Abstract
Patients with Alzheimer’s disease and other dementias often make poor financial decisions, but it remains unclear whether this reflects specific failures in decision-making or more general deficits in episodic and working memory. We investigated how patients with Alzheimer’s disease, behavioral variant frontotemporal dementia (bvFTD), and semantic variant primary progressive aphasia (svPPA) apply information in an intertemporal choice task between smaller intermediate and larger delayed rewards, with minimal memory demands. Multilevel modeling estimated subject-level sensitivities to three attributes of choice (the relative difference in reward magnitude, delay length, and absolute reward magnitudes) as well as baseline impulsivity. While baseline impulsivity in patients with Alzheimer’s disease did not differ from controls, patients with bvFTD and svPPA were more impulsive than controls overall. Patients with Alzheimer’s disease or bvFTD were less sensitive than controls to all three choice attributes, whereas patients with svPPA were less sensitive than controls to two attributes. Attenuated sensitivity to information presented during the choice was associated across all subjects with dorsomedial prefrontal atrophy for all three choice attributes. Given the minimal memory demands of our task, these findings suggest specific mechanisms underlying decision-making failures beyond episodic and working memory deficits in dementia.
Keywords: Alzheimer’s disease, delay discounting, frontotemporal dementia, intertemporal decision making, neuroeconomics
1. Introduction
Financial mismanagement is an early and particularly disabling feature of Alzheimer’s disease and other dementias (Pérès et al., 2008). Impairments in financial decision-making place patients at increased risk for financial abuse, which is the most common form of elder abuse, as well as for other financial losses that can have devastating consequences for their future ability to access care and for their families’ financial stability (Acierno et al., 2010; Marson, 2001). 30% of financial exploitation cases reported to protective services involve victims with dementia (Huang & Lawitz, 2016), and victims with Alzheimer’s disease lose twice as much money per case as those without dementia (Lichtenberg, 2016). Because financial abuse is often unreported and many patients with dementia either are unaware of having been exploited or are dismissed as unreliable reporters, these figures likely underestimate the true costs of impaired decision-making in illness.
In frontotemporal dementia, an umbrella designation encompassing related etiologies that together constitute the third- or fourth-most common form of dementia (Bang, Spina, & Miller, 2015), behavioral paradigms drawn from neuroeconomics and decision neuroscience have provided insights into the neural bases of patients’ financial impairments. This body of research has identified specific abnormalities in the evaluation of potential outcomes of action (Bertoux et al., 2014; Bertoux, de Souza, Zamith, Dubois, & Bourgeois-Gironde, 2015; Chiong et al., 2016), a cognitive process commonly associated with brain regions known to be affected by frontotemporal dementia such as the ventromedial prefrontal cortex and ventral striatum. However, this work has been less revealing about the bases of financial impairment in Alzheimer’s disease, the most common form of dementia, which is associated with temporoparietal and dorsal (rather than ventral) prefrontal atrophy and dysfunction. One potential explanation for financial impairment in Alzheimer’s disease patients is that their susceptibility can be explained entirely by general deficits in episodic and working memory that are well-documented cognitive features of this disorder; i.e., that patients simply forget financially relevant information, or fail to maintain this information in working memory for use in decision-making. An alternative hypothesis is that patients with Alzheimer’s disease also suffer from specific deficits in value-based decision-making analogous to those observed in frontotemporal dementia, in addition to documented deficits in memory. To date, neuroeconomic research paradigms have not yielded firm conclusions about the causes of decisional impairments in Alzheimer’s disease.
Several groups have studied the Iowa Gambling Task in Alzheimer’s disease (Bayard, Jacus, Raffard, & Gely-Nargeot, 2014; Bertoux, Funkiewiez, O’Callaghan, Dubois, & Hornberger, 2013; Kloeters, Bertoux, O’Callaghan, Hodges, & Hornberger, 2013; Sinz, Zamarian, Benke, Wenning, & Delazer, 2008; Torralva, Dorrego, Sabe, Chemerinski, & Starkstein, 2000). While this task was initially proposed as a test of ventromedial prefrontal function (Bechara, Damasio, Tranel, & Damasio, 1997), performance on this task does not reliably distinguish between patients with Alzheimer’s disease and patients with frontotemporal dementia (Bertoux et al., 2013; Kloeters et al., 2013), and poor performance in Alzheimer’s disease is associated with brain volumes in parietal and temporal cortex rather than prefrontal cortex (Kloeters et al., 2013).
Sinz and colleagues studied decision-making under risk in patients with mild Alzheimer’s disease using a gambling task in which subjects chose between (1) a sure gain or loss of 20€ and (2) a gamble in which they could either gain or lose 100€ with varying explicit probabilities (Sinz et al., 2008). There was no main effect of Alzheimer’s disease diagnosis on subjects’ propensity to gamble, but patients’ decisions were less strongly influenced than controls by the probability of winning. Thus, patients made less advantageous choices: they were more likely to gamble when the probability of winning was low, and less likely to gamble when the probability of winning was high. In their study, the probabilities of winning were represented explicitly at the time of choice during the task, and trials were independent. Unlike the Iowa Gambling Task, which has a significant learning component, impaired patient performance on this task by Sinz and colleagues cannot be explained by general deficits in episodic and working memory. This suggests more specific impairments in sensitivity to choice-relevant attributes in immediate value-based decision-making. However, this study was limited by the absence of a neurodegenerative disease comparison group and neuroimaging correlates of behavior, so a generic effect of diminished cognitive ability or neurodegenerative illness could not be excluded.
In previous work, we used a delay discounting paradigm with minimal memory demands to study intertemporal choice in patients with Alzheimer’s disease and in two variants of frontotemporal dementia: behavioral variant frontotemporal dementia (bvFTD) and semantic variant primary progressive aphasia (svPPA, also called semantic dementia). Our findings demonstrated that patients with svPPA, marked by temporal pole and ventromedial prefrontal atrophy (Gorno-Tempini et al., 2004), were more likely than controls to select smaller immediate rewards over larger delayed rewards. There was, similar to the finding by Sinz and colleagues, no significant main effect of Alzheimer’s disease or bvFTD diagnosis on subjects’ propensity to choose smaller immediate or larger delayed rewards. However, because our prior study did not evaluate behavior at the individual trial level, it was not possible to determine whether the patient groups were equally sensitive to choice attributes such as the relative difference in reward magnitude, the delay length, and the absolute magnitude of rewards, which may differentially engage the networks targeted by these different diseases (Greicius, Srivastava, Reiss, & Menon, 2004; Peters & Büchel, 2011; Seeley, Crawford, Zhou, Miller, & Greicius, 2009).
