Abstract
Background
Influenza is a major cause of morbidity and mortality in the elderly worldwide. Influenza vaccination can prevent morbidity/mortality from influenza infection. A gap of 1–2 years, before an epidemic strain is recommended by the World Health Organization (WHO) to be the vaccine strain in Southeast Asia, has been reported; this results in a high rate of vaccine mismatch and excess influenza-associated morbidity. The aim of the current study was to evaluate the effect of repeated vaccination on vaccine effectiveness (VE) among the elderly in Taiwan, during years with and without early appearance of antigenically drifted strains.
Methods
A historical cohort study was conducted to evaluate the impact of repeated vaccination on the reduction of influenza-associated hospitalization among persons older than 64 years over two influenza seasons: 2007–08, with all circulating virus strains mismatched, and 2008–09, with all virus strains matched with the vaccine strains, considering four exposure effects, namely current vaccine effect, sequential vaccination effect, residual protection effect and no vaccination effect. Propensity score matching on vaccination status was performed to ensure similar baseline characteristics between the groups that received and did not receive vaccination.
Results
Only current-year vaccination in combination with prior history of annual revaccination significantly reduced the risk of hospitalization, with adjusted hazard ratios of 0.68 (95% CI: 0.54, 0.85) and 0.74 (95% CI: 0.57, 0.95) during the 2007–08 and 2008–09 influenza seasons, respectively. Further stratification showed that even during the 2007–08 influenza season, when all vaccinations were mismatched with the circulating strains, sequential vaccinations still significantly reduced influenza-associated hospitalization in the female population aged 68–74 and 75–84 years, with adjusted VE of 25.2% (95% CI: −9.6, 49.0%) and 36.9% (95% CI: 17.1, 52.0%), respectively.
Conclusion
Our study supports the recommendation of annual revaccination against influenza in the elderly, even though the circulating strain of influenza virus was antigenically mismatched with the vaccine strains.
Keywords: vaccine effectiveness, trivalent influenza vaccine, elderly, prior vaccination history, repeated vaccination, propensity score matching
Introduction
Globally, seasonal influenza infection with three different strains (A/H1N1, A/H3N2 and B) is a major cause of morbidity and mortality in the elderly.1 H3N2 subtype typically caused more morbidity and mortality than either H1N1 or B virus, before the 2009 pandemic of A/H1N1pdm09.2,3 Influenza vaccination is the best tool for the prevention of influenza and its complications.4 The influenza strains currently used in the annual vaccine need to be altered yearly to target the strains that are predicted by the World Health Organization (WHO) to circulate in the upcoming season.5,6 However, the vaccine effectiveness (VE) varies year by year. A number of explanations for this variation have been suggested, including the antigenic match between the vaccine and circulating strains, the age and health status of vaccine recipients, and the influenza subtype in circulation.7,8 Studies comparing the hemagglutination (HA) sequence and antigenicity of influenza viruses isolated from Taiwan indicated a high rate of vaccine mismatch, and there is usually a gap of 1–2 years before an epidemic strain is recommended by the WHO to be the vaccine strain in Southeast Asia.9,10 Therefore, an excess influenza-associated morbidity, with a substantial trend toward higher rates of hospitalization due to pneumonia and influenza (P&I), was reported during the influenza season with early appearance of antigenically drifted strains.11
Although repeated immunization has been advised to ensure compliance with the antigenic drift of the viruses and the decrease in antibody level with time,12,13 several studies have suggested that VE may be influenced by prior season vaccination, owing to the observation of impaired vaccine seroresponse to influenza HA antigens among repeated vaccine recipients.14–19 Field vaccination efficacy trial studies are difficult to perform because of the poor predictability of influenza outbreaks, and require large numbers of participants to observe significant reductions in mortality or severe morbidity.12,20 Although the large randomized trials conducted during the 1980s found no differences in efficacy between primary and repeated vaccination against serologically confirmed influenza, the effect of repeated vaccination on VE remains inconclusive so far and a potential mechanism explaining the variable VE through different influenza seasons has been proposed.21 Despite the debates on the relevance of repeated vaccination, no influenza VE study has been conducted in countries with early appearance of vaccine-mismatched strains, considering the impact of repeated vaccination on the reduction of influenza-associated hospitalization among the elderly.
The free influenza vaccination program (FIVP) in Taiwan was implemented in 1996. It was initially targeted at people with underlying medical disorders and further expanded in 1998 to include all people older than 64 years. Given the universal recommendation for annual influenza vaccination among the elderly in Taiwan, a better understanding of the relationship between prior vaccination history and current-season VE is needed. Here, we conducted a historical cohort study to evaluate the impact of repeated vaccination on the reduction of influenza-associated P&I hospitalization among persons older than 64 years over two influenza seasons: 2007–08, with all virus strains mismatched, and 2008–09, with all matched with the vaccine strains.
Materials and Methods
Data Sources
The data used in this study were obtained from the National Health Insurance (NHI) Research Database (NHIRD) in Taiwan for the period 1999–2012. For each year, we acquired the records of those aged 65 or older from the NHIRD, and the process was repeated by year from 1999 to 2012, as previously described.11 Instead of using calendar year, annual influenza year was marked as the period beginning on 1 September and concluding on 31 August of the following year. Since the FIVP requires that all the elderly older than 64 years use an insurance card to receive vaccination, vaccination rate data among the elderly were obtained from three different codings of outpatient visit prescriptions from the complete NHI claims database in Taiwan during the period 1999–2012. The results are consistent with the vaccination rate data compiled from local health bureaus by Centers for Disease Control, Taiwan (Taiwan-CDC) without subject selection bias, as previously described.11 All personal identification numbers in the NHIRD were encrypted as scrambled numbers before data processing, for confidentiality and compliance with regulations on personal privacy in Taiwan.
Data on annual influenza vaccine strains were obtained from the WHO. The annual dominant types/subtypes of influenza viruses and monthly influenza isolation rates for the 1999–2000 through 2011–2012 influenza seasons were also collected by the Laboratory-based Influenza Virological Surveillance Network (Lab-ISN) from Taiwan-CDC.
