Abstract
In rodents, the anterior cingulate (ACC), prelimbic (PL), and infralimbic cortex (IL) comprise the medial prefrontal cortex (mPFC). Through extensive connections with cortical and subcortical structures, the mPFC plays a key modulatory role in the neuronal circuits underlying associative fear and reward learning. In this article, we have compiled the evidence that associative learning induces plasticity in both the intrinsic and synaptic excitability of mPFC neurons to modulate conditioned fear and cocaine seeking behavior. The literature highlights the accumulating evidence that plasticity in the intrinsic excitability of mPFC neurons represents a major cellular mechanism that interacts with synaptic changes to alter the impact of the mPFC on fear and reward circuits.
Keywords: prelimbic cortex, infralimbic cortex, intrinsic excitability, fear conditioning, cocaine
The medial prefrontal cortex (mPFC) in rodents includes the anterior cingulate, prelimbic, and infralimbic cortex. Extensive connections with both cortical and subcortical structures allow the mPFC to partake in the neuronal circuits that allow experience to modify behavior. In this review, we have compiled the evidence that associative learning of aversive and rewarding events induces plasticity in the intrinsic excitability of mPFC neurons. These intrinsic changes interact with synaptic plasticity in mPFC neurons to modulate conditioned fear and cocaine seeking behavior via changes in mPFC influence over fear and reward circuits.
Delay Fear Conditioning
The central neuronal circuit that rodents use to acquire, store, and update delay fear conditioning memories involves the amygdala, hippocampus, and mPFC (Paré et al., 2004; Giustino and Maren, 2015). During fear conditioning, incoming sensory information impinges on glutamatergic neurons in the lateral amygdala to induce synaptic strengthening of glutamatergic inputs from the cortex and thalamus which allows the association between the conditioned (CS) and unconditioned stimulus (US) to be stored in the amygdala circuits (Medina et al., 2002). Coincidently, information related to the context in which the animal receives fear conditioning activates hippocampal neurons to strengthen glutamatergic synapses on CA1 pyramidal neurons (Sun et al., 2016). As discussed in depth elsewhere (Oh and Disterhoft, 2015), trace eyeblink (Moyer Jr. et al., 1996; Thompson et al., 1996), delay (Sun et al., 2016), trace (McKay et al., 2009; Song et al., 2015) and contextual fear conditioning (McKay et al., 2009) increases the intrinsic excitability of CA1 and CA3 pyramidal neurons in the dorsal hippocampus of rabbits and rats. The strengthening of synaptic and intrinsic excitability of hippocampal neurons enables them to encode information about the context related to the CS. Subsequent exposure to the CS in the appropriate context and/or when appropriate cues are presented produces a more robust firing of the hippocampal neurons that signals to the amygdala to produce a fear response. The basal amygdala and ventral hippocampus also connect with regions of the mPFC which allows higher order integration and processing of internal and external signals to generate an appropriate response. Activation of the PL tends to increase fear responses to the CS while stimulation of the IL inhibits fear response (Milad and Quirk, 2012; Giustino and Maren, 2015). On the other hand, the ACC seems to modulate remote fear recall (Frankland et al., 2004; Bergstrom, 2016).
In contrast to enhanced excitability found in hippocampal neurons (Kaczorowski et al., 2007; McKay et al., 2009), the initial recordings of randomly selected mPFC neurons in brain slices found that auditory fear conditioning depressed the intrinsic excitability of IL pyramidal neurons of male adolescent rats (Santini et al., 2008). The IL neurons fired fewer repetitive spikes during prolonged depolarization. The depression was learning-dependent, since neurons from the unpaired controls did not show the same depression. Furthermore, neurons in the PL were not affected indicating some regional selectivity. A subsequent study found that contextual fear conditioning was sufficient to depress the intrinsic excitability of IL neurons suggesting that the depression may be encoding contextual information perhaps via direct connections from the ventral hippocampus (Soler-Cedeño et al., 2016). However, the possibility that information related to the cue also contributes to the depression cannot excluded. A recent paper demonstrated that auditory fear conditioning also depressed IL intrinsic excitability of juvenile, adolescent and young adult mice (Koppensteiner et al., 2019). Consistent with the slice recordings, single-unit recordings in awake behaving animals found that IL firing is suppressed during the recall of auditory fear conditioning (Giustino et al., 2016). In fact, the more depressed the IL firing was relative to PL firing in response to the CS correlated with the amount of freezing suggesting that IL suppression may drive fear expression (Giustino et al., 2016).