In the present study, we examined how individual trial-level choice attributes influence subjects’ intertemporal choices. We hypothesized that, in addition to changes in patients’ overall tendency to choose immediate or delayed rewards, another mechanism of specific disease-related impairment in decision-making is diminished sensitivity to choice-relevant information; and hypothesized also that behavioral insensitivity would be correlated with atrophy in brain regions involved in choice selection. Healthy older controls and patients with Alzheimer’s disease, bvFTD, and svPPA performed a delay discounting task with multiple trials in which the relative reward difference between a smaller immediate and a larger delayed reward, the length of time required to wait for the larger delayed reward, and the absolute magnitude of the delayed rewards were systematically varied and fully crossed as orthogonal task parameters. In this task, relevant information was explicitly presented at the time of choice and trials were independent, so aberrant behavior would suggest specific deficits in utilizing information to make advantageous choices, as opposed to more general failures of episodic or working memory. Subjects’ choice behavior was modeled with a multilevel mixed-effects regression to derive subject-level estimates of sensitivity to choice attributes, which were then correlated with regional brain volumes using voxel-based morphometry (VBM).
2. Materials and methods
2.1. Study subjects
All subjects or their legally authorized representatives gave written informed consent according to the Declaration of Helsinki, and the study was approved by the Committee on Human Research at the University of California, San Francisco. We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. No part of the study procedures or analysis plans was pre-registered prior to the research being conducted.
Patients were diagnosed by consensus among a multidisciplinary team of neurologists, neuropsychologists and nurses after a comprehensive evaluation including a clinical history, neurological examination, and extensive neuropsychological testing according to established research criteria (Gorno-Tempini et al., 2011; McKhann et al., 2011; Rascovsky et al., 2011). Healthy older subjects were verified as normal by a clinical interview, neurological examination, and neuropsychological testing. We recruited patients with mild to moderate severity of disease by consensus clinical assessment because of the cognitive demands of the intertemporal choice task. Furthermore, these patients are the most clinically relevant population, as patients with more advanced disease usually do not handle their own finances (Giebel, Challis, & Montaldi, 2015). Of the 139 subjects who met diagnostic criteria and completed the task, 17 (12%; seven patients with bvFTD, five with Alzheimer’s disease, two with svPPA, and three healthy controls) were excluded using control conditions (described below) designed to identify subjects with uninterpretable data. This yielded a cohort of 122 subjects (15 patients with Alzheimer’s disease, 18 with bvFTD, 17 with svPPA, and 72 healthy controls) included for analysis. Given that our previous work has demonstrated a wide range of normal behavior in healthy controls with respect to impulsive choice proportion (Chiong et al., 2016), we expected a large degree of variability in responsiveness to choice attributes as well. We used a larger control sample to avoid biasing behavioral model estimation of group differences by under-sampling the normal range of behavior in matched controls without neurologic disease. Research records for the study subjects were reviewed to obtain demographic characteristics, including age, gender, handedness, and years of education. We also obtained Mini-Mental Status Examination (MMSE) (Folstein et al., 1975) and Clinical Dementia Rating (Morris, 1993) scores collected within 365 days of the experimental task from existing research records. Demographic, clinical and neuropsychological data for the patients and control subjects included for analysis are summarized in Table 1.
Table 1.
Demographic, clinical, and neuropsychological characteristics of the 122 study subjects
| Healthy Control (n = 72) | Alzheimer (n = 15) | bvFTD (n = 18) | svPPA (n = 17) | P Value | |
|---|---|---|---|---|---|
| Demographic and Clinical | |||||
| Gender (m/f) | 39/33 | 6/9 | 9/9 | 8/9 | 0.77a |
| Age (years) | 69.3 (7.2) | 63.7 (9.4) | 63.6 (6.6) | 68.4 (7.6) | 0.01b |
| Education (years) | 18 (16 – 20) | 18 (16 – 18) | 16 (14 – 18) | 17 (14 – 18) | 0.03c |
| MMSE (score/30) | 30 (29 – 30) | 23 (14 – 26) | 26 (25 – 28) | 24 (22 – 26) | < 0.001c |
| Clinical Dementia Rating (total score) | 0 (0 – 0) | 1 (0.5 – 1) | 1 (0.5 – 1) | 0.5 (0.5 – 1) | < 0.001c |
| Clinical Dementia Rating (sum-of-boxes score) | 0 (0 – 0) | 4.5 (4 – 7) | 5.5 (3 – 8) | 4.5 (3.5 – 6.5) | < 0.001c |
| Memory | |||||
| Modified Rey-Osterrieth figure recall (score/17) | 12.9 (2.8) [62/72] | 3.6 (3.1) [14/15] | 8.9 (3.9) [17/18] | 5.7 (4.1) [16/17] | < 0.001b |
| Executive Function | |||||
| Backward digit span | 5.6 (1.3) [68/72] | 3.6 (1.2) [12/15] | 3.9 (1.2) [18/18] | 5.1 (1.4) [16/17] | < 0.001b |
| Stroop interference | 54.9 (12.3) [61/72] | 24.9 (10.6) [11/15] | 28.8 (15.7) [18/18] | 40.4 (17.1) [16/17] | < 0.001b |
| Design fluency | 11.7 (2.8) [60/72] | 4.8 (2.7) [13/15] | 7.7 (4.5) [18/18] | 6.9 (3.6) [16/17] | < 0.001b |
| Modified trails time (s) | 25.0 (11.4) [71/72] | 87.3 (35.1) [13/15] | 56.6 (39.0) [17/18] | 45.4 (21.2) [16/17] | < 0.001b |
| Verbal fluency (words) | 16.9 (4.6) [72/72] | 9.2 (6.4) [14/15] | 8.1 (4.5) [18/18] | 8.4 (4.0) [16/17] | < 0.001b |
| Category fluency (words) | 24.1 (5.0) [69/72] | 12.1 (7.9) [14/15] | 12.6 (5.9) [18/18] | 8.1 (4.0) [16/17] | < 0.001b |
| Language | |||||
| Boston naming test (score/15) | 14.8 (0.6) [65/72] | 10.9 (3.0) [14/15] | 13.1 (2.8) [17/18] | 4.9 (3.9) [15/17] | < 0.001b |
| Visuospatial | |||||
| Modified Rey-Osterrieth figure copy (score/17) | 15.5 (0.9) [62/72] | 11.4 (5.6) [14/15] | 14.7 (1.4) [17/18] | 15.5 (0.7) [16/17] | < 0.001b |
| Emotional Function | |||||
| Affect matching (score/16) | 12.6 (1.7) [45/65] | 12.1 (1.8) [12/15] | 10.5 (3.0) [17/18] | 9.8 (2.6) [15/17] | < 0.001b |
Values represent mean (standard deviation) when normally distributed or median (interquartile range) when non-normal. Bracketed values are the numbers of subjects with data available from neuropsychological tests. bvFTD = behavioral variant frontotemporal dementia; MMSE = Mini-Mental Status Examination; svPPA = sematic-variant primary progressive aphasia.