Study Design
To cope with a large number of potential confounders, we performed matching to create comparable cohorts with or without trivalent influenza vaccine (TIV) vaccination in the current influenza season based on individual propensity score obtained from the propensity score logistic regression model, as described previously.11 In brief, potential confounding variables incorporated in the model included both demographic and clinical confounders. The demographicconfounders included age, gender, geographic region of residence, urbanization level of residenceand individual socioeconomic status. The clinical confounders, as a proxy of health status, were acquired from the NHIRD based on the 5-year period preceding the vaccination, and included the number of outpatient visits, the indicator of having catastrophic illness (defined by NHI with more than 30 different types of diseases, such as malignant neoplasm, hereditary deficiency of clotting factors and hereditary anemia; with details available upon request), number of outpatient department (OPD) visits for upper respiratory infection (URI) (URI OPD#; ICD-9-CM code: 460–466); and the cumulative number of comorbidities of chronic conditions, such as existing cardiovascular and other heart diseases (treatment item 11) or chronic obstructive pulmonary disease (COPD) (treatment item 21), with details available upon request. Then, the probability (ie, propensity score) of receiving vaccination based on the model prediction was used to identify a matched control from the non-vaccination subjects each year for the vaccination subject based on an 8→1 digit greedy matching algorithm.22
Ninety-six percent of the vaccinations among the elderly were given in October or November, and 3.6% in December, in Taiwan. Therefore, the cumulative number of influenza vaccinations was determined between 1 September and 31 December of each calendar year. Exposure status of prior vaccination history was categorized into five mutually exclusive categories: non-exposed, first vaccination, sequential vaccination, interrupted vaccination and restart, following the definition from the previous publication.23 Consequently, after this time-varying search of exposure status, individuals in the studied cohort were grouped based on the TIV vaccination status during the current influenza season and five different exposure categories of prior season vaccination history during the follow-up disease outcome. Since the FIVP in Taiwan was only provided to the elderly older than the age of 64 years, an observation period of at least 3 years was needed to trace the vaccination history records from the database; therefore, the age group of 68–74 years was presented in this study, instead of 65–74 years.
Outcome Definition
Influenza-associated morbidities are difficult to quantify because influenza infections are typically not confirmed, and the true burden of influenza tends to be underestimated if choosing morbidities coded as influenza. To better evaluate the disease burden of influenza, we studied more broadly defined conditions by including all hospital discharge diagnoses due to P&I during the study period, excluding admissions of non-residents, transfers between institutions and readmissions within 1 week of discharge. For case ascertainment, we only included subjects with influenza-associated hospitalization during the peak period of the annual influenza season (Table S1), since the influenza-associated morbidity peaks during the periods when influenza virus activity increases, as suggested by the previous study.11
Statistical Analysis
The chi-squared test and Student’s t-test were used to examine differences in demographic variables, comorbid medical disorders and propensity scores between the vaccinated and non-vaccinated groups. Stratified Cox proportional hazard regression with individuals matched on propensity score was used to estimate the VE. The model included exposure variables for current-season vaccination and prior vaccination history, and the interaction term of the current and prior history of vaccination. VE was estimated for all combinations of vaccine exposure in the current and prior vaccination history since 1999, including first vaccination, revaccination, interrupted vaccination and restarted vaccination. Age group was set as a dummy variable in three categories: 68–74, 75–84 (reference group) and >85 years old. VEs were estimated as 100% (1 – hazard ratio [HR]) using Cox proportional hazard regression models within each matching stratum for each level of interaction, by further adjusting for different age groups and gender from three different exposure groups: with current-season vaccination but no prior vaccination history, with both current and prior history of sequential vaccination, and with prior sequential vaccination but with no current-season vaccination. Using this model, VE was estimated without being confounded by residual protection from the inclusion of subjects in the unvaccinated reference group who were vaccinated in prior influenza seasons. To further calculate the HR for the stratified group of women within the age groups of 68–74 and 75–84 years, a conventional Cox proportional hazard regression model was used to avoid the small sample size due to the propensity score matching. An alpha level of 0.05 was considered statistically significant for all analyses. The analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC).
Results
The vaccination coverage and status of revaccination for each study year are shown in Table 1. During the total study period, the annual vaccination rate was highest during the 2003–04 season and gradually decreased to 27% during the 2011–2013 influenza seasons among the elderly aged more than 65 years. The population with interrupted or restarted vaccination was commonly observed annually. A total of 41,655 eligible individuals (33%) never received influenza vaccination during the entire follow-up study years. Comparisons of the antigenic match between influenza vaccine strains and Taiwan’s dominant influenza epidemic strains are summarized in Supplementary Table S1, and the years with early appearance of antigenically drifted virus are also indicated. Generally, the peak activity of influenza-like illness (ILI) was during December and February each year. Occasional summer influenza occurred during June and August of the 2001–02 and 2003–04 influenza seasons with the circulation of H3N2 influenza type A virus. The majority of ILI was caused by type A influenza virus. Also, the vaccine strains and the predominant circulating strain (mainly A[H3N2]) were not well matched for most of the influenza seasons during the study years. Since the influenza A/H1N1pdm09 pandemic occurred during the spring of 2009, we focused our analysis on the influenza seasons before mid-2009 to avoid the aggravated effects due to A/H1N1pdm09 infection, as suggested by previous studies.2,24,25 In order to have at least 3 years of prior vaccination records to evaluate the effectiveness of repeated vaccination on the influenza-associated P&I hospitalization, we selected the 2007–08 season, with all three strains mismatched, and the 2008–09 season, with all three strains matched, for comparison. Since the major subtype circulating during both seasons was H1N1 influenza virus, the possible causes of different VE due to circulating subtype can be avoided in this study.
Table 1.
