Abstract
Elevated and sustained estradiol concentrations cause a gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) surge that is necessary for ovulation. In sheep, several different neural systems have been implicated in this stimulatory action of estradiol and this study focused on somatostatin (SST) neurons in the ventral lateral region of the ventral medial nucleus (vlVMN) which express c-Fos during the surge. First, we determined if increased activity of SST neurons could be related to elevated GnRH secretion by assessing SST synapses onto GnRH neurons and neurons coexpressing kisspeptin, neurokinin B, dynorphin (KNDy). We found that the percentage of preoptic area GnRH neurons that receive SST input increased during the surge compared with other phases of the cycle. However, since SST is generally inhibitory, and pharmacological manipulation of SST signaling did not alter the LH surge in sheep, we hypothesized that nitric oxide (NO) was also produced by these neurons to account for their activation during the surge. In support of this hypothesis we found that (1) the majority of SST cells in the vlVMN (>80%) contained neuronal nitric oxide synthase (nNOS); (2) the expression of c-Fos in dual-labeled SST-nNOS cells, but not in single-labeled cells, increased during the surge compared with other phases of the cycle; and (3) intracerebroventricular (ICV) infusion of the nitric oxide synthase inhibitor, N(G)-nitro-L-arginine methyl ester, completely blocked the estrogen-induced LH surge. These data support the hypothesis that the population of SST-nNOS cells in the vlVMN are a source of NO that is critical for the LH surge, and we propose that they are an important site of estradiol positive feedback in sheep.
Keywords: neuronal nitric oxide synthase, L-NAME, somatostatin, luteinizing hormone
Ovulation is caused by surge-type secretion of gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) induced by high and sustained concentrations of estradiol (E2). Early work using c-Fos, a common marker for neural activation (1), indicated that GnRH neurons in the rodent preoptic area (POA) are activated during the LH surge (2). In ewes, a similar approach demonstrated that approximately half of the GnRH neurons throughout the hypothalamus and POA contained c-Fos, indicating broad activation of GnRH cells (3). Additionally, neural deafferentation studies have demonstrated that neural substrates in the rostral hypothalamus/POA contribute to the LH surge in sheep (4).
The stimulatory actions of E2 (often termed positive feedback) occur via activation of estrogen receptor alpha (ERα). Since GnRH cells in numerous mammalian species do not contain ERα (5,6), neural afferents must convey the estrogenic signal to this critical cell population. It is now generally recognized that in rodents the positive feedback actions of E2 occurs via kisspeptin neurons in the rostral periventricular area of the third ventricle (7,8). A critical role for kisspeptin in the generation of the LH surge in sheep is also supported by several lines of evidence. First, kisspeptin cells in both the POA and arcuate nucleus (ARC) contain ERα (9) and express c-Fos during the LH surge (10–12). Kisspeptin neurons in the ARC are known as KNDy cells because they coexpress kisspeptin, neurokinin B (NKB), and dynorphin (13). Moreover, ICV administration of a kisspeptin receptor antagonist reduced the LH surge amplitude by ~50% (14,15). Although this points to a role for kisspeptin in the surge, it also indicates that other systems likely contribute to the generation of the full LH surge.
One system implicated in the LH surge in sheep resides in the ventral medial nucleus (VMN). E2 administered into the VMN, but not the POA, induced surge secretion of GnRH and LH (16), and acute systemic E2 treatment induced a robust increase in the number of cells that express c-Fos in the VMN (and ARC) (17). Thus, E2 positive feedback appears to occur in the VMN and somatostatin (SST) neurons in the VMN that contain ERα are a likely candidate to mediate this action of E2 (18). Moreover, c-Fos expression increases in SST cells in the VMN during both a naturally occurring (19) and an estrogen-induced (18) LH surge. This hypothesis is consistent with the report that a SST receptor 2 (SSTR2) agonist (octreotide) blunted the LH surge and prevented c-Fos expression in GnRH cells in rats (20). However, in our previous work, neither an agonist nor antagonist to this receptor altered the timing or amplitude of an E2-induced LH surge in sheep (21). Reports that SST cells are activated during LH surge secretion are also unexpected in light of the generally inhibitory effects of SST reported throughout the body (22). Moreover, SST suppresses the firing of GnRH neurons in rodents (23,24), and inhibits GnRH secretion from rat hypothalamic explants (25) and LH pulses in ovariectomized (OVX) ewes (26). Moreover, a SSTR2 antagonist was recently reported to restore LH pulses in lactating rats (27), further demonstrating an endogenous action of SST in the suppression of gonadotropin secretion.
There are 2 simple explanations for the discrepancy between the observations of SST cell activation during the LH surge, but no effect of pharmacological manipulation of SST–SSTR2 signaling. First, SST neurons may mediate other actions of E2 (eg, estrous behavior), but the increase in Fos expression in these neurons during the normal ovine estrous cycle did not correlate with estrous behavior (19). Alternatively, these SST neurons in the VMN may also contain another signaling molecule that does stimulate GnRH secretion during the LH surge. One likely possibility is nitric oxide (NO), which is produced by the enzyme neuronal nitric oxide synthase (nNOS), and is critical for the LH surge (28,29) and sexual behavior (30) in rodents. Interestingly, in rats, a subpopulation of SST cells in the VMN was reported to also produce nNOS (31) and approximately 70% of nNOS neurons in this nucleus contain ERα (32, 33).
In the present study, we first tested whether activation of ovine SST neurons during the LH surge was related to GnRH release by determining if input to GnRH or KNDy cells from SST neurons increased at the time of the surge. We next tested the possible role of NO by (1) examining colocalization of SST and nNOS in VMN neurons, (2) testing if the increase in c-Fos expression in SST neurons at the time of the surge occurs only in SST neurons that contain nNOS, and (3) attempting to block the E2-induced LH surge with the nonspecific NOS inhibitor, N(G)-nitro-L-arginine methyl ester (L-NAME).
Material and Methods
Animals
Adult, multiparous ewes (>3 years) of predominantly Suffolk breeding were housed in a light- and temperature-controlled research building. Ewes were fed a maintenance diet of Timothy Pellets (Standlee Hay Company, Inc., Kimberly, ID) and Triple Crown Complete Pellets (Triple Crown, Wayzata, MN) and had free access to mineral blocks and water. All experiments were performed in the breeding season, between October and December. Blood samples (3–5 mL) were collected via jugular venipuncture into heparinized tubes. Plasma was harvested and stored at –20°C until assayed. All procedures were approved by the West Virginia University Animal Care and Use Committee and were performed in accordance with the NIH guidelines for the care and use of research animals.
General methods
Surgical procedures.
Ovariectomy was performed with aseptic technique under isoflurane anesthesia (2–5% in oxygen) using a midventral approach as previously described (34). Ewes were treated with analgesic (carpofen, 4 mg/kg; Putney, Portland, ME) and antibiotic (ampicillin, 100 mg/kg; Boehringer Ingelheim, Ingelheim am Rhein, Germany) pre- and postoperatively. All veterinary drugs were purchased from Patterson Veterinary (Bessemer, AL). For Experiment 4, sheep were fitted with a permanent indwelling third ventricle cannula (34). Briefly, the tip of an 18-gauge needle was placed in the third ventricle using a stereotaxic procedure and cemented in place with dental acrylic. The hub of the needle was plugged and a plastic cap was fitted over the hub and plug. Animals were treated daily with dexamethasone (Vedco, Saint Joseph, MO) and antibiotics (gentamicin, 3.3 mg/kg intramuscularly, Phoenix, Burlingame, CA; and ampicillin intramuscularly) twice daily from 1 day before to 4 days after surgery. Carprofen and butorphanol (0.05 mg/kg; Zoetis, Parsippany-Troy Hills, NJ) were given intravenously on the day of surgery. The analgesic, gabapentin (4 mg/kg; McCracken Pharmacy, Waynesburg, PA) was given orally twice per day for 4 days after surgery. Animals were allowed to recover for at least 7 days from either surgery before any experimental treatments.