Since IL has direct projections to the amygdala (Vertes, 2004; Arruda-Carvalho and Clem, 2014) and the stimulation of the IL neurons or IL synapses in the amygdala reduces conditioned fear responses (Milad and Quirk, 2002; Adhikari et al., 2015), the prevailing model postulates that the IL inhibits the amygdala to reduce fear responses (Milad and Quirk, 2012; Giustino and Maren, 2015). Based on this model, the depression of IL intrinsic excitability after fear conditioning was interpreted to mean reduced inhibition of the amygdala by IL which would aide in increasing the conditioned fear responses (Santini et al., 2008). However, given that only about 8% of IL neurons project to the amygdala (Gabbott et al., 2005), randomly sampling IL pyramidal neurons would unlikely include many neurons with projections to the amygdala (Santini et al., 2008; Soler-Cedeño et al., 2016). Therefore, a later study directly examined the intrinsic excitability of IL neurons projecting to the amygdala (Bloodgood et al., 2018). This study found IL neurons projecting to the amygdala were not depressed after delay fear conditioning. Instead delay fear conditioning depressed IL neurons with projections to the nucleus accumbens (NAc) (Bloodgood et al., 2018). Similarly, delay fear conditioning also depresses the synaptic activation of BLA neurons that project to the NAc (Namburi et al., 2015). Since the NAc plays a central role in promoting rewarding behaviors (Kauer and Malenka, 2007), this suggests that reduced IL and BLA modulation of the reward circuit may contribute to enhancing fear responses.
Relatively little is known about the cellular mechanisms that are activated by delay fear learning to depress the intrinsic excitability of mPFC neurons. Both delay and contextual fear conditioning increased the slow afterhyperpolarizing potential (sAHP) (Santini et al., 2008; Soler-Cedeño et al., 2016) which are produced by a calcium-dependent potassium current (Sah and Faber, 2002; Abel et al., 2004; Tombaugh et al., 2005). Calcium influx through voltage-gated calcium channels that open during prolonged depolarization activates this potassium current leading to inhibition of additional action potentials (Disterhoft and Oh, 2006). Reducing the sAHP in IL neurons by stimulation of β-adrenergic receptors (Mueller et al., 2008), muscarinic receptors (Santini et al., 2012), α2-adrenergic receptors (Carr et al., 2007), or type 5 metabotropic glutamate receptors (mGluR5) (Fontanez-Nuin et al., 2011) increases their ability to fire barrages of action potentials. Therefore, the increase in sAHP could account for the depressed repetitive firing seen in IL neurons after delay fear conditioning. It is also possible that delay fear conditioning enhances M type potassium channels (Santini and Porter, 2010) or small calcium-activated (SK) potassium channels (Criado-Marrero et al., 2014) which also modulate IL repetitive firing. Although, future experiments are needed to test this possibility, reducing IL intrinsic excitability by acutely stimulating M channels did enhance the recall of conditioned fear (Santini and Porter, 2010). In contrast, stimulating SK potassium channels did not alter the fear recall (Criado-Marrero et al., 2014). M channel activation also reduced the input resistance of IL neurons, whereas SK channel stimulation did not. Therefore, increasing potassium channel opening at the resting membrane potential of IL neurons is sufficient to increase fear expression.
