SUMMARY
Cytosolic caspase-8 is a mediator of death receptor signaling. While caspase-8 expression is lost in some tumors, it is increased in others, indicating a conditional pro-survival function of caspase-8 in cancer. Here we show that tumor cells employ DNA damage-induced nuclear caspase-8 to override the p53-dependent G2/M cell cycle checkpoint. Caspase-8 is upregulated and localized to the nucleus in multiple human cancers correlating with treatment resistance and poor clinical outcome. Depletion of caspase-8 causes G2/M arrest, stabilization of p53, and induction of p53-dependent intrinsic apoptosis in tumor cells. In the nucleus, caspase-8 cleaves and inactivates the ubiquitin-specific peptidase 28 (USP28), preventing USP28 from de-ubiquitinating and stabilizing wildtype p53. This results in de facto p53 protein loss, switching cell fate from apoptosis towards mitosis. In summary, our work identifies a non-canonical role of caspase-8 exploited by cancer cells to override the p53-dependent G2/M cell cycle checkpoint.
Graphical Abstract

INTRODUCTION
Caspases, a family of cysteine-proteases, are key drivers of extrinsic and intrinsic apoptotic cell death. Inactivation of apoptotic signaling is a common event in cancer (Brown and Attardi, 2005; Suzuki and Matsubara, 2011). Therefore, vitalizing pro-apoptotic pathways by reactivation or overexpression of caspases has been widely entertained as potential therapeutic intervention strategies (Delbridge et al., 2016; Fiandalo and Kyprianou, 2012). Caspase-8 plays a cardinal role in transmitting signals from ligated death receptors to intracellular pro-apoptotic components. However, in the absence of caspase-8 cells can redirect signaling towards an alternative cell death pathway called necroptosis, which is mediated by the receptor-interacting protein kinase 1 and 3 (RIPK1/RIPK3), and the mixed lineage kinase domain-like (MLKL) (Declercq et al., 2009). Knockout of caspase-8 is embryonic lethal in mice, but animals double knockout for caspase-8/RIPK3 or caspase-8/MLKL develop normally (Kaiser et al., 2011; Oberst et al., 2011). In this context, caspase-8 acts to prevent RIPK3 activation and necroptotic cell death. Additional non-apoptotic functions have been ascribed to caspase-8, including roles in tissue homeostasis, post-injury recovery and tumor progression (Dabrowska et al., 2016; Lemmers et al., 2007; Li et al., 2010; Salmena et al., 2003; Shalini et al., 2015).
The role of caspase-8 in tumor progression and response to therapy remains controversial and has been associated with both, down- and upregulation of the protein (Shalini et al., 2015). Low caspase-8 expression seems to potentiate metastasis formation of neuroblastoma (Barbero et al., 2009; Stupack et al., 2006; Teitz et al., 2000) and neuroendocrine lung tumors (Harada et al., 2002), and is associated with a poor prognosis in ovarian cancer patients (Kim et al., 2016). By contrast, elevated caspase-8 expression has been implicated in promoting tumor cell motility of breast and pancreatic cancer (Frisch, 2008; Helfer et al., 2006), and is associated with poor survival of patients with hepatocellular carcinoma (Koschny et al., 2013). Interestingly, nuclear localization of caspase-8 has been observed in a number of cancer types (Koschny et al., 2013; Manzo-Merino et al., 2014), hinting at a potential non-apoptotic tumorigenic role for caspase-8 in malignant diseases. Here, we elucidated the contribution of caspase-8 to cancer progression and patient prognosis. Using melanoma as a cancer model we find that cells depleted for caspase-8 become stalled at the G2/M transition. Under these conditions the de-ubiquitinase USP28 stabilizes p53 through de-ubiquitination thereby facilitating apoptosis induction of genomically unstable cancer cells. In the presence of nuclear caspase-8, however, USP28 is inactivated through cleavage and, as a consequence, cells progress through mitotic cell division instead. Hence, caspase-8 expression and subcellular localization may be indicative of p53 proficient cancers.
RESULTS
Tumors with elevated and nuclear caspase-8 expression present with higher relapse rates and poor prognosis
Analyzing RNAseq data from The Cancer Genome Atlas (https://cancergenome.nih.gov/), we identified caspase-8 gene and protein expression levels (Figure 1, S1A and S1B) to be significantly elevated in tumor tissues compared to normal tissues of most cancer types (Rahman et al., 2015). Focussing on cancer types ranked the most common and aggressive by the WHO (https://gco.iarc.fr/today/fact-sheets-populations?population=900&sex=0), we found renal clear cell carcinoma, gastric adenocarcinoma, hepatocellular carcinoma, head and neck squamous cell carcinoma, glioblastoma multiforme, lung adenocarcinoma, urothelial carcinoma, and prostate adenocarcinoma, to show the most significant elevation of caspase-8 expression. For colon adenocarcinoma, a similar but non-significant tendency was observed while there was no increase in caspase-8 expression detectable in breast invasive carcinoma (Figure 1A). Bioinformatics analyses implied that elevated caspase-8 expression might play a role in cancer development and/or progression. A comprehensive immunohistochemical (IHC) analysis in different human tumors confirmed caspase-8 expression levels to be commonly elevated in cancers. Moreover, it revealed a frequent nuclear localization of caspase-8 in cancer cells as compared to tumor-adjacent normal cells and cells of benign lesions (Figures 1B, S2A and S2B), suggesting that caspase-8 might play a role in cancer beyond its cytosolic function in extrinsic apoptosis.
Figure 1. Tumors with elevated and nuclear caspase-8 expression present with higher relapse rates and poor prognosis.
(A) Caspase-8 gene expression was compared between human healthy and tumor samples derived from TCGA data base; ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05.
(B) IHC analysis of caspase-8 localization in primary versus metastatic melanoma and in prostate benign and cancer tissue, respectively. Scale bar = 100 μm.
(C) Quantification of caspase-8 expression level in TMAs of nevi (n=100) compared to melanoma (n=92); ***p ≤ 0.001.
(D) Kaplan-Meier-Plot displaying survival rate of patients suffering from primary (n=91) melanoma with low or high caspase-8 expression level; *p ≤ 0,01.
(E) Quantification of nuclear caspase-8 in TMAs of nevi (n=100), primary (n=92) and metastatic (n=48) melanoma.
(F) Overall survival (days) of melanoma patients showing either nuclear (n=16) or cytosolic (n=27) staining of caspase-8; *p ≤ 0.05.
(G) Ratio of caspase-8 expression derived from RNAseq analysis of patients with benign prostate phenotype (n=11), low (n=22) or high risk (n=47) prostate cancer, and NHT treated high risk prostate cancer (n=15); ***p ≤ 0.001, **p ≤ 0.005.
(H) Kaplan-Meier-Plot displaying PSA recurrence free survival of Tax (n=19) or NHT (n=51) treated prostate cancer patients with low or high caspase-8 expression; *p ≤ 0,01.
(I) Quantification of nuclear caspase-8 expression, in prostate cancer tissue cores of untreated (n=129/730) versus Tax-treated (n=59/107), castration resistant prostate cancer (CRPC/TURP, n=32/42) and neuroendocrine prostate cancer (NEPC, n=7/8) TMAs.
(J) Quantification of nuclear caspase-8 in TMAs of untreated (n=16/47) compared to NHT treated for 1-6 months (n=15/26) and 7-12 months (n=37/43), respectively. See also Figure S1 and S2.
In depth IHC analysis of caspase-8 protein expression in tissue microarrays (TMA) of prostate cancer (PCa) and melanoma (MM) patients, confirmed caspase-8 expression to be significantly elevated in tumor tissue of MM patients compared to untransformed nevi (Figure 1C), and to correlate with reduced survival (Figure 1D). Above this, tumors of metastatic progression stage appeared to accumulate nuclear caspase-8 compared to primary melanoma (Figure 1E). Accordingly, MM patients with nuclear caspase-8 localization, turned out to have a reduced overall survival as compared to patients expressing cytosolic caspase-8 (Figure 1F), demonstrating that expression levels and subcellular localization of caspase-8 have a prognostic value in malignant melanoma.
RNAseq analysis from PCa patients (Wyatt et al., 2014) showed that high-risk primary PCa tumors presented with increased caspase-8 expression, which was sustained after treatment with neoadjuvant hormone therapy (NHT) (Figure 1G). Importantly, elevated caspase-8 expression correlated with impaired recurrence-free survival of patients treated with standard-of-care docetaxel (TAX) plus NHT (Figure 1H). Intriguingly, tumors able to resist TAX treatment, analyzed after transurethral resection of the prostate (TURP), as well as the aggressive late stage neuroendocrine PCa (NEPC) tumors, also displayed high nuclear caspase-8 levels (Figure 1I), indicating that tumors expressing nuclear caspase-8 may have a survival advantage when challenged with chemotherapy. This became even more obvious by the finding that cells expressing elevated nuclear caspase-8 accumulated over time in PCa tumors previously subjected to NHT (Figure 1J) consistently hinting that nuclear caspase-8 plays a conditional pro-survival role in cancer.
Nuclear expression of caspase-8 is mediated by distinct NES and NLS sequences
Immunoblotting revealed that caspase-8 expression was also elevated in melanoma-derived cell lines compared to primary melanocytes (Figure 2A). Moreover, caspase-8 could be detected in both, the cytosolic and to a lesser extend in the nuclear compartment of melanoma cells (Figure 2B), suggesting that the caspase-8 amino acid sequence contains nuclear localization (NLS) and export (NES) signals. In silico analysis identified a putative NES, 21SLKFLSLDY29, located within the first death effector domain (DED), and a putative NLS, 468FTLRKKLVF476, in the C-terminal part of the protein. Hence, we created a panel of myc-tagged mutant and wild-type forms of caspase-8 (Figure 2C) and analyzed their localization in melanoma cells by immunocytochemistry. Wild-type caspase-8 (wtCasp-8) showed a diffuse distribution throughout cytosol and nucleus whereas NES-mutated (Casp-8-mNES) and death domain-deleted (Casp-8-ΔDED) caspase-8 presented with pronounced nuclear localization (Figure 2D). In contrast, NLS-mutated (Casp-8-mNLS) and, notably, also catalytically inactive caspase-8 (Casp-8-C360A (Barbero et al., 2009)) resided exclusively in the cytosol. These data imply that pro-caspase-8 shuttles between the cytosol and the nucleus in an NLS/NES-dependent manner, and only remains in the nucleus if the NES-containing DEDs have been removed by processing caspase-8 into its catalytically activate form.
Figure 2. Nuclear expression of caspase-8 is mediated by distinct NES and NLS sequences.
(A) Expression level of caspase-8 in primary melanocytes and different melanoma cell lines (WM115, WM35, MeWo, 451-LU, WM3211, A375) was determined by Western-blotting with GAPDH as loading control.
(B) Caspase-8 was detected in cytosolic and nuclear fractions of WM115 cells by Western-blotting, compared to whole cell lysates with IκBα as a marker for cytosolic, and Histone H3 for nuclear fraction.
(C) Based on a wild type myc-tagged caspase-8 expression plasmid (wtCasp-8) putative NES (Casp-8-mNES) and NLS (Casp-8-mNLS) sequences were mutated. A catalytically inactive (Casp-8-C360A) and a Death Effector Domain lacking mutant (Casp-8-ΔDED) were generated.
