Significance
A major question in ecology concerns how spatial variation in habitat conditions (habitat heterogeneity) affects species richness. The area–heterogeneity tradeoff proposes that increasing habitat heterogeneity within a fixed area may cause a decline in species richness because any increase in heterogeneity leads to a reduction in the effective area available per species, thereby increasing the likelihood of stochastic extinctions. Here, we show that such reduction in effective area may also promote richness by reducing the likelihood of deterministic competitive exclusions. Based on our experimental results, we propose that the effect of the area–heterogeneity tradeoff (and habitat heterogeneity in general) on species richness depends on the relative importance of deterministic vs. stochastic drivers of extinction.
Keywords: species richness, ecological drift hypothesis, habitat heterogeneity, mesocosm experiment, metacommunity ecology
Abstract
A fundamental property of ecosystems is a tradeoff between the number and size of habitats: as the number of habitats within a fixed area increases, the average area per habitat must decrease. This tradeoff is termed the “area–heterogeneity tradeoff.” Theoretical models suggest that the reduction in habitat sizes under high levels of heterogeneity may cause a decline in species richness because it reduces the amount of effective area available for individual species under high levels of heterogeneity, thereby increasing the likelihood of stochastic extinctions. Here, we test this prediction using an experiment that allows us to separate the effect of the area–heterogeneity tradeoff from the total effect of habitat heterogeneity. Surprisingly, despite considerable extinctions, reduction in the amount of effective area available per species facilitated rather than reduced richness in the study communities. Our data suggest that the mechanism behind this positive effect was a decrease in the probability of deterministic competitive exclusion. We conclude that the area–heterogeneity tradeoff may have both negative and positive implications for biodiversity and that its net effect depends on the relative importance of stochastic vs. deterministic drivers of extinction in the relevant system. Our finding that the area–heterogeneity tradeoff may contribute to biodiversity adds a dimension to existing ecological theory and is highly relevant for understanding and predicting biodiversity responses to natural and anthropogenic variations in the environment.
Area and heterogeneity are two fundamental determinants of species richness (1–5). Both factors are thought to promote richness. A large area supports larger populations than a smaller area, thereby reducing the likelihood of stochastic extinctions (6–8). A more heterogeneous area provides a wider range of ecological conditions, thereby allowing coexistence of more species with different ecological requirements (9–12). The former mechanism is a key element of stochastic theories of biodiversity (6, 13), and the latter is a key element of deterministic theories (9, 10, 14). Together, these processes constitute the heart of community ecology and have broad implications for biodiversity conservation [e.g., the design of effective nature reserves (15–17) and the prediction of diversity responses to habitat loss and fragmentation (18, 19)].
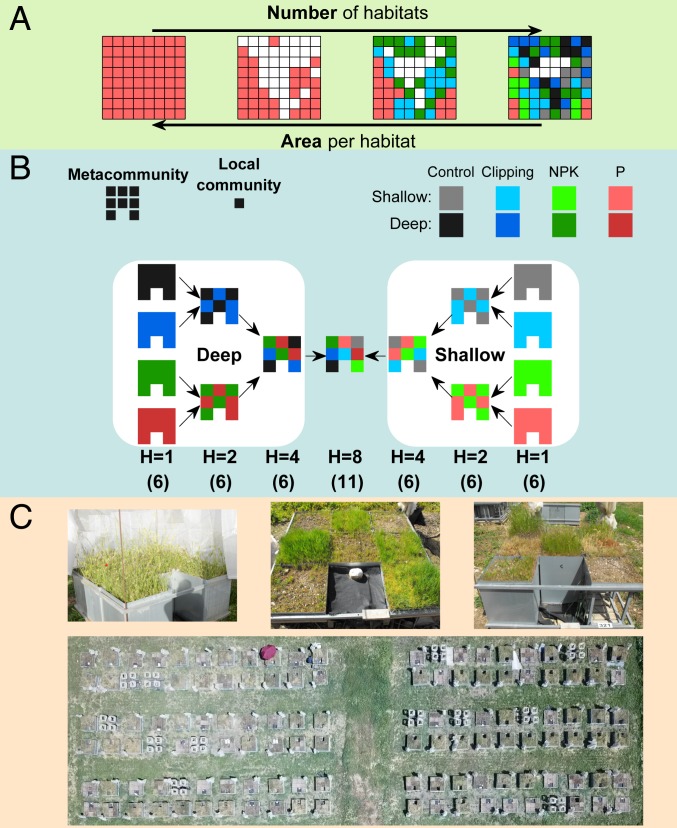
Yet, from the perspective of individual species, area and heterogeneity are not independent factors since any increase in habitat heterogeneity within a fixed area must lead to a reduction in the effective area available per species (Fig. 1A). This “area–heterogeneity tradeoff” (AHTO) has two important implications for biodiversity. First, a smaller effective area supports smaller populations. Second, a smaller effective area increases the likelihood that dispersed individuals would reach unsuitable habitats (12, 20, 21). Both mechanisms increase the likelihood of stochastic extinctions, and results from a variety of models suggest that they may cause a decline in species richness under high levels of heterogeneity (20–25). Such decline is predicted to be particularly pronounced in highly specialist species (22, 25). Thus, while classical niche theory predicts a positive effect of habitat heterogeneity on species richness (9, 10, 14), the AHTO proposes that this effect might be negative or unimodal (20, 22–24).
Fig. 1.
The AHTO and its experimental test. (A) A schematic illustration of the AHTO: increasing the number of habitats within a fixed area reduces the average area available per habitat and therefore, the amount of effective area available per species. (B) A schematic illustration of the experimental design. A metacommunity is composed of eight local communities grown in metal containers of 50 × 50 cm such that the total area of a metacommunity is 2 m2. Each local community is assigned to a combination of soil depth (shallow vs. deep) and treatment (control, clipping, NPK fertilization, or P fertilization), forming metacommunities with one (H = 1), two (H = 2), four (H = 4), or eight (H = 8) habitats. Each habitat combination in the first three levels (H = 1, H = 2, and H = 4) is replicated by six metacommunities, and level H = 8 is replicated by 11 metacommunities, adding up to 95 metacommunities (8 × 6 + 4 × 6 + 2 × 6 + 1 × 11). Three types of habitats (control, clipping, and NPK) have additional isolated local communities (16 replicates per habitats; for more information see Materials and Methods). (C) Photos of metacommunities with one, two, and eight habitats (Upper) and the overall experiment (Lower).
