Abstract
Differentiation of oligodendrocytes (OL) from progenitor cells (OPC) is the result of a unique program of gene expression, which is further regulated by the formation of topological domains of association with the nuclear lamina. In this study, we show that cultured OPC were characterized by progressively declining levels of endogenous Lamin B1 (LMNB1) during differentiation into OL. We then identify the genes dynamically associated to the nuclear lamina component LMNB1 during this transition, using a well established technique called DamID, which is based on the ability of a bacterially-derived deoxyadenosine methylase (Dam), to modify genomic regions in close proximity. We expressed a fusion protein containing Dam and LMNB1 in OPC (OPCLMNB1-Dam) and either kept them proliferating or differentiated them into OL (OLLMNB1-Dam) and identified genes that were dynamically associated to LMNB1 with differentiation. Importantly, we identified Lss, the gene encoding for lanosterol synthase, a key enzyme in cholesterol synthesis, as associated to the nuclear lamina in OLLMNB1-Dam. This finding could at least in part explain the lipid dysregulation previously reported for mouse models of ADLD characterized by persistent LMNB1 expression in oligodendrocytes.
Keywords: Nuclear lamina, Myelin, Brain, Leukodystrophy
Introduction
Oligodendrocytes (OL) are the myelinating cells of the central nervous system. They derive from oligodendrocyte progenitor cells (OPC), which are generated from stem cells, via a unique program of gene expression with specific sets of genes being either up- or down- regulated [1], due to binding of specific transcription factors, epigenetic modifications (histone and DNA modifications as well as microRNA and lncRNAs) and higher-order chromatin organization including genomic interactions with the nuclear lamina [2-6]. In eukaryotic cells the nuclear lamina forms a protein network located at the nucleoplasmic surface of the inner nuclear membrane [7, 8]. Its function is essential for maintaining the structural integrity of the nucleus [9], guaranteeing genomic integrity during DNA replication/repair [10, 11], and recruiting transcription factors [12, 13]. The nuclear lamina has also been shown to interact with regions of heterochromatin [14] and to respond to mechano-stimulation [15, 16]. Genome wide molecular mapping studies have suggested that the genome is organized in discrete lamina-associated domains (i.e. LADs) which contain thousands of genes enriched in repressive histone marks (i.e. H3K27me3 and H3K9me2/3) and may be stably silenced by further recruitment to the nuclear periphery [17]. Two major classes of proteins have been shown to form the nuclear lamina. A-type lamins include Lamin A and C, representing alternatively spliced isoforms of the same Lmna gene [7]. B-type lamins include Lamin B1 and B2, which are encoded by two separate genes Lmnb1 and Lmnb2 [18]. LMNB1 is expressed in proliferating and undifferentiated cells including OPC [19], and its expression declines with differentiation [8]. Although A-type and B-type lamins share 56% sequence homology, which explains the similar rod-like structure [18], they also retain important differences which may account for the different roles at distinct stages of differentiation. This manuscript investigates the genomic regions associated with LMNB1 during the differentiation of oligodendrocyte progenitors.
While in physiological conditions LMNB1 levels decline as progenitors differentiate, its continued expression in OL has been detected in patients with autosomal dominant leukodystrophy (i.e. ADLD), a devastating late onset human demyelinating disease of the central nervous system [20-22]. The importance of downregulation of LMNB1 levels during the process of OPC differentiation into OL was further highlighted by the discovery that decreased levels of a specific miRNA (i.e. miR-23) regulating the levels of Lmnb1 precluded differentiation [23]. At least two types of genetic mutations and clinical syndromes have been identified. Duplication of the LMNB1 gene, has been linked to a disease characterized by demyelination, pyramidal and cerebellar symptoms, muscle wasting and autonomic nervous system dysfunction [20-22]. Later studies identified deletions occurring in the upstream regulatory regions of LMNB1 gene in a number of cases of ADLD with demyelination and CNS symptoms, but lacking autonomic dysfunction [24]. Importantly, both duplications and upstream genetic deletions in patients [22, 24], were characterized by the detection of persistent LMNB1 levels in differentiated cells, but the resulting molecular changes and the potential for inducing ADLD, remain elusive. More conclusive evidence was provided by the observation that transgenic mice with Lmnb1 overexpression in mature oligodendrocytes was sufficient to induce a late-onset motor phenotype, characterized by severe demyelination, axonal damage and neuronal loss, which was associated with profound alterations in the myelin lipid profile [25], a phenotype which could not be reproduced by overexpression in neurons or astrocytes [26].
In this study we used the Lamin DamID method [27] to identify the LMNB1-associated genomic regions in oligodendrocytes. This technique is based on the cell-specific expression of a fusion protein between the protein of interest and a bacterially-derived deoxyadenosine methylase (Dam) which adds methyl groups to adenines in the DNA recruited in close proximity to the fusion protein [28]. The LMNB1-Dam ID method has been successfully used before for the identification of LMNB1 associated genomic regions in Embryonic Stem Cells (ESC) and astrocytes [6]. We employed the same method in primary cultures of proliferating OPC and either kept them proliferating or differentiated into OLLMNB1-Dam. We reasoned that the identification of LMNB1 associated genes in these OLLMNB1-Dam might shed some light on the potential mechanisms underlying the lipid dysregulation reported in the mouse model of ADLD.
Experimental Procedures
Cell Cultures
Primary mouse OPC were isolated from the brain cortex of C57BL/6 mice at postnatal day 7 as previously described [29]. Biochemical experiments were conducted in OliNeu cells, kept in the presence of mitogens or cultured in differentiation medium as in [29].
Dam-ID Method
Primary mouse OPC were plated on day 1 and then transduced either with lentiviral vectors expressing either tethered LMNB1-Dam or untethered Dam on day 2. OPC expressing LMNB1-Dam or Dam alone were kept proliferating in the presence of mitogens (PDGF-AA 20 ng/ml and FGF 10 ng/ml). OL were differentiated by mitogen removal and thyroid hormone (T3 30 ng/ml) supplementation in chemically defined medium. Cells were harvested for isolation of genomic DNA after 72 h.
DamID was performed as previously described [28]. Briefly, genomic DNA was isolated from harvested cells. Adenine-methylated fragments were amplified from genomic DNA using a methylation specific PCR amplification protocol, and fragments purified using Qiaquick columns (Qiagen). The DamID PCR products were then sheared to a range of 100–500 bp with a peak around 300 bp. After shearing, the DNA was further purified and concentrated using Agencourt magnetic beads. The DNA was then analyzed on an Agilent BioAnalyzer 2100 DNA 7500 chip. The sheared material was used to prepare Illumina sequencing libraries using TruSeq DNA HT Sample Prep Kit according to the manufacturer’s instructions. Libraries were quantified on an Agilent BioAnalyzer 2100 DNA 7500 chip and processed for sequencing, using an Illumina HiSeq2500.