(D) The localization of caspase-8 mutants was analyzed immune-cytochemically: anti-caspase-8 (green), anti-myc (orange), phalloidin (red), DAPI (blue). Percentage of cells (n=100) expressing mostly cytosolic (cyt) or nuclear (nuc) caspase-8 was scored. Scale bar = 20 μm.
(E) Cells were stimulated with izTRAIL (100 ng/ml), irradiated with sublethal UVB (200 J/m2), or stimulated with Cispatin (Cis; 10 μM) and Temozolomide (TMZ; 2 mM), respectively, for 30 min and analyzed by immunofluorescence. Scale bar = 50 μm.
(F) Cells were treated as in (E) for 1 h and the subcellular localization of cleaved caspase-8 aggregates analyzed by immunofluorescence (anti-caspase-8 or anti cleaved capsase-8 (green), DAPI (blue), phalloidin (red)). Quantification of nuclear and cytosolic cleaved caspase-8 aggregates is shown. Scale bar = 20 μm.
As expected, stimulation with the extrinsic caspase-8 activator tumor necrosis factor-related apoptosis inducing ligand (TRAIL) induced clustering of caspase-8 in the cytosol (Figure 2E). Intriguingly, caspase-8 translocated into the nucleus of tumor cells in response to DNA damage induced by UVB irradiation and treatment with chemotherapeutic drugs cisplatin (Cis) and temozolomide (TMZ), respectively. Correspondingly, enrichment of aggregates consisting of cleaved caspase-8 could be detected in treated versus untreated cells (Figure 2F). Importantly, cleaved caspase-8 accumulated preferably in the nucleus of cells exposed to DNA-damaging agents, whereas mostly cytosolic aggregates could be detected in TRAIL-treated cells. These data demonstrate that nuclear translocation of caspase-8 is triggered by DNA damage and suggests that cancer cells utilize caspase-8 activity in the nucleus to cope with genotoxic stress.
Caspase-8 knockdown induces intrinsic apoptosis in tumor cells
Most cancer cells display increased levels of constitutive DNA damage due to replication stress and mitotic abnormalities (Ricke et al., 2008). We therefore aimed to determine the impact of caspase-8 on cancer cell behavior under ambient conditions. Knockdown of caspase-8 using an individual siRNA or a smart-pool of siRNAs induced cell death in melanoma cells (Figure 3A) being significantly enhanced by exposure to a sublethal UVB dose (Figure 3B), supporting the concept that caspase-8 plays a survival role in tumor cells that have accumulated DNA damage. Cell death induced by caspase-8 depletion could be exclusively attributed to apoptosis, as the pan-caspase inhibitor QVD completely rescued caspase-8 silenced cells, whereas the RIP1 inhibitor necrostatin-1S (NEC-1S) or simultaneous silencing of either RIP1 or RIP3 had no effect (Figures 3C, 3D S3A and S3B). Moreover, depletion of caspase-8 resulted in robust stabilization and phosphorylation of p53, upregulation of the p53-regulated pro-apoptotic Bcl-2 family members Bax, Bak, Puma, and Noxa, and cleavage of caspase-9, −3 and PARP, while proteins of the extrinsic apoptosis machinery remained unaffected (Figures 3E and S3C). This indicates that tumor cells rely on caspase-8 to prevent canonical intrinsic apoptosis. Corroborating this finding, exclusively reconstitution of cells with wtCasp-8, but not with catalytically inactive mutCasp-8-C360A, was able to rescue cells from apoptosis induced by caspase-8 depletion which clearly correlated with diminished p53 expression and activation (Figure 3F). To unravel the contribution of nuclear versus cytosolic caspase-8 to melanoma cell survival, we generated single cell clones stably expression preferably nuclear (Casp-8-mNES; clones 30 and 150) or cytosolic (Casp-8-mNLS, clones 213 and 215) caspase-8 as compared to mock transfected cells (Figures 3G and S3D). Intriguingly, apoptosis induction in response to cisplatin and TMZ was significantly reduced only in Casp-8-mNES clones but not in Casp-8-mNLS clones compared to wildtype cells (Figure 3H). Collectively, these data indicate a possible relationship between nuclear localization of caspase-8, endogenous p53 level, and consequently the apoptotic response to DNA-damaging agents, suggesting that the catalytic activity of nuclear caspase-8 is required for its non-apoptotic survival function in cancer.
Figure 3. Caspase-8 knockdown induces intrinsic apoptosis in melanoma cells.
(A) WM115 cells were silenced for caspase-8 with siRNA (siCasp-8) or a siRNA smart pool (spCasp-8) for 72 h and apoptosis determined by Cell Death Detection ELISA (CDDE). Caspase-8 knockdown was documented by Western-blotting with GAPDH as loading control.
(B) Cells silenced for caspase-8 were irradiated with sub-lethal UVB (200 J/m2) and apoptosis assessed 24 h later using CDDE; **p ≤ 0.005.
(C) Cells were treated with QVD (5 μM), Necrostatin-1S (Nec-1S, 15 μM) or both for 1 h prior to caspase-8 silencing. After 72 h apoptosis was determined by CDDE; **p ≤ 0.005.
(D) Cells were silenced for caspase-8 alone or co-silenced for RIP1 and RIP3, respectively. After 72 h apoptosis was assessed by CDDE, and knockdown of RIP1, RIP3, and caspase-8 documented by Western-blotting with GAPDH as loading control.
(E) Expression of proteins involved in intrinsic apoptosis (p53, p-p53-Ser15, Bax, Bak, Puma, Noxa, caspase-9, caspase-3, PARP) in untreated, siCasp-8 and mitotic (MSO) cells was documented by Western-blotting with α-tubulin as loading control.
(F) Endogenous caspase-8 was silenced in cells stably expressing moTAP-tagged wtCasp-8 or mutCasp-8 (C360A) for 72 h and apoptosis determined by CDDE; **p ≤ 0.005. Expression of caspase-8 and p53 was determined by Western-blotting with GAPDH as loading control.
(G) Caspase-8 was detected in cytosolic and nuclear fractions of WM115 cell clones 30 and 150, stably expressing preferably nuclear caspase-8 and clones 213 and 215 expressing preferably cytosolic caspase-8 by Western-blotting. IκBα served as a marker for cytosolic, Histone H3 for nuclear fractions.
(H) The same clones as characterized in (G) were stimulated with cisplatin (10 μM) or temozolomide (2 mM). After 24 h apoptosis was assessed by CDDE; *p≤0.05; **p≤0.01; ***p≤0.005.
Error bars are shown as mean ± SD. See also Figure S3.
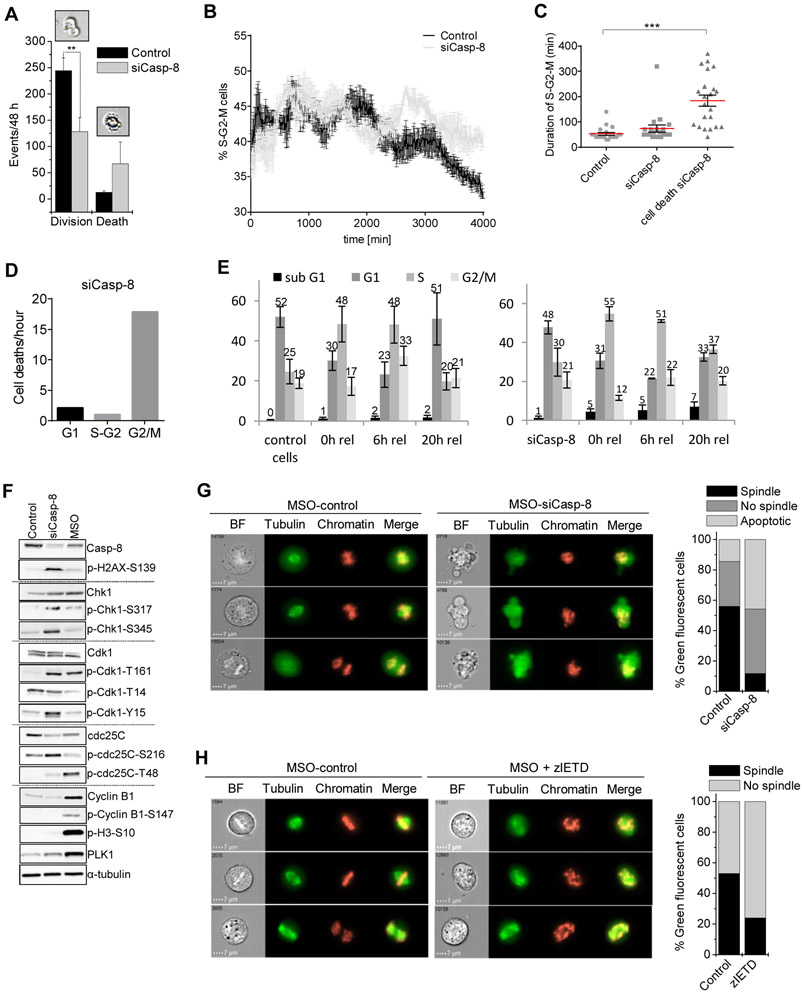
Cancer cells with nuclear caspase-8 overcome cell cycle arrest at the G2/M checkpoint
Noticing that p53 was stabilized and phosphorylated in tumor cells upon caspase-8 silencing, we reasoned that nuclear caspase-8 might have a function in regulating the p53-dependent cell cycle checkpoint machinery. Indeed, knockdown of caspase-8 reduced cell division while enhancing cell death as documented by live cell transmission microscopy (Figure 4A). To investigate whether apoptosis induced by caspase-8 depletion occurred in a specific cell cycle phase, we took advantage of FUCCI2 HeLa cells, which express mCherry-fused Cdt1 and mVenus-fused Geminin, labeling the nuclei of G1 phase cells red and cells in S/G2/M phase green (Sakaue-Sawano et al., 2011). As expected, silencing caspase-8 in FUCCI2 Hela cells induced apoptosis (Figure S4A) but also hampered cell proliferation and increased the population of cells in S/G2/M (Figure 4B), while the population of cells in G1 decreased (Figures S4B and S4C; Supplemental Video 1). Life cell imaging further revealed that in caspase-8 silenced cells undergoing apoptosis, the duration of S/G2/M was prolonged (Figures 4C and S4D), and cell death was specifically induced during G2/M (Figure 4D), suggesting that caspase-8 silenced cells are stalled in G2 prior to cell death induction. Correspondingly, caspase-8 depleted melanoma cells remained accumulated at the S/G2 transition 20 h after release from S-phase synchronization by a double thymidine block, and a subpopulation underwent apoptotic cell death (sub-G1 peak) instead of proceeding with mitotic cell division (Figure 4E).
Figure 4. Cancer cells with nuclear caspase-8 overcome cell cycle arrest at the G2/M checkpoint.
(A) siCasp-8 WM115 cells were subjected to live cell imaging and dividing versus dying cells were quantified morphologically; **p ≤ 0.01.
(B) FUCCI2-HeLa cells were transfected with siCasp-8 or siLacZ. After 24 h, cells were subjected to life cell imaging for 48 h. Percentage of green cells (S-G2-M) over time was determined by evaluating microscopic images (timeframe 10 min).
(C) Scatter dot blot showing the duration of S-G2-M phase of control versus siCasp-8 cells (n=25); ***p≤ 0.001.
(D) Number of cell deaths/hour of siCasp-8 transfected FUCCI2-HeLa in G1 (red), S-G2 (yellow), and G2/M (green) transition.
(E) WM115 cells were subjected to LacZ or caspase-8 knockdown and synchronized in S-phase using a double thymidine block. 0 h, 6 h and 20 h after release cell cycle analysis was performed. Average percentages of cells in the particular cell cycle phase are given.
