Abstract
Background
Pulmonary hypertension (PH) is a major comorbidity of chronic obstructive pulmonary disease (COPD). However, the association of PH detected by echocardiography and COPD-related outcome in longitudinal follow-up has not been elucidated. In this study, we aimed to investigate the relationship between clinical characteristics of COPD patients with PH detected by echocardiography and various outcome parameters such as COPD exacerbation and health status over a three-year observation period.
Methods
In this observational study, we analyzed patients with COPD who underwent chest computed tomography and echocardiography at baseline (n = 183).
Results
The prevalence of PH was 21.9% (40 patients). The median estimated systolic pulmonary artery pressure in patients with PH was 38.8 mmHg. COPD patients with PH were older, had a lower body mass index, scored worse in the COPD Assessment Test and St. George’s Respiratory Questionnaire, and exhibited a lower diffusing capacity of the lung for carbon monoxide in comparison to patients without PH. In computed tomography images, the percentages of low-attenuation areas (LAA%) and interstitial abnormalities were higher in COPD patients with PH than in those without PH. Higher values for LAA% (LAA ≥ 30%) and interstitial abnormalities independently increased the risk of PH. The ratio of main pulmonary diameter to aortic artery diameter was significantly correlated with estimated systolic pulmonary artery pressure. In the follow-up analysis, the frequency of exacerbations in three years was significantly higher in patients with PH compared to patients without PH.
Conclusion
In this study, we identified the clinical characteristics of COPD patients with PH detected by echocardiography. The presence of PH assessed by echocardiography was related to future COPD exacerbations and closely related to radiographical emphysema.
Keywords: comorbidity, COPD, echocardiography, pulmonary hypertension
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death in the world.1 Although COPD is defined by the presence of chronic airflow limitation, recent epidemiological studies have shown that COPD is frequently associated with comorbidities that modify disease expression, disease burden, and survival.2,3 Previously, we reported the importance of various comorbidities in Japanese COPD patients.4–7
Pulmonary hypertension (PH) is a serious comorbidity of COPD8 because it is associated with increased risk of hospitalization,9 decreased exercise capacity,10 and survival.11 Right-heart catheterization (RHC) remains the “gold standard” for the diagnosis of PH, but there are significant risks12 and cost issues associated with this procedure. Thus, it is difficult to justify an RHC in all COPD patients, especially in cases with mild-to-moderate disease. Actually, most reports about PH in COPD patients using RHC enrolled patients with severe airway obstruction such as lung-volume-reduction surgery13 or lung transplants.14
Transthoracic echocardiography is used as a substitute screening tool in suspected PH15 to estimate the pulmonary artery pressure from continuous-wave Doppler measurements.15 The agreement between the pulmonary pressure values determined by RHC and echocardiographic measurements has been confirmed in vascular heart diseases and PH.16,17 However, most studies on PH in COPD patients used RHC for its diagnosis, and only a few reports about clinical characteristics of COPD patients used echocardiography to diagnose PH.18–20 Furthermore, neither the association between PH detected by echocardiography and chest-related disorders such as emphysema or airway diseases verified by computed tomography (CT) nor the influence of PH presence on COPD-related outcomes in longitudinal follow-up studies has been elucidated. Thus, the aim of this study is to investigate the clinical characteristics of COPD patients with PH detected by echocardiography, as well as the association of PH with chest CT parameters and some outcomes, such as COPD exacerbation and health status, over a three-year observation period.
Materials and Methods
Study Population
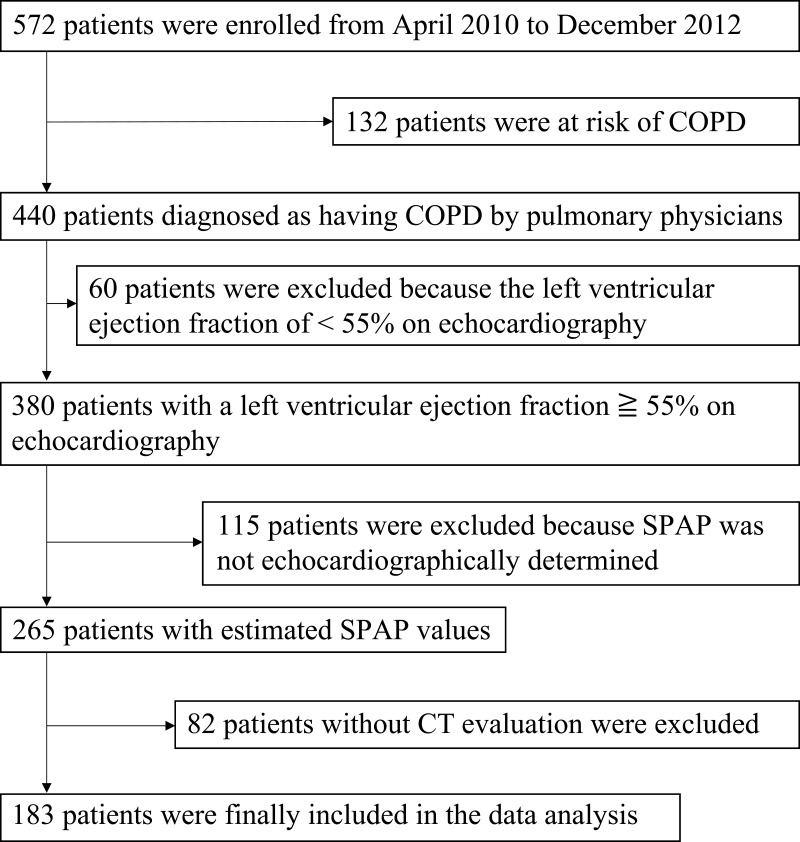
The overall design of the Keio COPD Comorbidity Research (K-CCR) has been published previously.4,21 The current study was a three-year, prospective, observational study that enrolled 572 men and women, aged 40–91 years, diagnosed by pulmonary physicians between April 2010 and December 2012 with COPD (n = 440) or as being at risk of COPD (n = 132). Data of COPD patients who underwent CT and echocardiography at baseline were analyzed (Figure 1). All patients were clinically stable at all assessments and had no exacerbations for at least one month before enrollment. Patients whose left ventricular ejection fraction (LVEF) was on echocardiography <55% (n = 60), or the tricuspid regurgitant jet could not be identified on echocardiography (n = 115) were excluded20; finally, the datasets of 183 patients were analyzed. Written informed consent for the use of data was obtained from each patient, and the study (University Hospital Medical Information Network; UMIN000003470) was approved by the ethics committees of Keio University and its affiliated hospitals (20090008). All aspects of this study conformed to the tenets of the Declaration of Helsinki.
Figure 1.
Process of patient selection in this study. Only data from COPD patients with spirometrically confirmed COPD (FEV1/FVC < 0.7), as well as CT and echocardiography measurements at baseline, were selected and analyzed.
