Abstract
The see-and-treat approach for cervical cancer screening (VIA followed by immediate cryotherapy) was first pilot tested in Botswana in 2009. Botswana’s Ministry of Health and the Botswana-UPenn Partnership collaborated to expand see-and-treat to 5 additional sites throughout the country in 2014. The purpose of this study was to evaluate whether nurses’ adherence to guideline-based screening was maintained during scale-up. Therefore, we compared nurses’ adherence between the pilot and scaled-up sites and determined main drivers of nonadherence across all sites.We conducted a retrospective review of 6644 medical charts from Botswana’s National Cervical Cancer Prevention Programme between February 2014 and October 2015. Using multivariable regression modeled with generalized estimating equations, we determined if nurses’ adherence to the see-and-treat guideline differed between the pilot and scale-up sites after controlling for significant covariates. Overall, adherence to the guideline was high (88.4%). Although the scaled-up sites had higher adherence compared to the pilot site (90.9% vs. 80.2%, respectively), the difference between sites was not statistically significant in the multivariable model (P=0.221). Of the non-adherent clinical encounters, the 3 most frequent visit types were VIA not performed (178, 23.3%), VIA negative: HIV unknown (163, 21.3%), and VIA negative: HIV negative (144, 18.9%). The most common reason for non-adherence was misspecification of follow-up times. Despite known challenges of scaling-up health innovations in resource-limited settings, our study shows that nurses maintained guideline-adherent care in Botswana’s national see-and-treat program. The successful scale-up may have been attributable to the program’s intensive quality assurance monitoring.
Keywords: cervical cancer, screening, guideline adherence, program implementation, sub-Saharan Africa
Introduction
Cervical cancer is the most commonly diagnosed and deadliest cancer among women in Botswana [1]. Although Pap testing has led to significant decreases in cervical cancer burden in the United States and Europe since the 1960s (as much as 80%) [2, 3], it is too complex and prohibitively expensive to sustain on a large scale in low resource settings [4]. Therefore, Botswana’s Ministry of Health has adopted the see-and-treat approach as part of its national prevention strategy [5, 6]. See-and-treat is an innovative method that is considered more contextually appropriate for implementation in resource-limited settings. It combines screening (visual inspection with acetic acid; VIA) and treatment (cryotherapy) in a single patient visit, greatly reducing opportunities for loss to follow-up [7]. Another advantage of see-and-treat is that it can be administered by non-physician providers and is less costly [7].
To achieve reductions in cervical disease, scale-up of see-and-treat at the population level is needed [7, 8]. Botswana is one of several low- and middle- income countries (LMICs) that have successfully implemented pilot or investigational screening programs and initiated scale-up efforts. However, scale-up of see-and-treat remains challenging, and no LMIC to-date has achieved nationwide coverage of their targeted population [8]. Botswana has not yet conducted a formal assessment of its scale-up efforts, but a recently published review has cited challenges from five other LMIC countries (Zambia, Bangladesh, Guatemala, Honduras, and Nicaragua) [8]. Common challenges include high staff attrition, lack of quality assurance programs, inefficient follow-up of cryotherapy-ineligible women, inadequate treatment capacity, and unsustainable government support [8, 9].
Fidelity, a key implementation outcome, is particularly helpful in assessing scale-up. Fidelity is the degree to which a practice or intervention is implemented as originally prescribed in the protocol and is a potential moderator of the relationship between interventions and their intended outcomes [10]. It is composed of two sub concepts, competence and adherence [11]. While competence refers to the skillfulness demonstrated in the delivery of the intervention, adherence is the extent to which the delivery conforms to the intervention protocol [11]. In the context of see-and-treat, competence has frequently been measured by comparing nurses’ VIA assessments to those of expert physicians [12–15]. Nurses’ adherence, however, has been understudied in this area.
In 2009, see-and-treat was successfully pilot tested [12] in Botswana’s capitol city, Gaborone, and later scaled-up to an additional 5 sites in 2014. The purpose of this study was to evaluate whether nurses’ adherence to guideline-based care was maintained during scale-up of see-and-treat in Botswana. Through medical record review, we compared nurses’ adherence between the pilot and scaled-up sites and determined main drivers of nonadherence across all sites.