Recently, there has been much progress in understanding the role of area and heterogeneity on species diversity (26, 27). Such studies have demonstrated the importance of dispersal processes, differences in competitive ability among species, and scale as moderators of the effects of area and heterogeneity on species diversity (28–31). However, explicit tests of the AHTO and its consequences for species richness are still rare. A decline in richness under high levels of heterogeneity was reported in some studies (22, 32–35), and one study (22) also confirmed the prediction that specialist species are more sensitive to the AHTO than generalist species. However, this evidence was criticized as being a result of confounding effects (36) or sampling bias (37). The only experimental evidence for the AHTO of which we are aware is a demonstration of a positive interaction between the effects of heterogeneity and habitat size on species richness (38). Certainly, there is a need for more direct tests of this fundamental tradeoff.
The most direct test of the AHTO would be an experiment in which the number of habitats is manipulated while the total area is kept constant. However, even such an experiment is insufficient because any diversity response to increasing heterogeneity reflects the net effect of habitat heterogeneity (the balance between its positive and negative components) rather than the effect of the AHTO. A robust test of the AHTO is challenging and requires a partitioning of the overall effect of heterogeneity into its positive and negative components.
Here, we present the results of a mesocosm experiment designed to decompose the effect of the AHTO from the total effect of heterogeneity on species richness. The basic experiment consisted of 95 mesocosm metacommunities of annual plants established in metal containers at the Hebrew University Botanical Garden in Jerusalem (Materials and Methods and Fig. 1 B and C). A metacommunity consisted of eight containers, each representing a combination of soil depth (10 or 70 cm) and treatment (control; clipping; nitrogen, phosphorus, and potassium [NPK] fertilization; or phosphorus-only fertilization) (Fig. 1B). With these eight treatments, we created metacommunities with one, two, four, or eight “habitats,” thereby achieving a wide range of heterogeneity levels (Materials and Methods and Fig. 1B).
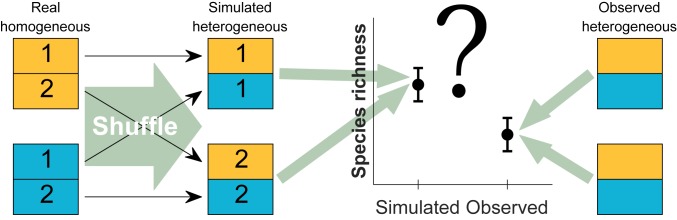
The effect of the AHTO on species richness was tested separately for each heterogeneity level using an approach that compares the observed number of species in a metacommunity with a “null” value indicating the number of species expected for the same level of heterogeneity at the absence of the AHTO. This null value was determined by constructing virtual (simulated) heterogeneous metacommunities from local communities that were part of homogeneous metacommunities (Materials and Methods and Fig. 2). Thus, for each level of heterogeneity, we had two values of species richness: the observed number (with AHTO) and the number of species in a set of simulated metacommunities representing the null expectation (without AHTO). The effect size of the AHTO at a given level of heterogeneity was calculated using the log response ratio (39): ESAHTO = log(SReal/SSimulated), where ESAHTO is the effect size of the AHTO, SReal is the mean number of species in real metacommunities, and SSimulated is the mean number of species in the corresponding set of simulated metacommunities (i.e., the null expectation). To prevent edge effects, all analyses were performed using data from an area of 25 × 25 cm at the center of the containers (Materials and Methods and SI Appendix, Fig. S1).
Fig. 2.
A schematic illustration of the procedure used to test the AHTO. In this example, each metacommunity (homogeneous or heterogeneous) is composed of two local communities, each occupying half of the metacommunity. Local communities from two homogeneous metacommunities (yellow and blue, each representing a different habitat entitled “real homogeneous”) are shuffled to create two virtual heterogeneous metacommunities (“simulated heterogeneous” metacommunities) with the same total area. The average number of species in the simulated metacommunities is used as a null expectation for the number of species expected in a heterogeneous metacommunity at the absence of the AHTO (since the species occurring in the homogeneous metacommunities have not experienced such a tradeoff). Our prediction is that average species richness in real heterogeneous metacommunities would be lower than that in the respective simulated metacommunities. This procedure can be applied to any level of heterogeneity or any habitat combination of interest.
Results
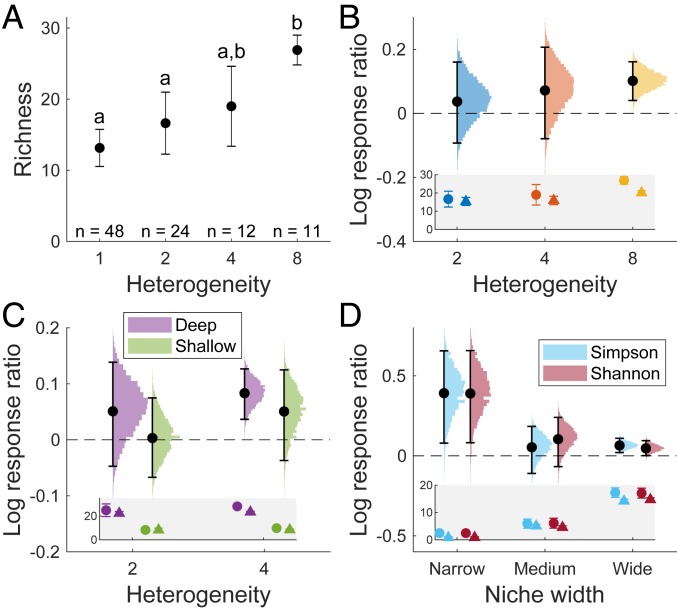
Our results can be summarized by three main findings. First, the overall (net) effect of heterogeneity on species richness was positive (Fig. 3A). There was no indication for a decline in richness at high levels of heterogeneity or even a reduction in the magnitude of the positive effect. Actually, the most pronounced increase in richness was observed at the highest level of heterogeneity (from H = 4 to H = 8) (Fig. 3A).
Fig. 3.
Effects of habitat heterogeneity and the AHTO on species richness. (A) Mean richness (±95% CI) of metacommunities representing different levels of habitat heterogeneity. Common letters indicate treatments that do not differ statistically in their richness (P > 0.05, Tukey’s test). (B) Effect size of the AHTO in metacommunities representing different levels of heterogeneity. Effect size was computed as log response ratio of mean richness in real vs. simulated (null) metacommunities. Error bars indicate ±95% CI based on 10,000 permutations (colored histograms). (C) Effect size of the AHTO in metacommunities representing deep vs. shallow soil conditions in metacommunities with two and four habitats. Error bars indicate ±95% CI based on 10,000 permutations (colored histograms). (D) Effect of niche width on effect size of the AHTO in metacommunities with eight habitats. Niche width was defined using two alternative indices: the Simpson index and the Shannon–Wiener index. For each measure, effect size was computed separately for each niche width category as log(S(J)Real/S(J)Simulated), where S(J) is the number of species in niche category J (J = narrow, medium, or wide). Error bars indicate ±95% CI based on 10,000 permutations (colored histograms). Insets in B–D present the raw data on species richness in real (circles) vs. null (triangles) metacommunities that were used for calculating the corresponding log response ratios.