Abbreviations: COPD, chronic obstructive pulmonary disease; SPAP, systolic pulmonary artery pressure; CT, computed tomography; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Assessment of Clinical Parameters
At enrollment and annually, a full medical and smoking history, including exacerbations22 and comorbidities4,21,22 was obtained. Spirometry was performed in all patients using an electronic spirometer (CHESTAC-9800; CHEST, Tokyo, Japan) according to the American Thoracic Society guidelines.23 Lung volumes were obtained using body plethysmography. Blood samples were analyzed for the assessment of leukocytes, C-reactive protein (CRP), and brain natriuretic peptide (BNP). The Japanese version of the COPD assessment test (CAT),24 the St. George’s Respiratory Questionnaire (SGRQ),25–27 and the Study Short-Form 36-Item (SF-36) version228 were completed at baseline and annually thereafter.
Echocardiography
Echocardiography was performed by four well-trained laboratory technicians, who each had at least 15 years of work experience. Echocardiography measurements were obtained using two commercially available echocardiography systems (GE Vivid7/Vivid9; GE Healthcare, Horten, Norway; and iE33/Sonos7500; Philips, Amsterdam, Netherlands). A 2.5-MHz transducer was used to obtain the images in the parasternal and apical views, corresponding to the standard long-axis, and two-chamber and four-chamber images, respectively. Standard two-dimensional and color Doppler data were collected. The estimated systolic pulmonary artery pressure (eSPAP) was calculated according to previously published guidelines.29 In summary, right atrial pressure was estimated to be 3, 8, or 15 mmHg from the diameter of the inferior vena cava (IVC) and the presence or absence of collapse with sniff. Then, the pressure gradient of the tricuspid valve estimated from the peak velocity of tricuspid regurgitation was added to the estimated right atrial pressure to calculate eSPAP. PH was defined by an eSPAP ≥36 mmHg.20
Assessment of Low-Attenuation Areas and Airway Wall Thickness on Chest CT
High-resolution CT was performed using four multi-detector CT scanners. All subjects underwent a volumetric CT at full inspiration and at the end of normal expiration on four CT scanners that were calibrated using a phantom at the start of the study.7 The emphysema extent was quantified as the ratio of the low-attenuation area to the total lung volume (LAA%) with Hounsfield units <−950 using a custom-made software (AZE, Ltd., Tokyo, Japan).7 The presence of an emphysema was defined as LAA% ≥10%, and it was classified as mild (≥10% to <20%), moderate (≥20% to <30%), or severe (≥30%).7,30 The percentage of airway wall area (WA%) was assessed in identified bronchi, as reported previously.31 Interstitial abnormalities were assessed using the sequential reading method.32 The main pulmonary artery diameter was measured at the widest portion of the pulmonary artery within 3 cm of the bifurcation. The descending aortic artery diameter was also measured on the same slice, and the ratio of main pulmonary artery diameter to aortic artery diameter (PA/Ao) was calculated.33
Statistical Analysis
Data are presented as the mean ± standard deviation (SD) or as the median (interquartile range [IQR]). Data between the two groups were compared using Student’s t-test, Mann–Whitney U-test, or χ2 test. Univariate and multivariate logistic regression analyses were performed to assess factors related to the presence of a PH. Receiver operating characteristics (ROC) curves were constructed to assess the areas under the curves (AUCs). We investigated the optimal cutoff value by maximizing the Youden index. Correlations between continuous variables were evaluated using Spearman’s rank correlation coefficient. For all tests, two-sided p-values <0.05 were considered significant. Data were analyzed using JMP 14 software (SAS Institute, Cary, NC).
Results
Clinical Features of the Study Population
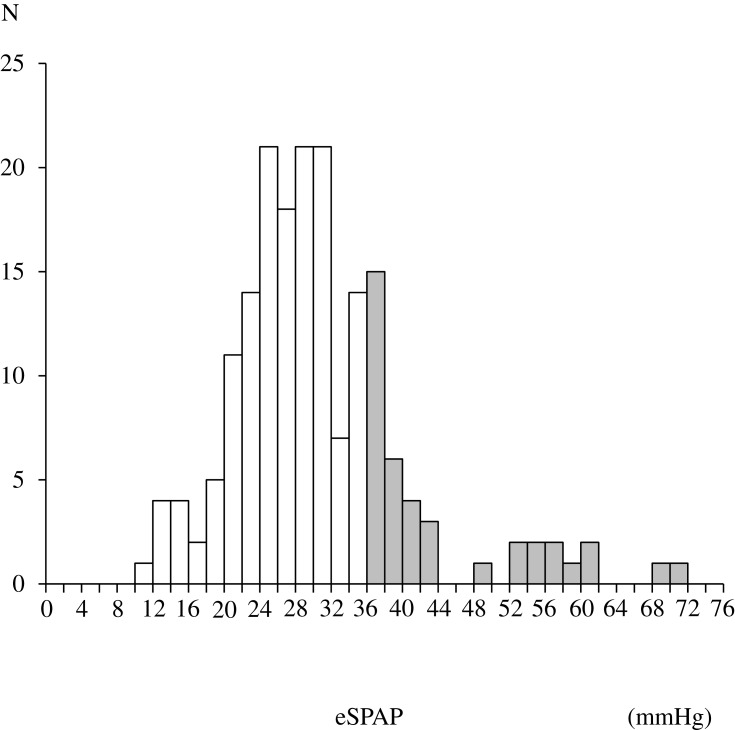
Table 1 shows the baseline characteristics of the study population. The median age of COPD patients was 74 years. The numbers of COPD patients according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades 1, 2, 3, and 4 were 56, 84, 34, and 9, respectively. COPD patients whose tricuspid regurgitant jet could not be identified on echocardiography (n = 115) had lower %FEV1 compared to those with estimated eSPAP (n=183) (p = 0.03). PH was present in 21.9% of the patients with estimated eSPAP. The distribution of eSPAP values in the population with PH is shown in Figure 2. The median eSPAP was 38.8 mmHg in COPD patients with PH.
Table 1.
Baseline Characteristics of the Study Population
| COPD Patients with Estimated eSPAP | COPD Patients Without Estimated eSPAP | p-value | |
|---|---|---|---|
| N = 183 | N=115 | ||
| Age, years | 74 (67–79) | 72(67–77) | 0.07 |
| Sex, female, n (%) | 23 (12.6) | 8(7.0) | 0.36 |
| Smoking index, pack-years | 46.5 (35.3–70.1) | 51(38–76.1) | 0.64 |
| Current smokers, n (%) | 17 (9.3) | 17(15.0) | 0.09 |
| FEV1/FVC, % | 55.2 (42.3–64.2) | 53.6(44.4–62.6) | 0.94 |
| %FEV1, % | 66.7 ± 21.7 | 58.2 ± 21.0 | 0.03 |
| COPD gradea 1/2/3/4, n (%) | 56/84/34/9 (30.6/45.9/18.6/4.9) | 17/55/31/12 (14.8/47.8/27.0/10.4) | 0.09 |
Notes: Data are presented as the mean ± SD, median (IQR), or number (%). aDefined by the Global Initiative for Chronic Obstructive Lung Disease.