DamID Sequencing Data Processing and Analysis
Raw FASTQs were first filtered for reads containing any portion of the DamID primer sequence using cutadapt (v1.9.1, https://cutadapt.readthedocs.io/en/stable/#), ‘cutadapt --no-trim -g "GGTCGCGGCCGAGGATC" --no-indels -O 4 -o primer_reads.fastq.gz -untrimmed-output=no_primer_reads.fastq.gz > find_gatc_metrics’. Subsequently, reads containing the primer sequence were fully trimmed to remove all primer sequence except for the DpnI-associated GATC recognition site, ‘cutadapt -g GGTCGCGGCCGAG -a GATCCTCGGCCGCGACC --no-indels -o trimmed_gatc_reads.fastq.gz primer_reads.fastq. gz > trim_gatc_metrics’. Trimmed reads were aligned to the mm10 reference using BWA-MEM (v0.7.17-r1194-dirty, https://bio-bwa.sourceforge.net) [30]. Reads were further removed from the resulting BAM unless they contained the GATC sequence signature expected at either end of the alignment. BAMs from all runs for each sample were merged (MergeSamFiles) and duplicate marked (MarkDuplicates) using Picard (v1.140, https://broadinstitute.github.io/picard/index.html).
Quality control was performed on final BAM’s using FastQC (v0.11.3,https://www.bioinformatics.babraham.ac.uk/projects/fastqc/).
The GATC-containing alignments were then assigned to DpnI-digested genomic fragments depending on the orientation of the alignment with respect to the GA∣TC recognition site. The read counts per fragment were normalized by total library size, counts per million (CPM). Log2 ratios of LMNB1-Dam to the associated untethered Dam counts were calculated. Smoothing was performed by taking the mean of the log2 ratio over 400 bp non-adjacent windows. To define lamin-associated domains (LADs), smoothed log2 CPM values were segmented per chromosome after wavelet smoothing using a 2-state Hidden Markov Model (HMM) as implemented by HMMSeq (https://noble.gs.washington.edu/proj/hmmseg/) [31] ‘java -jar HMMSeg. jar --input-bed --smooth 20000 --output_list output_files. txt --parse both --num-states 2 input_files.txt’. To facilitate sample-to-sample comparisons, smoothed log2 values were further quantile normalized across all samples using custom R scripts. To perform differential analysis between LMNB1 associated signal in differentiating OL (T3) versus proliferating OPC (P+F) samples, we used the mean smoothed, quantile normalized signals over gene regions (upstream 10 kb, downstream 2 kb) over the known canonical gene coordinates for mm10 downloaded from the UCSC Table Browser (https://genome.ucsc.edu) [32]. Student’s T-tests were performed comparing the average signal for each gene in proliferating (PDGF+FGF) and differentiating (T3) conditions and p-values were Benjamini–Hochberg corrected. Adjusted p-values less than or equal to 0.05 were deemed significant. Data were deposited into the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) with provisionary accession number GSE135834.
Comparative Analysis of DamID Sequencing Data and Previously Published ESC Datasets
ESCs DamID data were obtained from Peric-Hupkes et al. [6]. As the ESC data were obtained using a microarray with probes and fluorescent signals, we obtained raw data and normalized them in R, by transforming all the numbers to Z scores to standardize the values between the two datasets. Normalized microarray data tables from Peric-Hupkes et al. [6] were obtained through NCBI’s GEO database. Nimblegen raw data were already log2 transformed and normalized using locally estimated scatterplot smoothing (LOESS). Normalized scores and the Nimblegen design file were brought together to identify genomic ranges of design probes. The data were then arranged into bedgraph file format through basic R tools. After sorting by chromosome number, the overlaps were removed and the new scores merged by summation using bedtools v2.27.1 on the Galaxy platform. Finally, the bedgraph files were converted to bigwig format on the Galaxy platform using the UCSC’s wig/bedgraph-to-bigwig tool v1.1.1.
Gene attribution was determined by first assigning gene IDs to microarray probes based on the overlap of genomic ranges, using the IRanges package v2.18.1 in R. Finally, in order to make the original microarray data in ESC compatible with our mm10 database, we converted genomic ranges from 9 to 10 mm assembly, using the UCSC liftOver application. Files in bedgraph format were imported and genomic ranges were merged to assign genes from the 10 mm UCSC known gene database (TxDb.Mmusculus.UCSC.mm10. knownGene). This allowed normalized datasets from our experiments to be compiled in R with previously published ones [6] and to be centered and scaled with basic R functions. P-values were generated with t-test function and corrected by Benjamini–Hochberg method in R. Only genes with a significant differential association or disassociation between ESC and OPC (padj-val < 0.05) were used for further analysis.
Correlation Between Association or Disassociation to LMNB1 and Transcript Levels
Expression data in the oligodendrocyte lineage were obtained using the Zhang et al. [33] database (https://web.stanford.edu/group/barres_lab/brain_rnaseq.html) and performing an enrichment for mature OL (mOL = including newly differentiated and myelinating) specific genes relative to OPC, to define “upregulated genes in mOL” and an enrichment for OPC genes relative to mOL, to define “downregulated genes in mOL”. The list of genes with significant differential association or disassociation to LMNB1 (ΔLamOPCmOL) was then overlapped with each of the two lists of genes either up or downregulation during differentiation of OPC into mOL expression (ΔExprOPCmOL). Additional information regarding specificity of gene expression was obtained by using published reference lists, such as Sharma et al. and McKenzie et al. [34, 35].
Gene Ontology Analysis
Gene Ontology (GO) analyses were obtained at https://amp.pharm.mssm.edu/Enrichr/ using the Enrichr [36, 37].
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde (PFA) for 15 min at room temperature and non specific binding was blocked by incubation in PGBA (0.1 M PB pH 7.4, 0.1% Gelatin porcine type A (Sigma G1890), 1% BSA (Sigma A8806) and 0.002% Sodium Azide), 5% normal goat serum and 0.1% Triton X-100 at room temperature for 1 h. Cells were then incubated overnight at 4 °C with primary antibodies anti-LMNB1 (rabbit polyclonal; Abcam ab16048, 1 μg/mL), anti-OLIG2 (mouse monoclonal, Millipore MABN50, 2 μg/mL), anti-MBP (rat monoclonal, Millipore MAB386, 2 μg/mL) and anti-V5 (mouse monoclonal, Fisher R960-25, 10 μg/mF). Next, cells were washed with PBS and incubated for 2 h with Alexa Fluor-conjugated secondary antibodies (Invitrogen Alexa Fluor: goat anti-mouse 546 2 μg/mF, goat anti-rabbit 488 2 μg/mF and goat anti-rat 647 2 μg/mF). DAPI (4′,6-diamidino-2-phenylindole) was used as a nuclear counterstain. Confocal images were captured using the Zeiss LSM-800 system. Quantification of the immunofluorescent intensity was performed on captured images, using ImageJ. The FMNB1 nuclear pixel intensity was quantified in n = 50 OLIG2 + cells for each condition and three independent biological replicates.