Vaccination Status per Annual Influenza Season Among the Elderly Aged Older Than 65 Years in Taiwan
| 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eligible population, no. | |||||||||||
| Total | 63,000 | 68,634 | 74,348 | 80,508 | 86,860 | 93,545 | 100,522 | 107,438 | 114,366 | 121,450 | 128,511 |
| Vaccination coverage, no. | |||||||||||
| Not vaccinated | 28,454 | 26,323 | 34,303 | 39,594 | 49,168 | 57,112 | 59,820 | 71,437 | 84,381 | 87,851 | 93,244 |
| Vaccinated | 34,546 | 42,311 | 40,045 | 40,914 | 37,692 | 36,433 | 40,702 | 36,001 | 29,985 | 33,599 | 35,267 |
| Vaccination rate (%) | 54.83 | 61.65 | 53.86 | 50.82 | 43.39 | 38.95 | 40.49 | 33.51 | 26.22 | 27.66 | 27.44 |
| Vaccination status, no. | |||||||||||
| Not vaccinated | 28,454 | 20,804 | 20,527 | 21,069 | 23,154 | 25,816 | 28,056 | 30,566 | 34,867 | 38,396 | 41,655 |
| First | 34,546 | 13,284 | 5991 | 5618 | 4268 | 4023 | 4737 | 4407 | 2628 | 3555 | 3802 |
| Second | 29,027 | 8119 | 5365 | 5807 | 5223 | 6229 | 6327 | 4222 | 4956 | 7078 | |
| Third | 23,516 | 5797 | 3662 | 3861 | 3922 | 3871 | 3527 | 2899 | 3663 | ||
| Fourth | 19,067 | 4186 | 2687 | 3078 | 2719 | 2502 | 2627 | 2254 | |||
| Fifth | 15,007 | 3107 | 2180 | 2147 | 1895 | 1963 | 2085 | ||||
| Sixth | 11,908 | 2587 | 1590 | 1491 | 1537 | 1615 | |||||
| Seventh | 10,132 | 1889 | 1179 | 1225 | 1287 | ||||||
| Eighth | 7693 | 1406 | 1005 | 1034 | |||||||
| Ninth | 5740 | 1148 | 878 | ||||||||
| Tenth | 4689 | 960 | |||||||||
| Eleventh | 3909 | ||||||||||
| Interrupted | 5519 | 13,776 | 18,525 | 26,014 | 31,296 | 31,764 | 40,871 | 49,514 | 49,455 | 51,589 | |
| Restarted | 2419 | 5067 | 4762 | 5624 | 7837 | 5358 | 5395 | 7995 | 6702 |
Our previous study suggested that the TIV-receiving group had a higher propensity score of being vaccinated than the non-receiving group.11 To assess the true effect of revaccination on the influenza-associated hospitalization with minimum interference from unmeasured confounding factors, we then performed propensity score matching on both vaccination and non-vaccination groups. A total of 38,815 and 40,934 subjects were selected in the propensity score-matched non-vaccination groups in the 2007 and 2008 influenza seasons, respectively. The demographic and clinical characteristics of the vaccination and non-vaccination groups before propensity score matching are shown in Table 2. The vaccination group had a higher prevalence of certain pre-existing medical comorbidities, including number of comorbidities (p<0.0001), number of OPD visits (p<0.0001), recent intestinal disorder (p=0.0260) and URI OPD in a prior year (p<0.0001), than the non-vaccination group. In contrast, the vaccination group had a lower prevalence of catastrophic illness than the non-vaccination group, with statistical significance (12.4% vs 19.4% and 12.4% vs 21.4% for 2007–08 and 2008–09 influenza seasons, respectively). There were also significant differences in the distribution of monthly income, urbanization level and geographic region between the vaccination and non-vaccination groups. The vaccination groups had higher propensity score than the non-vaccination group (p<0.0001). After propensity score matching, the matched cohorts were well-balanced, with less than 2% difference between the vaccination and non-vaccination groups in terms of all observed covariates (Table 3), although statistically significant differences could still be observed in certain baseline characteristics, such as age and catastrophic illness, mainly because of the large sample size.
Table 2.
Demographic Characteristics and Comorbid Medical Disorders for the Trivalent Influenza Vaccine Vaccination and Non-Vaccination Groups Before Propensity Score Matching
| 2007 | 2008 | ||||||
|---|---|---|---|---|---|---|---|
| Vaccinated (36,433) | Non-Vaccinated (57,112) | p-value | Vaccinated (40,702) | Non-Vaccinated (59,820) | p-value | ||
| Gender | Male | 50.8% | 49.6% | 0.0003 | 50.4% | 49.6% | 0.012 |
| Female | 49.2% | 50.4% | 49.6% | 50.4% | |||
| Age (years) | 65–74 | 45.8% | 42.2% | <0.001 | 43.9% | 41.6% | <0.001 |
| 75–84 | 45.7% | 42.2% | 47.0% | 40.5% | |||
| >85 | 8.5% | 15.6% | 9.2% | 17.9% | |||
| Geographic region | North | 35.9% | 45.4% | <0.001 | 37.1% | 44.7% | <0.001 |
| Central | 29.2% | 23.0% | 28.7% | 23.1% | |||
| South | 30.5% | 27.3% | 29.9% | 27.7% | |||
| East | 4.4% | 4.4% | 4.3% | 4.5% | |||
| Urbanization level | 1 (Most urbanized) | 19.2% | 26.6% | <0.001 | 20.2% | 26.4% | <0.001 |
| 2 | 23.1% | 25.5% | 23.5% | 25.5% | |||
| 3 | 16.2% | 16.7% | 16.4% | 16.6% | |||
| 4 | 21.7% | 17.6% | 21.3% | 17.7% | |||
| 5 | 5.0% | 3.3% | 4.7% | 3.4% | |||
| 6 | 14.7% | 10.3% | 14.0% | 10.5% | |||
| Socioeconomic status | Low | 30.7% | 38.6% | <0.001 | 31.2% | 38.2% | <0.001 |
| Moderate | 19.5% | 20.5% | 18.5% | 19.8% | |||
| High | 49.8% | 40.9% | 50.3% | 42.0% | |||
| Number of comorbidities | 0 | 17.3% | 24.5% | <0.001 | 17.2% | 24.6% | <0.001 |
| 1–3 | 69.4% | 63.8% | 70.2% | 64.0% | |||
| >3 | 13.4% | 11.6% | 12.6% | 11.3% | |||
| URI OPD in prior year | 1.6 | 1.1 | <0.001 | 1.5 | 0.9 | <0.001 | |
| Number of outpatient visits | 29.1 | 18 | <0.001 | 28.8 | 16.6 | <0.001 | |
| Catastrophic illness | Yes | 12.4% | 19.4% | <0.001 | 12.4% | 21.4% | <0.001 |
| No | 87.6% | 80.6% | 87.6% | 78.7% | |||
| Recent intestinal disorder | Yes | 14.2% | 9.0% | <0.001 | 12.7% | 7.5% | <0.001 |
| No | 85.8% | 91.1% | 87.3% | 92.5% | |||
Abbreviation: URI OPD, outpatient department visit for upper respiratory infection.