Second, none of the heterogeneity levels showed a significant negative effect of the AHTO on species richness (Fig. 3B). This result was robust to differences in soil depth (Fig. 3C). In fact, under the highest level of heterogeneity (H = 8), where the negative effect of the AHTO was expected to be strongest, real metacommunities showed a significantly higher richness than the null expectation (t = 5.7, df = 17.06, P < 0.001), and the corresponding effect size (expressed by the log response ratio) was highly significant (P < 0.001) (Fig. 3B).
Third, in contrast to our expectation that the negative effect of the AHTO would increase from generalist to specialist species, the magnitude of its positive effect increased from the most generalist to the most specialist species (an increase by 14, 53, and 173% for wide-, medium-, and narrow-niche categories, respectively) (Fig. 3D). This result was robust to the measure of niche width (Fig. 3D).
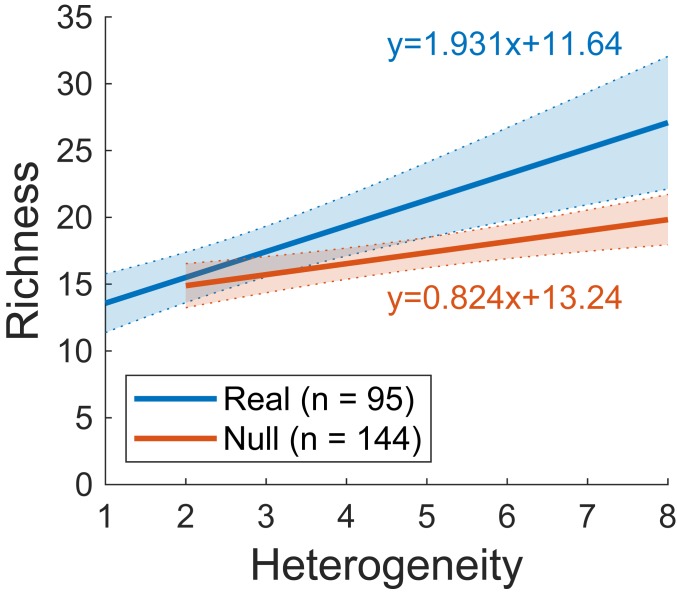
Thus, our overall results show that the effect of the AHTO on species richness in the experimental metacommunities was either neutral (nonsignificant) or positive. Moreover, the strongest positive effect was found under those conditions where we expected to find the strongest negative effect (high levels of heterogeneity and species with narrow niches). Consistent with these results, analysis of the pooled data from all metacommunities indicated that the slope of the heterogeneity–diversity relationship was higher than that expected at the absence of the AHTO (Fig. 4).
Fig. 4.
Effect of habitat heterogeneity on species richness at the level of individual metacommunities. Separate regressions were performed for real vs. simulated (null) metacommunities. Both slopes were statistically significant (real: t = 4.62, df = 93, P < 0.001; null: t = 3.60, df = 142, P < 0.001).
Discussion
Our null model approach has the ability to test the effect of the AHTO on species richness in a manner that is independent of the total effect of heterogeneity on species richness. Most importantly, our approach allows us to test the effect of the AHTO under both low levels and high levels of heterogeneity and even if the overall (net) effect of heterogeneity on species richness is positive. These features make our approach more powerful and more reliable than previous tests of the AHTO (22, 32, 35, 38).
Despite this power, our experimental results showed no evidence for the expected negative effect of the AHTO on species richness. How can these unexpected results be explained? One possible explanation is that the experiment was too short to obtain the magnitude of extinction required to generate the expected negative effect. However, our data show that nearly all local communities experienced considerable extinctions during the experiment (Fig. 5A). An alternative explanation is problems in our experimental design. These could include technical failures (e.g., edge effects), confounding effects (e.g., differences among treatments in the size of their species pools), or a failure to satisfy the assumptions of models predicting negative effects of the AHTO (e.g., insufficient heterogeneity or insufficient dispersal among local communities). A detailed analysis of the experimental data shows that none of these factors were an issue in our experiment (SI Appendix, Figs. S1–S7).
Fig. 5.
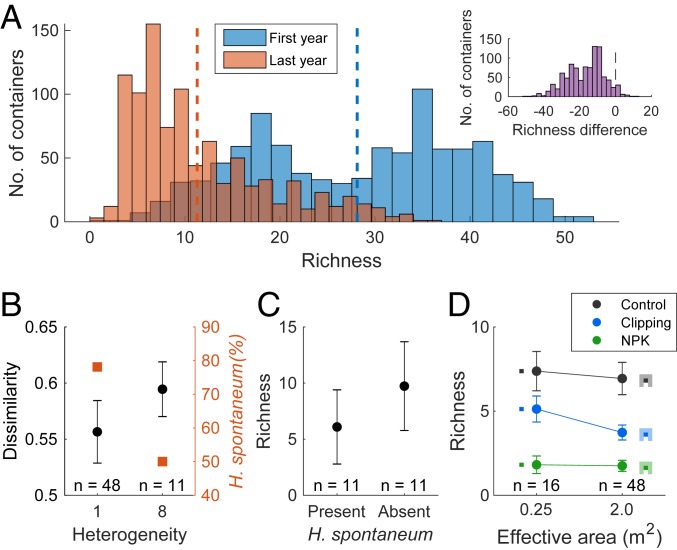
Evidence supporting the ecological drift hypothesis in the experiment. (A) Frequency distribution of the number of species per container in the first and last years of the experiment. Vertical dashed lines indicate mean values. Inset shows the distribution of the differences in richness between the last and first years of the experiment (note that the data are based on the species found in the whole container and may, therefore, involve some edge effects). (B) Effect of habitat heterogeneity on compositional dissimilarity among metacommunities quantified using the Jaccard index of dissimilarity (in black; P = 0.043, one-tailed randomization test for differences in means) and on the relative frequency of H. spontaneum (in red; P = 0.0037, one-tailed χ2 test). (C) Effect of the presence of H. spontaneum on the number of other species in local communities representing control and fertilized deep soils in heterogeneous metacommunities (P = 0.044, one-tailed t test). (D) Effect of increasing the effective area of local communities on species richness under the three treatments for which data were available (control, clipping, and NPK fertilization). Error bars indicate two SEs.