Abbreviations: PH, pulmonary arterial hypertension; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; %FEV1, forced expiratory volume in 1 second as a percentage of the predicted forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; SD, standard deviation; IQR, interquartile range.
Figure 2.
Distribution of eSPAP in the study population with PH assessed by echocardiography. Shaded bars indicate patients with PH.
Abbreviations: eSPAP, estimated systolic pulmonary artery pressure; PH, pulmonary arterial hypertension.
Characteristics of COPD Patients with PH Assessed by Echocardiography
The comparison of patient characteristics stratified by the presence of PH is shown in Table 2. Patients with PH were significantly older (75.5 years vs 73.0 years, p = 0.04) and had a lower body mass index (BMI; 21.6 ± 3.0 vs 22.8 ± 3.0, p = 0.02) than patients without PH. The BNP values were higher in patients with PH compared to those without PH (33.1 pg/mL vs 24.2 pg/mL, p = 0.01). There were no differences in the ratio of patients receiving long-term oxygen therapy, prescription of vasodilators, serum leucocytes, CRP levels, and LVEF by echocardiography between the two groups. The ratio of patients prescribed diuretics was higher in patients with PH compared to those without PH (5.6% vs 0.0%, p = 0.04).
Table 2.
Comparison of the Characteristics Stratified by the Presence of PH
| COPD Patients Without PH | COPD Patients with PH | p-value | |
|---|---|---|---|
| N | 143 | 40 | |
| Pulmonary artery pressure, mmHga | 27.0 (23.0–30.0) | 38.8 (36.0–53.0) | <0.01 |
| Age, yearsa | 73.0 (67.0–79.0) | 75.5 (71.3–80.0) | 0.04 |
| Sex, female, n (%)c | 17 (11.9) | 6 (15.0) | 0.60 |
| Smoking index, pack-yearsa | 46.0 (35.2–70.1) | 51.0 (37.9–68.9) | 0.84 |
| Current smokers, n (%)c | 15 (10.7) | 2 (5.1) | 0.29 |
| Cancer, n (%)c | 24 (21.4) | 11 (30.6) | 0.26 |
| Coronary artery disease, n (%)c | 15 (13.4) | 2 (5.6) | 0.20 |
| LTOT, n (%)c | 9 (11.0) | 2 (7.4) | 0.59 |
| Prescription of diuretics, n (%)c | 0(0%) | 8(5.6%) | 0.04 |
| Prescription of vasodilators, n (%)c | 0(0%) | 1(0.7%) | 0.48 |
| SpO2, %a | 96.0 (95.0–97.0) | 96.0 (95.0–97.0) | 0.75 |
| BMI, kg/m2b | 22.8 ± 3.0 | 21.6 ± 3.0 | 0.02 |
| CRP, mg/dla | 0.06 (0.03–0.20) | 0.07 (0.03–0.30) | 0.28 |
| WBC,/µLa | 6000 (5100–7400) | 6000 (5025–8000) | 0.60 |
| BNP, pg/mLa | 24.2 (11.9–47.2) | 33.1 (18.5–92.8) | 0.01 |
| LVEF, %a | 76.0 (72.0–80.0) | 74.0 (71.0–81.8) | 0.60 |
| IVC, cma | 1.3 (1.1–1.5) | 1.3 (1.0–1.4) | 0.97 |
| LVEDD, cmb | 4.4 ± 0.5 | 4.4 ± 0.7 | 0.96 |
| LVESD, cma | 2.7 (2.4–3.0) | 2.7 (2.4–3.2) | 0.64 |
Notes: Data are presented as the mean ± SD, median (IQR), or number (%). Data were compared between two groups using aMann–Whitney U-test, bStudent’s t-test, and cχ2 test.
Abbreviations: PH, pulmonary arterial hypertension; COPD, chronic obstructive pulmonary disease; LTOT, long-term oxygen therapy; SpO2, oxygen saturation of peripheral artery; BMI, body mass index; CRP, C-reactive protein; WBC, white blood cell count; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; IVC, inferior vena cava; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; SD, standard deviation; IQR, interquartile range.
Association of PH Assessed by Echocardiography and Health Status in COPD Patients
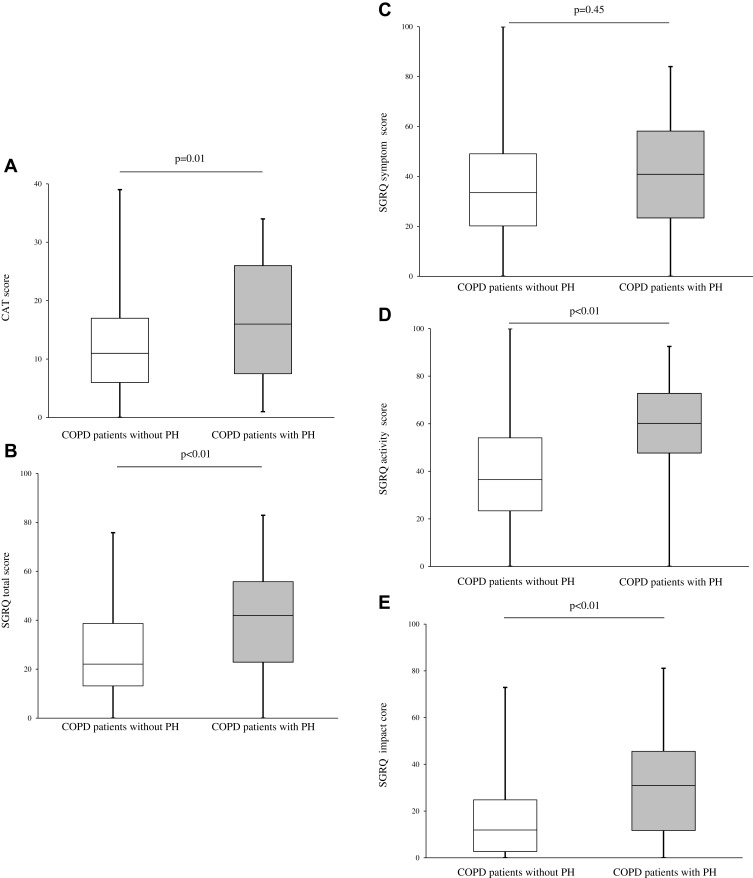
At baseline, COPD patients with PH presented a significantly higher total CAT score compared to those without PH (16.0 vs 11.0, p = 0.01; Figure 3A). Additionally, the SGRQ components total score, activity, and impact were significantly higher in COPD patients with PH compared to those without PH (total, 42.0 vs 22.1, p < 0.01; activity, 60.2 vs 36.5, p < 0.01; impact, 31.0 vs 11.9, p < 0.01; Figure 3B–E). The differences of CAT scores and SGRQ total scores between the groups with statistical difference exceeded 2 and 4 units, respectively.34,35 In the SF-36 at baseline, the items physical functioning, role physical, general health, and role emotional were worse in COPD patients with PH compared to those without PH (Supplementary Figure 1). The follow-up analysis showed that there was no significant difference in the annual change in CAT score (ΔCAT score/year, 0.2 vs 0.5, p = 0.78) and SGRQ total score (ΔSGRQ total score/year, 0.040 vs 0.002, p = 0.40) between the two groups (Supplementary Figure 2).
