Abstract
Objective:
While the effectiveness of medial branch nerve radiofrequency ablation (RFA) for lumbosacral facet pain has been described, little is known regarding patterns of repeat RFA utilization and prescription opioid use afterwards.
Design:
Retrospective cohort analysis.
Subjects:
Patients undergoing lumbosacral RFA in MarketScan from 2007 – 2016.
Methods:
The time until and number of staged RFAs (<180 days after initial RFA) and repeat RFAs (≥180 days after initial RFA), as well as opioid use at 90 and 180 days after RFA were assessed. Survival analyses were employed to estimate subsequent RFA rates, while subsequent RFA frequencies were estimated with inverse probability weighting. Repeated measures testing was performed comparing opioid use pre- and post-RFA.
Results:
Initial RFAs were identified in 44,936 patients. Staged RFAs were performed in 33.1% of patients. Repeat RFAs through 1, 3, and 7 years were performed for 14.6%, 33.5%, and 45.7% of patients, respectively. Within 3 years, 12.2% of patients underwent one repeat RFA, while 13.2% of patients underwent two or more. Post-RFA opioid use was examined in 128,310 patients, 32.2% of whom used opioids pre-RFA. By 180 days post-RFA, 8.1% of patients discontinued opioids and 6.7% started opioids (p<0.001). Exclusively examining pre-RFA opioid users, 24.9% stopped filling opioid prescriptions 180 days after RFA.
Conclusions:
This study delineates utilization rates of repeat RFA in the commercially insured population, with one-third undergoing repeat RFA within three years. Additionally, the present data indicate that lumbosacral RFA is associated with reduced filling of opioid prescriptions through 180 days.
Keywords: Zygapophyseal Joint, Low Back Pain, Analgesics, Opioid
Introduction:
Lumbosacral radiofrequency ablation (RFA) is a commonly used, minimally invasive intervention that involves selective destruction of medial branch nerves by thermal lesioning in order to disrupt afferent nociception from painful facet joints.(1,2) Outcomes after RFA have been described in controlled studies, but little is known about how RFA is used in actual clinical practice in the United States (US), particularly outside of the Medicare population. (2-8)
In the absence of patient reported outcomes from large cohorts, insights can be gleaned from objective measures recorded within insurance claims data.(9) This study focused on two such measures of treatment utilization after RFA in the US, repeat RFA rates and changes in opioid prescription fill rates after RFA.
Because of medial branch nerve regeneration, RFA is not expected to be a curative procedure for chronic low back pain.(10) MacVicar et al. found that the procedure provided a median benefit time of 13 months.(2) While repeat procedures are expected for a portion of patients as a long-term pain-management strategy, the exact timing and frequency is not well-described in real-world cohorts. Thus, the first aim of this study was to characterize repeat RFA utilization in the US. Furthermore, in the context of the current opioid crisis, determining which interventions decrease the likelihood of continued opioid use is critically important.(11) Accordingly, the second aim of this study was to assess the influence of RFA on opioid presciption practices.
Methods:
This study was exempt from review by the University of Washington Institutional Review Board. Deidentified data were collected from the IBM/Watson (formerly Truven Health Analytics) MarketScan® Commercial Claims and Encounters Databases from 2007 – 2016.(9) These databases include inpatient, outpatient, and pharmacy claims for patients covered by employer-sponsored commercial insurance plans from over 150 employers across the US.
Using these databases, all patients undergoing a lumbosacral RFA between 2007 and 2016 were identified with Current Procedural Terminology (CPT) codes (CPT 64622, 64623, 64635, and 64636). Patient demographic data included age, sex, year of index RFA, whether three or more facet joints were treated during index RFA, type of health insurance plan, Census Bureau geographic region, and Charlson Comorbidity Index.(12,13) The number of facet joints treated was based on billing codes, with three codes required starting in 2012. Before 2012, when codes represented nerves ablated and not facet joints, four codes were required for unilateral procedures, and five codes were required for bilateral procedures.
Subsequent Radiofrequency Ablation
In order to identify RFA procedures most likely to be the first RFA for a given patient, index RFAs were defined as a first recorded RFA with at least three years of continuous MarketScan enrollment preceding the RFA date. All lumbosacral RFA procedures performed on a single day were grouped into a single RFA event. Patients with index RFAs were included in the “index RFA cohort”, and their rates of second RFA procedures (“subsequent RFAs”) were assessed. Because private insurance plans typically cover repeat RFAs only if they are ≥180 days after a previous RFA, subsequent RFAs were divided into “staged RFAs” (<180 days after index RFA) and “repeat RFAs” (≥180 days after index RFA).(14-16)
Staged RFAs in typical practice often represent a bilateral or multi-level RFA that has been performed on different dates, due to insurance plan restrictions or provider preference. These are frequently performed within six weeks of an index RFA. However, in some cases, staged RFAs may be performed at different levels for new, worsening, or recurrent sources of pain.