An Alternative Hypothesis: A Positive Effect of the AHTO on Species Richness?
A fundamentally different perspective is that the AHTO itself has some inherent, unrecognized mechanism that enhances species richness, and this positive effect may exceed the negative effect caused by the reduction in the amount of effective area available per species. We propose that this mechanism might be an increase in the magnitude of ecological drift and a consequent reduction in the likelihood of deterministic competitive exclusions (hereby, the “ecological drift hypothesis”). This hypothesis emphasizes that effects of AHTO mediated by species interactions may be more important than previously considered.
Importantly, the negative effect of the AHTO on species richness is attributed to stochastic extinctions caused by the decrease in effective area, thereby ignoring the potential consequences of species interactions (20). However, a decrease in the amount of effective area also increases the magnitude of ecological drift, thereby reducing the likelihood of deterministic competitive exclusions. Two distinct mechanisms may contribute to this effect. First, a smaller effective area reduces population sizes, thereby increasing the relative importance of demographic stochasticity (13, 40). This mechanism may allow inferior competitors to escape from deterministic exclusion by superior competitors (40–43). If the number of species released from competitive exclusion is large, the positive effect of the AHTO may exceed its negative effect.
Second, a decrease in effective area may promote drift by equalizing the competitive ability of habitat specialists. Under uniform habitat conditions and no dispersal, the best competitor(s) in each habitat may exclude locally inferior competitors (44). Habitat heterogeneity coupled with dispersal reduces the competitive ability of such “habitat-specific competitors” because a higher fraction of their propagules reaches unsuitable habitat conditions (25). Thus, in addition to its well-recognized role as a stabilizing mechanism, habitat heterogeneity may function as an equalizing mechanism that reduces fitness differences among competing species. The effectiveness of this fitness equalization mechanism should increase with increasing dispersal. As with the first mechanism (a decrease in population sizes), it is expected to promote drift, thereby reducing the likelihood of deterministic competitive exclusions.
According to these theoretical considerations, the AHTO has two contrasting effects on species richness: it increases the likelihood of stochastic extinctions at high levels of heterogeneity, but at the same time, it reduces the likelihood of deterministic competitive exclusions. Both effects should increase with increasing heterogeneity, and their net outcome is expected to depend on the relative importance of stochastic vs. deterministic drivers of extinction in the relevant communities.
Evidence Supporting the Ecological Drift Hypothesis.
The ecological drift hypothesis generates several testable predictions. First, if habitat heterogeneity promotes drift, replicated heterogeneous metacommunities should undergo higher compositional divergence during the course of the experiment than replicated homogeneous metacommunities. This should lead to larger differences in species composition (higher beta diversity) among heterogeneous metacommunities at the end of the experiment (8, 45). Second, increasing drift should increase the likelihood that dominant competitors would go extinct in their preferred habitats (40, 43, 46). We, therefore, expect that high levels of heterogeneity would reduce the frequency of occurrence of superior competitors in local communities representing their preferred habitats. Third, absence of a dominant competitor from a local community representing its preferred habitat is expected to result in an increase of local richness (47).
Our results are consistent with this chain of cascading effects. First, compositional dissimilarity among the most heterogeneous metacommunities was significantly higher than that among homogeneous metacommunities, supporting the prediction of higher drift in heterogeneous metacommunities (Fig. 5B) (note that dissimilarity is quantified among metacommunities). Moreover, since all metacommunities have started from similar conditions and heterogeneous metacommunities had a much higher richness at the end of the experiment, the observed difference in drift between homogeneous and heterogeneous metacommunities can be regarded as a conservative one (45).
Second, we know from previous experiments focusing on the same system that the annual grass Hordeum spontaneum is by far the best competitor in deep soils and that N fertilization further contributes to its competitive dominance (47–49). We, therefore, expected that 1) the increase in drift in heterogeneous metacommunities would lead to a decrease in the frequency of occurrence of H. spontaneum in local communities representing control or fertilized deep soils and 2) that this decrease would lead to an increase in local richness. Consistent with these predictions, the frequency of occurrence of H. spontaneum in local communities representing control or fertilized deep soils decreased from 78% in homogeneous metacommunities to 50% in the most heterogeneous metacommunities (Fig. 5B), and absence of H. spontaneum from local communities representing these conditions increased richness by 91% (Fig. 5C). These overall findings support our hypothesis that the positive effect of the AHTO on species richness was related to an increase in ecological drift that allowed inferior competitors to escape from deterministic competitive exclusion.
Moreover, if extinctions were primarily driven by stochastic (as opposed to deterministic) processes, one would expect that increasing the effective size of local communities would increase richness (13, 41). We tested this prediction by comparing richness of isolated local communities with that of local communities embedded within larger metacommunities of the same habitat (Materials and Methods). Despite the considerable increase in effective area (from 0.25 to 2 m2), local communities embedded within metacommunities had similar or even fewer species than isolated local communities with the same ecological conditions (Fig. 5D). These results are consistent with our hypothesis that species loss in local communities was primarily driven by deterministic rather than stochastic forces.
Results from a number of previous experiments focusing on the same system provide further support for our interpretation. Segre et al. (47) tested the role of stochastic vs. deterministic drivers of competitive exclusion in the natural community used as a source of seeds for the present work and found that deterministic competitive exclusions were the main source of extinction at scales comparable with those examined in our mesocosm experiment. Subsequent experiments showed that removal of the dominant competitor in this community (H. spontaneum) facilitates richness by releasing a large number of competitively inferior species from light competition (49, 50). In a related mesocosm experiment focusing on a subset of our study species (a total of 51 species), Ron et al. (48) found that reducing the effective area of mesocosm communities from 2 to 0.25 m2 increased the magnitude of ecological drift and reduced the dominance of H. spontaneum (48). All of these findings are consistent with our conclusion that the main mechanism underlying the positive effect of the AHTO on species richness in our experiment was a reduction in the magnitude of deterministic competitive exclusions.
Still, an inherent limitation of our experiment (and many other microcosm and mesocosm experiments) is that a small system is used as a toy model for studying processes operating at larger scales. Since any mechanism affecting species diversity is scale dependent, generalizing from such small-scale experiments to real-world systems is not trivial and should be made with caution. Thus, although the observed positive effect of the AHTO and its explanation are compatible with theoretical considerations and are supported by independent results from our current and previous experiments, we do not attempt to argue that this positive effect is general. Probably it is not. Our main message is that the mechanism responsible for the negative effect of the AHTO on species richness (increasing demographic stochasticity due to reduction in the amount of effective area) may also generate a positive effect by increasing the likelihood of ecological drift, thereby reducing the relative importance of deterministic competitive exclusion. A major challenge for future studies is to theoretically and experimentally investigate how various attributes of scale (spatial extent, spatial grain) interact with habitat heterogeneity and dispersal in determining the balance between these opposite forces.