Chi-squared test
ANOVA test
Kruskal-Wallis test
2.2. Experimental task
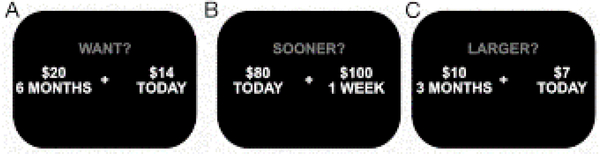
Study subjects completed a computer-based, intertemporal decision task that we have previously described (Chiong et al., 2016), adapted from prior work (Boettiger et al., 2007; Kayser, Allen, Navarro-Cebrian, Mitchell, & Fields, 2012). Analyses included data from subjects in which the main effect of diagnosis on delay discounting has been previously reported (Chiong et al., 2016) as well as from additional subjects who have performed the task since the prior study. In each of 128 trials (Fig. 1A), we presented subjects with hypothetical choices between a smaller immediate monetary reward ($3 to $90) and a larger reward ($5 to $100) delayed 7 to 180 days. The two options were randomly assigned to the left and right sides of a computer screen, and subjects indicated whether they preferred the left or right option by pressing a corresponding arrow key. A brief training session preceded each experimental session to ensure subjects understood the task.
Figure 1. Representative examples of stimuli presented to subjects.
(A) an intertemporal choice with a percent penalty of 30%, delay length of 180 days, and delayed reward magnitude of $20, and control conditions where subjects determined which of two options (B) paid sooner (left option correct) or (C) had a larger monetary value (left option correct).
Three attributes of the choice were varied systematically: percent penalty (i.e., the percent reduction in monetary value of the smaller immediate reward as compared to the larger delayed reward, either 10%, 20%, 30%, or 40%), delay length (i.e., length of time required to wait for the larger delayed reward option, either 7, 14, 90, or 180 days), and delayed reward magnitude (i.e., the monetary value of the larger delayed reward, either $5, $10, $20, or $100). Each of these three attributes thus comprised four levels that were fully crossed as orthogonal task parameters. Each choice was presented twice for a total of 4 × 4 × 4 × 2 = 128 trials, presented in a randomized order. Stimuli were presented and responses were recorded using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA).
Two control conditions resembling the task of interest were used to exclude subjects unable to provide interpretable data given the cognitive and semantic complexity of the task. In the first control condition, instead of asking which of two choices the subject would prefer, we asked which of two choices would pay sooner (Fig. 1B). In the second control condition, we asked which of two choices would pay a larger amount (Fig. 1C). These 20 trials (10 for each condition) were randomly interspersed with the 128 experimental trials for a total of 148 trials in the session. We excluded subjects who did not answer at least 80% (16 out of 20) of these questions correctly.
2.3. Behavioral choice modeling
While our earlier work addressed between-group differences in the overall tendency to choose smaller immediate or larger delayed rewards, in this study we focused on the influence of trial-by-trial information on subjects’ individual choices. We used multilevel mixed-effects logistic regression to assess the influence of three choice attributes (percent penalty, delay length, and delayed reward magnitude) on the likelihood of subjects’ selecting the smaller immediate reward over the larger delayed reward. This model enabled us to estimate the relative influence of each attribute on the likelihood of selecting the smaller immediate reward, and also to estimate subjects’ baseline impulsivity (de Water et al., 2017). This model does not rely on assumptions about the shape of the discount function (e.g., exponential, hyperbolic or quasihyperbolic), which could be distorted in neurological patients. The dependent variable in the model was whether the subject chose the smaller immediate reward instead of the larger delayed reward on a given trial. Independent variables included fixed effects of diagnosis and random effects of the three attributes of each choice trial. Subject-level random effects on the relationship between choice attributes and the log-odds of choosing the smaller immediate reward were included to account for between-subject differences in sensitivity to each attribute (A1. Supplementary Materials and methods). We also included interaction terms between diagnosis and the three choice attributes to model differing sensitivities to each attribute in each neurodegenerative disease.
2.4. Neuroimaging analyses
All images were acquired on a 3.0 Tesla Siemens (Siemens, Iselin, NJ) Tim Trio scanner equipped with a twelve-channel head coil using a magnetization prepared rapid gradient echo (MPRAGE) sequence (160 sagittal slices, slice thickness 1.0 mm, field of view 256 × 230 mm2, matrix 256 × 230, voxel size 1.0 × 1.0 × 1.0 mm3, repetition time 2,300 ms, echo time 2.98 ms, flip angle 9°).
VBM preprocessing and analyses were performed using Statistical Parametric Mapping 8 (Wellcome Department of Cognitive Neurology, London). To optimize intersubject registration, each participant’s image was warped to a template derived from 150 confirmed neurologically healthy older adults who had been scanned with one of three magnet strengths (1.5T, 3T, 4T), using affine and nonlinear transformations with the help of the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) method, as implemented in the toolbox (Ashburner, 2007; Ashburner & Friston, 2005). The developer’s suggested settings were used for all processing steps, and an 8 mm Full Width at Half Maximum (FWHM) kernel was used to smooth the images. For all neuroimaging analyses, we included only those subjects that had a structural T1 scan performed within 365 days of the task.
To characterize regional atrophy in the three disease cohorts (Alzheimer’s disease, bvFTD, and svPPA), we ran a VBM analysis comparing them to healthy controls recruited for our task. Each analysis controlled for the effects of age, gender, education, and total intracranial volume. After being thresholded at voxelwise P < 0.005 and then thresholded at P < 0.05 based on cluster size using a Monte Carlo simulation running 1,000 permutations, we overlaid resulting maps of statistical significance onto a template brain.
To address the hypothesis that distinct neural substrates might underlie sensitivity to choice attributes, model estimates of each subject’s baseline impulsivity and their sensitivities to percent penalty, delayed reward magnitude, and delay length were each associated with regional brain volumes using VBM across all subjects (healthy control, Alzheimer’s disease, bvFTD, and svPPA). We included age, gender, total intracranial volume, MMSE, education, and difference in days between scan date and task date as covariates. Statistical significance maps were thresholded at voxelwise P < 0.005 and then thresholded at P < 0.05 based on cluster size using a Monte Carlo simulation running 1,000 permutations. All voxel-based statistical analyses were conducted using voxel-based lesion–symptom mapping (VLSM) software, version 2.55 (Bates et al., 2003).
To ensure that brain-behavior relationships identified in these analyses were indeed generalizable (i.e., not driven exclusively by findings in a single diagnostic group), we performed a co-atrophy sensitivity analysis for diagnostic group effects (Sollberger et al., 2009). In VBM analyses combining patients from multiple neurodegenerative disease groups, there is a risk that significant findings may in fact hold true only in one diagnostic group rather than representing a generalizable brain-behavior relationship. (For instance, if diagnosis predicts regional atrophy, and diagnosis also predicts behavior, then atrophy may misleadingly appear to be directly correlated with behavior when this association actually depends on the common predictor, diagnosis.) For any brain-behavior associations found significant in our primary analyses, we constructed an additional generalized linear model adding three additional binary confounding variables, one for each diagnosis (Alzheimer’s disease, bvFTD and svPPA). In this co-atrophy sensitivity analysis, we accepted a voxelwise level of significance of P < 0.005 within the clusters previously identified in primary analyses.