Table 3.
Demographic Characteristics and Comorbid Medical Disorders for the Trivalent Influenza Vaccine Vaccination and Non-Vaccination Groups After Propensity Score Matching
| 2007 | 2008 | ||||||
|---|---|---|---|---|---|---|---|
| Vaccinated (31,943) | Non-Vaccinated (31,943) | p-value | Vaccinated (33,845) | Non-Vaccinated (33,845) |
p-value | ||
| Gender | Male | 50.5% | 49.0% | 0.0003 | 49.8% | 48.0% | <0.001 |
| Female | 49.5% | 51.0% | 50.2% | 52.0% | |||
| Age (years) | 65–74 | 45.8% | 48.0% | <0.001 | 44.7% | 47.8% | <0.001 |
| 75–84 | 45.1% | 44.3% | 45.1% | 43.9% | |||
| >85 | 9.1% | 7.7% | 10.35 | 8.3% | |||
| Geographic region | North | 38.1% | 39.1% | 0.0142 | 39.2% | 40.6% | 0.0023 |
| Central | 27.9% | 26.9% | 27.0% | 26.3% | |||
| South | 29.6% | 29.5% | 29.6% | 28.9% | |||
| East | 4.4% | 4.5% | 4.3% | 4.3% | |||
| Urbanization level | 1 (Most urbanized) | 20.5% | 21.4% | 0.0012 | 21.7% | 23.0% | <0.001 |
| 2 | 23.8% | 24.3% | 24.1% | 24.7% | |||
| 3 | 16.6% | 16.8% | 16.6% | 16.8% | |||
| 4 | 21.0% | 20.3% | 20.3% | 19.3% | |||
| 5 | 4.5% | 4.4% | 4.3% | 4.1% | |||
| 6 | 13.6% | 12.8% | 13.0% | 12.2% | |||
| Socioeconomic status | Low | 32.5% | 33.9% | <0.001 | 33.1% | 34.9% | <0.001 |
| Moderate | 19.5% | 18.3% | 18.2% | 17.1% | |||
| High | 48.1% | 47.8% | 48.7% | 48.0% | |||
| Number of comorbidities | 0 | 18.5% | 19.6% | 0.0005 | 18.7% | 19.8% | <0.001 |
| 1–3 | 69.1% | 67.5% | 69.7% | 67.0% | |||
| >3 | 12.4% | 12.8% | 11.7% | 12.2% | |||
| URI OPD in prior year | 1.5 | 1.5 | 0.4981 | 1.3 | 1.4 | 0.6487 | |
| Number of outpatient visits | 26.4 | 26.2 | 0.1199 | 25.7 | 25.2 | 0.0027 | |
| Catastrophic illness | Yes | 13.1% | 11.5% | <0.001 | 13.5% | 11.6% | <0.001 |
| No | 86.9% | 88.5% | 86.6% | 88.4% | |||
| Recent intestinal disorder | Yes | 12.9% | 12.8% | 0.7229 | 11.4% | 11.3% | 0.5528 |
| No | 87.1% | 87.2%% | 88.6% | 88.7% | |||
Abbreviation: URI OPD, outpatient department visit for upper respiratory infection.
Without matching, current-year vaccination was associated with increased P&I hospitalization during the 2007 and 2008 influenza seasons (HR=1.18 and 1.06, respectively) (Table 4). However, a reduced risk of P&I hospitalization was observed if prior vaccination histories were considered, including first vaccination, revaccination, interrupted vaccination or restart vaccination. After matching, those associations were not statistically significant, except for interrupted and restart vaccination, which were associated with increased risk of hospitalization. Multivariate Cox regression was performed to adjust the confounding effect from age and gender, with further consideration of the interaction between current and prior history of vaccination (Table 4). The results suggest that only current-year vaccination in combination with prior history of revaccination significantly reduced the risk of P&I hospitalization, with adjusted HRs of 0.68 (95% CI: 0.54, 0.85) and 0.74 (95% CI: 0.57, 0.95) during the 2007–08 and 2008–09 influenza seasons, respectively.
Table 4.