Theoretical Implications and Predictions.
A major question arising from our results is under what conditions should we expect to find negative vs. positive effects of the AHTO. Answering this question is crucial for understanding the mechanisms by which habitat heterogeneity affects species diversity. We propose that the balance between these contrasting effects is determined by three main factors: 1) the position of the system along environmental gradients, 2) the magnitude of among-species variation in competitive ability, and 3) the number of strong vs. weak competitors in the relevant species pool.
The position along environmental gradients is expected to influence the consequences of the AHTO by influencing the prevalence of competitive exclusions. For example, studies focusing on the response of plant communities to environmental gradients often show an increase in the likelihood of competitive exclusions from stressful to more favorable environmental conditions (51, 52). If (as we claim) the mechanism underlying the positive effect of the AHTO is a reduction in the likelihood of competitive exclusion, one would expect that such gradients would be associated with an increase in the positive effect of the AHTO.
Increasing the magnitude of variation in competitive ability among species increases the deterministic component of competitive interactions (43). We, therefore, expect that communities characterized by small differences in competitive ability (neutral communities can be regarded an extreme case) would show a dominance of negative AHTO effects, while communities characterized by large differences would show a dominance of positive effects. Size asymmetry of resource acquisition facilitates competitive exclusions (53) and is, therefore, expected to increase the positive effect of the AHTO.
It is also important to distinguish between “abundance-based” and “trait-based” mechanisms of extinction (54). The first mechanism refers to situations in which increasing competition leads to the exclusion of rare species independently of species traits, while the second refers to situations in which the outcome of competition is determined by species functional traits. Applying this framework to the AHTO, we expect that communities structured by abundance-based mechanisms should show a dominance of negative AHTO effects, while communities structured by trait-based mechanisms should show a dominance of positive effects.
The number of strong vs. weak competitors in the species pool is expected to affect the balance between negative and positive effects of the AHTO because it determines the potential magnitude of increase in diversity following local extinction of the dominant competitor(s). In general, the strongest positive effect of the AHTO is expected in systems where the species pool consists of a single or a few strong competitors and a large number of weak competitors.
Future Challenges.
Although the tradeoff between the number and size of habitats is a fundamental property of ecological systems, testing the consequences of this tradeoff has proved a great challenge. The most challenging issue is to separate the effect of the AHTO from other effects of habitat heterogeneity. As emphasized in the Introduction, a monotonic positive response of richness to increased heterogeneity cannot be interpreted as evidence that the AHTO does not affect richness. Similarly, a decline in richness under high levels of heterogeneity does not necessarily reflect a response to the AHTO since other mechanisms may also lead to such responses (12, 21, 55–57). A robust test of the AHTO requires null models that provide expectations for the patterns expected at the absence of the AHTO. Developing such models is a crucial requirement for empirical tests of the AHTO in natural communities.
A related challenge is to develop methodologies for separating the negative and positive effects of the AHTO. Since these effects are expected to covary (both increase with decreasing the amount of effective area), separating their effects might be challenging. One possible approach to overcome this difficulty is to develop and test predictions concerning the manner by which the net effect of these two components is expected to depend on properties of the landscape, the species, and the scale at which the data are analyzed.
A third challenge is to develop more realistic models of the AHTO. Existing models are rather simple and assume that species are identical in both their niche width and dispersal ability. Another critical assumption of current models is that species cannot select their preferred habitats. Future models of the AHTO should relax these simplifying assumptions and should allow for distinguishing between mechanisms generating negative vs. positive effects of the AHTO.
Finally, most previous models of the AHTO were either spatially implicit (20, 22, 58) or spatially explicit with random landscapes (23, 24). Such models have limited capability to analyze the consequences of spatial processes. Yet, recent models emphasize the role of habitat fragmentation (25), dispersal (28), and scale (30, 31) as moderators of the effect of habitat heterogeneity on species diversity. These factors may also affect the relative importance of deterministic vs. stochastic drivers of species diversity (29, 30, 48). We, therefore, recommend that future attempts to understand the ecological consequences of the AHTO should focus on spatially explicit rather than spatially implicit models and should distinguish between compositional and spatial (configurational) aspects of habitat heterogeneity.
Conclusions
All previous studies of the AHTO have emphasized its negative implications for biodiversity (20, 22–25, 38, 58). Our experiment adds a dimension to this work, demonstrating that the AHTO may have a positive effect on biodiversity and that such positive effect may exceed its negative effect. This finding has important implications for understanding and predicting diversity responses to natural and anthropogenic variations in the environment and opens directions for future theoretical and empirical studies of biodiversity.
Materials and Methods
Seed Collection.
The seeds required for the experiment were collected by sampling the seed bank of a Mediterranean annual grassland located in Beit Guvrin National Park (altitude is 420 m, mean annual rainfall is 420 mm, mean annual temperature is 19 °C). This community was chosen because it is dominated by annual species, shows exceptional taxonomic and functional diversity, and was heavily investigated in previous studies (47, 49, 50, 59, 60). The seed bank samples were obtained by scraping the top 1-cm layer of the soil in randomly stratified patches scattered over the entire area. Preliminary experiments have shown that this soil depth contains most of the grassland seed bank. The soil samples from all sites were well mixed to create a homogenized mixture of topsoil (a total of ∼4 m3).
Experimental Design.
The experimental system was established at the Hebrew University Botanical Garden in Jerusalem. The basic experiment consisted of an array of artificial metacommunities that were created by sowing random samples from the homogenized seed bank in metal containers filled with a mixture of peat, red loam soil, and tuff in equal proportions. Each container had holes at the bottom for drainage, and all containers were placed on wood plates to level them and disconnect them from the local ground (Fig. 1 B and C). Each metacommunity consisted of eight containers of 50 × 50 cm (“local communities”) that were attached to each other, allowing spontaneous dispersal of seeds among the containers (Fig. 1 B and C). Seed dispersal into the metacommunity and out from the metacommunity was prevented by positioning a 50-mesh vertical nylon net around each metacommunity during the period of seed dispersal. Thus, each metacommunity could be treated as an independent functional and experimental unit.