Collection of neural tissues at different stages of the estrous cycle.
To assess changes during the estrous cycle, tissue was collected during the luteal phase, early follicular phase, and LH surge (n = 5/group). Briefly, ovarian cycles were synchronized with prostaglandin (Lutayse; Upjohn, Kalamazoo, MI) and 2 intravaginal progesterone-containing CIDRs (controlled intravaginal drug release devices, Zoetis, Parsipanny, NJ) as previously described (10). Tissue was then collected at the following times after CIDR removal: 18 hours (early follicular phase), 60 hours (LH surge), or 10 days (luteal phase). Before euthanasia, jugular blood samples (3–4 mL) were collected at 12-minute intervals for 4 hours from animals in the follicular and luteal phase groups and at 2- to 4-hour intervals from 36 to 60 hours after CIDR removal in the LH surge group.
To collect tissue from all ewes, animals were treated with heparin (20 000 U) 10 minutes before and immediately before euthanasia via overdose (8–16 mL, intravenously) of Euthasol (Patterson Veterinary, Bessemer, AL). After the ewe had stopped breathing and had no eye-blink reflex, the head was removed, and the brain perfused via both carotids with 6 L of 4% paraformaldehyde in 0.1 M phosphate buffer (PB) with 0.1% sodium nitrite. A block of neural tissue including the hypothalamus and POA was removed and stored in paraformaldehyde solution overnight at 4°C. Tissue was then stored in 20% sucrose in PB at 4°C for at least a week. Tissue was frozen and 45-μm-thick coronal sections were cut with a microtome equipped with a freezing stage. Sections were collected in 10 series (450 μm apart) and stored at –20°C in cryoprotectant solution (35).
Experiments
Experiment 1: Does SST input onto GnRH or KNDy cells increase during the LH surge?
To determine whether the abundant SST fibers within the ovine hypothalamus (18, 36) synapse onto GnRH or KNDy cells, we employed immunohistochemistry (IHC) for SST, synaptophysin (a presynaptic marker (37)), and GnRH or NKB (as a marker for KNDy cells). Antibodies for GnRH (38), NKB (39), synaptophysin (40), and SST (18) have been used previously in sheep tissue.
The number of SST synapses onto GnRH cells was determined in 1 to 2 POA hemisections and 4 to 8 mediobasal hypothalamic (MBH) hemisections from tissue collected during the luteal phase, follicular phase, or LH surge. Tissue was rinsed in PB 8 times (all rinses were 15 minutes each), stored overnight in PB at 4°C, and rinsed 12 additional times in phosphate-buffered saline (PBS). Tissue was incubated in 1% H2O2 in PBS for 10 minutes, rinsed 4 times, then incubated in 4% normal goat serum (NGS; Jackson Immunoresearch, West Groove, PA) in PBS with 0.04% triton X-100 (PBST) for 1 hour followed by rabbit anti-GnRH serum (dilution: 1:3000 with 4% NGS in PBST, cat#: 20075, Immunostar, Hudson WI; RRID: AB_572248) (41) for 17 hours. Tissue was rinsed with PBS and the primary antibody amplified with biotinylated tyramide by incubating sequentially in biotinylated goat antirabbit IgG (dilution: 1:500 in PBST with 4% NGS; Vector Labs, Burlingame, CA) for 1 hour, in ABC elite (dilution: 1:500 in PBS; Vector Labs) for 1 hour, in biotinylated tyramide (1:250 in PBS with 1 µL of 3% H2O2 per mL; Perkin Elmer, Bridgeville, PA) for 10 minutes, and in Alexa 555 streptavidin (dilution: 1:200, cat#: S32355; Invitrogen, Carlsbad, CA) for 1 hour, with intervening rinsing steps. Tissue was next rinsed and incubated in 4% NGS in PBST for 1 hour and then placed in rabbit anti-SST serum (dilution 1:500, cat#: T-4103; Peninsula Laboratories, San Carlos, CA; RRID: AB_518614) (42) and monoclonal mouse antisynaptophysin (dilution 1:200, cat#: S5768; Sigma-Aldrich, Saint Louis, MO; RRID: AB_477523) (43) with 4% NGS in PBST for 17 hours. Tissue was rinsed and sequentially incubated with Dylight 488 goat antimouse IgG (dilution 1:200 in 4% NGS in PBST, cat#: 35503, Invitrogen) and Alexa 647 goat antirabbit IgG (dilution 1:200 with 4% NGS in PBST, cat#: A-21245, Invitrogen) for 1 hour each with intervening rinsing steps. Finally, tissue was incubated in Neurotrace 435/455 (dilution 1:200 in PBS, cat#: N21479, Invitrogen). Tissue was mounted on superfrost slides (Fisher Scientific, Pittsburgh, PA) and coverslipped with Gelvatol (44).
The number of SST synapses onto KNDy cells was determined using NKB as a marker in 1 to 2 hemisections from each the middle and caudal aspects of the ARC, using anatomical landmarks for these 2 regions as described previously (45). Immunohistochemistry was performed identically as described for SST synapses onto GnRH cells, except that rabbit anti-NKB serum (dilution 1:10 000 with 4% NGS in PBST, H-046-26; Phoenix Pharmaceuticals, Burlingame, CA; RRID: AB_2716809) (46) was used in place of the rabbit anti-GnRH serum.
Experiment 2: Do SST neurons in the ovine VMN contain nNOS?
To determine whether SST cells in the VMN also contained nNOS, dual label IHC was performed on tissue from 3 OVX ewes that were treated with E2 via a 1-cm-long Silastic (Dow Corning Corp., Midland, MI) subcutaneous implant (inner diameter 0.34 cm, outer diameter 0.46 cm,) and 2 CIDRs to mimic luteal phase steroid concentrations. Tissue was collected 2 hours after an ICV injection of saline (for a different experiment) that did not alter LH secretion (data not shown).
IHC was performed as described above, except rabbit anti-SST serum (dilution: 1:20 000 with 4% NGS in PBST) was used in the first incubation with tyramide amplification, and rabbit anti-nNOS serum (dilution 1:1000, cat#: 24287; Immunostar, Hudson, WI; RRID: AB_572256) (47) was used in the second incubation; they were detected with Dylight 488 streptavidin and Alexa 555 goat antirabbit IgG, respectively. Preadsorption of either antibody with the alternative immunogenic peptide (ie, SST antibody with nNOS immunogenic peptide and vice versa) did not alter patterns or density of immunostaining (data not shown).
Experiment 3: Is the increased c-Fos expression in SST neurons during the LH surge limited to SST neurons that also contain nNOS?
To determine whether the population of SST cells that expressed c-Fos during the LH surge also contained nNOS, triple-label IHC was performed using 2 to 3 hemisections containing the VMN. Neural tissue collected from ewes during the LH surge, early follicular phase and luteal phase of the estrous cycle for the first experiment was used for this experiment. Antisera for nNOS and c-Fos were both raised in rabbits, so an alternative SST antiserum was used in this study (48), which also required the use of an antigen retrieval procedure.