Materials and Methods
NCCPP and study population
Botswana’s National Cervical Cancer Prevention Programme (NCCPP) primarily focused on secondary prevention with the see-and-treat approach. Following pilot testing from 2009 to 2011 in the capital city of Gaborone, researchers concluded that see-and-treat was a “feasible, high-output, and high-efficiency” program. Details of the pilot have been reported elsewhere [12]. The Ministry of Health, in collaboration with the Botswana-UPenn Partnership (BUP), began scale-up of see-and-treat in 2014 with support of the President’s Emergency Plan for AIDS Relief (PEPFAR) funding [6, 12]. The NCCPP strategy aimed to screen 80% of women aged 30–49 at least once within 5 years [5, 6]. In addition to Gaborone (pilot site), NCCPP was scaled-up to include 5 new clinics in Lobatse, Selebi-Phikwe, Maun, Francistown, and Mahalapye. Each site was staffed with Ministry of Health nurses to conduct VIA and equipped with adequate supplies of liquid nitrogen gas for cryotherapy. A medical officer was also present at least one day of the week to perform colposcopy and LEEP procedures for referred women. Throughout scale-up, all sites participated in intensive quality assurance (QA) monitoring. On a weekly basis, copies of all clinical charts were sent to the pilot site to be reviewed by expert physicians, nurses, and data managers. On a monthly basis, the pilot site experts also visited each scaled-up site to provide continuous provider education and discuss discrepancies found in the reviewed charts [6].
NCCPP see-and-treat guideline
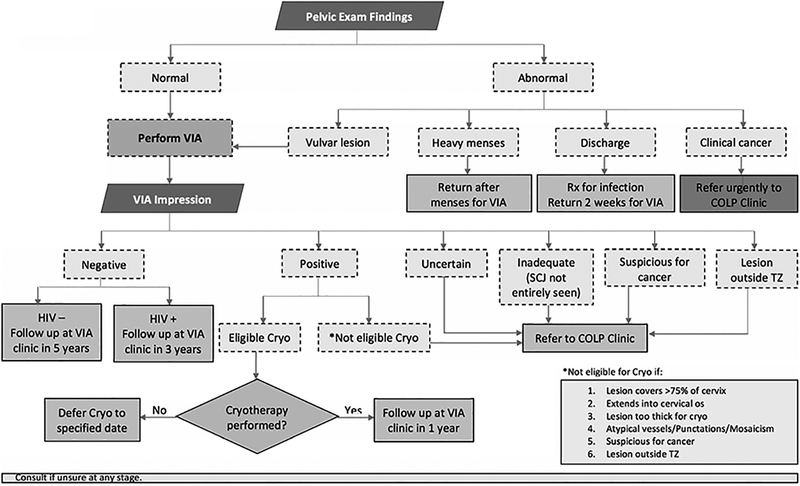
The protocol for screening and treating precancerous lesions across all sites, referred to hereafter as the NCCPP see-and-treat guideline (see Figure 1), was developed from WHO recommendations and modified with feedback from the pilot phase [5, 12, 16]. Although the target age range was 30–49 years, all women, regardless of age and HIV status, were eligible to participate. To begin, a trained nurse performs a pelvic examination to initially inspect the cervix and vulva. Abnormal findings, including heavy menses and infectious discharge, require women to return at a later date for reexamination. If cancer is suspected, the woman should be urgently referred to a colposcopy clinic. If the pelvic exam is normal, including the vulva, the nurse should proceed with conducting VIA. A normal VIA result indicates that the woman does not require treatment and should be followed up in 3 years if she is HIV positive/unknown or 5 years if she is HIV negative. Women with a positive VIA result are eligible for immediate cryotherapy if the lesion is inside the transformation zone, considered mild, covers <75% of the cervix, and does not extend into the cervical os. Women with VIA results that are inadequate, uncertain, or positive but cryotherapy-ineligible should be referred to the colposcopy clinic for further evaluation.
Figure 1.
