Significance
In some monogamous species, the oxytocin system facilitates mating preference for a familiar individual, irrespective of sex. In contrast, in nonmonogamous species, the mating strategy mostly differs between males and females; males tend to compete with rival males and court indiscriminately, whereas females choose their mates selectively. Here, we studied the role of oxytocin in mate choice in medaka fish, a nonmonogamous species. Analysis of oxytocin system mutants in medaka revealed that the oxytocin system regulates mate preference in a sex-specific manner, indicating that the oxytocin system is required for mate choice in both sexes, regardless of the differences in the mating strategy between monogamous and nonmonogamous species.
Keywords: sexual preference, mate guarding, social recognition, genome editing
Abstract
Oxytocin is a central neuromodulator required for facilitating mate preferences for familiar individuals in a monogamous rodent (prairie vole), irrespective of sex. While the role of oxytocin in mate choice is only understood in a few monogamous species, its function in nonmonogamous species, comprising the vast majority of vertebrate species, remains unclear. To address this issue, we evaluated the involvement of an oxytocin homolog (isotocin, referred herein as oxt) in mate choice in medaka fish (Oryzias latipes). Female medaka prefer to choose familiar mates, whereas male medaka court indiscriminately, irrespective of familiarity. We generated mutants of the oxt ligand (oxt) and receptor genes (oxtr1 and oxtr2) and revealed that the oxt-oxtr1 signaling pathway was essential for eliciting female mate preference for familiar males. This pathway was also required for unrestricted and indiscriminate mating strategy in males. That is, either oxt or oxtr1 mutation in males decreased the number of courtship displays toward novel females, but not toward familiar females. Further, males with these mutations exhibited enhanced mate-guarding behaviors toward familiar females, but not toward novel females. In addition, RNA-sequencing (seq) analysis revealed that the transcription of genes involved in gamma-amino butyric acid metabolism as well as those encoding ion-transport ATPase are up-regulated in both oxt and oxtr1 mutants only in female medaka, potentially explaining the sex difference of the mutant phenotype. Our findings provide genetic evidence that oxt-oxtr1 signaling plays a role in the mate choice for familiar individuals in a sex-specific manner in medaka fish.
Oxytocin is a neuropeptide considered to contribute to the biological basis of prosociality for familiar individuals. For example, the oxytocin system facilitates maternal behaviors and mother-kin bonding (1, 2). In some monogamous rodents (e.g., prairie voles), oxytocin has an essential role in both sexes to enhance the preference for familiar individuals (3–5). Little attention, however, has been paid to the function of oxytocin in mate choice in nonmonogamous species. Mate choice based on familiarity recognition is divergent and variable across species and, in most nonmonogamous species, females and males exhibit different mating strategies (3, 6–9). Thus, studies on the sexual difference in nonmonogamous species offering a comparative perspective will broaden our understanding of the role of oxytocin in mate choice. Medaka is a fascinating model for studying the molecular underpinnings of sexual dimorphism in mate choice because the male and female mating strategies are distinct. Under laboratory conditions, we previously demonstrated that female medaka prefer to mate with visually familiar males (10, 11); familiarization could facilitate the selection of dominant males that prominently exhibit mate-guarding behavior in triadic relationships (two males and one female) (12, 13). We also found that male medaka did not exhibit any preference based on familiarity. Here, we performed a functional dissection of the oxytocin system using a series of medaka mutants and demonstrated a sexually dimorphic role of the oxytocin signaling pathway (oxt-oxtr1) in mate choice.
Results
Generation of Medaka with Oxytocin and Oxytocin Receptor Gene Mutations.
Among teleost fish, the homolog of the nonapeptide oxytocin is isotocin, which has two amino acid substitutions at positions 4 (Gln to Ser) and 8 (Leu to Ile) in the mammalian OXT (SI Appendix, Fig. S1B). A series of medaka-carrying mutations of genes encoding oxt and its receptors were generated using TILLING (Targeting Induced Local Lesions In Genomes) (14, 15), TALEN (transcription activator-like effector nucleases) (16), and CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeat), which is the most widely used technology for genome editing using RNA-guided endonuclease (Cas) (17) (SI Appendix, Table S1). Using TILLING, we first generated oxt mutant fish (oxtI22F/I22F), in which a conserved isoleucine residue was changed to phenylalanine (SI Appendix, Fig. S1). A previous study (18) indicated that this single amino acid substitution decreases the affinity for the receptor and diminishes the biological efficacy of oxytocin. We also generated another oxt mutant fish (oxt−/−) using CRISPR/Cas9. A 4-bp deletion in the first exon in the mutant resulted in a frameshift upstream of oxt (SI Appendix, Fig. S2).
In addition, we created medaka mutants of genes for oxt receptors. Like in cichlid (19), we found two paralogous genes encoding oxt receptors (oxtr1 [Ensembl gene ID: ENSORLG00000000719] and oxtr2 [Ensembl gene ID: ENSORLG00000003297], respectively) in the medaka genome. As neither oxtr1 nor oxt2 expression has been reported in medaka fish, we confirmed the expression of these genes in the brain using RT-PCR (SI Appendix, Fig. S3A). Both oxtr1 and oxtr2 were expressed in the adult brain in both sexes. To evaluate the role of the oxtr, we generated oxtr1 and oxtr2 mutants: The oxtr1 mutants (oxtr1−/−, generated by TALEN) and oxtr2 mutants (oxtr2−/−, generated by CRISPR/Cas9) have 7-bp and 8-bp deletions in the first exon, respectively (SI Appendix, Fig. S3 B–D), and both have mutated transcripts encoding C-terminal–deleted proteins that lack at least six of the seven transmembrane domains. Considering that oxtr1 and oxtr2 are seven-transmembrane receptors and the topology of the deletions along the domain structures (SI Appendix, Fig. S3B), these deletions should lead to a loss of function.
oxt and oxtr1 Mutant Females Exhibited a Loss of Mate Preference for Visually Familiarized Males.