The degree of habitat heterogeneity within metacommunities was manipulated by using containers of different soil depths (10 and 70 cm) and applying different ecological treatments to the containers of each depth (Fig. 1 B and C). Four treatments common to Mediterranean grasslands were applied: control, clipping (mimicking cattle grazing), NPK fertilization, and phosphorus-only fertilization (hereafter P). The clipping treatment received additional NPK fertilization to mimic management of real grasslands. The resulting eight habitats (two soil depths × four treatments per soil depth) were used to construct metacommunities representing four levels of habitat heterogeneity (Fig. 1B): one habitat (H = 1, all containers of the metacommunity are subjected to the same soil depth and the same treatment), two habitats (H = 2, half of the containers are assigned into one treatment and the other half to another treatment), four habitats (H = 4, two containers are assigned into each of the four treatments), and eight habitats (H = 8, each container is assigned into a different soil depth–treatment combination). Thus, individual metacommunities differed in both their “habitat heterogeneity” (the number of habitats composing the metacommunity) and their “habitat composition” (the identity of the habitats used to achieve the required level of heterogeneity).
A major challenge in assigning the eight habitats to the various levels of heterogeneity was to prevent confounding effects caused by differences among habitats in the sizes of their species pools. This problem was critical because the habitats were known to differ considerably in their species pools (60), and not all combinations of habitats could be realized at each level of heterogeneity (potentially, there were 28 distinct combinations of two habitats and 70 combinations of four habitats). To this end, the actual allocation of habitats to each heterogeneity level was determined using a hierarchical approach such that each level of heterogeneity was composed from habitats that were already included in lower levels (Fig. 1B). Level H = 2 was represented by four types of two-habitat combinations (control/clipping with deep soil, NPK/P with deep soil, control/clipping with shallow soil, NPK/P with shallow soil) (Fig. 1B), and level H = 4 was represented by two types of four-habitat combinations (control/clipping/NPK/P with deep soil, control/clipping/NPK/P with shallow soil) (Fig. 1B). This procedure ensured that differences in average richness among metacommunities representing different levels of habitat heterogeneity would not be confounded by underlying differences in habitat-specific species pools.
Another challenge in constructing the metacommunities was to separate the effects of compositional heterogeneity (number of habitats in our experiment) and habitat fragmentation [the degree of spatial connectivity among local communities of the same habitat (56)]. In our experiment, such separation was possible in heterogeneity levels of H = 2 and H = 4. To achieve that, we defined “fragmentation” as the proportion of edges between adjacent local communities (of the nine edges) in which the local communities at the two sides of the edge were of different habitats. We then constructed the metacommunities such that each habitat combination of H = 2 and H = 4 will have one metacommunity with the lowest possible fragmentation, another metacommunity with the highest possible fragmentation, and four metacommunities with intermediate levels of fragmentation. Thus, each habitat combination of H = 2 and H = 4 had six metacommunities representing the entire range of possible fragmentation levels. Metacommunities of H = 8 were inherently identical in their fragmentation, and all 11 repetitions were constructed by randomly allocating the eight habitats to the eight local communities.
For three habitats (control, NPK, and clipping with deep soil), we established additional local communities in isolated containers of 50 × 50 cm that were not part of a larger metacommunity (16 independent repetitions per habitat). These isolated local communities were blocked for dispersal and were used to assess the number of species expected at the level of a local community at the absence of dispersal to or from the local community.
All 808 containers (95 × 8 + 3 × 16) were sown in September 2013, and the communities emerging in each container were monitored for presence/absence of all species. Following this initial, first-year monitoring, the communities were allowed to grow and interact without any intervention for 4 y, except for the annual application of the fertilizer and clipping treatments in the relevant containers. During peak flowering of the fourth year (2017), the communities were monitored again, this time using a spatially stratified manner (SI Appendix, Fig. S1) following visual impression for edge effects at the margins of some containers (containers were less dense at their margins, particularly at the periphery of the metacommunity). Analysis of the data obtained for the central area of 25 × 25 cm in the containers (SI Appendix, Fig. S1) confirmed that neither species richness (SI Appendix, Fig. S2) nor species composition (SI Appendix, Fig. S3) showed any sign of edge effects at this scale. We, therefore, performed all analyses using the data obtained for the center of the containers.
Data Analysis.
Quantifying the effect of the AHTO.
As emphasized in the text, even if a gradient of habitat heterogeneity is established experimentally (by varying the number of habitats while keeping the total area constant) (Fig. 1A), it does not allow us to test the AHTO because any response of species richness to such gradient reflects the net effect of heterogeneity (i.e., the balance between its positive and negative components) rather than the negative component predicted by the AHTO. A robust test of the AHTO requires a separation of the effect of the AHTO from the overall effect of habitat heterogeneity.
Our experiment was designed to allow such separation. The essence of our methodological approach is that data from uniform metacommunities (i.e., metacommunities evolved without AHTO, H = 1) are shuffled to construct virtual (simulated) heterogeneous metacommunities that are similar to real metacommunities in both the number and identity of their habitats (Fig. 2). Mean richness in such simulated metacommunities is then used as a null expectation for species richness at the absence of the AHTO. A comparison of species richness in real heterogeneous metacommunities with that of the simulated (null) metacommunities allows us to quantify the effect of the AHTO on species richness at any level of habitat heterogeneity.
To determine the number of species expected at the absence of the AHTO in metacommunities of H = 8, we randomly sampled a single local community from the 48 local communities of each of the eight habitat types (note that each uniform metacommunity consisted of 8 local communities and was replicated six times). This sampling resulted in a virtual metacommunity with eight local communities, each representing a different habitat. Each of these local communities was evolved in a uniform metacommunity with the same area of real metacommunities (2 m2), thereby removing any negative or positive effects caused by the AHTO. This procedure ensured that the only difference between simulated (null) metacommunities and real metacommunities was lack of the AHTO in the former (null) metacommunities. This sampling procedure was repeated 48 times without replacement, ensuring that a different (random) set of local communities is used to construct each simulated metacommunity. Based on the AHTO, we predicted that mean richness of the 11 real metacommunities of H = 8 will be lower than mean richness of the 48 simulated metacommunities with H = 8.
A similar procedure was used to construct null metacommunities with two (H = 2) and four (H = 4) habitats. For example, to calculate a null richness for a deep soil metacommunity where half of the local communities were assigned to the NPK treatment and the other half to the P treatment (one of the four combinations of H = 2) (Fig. 1B), we randomly sampled four local communities from uniform metacommunities subjected to the NPK treatment and four local communities from uniform metacommunities subjected to the P treatment and repeated this procedure 12 times without replacements. This sampling resulted in 12 simulated metacommunities that were similar in their habitat composition to real metacommunities representing these two habitats (NPK/P). A similar procedure was applied to construct null expectations for all other types of heterogeneous metacommunities.