(F) Expression of proteins involved in mitotic transition (caspase-8, p-H2AX-S139, Chk1, p-CHk1-S317, p-CHk1-S345, Cdk1, p-Cdk1-T161, p-Cdk1-T14, p-Cdk1-Y15, cdc25C, p-cdc25C-Ser216, p-cdc25C-T48, cyclin B1, p-cyclin B1-S147, p-H3-S10, PLK1) in untreated, siCasp-8 and mitotic (MSO) cells was documented by Western-blotting with α-tubulin as loading control.
(G and H) HKF1_H2B-mCherry_αTubulin-mEGFP cells (G) silenced for caspase-8 or (H) treated with the caspase-8 inhibitor zIETD (20 μM) for 72 h were harvested via mitotic shake off (MSO). Chromatin structure and spindle formation were evaluated by image stream analysis. 500 cells were quantified. Scale bar = 7 μm.
Error bars are shown as mean ± SD. See also Figure S4.
In line with this, caspase-8 silencing resulted in increased levels of γH2AX (pH2AX-Ser139) as a consequence of DNA damage accumulation. Accordingly, robust phosphorylation/activation of checkpoint kinase 1 (Chk1) occurred, being a central component of the canonical genome surveillance pathway directing cells with intact DNA towards G2/M transition and cells with damaged DNA towards p53-mediated apoptosis (Zhang and Hunter, 2014) (Figure 4F). The mitosis promoting factor Cdk1 became only partially activated in caspase-8 knockdown cells as compared to mitotic cells (MSO). Auto-phosphorylation of Cdk1 at T161 occurred in both caspase-8 silenced and in MSO cells, while the essential de-phosphorylation of Cdk1 at T14 and Y15 by the phosphatase cdc25C was only observed in mitotic cells. Consistent with this observation, de-phosphorylation of cdc25C at S216 and phosphorylation of T48 took place only in mitotic cells but not in caspase-8 depleted cells, indicating that cdc25C resided in the cytosol in an inactive state. Concomitantly, activation of the Cdk1 co-factor cyclin B1 remained low in caspase-8 silenced cells, indicating that mitotic transition was impaired in the absence of caspase-8. Thus, Polo like kinase 1 (PLK1), a marker of mitotic prophase, was only partially upregulated, and histone H3 phosphorylation, being indicative for chromatin condensation, remained absent in caspase-8 silenced cells compared to mitotic cells.
To further substantiate a role of caspase-8 in cell cycle regulation, we conducted time-lapse microscopy of H2B-mCherry- and αTubulin-mEGFP-expressing HeLa cells and confirmed that cancer cells lacking caspase-8 are deficient in proper cell division (Supplemental Video 2). Image stream analysis of potentially mitotic cells shaken off (MSO) cell culture flasks showed that caspase-8 depletion reduced the number of cells with mitotic spindle formation and metaphase chromatin alignment. Instead, chromatin condensation was accompanied by chromatin dis-integrity and an apoptotic phenotype (Figure 4G). Notably, chemical inhibition of caspase-8 activity using zIETD also lead to a reduced number of cells with mitotic spindle formation (Figure 4H). In aggregate, these data identify an essential role of caspase-8 at the G2/M checkpoint in tumor cells.
Caspase-8 regulates p53 stability at the G2/M checkpoint through cleavage of USP28
Being an essential component of the G2/M cell cycle checkpoint (Bieging et al., 2014) the tumor suppressor p53 gets ubiquitinated by the E3 ligase MDM2 and targeted for proteasomal degradation, allowing cells to progress to mitosis. Upon G2/M checkpoint activation, p53 is stabilized and activated to sustain cell cycle arrest and induce intrinsic apoptosis. Stabilization of p53 requires de-ubiquitination and this event has been proposed to involve the de-ubiquitinase (DUB) USP28 (Cuella-Martin et al., 2016). Indeed, recombinant USP28 efficiently removed MDM2-catalyzed K48 ubiquitin chains from p53 in an in vitro de-ubiquitination assay (Figure 5A). Strikingly, addition of recombinant caspase-8 caused cleavage of USP28 and prevented de-ubiquitination of p53, indicating USP28 to be a putative proteolytic target of caspase-8. Accordingly, recombinant caspase-8 produced a robust and concentration-dependent cleavage of recombinant USP28 in vitro and in whole cell lysates (Figures S5A and S5B). Thus, both, USP28 and p53 protein levels increased in response to caspase-8 silencing, while MDM2 levels remained unaffected (Figure 5B). To test whether caspase-8 and USP28, respectively, were able to alter cellular K48 ubiquitination along with the p53 level, we knocked down caspase-8 and USP28, individually. Intriguingly, exclusive knockdown of caspase-8 significantly reduced cellular ubiquitination levels, while concomitantly enhancing p53 levels, implying that the activity of caspase-8 is directly linked to the activity of a de-ubiquitinating enzyme. Conversely, depletion of USP28 resulted in significantly increased K48 ubiquitination and simultaneously diminished p53 level, which could be fully reversed by addition of recombinant USP28 (Figure 5C). This finding was substantiated by direct pull-down of ubiquitinated p53 using tandem ubiquitin binding entities (TUBEs). Here, knockdown of caspase-8 decreased the fraction of ubiquitinated p53 relative to total p53 present in melanoma cells. Vice versa, upon USP28 silencing cellular levels of p53 were significantly diminished, due to increased p53 ubiquitination (Figure 5D). Hence, silencing of USP28 antagonized apoptosis induced by caspase-8 depletion and also reverted accumulation and activation of p53 (Figure 5E). Consequently, also concurrent depletion of p53 largely prevented apoptosis induced by silencing of caspase-8 (Figure 5F).
Figure 5. Caspase-8 regulates p53 stability at the G2/M checkpoint through cleavage of USP28.
(A) Recombinant p53 was subjected to MDM2-mediated ubiquitination in vitro. De-ubiquitination of p53 by addition of recombinant USP28 (0.9 μM) was analyzed by immunoblotting. Addition of recombinant active caspase-8 (400 ng) resulted in cleavage and inactivation of USP28.
(B) Expression of p53, USP28 and MDM2 in untreated, siCasp-8 and mitotic (MSO) WM115 cells was documented by Western-blotting with GAPDH as loading control.
(C) Cells were treated with the proteasome inhibitor MG132 (10 μM), transfected with siCasp-8 or siUSP28. After 72 h recombinant USP28 (0.5 μM) was added to siUSP28 cell lysates. Level of p53 and K48-ubiquitin were analyzed in whole cell lysates by immunoblotting with β-actin as loading control. Ratio of p53 versus ubiquitin was calculated; *p ≤ 0.05; **p ≤ 0.005; ***p≤ 0.001.
(D) Cells were treated with siCasp-8, siUSP28 or sip53. After 72 h TUBE-assay identifying the ubiquitination status of p53 was performed; *p ≤ 0.05.
(E and F) Apoptosis of cells silenced for (E) USP28, Casp-8, or both, or (F) Casp-8, p53 or both was determined after 72 h by CDDE; ***p ≤ 0.001. Expression of caspase-8, USP28 and p53 was determined by Western-blotting with β-actin or GAPDH as loading controls.
(G) Cells were synchronized in S-Phase with a double aphidicolin block. 5 h and 6 h after release cells were harvested and the status of cleaved caspase-8, USP28 and p53 determined by Western-blotting with β-actin as loading control.
(H) Protein lysates of cells expressing recombinant wtUSP28, mutUSP28(D119E) or mutUSP28(D240E) were treated with recombinant Caspase-8 (400 ng). Cleavage of USP28 and caspase-8 was documented by Western-blotting with β-actin as loading control.
(I) Apoptosis of cells expressing wtUSP28 or mutUSP28(D119E) was determined by CDDE 24 h after transfection; ***p ≤ 0.005. Status of USP28, p53 and ub-K48 was documented by Western-blotting with β-actin as loading control.
(J) Caspase-8 was silenced in cells expressing wtUSP28 or mutUSP28(D119E). After 48 h apoptosis was assessed by CDDE; ***p ≤ 0.005.
(K and L) Casp-8-mNES cell clone 30 stably expressing preferably nuclear caspase-8 and Casp-8-mNLS cell clone 213 expressing preferably cytosolic caspase-8 were transfected with wtUSP28 or mutUSP28(D119E), respectively, and stimulated with UVB (300 J/m2), cisplatin (10 μM) or Temozolomide (2 mM). (K) After 24 h apoptosis was assessed by CDDE; **p ≤ 0.01, and (L) the protein status of USP28 and p53 documented by Western-blotting with β-actin as loading control.
Error bars are shown as mean ± SD. See also Figures S5 and S6.
Most importantly, cells that have progressed to G2 after release from synchronization by aphidicolin for 5/6 h (Figure S5C), showed both, caspase-8 activation and cleaved USP28 with concomitant loss of p53 (Figure 5G). Together, our data identify USP28 as a bona fide substrate of caspase-8 and demonstrate that caspase-8-mediated cleavage inactivates USP28 at the G2/M checkpoint leading to decreased p53 stability, allowing cells to escape p53-mediated apoptosis and to re-engage cell cycle progression.
Mass spectrometry analysis mapped two putative caspase-8 cleavage sites, 115IQAD120 and 236AALD241 (Stoehr et al., 2013), in the USP28 protein that, if processed, result in loss of a 30 kDa N-terminal fragment encoding the ubiquitin-associated domain (UBA) and the ubiquitin-interacting motive (UIM) (Lambrus and Holland, 2017) (Figure S6A). Mutation of D to E residues revealed 115IQAD120 but not 236AALD241 to be recognized for cleavage by caspase-8 (Figure 5H). Ectopic expression of either wtUSP28 or un-cleavable mutUSP28(D119E), induced mild apoptosis that coincided with reduced ubiquitination, and accumulation of p53 (Figure 5I), and significantly enhanced apoptosis induced by caspase-8 depletion (Figure 5J).
In line with or previous assumptions, melanoma cells preferably expressing nuclear Casp-8-mNES (cl 30) induced less apoptosis in response to DNA-damaging agents UVB, cisplatin or temozolomide compared to cells expressing preferably cytosolic Casp-8-mNLS (cl 213) and, hence, induced less p53 (Figures 5K and 5L (mock)). Ectopic expression of wtUSP28 and mutUSP28(D119E), respectively, caused significant enhancement of DNA damage-induced apoptosis by gradually increasing the levels of p53 only in nuclear caspase-8 (Casp-8-mNES) expressing cells. In contrast, both, apoptosis and p53 levels remained largely unaffected in cells expressing cytosolic caspase-8 (Casp-8-mNLS) (Figures 5K, 5L and S6B). Accordingly, ectopic wtUSP28 was pronouncedly cleaved in Casp-8-mNES cells strengthening that nuclear caspase-8 is required to target this de-ubiquitinase. Although our results have to be interpreted in the context of co-existence of endogenous caspase-8 and USP28 in the cell lines investigated, these results provide strong evidence that the nuclear caspase-8/USP28/p53 axis plays a crucial role in survival of cancer cells in response to DNA-damaging agents.