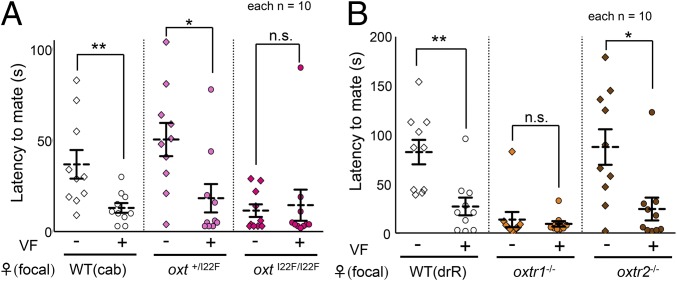
Wild-type (WT) medaka females can recognize and select visually familiarized males as their mating partners (10, 11). We quantified the effect of visual familiarization (VF; allowing a pair of fish can see each other through a transparent wall for a short-time: 1 d) on the degree of female receptivity in a series of mutants. This short period of VF decreased the latency to mate on the basis of the shorter time interval between the first male courtship behavior and the first mating in WT females (Mann–Whitney U test: Z = −2.608, P = 0.0091; Fig. 1A). The shorter latency correlated with higher female receptivity. The oxtI22F/I22F and oxtr1−/− females, however, had a short latency to mate even without VF and exhibited high receptivity for any male (Mann–Whitney U test: Z = −0.340, P = 0.733; Z = 0.340, P = 0.733, respectively, Fig. 1 A and B), suggesting a loss of mate preference. However, oxt+/I22F and oxtr2−/− females, like WT fish, exhibited a mate preference for familiar males (Mann–Whitney U test: Z = −2.494, P = 0.012; Z = −2.532, P = 0.011, respectively, Fig. 1 A and B). We confirmed that the other oxt mutant female (deletion mutant: oxt−/−) exhibited the same behavioral phenotype as the oxt I22F/I22F mutant (Mann–Whitney U test: Z = −0.265, P = 0.791; SI Appendix, Fig. S4A), indicating that this phenotype was due to loss of function. Moreover, we confirmed that none of these mutations affected visual responses or locomotion in the males (Kruskal–Wallis X2 [3, n = 16] = 5.801, P = 0.12; SI Appendix, Fig. S5). Taken together, these findings indicated that oxt and oxtr1 are required for mate preference in medaka females.
Fig. 1.
Mating preference of oxt and oxtrs mutant females. While visual familiarization (VF) increased the receptivity of WT, oxt+/I22F (pink), and oxtr2−/− (brown) females toward familiarized males, oxtI22F/I22F females (dark pink) (A) and oxtr1−/− females (orange) (B) showed high receptivity even toward unfamiliar males. Mean ± SEM, n = 10 per group, Mann–Whitney U test: *P < 0.05; **P < 0.01; n.s., not significant.  , female;
, female;  , male.
, male.
oxt and oxtr1 Males Preferred to Mate with Socially Familiarized Females.
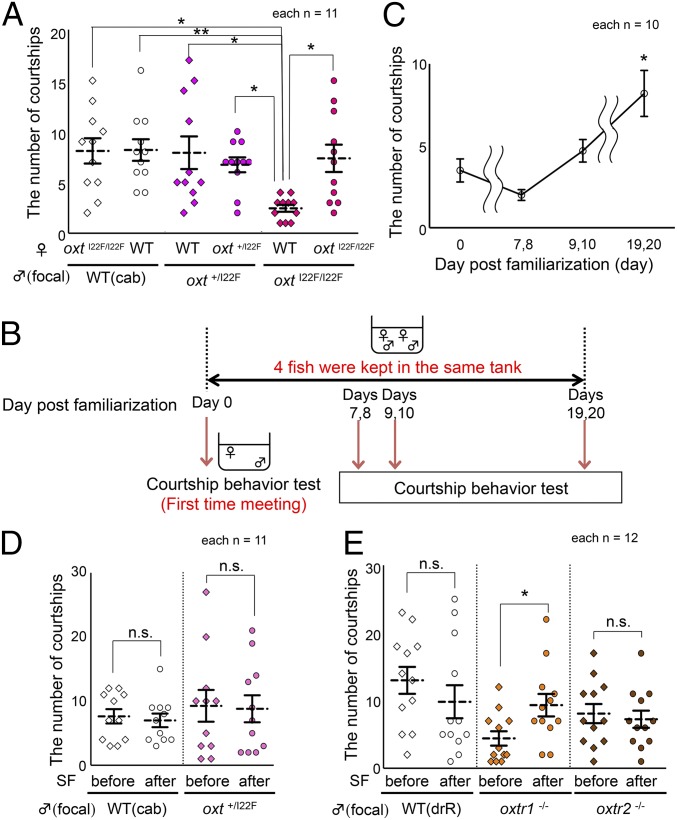
We previously reported that short-time VF did not influence male mating preference (10). Consistent with this observation, even long-time social familiarization (SF; group rearing in a single tank) had no effect on mate preference in WT males (Fig. 2A). Thus, WT males have no mate preference based on familiarity. To examine the effect of the oxt mutation on male courtship activity toward socially unfamiliar or familiar females, we performed a courtship behavior assay using females whose genotype was the same as that of the males (SF group) and females whose genotype differed from that of the males (non-SF group). In the experiments, individuals with the same genotype were group reared from birth, i.e., socially familiarized for a long period of time. Whereas in WT and oxt+/I22F males, neither SF nor the female genotype significantly affected the number of courtship behaviors (Steel–Dwass’s P > 0.999; Fig. 2A), the number of courtship behaviors of homozygous oxtI22F/I22F males in the non-SF group was significantly lower than that of WT males in the non-SF group (Kruskal–Wallis X2 [5, n = 66] = 19.602, P = 0.0014, WT male, oxtI22F/I22F female, non-SF vs. oxtI22F/I22F male, WT female, non-SF; Steel–Dwass’s P = 0.013; Fig. 2A).
Fig. 2.