Western Blot Analysis
Protein lysates were obtained using RIPA buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% Sodium Deoxycholate) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μM Trichostatin A (TSA), phosphatase inhibitor cocktail, protease inhibitor cocktail freshly prepared (at 4 °C for 15 min). Lysates were incubated for 1 h at 4 °C and then centrifuged at 13,000 rpm for 10 min at 4 °C and the pellet discarded. The soluble fraction was collected and protein concentration was determined by Lowry protein assay (Biorad, 5000112). 4X SDS-PAGE loading buffer (200 mM Tris–HCl pH 6.8, 400 mM DTT, 8% SDS, 40% Glycerol, 0.4% Bromophenol blue, and 10% β-mercaptoethanol) was added to the samples that were boiled at 95 °C for 5 min to denature the proteins. 10 μg of proteins were loaded onto a 4-20% SDS-PAGE precast gel (Sigma, PCG2012) for protein separation (80 V for 120 min) and transferred (100 V for 60 min) to a PVDF membrane (Millipore, IPVH00010) activated in methanol. The membranes were blocked in TBS-T (TBS, 0.1% Tween 20) containing 5% milk for 1 h at room temperature and incubated overnight at 4 °C with the primary antibodies against LMNB1 (rabbit polyclonal; Abcam, ab16048, 0.05 μg/mL) and GAPDH (mouse monoclonal, Abcam, ab8245; 0.2 μg/mL) or anti-V5 (mouse monoclonal antibody, Fisher R960-25, 1 μg/mL). The following day, membranes were washed 3 times for 10 min each in TBS-T and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (diluted in blocking solution) for 1 h at room temperature. Secondary antibodies were diluted in blocking solution at a dilution of 1:10,000, anti-rabbit (Jackson immunoresearch, 211-032-171) and anti-mouse (Jackson Immunoresearch, 115-035-166). Proteins were exposed with ECL Prime western blotting detection reagent kit (GE healthcare, RPN2232) at a ChemiDoc XRS imaging system (Bio-Rad). Quantification of the LMNB1 signal was obtained by selecting the LMNB1 immunoreactive bands in ImageJ, measuring their intensities and referring those valued to the intensity of the GAPDH immunoreactive protein bands.
RT-PCR
Total mRNA was extracted using the RNeasy Mini Kit (Qiagen, 74106). For reverse transcription-polymerase chain reaction (RT-PCR), cDNA was synthesized with the qScript cDNA Synthesis Kit (Quantabio, 95047). Quantitative realtime reverse transcriptase PCR (qRT-PCR) reactions were run in triplicates, using the PerfeCTa SYBR Green FastMix ROX reagent (Quantabio, 95072) at the quantitative PCR core facility at the Epigenetic Core facility of the Advanced Science Research Center of the City University of New York (ASRC-CUNY). Relative standard curves were generated for each gene to determine relative expression (CT values are converted to arbitrary quantities of initial template per sample). Expression levels were then obtained by normalizing the data to the GEOMEAN of three housekeeping genes: Gapdh, m18S and Wdr33. PCR primer sequences used: Lss (F 5′-CTCCAGAATGAGTTGGGTCGG-3′; R 5′-CGCTTTTGGTAAGTCCGTGAAA-3′), Lmnb1(F 5′-AGCTCACCGGGCTCAAGGCT-3′; R 5′-AGCAGCAGCTGGTCGTGCTC-3′), Gapdh (F 5′-ACCCAGAAGACTGTGGATGG-3′; R 5′-CACATTGGGGGTAGGAACAC-3′), m18S (F 5′-AGTCCCTGCCCTTTGTACACA-3′; R 5′-GATCCGAGGGCCTCACTAAAC-3′) and Wdr33 (F 5′-TGATCTGGTCCCACCAATAG-3′ and R 5′-TGACCAATCGTCTTCCTTCC-3′).
Results
LMNB1 Levels Decrease During Differentiation of Progenitors into Oligodendrocytes
The stability of lineage-specific transcriptional programs involves the interaction of genomic regions with the nuclear periphery [38]. Previous studies reported the importance of LMNB1 in the progression from ESC to astrocytes [6], while this study is focused on the expression of LMNB1 and the identification of the dynamic association with genes in oligodendrocyte lineage cells. OPC were cultured from mouse brains and then kept proliferating in the presence of mitogens or differentiated by removing the mitogens and adding T3 to the culture medium (Fig. 1a). In these conditions, LMNB1 transcript levels have been reported to be high in progenitors and decline during the process of differentiation [33]. To define whether decreasing transcript levels resulted also in decreased protein levels, we used immunocytochemistry. OLIG2 immunoreactivity was used to identify all oligodendroglial cells while MBP immunoreactivity was used to identify differentiated OL. Consistent with the transcriptional data, LMNB1 characteristic nuclear immunoreactivity was higher in OLIG2+/MBP− OPC cultured in the presence of mitogens than in cells differentiated into OLIG2+/MBP+ oligodendrocytes (Fig. 1b).
Fig. 1.
LMNB1 protein levels decrease during oligodendrocyte differentiation. a Schematics of the declining LMNB1 levels during the transition from proliferating OPC (purple) to differentiated OL (light blue). b Expression of LMNB1 protein (green) in OPC and mOL detected by immunofluorescence with antibodies specific for the panoligodendroglial lineage marker OLIG2 (red) and the differentiation marker MBP (white). DNA counterstained by DAPI (blue). A representative image is shown. c The scatter plots represent the LMNB1signal intensity measured in at least 50 OLIG2+ nuclei in each condition (P < 0.005, t test) (Color figure online)
Identification of Oligodendrocyte Lineage-Specific Patterns of Gene Association to the Nuclear Lamina Component LMNB1
To begin defining the genes associated to the nuclear lamina we used a very well established and characterized technique called DamID, which relies on the expression of a fusion protein between LMNB1 and the bacterial enzyme deoxyadenosine methylase (Dam) in the cells of interest. The underlying principle of this approach is that DNA methylation in eukaryotic cells occurs on cytosine residues, while bacteria, such as E. coli, have the ability to also methylate adenine residues. The proximity between the fusion protein and genomic DNA, allows the bacterial enzyme to methylate adenosines within GATC sequences, which can then be identified by deep sequencing and define the LMNB1 interacting genomic regions.