IHC was performed as described above, but with antigen retrieval, performed by incubating tissue in an unmasking solution (Vector Laboratories) at 80°C for 20 minutes and then cooling it for 30 minutes and rinsing with PBS before any antisera incubations. Tissue was first incubated with rabbit anti-c-Fos serum (dilution: 1:3000 with 4% NGS in PBST, cat#: SC-253, Santa Cruz Biotechnology, Dallas, TX; RRID: AB_2106783) (49) which was amplified with tyramide and subsequently detected with Dylight 488 streptavidin. Then tissue was coincubated with rabbit anti-nNOS (dilution 1:1000 with 4% NGS in PBST) and rat anti-SST (dilution: 1:200 with 4% NGS in PBST, cat#: MAB354, Millipore, San Billerica, MA; RRID: AB_2255365) (50), which were detected with Alexa 647 goat antirabbit and alexa 555 goat antirat IgG, respectively.
Experiment 4: Is nitric oxide necessary for the LH surge?
To determine whether NO is critical for generation of the LH surge in sheep, we infused an inhibitor of NOS (L-NAME) into the third ventricle during an estrogen-induced LH surge. In preliminary work, using a dose (0.1 mmol/hour) based on previous work in sheep (51), the animal showed clear signs of distress (ie, increased respiration rate after about 4 hours of infusion). Therefore, we tested increasing doses of L-NAME (0.01, 0.02, 0.04, and 0.06 mmol/hour for 10 hours) sequentially over several days in separate ewes and monitored body temperature, and heart and respiration rates. There were no adverse reactions to the 2 lower doses that were tested first, but we noticed distinct circling behavior after the end of the infusion. Since this was similar to that observed due to brain swelling after neurosurgeries, we hypothesized that there might be an abrupt increase in blood flow to the brain after L-NAME infusion stopped. Therefore, for the 2 higher doses the infusion rate was cut to 50% 1 hour before, and to 25% 30 minutes before the end of the infusion, which eliminated this behavior. There was no reaction to the 0.04 mmol/hour dose, but the highest dose increased heart and respiration rates half way through the infusion, so it was terminated early, and we used 0.04 mmol/hour for this experiment.
L-NAME was infused using a well-established artificial follicular phase model (52). On the day of surgery (OVX and third ventricle cannulation), sheep received 2 CIDRS and a subcutaneous 1-cm-long Silastic implant containing E2. Eleven days later, CIDRs were removed, and the next day 4 large E2-containing implants (3 cm long) were inserted subcutaneously. At the time of this implant insertion, ewes were placed in a narrow pen and were fitted with a backpack for holding a syringe pump. L-NAME (0.04 mmol/hour) or saline (60 µL/hour) was infused into the third ventricle for 10 hours (with tapering off of the dose during the last hour) starting 14 hours after insertion of the 4 E2 implants. Blood samples were collected at 4-hour intervals for the first 8 hours, at 2-hour intervals for the next 20 hours, and then at 4-hour intervals for the last 2 samples. This protocol typically induces an LH surge 20 to 24 hours after the insertion of the 4 E2 implants. After the blood sampling procedure, the large E2 implants were removed, 2 new CIDRs were inserted into each ewe and this protocol was repeated with a crossover of L-NAME and saline infusions.
Data analysis and statistical analyses
LH assay and statistical analysis.
Plasma samples (25–200-μL aliquots) were assayed in duplicate for LH using a double liquid phase radioimmunoassay that has been previously validated for use in sheep (53). Assay reagents were acquired from the National Hormone and Peptide Program and LH concentrations are expressed in terms of NIH S24. The sensitivity of the LH assays averaged 0.08 ± 0.02 ng/tube and the inter- and intra-assay coefficients of variation were 9.6% and 12.7%, respectively. LH pulses were detected as described previously (54).
A LH surge was defined as a sustained increase in LH for at least 6 hours (55) with a maximum point at least 10 times the baseline LH value (average of samples at time 0 and 4 hours). The time of maximal LH secretion after large E2 implant insertion and the average peak of the LH surge were calculated when appropriate. If no LH surge occurred with one treatment, LH concentrations between treatments at the time of the peak of the LH surge in the other treatment were compared by paired t test.
Microscopy and statistical analysis
All imaging and cell counts were made by a single observer blinded to treatment groups. For Experiment 1, all confocal images were acquired with a Nikon A1R laser-scanning microscope (Nikon Instruments, Tokyo, Japan) with a 60× objective (aperture: 1.4). Nissl, Dylight 488, Alexa 555, and Alexa 647 were imaged with 405, 488, 561, and 640 nm lasers, respectively. For SST contacts onto GnRH or NKB cells in Experiment 1, 7 to 11 neurons with complete cell bodies were randomly selected from each region for analysis from each animal. Confocal images were taken at 0.5-μm intervals in the z-axis. The number of SST inputs (ie, that contained synaptophysin) onto either GnRH or NKB cell bodies or proximal dendrites were counted; single labeled synaptophysin contacts onto GnRH or NKB cells were also counted. To be considered a close contact the SST or synaptophysin staining had to be in direct contact (no intervening dark pixels) with GnRH, NKB, or Nissl staining that also contained either GnRH or NKB. To minimize the risk of overcounting, markers were placed on contacts as they were counted in ImageJ. GnRH and NKB cell size was estimated by the number of optical sections required to image the entire cell; these did not differ among treatment groups (data not shown). The percentage of GnRH and NKB cells that had dual-labeled SST and synaptophysin contacts/cell was determined in each region. For cells that had dual labeled SST and/or synaptophysin contacts, the number of contacts was enumerated and averaged for each animal. The number of SST synapses/cell, total number of synapses/cell (synaptophysin, independent of presence of SST) on GnRH and NKB neurons and percentage of these cells that had SST synapses within each region were compared among treatment groups with 1-way analysis of variance. Where appropriate, Tukey’s multiple comparisons test was used to determine differences among treatment groups.
For experiments 2 and 3, immunostained neurons (each, single, double, or triple-labeled) were counted in images encompassing the entire VMN that were collected with a fluorescent microscope (VS-120, Olympus, Tokyo, Japan) with a 20× objective following minor adjustments to brightness and contrast. The VMN was defined as medial to and ventral to the fornix, excluding the ARC. Values were averaged per animal and, for experiment 3, compared among groups (mid-LH surge, early follicular and luteal) with 1-way analysis of variance and Tukey’s multiple comparisons test. For all experiments, P < .05 was considered statistically significant; data are presented as mean ± SEM.
Results
Experiment 1: Does SST input onto GnRH or KNDy cells increase during the LH surge?
For the LH surge group, the mean LH concentration at the time of euthanasia was 21.9 ± 11.9 ng/mL. Tissue was collected from 2 ewes during the rising phase of the surge and from 2 during the falling phase, but GnRH secretion was still elevated in the latter (56). The characteristics of LH secretion patterns for ewes euthanized during the luteal phase and early follicular phase are shown in Table 1.
Table 1.
Characteristics of LH pulses at different stages of estrous cycle.
| Mean LH, ng/mL | IPI, minutes | Amplitude, ng/mL | |
|---|---|---|---|
| Luteal | 1.24 ± 0.13 | 115.2 ± 8.98 | 3.16 ± 0.87 |
| Early follicular | 3.13 ± 1.04 | 46.0 ± 3.16 | 2.09 ± 0.41 |
Values are mean ± SEM.
Abbreviations: IPI interpulse interval; LH, luteinizing hormone.