Courtship behavior of oxt and oxtrs mutant males toward unfamiliar or familiar females. (A) Males and females with the same genotype were group reared from birth, i.e., socially familiarized for a long period of time. Neither female genotype nor SF influenced the number of courtship behaviors in WT and oxt+/I22F males (pink). In contrast, oxtI22F/I22F males (dark pink) exhibited courtship behaviors toward WT females less frequently than toward oxtI22F/I22F females. Mean ± SEM, n = 11 per group, Kruskal–Wallis: Steel–Dwass’s post hoc **P < 0.01, *P < 0.05. (B) Procedure for assessing the effect of social familiarization on courtship behavior in oxtI22F/I22F males. oxtI22F/I22F males and WT females were introduced for the first time during the courtship behavior test (day 0) and then two oxtI22F/I22F males and two WT females were kept in the same tank (familiarization) for 20 d. (C) The number of courtship behaviors of oxtI22F/I22F males toward unfamiliar WT females significantly increased following 19–20 d of SF. Mean ± SEM, n = 10 per group, Kruskal–Wallis: Steel’s post hoc: *P < 0.05 vs. “0 day post familiarization”. (D) WT and oxt+/I22F (pink) males exhibited courtship behavior toward familiarized WT females to the same extent as toward unfamiliar WT females. Mean ± SEM, n = 11 per group, Mann–Whitney U test. (E) WT and oxtr2−/− (brown) males exhibited courtship behavior toward familiarized WT females to the same extent as toward unfamiliar WT females. However, the number of courtship behaviors of oxtr1−/− males (orange) toward unfamiliar WT females was significantly lower than that toward familiarized females. Mean ± SEM, n = 12 per group, Mann–Whitney U test: *P < 0.05; n.s., not significant.  , female;
, female;  , male.
, male.
There are two possible interpretations of these results. The WT females could react oxtI22F/I22F males differently, which might decrease male courtship behaviors. Alternatively, the oxtI22F/I22F males could recognize socially familiarized females and had a mate preference for socially familiarized females, as the courtship behaviors of oxtI22F/I22F males in the SF group were not significantly different from that of WT (WT male, WT female, SF vs. oxtI22F/I22Fmale, oxtI22F/I22F female, SF; Steel–Dwass’s P = 0.989, WT male, oxtI22F/I22F female, non-SF vs. oxtI22F/I22F male, oxtI22F/I22F female, SF; Steel–Dwass’s P = 0.969; Fig. 2A). To evaluate these possibilities, we examined whether SF could enhance the courtship activities in oxtI22F/I22F males toward unfamiliar WT females that had been reared in other tanks. We group reared oxtI22F/I22F males and WT females in the same tank for a certain period of time (1–20 d) and examined the effect of SF on the number of courtship behaviors (Fig. 2B). Without SF, oxtI22F/I22F males exhibited courtship behaviors approximately three times within 5 min. In contrast, after SF for 19–20 d, the number of courtship behaviors significantly increased (Kruskal–Wallis X2 [3, n = 40] = 14.624, P = 0.0021, 0 d after familiarization vs. 19–20 d after familiarization; Steel’s P = 0.048; Fig. 2C). The other oxt mutant males (oxt−/−) also performed significantly more courtship behaviors toward WT females after SF for 19–20 d (Mann–Whitney U test: Z = −2.425, P = 0.015; SI Appendix, Fig. S4B). We then investigated behavioral changes in WT and oxt+/I22F males following SF for 19–20 d and revealed no effect in WT (Cab) or oxt+/I22F males (Mann–Whitney U test: Z = −0.427, P = 0.670; Z = 0, P > 0.999, respectively; Fig. 2D), consistent with previous observations that WT males exhibit courtship behaviors irrespective of SF. In addition, SF for 19–20 d facilitated the courtship activities of oxtI22F/I22F males to a similar level as WT (∼8 times within 5 min; Fig. 2 C and D), implying that 19–20 d was sufficient for SF. Next, we examined the possible involvement of oxtrs in the emergence of mate preference. SF influenced mating preference for females in oxtr1−/− males like oxt mutant males, whereas it did not influence mating preference in oxtr2−/− males (Mann–Whitney U test: Z = −2.281, P = 0.023; Z = −0.491, P = 0.624, respectively; Fig. 2E).
In addition, we compared social motivation of nonfocal females toward WT and mutant males. We performed simple behavioral tests to evaluate approaching behavior of a single fish toward the target fish (ref. 20 and SI Appendix, Fig. S6A). Indeed, WT females exhibited approaching behavior toward males, and this trend was not changed by the genotype of the male (Mann–Whitney U test: WT Z = −3.926, P < 0.0001; oxtI22F/I22F Z = −3.580, P = 0.0003, oxt−/− Z = −3.291, P = 0.0009; oxtr1−/− Z = −2.338, P = 0.019, respectively; SI Appendix, Fig. S6B). This finding suggested that females did not react differently to mutant and WT males. Taken together, our data indicated that the activation of oxtr1, but not oxtr2, by oxt ligands blocks the emergence of mate preference toward socially familiarized females.
oxt and oxtr1 Mutant Males Exhibit a Loss of Mate-Guarding Behaviors toward Unfamiliar Individuals.