Patients with index RFAs and at least 180 days of post-index RFA enrollment were included in the "repeat RFA cohort”, which was used to determine repeat RFA rates. By only considering repeat RFAs as those performed ≥180 days after an index RFA, these procedures are more likely to truly represent procedures performed on a previously treated facet joint. Nevertheless, a portion of the “repeat RFAs”, as defined in this study, may actually represent procedures performed at different levels. Additionally, there may have been some insurance exceptions which allowed repeat RFAs to be covered before 180 days.
To examine the number of staged RFAs patients received, two calculations were performed. First, the observed staged RFAs were counted only among patients enrolled for at least 180 days after index RFA. Second, using all patients, frequencies were estimated using inverse probability weighting and adjusting for all demographic covariates. To count the number of repeat RFAs patients received, similar methods were utilized. Repeat RFAs observed in patients with at least three years of enrollment after index RFA were counted. Then, inverse probability weighting and adjustment for demographic data was performed to estimate repeat RFA frequencies for the entire repeat RFA cohort over three years. Three years of follow-up was chosen because of prior work showing 75% of patients experienced complete pain relief for 2.4 years after RFA.(2)
Opioid Prescription Fills
Cohorts were also defined to examine prescription opioid use at 90 and 180 days after RFA, including all patients with continuous enrollment at least 60 days before and 90 or 180 days after an RFA, respectively. Pre-RFA prescription opioid use was defined by the filling of two opioid prescriptions between 1 – 60 days before the index RFA date. The threshold was set at two prescription fills to exclude patients who received a single prescription for a reason potentially unrelated to chronic low back pain, such as for a dental procedure. Post-RFA opioid use was defined by the filling of two opioid prescriptions between 31 – 90 days or 121 – 180 days after RFA. The first 30 days after RFA were excluded, because some patients are prescribed short courses of opioids specifically for a short-term increase in pain secondary to the procedure.
Statistical Analysis
To identify associations between demographic characteristics and subsequent RFA, staged RFA, and repeat RFA rates, log-rank tests were used. When analyzing the time to repeat RFA stratified by individual demographic characteristics, the median days to repeat RFA were compared with Wilcoxon-Mann-Whitney tests. To examine the overall times until staged and repeat RFAs, Kaplan-Meier survival curves were plotted, censoring at insurance plan disenrollment or the end of the database follow-up time (December 31, 2016).
Rates of staged and repeat RFAs were calculated, adjusting for all demographic variables collected. Staged and repeat RFA frequencies were calculated using inverse probability weighting based on the likelihood of remaining enrolled in MarketScan at 180 days and 3 years post-index RFA, respectively.(17) Pearson’s chi-squared test was used to examine associations between demographic covariates and pre- and post-RFA opioid prescription fills. McNemar’s chi-squared test was used to compare the proportions of patients who filled opioids pre- and post-RFA.
Results:
From 2007 – 2016, the MarketScan database included 149,831,011 unique patients, and lumbosacral RFA was performed for 165,734 patients (Figure 1).
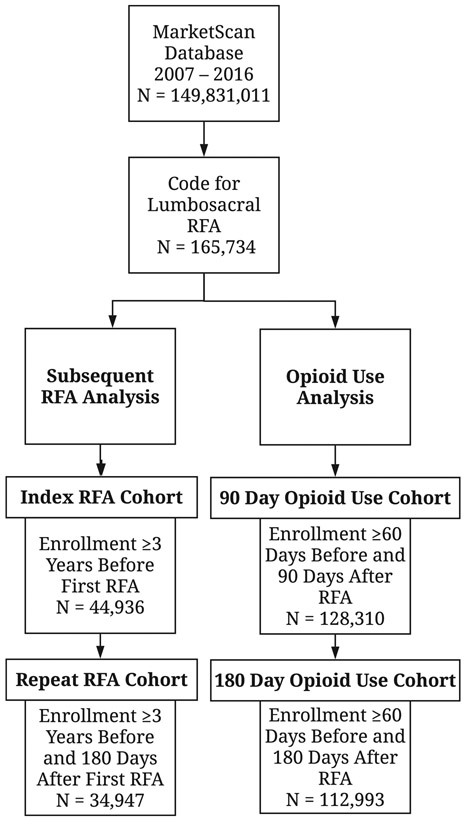
Figure 1:
Flow diagram of the study inclusion criteria and sample sizes. The index RFA cohort was used to determine staged RFA rates (<180 days after index RFA), while the repeat RFA cohort was used to determine repeat RFA rates (≥180 days after index RFA). Prescription opioid use was defined by the filling of two or more opioid prescriptions within 60 days prior to RFA.