Lastly, a conjunction analysis using minimum statistics against the comparative null methods was carried out to ascertain which brain regions were significantly associated with sensitivities to all three choice attributes (Nichols, Brett, Andersson, Wager, & Poline, 2005). In brief, this method identifies brain volumes that were significantly correlated with all three of the sensitivity estimates, which were then used to generate a mask to overlay on a template brain.
2.5. Statistical Analysis
Demographic characteristics were compared by parametric tests when normally distributed and non-parametric tests when not normally distributed. Parameters from the behavioral model were linearly combined to generate estimates of group-level fixed effects of choice attributes and compared by Wald test. Goodness of fit testing for the model is described in detail in A1. Supplementary Materials and methods. All statistical analyses were performed in STATA 14 (StataCorp, College Station, TX). Two-tailed P values < 0.05 were considered significant.
Three sensitivity analyses were undertaken. The first was to determine whether estimates generated by the behavioral model and subsequent neuroanatomic correlations were distorted by the inclusion of subjects who did not vary in their decisions. Thus, this sensitivity analysis excluded subjects if they consistently chose either smaller immediate or larger delayed rewards on every trial. This model was fit to the choice data using identical starting parameters to the full behavioral model. Estimates from the sensitivity analysis were inspected for divergence from the full model’s estimates and applied in brain-behavior correlation analyses using identical methods to those described above. A second analysis was performed excluding one patient who received an adjudicated clinical diagnosis of Alzheimer’s disease but subsequently underwent positron emission tomography (PET) imaging that was negative for amyloid deposition (A2.1 Supplementary results) and then refitting the behavioral model to examine whether their inclusion distorted group-level results or individual-level sensitivity estimates (A1.6 Supplementary Materials and methods). A third analysis was performed in which the main behavioral model was adjusted for age and education to observe whether any identified group-level results were confounded due to significant differences in these characteristics (Table 1).
3. Results
3.1. Voxel-based morphometry by diagnostic group
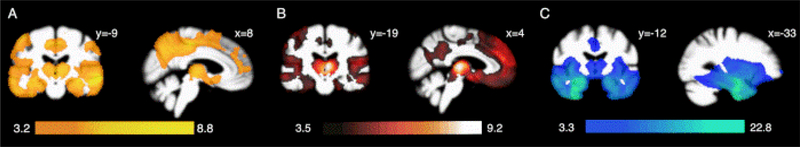
Of the 122 subjects included in the behavioral analysis, 105 had an MRI scan performed within 365 days of the intertemporal choice task (14 patients with Alzheimer’s disease, 18 with bvFTD, 15 with svPPA, and 58 healthy controls). Patient scans (n = 47) were obtained a median of 3 days (IQR 1 – 22) apart from the task, whereas healthy control scans (n = 58) were obtained a median of 132 days (IQR 35 – 273) from the task. Patients demonstrated distinct but overlapping patterns of atrophy that were consistent with clinical diagnoses (Fig. 2). In Alzheimer’s disease, atrophy was most prominent in the medial temporal lobes, medial and lateral parietal cortices, dorsal frontal cortices, and thalami. In bvFTD, atrophy was most prominent in the insulae, thalami, bilateral inferior and medial prefrontal cortices, and ventral basal ganglia. The svPPA cohort displayed bilateral anterior temporal and ventromedial prefrontal atrophy. Shared overlap in atrophy was observed in the temporal poles, putamina, hippocampi, and thalami proper.
Figure 2. Voxel-based morphometry maps of regional atrophy in patients.
(A) Alzheimer’s disease, (B) bvFTD, and (C) svPPA, as compared to healthy controls. Images are oriented by neurological convention.
3.2. Choice behavior by diagnostic group
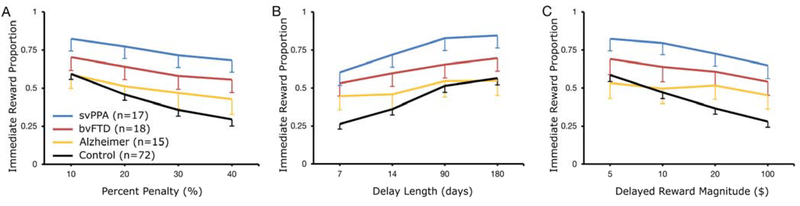
Healthy controls’ choices were sensitive to all three choice attributes in the behavioral model (Table 2). Specifically, controls were less likely to choose the smaller immediate reward as percent penalty (i.e., the relative difference in magnitude between the smaller immediate and larger delayed reward) increased (P < 0.001) and as the absolute magnitude of the delayed reward increased (P < 0.001), and more likely to choose the smaller immediate reward as delay length increased (P < 0.001) (Fig. 3), as predicted by canonical models of delay discounting.
Table 2.
Multilevel mixed-effects logistic regression model parameters describing the influence of choice attributes and diagnosis on the decision to choose smaller immediate rewards
| β (SE) | 95% CI | P valuea | |
|---|---|---|---|
| Fixed Effects | |||
| Diagnosisa | |||
| Alzheimer | 1.17 (0.79) | −0.38 – 2.72 | 0.14 |
| bvFTD | 2.23 (0.84) | 0.58 – 3.87 | 0.01 |
| svPPA | 4.84 (0.98) | 2.92 – 6.76 | < 0.001 |
| Random Effects | |||
| Intercept | −1.05 (0.45) | −1.92 – −0.17 | 0.02 |
| Choice Attributes | |||
| Percent Penaltya | |||
| Control | −2.13 (0.13) | −2.39 – −1.88 | |
| Alzheimer | −0.75 (0.24) | −1.21 – −0.28 | < 0.001 |
| bvFTD | −0.85 (0.24) | −1.33 – −0.38 | < 0.001 |
| svPPA | −1.22 (0.28) | −1.76 – −0.68 | 0.003 |
| Delay Lengtha | |||
| Control | 1.99 (0.18) | 1.64 – 2.33 | |
| Alzheimer | 0.49 (0.32) | −0.15 – 1.12 | < 0.001 |
| bvFTD | 1.09 (0.34) | 0.42 – 1.76 | 0.02 |
| svPPA | 2.72 (0.44) | 1.86 – 3.57 | 0.12 |
| Delayed Reward Magnitudea | |||
| Control | −2.58 (0.20) | −2.97 – −2.19 | |
| Alzheimer | −0.31 (0.36) | −1.01 – 0.39 | < 0.001 |
| bvFTD | −0.83 (0.36) | −1.53 – −0.13 | < 0.001 |
| svPPA | −1.51 (0.40) | −2.29 – −0.72 | 0.02 |
bvFTD = behavioral variant frontotemporal dementia; svPPA = sematic-variant primary progressive aphasia.