To control for differences in habitat heterogeneity when quantifying the effect of the AHTO on species richness, we determined the effect of the AHTO for each level of heterogeneity using the log response ratio as a measure of effect size: ESAHTO = log(SReal/SSimulated), where SReal is the mean number of species in real metacommunities and SSimulated is the mean number of species in simulated (null) metacommunities of the same heterogeneity level. Confidence intervals (CIs) of 95% were calculated to mean values of ESAHTO using bootstrapping by resampling 10,000 times with replacements the data of species richness obtained for each group of metacommunities (real vs. simulated), calculating ESAHTO for each set of data, sorting the resulting 10,000 values of ESAHTO from the smallest to the largest, and determining the boundaries of the lower 2.5% and upper 97.5% values of the sorted values. The statistical significance of the effect of the AHTO on species richness was evaluated for each heterogeneity level using two alternative methods: based on the confidence levels of the mean effect size and based on species richness in the relevant sets of real vs. simulated metacommunities (H0: SReal − SSimulated = 0, HA: SReal − SSimulated ≠ 0, a two-tailed independent samples t test).
Quantifying niche width.
A major prediction of AHTO models is that specialist species (species with narrow niches) should be more sensitive to the decrease in the amount of effective area at high levels of heterogeneity than generalist species (22, 58). To test this prediction, we quantified the niche width of each species based on its relative frequency of occurrence in containers of homogeneous metacommunities representing the eight types of habitats. This procedure had two advantages: 1) niche width was directly related to performance in the actual habitats used to construct the various levels of heterogeneity, and 2) the data used for quantifying niche width were independent of the data used for testing its consequences. Two alternative measures of niche width were quantified for each species: the Shannon–Wiener index and the Simpson index (61). For each measure, we ranked the species from the most specialist to the most generalist and based on this rank, categorized the species into three equal groups: narrow-niche species, medium-niche species, and wide-niche species. We then tested the effect of the AHTO separately for each group using the same procedure as described above. This analysis was limited to the highest level of heterogeneity (i.e., where the negative effect was expected to be strongest). Our prediction was that the negative effect of the AHTO on species richness would increase with decreasing niche width.
Testing for differences in beta diversity.
The prediction that habitat heterogeneity increases the magnitude of ecological drift was tested by quantifying the difference in beta diversity between the most heterogeneous metacommunities (H = 8) and homogeneous metacommunities (H = 1). Beta diversity was quantified as the mean value of the Jaccard index of dissimilarity among all pairwise combinations of metacommunities in the relevant group (note that the first group included only pairs subjected to the same treatment; i.e., we did not compare homogeneous metacommunities representing different treatments). The statistical significance of the differences was determined using one-tailed randomization test because the dissimilarity data were not independent.
Data Deposition.
Data are available at https://dx.doi.org/10.6084/m9.figshare.10282724.
Supplementary Material
Acknowledgments
We thank the Jerusalem Botanical Gardens for allocating the area for this research; R. Ron, T. Segal, D. Hirschfeld, and E. Beker for technical assistance in the field and in the laboratory; and O. Fragman-Sapir for botanic advising. The study was supported by Israel Science Foundation Grants 447/15 and 192/19, the Nature and Parks Authority, and Deutsche Forschungsgemeinschaft (DFG) Priority Program 1374 “Infrastructure—Biodiversity Exploratories.”
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. M.A.L. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited in Figshare, https://dx.doi.org/10.6084/m9.figshare.10282724.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911540117/-/DCSupplemental.
References
- 1.McGuinness K. A., Species-area curves. Biol. Rev. Camb. Philos. Soc. 59, 423–440 (1984). [Google Scholar]
- 2.Rosenzweig M. L., Species Diversity in Space and Time (Cambridge University Press, 1995). [Google Scholar]
- 3.Stein A., Kreft H., Terminology and quantification of environmental heterogeneity in species-richness research. Biol. Rev. Camb. Philos. Soc. 90, 815–836 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Kolasa J., Pickett S. T., Ecological Heterogeneity (Springer-Verlag, 1991). [Google Scholar]
- 5.Connor E. F., McCoy E. D., Species-area relationships. Encycl. Biodivers. 5, 397–411 (2001). [Google Scholar]
- 6.MacArthur R. H., Wilson E. O., The Theory of Island Biogeography (Princeton University Press, 1967). [Google Scholar]
- 7.Lande R., Extinction thresholds in demographic models of territorial populations. Am. Nat. 130, 624–635 (1987). [Google Scholar]
- 8.Gilbert B., Levine J. M., Ecological drift and the distribution of species diversity. Proc. Biol. Sci. 284, 20170507 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutchinson G. E., Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 22, 415–427 (1957). [Google Scholar]
- 10.Tilman D., Resource competition and community structure. Monogr. Popul. Biol. 17, 1–296 (1982). [PubMed] [Google Scholar]
- 11.Shmida A., Wilson M. V., Biological determinants of species diversity. J. Biogeogr. 12, 1–20 (1985). [Google Scholar]
- 12.Palmer M. W., The coexistence of species in fractal landscapes. Am. Nat. 139, 375–397 (1992). [Google Scholar]
- 13.Hubbell S. P., The Unified Neutral Theory of Biodiversity and Biogeography (Princeton University Press, 2001). [DOI] [PubMed] [Google Scholar]
- 14.Chase J. M., Leibold M. A., Ecological Niches: Linking Classical and Contemporary Approaches (University of Chicago Press, 2003). [Google Scholar]
- 15.Diamond J. M., The island dilemma: Lessons of modern biogeographic studies for the design of natural reserves. Biol. Conserv. 7, 129–146 (1975). [Google Scholar]
- 16.Benton T. G., Vickery J. A., Wilson J. D., Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 18, 182–188 (2003). [Google Scholar]
- 17.McCarthy M. A., Thompson C. J., Possingham H. P., Theory for designing nature reserves for single species. Am. Nat. 165, 250–257 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Rybicki J., Hanski I., Species-area relationships and extinctions caused by habitat loss and fragmentation. Ecol. Lett. 16 (suppl. S1), 27–38 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Fahrig L., Ecological responses to habitat fragmentation per Se. Annu. Rev. Ecol. Evol. Syst. 48, 1–23 (2017). [Google Scholar]