Abbreviations: RFA – radiofrequency ablation.
Subsequent Radiofrequency Ablation
Index RFAs were identified in 44,936 patients (index RFA cohort), and 34,947 of these patients were followed for at least 180 days after an index RFA (repeat RFA cohort). Demographic characteristics of the index RFA cohort, stratified by the performance of a subsequent RFA, are summarized in Table 1. Overall, 43.4% of patients underwent a subsequent RFA at any time while continuously enrolled in MarketScan. The median time to censorship was 497 days (IQR 156 – 851 days). Subsequent RFAs were associated with comprehensive or health maintenance organization (HMO) health plans, living in the North Central or South US, and having at least one comorbid medical condition.
Table 1:
Demographic characteristics of the index RFA cohort stratified by the performance of any subsequent RFA.
| Overall n = 44,936 |
No Subsequent RFA n = 25,449 |
Subsequent RFA n = 19,487 |
Log-Rank p-value |
|
|---|---|---|---|---|
| Age (n, %) | 0.134 | |||
| < 35 | 2,809 (6.3) | 1,593 (6.3) | 1,216 (6.2) | |
| 35 - 44 | 7,194 (16.0) | 3,979 (15.6) | 3,215 (16.5) | |
| 45 - 54 | 14,789 (32.9) | 8,181 (32.2) | 6,608 (33.9) | |
| 55 - 64 | 20,110 (44.8) | 11,672 (45.9) | 8,438 (43.3) | |
| 65+ | 34 (0.1) | 24 (0.1) | 10 (0.1) | |
| Male (n, %) | 17,982 (40.0) | 10,243 (40.3) | 7,739 (39.7) | 0.416 |
| Health Plan (n, %) | 0.004 | |||
| Comprehensive | 2,281 (5.1) | 1,186 (4.7) | 1,095 (5.6) | |
| Exclusive/ PPO | 27,559 (61.3) | 15,782 (62.0) | 11,777 (60.4) | |
| HMO | 5,624 (12.5) | 3,095 (12.2) | 2,529 (13.0) | |
| Point of Service | 4,205 (9.4) | 2,399 (9.4) | 1,806 (9.3) | |
| CD/ HD | 4,691 (10.4) | 2,670 (10.5) | 2,021 (10.4) | |
| Missing | 576 (1.3) | 317 (1.2) | 259 (1.3) | |
| Region (n, %) | < 0.001 | |||
| Northeast | 5,015 (11.2) | 3,117 (12.2) | 1,898 (9.7) | |
| North Central | 10,934 (24.3) | 5,945 (23.4) | 4,989 (25.6) | |
| South | 20,700 (46.1) | 11,565 (45.4) | 9,135 (46.9) | |
| West | 7,784 (17.3) | 4,531 (17.8) | 3,253 (16.7) | |
| Missing | 503 (1.1) | 291 (1.1) | 212 (1.1) | |
| Charlson Comorbidity | < 0.001 | |||
| Index (n, %) | ||||
| 0 | 25,155 (56.0) | 14,417 (56.7) | 10,738 (55.1) | |
| 1 - 2 | 15,091 (33.6) | 8,493 (33.4) | 6,598 (33.9) | |
| 3 - 4 | 3,406 (7.6) | 1,836 (7.2) | 1,570 (8.1) | |
| 5+ | 1,284 (2.9) | 703 (2.8) | 581 (3.0) | |
| 3+ Levels Treated (n, %) | 24,588 (54.7) | 14,106 (55.4) | 10,482 (53.8) | 0.447 |
| Days Until RFA or Censorshipa (median, IQR) | 201 (28 - 563) | 497 (156 - 851) | 28 (14 - 196) | NA |
Censoring occurred at insurance plan disenrollment or the end of the database follow-up time (December 31, 2016).
Abbreviations: CD/ HD – consumer directed/ high deductible; HMO – health maintenance organization; PPO – preferred provider organization; RFA – radiofrequency ablation.