Figure 3.
Association of PH assessed by echocardiography and health status in COPD patients. (A) Comparison of baseline CAT scores between the two groups. (B–E) Comparison of baseline SGRQ scores between the two groups. Data were compared between groups using the Mann–Whitney U-test.
Abbreviations: PH, pulmonary arterial hypertension; COPD, chronic obstructive pulmonary disease; CAT, COPD assessment test; SGRQ, St. George’s respiratory questionnaire.
Lung Function Parameters in COPD Patients with PH Assessed by Echocardiography
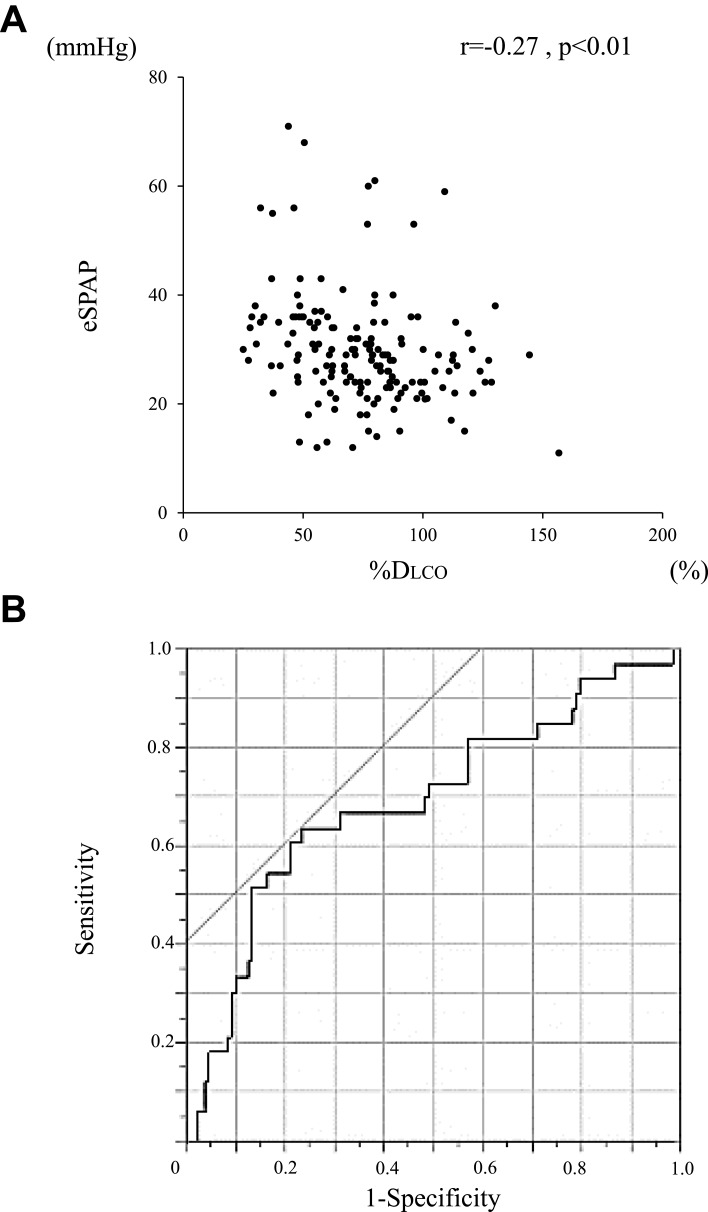
Comparison of lung function parameters between COPD patients with PH and those without PH is shown in Table 3. The forced expiratory volume in 1 second (FEV1) and the ratio inspiratory capacity (IC)/total lung capacity (TLC) were significantly lower in COPD patients with PH compared to those without PH (%FEV1, 68.1 ± 20.8% vs 61.4 ± 24.2%, p = 0.09; IC/TLC, 0.43 ± 0.08 vs 0.39 ± 0.07, p = 0.02). The residual volume ratio (RV)/TLC tended to be higher in COPD patients with PH compared to those without PH (0.39 (0.32–0.45) vs 0.34 (0.31–0.40), p = 0.06). The predicted diffusing capacity (%DLCO) was also significantly lower in COPD patients with PH compared to those without PH (61.6 ± 24.9% vs 77.9 ± 25.1%, p < 0.01). %DLCO was significantly correlated with eSPAP in the total study population (r = −0.27, p < 0.01; Figure 4A). ROC curves showed that the optimal %DLCO cutoff value for predicting the presence of PH was 60.2% (AUC, 0.69; sensitivity, 63.6%; specificity, 76.7%; Figure 4B).
Table 3.
Comparison of Lung Function Parameters Between COPD Patients with and Without PH
| COPD Patients Without PH | COPD Patients with PH | p-value | |
|---|---|---|---|
| N | 143 | 40 | |
| FEV1a | 1830 (1320–2250) | 1410 (912.5–1995.0) | <0.01 |
| %FEV1b | 68.1 ± 20.8 | 61.4 ± 24.2 | 0.09 |
| %DLCOb | 77.9 ± 25.1 | 61.6 ± 24.9 | <0.01 |
| %TLCb | 101.2 ± 14.7 | 98.8 ± 13.5 | 0.40 |
| %ICb | 117.3 ± 24.7 | 110.9 ± 24.4 | 0.20 |
| IC/TLCb | 0.43 ± 0.08 | 0.39 ± 0.07 | 0.02 |
| %RVa | 95.1 (82.4–112.8) | 101.1 (77.3–115.8) | 0.93 |
| RV/TLCa | 0.39 (0.32–0.45) | 0.34 (0.31–0.40) | 0.06 |
Notes: Data are presented as the mean ± SD or median (IQR). Data were compared between groups using athe Mann–Whitney U-test, or bthe Student’s t-test.
Abbreviations: COPD, chronic obstructive pulmonary disease; PH, pulmonary arterial hypertension; FEV1, forced expiratory volume in 1 second; %FEV1, forced expiratory volume in 1 second as a percentage of predicted forced expiratory volume in 1 second; DLCO, diffusing capacity of the lung for carbon monoxide; TLC, total lung capacity; IC, inspiratory capacity; RV, residual volume; SD, standard deviation; IQR, interquartile range.
Figure 4.
Relationship between PH assessed by echocardiography and lung diffusion capacity. (A) Correlation between eSPAP and %DLCO in COPD patients. (B) ROC curves of %DLCO for the prediction of PH assessed by echocardiography. Correlations between continuous variables were evaluated using Spearman’s rank correlation coefficient.
Abbreviations: PH, pulmonary arterial hypertension; eSPAP, estimated systolic pulmonary artery pressure; DLCO, diffusing capacity of lung carbon monoxide; COPD, chronic obstructive pulmonary disease; ROC, receiver operating characteristic.
