The tumor microenvironment, including the tumor immune microenvironment, has been recognized as a complex milieu where cancer cells interact with stromal cells via numerous biochemical and physical signals that are crucial for cancer progression and metastasis (1). Tumor stroma includes blood and lymphatic vasculatures, extracellular matrix (ECM), cancer-associated fibroblasts, and numerous immune cells of the innate and adaptive immune systems, such as T cells, B cells, antigen-presenting cells (e.g., dendritic cells), tumor-associated macrophages, neutrophils, natural killer cells, and myeloid-derived suppressor cells. The important molecules in these cell–cell communications include cytokines (e.g., IL-2, IL-6, IL-10, and IFNγ), chemokines (e.g., CCL2, CCL5, and CXCL12), growth factors (e.g., VEGF, HGF, EGF, TGF-β) and their receptors, and immune checkpoints expressed on cancer and immune cells, such as PD-1, PD-L1, CLTA-4, LAG3, OX40, TIM3, and TIGIT. Tumor cells orchestrate a complex network of immunosuppression to evade elimination by immune cells. Up-regulation of immune checkpoints is an important aspect of this process. In the last decade, immunotherapy in the form of immune checkpoint blockers (ICBs) has emerged as one of the most promising cancer therapies (2). However, the response rate to ICB across different cancer types is only around 13% (3), and the administration of ICB induces drug resistance (4); thus, there is an important unmet need to increase the response rate and also, to determine the signatures of cancer that reliably predict whether patients with these signatures (predictive biomarkers) would respond to a specific immunotherapy or combination therapies. Among different cancer types, tumors are sometimes classified as “cold” and poorly immunogenic or “hot,” inflamed, and immunogenic (5). Also there is significant intertumoral and intratumoral (spatial, cellular, genomic) heterogeneity; in fact, tumor heterogeneity is a hallmark of cancer.
The importance of tumor vasculature for tumor progression was demonstrated in studies of Judah Folkman in the 1970s (6). These studies were followed by the discovery of VEGF and subsequent development of therapeutic antiangiogenic agents, such as anti-VEGF and anti-VEGFR2 monoclonal antibodies and receptor tyrosine kinase inhibitors. In numerous animal and clinical studies, Rakesh Jain and coworkers (7, 8) demonstrated that the likely mechanism for antiangiogenic agents is not necessarily vascular elimination and starving tumors of oxygen and nutrients but rather, more subtle vascular normalization that leads to improved and more homogeneous intratumoral blood flow and oxygen delivery, and as a result, this improved drug delivery and better access of immune cells. At the same time, interesting questions were raised about the interactions between the tumor vascular and immune systems (8–10). In addition to a myriad of cellular and molecular interactions that involve the two systems, physical forces also contribute to the tumor microenvironment, namely interstitial fluid pressure, solid stress that results from both tumor growth and tension within the ECM, and also, the stiffness of the ECM. Numerous studies are devoted to the effects of mechanical forces on cell signaling (mechanotransduction) in cancer at the molecular, cellular, and tissue levels (11, 12). Jain and coworkers (13) have demonstrated in animal experiments that, in addition to normalizing the vasculature, antiangiogenic agents also normalize the stroma by decreasing the interstitial pressure and mechanical stress; in addition, there are agents that target cancer-associated fibroblasts and extracellular collagen and hyaluronan, which leads to alleviation of mechanical forces and normalization of the stroma. There is experimental and clinical evidence that antiangiogenic, stroma-normalizing, and ICB immunotherapies may synergize if administered in a specific sequence or combination.