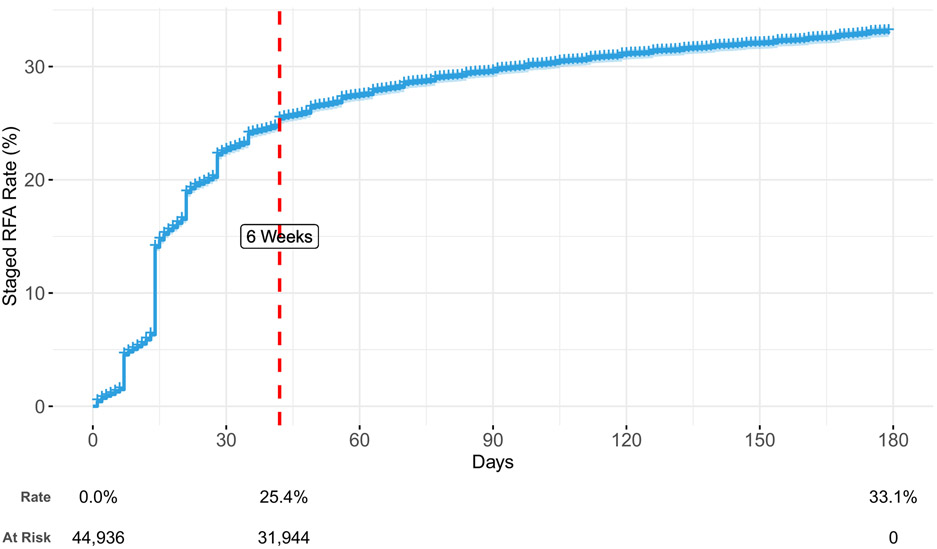
By 180 days after an index RFA, 33.1% of individuals received a staged RFA (Figure 2). Staged RFAs most frequently occurred within six weeks after an index RFA (76.7% of all staged RFAs), with large increases in staged RFA events at 7, 14, 21, and 28 days after an index RFA. These increases may reflect provider procedural days falling on the same days each week. Staged RFA performance was associated with a comprehensive health plan, living in the North Central or South US, having at least one comorbid medical condition, and treatment of less than three facet joints during index RFA (Supplemental Table 1).
Figure 2:
Time to event curve for staged RFA, using the index RFA cohort, adjusted for age, sex, health plan, region, Charlson Comorbidity Index, three or more levels treated during index RFA, and year of index RFA. Staged RFAs likely reflect the second occasion for a bilateral or multilevel RFA that is split over two or more occasions.
Abbreviations: RFA – radiofrequency ablation.
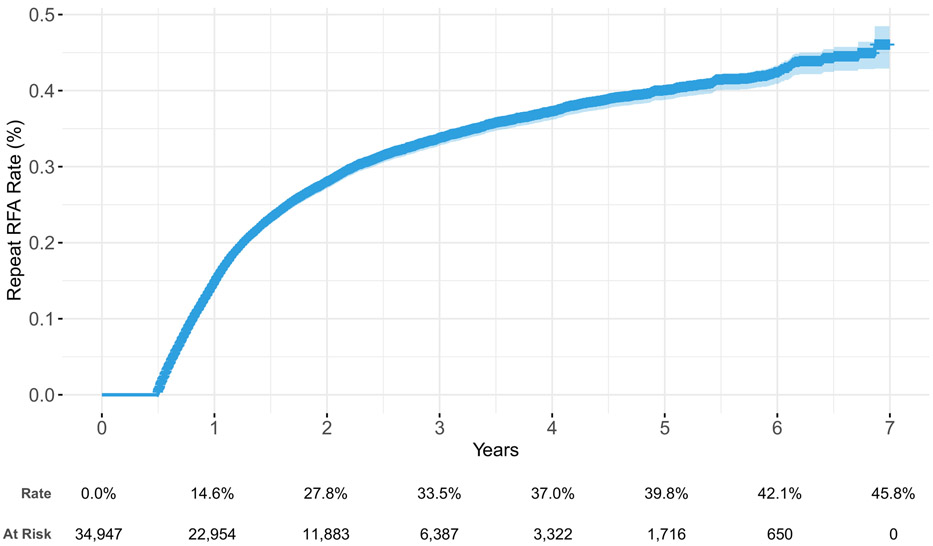
Through 1, 3, and 7 years, the repeat RFA rate was 14.6%, 33.5%, and 45.7%, respectively (Figure 3). The use of repeat RFA was associated with younger age (35 – 54 years old); comprehensive, HMO, or point of service health plans; living in the North Central or South US; receiving a staged RFA; and treatment of three or more facet joints during index RFA (Supplemental Table 2). Though staged RFAs were associated with an increased repeat RFA rate, they were not associated with reduced time to repeat RFA (p = 0.054). The median time to repeat RFA without a staged RFA was 355 days (IQR 252 - 551), compared to 350 days (IQR 252 - 504) in patients with staged RFAs. Treatment of three or more facet joints during index RFA was also not associated with earlier repeat RFA (p = 0.240). The median time to repeat RFA with less than three levels treated was 351 days (IQR 252 – 547), compared to 352 days (IQR 252 – 518) when more than three levels were treated.
Figure 3:
Time to event curve for repeat RFA, using the repeat RFA cohort, adjusted for age, sex, health plan, region, Charlson Comorbidity Index, three or more levels treated during index RFA, and year of index RFA
Abbreviations: RFA – radiofrequency ablation.