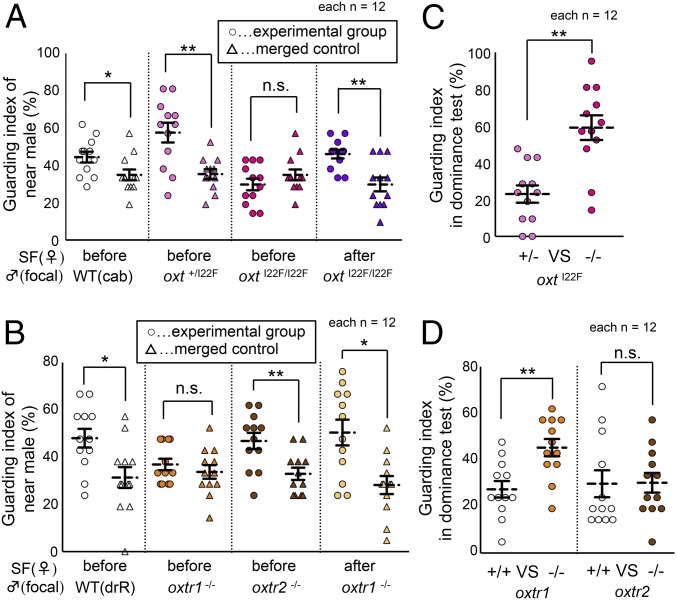
To further investigate whether oxt and oxtr1 mutant males lack sexual motivation toward unfamiliar females, we used another behavioral test to assess male mate-guarding behaviors. In triadic relationships (two males and one female), the two males compete and the male nearest the female dominantly exhibits mate-guarding behavior (occupying a dominant position near the female while interfering with the other male’s approach toward the female) (12, 13). Our previous study in medaka demonstrated that male sexual motivation toward a target female is essential to drive mate-guarding in triadic relationships (12, 13). Thus, we could indirectly test male sexual motivation toward a target female using this behavioral paradigm. We placed two males with the same allele and one unfamiliar female in the tank and quantified the guarding index of the dominant male. The guarding index correlates with the frequency of the male fish locating in the area between the rival male and female. If the guarding index is higher than that of the merged control groups (negative control, Materials and Methods), it is considered that mate-guarding emerged in the triadic relationship. Indeed, the guarding indices of the WT (oxt+/+), oxt+/I22F, and oxtr2−/− males were significantly higher than those of the merged control groups (Mann–Whitney U test: Z = 2.165, P = 0.030; Z = 2.800, P = 0.0051; Z = 2.656, P = 0.0074, respectively; Fig. 3 A and B), indicating that these males exhibited mate-guarding behavior toward unfamiliar females. However, oxtI22F/I22F and oxtr1−/− males did not exhibit mate-guarding behavior toward unfamiliar females (Mann–Whitney U test: Z = −0.953, P = 0.341; Z = 0.693, P = 0.489, respectively; Fig. 3 A and B). A 20-d period of SF facilitated the mate-guarding behavior in these mutants (Mann–Whitney U test: Z = 2.858, P = 0.0043; Z = 2.540, P = 0.011, respectively; Fig. 3 A and B). The other oxt mutant (oxt−/−) males exhibited the same behavioral phenotype (Mann–Whitney U test: before SF Z = 0.265, P = 0.791; after SF Z = 3.023, P = 0.0025, respectively; SI Appendix, Fig. S7A). Taken together, these findings suggested that oxt and oxtr1 mutant males might lack sexual motivation toward unfamiliar females, which could lead to decreased motivation for mate guarding.
Fig. 3.
Effect of social familiarization on mate-guarding behavior. (A) Although oxtI22F/I22F males did not exhibit mate-guarding behavior toward unfamiliar WT females (dark pink), social familiarization (SF) enhanced the mate-guarding behavior of these mutant males toward familiarized mates (purple). (B) oxtr2−/− males exhibited mate-guarding toward unfamiliar females (brown), whereas oxtr1−/− males did not (orange). oxtr1−/− males exhibited mate-guarding toward socially familiarized females (beige). (C) oxtI22F/I22F males (dark pink) tended to be dominant in the dominance test using familiar females. (D) oxtr1−/− males (orange) tended to be dominant in the dominance test using familiar females. (A–D) Mean ± SEM, n = 12 per group, Mann–Whitney U test: *P < 0.05; **P < 0.01; n.s., not significant.  , female;
, female;  , male.
, male.
oxt and oxtr1 Mutant Males Exhibit Dominant Mate-Guarding Behaviors toward Socially Familiarized Females.
Next, we examined whether familiarity with target females could affect the dominance of mutant males in the mate-guarding behaviors. To compare the dominance of oxt homozygous mutant males with that of heterozygous mutants, we performed the dominance test (Materials and Methods). We allowed two males with different alleles to compete for one female and examined the dominance of individual males in mate-guarding behavior. When we allowed one oxt+/I22F male and one oxtI22F/I22F male to compete for an unfamiliar female, the guarding index of the oxt+/I22F males was significantly higher than that of the oxtI22F/I22F males (Wilcoxon signed ranks: Z = −2.863, P = 0.0042; SI Appendix, Fig. S7B), indicating that oxt+/I22F males were dominant toward unfamiliar females (Movie S1). This observation was consistent with previous findings that oxtI22F/I22F males did not exhibit mate-guarding behavior toward unfamiliar females (Fig. 3A). Surprisingly, when we used long-time socially familiarized females instead of unfamiliar females, the tendency toward dominance was completely reversed (Wilcoxon signed ranks: Z = −2.628, P = 0.0086; Fig. 3C). The oxtI22F/I22F males exhibited dominant mate-guarding behavior toward socially familiarized females (Movie S2). In addition, oxtI22F/I22F males exhibited dominance when competing with WT males for socially familiarized females (Wilcoxon signed ranks: Z = −2.981, P = 0.0029; SI Appendix, Fig. S7C). We confirmed that other oxt mutant (oxt−/−) males had the same behavioral phenotype (Wilcoxon signed ranks: before SF Z = −2.089, P = 0.037; SI Appendix, Fig. S7D, after SF Z = −2.701, P = 0.0069; SI Appendix, Fig. S7E). Therefore, oxt mutations enhanced mate-guarding behavior toward familiar females, but not toward unfamiliar females. We next performed the dominance test using oxtr mutants and showed that oxtr1−/− males, but not oxtr2−/− males, exhibited dominant mate-guarding behavior toward socially familiarized females, similar to oxt mutants (Wilcoxon signed ranks: oxtr1 Z = −2.980, P = 0.0029, oxtr2 Z = −0.178, P = 0.859; Fig. 3D, oxtr1 Z = −2.275, P = 0.023, oxtr2 Z = −0.275, P = 0.784; SI Appendix, Fig. S7F). These findings indicated that oxt and oxtr1 signaling mediated the suppression of dominant mate-guarding behavior toward socially familiarized females.
Signaling Pathways Influenced by oxt System Defects.