Reference group for statistical comparison was healthy controls.
Figure 3. The proportion of trials in which smaller immediate reward was chosen.
Grouped by diagnosis and level of the three choice attributes that were systematically varied, including (A) percent penalty, (B) delay length, and (C) delayed reward magnitude. Attenuated slopes in Alzheimer’s disease as compared with controls indicate reduced sensitivity to each of the three choice attributes, whereas upward displacement of curves such as in svPPA reflects an increased baseline tendency to choose smaller immediate rewards. Values plotted are means, and error bars represent standard error of the mean. Error bars not displayed above curves for visual clarity.
The baseline tendency to choose smaller immediate rewards did not significantly differ between patients with Alzheimer’s disease and controls (P = 0.14). However, patients with Alzheimer’s disease were less sensitive than controls to all three choice attributes (three comparisons, all P < 0.001) (Fig. 3). Patients with bvFTD had a greater baseline tendency to choose smaller immediate rewards than healthy controls (P = 0.01) in the main model but not in a model controlling for age and education (Supplementary Table 1), and were also less sensitive than controls to all three choice attributes (percent penalty P < 0.001, delay length P = 0.02, delayed reward magnitude P < 0.001). Patients with svPPA had the largest increase in baseline tendency to choose smaller immediate rewards (P < 0.001 versus controls). They did not differ from controls in their sensitivity to delay length (P = 0.12) but were less sensitive than controls to percent penalty (P = 0.003) and delayed reward magnitude (P = 0.02).
Estimates of sensitivity to percent penalty were not significantly different between any two of the three patient groups (three comparisons, P values 0.19 to 0.75) (Fig. 3). Patients with Alzheimer’s disease and bvFTD were less sensitive to delay length than patients with svPPA (P < 0.001 & P = 0.003, respectively), while differences between those with Alzheimer’s disease and bvFTD were not statistically significant (P = 0.20). Patients with Alzheimer’s disease were also less sensitive than patients with svPPA to delayed reward magnitude (P = 0.03); there were no statistically significant differences between patients with bvFTD and patients with either svPPA (P = 0.21) or Alzheimer’s disease (P = 0.30). Baseline tendency to select smaller immediate rewards was elevated in patients with svPPA compared to patients with either Alzheimer’s disease (P < 0.001) or bvFTD (P = 0.02) but was not significantly different between patients with Alzheimer’s disease and patients with bvFTD (P = 0.28).
These findings were unchanged in a sensitivity analysis excluding one subject clinically diagnosed with Alzheimer’s disease but later found to have discrepant amyloid PET imaging (Supplementary Table 1). In a sensitivity analysis using a behavioral model adjusted for age and education, no other group-level comparisons were affected aside from the one described above.
Choice consistency, defined as the percent of trial pairs where subjects made the same decision on two trials presenting an identical choice, was 87.3% across the entire sample but significantly lower in patients with Alzheimer’s disease compared with healthy controls and patients with svPPA (A2. Supplementary Results).
Regarding goodness-of-fit, the behavioral model accounted for more variability in the choice data than an intercept-only model (Wald chi-squared = 415.4, P < 0.001) and outperformed an identically-specified logistic regression that did not include random effects (likelihood ratio test chi-squared = 10,595, P < 0.001). A sensitivity analysis indicated that estimates of the fixed and random effects, their variances, and covariances were not substantially affected by the inclusion of subjects who did not vary in their choices (i.e., who selected either smaller immediate or larger delayed rewards in all choice trials). The one exception was that exclusion of subjects who only chose either smaller immediate or larger delayed rewards decreased the variance estimate for the model intercept from 20.9 (95% CI 15.8 to 27.7) to 8.4 (95% CI 6.4 to 11.1).
3.3. Neuroanatomical correlates of behavior
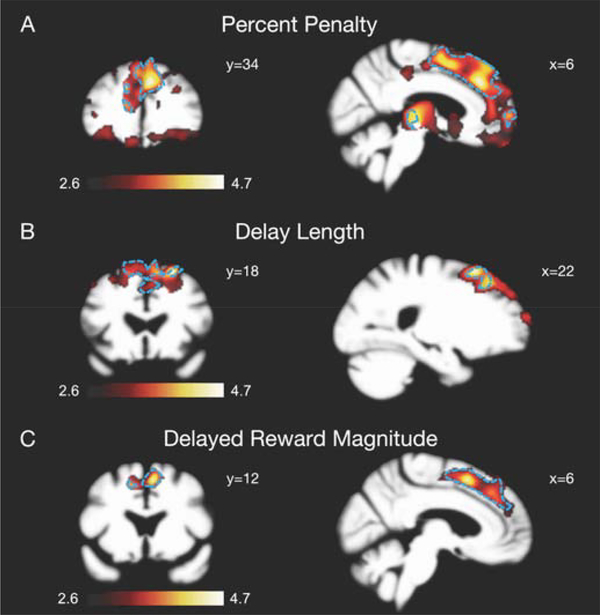
Across the same 105 subjects who had an MRI scan performed within 365 days of the intertemporal choice task (14 patients with Alzheimer’s disease, 18 with bvFTD, 15 with svPPA, and 58 healthy controls), grey matter volumes in the dorsomedial prefrontal cortex (dmPFC) were significantly associated with estimates of sensitivity to all three attributes of the choices (Fig. 4). In the co-atrophy sensitivity analysis controlling for diagnostic group effects, clusters in bilateral dmPFC with peaks in the right dmPFC remained significantly associated with all three attributes (Fig. 4), supporting a generalizable brain-behavior relationship. Additionally, a conjunction analysis to identify brain regions associated with sensitivity to all three choice attributes confirmed overlap in the dmPFC (Supplementary Fig. 5). The MNI coordinates and T values for clusters of voxels and associated regions of interest that were significantly associated with each of three attributes are summarized in Supplementary Tables 2 – 4. There were no significant relationships between baseline impulsivity estimates and brain volumes.
Figure 4. Neuroanatomic correlates of sensitivity to information presented in an intertemporal choice.
Voxel-based morphometry maps of grey matter regions associated with sensitivity to (A) percent penalty, (B) delay length, and (C) delayed reward magnitude across 105 subjects (14 Alzheimer’s disease, 18 bvFTD, 15 svPPA, and 58 healthy controls). Dotted blue lines indicate regions that remained significantly associated with choice attributes after co-atrophy analysis controlling for diagnostic group effects. Images are oriented by neurological convention.
In the sensitivity analyses using estimates generated from the version of the behavioral model that excluded subjects who did not vary in their responses (i.e., only chose smaller immediate or larger delayed rewards), the main finding that dmPFC volumes correlate with sensitivities to the three choice attributes was unchanged.