Delay fear conditioning does not induce a global change in glutamatergic inputs onto IL neurons (Pattwell et al., 2012; Sepulveda-Orengo et al., 2013) or in IL synapses onto amygdala neurons (Arruda-Carvalho and Clem, 2014). However, more subtle synaptic changes do occur. Optogenetic stimulation in brain slices found that delay fear conditioning reduces NMDA receptor currents at ventral hippocampal synapses onto randomly selected IL pyramidal neurons (Soler-Cedeño et al., 2019). Since NMDA receptors prolong the duration of the EPSPs, facilitate bursts of action potentials, and increase Ca2+ in the dendritic spines (Paoletti et al., 2013), this suggests that delay fear conditioning reduces ventral hippocampal activation of IL pyramidal neurons. Furthermore, ventral hippocampal projections produce a robust feedforward inhibition in IL (Marek et al., 2018) and delay fear conditioning did not affect GABAergic synapses onto IL neurons (Koppensteiner et al., 2019). Taken together, these findings suggests that the intrinsic and synaptic plasticity produced by delay fear conditioning would result in ventral hippocampal inhibition of IL projections during the recall of conditioned fear. In addition, the current literature suggests that delay fear conditioning induces projection-specific depression of intrinsic excitability with synapse-specific reductions in synaptic input to reduce IL activation of rewarding circuits relative to aversive circuits (Figure 1A).
Figure 1.
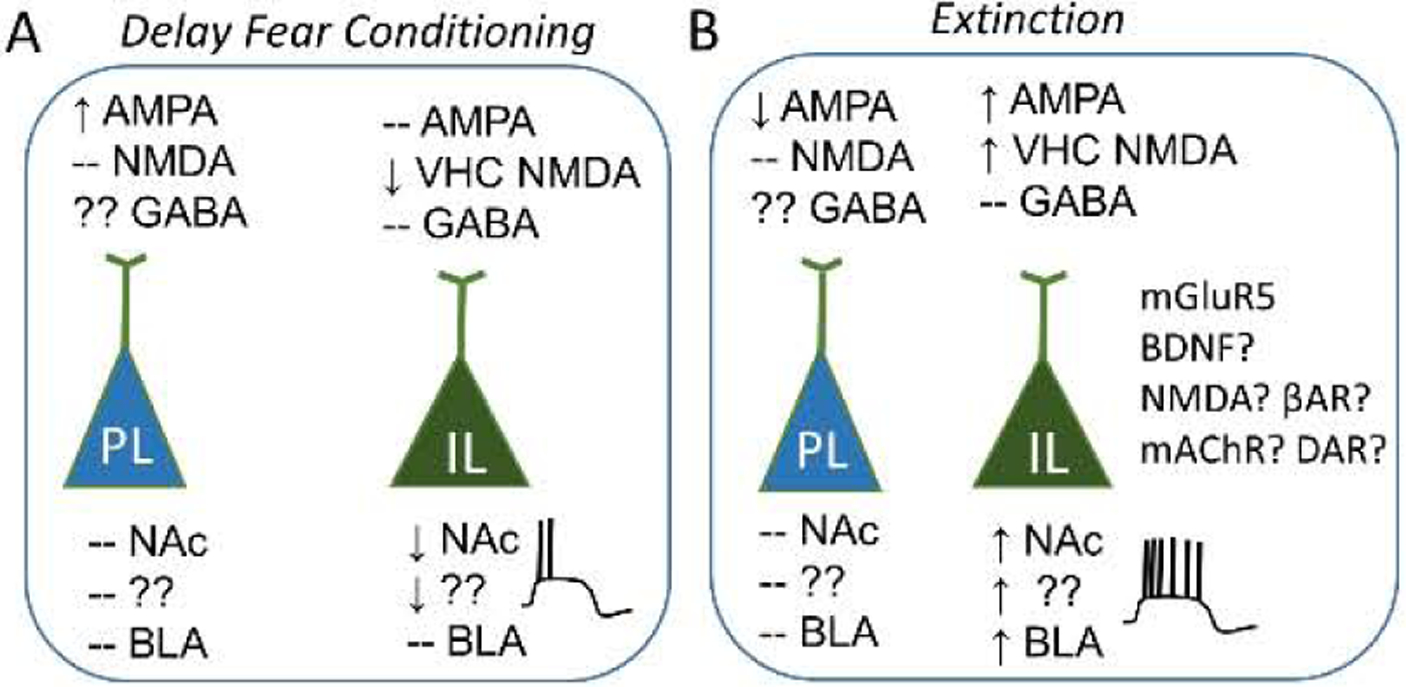
This model depicts the intrinsic and synaptic plasticity induced in PL and IL neurons by delay fear conditioning (A) and extinction (B). No observed changes are indicated by -- and undetermined changes or projections are indicted by question marks.