The sex differences in mating behavior induced by the oxt system led us to investigate the molecular basis of these differences. In the medaka brain, oxt is expressed mainly in the preoptic area, and there are no significant sex differences (21), but whether the oxtr1 expression pattern in medaka differs between sexes was unknown. To identify oxtr1-expressing neurons, we performed in situ hybridization and showed that oxr1 was expressed in the telencephalon, preoptic area, habenula, optic tectum, hypothalamus, and corpus glomerulosum. We found no significant sex differences in the distribution pattern (SI Appendix, Fig. S8).
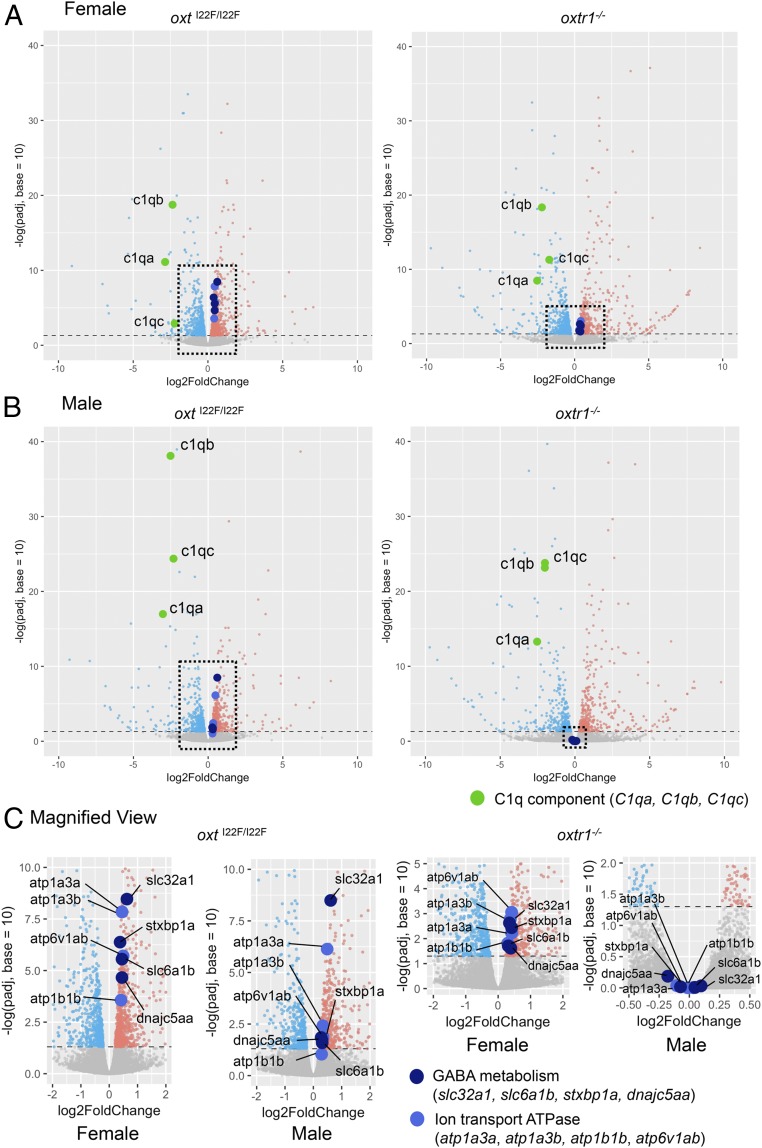
Next, to identify signaling pathways that could be affected by defects in the oxt-oxtr1 pathway and clarify the molecular basis underlying the sex differences, we performed RNA-sequencing (seq) (22). We used the whole adult brains, as oxtrs are widely distributed throughout the medaka brain, similar to what has been reported in the brains of other teleost fish and mammals (23). We compared gene expression profiles among six groups: 1) WT female, 2) WT male, 3) oxtI22F/I22F female, 4) oxtI22F/I22F male, 5) oxtr1−/− female, and 6) oxtr1−/− male. The oxt or oxtr1 mutations significantly changed the transcription levels of 1,389 and 1,181 genes, respectively, in females (SI Appendix, Fig. S9A and Dataset S1), and in 1,052 and 813 genes, respectively, in males (SI Appendix, Fig. S9B and Dataset S1). As oxt and oxtr1 mutants exhibited similar behavioral phenotypes, we focused on genes whose transcription levels significantly increased or decreased with both oxt and oxtr1 mutations. There were no genes regulated in an opposite manner between females and males (SI Appendix, Fig. S10 and Dataset S2). At first, we searched for genes associated with the oxt system in both sexes. Gene ontology (GO) analysis and pathway analysis revealed no enrichments of up-regulated genes (SI Appendix, Fig. S11A), whereas down-regulated gene clusters included the three genes for all of the components of C1q (Fig. 4 A and B and SI Appendix, Fig. S11B). Functional C1q is a heterotrimer complex consisting of the products of three genes: c1qa, c1qb, and c1qc. Next, as mutations in the oxt-oxtr1 system have different effects on mate preference between the sexes, we reasoned that the oxt system regulated signaling pathway in a sex-specific manner. GO analysis showed an enrichment of genes involved in cellular sodium ion homeostasis in a female-selective manner. The GO term (12 genes) included 4 genes encoding ion-transporting ATPase subunits (atp1a3a, atp1a3b, atp1b1b, and atp6v1ab), which were up-regulated (Fig. 4C and SI Appendix, Fig. S12A). Pathway analysis revealed an enrichment of a gene set involved in gamma-aminobutyric acid (GABA) metabolism, suggesting that the mutation prominently up-regulated the transcription of these genes (slc32a1, slc6a1b, stxbp1a, and dnajc5aa) in a female-selective manner (Fig. 4C and SI Appendix, Fig. S12A). There was no enrichment of female-specific down-regulated genes for any terms (SI Appendix, Fig. S12B).
Fig. 4.