For mouse primary OPCs we transduced the LMNB1-Dam tethered enzyme using lentiviral vectors and then either kept OPC proliferating in the presence of mitogens or induced them to differentiate by mitogen removal and addition of thyroid hormone T3 (Fig. 2a). Expression of untethered Dam in OPC cultured in the same conditions, was used to control for non-specific activity of the enzyme (Fig. 2a). To determine the effect of lentiviral transduction on the total LMNB1 levels in the cells, we conducted Western blot analysis of protein extracts from cells transduced with untethered or LMNB1 tethered Dam and compared the levels to those in uninfected cells (Fig. 2b). While endogenous levels of LMNB1 were found to decline during the differentiation of OPC into OL in control conditions, they remain elevated in cells transduced with the LMNB1-Dam fusion protein (Fig. 2b). The results were independently validated by immunocytochemistry, using an antibody against LMNB1 to detect the total levels and one against the V5 tag (present in the expression vector and therefore identifying the transduced cells) which revealed higher levels of immunoreactivity in differentiated OL (OLLMNB1-Dam) expressing LMNB1-Dam (Fig. 2c). The persistence of high levels of LMNB1 protein in differentiated OLLMNB1-Dam was reminiscent of cells from patients with adrenoleukodistrophy consequent to genetic mutations of LMNB1. Therefore, we sought to identify genes associated to LMNB1 in OPCLMNB1-Dam, as the ones detected in physiological conditions, and those associated to LMNB1 in OLLMNB1-Dam, as the ones possibly detected in pathological conditions consequent to genetic LMNB1 mutations resulting in the persistence of this nuclear lamina component in OL.
Fig. 2.
An approach using the bacterial enzyme Dam fused to LMNB1 to identify regions of genomic association in oligodendrocyte lineage cells. a Schematics of the experimental approach. Top panel: OPC transduced with lentiviruses containing untethered Dam expression vector, were used as controls for random methylation by the untethered deoxyadenosine methylase Dam. Bottom panel: OPC transduced with the tethered LMNB1-Dam expression vector to allow Dam to methylate adenines on DNA regions in close proximity to LMNB1. After transduction, OPC were either kept proliferating in the presence of mitogens or differentiated by mitogen withdrawal and T3 addition. b LMNB1 protein levels detected by Western Blot of protein extracts from uninfected OPC kept in proliferating conditions (mitogens) or differentiated into OL (T3), from OPC transduced with untethered Dam and from cultures transduced with tethered LMNB1-Dam. The intensity of the immunoreactive LMNB1 bands was referred to the protein levels of GAPDH in each sample for quantification and the numerical values of the ratios are shown. c Nuclear localization of LMNB1-Dam fused protein transiently expressed in the nucleus of OliNeu cells and detected by immunofluorescent labeling of the V5 epitope tag (present on the expression vector) in order to identify transduced cells (red). Total LMNB1 (green) immunoreactivity, recognizing both endogenous and exogenously expressed proteins is shown. DNA counterstained by Dapi (blue). Scale bar = 10um (Color figure online)
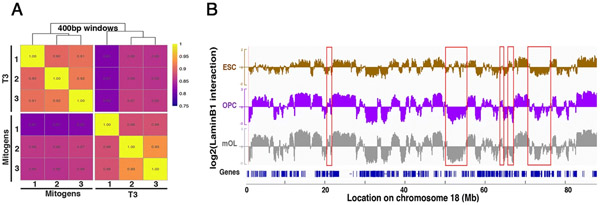
After genomic DNA isolation from OPCLMNB1-Dam, OLLMNB1-Dam and from control cultures expressing untethered Dam, DNA was amplified, digested with methylationsensitive enzymes, and subjected to deep sequencing [28]. The experiment was conducted in three biological replicates for each condition, and the high Pearson’s correlation coefficient among triplicates, revealed a high degree of consistency (Fig. 3a). Genomic regions of association with LMNB1 were defined using the value of the log2-transformed LMNB1-Dam over untethered Dam methylation ratio. Smoothed ratios, calculated by taking the mean over 400 bp adjacent windows, were visualized using Integrative Genomics Viewer (IGV). The presence of enriched methylated adenine signal in a given region of the genome of cells expressing LMNB1-Dam compared to the signal from cells expressing untethered Dam, would be represented as a positive log2-ratio and indicate association of that specific region to the nuclear LMNB1 (Fig. 2c). In contrast, a negative log2-ratio would be indicative of lack of association. As the DamID approach has been previously adopted to define the regions of nuclear lamina association in murine ESC, we also compared our data to this published dataset [6].
Fig. 3.
Chromosome 18 map of nuclear lamina interactions defines regions of dynamic LMNB1 association in oligodendroglial lineage cells compared to embryonic stem cells. a Heat map representation of the DamID data from three biological replicates each for OPC and mOL. b Genomic regions of chromosome 18 showing LMNB1 association in ESCs (brown), oligodendrocyte progenitors, OPC (purple) and mature oligodendrocyte, mOL (gray). For OPC and mOL the traces represent IGV curves of regions of LMNB1 association. The y axis indicates the log2-transformed ratio of the methylation signal in OPC or OL expressing LMNB1-Dam fusion protein over the signal detected in cells expressing only Dam of chromosome 18 of chromosome 18. Blue lines at the bottom represent genes on chromosome 18 and respective genomic coordinates are indicated below. The red boxes highlight lineage-specific chromosomal regions of reorganization (Color figure online)
Since in physiological conditions LMNB1 is expressed at high levels in both ESC and OPC [9, 19, 39], we reasoned that a comparison of the tethered LMNB1-Dam signal in these two cell types would help elucidate any genomic reorganization occurring during oligodendrocyte lineage commitment. Genes, such as Caln1, which encodes the calcium-binding protein calneuron-1 that is highly enriched in neurons [40] or Gypc that encodes glycophorin C a glycoprotein that provides mechanical stability to red blood cells [41] and CamK4 encoding the neuronal specific calcium/calmodulin dependent protein kinase IV implicated in transcriptional regulation [42] showed LMNB1 association only in lineage committed proliferating OPC and not in ESC (Fig. 4a). Interrogation of available transcriptional datasets confirmed the interpretation of these results (Supplemental Fig. 1). Genes involved in cell cycle regulation, such as E2f7 [43] or cell cycle checkpoint control such as Rad9b [44] in contrast, were not recruited to the nuclear LMNB1 (Fig. 4b), and this was consistent with the proliferative capacity of both cell types. Genes expressed during the late stages of oligodendrocyte differentiation, in contrast, such as Ptprd, the gene encoding for a protein phosphatase receptor type D [45], or Semaphorin 6a which is encoded by Sema6a, a gene required for proper development of the neural tissue [46] displayed a clear association to the nuclear lamin in ESC and detachment in OPC (Fig. 4c). Additional genes characteristic of the oligodendrocyte lineage, such as Olig1 and Olig2 (Supplemental Fig. 2) which have critical roles in regulating oligodendrocyte development, showed a similar pattern of association. From this analysis, we conclude that the association of genomic regions to the nuclear lamina is a dynamic process, dependent on the functional role of the gene product and the differentiation state of the cell.