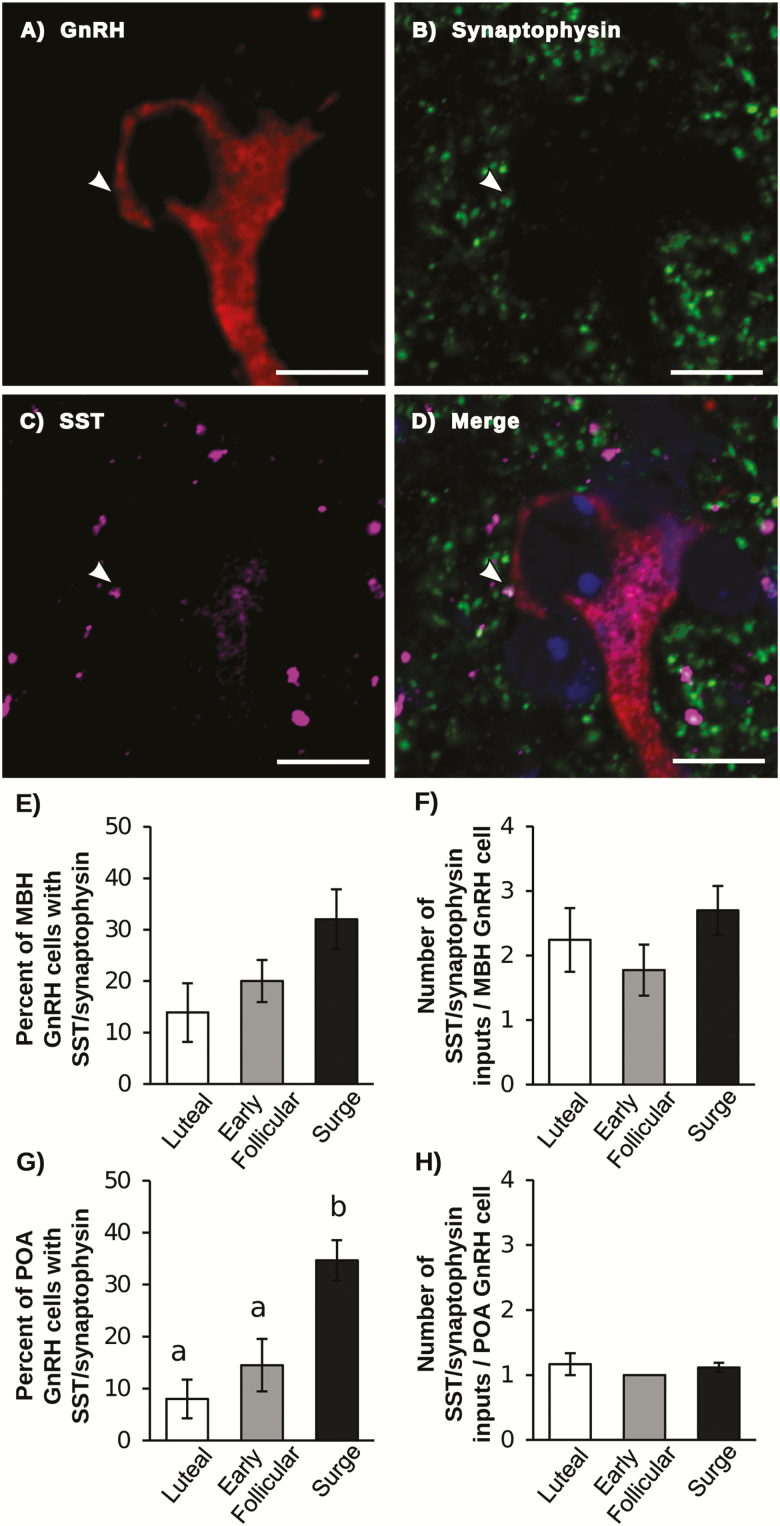
A small subpopulation of each of the MBH and POA GnRH neurons received SST/synaptophysin inputs (Fig. 1). There were no differences in the percentage of MBH GnRH neurons that received SST/synaptophysin inputs, nor the number of SST/synaptophysin inputs per cell among stages of the cycle. In contrast, the percentage of POA GnRH cells that received SST/synaptophysin inputs was greater in animals during the LH surge than in either the luteal or early follicular phase. However, the number of SST/synaptophysin inputs per cell did not differ between groups. There was a significant increase in the number of synaptophysin inputs (both with and without SST) onto POA GnRH cells during the LH surge compared to the luteal phase; the number of these contacts during the early follicular phase did not differ from the other groups (Table 2).
Figure 1.
Representative confocal image of a POA GnRH neuron (red; A) with synaptophysin (green; B) and SST (magenta; C) dual labeled contact (indicated by white arrow). (D) Merged image of GnRH, SST, synaptophysin and Neurotrace (blue). Scale bar = 10 μm. (E,G) Percentage of GnRH neurons in the MBH (E) and POA (G) that received at least 1 SST/synaptophysin contact. (F,H) Number of SST/synaptophysin inputs per GnRH neuron in the MBH (F) and POA (H). Bars with unique letters are significantly different (P < .05).
Table 2.
Number of synaptophysin contacts onto GnRH and NKB cells at different stages of the estrous cycle.
| Luteal | Early Follicular | Surge | |
|---|---|---|---|
| MBH GnRH | |||
| Synaptophysin only | 9.62 ± 1.16 | 7.72 ± 1.34 | 9.9 ± 1.10 |
| Total synaptophysin | 9.78 ± 1.18 | 7.90 ± 1.44 | 10.30 ± 1.06 |
| POA GnRH | |||
| Synaptophysin only | 7.22 ± 0.31a | 8.44 ± 0.68a,b | 9.93 ± 0.69b |
| Total synaptophysin | 7.32 ± 0.29a | 8.59 ± 0.65a,b | 10.32 ± 0.70b |
| mARC NKB | |||
| Synaptophysin only | 9.16 ± 0.25a,b | 9.81 ± 0.91a | 14.71 ± 1.51b |
| Total synaptophysin | 11.68 ± 0.88 | 13.16 ± 0.59 | 16.53 ± 1.77 |
| cARC NKB | |||
| Synaptophysin only | 8.30 ± 1.78a,b | 6.80 ± 0.38a | 11.80 ± 1.21b |
| Total synaptophysin | 10.93 ± 2.00a,b | 10.33 ± 0.31a | 14.85 ± 1.18 b |
Values are mean ± SEM. Rows with unique subscripts significantly differ (P < .05).