Botswana’s National Cervical Cancer Prevention Program (NCCPP) See-and-Treat Guideline
Adherence definition and measurement
Adherence is the extent to which practitioners’ behaviors align with the intervention protocol [11]. The behavior of central interest in this analysis was the treatment and referral action taken by the nurse during each patient visit. The intervention protocol used was the NCCPP see-and-treat guideline, as shown in the Appendix. Adherence, therefore, was operationalized as the concordance between the guideline and the nurses’ treatment and referral choice as documented on the initial visit form. Since adherence was only indicative of provider behavior, we could not determine patient adherence and whether they followed through with the nurses’ instructions.
Adherence was measured as a binary variable. If the nurse followed the guideline, the clinical encounter was classified as adherent. Any actions that deviated from the guideline were considered non-adherent. A coding scheme was developed through consensus of clinical and implementation experts to define adherence uniquely for each visit type (Table 1). As indicated in the guideline, treatment and referral plans were based on the following characteristics: pelvic exam findings (normal cervix, abnormal cervix) and VIA results (negative, positive cryotherapy eligible, positive cryotherapy ineligible, uncertain, inadequate, suspicious for cancer). Fourteen distinct visit types were created based on these characteristics. Because cell counts/frequencies for several visit types were low or zero, the 14 visit types were collapsed to create 5 larger categories to be able to run the regression analysis.
Table 1.
Adherence defined for each visit type
| Visit Type | Adherence Definition |
|---|---|
| VIA Negative: HIV Unknown | No treatment - 3 year follow-up |
| VIA Positive: Cryotherapy Ineligible | No cryotherapy, Refer |
| VIA Uncertain | Refer |
| VIA Squamous Cell Junction (SCJ) Not Seen | Refer |
| Other | Refer |
Data source and collection
Institutional review boards within Botswana’s Ministry of Health and the University of Pennsylvania provided study approval before data collection commenced. Data consisted of initial visit forms, which were included as part of each patient’s medical chart. Nurses used these standardized, paper-based forms during each patient visit to document information essential for clinical care and program evaluation. The same form was used at all clinics and included the following categories of information: demographics, medical/sexual history, pelvic/cervical exam findings, VIA result, and treatment/referral action taken by the nurse. All available forms from the pilot and scaled-up sites during the NCCPP scale-up period, February 2014 to October 2015, were included in the study. During this timeframe, the scaled-up sites were in their first year of operation and the pilot site was in its fifth year.
BUP was responsible for collecting the data through retrospective chart review. BUP data clerks, who were trained on proper methods for conducting data entry using a defined codebook, entered data from each initial visit form into an Access database. Although the data set was checked periodically for missing values by a supervisor, it was only entered into the Access database once by BUP data clerks. Therefore, double digitation was completed by Penn undergraduate research assistants on a computer-generated random sample of the data set (n = 67). Double digitation of the random sample yielded a satisfactory error rate of .01%, which did not warrant complete data reentry.
Statistical analysis
Sample Size.
All records from clinic visits at the pilot site and scaled-up sites from February 2014 to October 2015 were sampled. Based on available data, sample sizes were 1544 from the pilot site and 5100 from the scaled-up sites. Using the two-sided Z test with pooled variance and an alpha level of .05, these sample sizes achieved 80% power to detect a difference between group proportions of 0.0408 (PASS.14). Since no estimates were available from the literature or a pilot test, a conservative estimate of 0.5000 was used for the estimated adherence proportion at the pilot site. SAS 9.4 software was used to conduct all statistical analyses for this study.
Patient demographic and clinical characteristics.
Relevant patient characteristics documented on the initial visit forms were included as covariates within this study. Continuous variables (age, number of children, and age of sexual debut) were described by calculating mean, standard deviation, and range. Categorical variables (HIV status, smoking status, menopausal status, and previous cervical cancer screening) were described with frequencies and proportions. Baseline characteristics between pilot and scale-up sites were compared using two sample t-tests for continuous variables and chi-squared or Fisher’s exact tests for categorical variables.
Provider adherence.