- 20.Kadmon R., Allouche O., Integrating the effects of area, isolation, and habitat heterogeneity on species diversity: A unification of island biogeography and niche theory. Am. Nat. 170, 443–454 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Laanisto L., et al. , Microfragmentation concept explains non-positive environmental heterogeneity-diversity relationships. Oecologia 171, 217–226 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Allouche O., Kalyuzhny M., Moreno-Rueda G., Pizarro M., Kadmon R., Area-heterogeneity tradeoff and the diversity of ecological communities. Proc. Natl. Acad. Sci. U.S.A. 109, 17495–17500 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Souza Júnior M. B., Ferreira F. F., De Oliveira V. M., Effects of the spatial heterogeneity on the diversity of ecosystems with resource competition. Phys. A Stat. Mech. 393, 312–319 (2014). [Google Scholar]
- 24.Bar-Massada A., Immigration rates and species niche characteristics affect the relationship between species richness and habitat heterogeneity in modeled meta-communities. PeerJ 3, e832 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben‐Hur E., Kadmon R., Heterogeneity–diversity relationships in sessile organisms: A unified framework. Ecol. Lett. 23, 193–207 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Vellend M., The Theory of Ecological Communities (MPB-57) (Princeton University Press, 2016). [Google Scholar]
- 27.Leibold M. A., Chase J. M., Metacommunity Ecology (Princeton University Press, 2017), vol. 59. [Google Scholar]
- 28.Gilbert B., Joint consequences of dispersal and niche overlap on local diversity and resource use. J. Ecol. 100, 287–296 (2012). [Google Scholar]
- 29.Hart S. P., Usinowicz J., Levine J. M., The spatial scales of species coexistence. Nat. Ecol. Evol. 1, 1066–1073 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Viana D. S., Chase J. M., Spatial scale modulates the inference of metacommunity assembly processes. Ecology 100, e02576 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Fournier B., Mouquet N., Leibold M. A., Gravel D., An integrative framework of coexistence mechanisms in competitive metacommunities. Ecography 40, 630–641 (2017). [Google Scholar]
- 32.Bar-Massada A., Wood E. M., The richness-heterogeneity relationship differs between heterogeneity measures within and among habitats. Ecography 37, 528–535 (2014). [Google Scholar]
- 33.Martins I. S., Proença V., Pereira H. M., The unusual suspect: Land use is a key predictor of biodiversity patterns in the Iberian Peninsula. Acta Oecol. 61, 41–50 (2014). [Google Scholar]
- 34.Redon M., Bergès L., Cordonnier T., Luque S., Effects of increasing landscape heterogeneity on local plant species richness: How much is enough? Landsc. Ecol. 29, 773–787 (2014). [Google Scholar]
- 35.Chocron R., Flather C. H., Kadmon R., Bird diversity and environmental heterogeneity in North America: A test of the area-heterogeneity trade-off. Glob. Ecol. Biogeogr. 24, 1225–1235 (2015). [Google Scholar]
- 36.Hortal J., et al. , Species richness can decrease with altitude but not with habitat diversity. Proc. Natl. Acad. Sci. U.S.A. 110, E2149–E2150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carnicer J., Brotons L., Herrando S., Sol D., Improved empirical tests of area-heterogeneity tradeoffs. Proc. Natl. Acad. Sci. U.S.A. 110, E2858–E2860 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuler M. S., Chase J. M., Knight T. M., Habitat size modulates the influence of heterogeneity on species richness patterns in a model zooplankton community. Ecology 98, 1651–1659 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg M. S., Rothstein H. R., Gurevitch J., “Effect sizes: Conventional choices and calculations” in Handbook of Meta-Analysis in Ecology and Evolution, Koricheva J., Gurevitch J., Mengersen K., Eds. (Princeton University Press, Princeton, NJ, 2013), pp. 61–71. [Google Scholar]
- 40.Orrock J. L., Watling J. I., Local community size mediates ecological drift and competition in metacommunities. Proc. Biol. Sci. 277, 2185–2191 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grainger T. N., Gilbert B., Dispersal and diversity in experimental metacommunities: Linking theory and practice. Oikos 125, 1213–1223 (2016). [Google Scholar]
- 42.Pedruski M. T., Arnott S. E., The effects of habitat connectivity and regional heterogeneity on artificial pond metacommunities. Oecologia 166, 221–228 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Kramer A. M., Drake J. M., Time to competitive exclusion. Ecosphere 5, art52 (2014). [Google Scholar]
- 44.Mouquet N., Loreau M., Coexistence in metacommunities: The regional similarity hypothesis. Am. Nat. 159, 420–426 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Chase J. M., Myers J. A., Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 2351–2363 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurtt G. C., Pacala S. W., The consequences of recruitment limitation: Reconciling chance, history and competitive differences between plants. J. Theor. Biol. 176, 1–12 (1995). [Google Scholar]
- 47.Segre H., et al. , Competitive exclusion, beta diversity, and deterministic vs. stochastic drivers of community assembly. Ecol. Lett. 17, 1400–1408 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Ron R., Fragman-Sapir O., Kadmon R., Dispersal increases ecological selection by increasing effective community size. Proc. Natl. Acad. Sci. U.S.A. 115, 11280–11285 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeMalach N., Zaady E., Kadmon R., Light asymmetry explains the effect of nutrient enrichment on grassland diversity. Ecol. Lett. 20, 60–69 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Segre H., DeMalach N., Henkin Z., Kadmon R., Quantifying competitive exclusion and competitive release in ecological communities: A conceptual framework and a case study. PloS One 11, e0160798 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grime J. P., Competitive exclusion in herbaceous vegetation. Nature 242, 344–347 (1973). [Google Scholar]
- 52.Grace J. B., et al. , Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 529, 390–393 (2016). [DOI] [PubMed] [Google Scholar]
- 53.DeMalach N., Zaady E., Weiner J., Kadmon R., Size asymmetry of resource competition and the structure of plant communities. J. Ecol. 104, 899–910 (2016). [Google Scholar]
- 54.Suding K. N., et al. , Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl. Acad. Sci. U.S.A. 102, 4387–4392 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Z., et al. , The effect of environmental heterogeneity on species richness depends on community position along the environmental gradient. Sci. Rep. 5, 15723 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fahrig L., et al. , Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 14, 101–112 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Seiferling I., Proulx R., Wirth C., Disentangling the environmental-heterogeneity–Species-diversity relationship along a gradient of human footprint. Ecology 95, 2084–2095 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Hortal J., Triantis K. A., Meiri S., Thébault E., Sfenthourakis S., Island species richness increases with habitat diversity. Am. Nat. 174, E205–E217 (2009). [DOI] [PubMed] [Google Scholar]
- 59.DeMalach N., Ron R., Kadmon R., Mechanisms of seed mass variation along resource gradients. Ecol. Lett. 22, 181–189 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Ron R., Fragman-Sapir O., Kadmon R., The role of species pools in determining species diversity in spatially heterogeneous communities. J. Ecol. 106, 1023–1032 (2018). [Google Scholar]
- 61.Levins R., Evolution in Changing Environments: Some Theoretical Explorations (Princeton University Press, 1968). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.