Nuclear caspase-8 causes progression and survival of cancer cells harboring wild-type p53
Our findings predicted that nuclear caspase-8 is most relevant in p53-proficient cancer cells that are subjected to a frequent ubiquitin-dependent turnover. To challenge this prediction, we knocked down caspase-8 in 17 different tumor cell lines of which 6 harbored wtp53 and 11 mutp53 (Allende-Vega et al., 2013; Chappell et al., 2012; Frank et al., 2011; Hinata et al., 2003; Leroy et al., 2014; Lim et al., 2009; Muller et al., 1997; Okazawa et al., 1998; Sato et al., 2009). While caspase-8 depletion induced apoptosis to various degrees (>20%) in all wtp53 cancer cells, the mutp53 cells remained largely resistant against apoptosis induced by caspase-8 depletion (<10%) (Figure 6A and S7). To determine whether wtp53 expressing tumor cells required caspase-8 for cell cycle progression, we created stable p53-depleted melanoma cell clones engineered to express either wild type caspase-8 (wtCasp-8) or the catalytic inactive (C360A) mutant caspase-8 (mutCasp-8). Biochemical analysis of 50 wtCasp-8 and mutCasp-8 clones, respectively, revealed that only 9.6% of clones showing downregulation of p53 maintained expression of wtCasp-8, while 80% maintained mutCasp-8 expression (Figures 6B and 6C). This indicated that elevated expression of catalytically active caspase-8 is only beneficial for tumor cells harboring wtp53, but is dispensable for tumor cells expressing mutp53 or no p53. Accordingly, single cell clones that expressed lower endogenous p53 levels (cl79 and cl80) were less sensitive to apoptosis induced by caspase-8 silencing compared to cells expressing moderate to high levels of p53 (Figure 6D). Vice versa, single cell clones expressing high endogenous caspase-8 levels (cl15 and cl17) showed enhanced proliferation compared to clones expressing low caspase-8 levels (cl24 and cl52) (Figure 6E). Moreover, cultivation of tumor cells with the caspase-8 inhibitor zIETD resulted in reduced clonogenic outgrowth (Figure 6F). Tumor selectivity of the caspase-8/USP28/p53axis was evident by the observation that silencing of caspase-8 only induced apoptosis in p53-proficient melanoma metastases isolated from patients, but neither in metastatic lesions not expressing p53 nor in primary cells of the skin (Figures 6G and 6H). Interestingly, the majority of tumors catalogued in the TCGA database (cbioportal.org) (Cerami et al., 2012) expressed wtp53 (60-90%) although the general prevalence of wtp53 is estimated to be ~50%. Moreover, roughly half of the wtp53 tumors also expressed high caspase-8 levels, and 5-40% of the patients in this particular subpopulation succumbed to the disease (Figure 6I).
Figure 6. Nuclear caspase-8 causes progression and survival of cancer cells harboring wild-type p53.
(A) 17 different cancer cells lines (melanoma: WM115, 451-LU, A375, MeWo; prostate: PC3, DU145; breast: MCF-7, T47D; colon: HCT116, Colo-205; glioma: A172, T98G; pancreas: Panc89, liver: HepG3B, HUH-7, bladder: UM-UC-3, UC-1) expressing either wt or mut p53 were silenced for caspase-8. After 72 h apoptosis was assessed by CDDE.
(B) p53 was stably knocked down using shRNA in cells stably expressing moTAP-tagged wtCasp-8 or mutCasp-8 (C360A), followed by single cell clone selection. The percentage of cell clones still expressing either wtCasp-8 (n=50) or mutCasp-8 (n=50) was quantified.
(C) Examples of clones expressing different levels of caspase-8 and p53 was determined by immunoblotting with β-actin as loading control.
(D) Apoptosis of clones 79 and 80 expressing lower endogenous p53 level was determined 72 h after caspase-8 knockdown by CDDE; ***p ≤ 0.001. Expression of caspase-8 and p53 was determined by immunoblotting with GAPDH as loading control.
(E) Proliferation of clones 15, 17, 24 and 52 expressing different level of caspase-8 and p53, as indicated by immunoblotting (loading control: β-actin), was determined; ***p ≤ 0.001, **p ≤ 0.01, *p ≤ 0.05.
(F) Cells were treated with the caspase-8 inhibitor zIETD and clonogenic outgrowth determined after 72 h by cristal violet staining; **p ≤ 0.01.
(G) Caspase-8 was silenced in primary keratinocytes, fibroblasts and melanocytes, as well as in melanoma cells freshly isolated from patients metastases (M10, M20, M45). After 72 h apoptosis was determined by CDDE. The p53 mutation status in M10, M20 and M45 was assessed by sequence analysis.
(H) Expression of caspase-8 and p53 in keratinocytes, fibroblasts, melanocytes, M10, M20 and M45 was determined by Western-blotting with GAPDH as loading control.
(I) RNAseq data for Casp-8, the p53 mutation and survival status of patients as deduced from TCGA database were analyzed in a bioinformatics approach. Groups were split into high and low Casp-8 expression based on its median expression values. Percentages indicate the patient's vital status based on the Casp-8 expression and the corresponding p53 mutation status.
Error bars are shown as mean ± SD.
See also Figures S7.
Nuclear caspase-8 confers resistance to genotoxic stress in metastatic melanoma
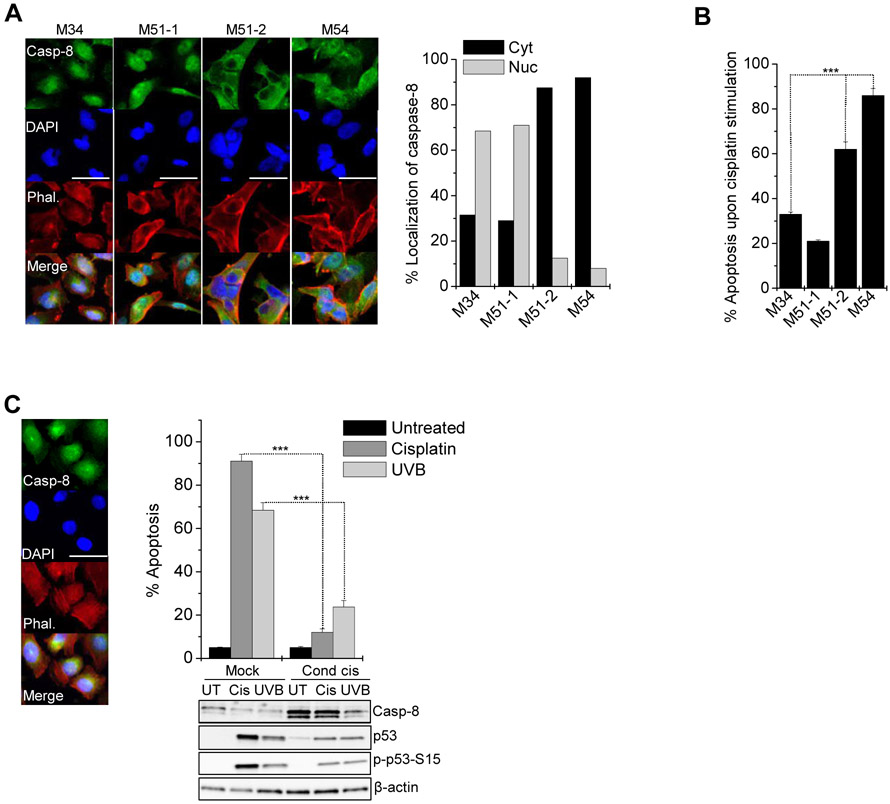
To investigate whether caspase-8 expression and localization was associated with therapy resistance and disease relapse we analyzed the responsiveness of melanoma cells isolated from metastatic lesions expressing mostly nuclear (M34, M51-1) or mostly diffuse/cytosolic (M51-2, M54) caspase-8 (Figure 7A) to genotoxic chemotherapy. Compared to cells with cytosolic caspase-8 cells with nuclear caspase-8 were significantly more resistant to cisplatin treatment (Figure 7B). Notably, metastases derived from the same patient (51-1, 51-2) showed completely opposing sub-cellular caspase-8 distribution and cisplatin responsiveness, illustrating the heterogeneity of tumor metastases.
Figure 7. Nuclear caspase-8 confers resistance to genotoxic stress in metastatic melanoma.
(A) Localization of endogenous caspase-8 in cells isolated from melanoma metastases (M34, M51-1, M51-2, M54) was analyzed immunocytochemically: anti-caspase-8 (green), phalloidin (red), DAPI (blue). Percentage of cells (n=100) expressing mostly cytosolic (cyt) or nuclear (nuc) caspase-8 was scored. Scale bar = 50 μm.
(B) Metastatic melanoma cells were treated with cisplatin (30 μM) for 24 h and apoptosis determined by CDDE; ***p ≤ 0.001.
(C) The localization of endogenous caspase-8 in A375 cells was analyzed immunocytochemically as in (A). Parental cells (mock) and cells conditioned to cisplatin (cond cis., 1 μM) for 6 months were treated with lethal doses of cisplatin (30 μM) or UVB (400 J/m2). After 24 h apoptosis was assessed by CDDE; ***p ≤ 0.001. Status of Caspase-8, p53 and phospho-p53 was determined by Western-blotting with β-actin as loading control. Scale bar = 50 μm.
Error bars are shown as mean ± SD.
To support the observation that elevated caspase-8 contributed to therapy resistance and tumor relapse, we conditioned A375 melanoma cells, mostly expressing nuclear caspase-8, to cisplatin over a period of six months. Compared to parental cells, conditioned cells had strongly accumulated caspase-8 and remained largely resistant against cisplatin and UVB-induced apoptosis (Figure 7C). As such, our work provides a clinical rationale for inhibiting caspase-8 activity in the context of genotoxic therapeutic interventions in p53 proficient tumors.
DISCUSSION
DNA damage accumulation and chromatin instability are hallmarks of the cancer cell phenotype, highlighting that cancer cells provide mechanisms allowing them to cope with genotoxic stress (Ricke et al., 2008). We have identified a molecular mechanism that explains how elevated and nuclear caspase-8 can sustain mitosis of cancer cells expressing wildtype p53. We found that melanoma and prostate cancer patients accumulated caspase-8 in the nucleus of tumor cells, which correlated with disease progression, treatment resistance, and poor recurrence-free survival. In line with our findings, elevated caspase-8 levels in triple-negative breast cancer cells (TNBC) was shown to correlate with treatment resistance and decreased cell growth after caspase-8 silencing (De et al., 2016). Consistently, high nuclear caspase-8 expression correlated with impaired overall survival of patients suffering from hepatocellular carcinoma (Koschny et al., 2013). These data support the concept that, within heterogeneous tumor tissues, sub-population of cells expressing high nuclear caspase-8 levels are able to resist therapeutic intervention and foster disease progression by facilitating mitotic cell division instead of committing cells to p53-induced apoptosis.
We describe that nuclear shuttling of caspase-8 in tumor cells is facilitated by distinct NLS/NES sequences. Although the molecular platform responsible for caspase-8 nuclear translocation remains to be determined, it is evident that translocation and nuclear retention is stimulated by genotoxic stress. Our finding is supported by recent work suggesting caspase-8 to be part of a proliferation-associated DNA damage sensing complex predisposing cells to transformation in HCC (Boege et al., 2017). Conventionally, cancer cells suffering from genotoxic stress are stalled post S-phase at the G2/M cell cycle checkpoint (Ricke et al., 2008). Here, Chk1 facilitates cell cycle arrest to allow time for cells to repair DNA or if unrepairable, induce p53-dependent apoptosis (Zhang and Hunter, 2014). Mutations in p53 is a hallmark of tumor development and found in ~50% of all tumors (Wang and Sun, 2010). As a consequence, cancer cells resist p53-dependent cell cycle checkpoints and intrinsic apoptosis. Accordingly, much effort is put into development of compounds that reconstitute wtp53 activity (Lane et al., 2010). Interestingly, the residual 50% of tumors can progress with wtp53 indicating that these tumor cells must be able to adapt alternative mechanisms suppressing wtp53. We show that stabilization of wtp53 is facilitated by the DUB USP28. We further provide evidence that tumor cells expressing high and nuclear levels of caspase-8 have defective p53-dependent apoptosis because caspase-8 cleaves and inactivates USP28 in cells with delayed or compromised mitosis (Fong et al., 2016). Supporting this, tumor cells silenced for USP28 or 53BP1 have previously been shown to prevent p53 elevation and growth arrest in response to prolonged prometaphase in cancer cells with increased propensity of mitotic errors (Lambrus et al., 2016; Meitinger et al., 2016).