Fig. 4.
Dynamic association of genes to the nuclear lamina during the transition from ESC to OPC. The figure shows the IGV curve profile of the log2-transformed LMNB1-Dam/ of chromosome 18 Dam methylation ratios in ESC (brown) and OPC (purple). Representative examples are provided. a The genomic region encoding for a neuronal gene (Caln1) becomes attached to the nuclear lamina during the transition from ESC to OPC. Similar trend occurs for the genomic region of glycophorin C (Gypc) and for the neuronal specific calcium/ calmodulin dependent protein kinase IV (Camk4). b Genomic regions encoding for cell cycle-regulatory genes (E2f7) and for the cell cycle checkpoint control gene (Rad9b) remain detached from the nuclear lamina, consistent with the proliferative state of these cells. c The genomic regions encoding for oligodendrocyte specific genes Ptprd and Sema6a lose their attachment to the nuclear LMNB1 during the transition from ESC to OPC (Color figure online)
To study the entity of the global changes of genome reorganization occurring during the transition from ESC to OPC we performed a comparative analysis between our data in OPCLMNB1-Dam, and the previously reported regions of LMNB1 association in ESC [6]. Since LMNB1 was expressed at high levels in OPC [19], we reasoned that the regions of dynamic association detected during the transition from ESC to OPCLMNB1-Dam, would be representative of those occurring in physiological conditions (Fig. 5a). Of the 21,618 genes detected in ESC cells, the progression into OPC was accompanied by 1251 genes recruited to LMNB1 and 1548 genes moving away from LMNB1. Importantly, the genes associated with LMNB1 when ESC were specified to the astrocytic lineage [6], were distinct from those detected in OPCLMNB1-Dam and suggested a divergent and lineage-specific association for each glial cell type.
Fig. 5.
Relationship between association of genes to the nuclear lamina component LMNB1 and their expression profile in oligodendrocyte lineage cells. a Bar graphs represent genes either unchanged (yellow) or showing a statistically significant (p-val < 0.05) association (red) or detachment (green) from LMNB1 during the transition from ESC to OPCLMNB1-Dam (left panel) or during the transition from OPCLMNB1-Dam to OLLMNB1-Dam (right panel). b Venn diagram of the overlap between genes with LMNB1-dynamic association (red) or not association (green) during the transition from OPCLMNB1-Dam to OLLMNB1-Dam and transcripts either upregulated (gray in upper panel) or downregulated (gray in lower panel) during normal OL differentiation (data from Zhang et al. [33]). c, d Bar graphs of genes with differential association to nuclear lamina (top) during the transition from OPCLMNB1-Dam to OLLMNB1-Dam and corresponding expression levels for the indicated genes according to Zhang et al. [33] (bottom). Positive ΔLam(OPCmOL) values (red bars) are indicative of gene attachment to nuclear lamina (c). Negative ΔLam(OPCmOL) values (green bars) are indicative of gene detachment from LMNB1 (d). The upward or downward direction of the gray bars (ΔExpr(OPCmOL) indicate upregulated or downregulated transcript during the l differentiation of OPC into mOL (data from Zhang et al. [33]) (Color figure online)
Identification of Nuclear Lamina Associated Genomic Regions in Oligodendrocytes Expressing LMNB1
We then sought to identify genes displaying a dynamic association to the nuclear lamina during the transition from OPCLMNB1-Dam to OLLMNB1-Dam. Since the expression Of LMNB1 dramatically declines during the process of differentiation but remains elevated in pathological conditions such as genetic mutations of regulatory regions or duplications of LMNB1 [21] we reasoned that such a comparison could provide some important leads to the molecular underpinning of the pathological process leading to dysmyelination in these specific forms of ADLD. Interestingly, a smaller number of genes showed dynamic association (n = 409) or disassociation (n = 262) during this transition while 18,441 genes remained unchanged (Fig. 5A).
To begin addressing the relationship between association to LMNB1 and expression levels in cultured OPC and OL, we intersected our data sets with genes either upregulated or downregulated during the process of oligodendrocyte differentiation. Of the 409 genes which associated to LMNB1 in OLLMNB1-Dam only 10 overlapped with transcripts characteristic of the differentiated state (including genes like Lss, regulating cholesterol biosynthesis) and 26 overlapped with transcripts characteristic of the progenitor state (including genes like Myt1). These results are consistent with the interpretation that the persistence of LMNB1 levels does not preclude the differentiation of OPCs into OLs, although it may alter the functional state of mature cells. The comparison between genes showing dynamic association to (Fig. 5c) or disassociation from (Fig. 5d) LMNB1 and transcripts that in physiological conditions are upregulated (Fig. 5c) or downregulated (Fig. 5d) during differentiation, further highlighted the complex and not linear relationship between nuclear lamina association and expression levels, which are modulated by transcription factor binding and epigenetic modifications.
Next, we conducted a Gene Ontology analysis (Enrichr) on the genes found to be associated to the nuclear lamina during the transition from OPCLMNB1-Dam to OLLMNB1-Dam which revealed several genes related to lipid metabolism (Fig. 6a). Among these genes we decided to focus on Lss, because it is a key enzyme in cholesterol biosynthesis (Fig. 6b) and its expression levels increase during the physiological process of OL differentiation (Fig. 6c). A comparison of the transcript levels in OLLMNB1-Dam compared to untransduced cells suggested an interesting relation between high levels of LMNB1 and low levels of Lss (Fig. 6d), and increased association to the nuclear lamina (Fig. 6e). Since OLLMNB1-Dam mimic a pathological condition characterized by persistent.
Fig. 6.