Association Between PH Assessed by Echocardiography and Chest CT Abnormalities in COPD Patients
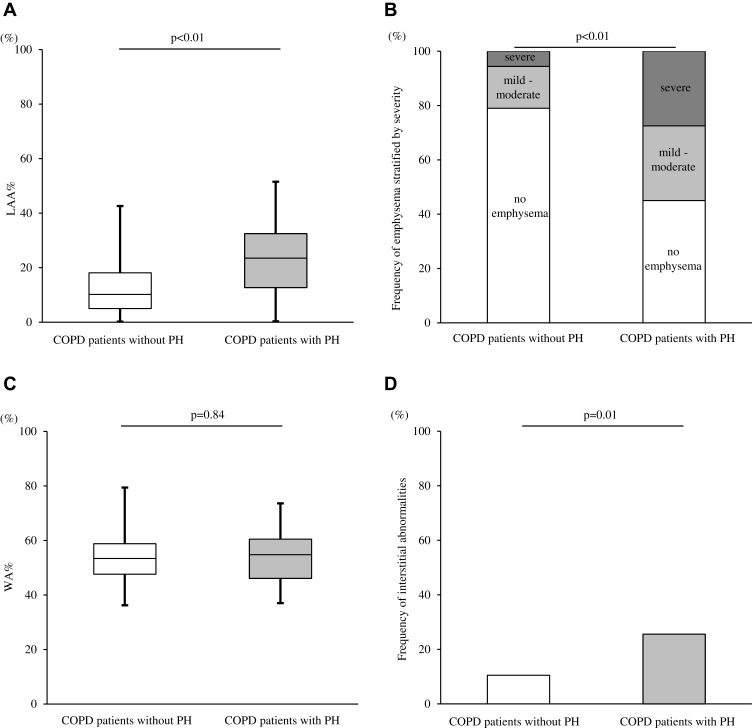
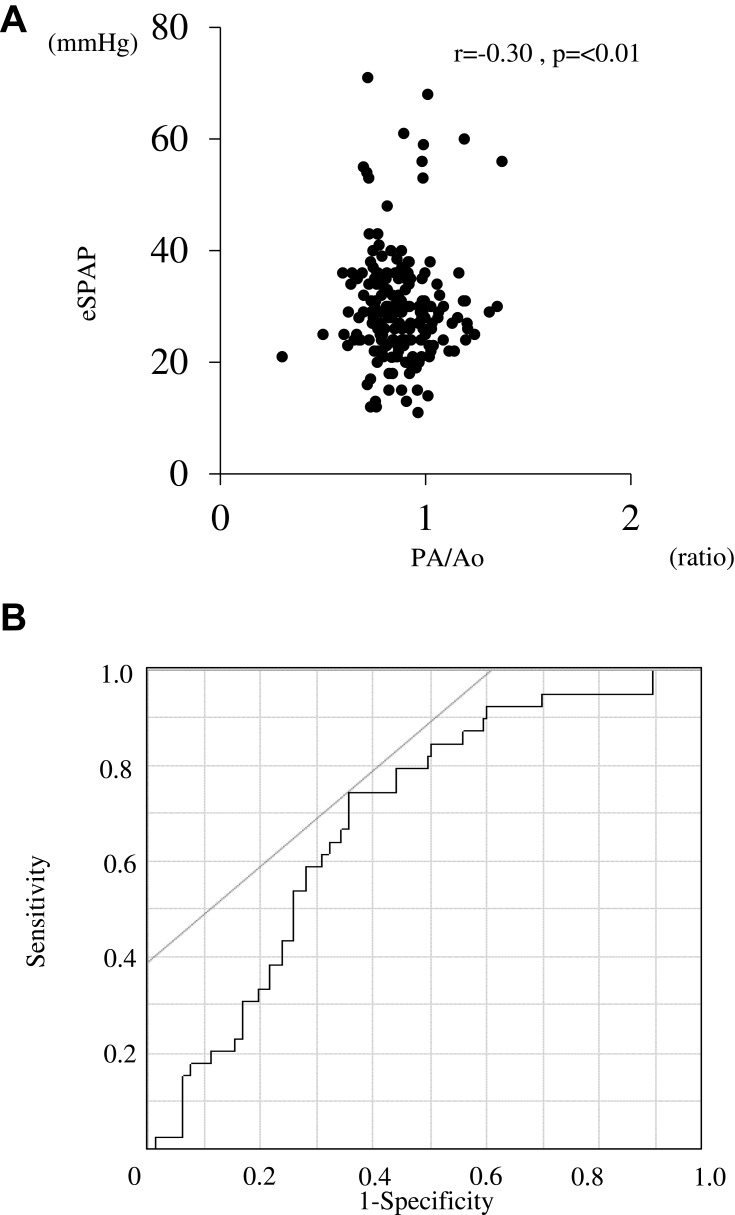
In chest CTs, LAA% was also significantly higher in COPD patients with PH in comparison to those without PH (23.5% vs 10.2%, p < 0.01; Figure 5A). The presence of severe emphysema was also more common in COPD patients with PH compared to those without PH (Figure 5B). On the other hand, WA% did not differ between the two groups (p = 0.84; Figure 5C). The incidence of interstitial abnormalities was higher in patients with PH compared to those without PH (25.6% vs 10.5%, p = 0.01; Figure 5D). The presence of severe emphysema (LAA% ≥30%; odds ratio [OR] = 7.1 [2.5–20.2], p < 0.01) or interstitial lung abnormalities (OR = 3.8 [1.5–9.6], p < 0.01) independently increased the likelihood of a PH presence (Table 4). Trends in LAA% change during the three years were compared between patients with PH and those without PH, but there was no significant difference in the annual change in LAA% (ΔLAA%/year; 0.5% vs 0.5%, p = 0.88; Supplementary Figure 3). PA/Ao was significantly correlated with eSPAP in the total study population (r = −0.30, p<0.01; Figure 6A). ROC curves showed that the optimal PA/Ao cutoff value for predicting the presence of PH was 20.7% (AUC, 0.69; sensitivity, 74.4%; specificity, 64%; Figure 6B).
Figure 5.
Relationship between PH assessed by echocardiography and baseline chest CT abnormalities in COPD patients. (A) Comparison of LAA% between the two groups. (B) Frequencies of emphysema severities in the two groups. (C) Comparison of WA% between the two groups. (D) Frequencies of interstitial abnormalities in the two groups. Data were compared between groups using the Mann–Whitney U-test and χ2 test.
Abbreviations: PH, pulmonary arterial hypertension; CT, computed tomography; COPD, chronic obstructive pulmonary disease; LAA%, ratio of the low-attenuation area to the total lung volume; WA%, percentage of airway wall area.
Table 4.
Predictors for the Presence of PH According to Multivariate Logistic Regression Analysis
| Parameters | Odds Ratio (95% CI) | p-value |
|---|---|---|
| LAA% ≥ 30% | 7.1 (2.5–20.2) | <0.01 |
| Interstitial lung abnormalities | 3.8 (1.5–9.6) | <0.01 |
Abbreviations: PH, pulmonary arterial hypertension; LAA%, ratio of the low-attenuation area to the total lung volume, CI, confidence interval.