The proportions of patients undergoing zero, one, or two or more staged or repeat RFAs within three years is shown in Table 2. After inverse probability weighting, adjusting for follow-up time and all demographic characteristics, 29.9% underwent one staged RFA, and 1.6% received two or more staged RFAs. Through three years, 12.2% received one repeat RFA and 13.2% underwent two or more repeat RFAs.
Table 2:
The proportions of patients undergoing staged RFA and repeat RFA within three years.
| Overall | Zero | One | Two+ | Mean (SD) | ||
|---|---|---|---|---|---|---|
| Staged | Observeda | 34,947 | 23,524 | 10,776 | 647 | 0.4 (0.5) |
| RFA | (67.3%) | (30.8%) | (1.9%) | |||
| Adjustedb | 44,936 | 68.5% | 29.9% | 1.6% | 0.3 (1.3) | |
| Repeat | Observedc | 9,278 | 5,978 | 1,287 | 2,013 | 1.0 (2.0) |
| RFA | (64.4%) | (13.9%) | (21.7%) | |||
| Adjustedd | 34,947 | 74.5% | 12.2% | 13.2% | 0.5 (1.6) |
Includes all patients who had at least 180 days of continuous follow-up.
Inverse probability weighted estimate, including patients followed for less than 180 days, adjusting for age, sex, health plan, region, Charlson Comorbidity Index, three or more levels treated during index RFA, and year of index RFA.
Includes all patients who had at least three years of continuous follow-up.
Inverse probability weighted estimate, including patients followed for less than three years, adjusting for age, sex, health plan, region, Charlson Comorbidity Index, three or more levels treated during index RFA, and year of index RFA.
Abbreviations: RFA – radiofrequency ablation.
Opioid Prescription Fills
The 90-day prescription opioid use cohort included 128,310 patients, and 112,993 patients remained in the 180-day prescription opioid use cohort. In the analyses of opioid prescription fills 90 and 180 days after index RFA, 67.8% and 67.7% of patients respectively did not fill two opioid prescriptions prior to index RFA. Filling opioid prescriptions prior to index RFA was associated with younger age (35 – 54); male sex; comprehensive, HMO, or consumer-directed/ high-deductible (CD/ HD) health plans; living outside of the Northeast US; and having at least one comorbid medical condition (Table 3).
Table 3:
Demographic characteristics of the 90-day prescription opioid use cohort stratified by pre-RFA opioid use.
| Overall n = 128,310 |
No Pre-RFA Opioid Usea n = 86,965 |
Pre-RFA Opioid Use n = 41,345 |
Chi-Square p-value |
|
|---|---|---|---|---|
| Age (n, %) | < 0.001 | |||
| < 35 | 11,223 (8.8) | 7,125 (8.2) | 4,098 (9.9) | |
| 35 - 44 | 24,038 (18.7) | 15,106 (17.4) | 8,932 (21.6) | |
| 45 - 54 | 43,688 (34.1) | 28,881 (33.2) | 14,807 (35.8) | |
| 55 - 64 | 49,350 (38.5) | 35,846 (41.2) | 13,504 (32.7) | |
| 65+ | 11 (0.0) | 7 (0.0) | 4 (0.0) | |
| Male (n, %) | 51,234 (39.9) | 34,383 (39.5) | 16,851 (40.8) | < 0.001 |
| Health Plan (n, %) | < 0.001 | |||
| Comprehensive | 4,317 (3.4) | 2,728 (3.1) | 1,589 (3.8) | |
| Exclusive/ PPO | 82,896 (64.6) | 56,984 (65.5) | 25,912 (62.7) | |
| HMO | 15,585 (12.1) | 9,821 (11.3) | 5,764 (13.9) | |
| Point of Service | 11,107 (8.7) | 7,551 (8.7) | 3,556 (8.6) | |
| CD/ HD | 9,308 (7.3) | 6,095 (7.0) | 3,213 (7.8) | |
| Missing | 5,097 (4.0) | 3,786 (4.4) | 1,311 (3.2) | |
| Region (n, %) | < 0.001 | |||
| Northeast | 15,812 (12.3) | 12,065 (13.9) | 3,747 (9.1) | |
| North Central | 28,872 (22.5) | 19,526 (22.5) | 9,346 (22.6) | |
| South | 59,913 (46.7) | 39,429 (45.3) | 20,484 (49.5) | |
| West | 21,101 (16.4) | 13,902 (16.0) | 7,199 (17.4) | |
| Missing | 2,612 (2.0) | 2,043 (2.3) | 569 (1.4) | |
| Charlson Comorbidity | < 0.001 | |||
| Index (n, %) | ||||
| 0 | 78,726 (61.4) | 53,853 (61.9) | 24,873 (60.2) | |
| 1 - 2 | 39,065 (30.5) | 26,278 (30.2) | 12,787 (30.9) | |
| 3 - 4 | 7,636 (6.0) | 4,974 (5.7) | 2,662 (6.4) | |
| 5+ | 2,883 (2.3) | 1,860 (2.1) | 1,023 (2.5) |
Pre-RFA opioid use defined by filling two opioid prescriptions within 1 – 60 days before RFA.