Volcano plots of up-regulated/down-regulated genes in oxt and oxtr1 mutants. Log2foldChange of gene transcription levels in female mutants (A) and in male mutants (B) compared with those in WT fish. (C) Magnified views of the graph outlined by the dotted line in A and B. Each dot represents one gene. Gray dots indicate genes whose transcription levels were not significantly changed in mutants compared with those in WT (FDR > 0.05). Coral and light blue dots represent significantly up-regulated/down-regulated genes in mutants, respectively. Dark blue and blue dots indicate genes up-regulated in female mutants and involved in the GABA system and sodium ion homeostasis, respectively. Green dots represent genes down-regulated in both sexes of mutants and involved in the complement activation system. Horizontal dotted line represents a padj of 0.05.
We determined 20 male-specific up-regulated genes and 39 male-specific down-regulated genes (SI Appendix, Fig. S13 and Dataset S2). Pathway analysis revealed the enrichment of extracellular matrix-related genes up-regulated in a male-specific manner that are reported to regulate the outgrowth of neuronal dendrites (24). In contrast, these analyses showed no enrichment of male-specific down-regulated genes for any terms (SI Appendix, Fig. S13B).
Discussion
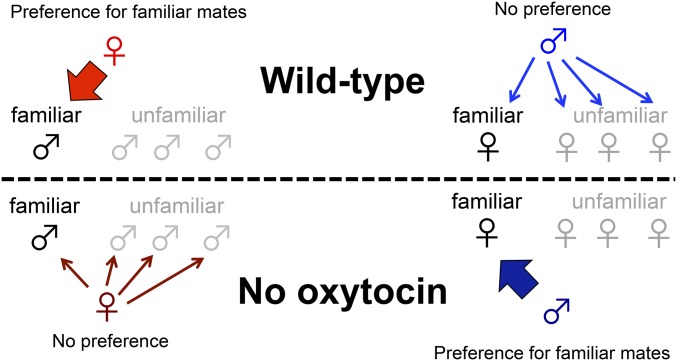
The findings of the present study demonstrated that the oxt system facilitates mate choice in a sexually dimorphic manner in a nonmonogamous species (medaka fish) in which males and females have different mating strategies. Here, we showed that the oxt-oxtr1 pathway is required in females for mate preference for familiar mates, and in males for an unrestricted and indiscriminate mating strategy (Fig. 5). Our findings also revealed that mutant males have the ability to discriminate between SF and non-SF females. Previously, we demonstrated that medaka females primarily use visual cues from “faces (head part)” to discriminate visually familiarized males from unfamiliar males (11, 25). However, SF (group rearing) provides exposure to both visual and olfactory cues. Reproductive hormones, such as 17α,20β-dihydroxy-4-pregnen-3-one and prostaglandin F2α, which act as pheromones released by females, modulate male behaviors in some fishes (26, 27). These pheromones also might be candidate substances that affect male mating preference in medaka fish. Future studies should investigate if, like females, mutant males use visual cues and/or chemical substances to recognize SF females.
Fig. 5.
Requirement of the oxt system for the mate preference for familiar mates. WT medaka females, but not males, have a sexual preference for familiar mates (red and blue arrows). This tendency was reversed, however, in oxt and oxtr1 mutant fish. Females lost mate preference (dark red arrows) and the mutant males gained mate preference for familiar mates (dark blue arrow).  , female;
, female;  , male.
, male.
The Different oxt Functions Between Sexes in Medaka Fish.
Among monogamous rodents, the oxt system facilitates sexual preference for a familiar mate, irrespective of sex (1–5). Generally, sexual dimorphism of the mating strategy in monogamous species is reduced, providing advantages for shared parenting, protection of resources, and social support (28). Our findings suggest that the oxt system influences mate choice in both sexes regardless of the mating strategy, and that sexual dimorphism in the mating strategy might relate to functional differences in the oxt system between sexes (Fig. 5). Here, transcriptome analysis showed that an oxt/oxtr1 mutation led to up-regulation of gene expression associated with GABA metabolism/ion-transport ATPase in a female-selective manner and down-regulation of genes encoding complement component C1q in both sexes. In human studies, abnormalities in glutamate/GABA signaling were recently hypothesized to underlie a neurodevelopmental syndrome, autism spectrum disorder (29). As atp1a3 are prominently expressed in GABAergic interneurons (30) in the mammalian brain, the enhanced expression of the ATPases might be associated with up-regulation of GABA metabolism. In addition, rodent and human studies suggest the involvement of complement component C1q in fundamental neurodevelopmental pathways (axon pruning) and the pathogenesis of autism spectrum disorders (31). Therefore, it might be that oxt/oxtr1 mutation in medaka fish impairs the neural mechanism underlying such a neurodevelopmental disorder. One possibility is that the oxt/oxtr1 mutation causes a developmental defect in TN-GnRH3 neurons, because TN-GnRH3 neurons are required for the suppression of female receptivity toward unfamiliar males. Two mutants (cxcr4 and cxcr7), which have an abnormal TN-GnRH3 neuron morphology, exhibit the same behavioral phenotype as oxt/oxtr1 mutant females (10). Further studies might clarify the involvement of the oxt system in the development of TN-GnRH3 neurons.
oxt Function in Mate Preference in the Ray-Finned Fish.
In the monogamous convict cichlid (Amatitlania nigrofasciata), administration of an arginine vasotocin (homolog of vasopressin)/oxt pathway antagonist into the brain impaired both affiliative behavior toward a potential mate and aggression toward neighbors (32). The oxt-selective function, however, has not been elucidated by pharmacological methods because of the cross-talk between the oxt and vasopressin systems (33). Thus, evaluation of the possible involvement of the oxt system on neurodevelopment cannot be performed by pharmacological studies (antagonist and agonist administration into the adult brain). Furthermore, most teleost fish have two paralogue genes for oxtrs, and it would be impossible to functionally dissect oxtrs without genetic techniques. The present genetic dissection using medaka fish showed their respective functions. In the African cichlid fish, the presence of rival males increases oxtr1 (istr2) expression in the preoptic area of the male brain (34), suggesting the possible involvement of oxtr1 (istr2) in social habituation. In contrast, among African cichlids, the expression pattern of two paralogue genes in the brain exhibits considerable species specificity, irrespective of sociality (19). Therefore, we cannot exclude the possibility that the function of oxtrs in medaka fish is species specific.