Figure 6.
Relationship between PH assessed by echocardiography and ratio of pulmonary artery diameter to aortic artery diameter. (A) Correlation between eSPAP and PA/Ao in COPD patients. (B) ROC curves of PA/Ao for the prediction of PH assessed by echocardiography. Correlations between continuous variables were evaluated using Spearman’s rank correlation coefficient.
Abbreviations: PH, pulmonary arterial hypertension; eSPAP, estimated systolic pulmonary artery pressure; PA/Ao, ratio of pulmonary artery diameter to aortic artery diameter; COPD, chronic obstructive pulmonary disease; ROC, receiver operating characteristic.
Association Between PH Assessed by Echocardiography and COPD Exacerbations Over Three Years
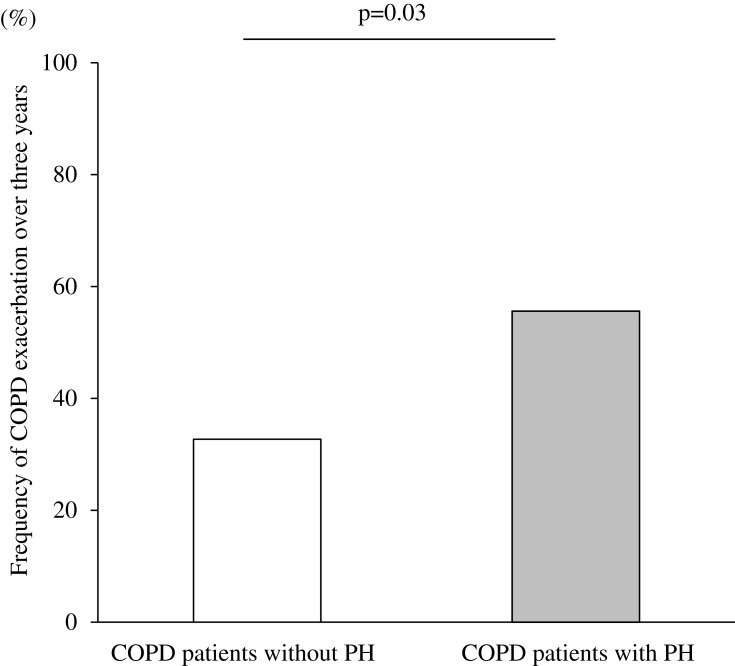
The incidence of moderate-to-severe exacerbations over three years was significantly higher in COPD patients with PH compared to those without PH (55.6 vs 32.7, p = 0.03; Figure 7). Next, we analyzed with the confounder which was reported as a predictor of exacerbation.36 After adjusting for age ≥75 years, sex, and %FEV1 < 50%, the presence of PH increased the likelihood of moderate-to-severe exacerbations over three years (OR, 2.9 [1.1–7.9], p = 0.04).
Figure 7.
Frequency of COPD exacerbations over three years according to the presence or absence of PH assessed by echocardiography. Data were compared between groups using χ2 test.
Abbreviations: COPD, chronic obstructive pulmonary disease; PH, pulmonary arterial hypertension.
Discussion
To the best of our knowledge, this is the first longitudinal study in COPD patients to evaluate the relationships between PH assessed by echocardiography and patient-centered outcomes. In this study, COPD patients with PH assessed by echocardiography presented worse CAT, SGRQ, and SF-36 scores compared to those without PH. In addition, exacerbations were over the three-year study period more frequent in COPD patients with PH than in those without PH. These results suggest that the treatment of PH may improve dyspnea and the overall health status of COPD patients.
We and others reported that COPD exacerbations are associated with a worse health status22 and a poorer prognosis37 of COPD patients. According to our study results, echocardiographically diagnosed PH is related to future COPD exacerbations independently of other known risk factors. This result is consistent with findings of a previous study using RHC in COPD patients; this study showed that a PH diagnosis by RHC increased the risk of a future hospitalization caused by COPD exacerbation.9 The precise link between exacerbations and chronic PH in COPD patients is not known. A previous report demonstrates that some COPD patients have increased pulmonary artery pressure during an exacerbation, and the acute respiratory failure, as well as the pulmonary artery pressure, return to their baselines after the recovery from exacerbation.38 Thus, it can be speculated that repeated exacerbations associated with transient increases in pulmonary artery pressure may lead to chronic PH.8 Whether medication for prevention COPD exacerbations can improve PH and whether PH treatment can reduce COPD exacerbation rates, improve dyspnea, and impact the overall health status is unknown. Future prospective studies are needed.
The current study is the first study showing a relationship between PH assessed by echocardiography and radiographic emphysema in COPD patients. In end-stage COPD patients undergoing lung transplant, the patients with PH showed more severe morphological signs of pulmonary emphysema compared to the patients without PH.39 Common mechanisms linking PH and emphysema induced by cigarette smoking are widely inferred. Increased pulmonary vascular endothelial death and decreased expression of vascular endothelial growth factor (VEGF) have been described in patients with emphysema.40 In addition, chronic blockade of VEGF receptors induces apoptosis of alveolar cells and emphysema in a rat model.41 Recent reports have shown that the nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) signaling pathway plays an important role in PH and emphysema.42–44 In tobacco smoke-exposed mice, inducible nitric oxide synthase inhibitor43 and riociguat,44 which promotes the NO–cGMP pathway, reversed existing lung emphysema and PH. Further, pulmonary vascular resistance and airway resistance have been shown to decrease in COPD patients with PH treated with riociguat.44 Our study also showed that the presence of interstitial abnormalities is a risk factor for PH assessed by echocardiography. This result is in line with previous reports demonstrating a high prevalence of PH in patients with combined pulmonary fibrosis and emphysema.45 The necessity to aggressively screen for PH in COPD patients with emphysema or interstitial abnormalities should be emphasized.
In this study, echocardiography revealed that the PH prevalence was 21.9% in COPD patients with the PH severity ranging from mild to moderate; these results are in line with previous reports showing that out-of-proportion or severe PH is relatively rare (1.1–3.7%).46,47 Currently, there are no conclusive data to support or completely reject the effectiveness of specific PH therapies in COPD patients, especially in mild-to-moderate PH.48 In COPD patients, nocturnal hypoxemia often exists without daytime hypoxemia, and this sleep-related hypoxemia may be related to chronic PH.49,50 Moreover, long-term oxygen therapy improves pulmonary vascular resistance, pulmonary arterial pressure, and stroke volume index in COPD patients.51 Screening of nocturnal hypoxemia and LTOT in COPD patients with mild-to-moderate PH may improve the clinical outcome of COPD patients.