The molecular mechanism uncovered here is likely a general phenomenon because we could identify nuclear translocation of caspase-8 in a large number of tumor tissues. It seems, however, to be of particular importance in tumors that have maintained wtp53, because only tumor cells with wtp53 but not mutp53, require protection from proteasomal turnover (Berglind et al., 2008). Our work provides an explanation of how tumors proficient in the intrinsic apoptotic machinery can maintain the ability to progress through mitosis by employing nuclear caspase-8 to eliminate USP28. Our results furthermore explain how USP28 may modulate the p53-dependent cell cycle checkpoint, thereby controlling tumor cell fate decisions as previously described (Cuella-Martin et al., 2016; Lottersberger et al., 2015).
In summary, we have identified a non-canonical role of caspase-8 in the nucleus that allows tumor cells with proficient p53 activity to establish a de facto p53 protein loss shifting cell fate from cell cycle arrest and apoptosis towards mitotic cell division. Consequently, cells that accumulate nuclear caspase-8 have an increased probability of surviving genotoxic chemotherapy such as TAX or cisplatin. As such, specific caspase-8 inhibition may be beneficial for patients that express elevated and nuclear caspase-8, which appears to be a clinical scenario commonly seen among aggressive human cancers.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
As Lead Contact, Dagmar Kulms is responsible for all reagent and resource requests. All requests for non-commercial reagents will be fulfilled. Please contact Dagmar Kulms (dagmar.kulms@uniklinikum-dresden.de) with requests and inquiries.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cells and Reagents
Human cell lines representing melanoma (WM115, A375, MeWo, 451-LU, WM35, WM3211), colon cancer (HCT116, Colo-205), pancreas carcinoma (Panc89), prostate cancer (PC3, DU145,), glioma (kindly provided by Dr. Debatin, Universtiy Hospital of Ulm, Germany: A172, T98G), breast cancer (kindly provided by Dr. Olayioye, Universtiy of Stuttgart, Germany: MCF-7, T47D), bladder cancer (UM-UC-3, UC-1), and hepatocellular carcinoma (kindly provided by Dr. Bantel, University of Hannover, Germany: HepG3B, HUH-7) were maintained in RPMI 1640 or DMEM medium (Invitrogen, Karlsruhe, FRG) with 10% FCS in a humified atmosphere of 5% CO2 at 37°C. U2OS cell line (kindly provided by Dr. Jäättelä, Danish Cancer Society Research Center, Denmark), FUCCI-HeLa2 (kindly provided by Dr. Miyawaki, RIKEN Brain Science Institute, Japan) and HKF1_H2B-mCherry_αTubulin-mEGFP (kindly provided by Dr. Gerlich, Institute of Molecular Biotechnology, Vienna, Austria) cells were maintained in DMEM with 10% FCS. All cell lines were tested every other month to be mycoplasma-negative as judged by the MocyAlert Mycoplasma Detection Kot (Lonza, LT-07). Primary cells were purchased from Cell Systems (Troisdorf, Germany) and used at passage 4. Keratinocytes were maintained in Keralife medium (Cell Systems), fibroblasts in DMEM and melanocytes in Melanocyte Growth Medium (M2, Promocell, Heidelberg, Germany). Primary melanoma cells were freshly isolated from chopped patients metastasis (M2 = m (65); M10 = m (63); M20 = f (87)) incubated in HBBS (w/o Ca2+ and Mg2+) containing 0.05% collagenase; 0.1% hyaluronidase; 1.25 U/ml dispase; 20 mM HEPES, 100g/ml gentamycin; 100 U/ml penicillin and 100 g/ml streptomycin for 60 min at 37°C. After centrifugation cell pellets were washed in HBSS / 20 mM HEPES and maintained in RPMI + 10% FCS. The usage of patient material (metastasis) for biochemical analysis was approved by the ethics committee of the TU-Dresden (EK 65032013) and informed consent was obtained from all patients.
The pan-caspase inhibitor QVD (Novus Biologicals, Littleton, CO, USA) was added at 5 μM, the caspase-8 inhibitor zIETD (R&D Systems, Minneapolis, MN, USA), the RIP1 inhibitor Nec1S (BioVision, Hannover, Germany) at 15 μM, the proteasome inhibitor MG-132 (Merck Millipore, Darmstadt, Germany) at 10 μM. cisplatin (GYR-Pharma GmBH) at 30 μM, temozolomide (Sigma) at 2 mg/ml, and izTRAIL at 100 ng/ml to cells. Cells were irradiated with UVB (200 J/m2) using TL12 fluorescent bulbs (290-320 nm, Philips; Eindthoven, Netherlands).
METHOD DETAILS
Cell Cycle Analysis
Synchronization of WM115 cells in G2/M was facilitated with an aphidicolin (5 μg/ml, Sigma, St. Louis, MS, USA) block (17 h - 6 h release - 19 h) at the G1/S transition and subsequent release for 5 and 6 h respectively. Synchronization in S-phase was facilitated with a thymidine (1 mM, Sigma) block (17 h - 6 h release - 19 h). Cells were fixed in EtOH (70%) over night at 4°C followed by extraction of DNA in DNA-Extraction buffer (0.2 M Na2HPO4, pH 7.8; 0.1% Triton) for 5 min at RT, and staining of DNA in staining-buffer (20μg/ml PI + 200 μg RNaseA) for 15 min at 37°C. Cell cycle phase was checked by flow cytometry analysis (FACSAria III, Becton Dickinson, Franklin Lakes, NJ, USA).
Plasmids, Cloning, DNA sequencing, and siRNA transfection
Based on a pcDNA3.1(+)−caspase8-myc tagged plasmid the potential NES (L25/27P-L65/66P-F67A), NLS (R471G-K472/473N), and (C360A) sequences were altered by site directed mutagenesis using Pfu-ultra polymerase (Promega, Madison, VC, USA) followed by DpnI digestion (Thermo Scientific, Waltham, MA, USA) according to the manufacturers instruction. For transient expression 6.5 x 106 cells were electroporated with 25 μg of respective plasmids in 600 μl RPMI + 10% FCS + 1.25% DMSO and investigated 24 h later. Single clones stably expressing cDNA or shRNA were selected after transfection by culturing in RPMI containing 1.6 mg/ml G418 (#sc-29065B, Santa Cruz Biotechnology, Heidelberg, Germany) for 8 weeks. wtUSP28 and mutUSP28(D119E) and (D240E) were synthesized by Eurofins (Ebersberg, Germany) and cloned into pRetroX-IRES-ZsGreen1 vectors (Clontech, Goeteborg, Sweden). A pRetroSuper-blasto-p53i plasmid was used to stably knockdown p53 in WM115 cells(Muller et al., 2015). Single cell clones were selected by culturing cells in RPMI containing 5 μg/ml blasticidin (Thermo Scientific). Caspase-8 (11 silent mismatches) was cloned into a MSCV-GFP-moTAPtag-vector via HpaI and MfeI restriction, and GFP-positive clones selected by sorting (FACSAria III, Becton Dickinson). For sequence analysis of the p53 gene of freshly isolated melanoma metastasis (M10, M20, M45) p53 was reverse transcribed from RNA using First Strand cDNA Synthesis Kit (Thermo Scientific) and amplified via PCR using forward: 5’-CTAGCTAGCATGGAGGAGCCGCAG-3’ and reverse primer: 5’-GCATCTAGAGTCTGACTGAGGCCCTTC-3’. cDNA was cloned via NheI/XbaI restriction into pcDNA3.1(+) and subjected to Sanger Sequencing (GATC, Konstanz, Germany) using pcDNA3.1-FP (5’-CTCTGGCTAACTAGAGAAC-3’) and pcDNA3.1-RP (5’-CAAACAACAGATGGCTGGC-3’) primer within the vector and p53for (5’-ATGACGGAGGTTGTGAG-3’) and p53rev (5’-ACTCGGATAAGATGCTGAGG-3’) primer designed to match with the p53 cDNA. Sequences were subjected to BLAST analysis compared to wtp53 transcript variant 1 (https://www.ncbi.nlm.nih.gov/nuccore/NM_000546.4).
Transient knockdown was facilitated by transfecting 5x104 cells with 40 pmol of the respective siRNA for caspase-8- 5’-GCAAUCUGUCCUUCCUGAAUUTT-3’, lacZ-5’-GCGGCUGCCGGAAUUUACCTT-3’ or USP28-5’- GUACUACUCAGACUAUUGATT-3’ (Eurofins) or respective ON-Target plus SMART pools using Lipofectamine 2000 (Thermo Scientific) 48 or 72 h prior to stimulation. For p53 knockdown custom made siRNA 5’-GGGUUAGUUUACAAUCAGCTT-3’ was purchased from Thermo Scientific.
Proliferation and clonogenic outgrowth
For proliferation analysis 105 cells were seeded into 6-well plates, counted, and 105 cells re-seeded every third day over a period of two weeks. Clonogenic outgrowth was determined by seeding 1.5x105 cells into 6-well plates with or without zIETD for 72 h. Subsequently, cells were stained with crystal violet (0.1 w/v in 20% methanol) for 15 min at RT. After washing in PBS crystal violet dissolved from cells with 0.1 M KH2PO4/EtOH for 5 min at RT and color intensity of supernatants measured at 595 nm (Tecan M200, Tecan, Männedorf, Switzerland).
Determination of cell death
72 h after transfection of cells with respective siRNAs cells were harvested and apoptosis determined in a Cell Death Detection ELISA (CDDE, Roche, Mannheim, FRG). The enrichment of mono- and oligonucleosomes released into the cytosol is calculated: absorbance of samples/absorbance of control cells (Tecan M200). An enrichment factor of 2 corresponds to 10% apoptosis as determined by AnnexinV FACS analysis (FACSAria III, Becton Dickinson).
In vitro cleavage and de-ubiquitination assay
In vitro cleavage assay was performed by co-incubating recombinant active caspase-8 (705-C8-010; R&D Systems) and recombinant USP28 (ab188695; Abcam, Camebridge, UK) in CHAPS buffer (25 mM HEPES, 0.1% CHAPS; 1 mM DTT; pH 7.5) at 37°C for 3 h.
For the in vitro de-ubiquitination assay recombinant p53 protein was ubiquitinated using the Human MDM2/HDM2 Ub Ligase Kit (BostonBiochem, Camebridge, MA, USA) according to the manufacturer’s instructions. Subsequently recombinant USP28 (0.9 μM) was added for 3 h at 37°C and samples subjected to Western-blotting.