Our findings provide genetic evidence that oxt function is required for mate choice based on familiarity recognition in ray-finned fish. In medaka fish, oxt/oxtr1 mutant females lost their mate preference for familiar males, which may be due to the loss of an aversion for unfamiliar males rather than an impaired affiliation toward familiar males. The interpretation that OXT mediates its effect via the modulation of a negative response (rejection) rather than a positive response differs from the typical interpretation that OXT systems promote social affiliation in mammals. Therefore, our findings could suggest a divergence in oxt system function across species. Various ray-finned fish such as the African cichlid (Neolamprologus pulcher) (35), the Trinidadian guppy (Poecilia reticulata) (1), bluegill sunfish (Lepomis macrochirus) (36), and threespine stickleback (Gasterosteus aculeatus) (37) are able to recognize familiar mates, an ability that may be important for selecting sexual and social partners. Further studies of oxt function in other ray-finned fish could shed light on the evolutionary origin of oxt central brain function in mate choice, dating back 400 million years with the emergence of Actinopterygii on earth.
Materials and Methods
Ethics Statement.
The experiments described herein were conducted using protocols approved by the Animal Care and Use Committee of the Hokkaido University (permit no. 17-0130). Surgeries were performed under anesthesia using MS-222, and all efforts were made to minimize suffering following the NIH Guide for the Care and Use of Laboratory Animals (38).
Fish and Breeding Conditions.
Medaka fish (Oryzias latipes; drR strain, cab strain and mutants) were maintained in groups in plastic aquariums (13 cm × 19 cm × 12 cm [height]). All fish were hatched and bred in our laboratory. WT fish and each mutant fish type were bred in different tanks (only oxt heterozygous and oxt homozygous mutants were bred in the same tanks because they were born to the same parents). Sexually mature (4-5 mo of age) male (2.8〜3.5 cm) and female (3.0〜3.5 cm) medaka producing fertilized eggs every morning were used. The water temperature was ∼28 °C, and light was provided by standard fluorescent lamps for 14 h per day (0800–2200).
Female Mating Receptivity Test.
To quantify the motivation of a female to mate with a male of interest, a female mating receptivity assay was performed as previously described (10). Please see SI Appendix, Methods.
Courtship Behavior Assay.
This procedure was performed as previously described (13). Males and females were separated in the evening (1800–1900) the day before the assay. The mating pair was then placed together in a single tank the next morning, and mating behavior was recorded for 5 min. We counted the number of courtship displays.
Social Familiarization.
Four fish (two focal males and two WT females) were reared in a single tank for SF. The focal males and WT females were unfamiliar to each other before this procedure. For this behavioral test, we used the same fish before and after the SF procedure (20 d) as “before SF” fish and “after SF” fish, respectively.
Mate-Guarding Test.
To determine whether males exhibited mate guarding, a mate-guarding test was performed as previously described (13). One female and two males (all male pairs were size-matched) were placed in the tank, and their behavior was recorded from the bottom of the tank in the morning (1000–1200). As a negative control group (merged group), we performed the same experiment using virtually merged trios, recording one female and two males one by one, each placed in a separate aquarium. For details, please see SI Appendix, Methods.
Dominance Test.
We used one genotype A male and one genotype B male and allowed them to compete for the female to evaluate their dominance in mate-guarding behavior as previously described (13). All male pairs were size matched. The relative locations of the three fish were measured and guarding indices of genotype A and genotype B were calculated. Their guarding indices were compared with a higher guarding index indicating higher dominance in the mate-guarding behavior compared with the other male.
RNA-Seq and Data Analyses.
Total RNA was extracted from the whole brains of two adult females or males for each sample (n = 3 per group). RNA-seq libraries were sequenced on HiSeq 4000 (Illumina). Significantly changed transcripts were defined as false discovery rate (FDR) < 0.05. For GO and pathway analysis, the transcript ID was converted into zebrafish homologs and then run on GO-MWU and PANTHER, respectively. See SI Appendix, Methods for details.
Statistical Analysis.
In the female mating preference test, the male courtship test and the male mate-guarding test, each behavioral index was compared using Mann–Whitney U test implemented in Statcel (OMS Ltd.). In the dominance test, we compared the guarding index of two males using the Wilcoxon signed ranks test implemented in Statcel (OMS Ltd.). To compare the behavioral index of multiple groups, we used Kruskal–Wallis and Steel–Dwass’s post hoc test or Steel’s post hoc test implemented in the R system. All statistical tests are two-tailed, and P values <0.05 were considered significant. See SI Appendix, Methods for details.
Data Availability.
RNA-seq data used in this study have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE139158).
Supplementary Material
Acknowledgments
We thank Profs. T. Kubo, T. Kikusui, Y. Taniguchi, T. Miyatake, and M. Kawata for constructive discussion; the National BioResource Project Medaka for supplying the medaka strains (https://shigen.nig.ac.jp/medaka); Drs. T. Sakuma and T. Yamamoto for providing the pFUS_A2A and pFUS_A2B vectors; I. Hara for technical assistance in TILLING methods; T. Yamazaki for breeding the mutant fish; and M. Takei and M. Endo for developing the optomotor response (OMR) apparatus. This work was supported by the National Institute for Basic Biology Priority Collaborative Research Project 10-104 and Cooperative Research Project 19-347; Joint Research Grant 01111904 by the National Institutes of Natural Sciences; Japan Society for the Promotion of Science (JSPS) KAKENHI Grants 16K18369 (to S.Y.), 19K16247 (to S.Y.), 16H06524 (to S.Y.), 18H02479 (to H.T.), 16H01276 (to H.T.); Grant-in-Aid for JSPS fellows (S.Y.); and Sumitomo Foundation (S.Y.). The contribution by L.J.Y. was supported by NIH Grant P50MH100023 (to L.J.Y.) and Yerkes National Primate Research Center (YNPRC) Base Grant P51OD11132 to YNPRC.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: RNA-seq data used in this study have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE139158).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921446117/-/DCSupplemental.