Similar to the initial report (Santini et al., 2008), PL neurons projecting to the NAc or amygdala did not show intrinsic changes after delay fear conditioning (Bloodgood et al., 2018). However, delay fear conditioning strengthens glutamatergic synapses onto PL neurons (Pattwell et al., 2012) and enhances PL synapses on to amygdala neurons (Arruda-Carvalho and Clem, 2014). Together these findings suggest that enhanced synaptic input is the primary driver of increased PL activity during recall of delay fear conditioning.
Extinction of Delay Fear Conditioning
Although extinction does not erase the fear memory (Quirk et al., 2006), extinction does tend to reverse the conditioning-induced changes at the cellular level. Extinction of delay fear conditioning reversed the conditioning-induced depression of IL neuronal firing in juvenile, mid-adolescent, and adults (Santini et al., 2008; Koppensteiner et al., 2019). Extinction also reversed the enhanced sAHP induced by delay fear conditioning (Santini et al., 2008). The repetitive firing of IL neurons did not reverse until four hours after extinction training suggesting that the reversal was important for consolidation of the extinction memory (Cruz et al., 2014). In support of this interpretation, early adolescent rats that fail to recall their extinction memory also fail to reverse their depressed IL intrinsic excitability (Koppensteiner et al., 2019). Similar to acquisition of delay fear conditioning, extinction induced projection-specific changes in intrinsic excitability. The conditioning-induced depression of IL neurons with projections to the NAc was reversed by extinction suggesting a return of IL modulation of reward circuits (Bloodgood et al., 2018). Therefore, extinction reverses the depression of repetitive firing induced by delay fear conditioning during the consolidation phase.
In addition to reversing the effects of delay fear conditioning, extinction also induces some specific intrinsic changes. In unidentified IL projections, extinction reduces the fast afterhyperpolarization (fAHP) between sequential spikes (Santini et al., 2008; Cruz et al., 2014) and increased burst firing (Santini et al., 2008). The reduction in the fAHP effectively reduces the time between spikes and may underlie the increase in bursting of IL neurons in vivo that is needed for consolidation of the extinction memory (Burgos-Robles et al., 2007). Although delay fear conditioning did not affect the intrinsic excitability of IL-to-BLA projecting neurons, extinction did enhance repetitive firing of IL-to-BLA projecting neurons immediately after extinction training (Bloodgood et al., 2018) suggesting that activity of this projection contributes to reduced fear after extinction. Consistent with this, chemogenic inhibition of IL projections to BLA reduces recall of fear extinction (Bloodgood et al., 2018).
Several findings suggest that the extinction-induced changes in IL intrinsic excitability contribute to the extinction memory. First, animals which fail to recall extinction memory also fail to alter the repetitive firing of IL neurons (Koppensteiner et al., 2019). This extinction failure occurred in early adolescent mice (P27) but not in preadolescent (P24) or adult mice suggesting that the mechanisms responsible for reversing the depressed IL firing is impaired during early adolescence. Second, impairing extinction memory by blocking mGluR5 receptors also prevents the intrinsic excitability changes in IL (Sepulveda-Orengo et al., 2013). Third, the repetitive firing of IL neurons reverses with the spontaneous return of fear after extinction leaving the IL neurons depressed relative to age-matched controls (Cruz et al., 2014).