4. Discussion
We present evidence for specific failures to integrate quantitative information in value-based decision-making in Alzheimer’s disease and other dementias, distinct from previously-characterized deficits in episodic and working memory. Specifically, patients with Alzheimer’s disease did not differ from controls in their baseline tendency to choose smaller immediate over larger delayed rewards. However, at the individual-trial level, the decisions of patients with Alzheimer’s disease were less influenced by relevant choice attributes such as the percent penalty, delay length, and absolute magnitude of rewards. By contrast, patients with svPPA had a greater baseline tendency than controls to choose smaller immediate over larger delayed rewards, but their sensitivity to individual choice attributes was attenuated for some but not all attributes. Patients with bvFTD presented an intermediate phenotype, with less extreme estimates of baseline impulsivity than in svPPA that no longer differed from controls after adjustment for age and education, and less attenuated estimates of sensitivity to all three individual trial attributes than in Alzheimer’s disease. In this task, relevant trial attributes are represented explicitly at the time of choice, and trials are independent. Thus, there is no learning component to task performance, and alterations in patient performance are not explained by deficits in memory alone.
It is also noteworthy that patterns of attenuated sensitivity were quite similar across the independently varied trial attributes of percent penalty, delay length, and delayed reward magnitude (Fig. 3). Sinz and colleagues have described a similar pattern of risk attitudes in Alzheimer’s disease, with preservation of the baseline tendency to gamble but with reduced individual trial-level sensitivity to the probability of winning (Sinz et al., 2008). Together, these findings suggest that disease-related insensitivity to relevant choice attributes likely involve common mechanisms across different dimensions of choice.
Such deficits in information sensitivity would have functionally significant consequences in the real world. For example, our intertemporal choice task can be analogized to a decision about whether to take out a payday loan, in which accepting a smaller immediate payment requires one to forgo a larger future payment. When other attributes are held constant, different values of the percent penalty (or conversely, the delay length) represent more and less advantageous interest rates, and patients whose choices are insensitive to such variations would be more likely to accept loans with higher interest rates and more likely to decline loans with lower interest rates. In real-world population-level data, Agarwal and colleagues have reported that higher loan interest rates and other disadvantageous uses of credit are associated with advanced age (Agarwal, Driscoll, Gabaix, & Laibson, 2009), which is the strongest population-level predictor of dementia. Our findings thus have implications for clinical and policy efforts to prevent financial losses by patients with Alzheimer’s disease and related dementias. As choice attribute insensitivity is observed even when these attributes are made explicit at the time of choice (minimizing memory demands), this aspect of disadvantageous decision-making may not be remedied by memory aids or other decision support tools focused on the availability of relevant information when needed.
In prefrontal cortex, Alzheimer’s disease is marked by principally dorsal atrophy; bvFTD by dorsal and ventral atrophy; and svPPA by predominantly ventral atrophy (Fig. 2). Our findings across these varied neurodegenerative conditions suggest a general role for the dmPFC in modulating economic choices based upon choice-specific information. Brain regions associated with sensitivity to percent penalty, delay length, and absolute reward magnitude were overlapping with shared representation in dmPFC (Fig. 4, Supplementary Fig. 5). These choice attributes were fully crossed across trials in the intertemporal choice task that was used to derive estimates of attribute sensitivity. These findings suggest shared mechanisms in dmPFC for integrating quantitative attributes of choice in a given decision.
Our structural neuroimaging findings in disease are congruent with recent proposals based on functional neuroimaging in healthy subjects regarding the role of the dmPFC in intertemporal choice, and in economic decision-making more broadly. Early studies indicated greater dmPFC activity during “difficult” intertemporal choices (i.e., choices closer to the subject’s indifference point) (Hoffman et al., 2008; Marco-Pallarés, Mohammadi, Samii, & Münte, 2010; Pine et al., 2009), which has been interpreted as a marker of response conflict. However, an alternative explanation is that dmPFC activation in these hard choices reflects the allocation of cognitive resources when less computationally demanding heuristics are unavailable or inappropriate. Outside of intertemporal choice, dmPFC activation has been associated with decisions that are contrary to simplifying heuristics such as framing effects (De Martino, Kumaran, Seymour, & Dolan, 2006), status quo bias (Fleming, Thomas, & Dolan, 2010), and defaulting to an individual’s own dominant choice tendency (Venkatraman, Payne, Bettman, Luce, & Huettel, 2009). Recently, Rodriguez and colleagues have proposed a value-accumulation model for intertemporal choice, in which subjective value signals from ventromedial prefrontal cortex regarding available options are integrated and accumulated in a frontoparietal network of brain regions, principally the dmPFC (Rodriguez, Turner, Van Zandt, & McClure, 2015).
The neuroanatomical associations between dmPFC volume and choice attribute sensitivity remained significant in a co-atrophy sensitivity analysis for diagnostic group effects. Thus, these associations are not driven by a single diagnostic group, but instead suggest a generalizable brain-behavior relationship. This finding supports the conjecture that between-group differences in behavior (i.e., diminished sensitivity to choice-specific information in Alzheimer’s disease) are attributable to disease-related neural changes in the dmPFC.
Our neuroanatomic analyses also identified that estimated sensitivity to percent penalty was associated with regions that are involved in classic reward circuitry such as orbitofrontal cortex, ventral striatum, and subgenual anterior cingulate cortex, which in intertemporal choice are thought to hold subjective valuations of the discounted future reward (Peters & Büchel, 2011). That we observed an association between these regions and sensitivity to percent penalty, but not delayed reward magnitude or delay length, could suggest that an individual’s estimate of percent penalty sensitivity, but not delay length or reward magnitude, might partially reflect the integrity of systems encoding subjective value for comparisons by the dmPFC and other frontoparietal structures in a valuation accumulation model such as that proposed by Rodriguez and colleagues (Rodriguez et al., 2015). However, it is alternatively possible this finding was due to network degeneration effects given that the peak voxels were consistently in the dmPFC and these regions are interconnected.
The principal limitation of this study is that the cognitive demands of our intertemporal choice task (and the stringency of our control conditions to ensure subject comprehension) restricted participation to patients in mild to moderate stages of illness (Table 1). While this approach was consistent with ecological validity, as patients with more advanced disease usually do not handle their own finances, it limited the sample sizes available for our between-group behavioral comparisons (our VBM analyses were performed across groups, with a sample size of 105). Small sample size is a contributor to low power and replication failure in neuroscience (Button et al., 2013); however, power is a function of both sample size and effect size (Ioannidis, 2005). In the case of the present study, impairments in financial decision-making are recognized clinical features of mild to moderate Alzheimer’s disease and related dementias, and sizeable differences between disease populations and controls could be anticipated. Concerning the neuroanatomic analysis, another potential limitation is the inclusion of the heavily memory influenced MMSE score as a covariate. While methodologically common and beneficial to ensure brain-behavior relationships are due to the parameter of interest as opposed to global cognitive or functional decline, this approach risks underestimating associations with neuroanatomic structures related to both memory and intertemporal choice such as the medial temporal lobes (Lempert, Speer, Delgado, & Phelps, 2017; Peters & Büchel, 2010).
