchyard in Campeche, Mexico: Diet, place of origin, and age. Curr. Anthropol. 53, 396–433 (2012). [Google Scholar]
- 50.Wright L. E., Immigration to Tikal, Guatemala: Evidence from stable strontium and oxygen isotopes. J. Anthropol. Archaeol. 31, 334–352 (2012). [Google Scholar]
- 51.Flockhart D. T. T., Kyser T. K., Chipley D., Miller N. G., Norris D. R., Experimental evidence shows no fractionation of strontium isotopes (87Sr/86Sr) among soil, plants, and herbivores: Implications for tracking wildlife and forensic science. Isotopes Environ. Health Stud. 51, 372–381 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Graustein W. C., “87Sr/86Sr ratios measure the sources and flow of strontium in terrestrial ecosystems” in Stable Isotopes in Ecological Research, Ecological Studies, Rundel P. W., Ehleringer J. R., Nagy K. A., Eds. (Springer New York, 1989), pp. 491–512. [Google Scholar]
- 53.Lewis J., Pike A. W. G., Coath C. D., Evershed R. P., Strontium concentration, radiogenic (87Sr/86Sr) and stable (δ88Sr) strontium isotope systematics in a controlled feeding study. Sci. Technol. Archaeol. Res. 3, 53–65 (2017). [Google Scholar]
- 54.DeNiro M. J., Epstein S., Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42, 495–506 (1978). [Google Scholar]
- 55.Lee-Thorp J. A., Sealy J. C., van der Merwe N. J., Stable carbon isotope ratio differences between bone collagen and bone apatite, and their relationship to diet. J. Archaeol. Sci. 16, 585–599 (1989). [Google Scholar]
- 56.Smith B. N., Epstein S., Two categories of 13C/12C ratios for higher plants. Plant Physiol. 47, 380–384 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Longinelli A., Oxygen isotopes in mammal bone phosphate: A new tool for paleohydrological and paleoclimatological research? Geochim. Cosmochim. Acta 48, 385–390 (1984). [Google Scholar]
- 58.Luz B., Kolodny Y., Horowitz M., Fractionation of oxygen isotopes between mammalian bone-phosphate and environmental drinking water. Geochim. Cosmochim. Acta 48, 1689–1693 (1984). [Google Scholar]
- 59.Pederzani S., Britton K., Oxygen isotopes in bioarchaeology: Principles and applications, challenges and opportunities. Earth Sci. Rev. 188, 77–107 (2019). [Google Scholar]
- 60.Baayen R. H., Analyzing Linguistic Data. A Practical Introduction to Statistics (Cambridge University Press, 2008). [Google Scholar]
- 61.McCullagh P., Nelder J. A., Generalized Linear Models (CRC Press, ed. 2, 1989). [Google Scholar]
- 62.Lee K. M., Appleton J., Cooke M., Keenan F., Sawicka-Kapusta K., Use of laser ablation inductively coupled plasma mass spectrometry to provide element versus time profiles in teeth. Anal. Chim. Acta 395, 179–185 (1999). [Google Scholar]
- 63.Tacail T., Kovačiková L., Brůžek J., Balter V., Spatial distribution of trace element Ca-normalized ratios in primary and permanent human tooth enamel. Sci. Total Environ. 603–604, 308–318 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Kohn M. J., Morris J., Olin P., Trace element concentrations in teeth–A modern Idaho baseline with implications for archeometry, forensics, and palaeontology. J. Archaeol. Sci. 40, 1689–1699 (2013). [Google Scholar]
- 65.Nakagawa S., Johnson P. C. D., Schielzeth H., The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 14, 20170213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Cerling T. E., A model of fossil tooth and bone diagenesis: Implications for paleodiet reconstruction from stable isotopes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 107, 281–289 (1994). [Google Scholar]
- 67.Hedges R. E. M., Bone diagenesis: An overview of processes. Archaeometry 44, 319–328 (2002). [Google Scholar]
- 68.Kurt F., “Muntjac deer” in Grzimek’s Encyclopedia of Mammals, Parker S. P., Ed. (McGraw-Hill Publishing Company, 1990), pp. 137–139. [Google Scholar]
- 69.Jackson A., Muntiacus muntjak (Indian muntjac). Animal Diversity Web. https://animaldiversity.org/accounts/Muntiacus_muntjak/. Accessed 13 December 2018.
- 70.Bataille C. P., et al. , A bioavailable strontium isoscape for Western Europe: A machine learning approach. PLoS One 13, e0197386 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tejada-Lara J. V., et al. , Body mass predicts isotope enrichment in herbivorous mammals. Proc. Biol. Sci. 285, 20181020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.R Core Team , R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria, 2018).
- 73.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 74.Maréchal C. N., Télouk P., Albarède F., Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. Chem. Geol. 156, 251–273 (1999). [Google Scholar]
- 75.Jackson A. L., Inger R., Parnell A. C., Bearhop S., Comparing isotopic niche widths among and within communities: SIBER-Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.