TUBE assay and Western blot analysis
Cells were lysed in lysis buffer (20 mM Na2HPO4; 20 mM NaH2PO4; 1% NP40, 2 mM EDTA; phosSTOP®; Complete® (Roche, Mannheim, Germany); 10 μM PR-619; 1,10-phenoanthroline; 1 mM DTT) and TUBE pull down performed according to the protocol supplied (UM401, Life Sensors, Philadelphia, PN, USA). Pulled down proteins were subsequently subjected to Western-blot analysis.
For cellular fractionation NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific) were used according to the manufacturer’s instructions. For whole cell lysates cells were lysed in lysis buffer as above. After centrifugation, supernatants were collected and the protein content determined by DC Protein assay kit (BioRad, Hercules, USA). 60-80 μg of protein extracts were subjected to 4-15% gradient SDS-PAGE (BioRad, Hercules, USA), blotted onto nitrocellulose membranes and incubated with antibodies directed against PARP, bak, bax, p53, XIAP (#551025, #556382, #556467, #554293, #610717, BD-Biosciences), caspase-3, caspase-9, caspase-10, cleaved caspase-8, FADD, RIP1, Puma, p-p53(Ser15), Cdk1, p-Cdk1(T161), p-Cdk1(T14), p-Cdk1(Y15), cdc25C, p-cdc25C(S216), p-cdc25C(T48), Chk1, p-Chk1(S317), p-Chk1(S345), cyclinB1, p-cyclinB1(S147), γH2AX, IκBα, PLK1 (#9665, #9502, #9752, #9496, #2782, #3493, #12450, #9284, #9112, #9114, #2543, #9111, #4688, #4901, #12028, #2360, #2344, #2348, #4138, #4131, #9718, #4814, #4513, Cell Signaling, Camebridge, UK), cIAP1, cIAP2, RIP3, USP28 (#ab108361, #ab23423, #ab71206, #ab126604, Abcam), p-H3(S10), FLIP, Noxa (#H0412, #F6550, #PRS2437, Sigma), caspase-8 (#AG-20B-0057, Adipogen, Liestal, Switzerland), Ubiquitin-K48 (#05-1307, Merck Millipore, Darmstadt, Germany), and MDM2 (#MA1-113, Thermo Scientific), respectively. Equal loading was monitored by re-probing membranes with antibodies against α-tubulin (#MS-581-P1, Thermo Scientific), β-actin or GAPDH (#4970, #2118, Cell Signaling). HRP-conjugated secondary antibodies (mouse: #NA931; rabbit: #NA934) were purchased from GE-Healthcare (Buckinghamshire, UK). Bands were visualized by applying chemiluminescense SuperSignal® detection systems (Thermo Scientific).
Immunohistochemistry, immunofluorescence and microscopy
Tissue samples were fixed in 4% buffered formaldehyde overnight and embedded in paraffin. 3 μm sections were blocked 20 mg/ml BSA and 1 mg/ml human gamma globulin (Gamma-Venin, Aventis Behring) in PBS for 20 min. Primary caspase-8 antibody (#ALX-804-429; Enzo Life Science) was incubated for 1 h at RT, sections washed twice in PBS, incubated with second blocking solution (20% NGS, #005-000-121 in PBS, Dianova, Hamburg, Germany) for 20 min, followed by incubation with biotinylated secondary antibody (#115-065-068, Dianova) for 30 min at RT, rinsed twice in PBS, and incubated with streptavidin-alkaline phosphatase (#HK321-UK, BioGenex, Trnava, Slowakia) for 30 min at RT. The staining reaction was developed using Liquid Permanent Red (#K0640, DAKO, Santa Clara, CA, USA). To block endogenous alkaline phosphatase, Levamisole (#X3021, DAKO) was added to the substrate buffer. After counterstaining with hematoxylin (#S2020, DAKO) sections were mounted in glycergel (#C0563, DAKO) and images taken with an Axiovert 200M, AxioCam HRc unit (Zeiss, Oberkochen, Germany).
Freshly cut prostate cancer TMA sections were incubated in Tris EDTA buffer (cell conditioning 1; CC1) at 95°C for 36 min to retrieve antigenicity, followed by incubation with primary caspase-8 antibody (#NB100-56527, Novus Biologicals, dilution 1:250) at 37°C for 1 h followed by universal secondary antibody at 37°C for 30 min and visualized using DAB Map detection kit (#760-124, Ventana, Rotkreuz, Switzerland). Caspase-8 expression was determined as the product of staining intensity and % of stained cancer cells as well as the localization. An overall score of 2 and 3 was considered as high caspase-8.
Non-transfected call lines and primary melanoma metastasis and cells transfected with respective caspase-8-myc constructs, respectively, were grown on coverslips, washed with PBS and fixed in 4% paraformaldehyde for 15 min at RT and permeabilized with 0.1% Triton X-100 in PBS (+1 mM MgCl2 + 0.1 mM CaCl2). After blocking with 5% FCS in PBS (++) for 30 min at RT, primary antibodies against caspase-8 (#PA5-21336, Thermo Scientific), cleaved caspase-8 (#sc-166320; Santa Cruz), and myc-tag (#ab18185, Abcam), respectively, were incubated for at RT 2 h. After washing, Alexa-488 or −546 coupled isotype-specific secondary antibodies (#A11034, #A11030, Life Technologies) were incubated at RT for 1 h, coverslips washed, and mounted in Mowiol (#D2522, Sigma). Nuclei were labelled with DAPI (1μg/ml, Thermo Scientific), and the cellular actin skeleton visualized with Alexa-633-coupled phalloidine (0.2 μM; Thermo Scientific). Images were taken with an LSM 710 confocal fluorescence microscope (Zeiss) and analysed using ZEN 2 lite software (Zeiss).
FUCCI2-HeLa and HKF1_H2B-mCherry_αTubulin-mEGFP cells were imaged for 72 h at the Zeiss Cell Observer using the Colibri-System to excite mCherry and mVenus. Additionally transmitted light was used. The MatLab based Fucci Analysis software, developed by Matthias Lorenzen (IST, University of Stuttgart, Germany), was used for further analyses.
HKF1_H2B-mCherry_αTubulin-mEGFP cells were harvested by mitotic shake off, resuspended in PBS/2%FCS, and subjected to ImageStreamX Mark II analysis.
RNAseq analysis
Prostate tissue was collected from high-risk patients undergoing radical prostatectomy and snap frozen according to the current Vancouver General Hospital pathology protocol. All patients signed a formal consent form approved by the ethics board, and in accordance with the Helsinki Declaration. High-risk cases were selected for this study by meeting any of the following criteria: Gleason ≥8, PSA ≥20, or clinical stage T3a and above. RNA from snap-frozen tissue was isolated using the mirVana Isolation Kit from Ambion (AM 1560). RNA sequencing was performed on Illumina HiSeq 2000 at BCCA Michael Smith Genome Sciences Centre according to standard protocols. Sequence data processing was performed as previously described (Wyatt et al., 2014). Raw read counts were normalized by R package DESeq, which was designed for gene expression analysis of RNA-seq data across all samples. Data is presented as ratio to benign. Statistical analysis was performed on normalized RNA-seq read counts.
Mass spectrometry analysis
Coomassi Brilliant Blue-stained protein bands were excised, cysteine residues reduced with DTE and alkylated with iodacetamide. Subsequently, proteins were digested in-gel with either trypsin or chymotrypsin in 10 mM NH4HCO3. Samples were analyzed by C-18 reversed phase HPLC-MS/MS at a flow rate of 220 nl/min (Figure S6A, Dionex Ultimate 3000 RLSC, LTQ-Orbitrap XL ETD, Acclaim Pepmap RLSC 75pm x 15cm, all Thermo Scientific). The mass spectrometer was operated in data dependent acquisition mode with acquisition of intact peptide ions in the orbitrap (R60000 @ m/z 400 Th) and acquisition of fragment spectra in the linear ion trap. Peptide fragment spectra were extracted from the raw data files with the MS data file conversion tool V3.9 (Massmatrix, USA) and peptides were identified with the Mascot software V2.6 (Matrixscience, London, UK). Peptide signals were quantified manually by peak integration over time and peptide abundances were compared.
Bioinformatics und statistical analysis
The processed Fragments Per Kilobase of transcript per Million mapped reads (FPKM) values from healthy and tumor tissues taken from The Cancer Genome Atlas (TCGA) were log2 transformed and implemented to the Linear Models for Microarray Data (limma) for differential expression analysis (Ritchie et al., 2015). The limma-trend method was used by inputting the log2-FPKM values into limma’s standard pipeline, with trend=TRUE for the empirical Bayes function (Law et al., 2014).
For correlation analysis of caspas-8 expression and p53 mutation status publicly available RNA sequencing data from TCGA were downloaded from cbioportal.org (Cerami et al., 2012). RNAseq data for caspase-8 as well as clinical variables for p53 mutation and the survival status of patients were used. Patients were split into high and low caspase-8 expression groups based on the median values of caspase-8 expression. For analysis R version 3.3.3, Bioconductor version 3.4 and the software packages limma 3.30.8 were used.
Statistical analysis of biochemical data was performed using students T-test. For statistical analysis of patient data one-way ANOVA, Turkey’s multiple comparison test was performed. Mantel-Cox (log-rank)-analysis was performed to compare two populations in Kaplan Meier plots.
DATA AND CODE AVAILABILITY
Original uncropped images for all IF, IHC and WB analyses presented in the paper and in the supplemental information are available at DOI: 10.17632/hnzf9h65p.
Supplementary Material
Supplemental Video 1. Related to Figure 4. Cell cycle analysis of S/G2/M phase (green) versus G1 phase (red) in FUCCI2 Hela cells silenced for caspase-8.