Unadjusted and Adjusted Hazard Ratios of Various Influenza Vaccination Histories Against Influenza-Associated Hospitalization Among the Elderly During 2007–08 and 2008–09 Influenza Seasons
| Parameter | 2007–08 | 2008–09 | ||||
|---|---|---|---|---|---|---|
| Unmatched Analysis | Matched Analysis | Unmatched Analysis | Matched Analysis | |||
| Univariate (95% CI) | Univariate (95% CI) | Multivariate (95% CI) | Univariate (95% CI) | Univariate (95% CI) | Multivariate (95% CI) | |
| Current Season Vaccination | ||||||
| No | Refa | Ref | Ref | Ref | Ref | Ref |
| Yes | 1.18 (1.10, 1.26)* | 0.95 (0.88, 1.03) | 1.13 (0.88, 1.47) | 1.06 (0.99, 1.13) | 0.99 (0.92, 1.08) | 1.10 (0.86, 1.41) |
| Prior Status | ||||||
| Never | Ref | Ref | Ref | Ref | Ref | Ref |
| First | 0.79 (0.68, 0.91)* | 1.09 (0.86, 1.39) | 1.26 (0.85, 1.88) | 0.72 (0.63, 0.83)* | 1.16 (0.92, 1.48) | 1.21 (0.80, 1.81) |
| Sequential | 0.84 (0.78, 0.92)* | 1.04 (0.91, 1.18) | 1.19 (0.93, 1.53) | 0.74 (0.68, 0.80)* | 1.00 (0.88, 1.13) | 1.05 (0.80, 1.38) |
| Interrupted | 0.71 (0.65, 0.77)* | 1.36 (1.15, 1.60)* | 1.10 (0.88, 1.36) | 0.74 (0.67, 0.80)* | 1.24 (1.06, 1.46)* | 0.95 (0.76, 1.18) |
| Restarted | 0.62 (0.53 ,0.73)* | 1.55 (1.16 ,2.07)* | 1.04 (0.65, 1.68) | 0.60 (0.51, 0.69)* | 1.51 (1.15 ,1.99)* | 1.44 (0.89, 2.33) |
| Interaction of Current and Prior Vaccination Status | ||||||
| Vac x Never | Ref | Ref | ||||
| Vac x First | 0.76 (0.44, 1.34) | 0.82 (0.47, 1.44) | ||||
| Vac x Sequential | 0.60 (0.42, 0.86)* | 0.67 (0.46, 0.97)* | ||||
| Vac x Interrupted | 0.96 (0.65, 1.41) | 1.09 (0.74, 1.60) | ||||
| Vac x Restarted | 1.07 (0.55, 2.06) | 0.70 (0.37, 1.32) | ||||
| Age Group | ||||||
| 68–74 years | 0.45 (0.39, 0.53)* | 0.45 (0.39, 0.53)* | ||||
| 75–84 years | Ref | Ref | ||||
| >85 years | 2.12 (1.71, 2.62)* | 2.55 (2.06, 3.18)* | ||||
| Gender | ||||||
| Male | Ref | Ref | ||||
| Female | 0.63 (0.55, 0.72)* | 0.61 (0.54, 0.71)* | ||||
Notes: *p<0.05; aRef: reference group.
Abbreviations: CI, confidence interval; Vac, vaccination.
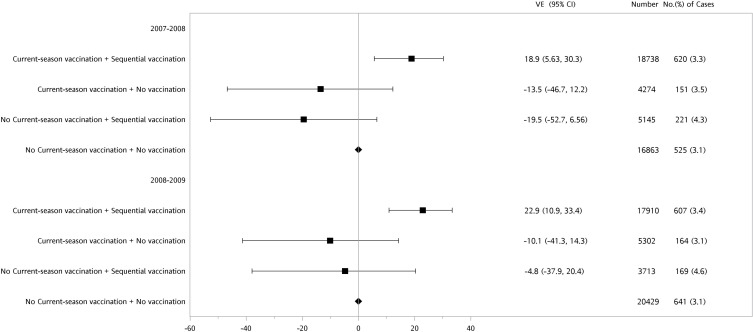
A forest plot of VE for the three different exposure groups is shown in Figure 1. Compared to the reference group with no current or prior vaccination history, the group with both current and sequential prior season vaccinations had the highest VE, with 18.9% (95% CI: 5.6, 30.3%) and 22.9% (95% CI: 10.9, 33.4%) during the 2007–08 and 2008–09 influenza seasons, respectively (Figure 1). The other two exposure groups, either receiving current-season vaccination only (current vaccine effect) or not receiving current vaccination but having prior sequential vaccination history (residual protection effect), demonstrated negative VE, with no statistical significance, in reducing P&I hospitalization during both influenza seasons.
Figure 1.
Adjusted vaccine effectiveness (VE) of sequential or never vaccination history among the elderly with or without current-season vaccination during 2007–08 or 2008–09 influenza seasons.
Abbreviation: CI, confidence interval.
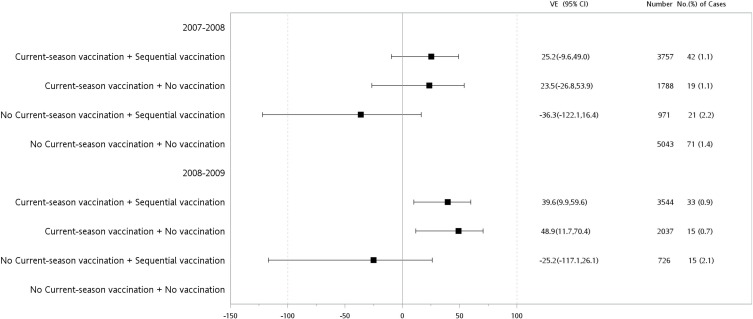
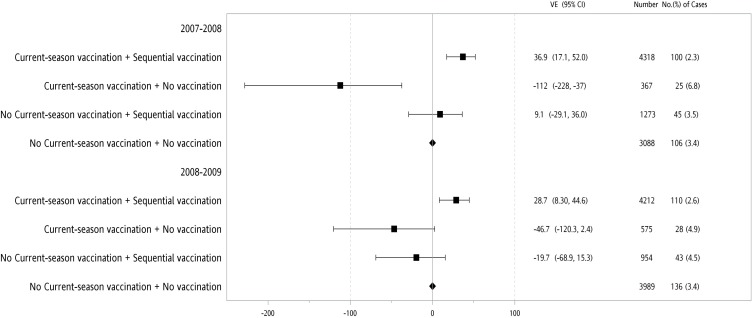
Since both gender and age group were independently associated with lower P&I hospitalization in the multivariate Cox proportional hazard model (Table 4), further stratification by gender and age group was performed. Our data showed that even during the 2007–08 influenza season, when the vaccine strains were unmatched with the circulation strains, sequential vaccinations reduced the P&I hospitalization in the female population aged 75–84 and 68–74 years, with adjusted VE values of 36.9% (95% CI: 17.1, 52.0%), with statistical significance, and 25.2% (95% CI: −9.6, 49.0%), without statistical significance, respectively (Figures 2 and 3).
Figure 2.
Adjusted vaccine effectiveness (VE) of sequential or never vaccination history among the female elderly aged 68–74 years with or without current-season vaccination during 2007–08 or 2008–09 influenza seasons.
Abbreviation: CI, confidence interval.
Figure 3.
Adjusted vaccine effectiveness (VE) of sequential or never vaccination history among the female elderly aged 75–84 years with or without current-season vaccination during 2007–08 or 2008–09 influenza seasons.