Using 2×2 contingency tables (cross-tabulations), we determined proportions of guideline adherence for the total sample, pilot site, and scaled-up sites. We then used chi-squared test to determine if a statistically significant difference in proportions of adherence existed between pilot and scaled-up sites. To add robustness to the analysis, we also conducted a multivariable regression modeled using generalized estimating equations (GEE) to determine if differences between the pilot and scale-up sites still existed after controlling for significant covariates. Since clinical encounters are clustered by provider, GEE using the exchangeable working correlation matrix was an appropriate choice for this data set to account for within-provider correlation. We used log-binomial regression given that the outcome variable (adherence) is binary and the incidence of nonadherence in this study was 11.61%. It is suggested that odds ratios (ORs) do not adequately estimate risk ratios (RRs) when incidence of the outcome of interest is common or >10% [17].
To determine which covariates would be included to create the most parsimonious multivariable model, we used purposeful covariate selection as described in [18, 19]. The primary exposure variable (site) and each covariate was tested in a univariable analysis to determine if it was significantly correlated to adherence. Variables with p<.25 and/or known clinical importance met criteria for inclusion in the initial multivariable model. Significance of each variable was reassessed once fitted in the initial model to see if relationships changed in the presence of other variables. Starting with the highest p value, variables with p>.10 were deleted from the initial model one-by-one. After a variable was deleted, coefficients of the remaining variables were assessed. Any change in coefficients >20% indicated that the deleted variable was important to the model in terms of adjustment and was added back. Variables with p<.10 remained in the final model.
Main drivers of nonadherence.
In addition to comparing guideline adherence between sites, we also conducted an exploratory analysis to determine the main drivers of nonadherence. We used frequency tables to show which referral and treatment options were most frequently chosen by nurses when they did not adhere to the guideline. We have reported results for the 3 visit types with the highest nonadherence rates.
Results
Summary of Baseline Demographic and Clinical Characteristics
As part of NCCPP, 6,644 total clinical encounters from 44 providers took place between February 2014 and October 2015. Several encounters were second or third rescheduled visits for the same patient. Therefore, the sample was from 6,257 unique patients. The most frequent visit types were VIA negative (3293, 49.6%) and VIA positive (2327, 35.0%). Less frequent visits included Abnormal cervix (464, 7.0%), VIA not performed (317, 4.8%), and VIA other (179, 2.7%). Due to missing data, 64 visits (1.0%) were unable to be classified. Most patient visits (n=5100, 76.8%) were conducted at the scaled-up sites. The remaining 23.2% (n=1544) occurred at the pilot site in Gaborone. Of the scaled-up sites, 1605 (31.5%) encounters were in Francistown, 1092 (21.4%) in Mahalapye, 1091 (21.4%) in Selebi-Phikwe, 821 (16.1%) in Lobatse, and 491 (9.6%) in Maun.
Baseline demographic and clinical characteristics of the 6,257 screened women are summarized in Table 2. Women in the study were on average aged 36.3 with 2.3 children and sexually debuted at 18.9 years old. Most women, 5278 (84.4%), were within the targeted age range of 30–49. There were 885 women (14.1%) younger than 30 years, and 94 women (1.5%) were 50 years or older. Approximately half of the sample had been previously screened for cervical cancer (2946, 47.1%): 2904 with Pap smear, 21 with VIA, and 21 could not recall the screening modality. Approximately half of the sample (3200, 51.1%) were also HIV positive. If only considering women with known HIV status, the HIV rate in the study sample increases to 56.2%.
Table 2.
Baseline demographic and clinical characteristics for total sample (n=6257 women)
| Pilot Site (n=1432) |
Scaled-up Sites (n=4825) |
T test or Chi X2 Test | |||
|---|---|---|---|---|---|
| Characteristics | Range or % | Range or % | |||
| Age | 20–56 | 17–59 | p<.001 | ||
| Number of Children | 0–10 | 0–12 | p<.001 | ||
| Age of Sexual Debut | 12–44 | 5–45 | p=.002 | ||
| Not reported | |||||
| Menopausal | p=.853 | ||||
| Yes | 1.5% | 1.5% | |||
| No | 98.5% | 98.4% | |||
| Not reported | 0.0% | 0.1% | |||
| HIV | p<.001 | ||||
| Positive | 47.4% | 52.3% | |||
| Negative | 36.5% | 40.9% | |||
| Unknown | 16.1% | 6.8% | |||
| Smoker | p=.667 | ||||
| Yes | 1.5% | 1.7% | |||
| No | 98.5% | 98.2% | |||
| Not reported | 0.0% | 0.1% | |||
| Previously Screened | p<.001 | ||||
| Yes | 37.4% | 50.0% | |||
| No | 62.1% | 49.5% | |||
| Unknown | 0.5% | 0.2% | |||
| Not reported | 0.0% | 0.3% | |||
Comparisons between the pilot site and scaled-up sites show statistically significant differences in all baseline characteristics except menopause (p=.853) and smoking status (p=.667). Although statistically significant, observed differences between groups for several variables (age, number of children, age of sexual debut) are quite small and have limited clinical significance. Compared to the pilot site, women from the scaled-up sites were older (p<.001), had more children (p<.001), and sexually debuted at an earlier age (p=.002). Scaled-up sites also had a larger proportion of women with HIV (p<.001), VIA positive results (p<.001), and previous cervical cancer screening (p<.001).