Abbreviations: CD/ HD – consumer directed/ high deductible; HMO – health maintenance organization; PPO – preferred provider organization; RFA – radiofrequency ablation.
Ninety days after RFA, 9,126 patients (7.1%) discontinued filling their opioid prescriptions, while 7,616 patients (5.9%) started filling new opioid prescriptions (p < 0.001) (Table 4). Similar results were seen at 180 days after RFA, with 9,096 patients (8.1%) no longer filling opioid prescriptions and 7,601 patients (6.7%) starting to fill new opioid prescriptions (p < 0.001). Exclusively examining pre-RFA prescription opioid users, 22.1% and 24.9% stopped filling opioid prescriptions at 90 and 180 days after RFA, respectively.
Table 4:
The proportions of patients using prescription opioids before and after RFA.
| No Pre-RFA Opioid Usea |
Pre-RFA Opioid Use |
Total | McNemar p-value |
||
|---|---|---|---|---|---|
| 90 Days After RFA | No Post-RFA | 79,349 (61.8) | 9,126 (7.1) | 88,475 (69.0) | |
| Opioid Useb(n, %) | |||||
| Post-RFA | 7,616 (5.9) | 32,219 (25.1) | 39,835 (31.0) | < 0.001 | |
| Opioid Use (n, %) | |||||
| Total | 86,965 (67.8) | 41,345 (32.2) | 128,310 | ||
| 180 Days After RFA | No Post-RFA | 68,857 (60.9) | 9,096 (8.1) | 77,953 (69.0) | |
| Opioid Usec (n, %) | |||||
| Post-RFA | 7,601 (6.7) | 27,439 (24.3) | 35,040 (31.0) | < 0.001 | |
| Opioid Use (n, %) | |||||
| Total | 76,458 (67.7) | 36,535 (32.3) | 112,993 |
Pre-RFA opioid use defined by the filling of two opioid prescriptions between 1 – 60 days pre-RFA.
Post-RFA opioid use defined by the filling of two opioid prescriptions between 31 – 90 days post-RFA.
Post-RFA opioid use defined by the filling of two opioid prescriptions between 121 – 180 days post-RFA.
Abbreviations: RFA – radiofrequency ablation.
Filling opioid prescriptions at 90 and 180 days was associated with younger age (< 55), male sex, a nonexclusive/ preferred provider organization (PPO) health plan, living outside of the Northeast US, having at least one comorbid medical condition, pre-RFA opioid use, and treatment of less than three facet joints during index RFA (Supplemental Table 3).
Discussion:
To our knowledge, this is the first study to quantify repeat RFA procedures and opioid use in a large, national cohort beyond one year after RFA. Approximately one-third of patients in a commercially insured population undergo repeat RFA procedures within three years of an index RFA, and approximately half of those patients will receive more than one repeat RFA during that time. Furthermore, RFA was associated with a reduction in prescription opioid fills through 180 days after RFA.
The timing and frequency of repeat procedures observed in this national cohort were similar to results from prior, smaller studies outside the US. For example, the median time to repeat RFA of 351 days in the current study approximates the 13-month median benefit to RFA found in a clinical audit conducted in New Zealand, as well as the median time to repeat RFA of 312 days in a retrospective cohort study conducted in Ontario, Canada.(2,18)
The opioid discontinuation rates observed in the present study were also similar to the rates in Ontario. Loh et al. found that 19.7 - 24.1% of patient using opioids pre-RFA discontinued their prescriptions after RFA, depending on the time frame examined.(18) Interestingly, treatment of three or more facet joints was also associated with reduced post-RFA opioid use, suggesting a possible dose-response relationship. Taken together, these results suggest that lumbosacral RFA is associated with reduced filling of opioid prescriptions through 180 days. However, because some patients started using opioids after RFA, the net reduction in opioid use after RFA is less robust. (19)
Interestingly, 33.1% of patients had a staged RFA, with a median timing of 19 days after index RFA. This suggests that the practice of splitting bilateral or multilevel RFAs into two sessions is quite common. A portion of these split sessions may be performed for specific clinical or diagnostic purposes, for example, to determine if treating one side provides enough relief to forego treating the other side. It is likely, however, that the observed practice patterns may be motivated in part by insurance restrictions, scheduling limitations, and reimbursement considerations. These non-patient-care related motivations may create waste and require patients to coordinate additional, unnecessary visits.