The cholesterol pathway regulator Lss is retained at the nuclear periphery by LMNB1 interaction when its expression persists in mOL. a The scale of green to red is representative of gene castegories with statistically significant LMNB1 association in mOLLMNB1-Dam compared to OPC LMNB1-Dam. GO categories involved in lipid metabolism show increased association to LMNB1. b Among these genes, the lanosterol synthase Lss which catalyzes the conversion of squalene epoxide to lanosterol in the biosynthesis of cholesterol, was identified. c In physiological conditions, Lss transcript levels increase during OPC differentiation into mOL according to Zhang et al. [33]. d Bar graph shows preliminary data on the transcript levels of Lss relative to Gapdh conducted in triplicate on a single biological replicate. The levels of transcripts in untransduced oligodendrocytes (mOL) differentiated from OPC are shown in comparison to the levels detected in OL with persistent expression of LMNB1 (mOL LMNB1), differentiated from OPCLMNB1-Dam. e Visualization of IGV curves showing the LMNB1 association profile of the Lss gene in mOLLMNB1-Dam compared to OPCLMNB1-Dam and to ESC (Color figure online)
LMNB1 expression in mature OL, and transgenic mice expressing Lmnb1 in OL are characterized by a late onset dysmyelinating pathology, driven by altered lipid metabolism, we suggest that our data may provide a molecular explanation to the pathophysiology of genetic form of ADLD related to LMNB1 levels.
Discussion
The generation of cells with a unique functional identity is essential for multi-organ diversity. This is achieved by the integration of transcriptional and epigenetic events (which include histone and DNA modifications and non-coding RNAs). As the stability of cell identity is paramount for the physiological function of the organism, cells have developed molecular mechanisms that guarantee the stability of each unique and specific transcriptional program. These mechanisms include the topological arrangement of chromatin in contiguous transcriptionally active and inactive domains, called TADs (for “topologically associated domains”) [46]. The TADs containing transcriptionally silent genes show a particular propensity to localize at the nuclear periphery and associate to the nuclear lamina, and have therefore been named LADs (for “lamina associated domains”) and were initially identified in D. melanogaster [47]. Studies in C. elegans identified regions of association to the nuclear lamina as enriched in repressive histone marks [48], a concept which was later validated in mammalian cells [49-51]. Further distinction between constitutive and facultative LADs led to a conceptual extension of their functional role [52]. “Constitutive LADs” [53] define regions of stable contact between the genome and the nuclear lamina, and have been proposed to serve as “anchoring points” for chromosomal territories [54] while “facultative LADs” define cell-state specific regions of association [6, 55].
The nuclear lamina is the 30–100 nm-thick filamentous network lining the inner side of the nuclear envelope. It is formed by filamentous proteins called lamins, whose structure is composed of a short N-terminus (forming a globular head), a central alpha-helix (forming the rod domain) and a C-terminus containing a nuclear localization signal and potential regions of protein interaction [9, 18]. Lamins of B and A type differ by amino acid sequence, as they only share 56% homology. They are encoded by distinct genes [56], are characterized by a cell-specific pattern of gene expression, with LMNB1 being expressed in progenitors and LMNA in differentiated cells [19]. Mutations of their genes are associated with very distinct pathologies called laminopathies [57]. Human studies on LMNA gene mutations, for instance, have been associated with 12 distinct types of laminopathies, including progeria [58], LMNB2 mutations have been associated with lipodystrophy and epilepsy [59, 60] and LMNB1 mutations associated with the late onset ADLD. At least two types of human genetic mutations have been associated with this fatal disease, which results in severe myelin damage starting in the fourth or fifth decade of life [61]. They include duplication [21] or 600 kb upstream deletion of regulatory elements of the LMNB1 gene [24], both resulting in the persistence of LMNB1 protein in differentiated cells [21, 24, 43].
Transgenic mouse models overexpressing Lmnb1 in oligodendrocytes were published by three groups [25, 26, 62], and all reported late onset myelin degeneration. However, the underlying cause of the pathology remained elusive. There was general consensus on the fact that oligodendrocyte survival was not affected in Plp overexpressing transgenic mice [63], but distinct hypotheses were suggested to explain the phenotype. The group of Heng et al. [26], for instance, suggested that dysmyelination could be consequent to lower transcript levels of myelin genes and consequent aberrant myelin formation. However, no changes in myelin gene transcripts were detected by the group of Rolyan et al. [25], who identified alterations in lipid composition of myelin and aberrant histone marks.
Mechanisms of epigenetic regulation requiring repressive histone marks and the interaction between cytoplasm and nuclear envelope, have been highlighted in previous reviews discussing the molecular basis for the acquisition of oligodendrocyte cell identity [64-69]. The phenotype of the transgenic mice and the link between chromatin repressive marks and nuclear lamins, prompted us to use the well-established technique DamID, to identify the specific regions of interaction between nuclear LMNB1 and genomic DNA in oligodendrocyte cells [27, 28]. This approach is well accepted for the identification of regions of attachment to the nuclear lamina and was previously used to study the reorganization of the genome in astrocytes differentiated from murine ESC [6] and also during the process of cardiac stem cell lineage restriction [55]. Our data identify differential regions of association to the nuclear LMNB1 between ESC and OPC with the recruitment of thousands of genes, presumably for the establishment of chromosomal territories.
In addition, we identify genes associated to LMNB1 in OL (Fig. 7) with persistent expression of this nuclear lamina component (OLLMNB1-Dam), and reflecting a pathological state previously described for specific forms of ADLD. Intriguingly, the analysis of the genomic regions of nuclear lamina association in OLLMNB1-Dam identified genes with a critical role in lipid metabolism, whose expression was inversely related to LMNB1 levels. We found this finding quite intriguing, since mouse models of ADLD caused by persistent expression of LMNB1 in OL showed clear signs of myelin damage consequent to altered lipid metabolism [25].
Fig. 7.
Model representing the dynamic genomic regions of association to the nuclear Lamin B1 in oligodendrocyte lineage cells in health and disease. Upper panel depicts the physiological state characterized by decreasing levels of LMNB1 (gray triangle) as progenitors (purple cells) differentiate into oligodendrocytes (pale blue on the upper right). In red are regions of attachment corresponding to genes that are silenced in progenitor cells (e.g. pluripotency genes) or in mature oligodendrocytes (e.g. cell cycle genes). In green are regions of “detachment” from the nuclear lamina, corresponding to active transcription in progenitors (e.g. RNA processing) or in oligodendrocytes (e.g. lipid metabolism genes). The lower panel indicate our results obtained in oligodendrocytes that are forced to continue to express LMNB1 during differentiation into oligodendrocytes (yellow bar). Note that, in these conditions, which mimic those detected in leukodystrophy, genes involved in lipid metabolism (e.g. Lss) remain attached to the nuclear lamina (Color figure online)
In conclusion, this manuscript provides the first molecular map of genomic reorganization and interaction with the nuclear lamina occurring at the early stages of oligodendrocyte lineage commitment from ESC cells, while also identifying regions of genomic association to LMNB1 occurring when the expression of LMNB1 persists in differentiated cells, a condition with resembles the one reported in models of ADLD caused by genetic mutations of LMNB1.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Bas van Steensel and Daan Peric-Hupkes for generous sharing of reagents and protocols and for providing invaluable suggestions. This work was supported by Grant R35 NS111604 to PC.