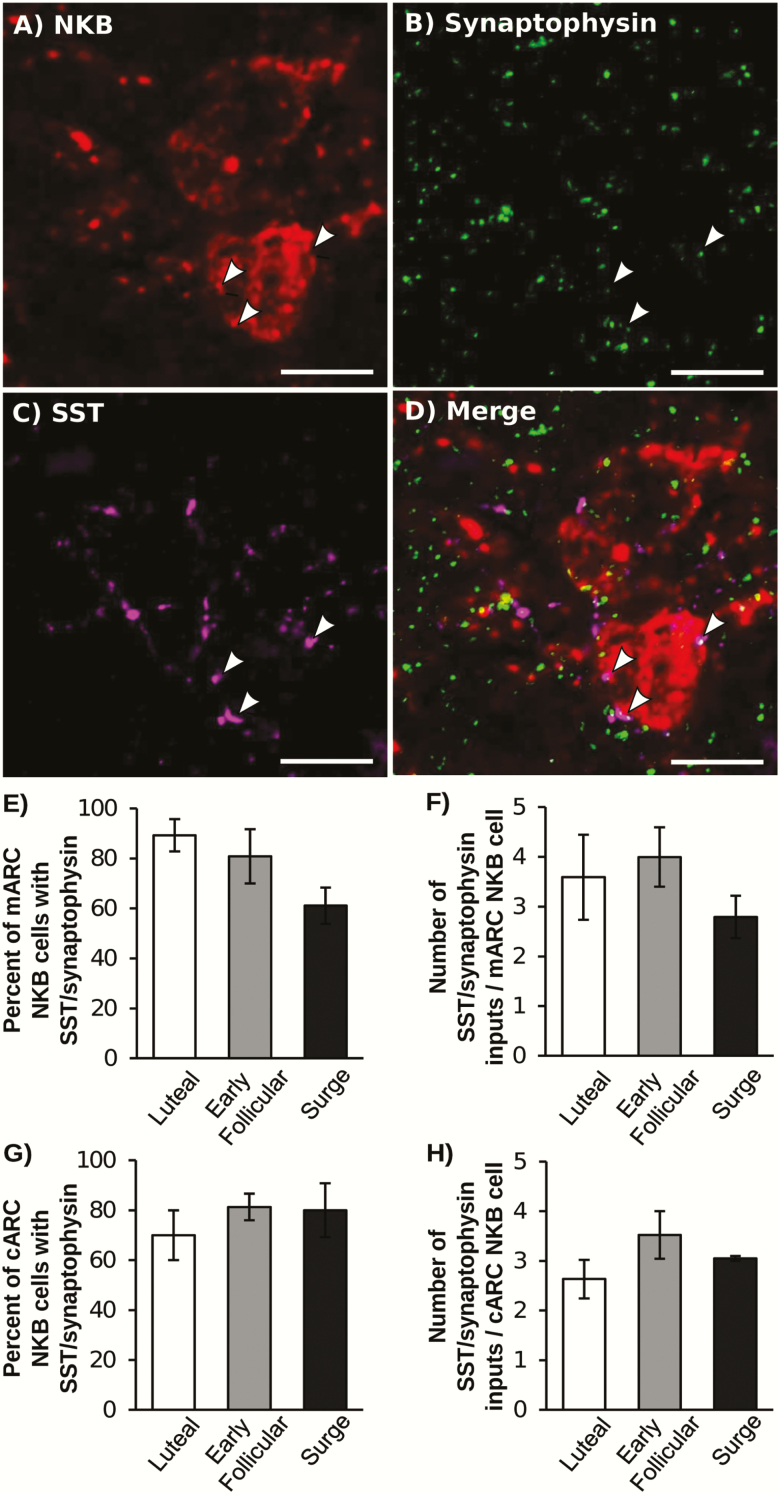
The majority of NKB cells in both the middle and caudal ARC received SST/synaptophysin inputs (Fig. 2). There were no differences in the percentage of NKB cells that had SST/synaptophysin contacts or the number of SST/synaptophysin contacts per cell in either the middle or caudal ARC among treatments. In the middle ARC, the number of synaptophysin only inputs onto NKB cells (devoid of SST) was increased during the LH surge compared with the early follicular phase (Table 2). Similarly, the total number of synaptophysin contacts and the number of synaptophysin only contacts onto NKB cells in the caudal ARC was increased during the LH surge compared with the early follicular phase.
Figure 2.
Representative confocal image of an NKB neuron from the cARC (red; A) with a synaptophysin (green; B) and SST (magenta; C) dual-labeled contacts (indicated by white arrows). (D) Merged image of NKB, SST, and synaptophysin. Scale bar = 10 μm. (E,G) Percentage of NKB neurons in the middle (E) and caudal (G) ARC that received at least 1 SST/synaptophysin contact. (F,H) number of SST/synaptophysin inputs per NKB neuron in the middle (F) and caudal (H) ARC. There were no significant differences among stages of the estrous cycle.
Experiment 2: Do SST neurons in the ovine VMN contain nNOS?
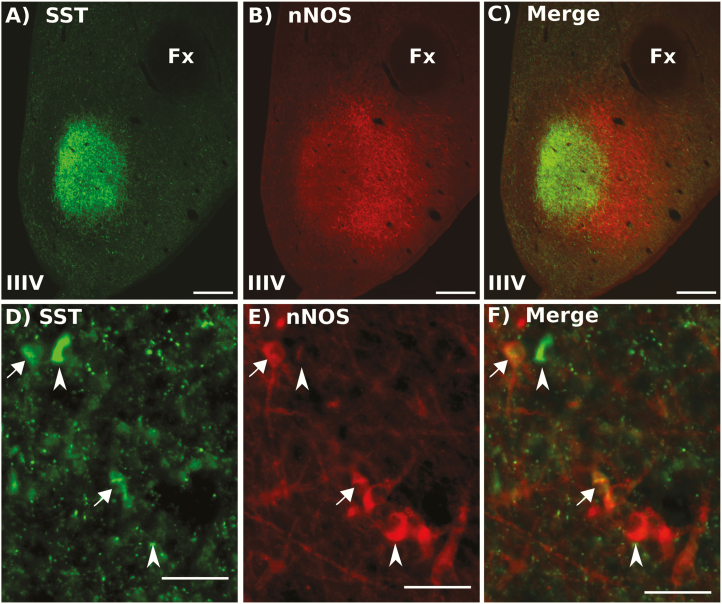
Numerous SST-containing fibers and neuronal cell bodies were observed throughout the MBH with many SST cells clustered in the ventral lateral aspect of the VMN (vlVMN). As reported previously (57), we observed a dense plexus of SST fibers in the central VMN; the majority of SST neurons were located immediately lateral to and slightly ventral to this dense fiber staining. The SST neurons in the vlVMN overlapped with a larger population of neurons that contained nNOS (Fig. 3). Immunostaining with the nNOS antiserum produced well-defined and very clear soma. Within the vlVMN, 81.7 ± 7.5% of SST neurons contained nNOS and 73.1 ± 2.5% of the nNOS-containing neurons also expressed SST. Somatostatin-ir fibers and soma were also evident throughout the rest of the MBH, but few of them contained nNOS. No colocalization of nNOS-ir and SST-ir cells was observed in the ARC, though few SST cells (<10 per hemisection) were observed in this nucleus.
Figure 3.
Representative image of SST (green, A + D) and nNOS (red, B + E) immunolabeling in the VMN. Merged images shown in C and F. Scale bar in A–C = 500 μm, Scale bar for D–F = 50 μm. Arrows indicate dual-labeled cells; arrowheads indicate single labeled SST or nNOS cells. Fx, fornix; IIIV, third ventricle.
Experiment 3: Is the increased c-Fos expression in SST neurons during the LH surge limited to SST neurons that also contain nNOS?