Abbreviation: CI, confidence interval.
Discussion
In this study using longitudinal influenza vaccination records, we examined the independent and combined effectiveness of current-season vaccination and prior vaccination history over multiple seasons in reducing the risk of P&I hospitalization. Although current vaccination alone did not significantly reduce P&I hospitalization, combined sequential prior and current influenza vaccinations significantly reduced the risk of P&I hospitalization among the elderly, by 18.9% and 22.9% during the 2007–08 and 2008–09 influenza seasons, respectively, whether the vaccine strains were matched with the currently circulating viruses or not. Our data support annual revaccination as an effective strategy to reduce P&I hospitalization during each influenza season, even though the vaccine strains were mismatched with the currently circulating strains.
The effect of influenza vaccination in reducing the risk of disease severity and further hospitalization remains inconclusive. The discrepancies in previous studies showed that the protective effect could be due to case ascertainment bias by using laboratory-confirmed cases when admitted into the hospital or intensive care unit (ICU), or even upon death.26–28 Further adjustment for confounding factors, including numberof medical visits, use of anti–viral treatment or length of stay in the ICU, and prior history of vaccination could also affect the results.29 Our study compared the VE of influenza vaccination by considering the following exposure groups: (1) current vaccine effect for the group with current-season vaccination with no prior vaccination history; (2) sequential protection effect for the group with both current and prior history of sequential vaccination; and (3) residual protection effect for the group with prior sequential vaccination with no current-season vaccination. Using this model, VE was estimated without being confounded by residual protection from the inclusion of subjects into the unvaccinated reference group who were vaccinated in prior influenza seasons, as suggested by the previous study.30 Based on our study, only sequential influenza vaccination significantly reduced the risk of P&I hospitalization after adjusting for gender and age confounding effects and propensity score matching.
Epidemiological studies have yielded inconsistent results on the clinical effectiveness of current-season influenza vaccination among persons with and without a prior history of influenza vaccination.12,23 The variability of revaccination efficacy may be due to antigenic differences between the vaccine and epidemic strains, which were rarely considered in epidemiological settings before the A/H1N1pdm09 pandemic in 2009.17,23,31,32 The current study considered the effect of vaccinations received in prior seasons on the VE of current-season vaccination during the years when the vaccine strains match or do not match with the circulating strains. Our data support that combined prior sequential and current influenza vaccinations could reach a significant VE of 22.9% when all three vaccine strains were completely matched during the 2008–09 influenza season; even though the three vaccine strains were completely mismatched, a similar VE of 18.9% among the elderly aged 65 years and older was shown. Our results are consistent with publications using meta-analysis, which suggested no significant reduction in VE when participants received TIV in both current and prior seasons, with the overall VE ranging from 17% to 67%, depending on the subtypes.33–36 Furthermore, influenza VE varies by influenza A subtype, with typically higher estimates against A (H1N1) viruses compared with A (H3N2).3 Since both 2007–08 and 2008–09 influenza seasons in Taiwan were dominated by H1N1 subtype A, the results were not affected by the circulating influenza subtype regardless of matching of the vaccine strains.
A unifying antigenic distance hypothesis, proposed by Smith et al, has been the widely accepted theoretical framework to explain the variability in VE after repeated TIV vaccination.21 Based on this hypothesis, vaccination by the current-season TIV strain (v2) with a history of receiving vaccine from a prior season (v1) represents an immunological outcome between pre-existing v1-induced antibody potentially interfering with v2 antigen and v2 stimulation of rapid v1 memory responses potentially protective against the current season’s epidemic strain (e). The profound negative effect of prior season vaccination (v1) on VE will be observed when the antigenic distance of v1 and v2 is small, but that of v1 and e is very large.37 Induction of cross-reactive antibodies preferentially focused toward epitopes selected from the memory pool through repeated vaccination could exacerbate the disease outcome owing to the poor neutralizing activity against the current season’s epidemic strain.38,39 However, such a hypothesis can be applied to only one prior season of vaccination history and is based entirely on hemagglutination inhibition (HI) antibody response.21 The adaptive immune response to other virus components (eg, HA2 or neuraminidase) is likely to contribute to the protective effect.40,41 The preferential induction or recruitment of regulatory T cells may also contribute to antagonistic T-cell responses with repeat influenza vaccination.42
An excess reduction of P&I hospitalization was observed in females aged 68–74 years, who received annual TIV vaccination sequentially (Figure 2). The different risks of hospitalization among different age categories of the elderly may reflect differences in age-related baseline health conditions. The elderly in the age group of 68–74 years had a lower rate of underlying diseases, and probably gained more benefit from revaccination than those in the highest age groups of older than 75 years. Further, the difference in the risk of P&I hospitalization after receiving vaccination between males and females could be due to the different immune responses by gender.43 Previous studies suggested that estrogen levels tend to promote stronger inflammatory, cellular and humoral immune responses in women than in men, and hence induce better protection among the female population.44,45
Our study has several limitations. First, P&I hospitalization without laboratory confirmation could dilute the VE if other respiratory-borne viral infections were concurrently circulating during the influenza season. Second, information bias may have occurred if the vaccination was not recorded. However, misclassification would likely be random because exposure was prospectively recorded before hospitalization occurred. This random misclassification would tend to reduce the size of the estimate, suggesting that the real protective effect could be even greater. Although both vaccinated and non-vaccinated groups presented similar distributions in most of the variables, some residual confounders by indication cannot be excluded in this study. Third, perceived good health has been reported as a reason for not receiving influenza vaccination,46 which would be likely to reduce the effect of HRs in this study. The potential bias due to healthy vaccine effect cannot be excluded but could be minimal since the group receiving current TIV showed higher numbers of comorbidities and higher risk of P&I hospitalization before matching. Lastly, since we focused our analysis on the influenza seasons before the A (H1N1)pdm09 pandemic in mid-2009, it is not possible to choose the H3N2 dominant seasons with at least 5 years' history of vaccination for comparison (Table S1).