Footnotes
Special Issue: In Honor of Professor Vittorio Gallo.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11064-019-02941-y) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Young RA (2011) Control of the embryonic stem cell state. Cell 144:940–954. 10.1016/j.cell.2011.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaret KS, Carroll JS (2011) Pioneer transcription factors : establishing competence for gene expression Parameters affecting transcription factor access to target sites in chromatin Initiating events in chromatin : pioneer factors bind first. Genes Dev. 10.1101/gad.176826.111.GENES [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohn F, Schubeler D (2009) Genetics and epigenetics: stability and plasticity during cellular differentiation. Trends Genet 25:129–136. 10.1016/j.tig.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 4.Catalanotto C, Cogoni C, Zardo G (2016) MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. 10.3390/ijms17101712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, Reinberg D (2011) Chromatin higher-order structures and gene regulation. Curr Opin Genet Dev 21:175–186. 10.1016/j.gde.2011.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peric-Hupkes D, Meuleman W, Pagie L et al. (2010) Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 10.1016/j.molcel.2010.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dittmer T, Misteli T (2011) The lamin protein family. Genome Biol. 10.1186/gb-2011-12-5-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung H-J, Nobumori C, Goulbourne CN et al. (2013) Farnesylation of lamin B1 is important for retention of nuclear chromatin during neuronal migration. Proc Natl Acad Sci USA 110:E1923–E1932. 10.1073/pnas.1303916110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dechat T, Pfleghaar K, Sengupta K et al. (2008) Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 22:832–853. 10.1101/gad.1652708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalo S (2014) DNA Damage and Lamins In: Schirmer EC, de las Heras JI (eds) Cancer biology and the nuclear envelope. Advances in experimental medicine and biology, vol 773 Springer, pp 377–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butin-Israeli V, Adam SA, Jain N et al. (2015) Role of lamin B1 in chromatin instability. Mol Cell Biol 35:884–898. 10.1128/mcb.01145-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrés V, González JM (2009) Role of A-type lamins in signaling, transcription, and chromatin organization. J Cell Biol 187:945–957. 10.1083/jcb.200904124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camozzi D, Capanni C, Cenni V, et al. (2014) Diverse lamindependent mechanisms interact to control chromatin dynamics. Nucleus 5(5):427–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naetar N, Ferraioli S, Foisner R (2017) Lamins in the nuclear interior—life outside the lamina. J Cell Sci 130:2087–2096. 10.1242/jcs.203430 [DOI] [PubMed] [Google Scholar]
- 15.Swift J, Ivanovska IL, Buxboim A, et al. (2013) Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science (80- ) 341:. 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swift J, Discher DE (2014) The nuclear lamina is mechanoresponsive to ECM elasticity in mature tissue. J Cell Sci 127:3005–3015. 10.1242/jcs.149203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lochs SJA, Kefalopoulou S, Kind J (2019) Lamina associated domains and gene regulation in development and cancer. Cells 8:271 10.3390/cells8030271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dechat T, Adam SA, Taimen P et al. (2010) Nuclear lamins (review). Cold Spring Harb Perspect Biol 2:1–23. 10.1101/cshperspect.a000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takamori Y, Hirahara Y, Wakabayashi T et al. (2018) Differential expression of nuclear lamin subtypes in the neural cells of the adult rat cerebral cortex. IBRO Rep 5:99–109. 10.1016/j.ibror.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffeen CM (2000) Genetic localization of an autosomal dominant leukodystrophy mimicking chronic progressive multiple sclerosis to chromosome 5q31. Hum Mol Genet 9:787–793. 10.1093/hmg/9.5.787 [DOI] [PubMed] [Google Scholar]
- 21.Padiath QS, Saigoh K, Schiffmann R et al. (2006) Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet 38:1114–1123. 10.1038/ng1872 [DOI] [PubMed] [Google Scholar]
- 22.Padiath QS (2016) Lamin B1 mediated demyelination: linking lamins, lipids and leukodystrophies. Nucleus 7:547–553. 10.1080/19491034.2016.1260799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S-T, Fu Y-H (2009) miR-23 regulation of lamin B1 is crucial for oligodendrocyte development and myelination. Dis Model Mech 2:178–188. 10.1242/dmm.001065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giorgio E, Robyr D, Spielmann M et al. (2014) A large genomic deletion leads to enhancer adoption by the lamin B1 gene: a second path to autosomal dominant adult-onset demyelinating leukodystrophy (ADLD). Hum Mol Genet 24:3143–3154. 10.1093/hmg/ddv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolyan H, Nmezi BC, Chen J et al. (2015) Defects of lipid synthesis are linked to the age-dependent demyelination caused by Lamin B1 overexpression. J Neurosci 35:2002–12017. 10.1523/JNEUROSCI.1668-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heng MY, Lin ST, Verret L et al. (2013) Lamin B1 mediates cell-autonomous neuropathology in a leukodystrophy mouse model. J Clin Invest 123:2719–2729. 10.1172/JCI66737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Steensel B, Henikoff S (2000) Steensel.Henikoff.DamID. NatBiotech.2000 18 [Google Scholar]
- 28.Vogel MJ, Peric-Hupkes D, van Steensel B (2007) Detection of in vivo protein - DNA interactions using DamID in mammalian cells. Nat Protoc 2:1467–1478. 10.1038/nprot.2007.148 [DOI] [PubMed] [Google Scholar]
- 29.Scaglione A, Patzig J, Liang J et al. (2018) PRMT5-mediated regulation of developmental myelination. Nat Commun. 10.1038/s41467-018-04863-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595. 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Day N, Hemmaplardh A, Thurman RE et al. (2007) Unsupervised segmentation of continuous genomic data. Bioinformatics 23:1424–1426. 10.1093/bioinformatics/btm096 [DOI] [PubMed] [Google Scholar]