In this study, eSPAP was measurable in 69.7% of all COPD patients; this is a relatively higher percentage compared to previously reported values that eSPAP can be determined by echocardiography in about 40% of the COPD patients.13,52 The reason for this discrepancy might be differences in COPD severity between this study and previous studies. In our study, the severity was relatively low, with GOLD grade 1 or 2 in almost half of the study population. Since lung hyperinflation seen in higher GOLD grades prevents the optimal visualization of the heart by echocardiography, this might be the reason for the relatively higher percentage of patients with measurable eSPAP value seen in our study in comparison to previous publications.13,52 Pulmonary artery diameter in chest CT has been reported as a useful diagnostic tool for pulmonary hypertension diagnosed by RHC.53 In our study, pulmonary artery diameter was correlated with eSPAP values determined by echocardiography. Based on the results of our study, echocardiography may be a useful tool for the screening of PH in COPD patients with mild severity, but the correlation of eSPAP values determined by echocardiography and RHC parameters in these patients remains unknown.
Our study had several limitations. First, although the presence of PH was associated with future COPD exacerbations, other outcomes such as hospitalization and mortality could not be evaluated. Previous reports show that PH diagnosed by RHC increase mortality54 and hospitalization due to exacerbations,9 but the influence of PH assessed by echocardiography on these outcomes is unknown. Second, the dropout rate in this study population was 15.6%, and we could not follow up the dropped-out patients. Moreover, patients whose tricuspid regurgitant jet could not be identified on echocardiography were excluded. Therefore, the impact of PH presence on health status and exacerbation rate might be underestimated. Third, the average age of our study participants was higher than that in other COPD cohort studies.55 In the present study, patients with PH were older compared to those without PH; thus, the prevalence of PH assessed by echocardiography might be overestimated.
Conclusions
COPD patients with PH assessed by echocardiography were older, had a lower BMI, and presented with a worse health status compared to those without PH. The presence of PH assessed by echocardiography was related to future COPD exacerbations in COPD patients, and emphysema was closely related to PH assessed by echocardiography.
Acknowledgments
The authors would like to acknowledge Tomoko Betsuyaku and Chiyomi Uemura for their contributions to collecting data and analysis. The authors acknowledge all the members of the K-CCR group who participated in this study, including Saiseikai Utsunomiya Hospital, Eiju General Hospital, Tokyo Saiseikai Central Hospital, Sano Public Welfare General Hospital, Nihon Kokan Hospital, Saitama Social Insurance Hospital, Kawasaki City Ida Hospital, Saitama City Hospital, Tokyo Medical Center, Tokyo Dental College Ichikawa General Hospital, Tokyo Electric Power Company Hospital, and the International Medical Welfare College Shioya Hospital.
Abbreviations
%DLCO, predicted diffusing capacity of the lung for carbon monoxide; %FEV1, predicted forced expiratory volume in 1 second; AUC, area under the curve; BMI, body mass index; BNP, brain natriuretic peptide; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CT, computed tomography; FEV1, forced expiratory volume in 1 second; eSPAP, estimated systolic pulmonary artery pressure; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IC, inspiratory capacity; IQR, interquartile range; K-CCR, Keio COPD Comorbidity Research; LAA%, ratio of the low-attenuation area to the total lung volume; LTOT, long-term oxygen therapy; LVEF, left ventricular ejection fraction; OR, odds ratio; PH, pulmonary hypertension; RHC, right-heart catheterization; ROC, receiver operating characteristics; SD, standard deviation; SF-36, Short-Form 36-Item; SGRQ, St. George’s respiratory questionnaire; TLC, total lung capacity; VEGF, vascular endothelial growth factor; WA%, percentage of airway wall area.
Author Contributions
Shingo Nakayama participated in the design of the study, performed the statistical analyses, and was a major contributor in writing the manuscript. Shotaro Chubachi planned the study design and contributed to interpretation of results. Hidetoshi Nakamura and Koichiro Asano conceived the study, participated in its design and coordination, and helped to draft the manuscript. Kaori Sakurai, Hidehiro Irie, Akihiro Tsutsumi, Mizuha Hashiguchi, Yuji Itabashi, Mitsushige Murata, and Koichi Fukunaga contributed to collection of data, interpretation of results and helped to draft the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Dr Koichiro Asano reports personal fees from Teijin Pharma Ltd, Novartis Pharma, Sanofi, Astellas Pharma, AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Kyorin Pharma, and MSD, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2019. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed March25, 2019.
- 2.Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1:73–83. doi: 10.1016/S2213-2600(12)70060-7 [DOI] [PubMed] [Google Scholar]
- 3.Vanfleteren L, Spruit MA, Wouters EFM, Franssen FME. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med. 2016;4:911–924. doi: 10.1016/S2213-2600(16)00097-7 [DOI] [PubMed] [Google Scholar]
- 4.Chubachi S, Sato M, Kameyama N, et al; Keio COPD Combordity Research (K-CCR) group. Identification of five clusters of comorbidities in a longitudinal Japanese chronic obstructive pulmonary disease cohort. Respir Med. 2016;117:272–279. doi: 10.1016/j.rmed.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 5.Irie H, Chubachi S, Sato M, et al. Impact of cataract on health-related quality of life in a longitudinal Japanese chronic obstructive pulmonary cohort. Chron Respir Dis. 2018;15:329–338. doi: 10.1177/1479972317745735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chubachi S, Takahashi S, Tsutsumi A, et al. Radiologic features of precancerous areas of the lungs in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1613–1624. doi: 10.2147/COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chubachi S, Nakamura H, Sasaki M, et al; Keio COPD Combordity Research(K-CCR) Group. Polymorphism of LRP5 gene and emphysema severity are associated with osteoporosis in Japanese patients with or at risk for COPD. Respirology. 2015;20:286–295. doi: 10.1111/resp.12429 [DOI] [PubMed] [Google Scholar]
- 8.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32:1371–1385. doi: 10.1183/09031936.00015608 [DOI] [PubMed] [Google Scholar]
- 9.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:158–164. doi: 10.1164/ajrccm.159.1.9803117 [DOI] [PubMed] [Google Scholar]
- 10.Sims MW, Margolis DJ, Localio AR, Panettieri RA, Kawut SM, Christie JD. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest. 2009;136:412–419. doi: 10.1378/chest.08-2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oswald-Mammosser M, Weitzenblum E, Quoix E, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107:1193–1198. doi: 10.1378/chest.107.5.1193 [DOI] [PubMed] [Google Scholar]
- 12.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 13.Minai OA, Fessler H, Stoller JK, et al; NETT Research Group. Clinical characteristics and prediction of pulmonary hypertension in severe emphysema. Respir Med. 2014;108:482–490. doi: 10.1016/j.rmed.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 14.Cuttica MJ, Kalhan R, Shlobin OA, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med. 2010;104:1877–1882. doi: 10.1016/j.rmed.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 15.Galie N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 37;2016:67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 16.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–756. doi: 10.1016/S0735-1097(85)80477-0 [DOI] [PubMed] [Google Scholar]