Extinction of delay fear conditioning also induces synaptic plasticity in IL neurons (Pattwell et al., 2012; Sepulveda-Orengo et al., 2013). Electrical stimulation found evidence for an increase in rectifying AMPA receptor current at synapses in IL suggesting that extinction induced the insertion of calcium-permeable AMPA receptors (Pattwell et al., 2012; Sepulveda-Orengo et al., 2013). Similar to the intrinsic plasticity these synaptic changes also required mGluR5 activation (Sepulveda-Orengo et al., 2013) suggesting that both intrinsic and synaptic plasticity share a common mechanism in IL. In addition, fear extinction also reversed the reduced NMDA receptor currents at ventral hippocampal synapses in IL pyramidal neurons (Soler-Cedeño et al., 2019). Extinction of Delay fear conditioning did not affect GABAergic synapses onto IL neurons (Koppensteiner et al., 2019). Overall, the current literature suggests that extinction of delay fear conditioning enhances the intrinsic and synaptic excitability of IL pyramidal neurons through projection- and synapse-specific mechanisms to increase IL modulation of rewarding and aversive circuits (Figure 1B).
Trace Fear Conditioning and Extinction
In trace fear conditioning the CS and US do not overlap in time as in delay fear conditioning. The temporal separation of the CS and US reduces the dependency of the learning on the amygdala while maintaining the importance of the hippocampus and mPFC (Gilmartin and Helmstetter, 2010; Raybuck and Lattal, 2011, 2014; Kwapis et al., 2014). Consistent with the differences between delay and trace fear conditioning, trace fear conditioning has different effects on IL intrinsic excitability. In contrast to delay fear conditioning (Bloodgood et al., 2018), trace fear conditioning increased the intrinsic excitability of IL-to-amygdala projecting neurons by reducing spike threshold and increasing HCN currents (Song et al., 2015). Based on the evidence that IL inhibits the amygdala (Quirk et al., 2003; Amano et al., 2010), this suggests that IL may inhibit the amygdala after trace fear conditioning which should reduce fear expression. However, trace fear conditioning and extinction can occur independent of the amygdala (Raybuck and Lattal, 2011; Kwapis et al., 2014), so the behavioral relevance of increased IL inputs to the amygdala for trace fear conditioning is unclear. Trace fear conditioning increased the intrinsic excitability of PL bursting neurons by increasing input resistance, while depressing the intrinsic excitability of PL regular firing neurons by reducing input resistance (Song et al., 2015). These findings suggest that regular spiking and bursting PL neurons may activate different amygdala circuits. Extinction reversed the intrinsic plasticity induced by trace fear conditioning in both IL and PL neurons projecting to the amygdala (Song et al., 2015). Overall, this study suggests that trace fear conditioning increases IL and decrease PL modulation of the amygdala. After extinction, the intrinsic changes are reversed leading to a relative decrease in IL and a relative increase in PL influences on the amygdala.
Drug Seeking Behavior
Similar to fear conditioning, substances of abuse also induce neuroadaptations in the mPFC, which modifies its influence on the reward circuitry that includes the NAc, ventral tegmental area (VTA), and hippocampus. These neuroadaptations lead to dysregulated communication between regions of the reward circuitry that contributes to the development of substance use disorders.
Clinical and preclinical studies show that the direction of change in neuronal firing in the mPFC depends on the stage of drug addiction under investigation (i.e. drug consumption, withdrawal, or relapse/reinstatement). For example, human studies found that drug intake or presentation of drug-related cues activates the ACC region of the mPFC (Goldstein and Volkow, 2002). On the other hand, ACC hypoactivity is observed during drug abstinence and cognitive task performance in human addicts (Goldstein and Volkow, 2002, 2011). Below, we discuss the effects of cocaine and opiates on the intrinsic excitability (firing rate) of the mPFC neurons during the development of substance use disorder.