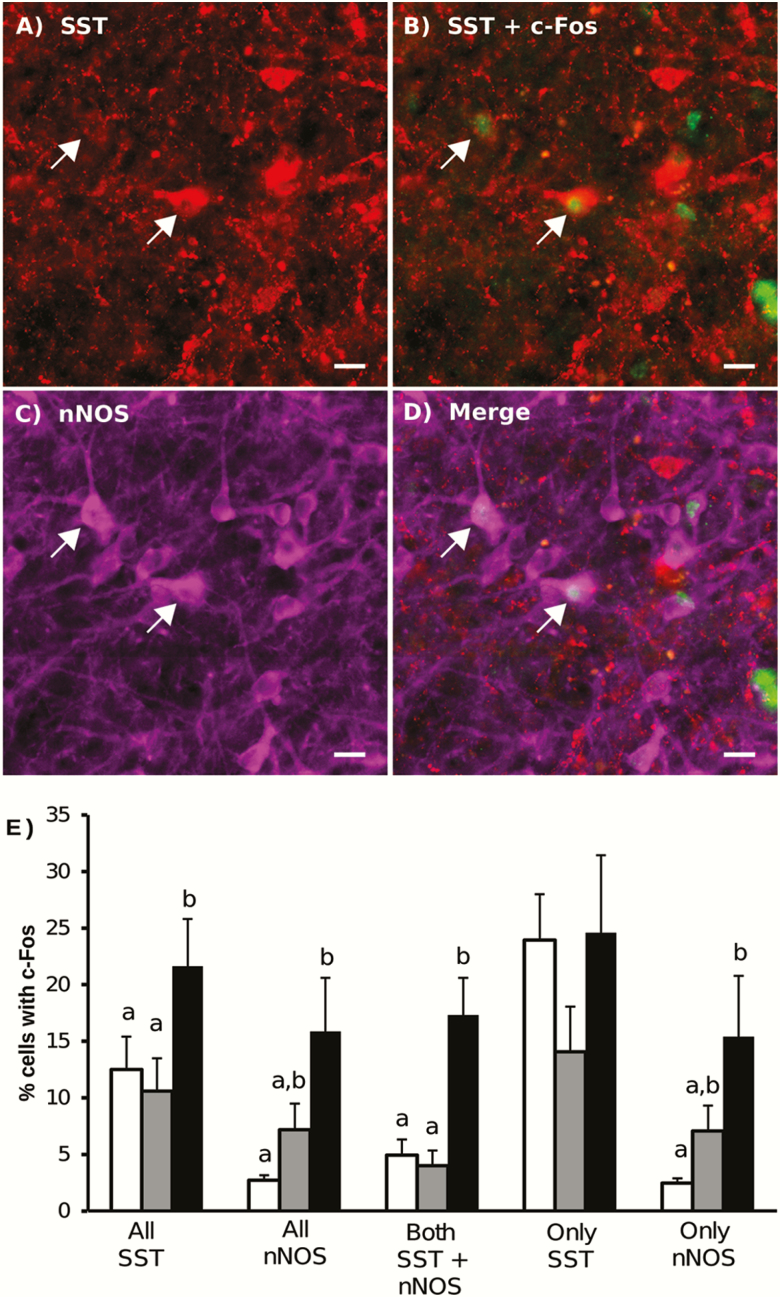
Similar to previous reports (18,19), we observed a significant increase in the percentage of all SST cells in the vlVMN that expressed c-Fos during the surge compared to other phases of the cycle. However, this selective increase only occurred in SST neurons that also contained nNOS: the percentage of these dual-labeled cells that contained c-Fos during the LH surge was significantly higher than in the early follicular or luteal phases (Fig. 4). In contrast, no differences in c-Fos content across the cycle were observed in the population of SST cells that did not contain nNOS. In both the population of all nNOS cells and the nNOS-only cells, the percentage of these cells that contained c-Fos was significantly greater during the surge than the luteal phase, but neither differed from the early follicular phase.
Figure 4.
Representative image of SST (red, A,B), c-Fos (green, B), nNOS (magenta, C) immunolabeling in the vlVMN in tissue collected during the LH surge. Merged images are shown in D. Scale bar = 20 μm. Arrows indicate dual-labeled SST and nNOS cells that contain c-Fos. (E) Quantification of SST and nNOS immunoreactive cells that contain c-Fos throughout the estrous cycle (white bars: luteal phase, grey bars: early follicular phase, black bars: LH surge). Bars with unique letters are significantly different (P < 0.05) within cell classification
Experiment 4: Is nitric oxide necessary for the LH surge?
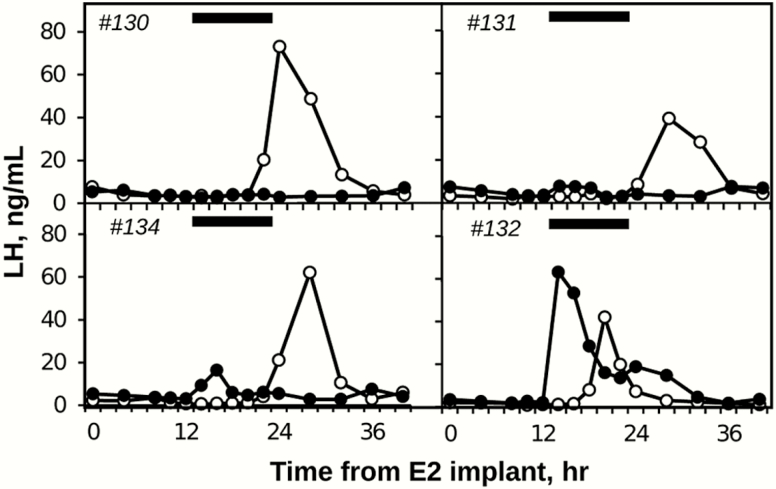
E2 treatment induced an LH surge during saline infusion in 4 out of 5 ewes; the animal that did not have an LH surge was removed from analysis. The L-NAME infusion completely blocked the LH surge in 3 out of 4 of the remaining ewes (Fig. 5). The fourth ewe had an LH surge that began before the L-NAME infusion and LH data from this ewe were not included in further analysis. During saline infusion, the peaks of the LH surge occurred 26.7 ± 1.3 hours after insertion of the large E2 implants. During L-NAME infusions, LH concentrations at this time (ie, ~27 hours) averaged 6.3 ± 3.3 ng/mL and were significantly (P = .047) less than control values (54.4 ± 9.4 ng/mL).
Figure 5.
Effects of L-NAME (black circles) and saline (white circles) infusions on LH secretion during an E2-induced LH surge. Black bars indicate time of infusions of both saline and L-NAME. Animal #132 was removed from analysis because the LH surge began before infusion started.
Discussion
This report demonstrates a role for NO in the generation of the LH surge in sheep. Specifically, consistent with data from other species (31), we found that a subpopulation of SST cells in the vlVMN contain nNOS in ewes and demonstrated that this subpopulation of dual-labeled cells showed increased c-Fos expression during the LH surge. Moreover, unlike pharmacological manipulation of SSTR2 in our previous work (21), inhibition of NO blocked the LH surge in sheep.
These results confirm previous work in sheep indicating increased activity (ie, c-Fos expression) of VMN SST neurons at the time of the LH surge (18,19), which has pointed to a role for these neurons in the generation of the LH surge. We have also extended this work by demonstrating increased input from SST-containing neurons to POA GnRH cells during the surge. In addition, our results provide a simple explanation for the apparent paradox of a system with an inhibitory neurotransmitter (SST) being activated during the LH surge. Specifically, we found that the population of SST neurons that also contained nNOS (but not either population of single labeled SST or nNOS cells) were specifically activated during the LH surge. This correlational data on the role of NO is supported by the observation that infusion of the NOS inhibitor, L-NAME, into the third ventricle completely blocked the LH surge in sheep. Although this analysis includes relatively few animals, a clear effect was observed.
It should be noted that the system responsible for the GnRH/LH surge in ewes is complex and, in addition to these SST/nNOS neurons in the vlVMN and GnRH cells in the POA and hypothalamus, includes (1) kisspeptin neurons in both the ARC (KNDy neurons) and POA (10–12), (2) NKB-responsive neurons in the retrochiasmatic area (15,58), and (3) noradrenergic neurons in the brain stem (59). However, it is important to note that, in contrast to the complete blockade of the LH surge produced by L-NAME, disrupting kisspeptin (14) or NKB (15, 58) signaling only reduced the LH surge amplitude by 40% to 50%, and an α-adrenergic antagonist produced inconsistent effects on surge secretion (60). This difference raises the possibility that these SST/nNOS neurons play a key role in the generation of the LH surge in the ewe. Previous data have implicated the VMN as the site of E2 positive feedback in ewes (16,17) and it is likely that the neurons containing SST and nNOS are the estrogen-responsive neurons in this region because subpopulations of each have been demonstrated to contain ERα in sheep (18,61). If so, disruption of the initial step in the process leading to the surge might explain the more dramatic effects of L-NAME than that of antagonists to receptors for other neurotransmitters implicated in the surge. It should also be noted that L-NAME inhibits all 3 subtypes of NOS, but the c-Fos data from this study points to nNOS as the critical subtype for the LH surge.