Providers’ Adherence to the NCCPP See-and-Treat Algorithm
Due to missing data, determinations of adherence vs. nonadherence could not be made for 64 (1.0%) of the 6644 total encounters. Of the remaining 6580 encounters, 5816 (88.4%) were documented with the correct treatment and referral as indicated in the NCCPP guideline. 764 (11.6%) deviated from guideline recommendations. When comparing adherence rates by site (Table 3), 80.2% of encounters from the pilot site were adherent compared to 90.9% at the scaled-up sites (chi-square statistic, P<0.001). Individual adherence rates for each scaled-up site were as follows: 95.5% (Francistown), 95.2% (Selebi-Phikwe), 92.1% (Mahalapye), 85.0% (Maun), and 77.8% (Lobatse).
Table 3.
Adherence by site
| Adherence | Nonadherence | |
|---|---|---|
| Pilot site (n=1525) |
1223 (80.2%) |
302 (19.8%) |
| Scale-up sites (n=5055) | 4593 (90.9%) |
462 (9.1%) |
| Total sample (n=6580) |
5816 (88.4%) |
764 (11.6%) |
Table 4 summarizes results from the univariable and multivariable analyses associating site and additional covariates with guideline adherence. In addition to site, the multivariable regression model included age, parity, menopausal status, HIV status, and visit type according to purposeful covariate selection. The crude and adjusted risk ratios reveal that site was not significantly associated with guideline adherence. When controlling for significant covariates, scaled up sites were 0.985 times as likely to be adherent to the guideline compared to the pilot site (P=0.221).
Table 4.
Results from Univariable and Multivariable Analysis Associating Covariates and Guideline Adherence
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| p-value | p-value | |||||
| Site | 0.115 | 0.221 | ||||
| Pilot | -- | -- | ||||
| Scaled-up | 0.115 | 0.221 | ||||
| Age | 0.005 | 0.151 | ||||
| Parity | 0.078 | 0.050 | ||||
| Age of Debut | 0.125 | -- | ||||
| Menopause | 0.235 | 0.409 | ||||
| No | -- | -- | ||||
| Yes | 0.235 | 0.409 | ||||
| Smoking history | 0.934 | -- | ||||
| No | -- | -- | ||||
| Yes | 0.934 | -- | ||||
| HIV | <.001 | <.001 | ||||
| Unknown | -- | -- | ||||
| Positive | 0.002 | 0.002 | ||||
| Negative | <.001 | <.001 | ||||
| Previous screening | 0.319 | -- | ||||
| Unknown | -- | -- | ||||
| No | 0.914 | -- | ||||
| Yes | 0.985 | -- | ||||
| Visit type | <.001 | <.001 | ||||
| Abnormal | -- | -- | ||||
| VIA neg | 0.691 | 0.060 | ||||
| VIA pos | 0.011 | 0.034 | ||||
| VIA unk | 0.453 | 0.551 | ||||
| VIA oth | <.001 | <.001 | ||||
Main drivers of Nonadherence
There were 764 encounters (11.6%) that did not align with the NCCPP guideline. Of these non-adherent encounters, the 3 visit types with the highest nonadherence counts were VIA not performed (178, 23.3%), VIA negative: HIV unknown (163, 21.3%), and VIA negative: HIV negative (144, 18.9%). Reasons for nonadherence have been summarized for these 3 visit types.