There are several limitations to this study. The MarketScan population encompasses a nationally representative sample of employer-covered patients in the US, however, it is not necessarily representative of the entire US population.(9) Patients covered by Medicaid and Medicare, as well as those who self-pay, are excluded.(9) There are also potential inaccuracies with any administrative dataset, though datasets related to payments for procedures are generally considered to be highly accurate.(20) Another concern is the labelling of all RFAs ≥180 days after index RFAs as “repeat RFAs” As discussed in the methods, it cannot be determined if RFAs are actually targeted towards previously treated facet joints, so differentiating between true repeat RFAs and RFAs performed at different levels is imprecise. Lastly, there is no consensus on the ideal definition of opioid use as ascertained from medical records, and opioid prescription fills are not the same as prescription opioid consumption, though opioid use rates in this study were consistent with prior studies.(18) Importantly, indications for opioid prescriptions were also unknown. Isolating opioid prescriptions for chronic low back pain would be ideal, but that was not possible in the data available, beyond requiring two prescription fills instead of one to limit the inclusion of acute-pain related prescriptions.
Given these limitations, the data presented do not represent the natural history of facet disease or absolute longevity of RFA procedures. Rather, these results provide insight into actual clinical practice patterns in the United States, which are influenced by patient outcomes but also by a myriad of external factors.
Conclusions:
Using a large, private insurance claims database, this study quantified the rates of repeat procedures and opioid use after lumbosacral RFA. Repeat RFA is performed in one-third of patients over three years, and RFA is associated with reduced opioid prescription fill rates through 180 days. These results shed new light on real-world utilization of treatment in the US after lumbosacral RFA, an increasingly performed procedure for one of the most ubiquitous health conditions, chronic low back pain. (1,21-24)
Supplementary Material
Acknowledgments
Funding Sources: Research reported in this publication was supported by the University of Washington CLEAR Center. The UW CLEAR Center is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health under Award Number P30AR072572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interests: All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Starr JB, Gold L, McCormick Z, Suri P, Friedly J. Trends in lumbar radiofrequency ablation utilization from 2007 - 2016. Spine J 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacVicar J, Borowczyk JM, MacVicar AM, Loughnan BM, Bogduk N. Lumbar medial branch radiofrequency neurotomy in New Zealand. Pain Med 2013; 14: 639–45. [DOI] [PubMed] [Google Scholar]
- 3.Dreyfuss P, Halbrook B, Pauza K, Joshi A, McLarty J, Bogduk N. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine 2000; 25: 1270–7. [DOI] [PubMed] [Google Scholar]
- 4.Juch JNS, Maas ET, Ostelo, Raymond WJG, Groeneweg JG, Kallewaard J, Koes BW, Verhagen AP, van Dongen JM, Huygen, Frank JPM, van Tulder MW. Effect of Radiofrequency Denervation on Pain Intensity Among Patients With Chronic Low Back Pain: The Mint Randomized Clinical Trials. JAMA 2017; 318: 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leggett LE, Soril LJJ, Lorenzetti DL, Noseworthy T, Steadman R, Tiwana S, Clement F. Radiofrequency ablation for chronic low back pain: a systematic review of randomized controlled trials. Pain Res Manag 2014; 19: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas ET, Ostelo, Raymond WJG, Niemisto L, Jousimaa J, Hurri H, Malmivaara A, van Tulder MW. Radiofrequency denervation for chronic low back pain. Cochrane Database Syst Rev 2015: CD008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormick ZL, Marshall B, Walker J, McCarthy R, Walega DR. Long-Term Function, Pain and Medication Use Outcomes of Radiofrequency Ablation for Lumbar Facet Syndrome. Int J Anesth Anesth 2015; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Tilburg CWJ, Stronks DL, Groeneweg JG, Huygen FJPM. Randomized sham-controlled, double-blind, multicenter clinical trial on the effect of percutaneous radiofrequency at the ramus communicans for lumbar disc pain. Eur J Pain 2017; 21: 520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen LG, Chang S. Health research for the real world: the marketscan databases. 2011: 1–32. [Google Scholar]
- 10.Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, Kim KH. Neural Ablation and Regeneration in Pain Practice. 2016; 29: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnie RJ, Kesselheim AS, Clark DJ. Both Urgency and Balance Needed in Addressing Opioid Epidemic: A Report From the National Academies of Sciences, Engineering, and Medicine 2017; 318: 423–4. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 13.Bureau Census. Census Bureau Regions and Divisions with State FIPS Codes 2018. [Google Scholar]
- 14.BlueCross BlueShield. Facet Joint Denervation 2018.
- 15.United Healthcare. Ablative Treatment for Spinal Pain 2019.