References
- 1.Numan M., Young L. J., Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm. Behav. 77, 98–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rilling J. K., Young L. J., The biology of mammalian parenting and its effect on offspring social development. Science 345, 771–776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walum H., Young L. J., The neural mechanisms and circuitry of the pair bond. Nat. Rev. Neurosci. 19, 643–654 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGraw L. A., Young L. J., The prairie vole: An emerging model organism for understanding the social brain. Trends Neurosci. 33, 103–109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson Z. V., et al. , Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Horm. Behav. 79, 8–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes K. A., Du L., Rodd F. H., Reznick D. N., Familiarity leads to female mate preference for novel males in the guppy, Poecilia reticulata. Anim. Behav. 58, 907–916 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Bull C. M., Monogamy in lizards. Behav. Processes 51, 7–20 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Klatt J. D., Goodson J. L., Oxytocin-like receptors mediate pair bonding in a socially monogamous songbird. Proc. Biol. Sci. 280, 20122396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheetham S. A., Thom M. D., Beynon R. J., Hurst J. L., “The effect of familiarity on mate choice” in Chemical Signals in Vertebrates 11, Hurst J. L., Beynon R. J., Roberts S. C., Wyatt T. D., Eds. (Springer New York, New York, NY, 2008), pp. 271–280. [Google Scholar]
- 10.Okuyama T., et al. , A neural mechanism underlying mating preferences for familiar individuals in medaka fish. Science 343, 91–94 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Wang M. Y., Takeuchi H., Individual recognition and the ‘face inversion effect’ in medaka fish (Oryzias latipes). eLife 6, e24728 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoi S., et al. , Mate-guarding behavior enhances male reproductive success via familiarization with mating partners in medaka fish. Front. Zool. 13, 21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoi S., et al. , An essential role of the arginine vasotocin system in mate-guarding behaviors in triadic relationships of medaka fish (Oryzias latipes). PLoS Genet. 11, e1005009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taniguchi Y., et al. , Generation of medaka gene knockout models by target-selected mutagenesis. Genome Biol. 7, R116 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishikawa T., et al. , High-resolution melting curve analysis for rapid detection of mutations in a Medaka TILLING library. BMC Mol. Biol. 11, 70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansai S., et al. , Efficient targeted mutagenesis in medaka using custom-designed transcription activator-like effector nucleases. Genetics 193, 739–749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansai S., Kinoshita M., Targeted mutagenesis using CRISPR/Cas system in medaka. Biol. Open 3, 362–371 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter R., Smith C. W., Roy J., Importance of the third amino acid residue of oxytocin for its action on isolated rat uterus: Study of relationship between hormone conformation and activity. Proc. Natl. Acad. Sci. U.S.A. 73, 3054–3058 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor C. M., Marsh-Rollo S. E., Ghio S. C., Balshine S., Aubin-Horth N., Is there convergence in the molecular pathways underlying the repeated evolution of sociality in African cichlids? Horm. Behav. 75, 160–168 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Isoe Y., Konagaya Y., Yokoi S., Kubo T., Takeuchi H., Ontogeny and sexual differences in swimming proximity to conspecifics in response to visual cues in medaka fish. Zool. Sci. 33, 246–254 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Kawabata Y., Hiraki T., Takeuchi A., Okubo K., Sex differences in the expression of vasotocin/isotocin, gonadotropin-releasing hormone, and tyrosine and tryptophan hydroxylase family genes in the medaka brain. Neuroscience 218, 65–77 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Yokoi S., Mito M., Iwasaki S., Nakagawa S., Takeuchi H., The gene expression of the brain/pituitary from oxt/oxtr1 homozygous mutants and WT medaka in both sexes. GEO. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE139158. Deposited 21 October 2019.
- 23.Huffman L. S., et al. , Distribution of nonapeptide systems in the forebrain of an African cichlid fish, Astatotilapia burtoni. J. Chem. Neuroanat. 44, 86–97 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Novak U., Kaye A. H., Extracellular matrix and the brain: Components and function. J. Clin. Neurosci. 7, 280–290 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Wang M. Y., Takeuchi H.. Individual recognition and the ‘face inversion effect’ in medaka fish (Oryzias latipes). elife 6, e24728 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi M., Sorensen P. W., Stacey N. E., Hormonal and pheromonal control of spawning behavior in the gold fish. Fish Physiol. Biochem. 26, 71–84 (2002). [Google Scholar]
- 27.Yabuki Y., et al. , Olfactory receptor for prostaglandin F2α mediates male fish courtship behavior. Nat. Neurosci. 19, 897–904 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Trivers R. L., “Parental investment and sexual selection” in Sexual Selection and the Descent of Man, Campbell B., Ed. (Heinemann, 1972), pp. 136–179. [Google Scholar]
- 29.Kang E., et al. , Interplay between a mental disorder risk gene and developmental polarity switch of GABA action leads to excitation-inhibition imbalance. Cell Rep. 28, 1419–1428.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paciorkowski A. R., et al. , Novel mutations in ATP1A3 associated with catastrophic early life epilepsy, episodic prolonged apnea, and postnatal microcephaly. Epilepsia 56, 422–430 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang C., C1q as a regulator of brain development: Implications for autism spectrum disorders. Brain Disord. Ther. 4, 1 (2015). [Google Scholar]
- 32.Oldfield R. G., Hofmann H. A., Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol. Behav. 102, 296–303 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Song Z., Albers H. E., Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol. 51, 14–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weitekamp C. A., et al. , A role for oxytocin-like receptor in social habituation in a teleost. Brain Behav. Evol. 89, 153–161 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Balshine-Earn S., Lotem A., Individual recognition in a cooperatively breeding cichlid: Evidence from video playback experiments. Behav 135, 369–386 (1998). [Google Scholar]
- 36.Brown J. A., Colgan P. W., Individual and species recognition in centrarchid fishes: Evidence and hypotheses. Behav. Ecol. Sociobiol. 19, 373–379 (1986). [Google Scholar]
- 37.FitzGerald G. J., Morrissette J., Kin recognition and choice of shoal mates by threespine sticklebacks. Ethol. Ecol. Evol. 4, 273–283 (1992). [Google Scholar]
- 38.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data used in this study have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE139158).