- 32.Karolchik D (2003) The UCSC Table Browser data retrieval tool. Nucleic Acids Res 32:493D–496. 10.1093/nar/gkh103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Chen K, Sloan SA et al. (2014) An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 34:11929–11947. 10.1523/jneurosci.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma K, Schmitt S, Bergner CG et al. (2015) Cell type- and brain region-resolved mouse brain proteome. Nat Neurosci 18:1819–1831. 10.1038/nn.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenzie AT, Wang M, Hauberg ME et al. (2018) Brain cell type specific gene expression and co-expression network architectures. Sci Rep 8:1–19. 10.1038/s41598-018-27293-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen EY, Tan CM, Kou Y et al. (2013) Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 10.1186/1471-2105-14-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuleshov MV, Jones MR, Rouillard AD et al. (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44:W90–W97. 10.1093/nar/gkw377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorkin DU, Leung D, Ren B (2014) The 3D genome in transcriptional regulation and pluripotency. Cell Stem Cell 14:762–775. 10.1016/j.stem.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu YQ, Lin X, Liu CM et al. (2001) Identification of a human brain-specific gene, calneuron 1, a new member of the calmodulin superfamily. Mol Genet Metab 72:343–350. 10.1006/mgme.2001.3160 [DOI] [PubMed] [Google Scholar]
- 40.Swiss VA, Nguyen T, Dugas J et al. (2011) Identification of a gene regulatory network necessary for the initiation of oligodendrocyte differentiation. PLoS ONE. 10.1371/journal.pone.0018088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Den KR, Raymond Y, Ramaekers FCS et al. (1997) A- and B-type lamins are differentially expressed in normal human tissues. Histochem Cell Biol 107:505–517 [DOI] [PubMed] [Google Scholar]
- 42.Takamori Y, Tamura Y, Kataoka Y et al. (2007) Differential expression of nuclear lamin, the major component of nuclear lamina, during neurogenesis in two germinal regions of adult rat brain. Eur J Neurosci 25:1653–1662. 10.1111/j.1460-9568.2007.05450.x [DOI] [PubMed] [Google Scholar]
- 43.Giorgio E, Rolyan H, Kropp L et al. (2013) Analysis of LMNB1 duplications in autosomal dominant leukodystrophy provides insights into duplication mechanisms and allele-specific expression. Hum Mutat 34:1160–1171. 10.1002/humu.22348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Y, Casaccia P, Richard LuQ (2010) Shaping the oligodendrocyte identity by epigenetic control. Epigenetics 5:124–128. 10.4161/epi.5.2.11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emery B (2010) Regulation of oligodendrocyte differentiation and myelination. Science (80-) 330:779–782. 10.1126/science.1190927 [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Sandoval A, Gasser SM (2016) On TADs and LADs: spatial control over gene expression. Trends Genet 32:485–495. 10.1016/j.tig.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 47.Pickersgill H, Kalverda B, De Wit E et al. (2006) Characterization of the Drosophila melanogaster genome at the nuclear lamina. Nat Genet 38:1005–1014. 10.1038/ng1852 [DOI] [PubMed] [Google Scholar]
- 48.Towbin BD, González-Aguilera C, Sack R et al. (2012) Stepwise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150:934–947. 10.1016/j.cell.2012.06.051 [DOI] [PubMed] [Google Scholar]
- 49.Harr JC, Luperchio TR, Wong X et al. (2015) Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J Cell Biol 208:33–52. 10.1083/jcb.201405110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harr JC, Gonzalez-Sandoval A, Gasser SM (2016) Histones and histone modifications in perinuclear chromatin anchoring: from yeast to man. EMBO Rep 17:139–155. 10.15252/embr.201541809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solovei I, Wang AS, Thanisch K et al. (2013) LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 152:584–598. 10.1016/j.cell.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 52.van Steensel B, Belmont AS (2017) Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell 169:780–791. 10.1016/j.cell.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meuleman W, Peric-Hupkes D, Kind J et al. (2013) Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence. Genome Res 23:270–280. 10.1101/gr.141028.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yáñez-Cuna JO, van Steensel B (2017) Genome–nuclear lamina interactions: from cell populations to single cells. Curr Opin Genet Dev 43:67–72. 10.1016/j.gde.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 55.Poleshko A, Shah PP, Gupta M et al. (2017) Genome-nuclear lamina interactions regulate cardiac stem cell lineage restriction. Cell 171:573–587.e14. 10.1016/j.cell.2017.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gruenbaum Y, Foisner R (2015) Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem 84:131–164. 10.1146/annurev-biochem-060614-034115 [DOI] [PubMed] [Google Scholar]
- 57.Worman HJ, Bonne G (2007) “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res 313:2121–2133. 10.1016/j.yexcr.2007.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobrzynska A, Gonzalo S, Shanahan C, Askjaer P (2016) The nuclear lamina in health and disease. Nucleus 7:233–248. 10.1080/19491034.2016.1183848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hegele RA, Cao H, Liu DM et al. (2006) Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am J Hum Genet 79:383–389. 10.1086/505885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damiano JA, Afawi Z, Bahlo M et al. (2015) Mutation of the nuclear lamin gene LMNB2 in progressive myoclonus epilepsy with early ataxia. Hum Mol Genet 24:4483–4490. 10.1093/hmg/ddv171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Knaap MS, Bugiani M (2017) Leukodystrophies: a proposed classification system based on pathological changes and pathogenetic mechanisms. Springer, Berlin: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lo Martire V, Alvente S, Bastianini S et al. (2018) Mice overexpressing lamin B1 in oligodendrocytes recapitulate the age-dependent motor signs, but not the early autonomic cardiovascular dysfunction of autosomal-dominant leukodystrophy (ADLD). Exp Neurol 301:1–12. 10.1016/j.expneurol.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Padiath QS (2019) Autosomal dominant leukodystrophy: a disease of the nuclear lamina. Front Cell Dev Biol 7:1–6. 10.3389/fcell.2019.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moyon S, Liang J, Casaccia P (2016) Epigenetics in NG2 glia cells. Brain Res 1638:183–198. 10.1016/j.brainres.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li H, He Y, Richardson WD, Casaccia P (2009) Two-tier transcriptional control of oligodendrocyte differentiation. Curr Opin Neurobiol 19:479–485. 10.1016/j.conb.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J, Casaccia P (2010) Epigenetic regulation of oligodendrocyte identity. Trends Neurosci 33:193–201. 10.1016/j.tins.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hernandez M, Casaccia P (2015) Interplay between transcriptional control and chromatin regulation in the oligodendrocyte lineage. Glia 63:1357–1375. 10.1002/glia.22818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsai E, Casaccia P (2019) Mechano-modulation of nuclear events regulating oligodendrocyte progenitor gene expression. Glia 67:1229–1239. 10.1002/glia.23595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu J, Moyon S, Hernandez M, Casaccia P (2016) Epigenetic control of oligodendrocyte development: Adding new players to old keepers. Curr Opin Neurobiol 39:133–138. 10.1016/j.conb.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.