- 16.HealthPartners. Radiofrequency ablative (RFA) denervation procedures for chronic facet-mediated neck and back pain 2019.
- 17.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. 2015; 168: 656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loh E, Reid JN, Alibrahim F, Welk B. Retrospective cohort study of healthcare utilization and opioid use following radiofrequency ablation for chronic axial spine pain in Ontario, Canada. 2019; 44: 398–405. [DOI] [PubMed] [Google Scholar]
- 19.Pezalla EJ, Rosen D, Erensen JG, Haddox JD, Mayne TJ. Secular trends in opioid prescribing in the USA 2017; 10: 383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher ES, Whaley FS, Krushat WM, Malenka DJ, Fleming C, Baron JA, Hsia DC. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health 1992; 82: 243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, Woolf A, Vos T, Buchbinder R. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012; 64: 2028–37. [DOI] [PubMed] [Google Scholar]
- 22.Martin BI, Turner JA, Mirza SK, Lee MJ, Comstock BA, Deyo RA. Trends in health care expenditures, utilization, and health status among US adults with spine problems, 1997-2006. Spine 2009; 34: 2077–84. [DOI] [PubMed] [Google Scholar]
- 23.Murray CJL, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, Dellavalle R, Danaei G, Ezzati M, Fahimi A, Flaxman D, Foreman n, Gabriel S, Gakidou E, Kassebaum N, Khatibzadeh S, Lim S, Lipshultz SE, London S, Lopez n, MacIntyre MF, Mokdad AH, Moran A, Moran AE, Mozaffarian D, Murphy T, Naghavi M, Pope C, Roberts T, Salomon J, Schwebel DC, Shahraz S, Sleet DA, Murray n, Abraham J, Ali MK, Atkinson C, Bartels DH, Bhalla K, Birbeck G, Burstein R, Chen H, Criqui MH, Dahodwala n, Jarlais n, Ding EL, Dorsey ER, Ebel BE, Ezzati M, Fahami n, Flaxman S, Flaxman AD, Gonzalez-Medina D, Grant B, Hagan H, Hoffman H, Kassebaum N, Khatibzadeh S, Leasher JL, Lin J, Lipshultz SE, Lozano R, Lu Y, Mallinger L, McDermott MM, Micha R, Miller TR, Mokdad AA, Mokdad AH, Mozaffarian D, Naghavi M, Narayan KMV, Omer SB, Pelizzari PM, Phillips D, Ranganathan D, Rivara FP, Roberts T, Sampson U, Sanman E, Sapkota A, Schwebel DC, Sharaz S, Shivakoti R, Singh GM, Singh D, Tavakkoli M, Towbin JA, Wilkinson JD, Zabetian A, Murray n, Abraham J, Ali MK, Alvardo M, Atkinson C, Baddour LM, Benjamin EJ, Bhalla K, Birbeck G, Bolliger I, Burstein R, Carnahan E, Chou D, Chugh SS, Cohen A, Colson KE, Cooper LT, Couser W, Criqui MH, Dabhadkar KC, Dellavalle RP, Jarlais n, Dicker D, Dorsey ER, Duber H, Ebel BE, Engell RE, Ezzati M, Felson DT, Finucane MM, Flaxman S, Flaxman AD, Fleming T, Foreman n, Forouzanfar MH, Freedman G, Freeman MK, Gakidou E, Gillum RF, Gonzalez-Medina D, Gosselin R, Gutierrez HR, Hagan H, Havmoeller R, Hoffman H, Jacobsen KH, James SL, Jasrasaria R, Jayarman S, Johns N, Kassebaum N, Khatibzadeh S, Lan Q, Leasher JL, Lim S, Lipshultz SE, London S, Lopez n, Lozano R, Lu Y, Mallinger L, Meltzer M, Mensah GA, Michaud C, Miller TR, Mock C, Moffitt TE, Mokdad AA, Mokdad AH, Moran A, Naghavi M, Narayan KMV, Nelson RG, Olives C, Omer SB, Ortblad K, Ostro B, Pelizzari PM, Phillips D, Raju M, Razavi H, Ritz B, Roberts T, Sacco RL, Salomon J, Sampson U, Schwebel DC, Shahraz S, Shibuya K, Silberberg D, Singh JA, Steenland K, Taylor JA, Thurston GD, Vavilala MS, Vos T, Wagner GR, Weinstock MA, Weisskopf MG, Wulf S, Murray n. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013; 310: 591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manchikanti L, Hirsch JA, Pampati V, Boswell MV. Utilization of Facet Joint and Sacroiliac Joint Interventions in Medicare Population from 2000 to 2014: Explosive Growth Continues! Curr Pain Headache Rep 2016; 20: 58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.