In summary, our study supports the recommendation of yearly influenza vaccination for elderly individuals, not only for those with comorbid illnesses but also for those without comorbidity, specifically for female inpatients aged 65 years or older. Because influenza vaccination is inexpensive and safe, clinicians should recommend annual influenza revaccination for such patients.
Conclusions
This study evaluated the impact of repeated TIV vaccination on the reduction of influenza-associated P&I hospitalization among persons older than 64 years, considering the interaction between prior vaccination history and current-season vaccination over two influenza seasons: 2007–08, with all circulating virus strains mismatched, and 2008–09, with all virus strains matched with the vaccine strains. Our data support that annual revaccination is an effective strategy to reduce P&I hospitalization during each influenza season, even though the vaccine strains were mismatched with the currently circulating strains.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institute (registered number 97113). The interpretation and conclusion contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or National Health Research Institute. We are thankful for Gielenny Salem’s editing of the English for this manuscript.
Abbreviations
WHO, World Health Organization; VE, vaccine effectiveness; HA, hemagglutination; P&I, pneumonia and influenza; FIVP, free influenza vaccination program; NHI, National Health Insurance; NHIRD, National Health Insurance Research Database; Taiwan-CDC, Centers for Disease Control, Taiwan; Lab-ISN, Laboratory-based Influenza Virological Surveillance Network; TIV, trivalent influenza vaccine; OPD, outpatient department; URI, upper respiratory infection; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; CI, confidence interval; ILI, influenza-like illness; ICU, intensive care unit.
Ethics Statement
The database used consists of de-identified secondary data released for research purposes, which complies with the Personal Information Protection Act in Taiwan. Also, this study was approved by the Institutional Review Board of Taichung Hospital, Ministry of Health and Welfare, Taiwan (IRB number I102017).
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annu Rev Med. 2005;51:407–421. doi: 10.1146/annurev.med.51.1.407 [DOI] [PubMed] [Google Scholar]
- 2.Minney-Smith C, Selvey LA, Levy A, Smith DW. Post-pandemic influenza A/H1N1pdm09 is associated with more severe outcomes than A/H3N2 and other respiratory viruses in adult hospitalisations. Epidemiol Infect. 2019;147:e310. doi: 10.1017/S095026881900195X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson W, Shay D, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179 [DOI] [PubMed] [Google Scholar]
- 4.Houser K, Subbarao K. Influenza vaccines: challenges and solutions. Cell Host Microbe. 2015;17(3):295–300. doi: 10.1016/j.chom.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiore A, Bridges C, Cox N. Seasonal influenza vaccines. Curr Top Microbiol Immunol. 2009;333:43–82. doi: 10.1007/978-3-540-92165-3_3 [DOI] [PubMed] [Google Scholar]
- 6.Russell C, Jones T, Barr I, et al. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine. 2008;26(Suppl 4):D31–D34. doi: 10.1016/j.vaccine.2008.07.078 [DOI] [PubMed] [Google Scholar]
- 7.Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25:6852–6862. doi: 10.1016/j.vaccine.2007.07.027 [DOI] [PubMed] [Google Scholar]
- 8.Osterholm M, Kelley N, Sommer A, Belongia E. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X [DOI] [PubMed] [Google Scholar]
- 9.Shih S, Chen G, Yang C, et al. Laboratory-based surveillance and molecular epidemiology of influenza virus in Taiwan. J Clin Microbiol. 2005;43(4):1651–1661. doi: 10.1128/JCM.43.4.1651-1661.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Lee Y, Chan Y, et al. Influenza A virus in Taiwan, 1980–2006: phylogenetic and antigenic characteristics of the hemagglutinin gene. J Med Virol. 2009;81(8):1457–1470. doi: 10.1002/jmv.v81:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lian I, Wu H, Chang W, Chao D. The temporal trend of influenza-associated morbidity and the impact of early appearance of antigenic drifted strains in a Southeast Asian country. PLoS One. 2014;9(1):e84239. doi: 10.1371/journal.pone.0084239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keitel W, Cate T, Couch R, Huggins L, Hess K. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997;15(10):1114–1122. doi: 10.1016/S0264-410X(97)00003-0 [DOI] [PubMed] [Google Scholar]
- 13.Keitel W, Cate T, Couch R. Efficacy of sequential annual vaccination with inactivated influenza virus vaccine. Am J Epidemiol. 1998;127(2):353–364. doi: 10.1093/oxfordjournals.aje.a114809 [DOI] [PubMed] [Google Scholar]
- 14.Ohmit S, Petrie J, Malosh R, et al. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis. 2013;56(10):1363–1369. doi: 10.1093/cid/cit060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohmit S, Thompson M, Petrie J, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319–327. doi: 10.1093/cid/cit736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohmit S, Petrie J, Malosh R, Fry A, Thompson M, Monto A. Influenza vaccine effectiveness in households with children during the 2012–2013 season: assessments of prior vaccination and serologic susceptibility. J Infect Dis. 2015;211(10):1519–1528. doi: 10.1093/infdis/jiu650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLean H, Thompson M, Sundaram M, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. 2014;59(10):1375–1385. doi: 10.1093/cid/ciu680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohmit S, Petrie J, Malosh R, et al. Substantial influenza vaccine effectiveness in households with children during the 2013–2014 influenza season, when 2009 pandemic influenza A(H1N1) virus predominated. J Infect Dis. 2016;213(8):1229–1236. doi: 10.1093/infdis/jiv563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrie J, Ohmit S, Johnson E, Truscon R, Monto A. Persistence of antibodies to influenza hemagglutinin and neuraminidase following one or two years of influenza vaccination. J Infect Dis. 2015;212(12):1914–1922. doi: 10.1093/infdis/jiv313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rondy M, Launay O, Castilla J, et al. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine. 2017;35(34):4298–4306. doi: 10.1016/j.vaccine.2017.06.088 [DOI] [PubMed] [Google Scholar]