Supplemental Video 2. Related to Figure 4. Time-lapse microscopy of H2B-mCherry-and αTubulin-mEGFP-expressing HeLa cells silenced for caspase-8.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| β-Actin | Cell Signaling | RRID:AB_2223172 |
| Bak | BD-Biosciences | RRID:AB_2060956 |
| Bax | BD-Biosciences | RRID:AB_396430 |
| Caspase-3 | Cell Signaling | RRID:AB_2069872 |
| Caspase-8 - Western-blot analysis | Adipogen | RRID:AB_2490271 |
| Caspase-8 - Immunocytochemistry | Thermo Scientific | Cat# PA5-21336 |
| Caspase-8 - Immunohistochemistry (TMAs) | Novus Biologicals | RRID:AB_837869 |
| Cleaved Caspase-8 | Santa Cruz Biotech | RRID:AB_2068327 |
| Cleaved Caspase-8 | Cell Signaling | RRID:AB_561381 |
| Caspase-9 | Cell Signaling | RRID:AB_2068621 |
| Caspase-10 | Cell Signaling | RRID:AB_2275152 |
| Cdk1 | Cell Signaling | RRID:AB_2074654 |
| p-Cdk1 (T161) | Cell Signaling | RRID:AB_2074652 |
| p-Cdk1 (T14) | Cell Signaling | RRID:AB_823465 |
| p-Cdk1 (Y15) | Cell Signaling | RRID:AB_331460 |
| cdc25C | Cell Signaling | RRID:AB_560956 |
| p-cdc25C (S216) | Cell Signaling | RRID:AB_331215 |
| p-cdc25C (T48) | Cell Signaling | RRID:AB_331486 |
| Chk1 | Cell Signaling | RRID:AB_2080320 |
| p-Chk1 (S317) | Cell Signaling | Cat# 2344 |
| p-Chk1 (S345) | Cell Signaling | RRID:AB_331212 |
| Cyclin B1 | Cell Signaling | RRID:AB_2072132 |
| p-Cyclin B1 (S147) | Cell Signaling | RRID:AB_2128140 |
| GAPDH | Cell Signaling | RRID:AB_561053 |
| p-Histone H3 (S10) | Sigma | RRID:AB_477043 |
| cIAP1 | Abcam | RRID:AB_10862855 |
| cIAP2 | Abcam | RRID:AB_2063933 |
| IκBα | Cell Signaling | Cat# 4814 |
| FADD | Cell Signaling | RRID:AB_2100484 |
| FLIP | Sigma | Cat# F6550 |
| MDM2 | Thermo Scientific | RRID:AB_2536824 |
| Myc-tag | Abcam | RRID:AB_444307 |
| Noxa | Sigma | RRID:AB_1854576 |
| PARP | BD-Biosciences | RRID:AB_394009 |
| p53 | BD-Biosciences | RRID:AB_395348 |
| p-p53 (S15) | Cell Signaling | Cat# 9284 |
| PUMA | Cell Signaling | RRID:AB_2797920 |
| PLK1 | Cell Signaling | RRID:AB_2167409 |
| RIP1 | Cell Signaling | RRID:AB_2305314 |
| RIP3 | Abcam | RRID:AB_1270322 |
| α-tubulin | Thermo Scientific | Cat# MS-581-P1 |
| Ubiquitin-K48 | Merck Millipore | Cat# 05-1307 |
| USP28 | Abcam | RRID:AB_11127442 |
| XIAP | BD Biosciences | RRID:AB_398040 |
| Anti-mouse-HRP | GE-Healthcare | RRID:AB_772210 |
| Anti-rabbit-HRP | GE-Healthcare | RRID:AB_772206 |
| Anti-mouse-Biotin | Dianova | Cat# 115-065-068 |
| Anti-rabbit-A488 | Thermo Scientific | Cat# A11034 |
| Anti-mouse-A546 | Thermo Scientific | Cat# A11030 |
| Biological Samples | ||
| Human melanoma metastasis tissue | Department of Dermatology, Medical Faculty, TU-Dresden | https://www.uniklinikum-dresden.de/de/dasklinikum/klinikenpolikliniken-institute/der |
| Primary Keratinocytes | CellSystems | Cat# FC-0007 |
| Primary Fibroblasts | CellSystems | Cat# FC-0001 |
| Primary Melanocytes | CellSystems | Cat# FC-0030 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Agarose-TUBEs | Life Sensors | Cat# UM401 |
| Annexin V-FITC | BioLegend | Cat# 640906 |
| Aphidicolin | Sigma | Cat# A0781 |
| Blasticidin | Thermo Scientific | Cat# R2101 |
| Complete™ Protease Inhibitor Cocktail | Roche | Cat# 04693116001 |
| DAPI | Thermo Scientific | Cat# 62248 |
| Dermalife®K Complete Medium | CellSystems | Cat# LN-0027 |
| DpnI | Thermo Scientific | Cat# ER1701 |
| Glycergel | DAKO | Cat# C0563 |
| G418 | Santa Cruz | Cat# sc-29065B |
| Hematoxylin | DAKO | Cat# S2020 |
| Levamisole | DAKO | Cat# X3021 |
| Lipofectamine® 2000 | Thermo Scientific | Cat# 11668019 |
| Liquid Permanent Red | DAKO | Cat# K0640 |
| M2 Melanocyte Growth Medium | Promo Cell | Cat# C-24300 |
| MG-132 | Merck Millipore | Cat# 474790 |
| Mowiol | Sigma | Cat# 81381 |
| Nec1S | BioVision | Cat# 2263-1 |
| NGS | Dianova | Cat# 005-000-121 |
| Paraformaldehyde (PFA) | Sigma | Cat# P6148 |
| Pfu-ultra polymerase | Promega | Cat# M7745 |
| Phalloidin-A633 | Thermo Scientific | Cat# A22284 |
| 1,10-phenoanthroline | Life Sensors | Cat# SI9649 |
| PhosSTOP™ | Roche | Cat# 04906837001 |
| PR-619 | Sigma | Cat# SML0430 |
| Q-VD-OPH (QVD) | Novus Biological | Cat# NBP2-29391 |
| Restriction enzymes (HpaI / MfeI) | Thermo Scientific | Cat# ER1031 / ER0751 |
| Rec. caspase-8 | R&D Systems | Cat# 705-C8-010 |
| Rec. USP28 | Abcam | Cat# ab188695 |
| ON-Target plus SMART pool, human caspase-8 | Thermo Scientific | Cat# 003466-00-005 |
| ON-Target plus SMART pool, human RIP1 | Thermo Scientific | Cat# 004445-00-005 |
| ON-Target plus SMART pool, human RIP3 | Thermo Scientific | Cat# 003534-00-005 |
| Thymidine | Sigma | Cat# T1895 |
| Streptavidine-alkaline phosphatase | BioGenex | Cat# HK321-UK |
| Critical Commercial Assays | ||
| Cell Death Detection ELISAPLUS | Roche | Cat# 11920685001 |
| NE-PER® Extraction kit | Thermo Scientific | Cat# 78835 |
| SuperSignal® West Pico Chemil. Substrate | Thermo Scientific | Cat# 34087 |
| SuperSignal® West Dura Ext. Dur. Substrate | Thermo Scientific | Cat# 34076 |
| DAB Map detection kit | Ventana | Cat# 760-124 |
| Human MDM2/HDM2 Ub Ligase Kit | BostonBiochem | Cat# K200B |
| First Strand cDNA synthesis Kit | Thermo Scientific | Cat# K1612 |
| RNeasy Mini Kit | Qiagen | Cat# 74104 |
| TUBE pull down kit | Life Senssors | Cat# UM401 |
| Deposited Data | ||
| Unprocessed and uncompressed imaging data (WB, IF, IHC) | Mendeley Dataset | DOI: 10.17632/hnzf9h65py.1 |
| Experimental Models: Cell Lines | ||
| Human melanoma WM115, A375, MeWo, 451-LU, WM35, WM3211 | The Wistar Institute Melanoma Research Center | https://www.wistar.org/our-science/wistar-centers/melanoma-research-center |
| Human colon cancer HCT116, Colo 205 | Institute of Cell Biology and Immunology University of Stuttgart, Germany | Dr. Angelika Hausser |
| Human pancreas carcinoma Panc89 | Institute of Cell Biology and Immunology University of Stuttgart, Germany | Dr. Angelika Hausser |
| Human prostate cancer PC3, DU145 | Vancouver Prostate Centre, Faculty of Medicine University of British Columbia, Vancouver | Dr. Mads Daugaard |
| Human glioma A172, T98G | University Hospital Ulm, Germany | Prof. Dr. Klaus-Michael Debatin |
| Human breast cancer MCF7, T47D | Institute of Cell Biology and Immunology University of Stuttgart, Germany | Prof. Dr. Monilola Olaiyoye |
| Human hepatocellular carcinoma HepG3B, HUH-7 | Department of Gastroenterology, Medical University of Hannover, Germany | Prof. Dr. Heike Bantel |
| Human bladder cancer UM-UC-3, UC-1 | Vancouver Prostate Centre, Faculty of Medicine University of British Columbia, Vancouver | Dr. Mads Daugaard |
| FUCCI-HeLa2 | Institute of Cell Biology and Immunology University of Stuttgart, Germany | Dr. Angelika Hausser |
| HKF1_H2B-mCherry_αTubulin-mEGFP | Austrian Academy of Science, Vienna, Austria | Prof. Dr. Daniel W. Gerlich |
| U2OS | Danish Cancer Society Research Center, Denmark | Prof. Dr. Marja Jaattela |
| Oligonucleotides | ||
| siRNA caspase8 5’-GCAAUCUGUCCUUCCUGAAUUTT-3’ | MWG Eurofins | N/A |
| siRNA lacZ 5’-GCGGCUGCCGGAAUUUACCTT-3’ | MWG Eurofins | N/A |
| siRNA USP28 5’- GUACUACUCAGACUAUUGATT-3’ | MWG Eurofins | N/A |
| siRNA p53 5’-GGGUUAGUUUACAAUCAGCTT-3’ | Thermo Scientific | AM51333 |
| pcDNA3.1-FP 5’-CTCTGGCTAACTAGAGAAC-3’ | MWG Eurofins | N/A |
| pcDNA3.1-RP 5’-CAAACAACAGATGGCTGGC-3’ | MWG Eurofins | N/A |
| p53for 5’-ATGACGGAGGTTGTGAG-3’ | MWG Eurofins | N/A |
| p53rev 5’-ACTCGGATAAGATGCTGAGG-3’ | MWG Eurofins | N/A |
| Recombinant DNA | ||
| pcDNA3.1(+)-Caspase8-myc | This study | N/A |
| pcDNA3.1(+)-Caspase8-myc(L25/27P-L65/66P-F(67A) | This study | N/A |
| pcDNA3.1(+)-Caspase8-myc(R471G-K472/473N) | This study | N/A |
| pcDNA3.1(+)-Caspase8-myc(C360A) | This study | N/A |
| pcDNA3.1(+)-Caspase8-myc(ΔDED) | This study | N/A |
| MSCV-GFP- Caspase8(11mm)-moTAP-tagged | This study | N/A |
| pRetroSuper-blasto-p53i | Previous collaboration | Dr. Moshe Oren |
| pRetroX-IHRES-ZsCreen1-wtUSP28 | This study | N/A |
| pRetroX-IHRES-ZsCreen1-mutUSP28(D119E) | This study | N/A |
| pRetroX-IHRES-ZsCreen1-mutUSP28(D240E) | This study | N/A |
| Software and Algorithms | ||
| BD FACSDiva™ Software Version 8 | BD Biosciences | https://bdbiosciences.com |
| Bioconductor 3.4 | R / RStudio | http://bioconductor.org/ |
| Fiji | Fiji | https://fiji.sc |
| FlowJo | FlowJo LLC | https://www.flowjo.com/ |
| Image Quant LAS 4000 | GE-Healthcare | http://gelifesciences.com |
| Image Quant TL | GE-Healthcare | http://gelifesciences.com |
| i-control™ | Tecan | http://www.tecan.com |
| JuLI™ Image View | Digital Bio | www.bulldog-bio.com/juli.html |
| Limma 3.30.8 | R / RStudio | http://bioconductor.org/packages/release/bioc/html/limma.html |
| R 3.3.3 | R | https://cran.r-project.org/ |
| RStudio 1.0.143 | RStudio | https://www.rstudio.com/ |
| SCAN.UPC 3.5 | R / RStudio | https://bioconductor.org/packages/release/bioc/html/SCAN.UPC.html |
| Tidyverse 1.1.1 | R / RStudio | http://tidyverse.org/ |
| ZEN 2 lite | Zeiss | https://www.zeiss.com/microscopy/int/downloads/zen-2-core.html |
| MS data file conversion tool V3.9 | MassMatrix | http://www.massmatrix.bio/ |
| Mascot V2.6 | Matrixscience | http://www.matrixscience.com/ |
HIGHLIGHTS.
High nuclear expression of caspase-8 occurs in cancers with poor prognosis
Nuclear caspase-8 in cancer cells accumulates in response to treatment
Caspase-8 cleaves USP28 to prevent p53-driven apoptosis of cancer cells
Nuclear caspase-8 promotes progression of wildtype p53 expressing tumors
ACKNOWLEDGEMENTS
We thank Zhaoyue He for melanoma TMA analysis; Jaromir Sykora and Aurélien Ginolhac for technical support. This work was supported by the Federal Ministry of Education and Research (FKZ 031A423A), the Luxembourg National Research Fund (BMBF/BM/7643621), Prostate Cancer Canada (RS2014-02), and the Vancouver Prostate Centre.