- 17.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359–365. doi: 10.1016/S0735-1097(85)80172-8 [DOI] [PubMed] [Google Scholar]
- 18.Gartman EJ, Blundin M, Klinger JR, Yammine J, Roberts MB, Dennis McCool F. Initial risk assessment for pulmonary hypertension in patients with COPD. Lung. 2012;190:83–89. doi: 10.1007/s00408-011-9346-8 [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Liu C, Lu W, et al. Clinical characteristics and risk factors of pulmonary hypertension associated with chronic respiratory diseases: a retrospective study. J Thorac Dis. 2016;8:350–358. doi: 10.21037/jtd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayngersh V, Drakopanagiotakis F, Dennis McCool F, Klinger JR. Pulmonary hypertension in a stable community-based COPD population. Lung. 2011;189:377–382. doi: 10.1007/s00408-011-9315-2 [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki M, Nakamura H, Chubachi S, et al; Keio COPD Combordity Research(K-CCR)Group. Analysis of comorbid factors that increase the COPD assessment test scores. Respir Res. 2014;15:13. doi: 10.1186/1465-9921-15-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato M, Chubachi S, Sasaki M, et al. Impact of mild exacerbation on COPD symptoms in a Japanese cohort. Int J Chron Obstruct Pulmon Dis. 2016;11:1269–1278. doi: 10.2147/COPD.S105454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS task force, standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 24.Kwon N, Amin M, Hui DS, et al. Validity of the COPD assessment test translated into local languages for Asian patients. Chest. 2013;143:703–710. doi: 10.1378/chest.12-0535 [DOI] [PubMed] [Google Scholar]
- 25.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1185–1189. doi: 10.1164/ajrccm.158.4.9802091 [DOI] [PubMed] [Google Scholar]
- 26.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Comparison of discriminative properties among disease-specific questionnaires for measuring health-related quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:785–790. doi: 10.1164/ajrccm.157.3.9703055 [DOI] [PubMed] [Google Scholar]
- 27.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 28.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol. 1998;51:1037–1044. doi: 10.1016/S0895-4356(98)00095-X [DOI] [PubMed] [Google Scholar]
- 29.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713; quiz 786–688. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 30.Mishima M, Hirai T, Itoh H, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 1999;96:8829–8834. doi: 10.1073/pnas.96.16.8829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med. 2000;162:1102–1108. doi: 10.1164/ajrccm.162.3.9907120 [DOI] [PubMed] [Google Scholar]
- 32.Washko GR, Hunninghake GM, Fernandez IE, et al; COPD Gene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 364;2011:897–906. doi: 10.1056/NEJMoa1007285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Liu K, Wang Z, et al. Computed tomography measurement of pulmonary artery for diagnosis of COPD and its comorbidity pulmonary hypertension. Int J Chron Obstruct Pulmon Dis. 2015;10:2525–2533. doi: 10.2147/COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer S, Calverley PM, Sherwood Burge P, Jones PW; ISOLDE Study Group Inhaled Steroids in Obstructive Lung Disease. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:122–128. doi: 10.1164/ajrccm.163.1.2005009 [DOI] [PubMed] [Google Scholar]
- 35.Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2:195–203. doi: 10.1016/S2213-2600(14)70001-3 [DOI] [PubMed] [Google Scholar]
- 36.de Torres JP, Casanova C, Hernández C, Abreu J, Aguirre-Jaime A, Celli BR. Gender and COPD in patients attending a pulmonary clinic. Chest. 2005;128:2012–2016. doi: 10.1378/chest.128.4.2012 [DOI] [PubMed] [Google Scholar]
- 37.Soler-Cataluña JJ, Martinez-Garcia MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abraham AS, Cole RB, Green ID, Hedworth-Whitty RB, Clarke SW, Bishop JM. Factors contributing to the reversible pulmonary hypertension of patients with acute respiratory failure studies by serial observations during recovery. Circ Res. 1969;24:51–60. doi: 10.1161/01.RES.24.1.51 [DOI] [PubMed] [Google Scholar]
- 39.Peinado VI, Gomez FP, Barbera JA, et al. Pulmonary vascular abnormalities in chronic obstructive pulmonary disease undergoing lung transplant. J Heart Lung Transplant. 2013;32(12):1262–1269. doi: 10.1016/j.healun.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163:737–744. doi: 10.1164/ajrccm.163.3.2002117 [DOI] [PubMed] [Google Scholar]
- 41.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weissmann N. Chronic obstructive pulmonary disease and pulmonary vascular disease. A comorbidity? Ann Am Thorac Soc. 2018;15(Suppl 4):S278–S281. doi: 10.1513/AnnalsATS.201808-532MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seimetz M, Parajuli N, Pichl A, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147(2):293–305. doi: 10.1016/j.cell.2011.08.035 [DOI] [PubMed] [Google Scholar]
- 44.Pichl A, Sommer N, Bednorz M, et al. Riociguat for treatment of pulmonary hypertension in COPD: a translational study. Eur Respir J. 2019;53(6). doi: 10.1183/13993003.02445-2018 [DOI] [PubMed] [Google Scholar]
- 45.Cottin V, Le Pavec J, Prévot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35:105–111. doi: 10.1183/09031936.00038709 [DOI] [PubMed] [Google Scholar]
- 46.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–194. doi: 10.1164/rccm.200401-006OC [DOI] [PubMed] [Google Scholar]
- 47.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–1536. doi: 10.1378/chest.127.5.1531 [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee K, Tarawneh AR, Alam S. Out of proportion pulmonary hypertension in obstructive lung diseases. Curr Opin Pulm Med. 2018;24:161–172. doi: 10.1097/MCP.0000000000000457 [DOI] [PubMed] [Google Scholar]
- 49.Levi-Valensi P, Weitzenblum E, Rida Z, et al. Sleep-related oxygen desaturation and daytime pulmonary haemodynamics in COPD patients. Eur Respir J. 1992;5:301–307. [PubMed] [Google Scholar]
- 50.Boysen PG, Block AJ, Wynne JW, Hunt LA, Flick MR. Nocturnal pulmonary hypertension in patients with chronic obstructive pulmonary disease. Chest. 1979;76:536–542. doi: 10.1378/chest.76.5.536 [DOI] [PubMed] [Google Scholar]
- 51.Timms RM, Khaja FU, Williams GW. Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med. 1985;102:29–36. doi: 10.7326/0003-4819-102-1-29 [DOI] [PubMed] [Google Scholar]
- 52.Arcasoy SM, Christie JD, Ferrari VA, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med. 2003;167:735–740. doi: 10.1164/rccm.200210-1130OC [DOI] [PubMed] [Google Scholar]
- 53.Mohamed Hoesein FA, Besselink T, Pompe E, et al. Accuracy of CT pulmonary artery diameter for pulmonary hypertension in end-stage COPD. Lung. 2016;194(5):813–819. doi: 10.1007/s00408-016-9926-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J. 2013;41:1292–1301. doi: 10.1183/09031936.00079512 [DOI] [PubMed] [Google Scholar]
- 55.Haraguchi M, Nakamura H, Sasaki M, Keio COPD combordity Research(K-CCR) Group, et al. Determinants of chronic obstructive pulmonary disease severity in the late-elderly differ from those in younger patients. BMC Res Notes. 9;2016:7. doi: 10.1186/s13104-015-1810-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of COPD. 2019. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed March25, 2019.