- 21.Smith D, Forrest S, Ackley D, Perelson A. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A. 1999;96(24):14001–14006. doi: 10.1073/pnas.96.24.14001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. In: Proceedings of the Twenty-sixthAnnual SAS Users group international conference: SAS Institute Inc; 2001:214–226. Available from: http://www212.sas.com/proceedings/sugi226/p214-226.pdf. [Google Scholar]
- 23.Voordouw A, Sturkenboom M, Dieleman J, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA. 2004;292(17):2089–2095. doi: 10.1001/jama.292.17.2089 [DOI] [PubMed] [Google Scholar]
- 24.Skowronski D, De Serres G, Crowcroft N, et al. Association between the 2008–09 seasonal influenza vaccine and pandemic H1N1 illness during spring-summer 2009: four observational studies from Canada. PLoS Med. 2010;7(4):e1000258. doi: 10.1371/journal.pmed.1000258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pebody R, Andrews N, Waight P, et al. No effect of 2008/09 seasonal influenza vaccination on the risk of pandemic H1N1 2009 influenza infection in England. Vaccine. 2011;29(14):2613–2618. doi: 10.1016/j.vaccine.2011.01.046 [DOI] [PubMed] [Google Scholar]
- 26.Arriola C, Anderson E, Baumbach J, et al. Does influenza vaccination modify influenza severity? Data on older adults hospitalized with influenza during the 2012–2013 season in the United States. J Infect Dis. 2015;212(8):1200–1208. doi: 10.1093/infdis/jiv200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean H, Meece J, Belongia E. Influenza vaccination and risk of hospitalization among adults with laboratory confirmed influenza illness. Vaccine. 2014;32:453–457. doi: 10.1016/j.vaccine.2013.11.060 [DOI] [PubMed] [Google Scholar]
- 28.Simonsen L, Taylor R, Viboud C, Miller M, Jackson L. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7:658–666. doi: 10.1016/S1473-3099(07)70236-0 [DOI] [PubMed] [Google Scholar]
- 29.Castilla J, Godoy P, Domínguez A, et al. Influenza vaccine effectiveness in preventing outpatient, inpatient, and severe cases of laboratory-confirmed influenza. Clin Infect Dis. 2013;57:167–175. doi: 10.1093/cid/cit194 [DOI] [PubMed] [Google Scholar]
- 30.Sullivan S, Kelly H. Stratified estimates of influenza vaccine effectiveness by prior vaccination: caution required. Clin Infect Dis. 2013;57(3):474–476. doi: 10.1093/cid/cit255 [DOI] [PubMed] [Google Scholar]
- 31.Beyer W, de Bruijn I, Palache A, Westendorp R, Osterhaus A. Protection against influenza after annually repeated vaccination: a meta-analysis of serologic and field studies. Arch Intern Med. 1999;159(2):182–188. doi: 10.1001/archinte.159.2.182 [DOI] [PubMed] [Google Scholar]
- 32.Looijmans-van den Akker I, Verheij T, Buskens E, Nichol K, Rutten G, Hak E. Clinical effectiveness of first and repeat influenza vaccination in adult and elderly diabetic patients. Diabetes Care. 2006;29(8):1771–1776. doi: 10.2337/dc05-2517 [DOI] [PubMed] [Google Scholar]
- 33.Bartoszko J, McNamara I, Aras O, et al. Does consecutive influenza vaccination reduce protection against influenza: a systematic review and meta-analysis. Vaccine. 2018;36(24):3434–3444. doi: 10.1016/j.vaccine.2018.04.049 [DOI] [PubMed] [Google Scholar]
- 34.Belongia E, Skowronski D, McLean H, Chambers C, Sundaram M, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines. 2017;16(7):1–14. doi: 10.1080/14760584.2017.1334554 [DOI] [PubMed] [Google Scholar]
- 35.Belongia E, Simpson M, King J, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–951. doi: 10.1016/S1473-3099(16)00129-8 [DOI] [PubMed] [Google Scholar]
- 36.Tricco A, Chit A, Soobiah C, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skowronski D, Chambers C, De Serres G, et al. Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A(H3N2) epidemics in Canada, 2010–2011 to 2014–2015. J Infect Dis. 2017;215(7):1059–1099. doi: 10.1093/infdis/jix074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skowronski D, Janjua N, De Serres G, et al. Cross-reactive and vaccine-induced antibody to an emerging swine-origin variant of influenza A virus subtype H3N2 (H3N2v). J Infect Dis. 2012;206(12):1852–1861. doi: 10.1093/infdis/jis500 [DOI] [PubMed] [Google Scholar]
- 39.Miller M, Gardner T, Krammer F, et al. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med. 2013;5(198):198ra107. doi: 10.1126/scitranslmed.3006637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monto A, Petrie J, Cross R, et al. Antibody to influenza virus neuraminidase: an independent correlate of protection. J Infect Dis. 2015;212(8):1191–1199. doi: 10.1093/infdis/jiv195 [DOI] [PubMed] [Google Scholar]
- 41.Christensen S, Toulmin S, Griesman T, et al. Assessing the protective potential of H1N1 influenza virus hemagglutinin head and stalk antibodies in humans. J Virol. 2019;93(8):e02134–E02118. doi: 10.1128/JVI.02134-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodewes R, Fraaij P, Kreijtz J, et al. Annual influenza vaccination affects the development of heterosubtypic immunity. Vaccine. 2012;30(51):7407–7410. doi: 10.1016/j.vaccine.2012.04.086 [DOI] [PubMed] [Google Scholar]
- 43.Klein S, Marriott I, Fish E. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15. doi: 10.1093/trstmh/tru167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furman D, Hejblum B, Simon N, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111(2):869–874. doi: 10.1073/pnas.1321060111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khurana S, Verma N, Talaat K, Karron R, Golding H. Immune response following H1N1pdm09 vaccination: differences in antibody repertoire and avidity in young adults and elderly populations stratified by age and gender. J Infect Dis. 2012;205(4):610–620. doi: 10.1093/infdis/jir791 [DOI] [PubMed] [Google Scholar]
- 46.van Essen G, Kuyvenhoven M, de Melker R. Why do healthy elderly people fail to comply with influenza vaccination? Age Ageing. 1997;26(4):275–279. doi: 10.1093/ageing/26.4.275 [DOI] [PubMed] [Google Scholar]