We also note that clinical research is generally limited by a lack of representative diversity (Oh et al., 2015), and corresponding socioeconomic or cultural factors likely play a role in decision-making but may not be well-reflected in this study (A3. Supplementary Discussion). Additionally, groups were not completely matched on age and education. While our main behavioral findings were largely unaffected in a sensitivity analysis, further work is needed to understand their roles in intertemporal choice. One patient with a clinical diagnosis of Alzheimer’s disease had a negative amyloid PET scan, but their exclusion did not affect the study’s results—recent work indicates that the Alzheimer’s clinical syndrome is more neuropathologically heterogeneous than previously supposed (Nelson et al., 2019; Robinson et al., 2018), and the prevalence of amyloid PET negativity in our cohort is consistent with other cohorts of clinically-defined Alzheimer’s disease (A3. Supplementary Discussion). Lastly, considerable variability exists among different estimation procedures for multi-level mixed effects modeling and among different statistical analysis programs, both of which can influence estimates of fixed and random effects. As a result, ensuring that methods are transparent and robust to different procedures is an important consideration for this novel approach to delay discounting behavior estimation (A1. Supplementary Materials and methods, and A3.Supplementary Discussion).
5. Conclusion
In the current study, we examined the influence of cognitive factors besides impaired memory for decision-making in Alzheimer’s disease and other dementias, utilizing an intertemporal choice task with minimal memory demands. While patients with Alzheimer’s disease did not differ from controls in their overall tendency to choose smaller immediate rewards over larger delayed rewards, their choices were less influenced than controls’ by choice-relevant information (the relative difference in reward magnitude, the delay length, and the absolute reward magnitudes), though all information was explicitly presented at the time of choice. Across all subjects, attenuated sensitivity to such information was associated with dorsomedial prefrontal atrophy. These findings are congruent with population-level studies documenting disadvantageous uses of credit in advancing age, and with recent proposals on the role of dorsomedial prefrontal cortex in economic decision-making.
Supplementary Material
7. Acknowledgments
The authors thank the patients, patient families, and healthy control volunteers of the Memory and Aging Center for their gracious contributions to our research. We also thank Chuck McCullogh for his support with statistical analyses, Jan Peters for helpful discussion, and Kristie Wood for her support with task programming and data collection. VBM analyses were performed in the Brainsight system, developed at UCSF by Katherine P. Rankin, Cosmo Mielke, and Paul Sukhanov, and powered by the VLSM script written by Stephen M. Wilson, with funding from the Rainwater Charitable Foundation and the UCSF Chancellor’s Fund for Precision Medicine. We also thank Lauren Edwards and Renaud La Joie for assistance with amyloid imaging data, and Joanne Taylor for assistance with genetic data.
6. Funding
This work was supported by the National Institutes of Health, National Institutes on Aging (grant numbers K23AG043553, R01AG022983, P01AG019724, P50AG023501, R01AG032306, R01AG058817); the National Institutes of Health, National Institute on Mental Health (grant number R01MH098023) and the Larry Hillblom Foundation.
Abbreviations
- bvFTD
behavioral variant frontotemporal dementia
- dmPFC
dorsomedial prefrontal cortex
- MMSE
Mini-Mental Status Examination
- svPPA
sematic-variant primary progressive aphasia
- VBM
voxel-based morphometry
Footnotes
Declarations of interest: none.
Data Access
De-identified behavioral data and code for the experimental task are available at https://osf.io/x2v9z. Policies for sharing of potentially identifying participant-level data, including neuroimaging, are described in detail at https://memory.ucsf.edu/research-trials/professional/open-science#Human-Studies, and are governed by concern for the sensitivity and potential discrimination/exploitation risks associated with a dementia diagnosis. In summary, academic, not-for-profit investigators may request access to human data, subject to approval from the UCSF Human Research Protection Program and the UCSF Memory and Aging Center Executive Committee. Applications can be made via an online resource request form accessible from the URL above, and also require completion of a Data Use Agreement accessible at the same address.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. References
- Acierno R, Hernandez MA, Amstadter AB, Resnick HS, Steve K, Muzzy W, & Kilpatrick DG (2010). Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: The national elder mistreatment study. American Journal of Public Health, 100(2), 292–297. 10.2105/AJPH.2009.163089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Driscoll JC, Gabaix X, & Laibson D (2009). The age of reason: Financial decisions over the life cycle and implications for regulation. Brooking Papers on Economic Activity, 2, 51–117. [Google Scholar]
- Ashburner J (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2005). Unified segmentation. NeuroImage, 26(3), 839–851. 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Bang J, Spina S, & Miller BL (2015). Frontotemporal dementia. The Lancet, 386(10004), 1672–1682. 10.1016/S0140-6736(15)00461-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, & Dronkers NF (2003). Voxel-based lesion–symptom mapping. Nature Neuroscience, 6(5), 448–450. 10.1038/nn1050 [DOI] [PubMed] [Google Scholar]
- Bayard S, Jacus J-P, Raffard S, & Gely-Nargeot M-C (2014). Apathy and Emotion-Based Decision-Making in Amnesic Mild Cognitive Impairment and Alzheimer’s Disease. Behavioural Neurology, 2014, 231469 10.1155/2014/231469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, & Damasio AR (1997). Deciding advantageously before knowing the advantageous strategy. Science (New York, N.Y.), 275(5304), 1293–1295. 10.1126/science.275.5304.1293 [DOI] [PubMed] [Google Scholar]
- Bertoux M, Cova F, Pessiglione M, Hsu M, Dubois B, & Bourgeois-Gironde S (2014). Behavioral variant frontotemporal dementia patients do not succumb to the Allais paradox. Frontiers in Neuroscience, 8(287), 287 10.3389/fnins.2014.00287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoux M, de Souza LC, Zamith P, Dubois B, & Bourgeois-Gironde S (2015). Discounting of future rewards in behavioural variant frontotemporal dementia and Alzheimer’s disease. Neuropsychology, 29(6), 933–939. 10.1037/neu0000197 [DOI] [PubMed] [Google Scholar]