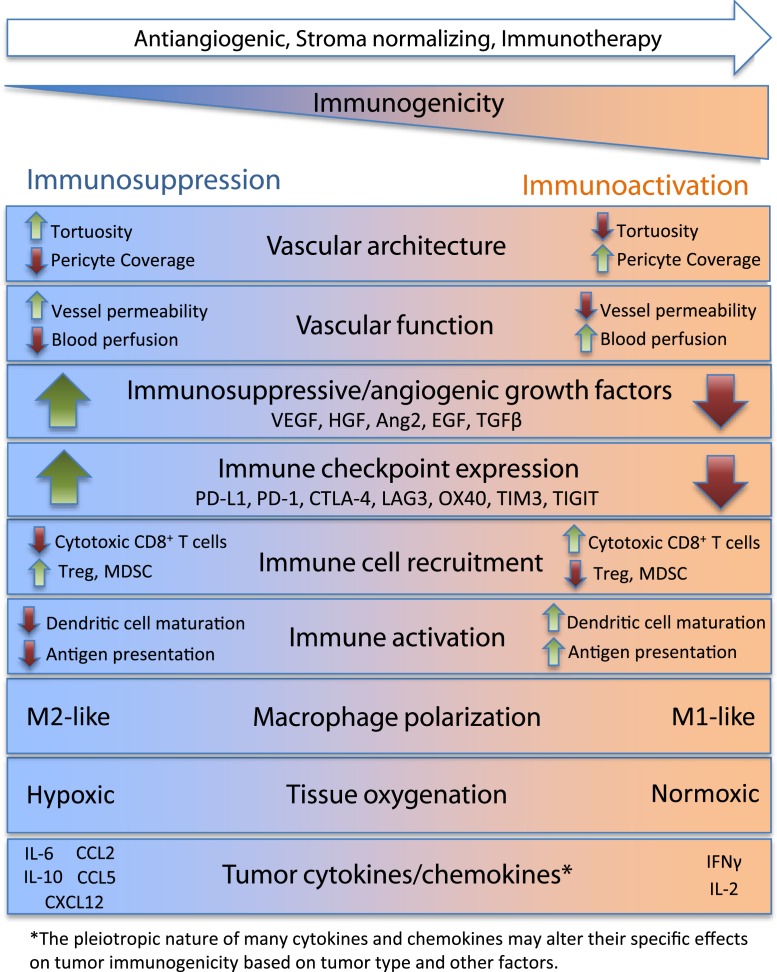
Given the complexity of the system with its multiscale nature and spatiotemporal dynamics, how can one integrate the knowledge of the interactions between the parts to understand the system response to therapeutics and make reliable predictions (e.g., for drug combinations, patient cohort selection, and drug regimens)? It is not possible without the advent of modern systems biology, specifically computational systems biology and quantitative systems pharmacology, which is being recognized as a necessary methodology in academia and pharmaceutical industry (14–17). In PNAS, Mpekris et al. (18) formulate an integrative computational model of tumor that includes multiple elements described above and explore the behavior of the system under different conditions. The model is based on several animal experiments from the authors’ laboratory as well as data from the literature. Fig. 1 shows selected factors that are associated with immunoactivation or immunosuppression of the tumor microenvironment based on literature data and current knowledge. Many of the factors shown are included in the computational model.
Fig. 1.
Selected factors playing a role in the immunoactivation and immunosuppression of the tumor microenvironment. MDSC, myeloid-derived suppressor cell.
The model comprises two interacting parts: tumor components (including cancer and stroma cells) and tumor vascular components. The tumor components include cancer cells (nonstem and stem like) and immune cells (CD8+ and CD4+ T cells, regulatory T cells [Treg], natural killer cells, and tumor-associated macrophages divided into M1 like and M2 like). The dynamics of these cells is modeled using mass balance ordinary differential equations for cells considered not motile and diffusion-type spatiotemporal partial differential equations for cancer cells considered motile. To calculate mechanical stress and strain as well as interstitial pressure distribution, tumor is modeled using a biphasic (incompressible fluid and elastic solid) continuum mechanics approach; the total stress is locally composed of the contribution from the fluid pressure and the solid-phase stress. In turn, the solid stress comprises a contribution from the ECM and associated cells and the component due to the cell proliferation and tissue growth. Oxygen concentration is modeled by a transport equation with a tissue consumption term and a source term reflecting the vasculature. For the vascular component, endothelial cell and pericyte density distributions are modeled as well as VEGF transport, stromal cell-derived factor 1 (SDF1α or CXCL12), PDGF-B, angiopoietin-1 and -2, and IFNγ. These coupled equations are solved numerically. Parameters of the model are estimated from the authors’ own animal experiments as well as data from the literature.
The model is systematically applied to simulate the experimental conditions. The pharmacodynamics for different drugs is simulated as certain impacts on the different variables. For example, stroma normalization is modeled as a decrease in the tumor elastic modulus or softening of the tumor; the ICB application is modeled as an increase in CD8+ T cells for anti–PD-1 therapy and a decrease of Tregs for anti–CTLA-4 therapy. Model predictions agree with experimental findings, which include the number of CD4+ and CD8+ T cells, Tregs, IFNγ level, and tumor volume. In another simulation, application of high and low doses of anti-VEGF (antiangiogenic) treatment is modeled as an effect on macrophage polarization from an immune inhibitory M2-like phenotype to an immune stimulatory M1-like phenotype; the results suggest that low doses of anti-VEGF are superior to high doses, in agreement with experimental findings. The effects of anti-VEGF treatment were modeled as changes in endothelial cells and VEGF degradation. In yet another simulation, anti-VEGF treatment was administered first, and immunotherapy was 4 d later. The anti-VEGF treatment was modeled as normalizing vascular density, blood perfusion, and elimination of hypoxia. The results show that the combination of anti-VEGF treatment with immunotherapy was more efficacious than immunotherapy alone as long as vascular function is improved, leading to a less heterogeneous blood perfusion. The authors continued systematically to explore all experiment-based combinations, including triple combination of antiangiogenic, stroma normalizing, and immunotherapy. It should be noted that most model parameters were selected and fixed in the parameterization process prior to the simulations, and in the application to each experimental therapeutic dataset, only a few parameters were varied. Thus, the comparison with experimental results does not constitute curve fitting but rather, reflects the qualitative behavior of the complex system.