There is also strong evidence in rodents implicating NO in the generation of the GnRH surge (28). In mice, nNOS knockout animals are infertile and, although some ovarian defects have been reported, the primary deficit appears to be in the brain (62). Moreover, estrogen did not induce an LH surge in nNOS knockout animals or in wild-type mice treated with L-NAME (29). Although much of the data point to POA nNOS neurons in mice (29), VMN nNOS neurons in rodents contain ERα (32,33) and have been proposed to mediate estrogen positive feedback (28). It should be noted, however, that the effects of NO in rodents are complex (63,64) and this highly diffusible molecule can also inhibit GnRH secretion under some circumstances (29,65).
Our findings that SST cells synapse directly onto GnRH cells raises the possibility that the dual-labeled cells directly alter GnRH secretion. The SST synapses onto GnRH cells that we observed have also been observed in mice (23), but not rats (24). Our data, which confirm a recent report in ewes (66), also demonstrate that SST cells synapse onto KNDy cells. Complimentary evidence for SST regulation of KNDy cells is provided by our recent report that ICV treatment with a SSTR2 antagonist increases c-Fos in KNDy cells in the caudal aspect of the ARC (21). Thus it is possible that SST acts directly on both GnRH and KNDy cells, but the latter are more likely involved in control of LH pulses than the LH surge (21). In addition to changes in SST-positive synaptic inputs, we did observe changes in overall synaptic inputs to GnRH and KNDy cells evident by changes in the total number of synaptophysin contacts on to these cells, which is consistent with previous reports of altered synaptic contacts onto GnRH and KNDy cells during the LH surge (40,67–69).
In summary, these data demonstrate that NO signaling is necessary for the LH surge in ewes. Our observation that a subpopulation of neurons in the vlVMN coexpress SST and nNOS and that this population of dual labeled cells is activated during the surge, supports the hypothesis that these vlVMN cells are the source of NO during the LH surge and together with earlier data point to them as a critical site of E2 positive feedback in ewes.
Acknowledgments
We thank Miroslav Valent for technical assistance and Gail Sager for assistance with animal surgery. We also thank Dr. Jennifer Fridley and Dr. Katie Knapek for veterinary care, Heather Bungard for routine care, and Dr. Al Parlow and the National Hormone and Peptide Program for reagents used for LH assay. Imaging experiments and analysis were performed in the West Virginia University Microscope Imaging Facility.
Glossary
Abbreviations
- ARC
arcuate nucleus
- CIDR
controlled intravaginal drug release device
- E2
estradiol
- ERα
estrogen receptor alpha
- GnRH
gonadotropin-releasing hormone
- ICV
intracerebroventricular
- IHC
immunohistochemistry
- IM
intramuscularly
- IV
intravenously
- KNDy
kisspeptin, neurokinin B, dynorphin
- LH
luteinizing hormone
- L-NAME
N(G)-nitro-L-arginine methyl ester
- MBH
mediobasal hypothalamic
- NGS
normal goat serum
- NKB
neurokinin B
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- OVX
ovariectomized
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- POA
preoptic area
- SST
somatostatin
- SSTR2
SST receptor 2
- vlVMN
ventral lateral region of the ventral medial nucleus
Financial Support: This work was supported by NIH grants R01 HD039916 and R01 HD082135 to R.L.G. and M.N.L. and NIH grant P20-GM103434 to the West Virginia IDeA Network for Biomedical Research Excellence. The West Virginia University Microscope Imaging Facility is supported by the Mary Babb Randolph Cancer Center and NIH grants U54 GM104942.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in the references.
References
- 1. Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296(4):517–530. [DOI] [PubMed] [Google Scholar]
- 2. Lee WS, Smith MS, Hoffman GE. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci U S A. 1990;87(13):5163–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133(2):896–903. [DOI] [PubMed] [Google Scholar]
- 4. Jackson GL, Kuehl D, McDowell K, Zaleski A. Effect of hypothalamic deafferentation on secretion of luteinizing hormone in the ewe. Biol Reprod. 1978;18(5):808–819. [DOI] [PubMed] [Google Scholar]
- 5. Lehman MN, Robinson JE, Karsch FJ, Silverman AJ. Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol. 1986;244(1):19–35. [DOI] [PubMed] [Google Scholar]
- 6. Herbison AE. Physiology of the adult gonadotropin-releasing hormone neuronal network. In: Plant TM, Zeleznik AJ, eds. Knobil and Neill’s Physiology of Reproduction. 4th ed Vol 1 Amsterdam: Elsevier; 2015:399–467. [Google Scholar]
- 7. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57(2):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. J Neuroendocrinol. 2012;24(1):131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225–230. [DOI] [PubMed] [Google Scholar]
- 10. Merkley CM, Porter KL, Coolen LM, et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. Expression of fos and in vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Endocrinology. 2011;152(1):214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150(12):5530–5538. [DOI] [PubMed] [Google Scholar]
- 13. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 14. Smith JT, Li Q, Yap KS, et al. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–1012. [DOI] [PubMed] [Google Scholar]
- 15. Goodman RL, He W, Lopez JA, et al. Evidence that the lh surge in ewes involves both neurokinin B-dependent and -independent actions of kisspeptin. Endocrinology. 2019;160(12):2990–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caraty A, Fabre-Nys C, Delaleu B, et al. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139(4):1752–1760. [DOI] [PubMed] [Google Scholar]
- 17. Clarke IJ, Pompolo S, Scott CJ, et al. Cells of the arcuate nucleus and ventromedial nucleus of the ovariectomized ewe that respond to oestrogen: a study using Fos immunohistochemistry. J Neuroendocrinol. 2001;13(11):934–941. [DOI] [PubMed] [Google Scholar]
- 18. Scanlan N, Dufourny L, Skinner DC. Somatostatin-14 neurons in the ovine hypothalamus: colocalization with estrogen receptor alpha and somatostatin-28(1-12) immunoreactivity, and activation in response to estradiol. Biol Reprod. 2003;69(4):1318–1324. [DOI] [PubMed] [Google Scholar]
- 19. Fergani C, Routly JE, Jones DN, Pickavance LC, Smith RF, Dobson H. Activation of cells containing estrogen receptor alpha or somatostatin in the medial preoptic area, arcuate nucleus, and ventromedial nucleus of intact ewes during the follicular phase, and alteration after lipopolysaccharide. Biol Reprod. 2014;91(6):141. [DOI] [PubMed] [Google Scholar]
- 20. Van Vugt HH, Swarts HJ, Van de Heijning BJ, Van der Beek EM. Centrally applied somatostatin inhibits the estrogen-induced luteinizing hormone surge via hypothalamic gonadotropin-releasing hormone cell activation in female rats. Biol Reprod. 2004;71(3):813–819. [DOI] [PubMed] [Google Scholar]
- 21. McCosh RB, Szeligo BM, Bedenbaugh MN, et al. Evidence that endogenous somatostatin inhibits episodic, but not surge, secretion of LH in female sheep. Endocrinology. 2017;158(6):1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16(4):427–442. [DOI] [PubMed] [Google Scholar]
- 23. Bhattarai JP, Kaszás A, Park SA, et al. Somatostatin inhibition of gonadotropin-releasing hormone neurons in female and male mice. Endocrinology. 2010;151(7):3258–3266. [DOI] [PubMed] [Google Scholar]