VIA negative: HIV negative.
According to the guideline, these patients should not be treated and recommended to follow-up with screening in 5 years. A total of 144 clinical encounters from this visit type were non-adherent. Reasons for nonadherence included either the nurse recommending the wrong follow-up time (n=84) or not specifying a follow-up time at all (n=56). Four encounters could not be classified due to missing data. Misspecifications for follow-up times were 3 years (n=78), 1 year (n=4), 6 years (n=1), or 15 years (n=1). Among the encounters that did not specify a follow-up time, many of those patients (n=27) had been treated with cryotherapy for ectopy. While cryotherapy for ectopy is a common opportunistic co-morbidity treatment, the follow-up time was not included in the guideline.
VIA negative: HIV unknown.
Patients in this category should not be treated and should follow-up for screening in 3 years, essentially treating them as a higher risk group than HIV negative. The follow-up timing was not specified in the guideline but was confirmed to be taught during training by the implementing team. HIV testing should also be recommended. A total of 163 clinical encounters for this visit type were non-adherent. Like VIA negative: HIV negative visits, the nurse either recommended the wrong follow-up time (n=148) or did not specify a follow-up time at all (n=15). All encounters that specified the wrong follow-up recommended patients to return in 5 years rather than 3. Six of the 15 encounters that did not specify follow-up were treated with cryotherapy for ectopy.
VIA not performed.
Nurses should proceed with performing VIA if patients have a normal cervical exam. Contraindications for VIA include discharge consistent with infection, heavy menses, or suspicion for cancer and would classify the encounter an abnormal cervical exam. This category included 178 visits where the patient had a normal cervix, but the nurse did not perform VIA. Instead, 90 were deferred, 43 referred, 24 treated with cryotherapy, and 21 had no specified action.
Discussion
Clinical practice guidelines (CPGs) are “statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative case options” [20]. CPGs have the potential to improve the quality, efficiency, consistency, and equitable distribution of healthcare. However, these benefits can only be assured if providers are adhering to CPGs in their daily practice. While development of CPGs has skyrocketed since the push for evidence-based medicine, their use in practice is less known and not guaranteed.
Despite known challenges of scaling up health innovations in resource-limited settings, our study shows that providers maintained guideline-adherent care in Botswana’s national see-and-treat program. Overall provider adherence in our study sample was high (88%). Furthermore, there was no significant difference in guideline adherence between the pilot site and scaled-up sites when controlling for covariates. Although adherence was high in our study, it is important to recognize that adherence alone does not guarantee complete fidelity. Providers can follow the outlined steps of the guideline, however, competence (or level of skill) when conducting VIA and cryotherapy is also important to ensure evidence-based, quality care is reaching patients [10, 11].
The success of Botswana in maintaining high adherence during scale-up may have been attributable to the intensive quality assurance (QA) monitoring of the program. VIA is a subjective, visual skill that requires frequent supervision and refresher courses to maintain providers’ skill level and minimize performance variability. Therefore, experts from the pilot site reviewed all cervical images centrally, and mentored providers during monthly site visits. While this QA monitoring was feasible for 5 sites, it may not be sustainable as the program continues to expand. Each scaled-up site was monitored monthly, which equates to 5 trips per month for 2–3 days at a time, occasionally lasting a week if specific training was needed. Oftentimes, the less experienced nurses remained at the pilot site while the experts made these frequent trips across the country, contributing to a “local brain drain”. A potential solution for overcoming this limitation is automating the QA process with outside trainers or online modules, which has been explored in [21]. Future studies are needed to develop valid and reliable quality assurance methods that will also be sustainable.
When seeking to better understand nonadherence, it is important to assess deviations from the NCCPP algorithm in terms of potential harm to patients. There were 3 visit types that were explored to determine reasons for provider nonadherence: VIA negative: HIV negative, VIA negative: HIV unknown, and VIA not performed.