Any model, whether in vitro, animal, or computational, has limitations that need to be clearly recognized in order not to overstep its limitations. However, if used judiciously and intelligently, computational models could be of enormous value in gaining quantitative and mechanistic understanding of the system. The model of Mpekris et al. (18) is built on solid foundation of fundamental principles of chemical kinetics, biological transport and tissue mechanics, and modern knowledge and understanding of vascular biology and tumor immunology. Therefore, its predictions have the potential to guide clinical trials and drug design.
In summary, Mpekris et al. (18) describe a computational model that builds on the previous studies from this group of coauthors that focus on descriptions of the tumor microenvironment, including its immune and vascular components, and intratumoral mechanical stress. The study provides a broad coverage of these important phenomena and their cross talks. The study is based on extensive experimental data. The authors model the effects of therapeutic agents that affect each of the three components and then, simulate their combinations to come up with predictions of optimal strategies for immunotherapy. The study is a significant advance in the field of cancer systems biology and specifically, cancer immunotherapy. That said, additional work needs to be done for the results to be applicable to predict outcome of clinical trials or standard of care, to identify predictive biomarkers, and to explore drug combinations. This and other models of tumor growth and cancer progression need to be thoroughly validated against clinical data using rigorous statistical tests and the arsenal of methodologies developed for calibration and validation of multiscale computational models, such as global sensitivity analysis against parameters of the model, uncertainty quantification, and parameter identifiability. Eventually, these developments should lead to in silico virtual clinical trials and contribute to the emerging field of personalized or precision medicine; the paper of Mpekris et al. (18) is an important step in this direction.
Acknowledgments
My research on cancer is supported by NIH Grants R01CA138264, U01CA212007, and R01CA196701 and grants from AstraZeneca and Boehringer Ingelheim. I thank Drs. A. C. Mirando, R. J. Sové, and M. Yarchoan for useful comments.
Footnotes
The author declares no competing interest.
See companion article on page 3728 in issue 7 of volume 117.
References
- 1.Binnewies M., et al. , Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popovic A., Jaffee E. M., Zaidi N., Emerging strategies for combination checkpoint modulators in cancer immunotherapy. J. Clin. Invest. 128, 3209–3218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haslam A., Prasad V., Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open 2, e192535 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fares C. M., Van Allen E. M., Drake C. G., Allison J. P., Hu-Lieskovan S., Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? Am. Soc. Clin. Oncol. Educ. Book 39, 147–164 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Galon J., Bruni D., Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 18, 197–218 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Folkman J., Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971). [DOI] [PubMed] [Google Scholar]
- 7.Jain R. K., Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Palma M., Jain R. K., CD4+ T cell activation and vascular normalization: Two sides of the same coin? Immunity 46, 773–775 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Schmittnaegel M., De Palma M., Reprogramming tumor blood vessels for enhancing immunotherapy. Trends Cancer 3, 809–812 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Tian L., et al. , Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong C., Zahir N., Konstantopoulos K., Biomechanics in Oncology (Springer Nature, 2018). [Google Scholar]
- 12.Northcott J. M., Dean I. S., Mouw J. K., Weaver V. M., Feeling stress: The mechanics of cancer progression and aggression. Front. Cell Dev. Biol. 6, 17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y., et al. , Losartan treatment enhances chemotherapy efficacy and reduces ascites in ovarian cancer models by normalizing the tumor stroma. Proc. Natl. Acad. Sci. U.S.A. 116, 2210–2219 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai J. P. F., Earp J. C., Pillai V. C., Translational quantitative systems pharmacology in drug development: From current landscape to good practices. AAPS J. 21, 72 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y., et al. , QSP toolbox: Computational implementation of integrated workflow components for deploying multi-scale mechanistic models. AAPS J. 19, 1002–1016 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Norton K. A., Gong C., Jamalian S., Popel A. S., Multiscale agent-based and hybrid modeling of the tumor immune microenvironment. Processes (Basel) 7, 37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szeto G. L., Finley S. D., Integrative approaches to cancer immunotherapy. Trends Cancer 5, 400–410 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mpekris F., et al. , Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc. Natl. Acad. Sci. U.S.A. 117, 3728–3737 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]