- 24. Koyama M, Yin C, Ishii H, Sakuma Y, Kato M. Somatostatin inhibition of GnRH neuronal activity and the morphological relationship between GnRH and somatostatin neurons in rats. Endocrinology. 2012;153(2):806–814. [DOI] [PubMed] [Google Scholar]
- 25. Rotsztejn WH, Drouva SV, Epelbaum J, Kordon C. Somatostatin inhibits in vitro release of luteinizing hormone releasing hormone from rat mediobasal hypothalamic slices. Experientia. 1982;38(8):974–975. [DOI] [PubMed] [Google Scholar]
- 26. Pillon D, Caraty A, Fabre-Nys C, Lomet D, Cateau M, Bruneau G. Regulation by estradiol of hypothalamic somatostatin gene expression: possible involvement of somatostatin in the control of luteinizing hormone secretion in the ewe. Biol Reprod. 2004;71(1):38–44. [DOI] [PubMed] [Google Scholar]
- 27. Sugimoto A, Tsuchida H, Ieda N, et al. Somatostatin-somatostatin receptor 2 signaling mediates LH pulse suppression in lactating rats. Endocrinology. 2019;160(2):473–483. [DOI] [PubMed] [Google Scholar]
- 28. Dhandapani KM, Brann DW. The role of glutamate and nitric oxide in the reproductive neuroendocrine system. Biochem Cell Biol. 2000;78(3):165–179. [PubMed] [Google Scholar]
- 29. Hanchate NK, Parkash J, Bellefontaine N, et al. Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. J Neurosci. 2012;32(3):932–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hellier V, Brock O, Candlish M, et al. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat Commun. 2018;9(1):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zou S, Somvanshi RK, Paik S, Kumar U. Colocalization of cannabinoid receptor 1 with somatostatin and neuronal nitric oxide synthase in rat brain hypothalamus. J Mol Neurosci. 2015;55(2):480–491. [DOI] [PubMed] [Google Scholar]
- 32. Okamura H, Yokosuka M, McEwen BS, Hayashi S. Colocalization of NADPH-diaphorase and estrogen receptor immunoreactivity in the rat ventromedial hypothalamic nucleus: stimulatory effect of estrogen on NADPH-diaphorase activity. Endocrinology. 1994;135(4):1705–1708. [DOI] [PubMed] [Google Scholar]
- 33. Chachlaki K, Malone SA, Qualls-Creekmore E, et al. Phenotyping of nNOS neurons in the postnatal and adult female mouse hypothalamus. J Comp Neurol. 2017;525(15):3177–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842. [DOI] [PubMed] [Google Scholar]
- 35. Watson RE Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7(1):155–159. [DOI] [PubMed] [Google Scholar]
- 36. Robinson J. Prenatal programming of the female reproductive neuroendocrine system by androgens. Reproduction. 2006;132(4):539–547. [DOI] [PubMed] [Google Scholar]
- 37. Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological plasticity in the neural circuitry responsible for seasonal breeding in the ewe. Endocrinology. 2006;147(10):4843–4851. [DOI] [PubMed] [Google Scholar]
- 38. Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. κ-Opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157(6):2367–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153(6):2756–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. RRID:AB_572248 https://scicrunch.org/resolver/AB_572248.
- 42. RRID:AB_518614 https://scicrunch.org/resolver/AB_518614.
- 43. RRID:AB_477523 https://scicrunch.org/resolver/AB_477523.
- 44. Harlow E, Lane D. Mounting samples in Gelvatol or Mowiol. CSH protocols. 2006. doi: 10.1101/pdb.prot4461. [DOI] [PubMed] [Google Scholar]
- 45. Merkley CM, Porter KL, Coolen LM, et al. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. RRID:AB_2716809 https://scicrunch.org/resolver/AB_2716809.
- 47. RRID:AB_572256 https://scicrunch.org/resolver/AB_572256.
- 48. Molgaard S, Ulrichsen M, Boggild S, et al. Immunofluorescent visualization of mouse interneuron subtypes. F1000Res. 2014;3:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. RRID:AB_2106783 https://scicrunch.org/resolver/AB_2106783.
- 50. RRID:AB_2255365 https://scicrunch.org/resolver/AB_2255365.
- 51. Castro M, Muñoz JM, Arruebo MP, et al. Involvement of neuronal nitric oxide synthase (nNOS) in the regulation of migrating motor complex (MMC) in sheep. Vet J. 2012;192(3):352–358. [DOI] [PubMed] [Google Scholar]
- 52. Skinner DC, Harris TG, Evans NP. Duration and amplitude of the luteal phase progesterone increment times the estradiol-induced luteinizing hormone surge in ewes. Biol Reprod. 2000;63(4):1135–1142. [DOI] [PubMed] [Google Scholar]
- 53. Goodman RL, Coolen LM, Anderson GM, et al. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 54. Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology. 1980;107(5):1286–1290. [DOI] [PubMed] [Google Scholar]
- 55. Stormshak F, Estill CT, Resko JA, Roselli CE. Changes in LH secretion in response to an estradiol challenge in male- and female-oriented rams and in ewes. Reproduction. 2008;135(5):733–738. [DOI] [PubMed] [Google Scholar]
- 56. Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127(3):1375–1384. [DOI] [PubMed] [Google Scholar]
- 57. Robinson JE, Grindrod J, Jeurissen S, Taylor JA, Unsworth WP. Prenatal exposure of the ovine fetus to androgens reduces the proportion of neurons in the ventromedial and arcuate nucleus that are activated by short-term exposure to estrogen. Biol Reprod. 2010;82(1):163–170. [DOI] [PubMed] [Google Scholar]
- 58. Porter KL, Hileman SM, Hardy SL, Nestor CC, Lehman MN, Goodman RL. Neurokinin-3 receptor activation in the retrochiasmatic area is essential for the full pre-ovulatory luteinising hormone surge in ewes. J Neuroendocrinol. 2014;26(11):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rawson JA, Scott CJ, Pereira A, Jakubowska A, Clarke IJ. Noradrenergic projections from the A1 field to the preoptic area in the brain of the ewe and Fos responses to oestrogen in the A1 cells. J Neuroendocrinol. 2001;13(2):129–138. [DOI] [PubMed] [Google Scholar]
- 60. Jackson GL. Effect of adrenergic blocking drugs on secretion of luteinizing hormone in the ovariectomized ewe. Biol Reprod. 1977;16(4):543–548. [PubMed] [Google Scholar]
- 61. Bedenbaugh MN, O’Connell RC, Lopez JA, McCosh RB, Goodman RL, Hileman SM. Kisspeptin, gonadotrophin-releasing hormone and oestrogen receptor alpha colocalise with neuronal nitric oxide synthase neurones in prepubertal female sheep. Journal of Neuroendocrinology. 2018. doi: 10.1111/jne.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology. 2002;143(7):2767–2774. [DOI] [PubMed] [Google Scholar]
- 63. Bellefontaine N, Hanchate NK, Parkash J, et al. Nitric oxide as key mediator of neuron-to-neuron and endothelia-to-glia communication involved in the neuroendocrine control of reproduction. Neuroendocrinology. 2011;93(2):74–89. [DOI] [PubMed] [Google Scholar]
- 64. Chachlaki K, Garthwaite J, Prevot V. The gentle art of saying NO: how nitric oxide gets things done in the hypothalamus. Nat Rev Endocrinol. 2017;13(9):521–535. [DOI] [PubMed] [Google Scholar]
- 65. Clasadonte J, Poulain P, Beauvillain JC, Prevot V. Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin-releasing hormone neurons: a possible local synchronizing signal. Endocrinology. 2008;149(2):587–596. [DOI] [PubMed] [Google Scholar]
- 66. Dufourny L, Lomet D. Crosstalks between kisspeptin neurons and somatostatin neurons are not photoperiod dependent in the ewe hypothalamus. Gen Comp Endocrinol. 2017;254:68–74. [DOI] [PubMed] [Google Scholar]
- 67. Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology. 2003;144(8):3663–3676. [DOI] [PubMed] [Google Scholar]
- 68. Xiong JJ, Karsch FJ, Lehman MN. Evidence for seasonal plasticity in the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in synaptic inputs onto GnRH neurons. Endocrinology. 1997;138(3):1240–1250. [DOI] [PubMed] [Google Scholar]
- 69. Smith JT, Coolen LM, Kriegsfeld LJ, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]