For VIA negative visits, nurses often suggested the incorrect follow-up timeframes or did not recommend follow-up at all. During VIA negative: HIV negative visits, nurses frequently recommended repeat screening in 3 years, rather than 5 years. Asking these low risk patients to come back earlier does not have adverse implications for patient care. However, it can lead to wasted resources, which is significant for resource-limited settings like Botswana and can be better allocated to higher risk patients. During VIA negative: HIV unknown visits, patients were typically told follow-up with screening in 5 years, instead of 3 years. In regions with high HIV prevalence, guidelines recommend screening these patients earlier given the association between HIV and higher risk for developing cervical cancer. Although taught as part of the initial training, treatment and referral plans for VIA negative: HIV unknown visits were not explicitly stated in the algorithm, which could have contributed to the high nonadherence for this visit type. Another missing element in the algorithm for VIA negative visits was the appropriate follow-up time for patients treated with cryotherapy for ectopy, which is a valid opportunistic co-morbidity treatment. Reassessment and revisions of the algorithm are needed to avoid providers having to rely on their own judgment when necessary actions are not specified.
Patients from the VIA not performed group had normal cervixes, and VIA should have been performed. Most of these patients were referred or deferred. Unfortunately, there was not enough information provided in the data set to determine why. The algorithm notes that nurses should consult when unsure, which is presumably what occurred. If these patients were eligible for VIA screening, however, time and effort spent reassessing them at the referral clinic could have been better allocated.
There were several limitations to this study. Adherence was determined based on self-reported treatment and referral actions as documented in the medical chart. However, reported action does not always equate to actual action taken. Adherence was also measured as a binary variable, indicating only whether the nurse completely followed the guideline. The variable does not account for differing levels of adherence, which we would anticipate even higher estimates for adherence with such a measure. Furthermore, adherence was only measured for the provider. Due to data constraints, we were unable to assess and account for adherence at the patient-level. Data was collected through retrospective chart review, which has limitations [22, 23]. Data from medical charts are intended primarily for clinical use so the information collected is usually most pertinent to daily practice and can be limited in scope given time restraints during patient visits. Provider and organizational-level factors are rarely tracked in the patients’ medical chart, which limited the analysis and what could be controlled for in the regression model (i.e. providers’ clinical experience and volume of screenings). Lastly, the high HIV rate in this sample (~56%) may limit the generalizability of our results. This rate is more than twice the population prevalence rate for adult women (20.8%) estimated in 2013 [24]. The higher than average HIV rate may be due to the PRRR funding source for NCCPP, which uses resources from already existing HIV treatment infrastructure and promotes screenings within this population given their increased risk of developing cervical cancer. Although screenings were open to all women, regardless of HIV status, women with HIV were largely targeted due to the programs’ infrastructure.
Conclusion
The landscape of cervical cancer screening is rapidly changing. As affordable HPV DNA tests have become available on the market, some researchers question whether VIA will have a future place in cervical cancer screening for resource-limited settings [25]. The World Health organization recommends, where feasible, that HPV tests should be utilized as primary screening [16]. However, the relatively low specificity of HPV tests requires women to be further triaged by either VIA or cytology [26]. In the meantime, VIA is still the reality for many countries and there is benefit to reflecting upon the lessons learned from implementing and scaling up see-and-treat. Some suggest VIA-based see-and-treat infrastructure will be well suited for introducing HPV DNA testing [27]. There is an urgent need for further developing and validating reliable interventions - such as QA monitoring - to ensure provider adherence, especially since the screening guidelines are becoming ever more complex.
Acknowledgements
We thank the staff at each screening site for their commitment to caring for the patients in this study and supporting the expansion of Botswana’s National Cervical Cancer Screening Programme. In addition, we thank Jesse Chittams and the BECCA Lab for their statistical support.
Financial Support: This study was supported in part by the National Cancer Institute (U54-CA190158-03S1), American Cancer Society (DSCN-16-112-01-SCN), Penn Center for AIDS Research (P30 AI 045008), and Penn Mental Health AIDS Research Center (P30 MH097488). LGJ was supported by the Rita and Alex Hillman Foundation through the BSN-PhD Hillman Scholars Program in Nursing Innovation.