- Bertoux M, Funkiewiez A, O’Callaghan C, Dubois B, & Hornberger M (2013). Sensitivity and specificity of ventromedial prefrontal cortex tests in behavioral variant frontotemporal dementia. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association, 9(5 Suppl), S84–94. 10.1016/j.jalz.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D’Esposito M, & Fields HL (2007). Immediate Reward Bias in Humans: Fronto-Parietal Networks and a Role for the Catechol-O-Methyltransferase 158Val/Val Genotype. Journal of Neuroscience, 27(52), 14383–14391. 10.1523/JNEUROSCI.2551-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, & Munafò MR (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews. Neuroscience, 14(5), 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Chiong W, Wood KA, Beagle AJ, Hsu M, Kayser AS, Miller BL, & Kramer JH (2016). Neuroeconomic dissociation of semantic dementia and behavioural variant frontotemporal dementia. Brain, 139(2), 578–587. 10.1093/brain/awv344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino B, Kumaran D, Seymour B, & Dolan RJ (2006). Frames, biases, and rational decision-making in the human brain. Science (New York, N.Y.), 313(5787), 684–687. 10.1126/science.1128356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Water E, Mies GW, Figner B, Yoncheva Y, van den Bos W, Castellanos FX, … Scheres A (2017). Neural mechanisms of individual differences in temporal discounting of monetary and primary rewards in adolescents. NeuroImage 10.1016/j.neuroimage.2017.04.013 [DOI] [PubMed] [Google Scholar]
- Fleming SM, Thomas CL, & Dolan RJ (2010). Overcoming status quo bias in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 107(13), 6005–6009. 10.1073/pnas.0910380107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Giebel CM, Challis D, & Montaldi D (2015). Understanding the cognitive underpinnings of functional impairments in early dementia: a review. Aging & Mental Health, 19(10), 859–875. 10.1080/13607863.2014.1003282 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, … Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. 10.1212/WNL.0b013e31821103e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini Maria Luisa, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, … Miller BL (2004). Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology, 55(3), 335–346. 10.1002/ana.10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, & Menon V (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 101(13), 4637–4642. 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, & Mitchell SH (2008). Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology, 201(2), 183–193. 10.1007/s00213-008-1261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, & Lawitz A (2016). The New York State Cost of Financial Exploitation Study. Retrieved from https://ocfs.ny.gov/main/reports/ [Google Scholar]
- Ioannidis JPA (2005). Why most published research findings are false. PLoS Medicine, 2(8), e124 10.1371/journal.pmed.0020124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser AS, Allen DC, Navarro-Cebrian A, Mitchell JM, & Fields HL (2012). Dopamine, Corticostriatal Connectivity, and Intertemporal Choice. Journal of Neuroscience, 32(27), 9402–9409. 10.1523/JNEUROSCI.1180-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloeters S, Bertoux M, O’Callaghan C, Hodges JR, & Hornberger M (2013). Money for nothing - Atrophy correlates of gambling decision making in behavioural variant frontotemporal dementia and Alzheimer’s disease. NeuroImage. Clinical, 2(1), 263–272. 10.1016/j.nicl.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert KM, Speer ME, Delgado MR, & Phelps EA (2017). Positive autobiographical memory retrieval reduces temporal discounting. Social Cognitive and Affective Neuroscience, 12(10), 1584–1593. 10.1093/scan/nsx086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg PA (2016). Financial exploitation, financial capacity, and Alzheimer’s disease. American Psychologist, 71(4), 312–320. 10.1037/a0040192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco-Pallarés J, Mohammadi B, Samii A, & Münte TF (2010). Brain activations reflect individual discount rates in intertemporal choice. Brain Research, 1320, 123–129. 10.1016/j.brainres.2010.01.025 [DOI] [PubMed] [Google Scholar]
- Marson DC (2001). Loss of financial competency in dementia: Conceptual and empirical approaches. Aging Neuropsychology and Cognition, 8(3), 164–181. [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, … Phelps CH (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia : The Journal of the Alzheimer’s Association, 7(3), 263–269. 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43(11), 2412–2414. 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, … Schneider JA (2019). Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain : A Journal of Neurology, 142(6), 1503–1527. 10.1093/brain/awz099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, & Poline J-BB (2005). Valid conjunction inference with the minimum statistic. NeuroImage, 25(3), 653–660. 10.1016/j.neuroimage.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Oh SS, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, … Burchard EG (2015). Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled. PLoS Medicine, 12(12), e1001918 10.1371/journal.pmed.1001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérès K, Helmer C, Amieva H, Orgogozo J-M, Rouch I, Dartigues J-F, & Barberger-Gateau P (2008). Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population-based study. Journal of the American Geriatrics Society, 56(1), 37–44. 10.1111/j.1532-5415.2007.01499.x [DOI] [PubMed] [Google Scholar]
- Peters J, & Büchel C (2010). Episodic Future Thinking Reduces Reward Delay Discounting through an Enhancement of Prefrontal-Mediotemporal Interactions. Neuron, 66(1), 138–148. 10.1016/j.neuron.2010.03.026 [DOI] [PubMed] [Google Scholar]
- Peters J, & Büchel C (2011). The neural mechanisms of inter-temporal decision-making: understanding variability. Trends in Cognitive Sciences, 15(5), 227–239. 10.1016/j.tics.2011.03.002 [DOI] [PubMed] [Google Scholar]
- Pine A, Seymour B, Roiser JP, Bossaerts P, Friston KJ, Curran HV, & Dolan RJ (2009). Encoding of Marginal Utility across Time in the Human Brain. Journal of Neuroscience, 29(30), 9575–9581. 10.1523/JNEUROSCI.1126-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, … Miller BL (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain : A Journal of Neurology, 134(Pt 9), 2456–2477. 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Corrada MM, Kovacs GG, Dominique M, Caswell C, Xie SX, … Trojanowski JQ (2018). Non-Alzheimer’s contributions to dementia and cognitive resilience in The 90+ Study. Acta Neuropathologica, 136(3), 377–388. 10.1007/s00401-018-1872-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CA, Turner BM, Van Zandt T, & McClure SM (2015). The neural basis of value accumulation in intertemporal choice. The European Journal of Neuroscience, 42(5), 2179–2189. 10.1111/ejn.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, & Greicius MD (2009). Neurodegenerative Diseases Target Large-Scale Human Brain Networks. Neuron, 62(1), 42–52. 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinz H, Zamarian L, Benke T, Wenning GK, & Delazer M (2008). Impact of ambiguity and risk on decision making in mild Alzheimer’s disease. Neuropsychologia, 46(7), 2043–2055. 10.1016/j.neuropsychologia.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Sollberger M, Stanley CM, Wilson SM, Gyurak A, Beckman V, Growdon M, … Rankin KP (2009). Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia, 47(13), 2812–2827. 10.1016/j.neuropsychologia.2009.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralva T, Dorrego F, Sabe L, Chemerinski E, & Starkstein SE (2000). Impairments of social cognition and decision making in Alzheimer’s disease. International Psychogeriatrics, 12(3), 359–368. 10.1017/S1041610200006463 [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Payne JW, Bettman JR, Luce MF, & Huettel SA (2009). Separate Neural Mechanisms Underlie Choices and Strategic Preferences in Risky Decision Making. Neuron, 62(4), 593–602. 10.1016/j.neuron.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.