Footnotes
DECLARATION OF INTERESTS
The authors declare no conflict of interest.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures and two videos.
REFERENCES
- Allende-Vega N, Dayal S, Agarwala U, Sparks A, Bourdon JC, and Saville MK (2013). p53 is activated in response to disruption of the pre-mRNA splicing machinery. Oncogene 32, 1–14. [DOI] [PubMed] [Google Scholar]
- Barbero S, Mielgo A, Torres V, Teitz T, Shields DJ, Mikolon D, Bogyo M, Barila D, Lahti JM, Schlaepfer D, and Stupack DG (2009). Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 69, 3755–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind H, Pawitan Y, Kato S, Ishioka C, and Soussi T (2008). Analysis of p53 mutation status in human cancer cell lines: a paradigm for cell line cross-contamination. Cancer Biol. Ther 7, 699–708. [DOI] [PubMed] [Google Scholar]
- Bieging KT, Mello SS, and Attardi LD (2014). Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 14, 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boege Y, Malehmir M, Healy ME, Bettermann K, Lorentzen A, Vucur M, Ahuja AK, Bohm F, Mertens JC, Shimizu, et al. (2017). A Dual Role of Caspase-8 in Triggering and Sensing Proliferation-Associated DNA Damage, a Key Determinant of Liver Cancer Development. Cancer Cell 32, 342–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, and Attardi LD (2005). The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer 5, 231–237. [DOI] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson, et al. (2012). The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell WH, Lehmann BD, Terrian DM, Abrams SL, Steelman LS, and McCubrey JA (2012). p53 expression controls prostate cancer sensitivity to chemotherapy and the MDM2 inhibitor Nutlin-3. Cell Cycle 11, 4579–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuella-Martin R, Oliveira C, Lockstone HE, Snellenberg S, Grolmusova N, and Chapman JR (2016). 53BP1 Integrates DNA Repair and p53-Dependent Cell Fate Decisions via Distinct Mechanisms. Mol. Cell 64, 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska C, Li M, and Fan Y (2016). Apoptotic Caspases in Promoting Cancer: Implications from Their Roles in Development and Tissue Homeostasis. Adv. Exp. Med. Biol 930, 89–112. [DOI] [PubMed] [Google Scholar]
- De BA, Di FR, Morreale M, Carlisi D, Drago-Ferrante R, Montalbano M, Scerri C, Tesoriere G, and Vento R (2016). Unusual roles of caspase-8 in triple-negative breast cancer cell line MDA-mB-231. Int. J. Oncol 48, 2339–2348. [DOI] [PubMed] [Google Scholar]
- Declercq W, Vanden Berghe T, and Vandenabeele P (2009). RIP kinases at the crossroads of cell death and survival. Cell 138, 229–232. [DOI] [PubMed] [Google Scholar]
- Delbridge AR, Grabow S, Strasser A, and Vaux DL (2016). Thirty years of BCL-2: translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 16, 99–109. [DOI] [PubMed] [Google Scholar]
- Fiandalo MV, and Kyprianou N (2012). Caspase control: protagonists of cancer cell apoptosis. Exp. Oncol 34, 165–175. [PMC free article] [PubMed] [Google Scholar]
- Fong CS, Mazo G, Das T, Goodman J, Kim M, O'Rourke BP, Izquierdo D, and Tsou MF (2016). 53BP1 and USP28 mediate p53-dependent cell cycle arrest in response to centrosome loss and prolonged mitosis. Elife. 5, e16270, 2016 July 2, DOI: 10.7554/eLife.16270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank AK, Pietsch EC, Dumont P, Tao J, and Murphy ME (2011). Wild-type and mutant p53 proteins interact with mitochondrial caspase-3. Cancer Biol. Ther 11, 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM (2008). Caspase-8: fly or die. Cancer Res. 68, 4491–4493. [DOI] [PubMed] [Google Scholar]
- Harada K, Toyooka S, Shivapurkar N, Maitra A, Reddy JL, Matta H, Miyajima K, Timmons CF, Tomlinson GE, Mastrangelo D, et al. (2002). Deregulation of caspase 8 and 10 expression in pediatric tumors and cell lines. Cancer Res. 62, 5897–5901. [PubMed] [Google Scholar]
- Helfer B, Boswell BC, Finlay D, Cipres A, Vuori K, Bong KT, Wallach D, Dorfleutner A, Lahti JM, Flynn DC, et al. (2006). Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 66, 4273–4278. [DOI] [PubMed] [Google Scholar]
- Hinata N, Shirakawa T, Zhang Z, Matsumoto A, Fujisawa M, Okada H, Kamidono S, and Gotoh A (2003). Radiation induces p53-dependent cell apoptosis in bladder cancer cells with wild-type- p53 but not in p53-mutated bladder cancer cells. Urol. Res 31, 387–396. [DOI] [PubMed] [Google Scholar]
- Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, and Mocarski ES (2011). RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Hernandez L, and Annunziata CM (2016). Caspase 8 expression may determine the survival of women with ovarian cancer. Cell Death. Dis 7, e2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschny R, Brost S, Hinz U, Sykora J, Batke EM, Singer S, Breuhahn K, Stremmel W, Walczak H, Schemmer P, et al. (2013). Cytosolic and nuclear caspase-8 have opposite impact on survival after liver resection for hepatocellular carcinoma. BMC. Cancer 13, 532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrus BG, Daggubati V, Uetake Y, Scott PM, Clutario KM, Sluder G, and Holland AJ (2016). A USP28-53BP1-p53-p21 signaling axis arrests growth after centrosome loss or prolonged mitosis. J. Cell Biol 214, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrus BG, and Holland AJ (2017). A New Mode of Mitotic Surveillance. Trends Cell Biol. 27, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DP, Cheok CF, and Lain S (2010). p53-based cancer therapy. Cold Spring Harb. Perspect. Biol 2, 2010 May 12, DOI: 10.1101/cshperspect.a001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CW, Chen Y, Shi W, and Smyth GK (2014). voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, 2014 February 3, DOI: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers B, Salmena L, Bidere N, Su H, Matysiak-Zablocki E, Murakami K, Ohashi PS, Jurisicova A, Lenardo M, Hakem R, et al. (2007). Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J. Biol. Chem 282, 7416–7423. [DOI] [PubMed] [Google Scholar]
- Leroy B, Girard L, Hollestelle A, Minna JD, Gazdar AF, and Soussi T (2014). Analysis of TP53 mutation status in human cancer cell lines: a reassessment. Hum. Mutat. 35, 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, and Li CY (2010). Apoptotic cells activate the "phoenix rising" pathway to promote wound healing and tissue regeneration. Sci. Signal 3, 2010 February 23, DOI: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LY, Vidnovic N, Ellisen LW, and Leong CO (2009). Mutant p53 mediates survival of breast cancer cells. Br. J. Cancer 101, 1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottersberger F, Karssemeijer RA, Dimitrova N, and de LT (2015). 53BP1 and the LINC Complex Promote Microtubule-Dependent DSB Mobility and DnA Repair. Cell 163, 880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo-Merino J, Massimi P, Lizano M, and Banks L (2014). The human papillomavirus (HPV) E6 oncoproteins promotes nuclear localization of active caspase 8. Virology 450-451, 146–152. [DOI] [PubMed] [Google Scholar]
- Meitinger F, Anzola JV, Kaulich M, Richardson A, Stender JD, Benner C, Glass CK, Dowdy SF, Desai A, Shiau AK, et al. (2016). 53BP1 and USP28 mediate p53 activation and G1 arrest after centrosome loss or extended mitotic duration. J. Cell Biol 214, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller I, Beissert S, and Kulms D (2015). Anti-apoptotic NF-kappaB and "gain of function" mutp53 in concert act pro-apoptotic in response to UVB+IL-1 via enhanced TNF production. J. Invest Dermatol 135, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Strand S, Hug H, Heinemann EM, Walczak H, Hofmann WJ, Stremmel W, Krammer PH, and Galle PR (1997). Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J. Clin. Invest 99, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, and Green DR (2011). Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa M, Shiraki T, Ninomiya H, Kobayashi S, and Masaki T (1998). Endothelin-induced apoptosis of A375 human melanoma cells. J. Biol. Chem 273, 12584–12592. [DOI] [PubMed] [Google Scholar]
- Rahman M, Jackson LK, Johnson WE, Li DY, Bild AH, and Piccolo SR (2015). Alternative preprocessing of RNA-Sequencing data in The Cancer Genome Atlas leads to improved analysis results. Bioinformatics. 31, 3666–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke RM, van Ree JH, and van Deursen JM (2008). Whole chromosome instability and cancer: a complex relationship. Trends Genet. 24, 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, 2015 January 20, DOI: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue-Sawano A, Kobayashi T, Ohtawa K, and Miyawaki A (2011). Drug-induced cell cycle modulation leading to cell-cycle arrest, nuclear mis-segregation, or endoreplication. BMC. Cell Biol 12, 2011 January 13, DOI: 10.1186/1471-2121-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, Tamblyn L, Shehabeldin A, Migon E, et al. (2003). Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 17, 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Kurose A, Ogawa A, Ogasawara K, Traganos F, Darzynkiewicz Z, and Sawai T (2009). Diversity of DNA damage response of astrocytes and glioblastoma cell lines with various p53 status to treatment with etoposide and temozolomide. Cancer Biol. Ther 8, 452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalini S, Dorstyn L, Dawar S, and Kumar S (2015). Old, new and emerging functions of caspases. Cell Death. Differ 22, 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoehr G, Schaab C, Graumann J, and Mann M (2013). A SILAC-based approach identifies substrates of caspase-dependent cleavage upon TRAIL-induced apoptosis. Mol. Cell Proteomics 12, 1436–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG, Teitz T, Potter MD, Mikolon D, Houghton PJ, Kidd VJ, Lahti JM, and Cheresh DA (2006). Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature 439, 95–99. [DOI] [PubMed] [Google Scholar]
- Suzuki K, and Matsubara H (2011). Recent advances in p53 research and cancer treatment. J. Biomed. Biotechnol 2011, 2011 June 16, DOI: 10.1155/2011/978312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, and Kidd VJ (2000). Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat. Med 6, 529–535. [DOI] [PubMed] [Google Scholar]
- Wang Z, and Sun Y (2010). Targeting p53 for Novel Anticancer Therapy. Transl. Oncol 3, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt AW, Mo F, Wang K, McConeghy B, Brahmbhatt S, Jong L, Mitchell DM, Johnston RL, Haegert A, Li E, et al. (2014). Heterogeneity in the inter-tumor transcriptome of high risk prostate cancer. Genome Biol. 15, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, and Hunter T (2014). Roles of Chk1 in cell biology and cancer therapy. Int. J. Cancer 134, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1. Related to Figure 4. Cell cycle analysis of S/G2/M phase (green) versus G1 phase (red) in FUCCI2 Hela cells silenced for caspase-8.
Supplemental Video 2. Related to Figure 4. Time-lapse microscopy of H2B-mCherry-and αTubulin-mEGFP-expressing HeLa cells silenced for caspase-8.
Data Availability Statement
Original uncropped images for all IF, IHC and WB analyses presented in the paper and in the supplemental information are available at DOI: 10.17632/hnzf9h65p.