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
References
- 1.Cancer, I.A.F.R.o. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012. [cited 2018 March 20]; Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
- 2.Chrysostomou AC, et al. , Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses, 2018. 10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGraw SL and Ferrante JM, Update on prevention and screening of cervical cancer. World J Clin Oncol, 2014. 5(4): p. 744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahasrabuddhe VV, et al. , Cervical cancer prevention in low- and middle-income countries: feasible, affordable, essential. Cancer Prev Res (Phila), 2012. 5(1): p. 11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Health R, National Cervical Cancer Prevention Programme: Five-year comprehensive prevention and control strategy 2012–2016. 2012: Gaborone, Botswana. [Google Scholar]
- 6.Grover S, et al. , Cervical Cancer in Botswana: Current State and Future Steps for Screening and Treatment Programs. Front Oncol, 2015. 5: p. 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catarino R, et al. , Cervical cancer screening in developing countries at a crossroad: Emerging technologies and policy choices. World J Clin Oncol, 2015. 6(6): p. 281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holme F, et al. , Scaling up proven innovative cervical cancer screening strategies: Challenges and opportunities in implementation at the population level in low- and lower-middle-income countries. Int J Gynaecol Obstet, 2017. 138 Suppl 1: p. 63–68. [DOI] [PubMed] [Google Scholar]
- 9.Chary AN and Rohloff PJ, Major challenges to scale up of visual inspection-based cervical cancer prevention programs: the experience of Guatemalan NGOs. Glob Health Sci Pract, 2014. 2(3): p. 307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll C, et al. , A conceptual framework for implementation fidelity. Implement Sci, 2007. 2: p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitenstein SM, et al. , Implementation fidelity in community-based interventions. Res Nurs Health, 2010. 33(2): p. 164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramogola-Masire D, et al. , Cervical cancer prevention in HIV-infected women using the “see and treat” approach in Botswana. J Acquir Immune Defic Syndr, 2012. 59(3): p. 308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinley KE, et al. , Use of mobile telemedicine for cervical cancer screening. J Telemed Telecare, 2011. 17(4): p. 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asgary R, et al. , mHealth to Train Community Health Nurses in Visual Inspection With Acetic Acid for Cervical Cancer Screening in Ghana. J Low Genit Tract Dis, 2016. 20(3): p. 239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firnhaber C, et al. , Evaluation of a cervicography-based program to ensure quality of visual inspection of the cervix in HIV-infected women in Johannesburg, South Africa. J Low Genit Tract Dis, 2015. 19(1): p. 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Organization WH, WHO guidelines for screening and treatment of precancerous lesoin for cervical cancer prevention. 2013: Geneva, Switzerland. [Google Scholar]
- 17.Zhang J and Yu KF, What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA, 1998. 280(19): p. 1690–1. [DOI] [PubMed] [Google Scholar]
- 18.Bursac Z, et al. , Purposeful selection of variables in logistic regression. Source Code Biol Med, 2008. 3: p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S, and Sturdivant RX, Applied logistic regression Third edition / ed. Wiley series in probability and statistics. 2013, Hoboken, New Jersey: Wiley; xvi, 500 pages. [Google Scholar]
- 20.Medicine I.o., Clinical Practice Guidelines We Can Trust. 2011: Washington DC. [Google Scholar]
- 21.Mink J and Peterson C, MobileODT: a case study of a novel approach to an mHealth-based model of sustainable impact. Mhealth, 2016. 2: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vassar M and Holzmann M, The retrospective chart review: important methodological considerations. J Educ Eval Health Prof, 2013. 10: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worster A and Haines T, Advanced statistics: understanding medical record review (MRR) studies. Acad Emerg Med, 2004. 11(2): p. 187–92. [PubMed] [Google Scholar]
- 24.Botswana AIDS Impact Survey Summary Results 2013, Statistics Botswana: Gaborone, Botswana. [Google Scholar]
- 25.Domgue JF and Valea FA, Is It Relevant to Keep Advocating Visual Inspection of the Cervix With Acetic Acid for Primary Cervical Cancer Screening in Limited-Resource Settings? J Glob Oncol, 2018(4): p. 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toliman PJ, et al. , Innovative approaches to cervical cancer screening in low- and middle-income countries. Climacteric, 2018. 21(3): p. 235–238. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn L and Denny L, The time is now to implement HPV testing for primary screening in low resource settings. Prev Med, 2017. 98: p. 42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]