Cocaine
Human studies show that cocaine intake activates the ACC, while withdrawal induces hypofunction that correlates with more craving symptoms (Jentsch and Taylor, 1999; Goldstein and Volkow, 2011). Milella and colleagues found increased dopamine release in frontal and striatal regions when cocaine-dependent participants were exposed to drug-related cues (Milella et al., 2016). Similarly, preclinical studies also showed that cocaine stimulates dopaminergic projections from the VTA to the mPFC which increase extracellular dopamine in the mPFC to subsequently enhance glutamatergic signaling from the mPFC to other regions (Cornish and Kalivas, 2000, 2001; Kalivas et al., 2003, 2009; McFarland et al., 2003). Dopaminergic projections innervate mPFC neurons (Goldman-Rakic et al., 1989; Carr and Sesack, 1996). Moreover, pyramidal neurons and GABAergic interneurons contain D1 and D2 dopamine receptors, which suggests that mPFC neuronal properties are modulated during the development of cocaine addiction (Le Moine and Gaspar, 1998; Xu and Yao, 2010).
Cocaine exposure affects the intrinsic excitability of mPFC neurons. Chen and colleagues observed a decrease of intrinsic excitability in the PL pyramidal neurons in rats exposed to prolonged (two months) cocaine self-administration combined with punishment (shocks) on the last 4 days (Chen et al., 2013). Rats that continued to self-administer high levels of cocaine despite the shocks showed more depressed repetitive firing than those that reduced their cocaine intake in the presence of the shocks. However, PL pyramidal neurons from both groups were severely depressed compared to rats that never received cocaine (Chen et al., 2013). In the rats that resisted the punishment, a reduction in input resistance caused the depressed excitability. In this study, the animals were not exposed to withdrawal. In contrast, moderate exposure to cocaine (two weeks or less) with a withdrawal period increased the intrinsic excitability of the PL pyramidal neurons (Dong et al., 2005; Nasif et al., 2005a, 2005b; Sepulveda-Orengo et al., 2018). Taken together, these findings suggest the possibility that prolonged self-administration of cocaine depresses PL repetitive firing and during withdrawal PL excitability increases. However, it is also possible that the duration of cocaine self-administration or the addition of punishment induces the depression of PL intrinsic excitability (Chen et al., 2013).
Several findings suggest that the changes in PL intrinsic excitability contribute to the cocaine-induced memories. Pharmacological reversal of the intrinsic changes prevents the cocaine-induced memories (Chen et al., 2013; Otis et al., 2018; Parrilla-Carrero et al., 2018; Sepulveda-Orengo et al., 2018). Furthermore, Parrilla-Carrero and colleagues found that PL pyramidal neurons from rats trained to self-administer cocaine show increased intrinsic excitability relative to the cocaine-yoked group, suggesting that the increased excitability is caused by associative learning (lever presses paired with cues), as opposed to being a direct effect of cocaine (Parrilla-Carrero et al., 2018). In addition, rats which fail to retrieve cocaine conditioned place preference also fail to show enhanced PL intrinsic excitability (Otis et al., 2018).
Moderate cocaine exposure also produced opposite physiological effects on pyramidal neurons from the PL and IL cortices (Dong et al., 2005; Nasif et al., 2005b; Gipson et al., 2013; Hearing et al., 2013; Otis et al., 2018; Parrilla-Carrero et al., 2018; Sepulveda-Orengo et al., 2018). Cocaine self-administration followed by extinction enhanced the intrinsic excitability of PL and depressed IL neurons (Sepulveda-Orengo et al., 2018). Sepulveda-Orengo and colleagues showed that the increased intrinsic excitability in PL neurons results from a reduction in the fAHP, which is largely regulated by voltage-gated and Ca2+-dependent potassium channels (Oh et al., 2010). On the other hand, the fAHP was not affected in IL, suggesting a different cellular mechanism depressed IL repetitive firing.
Other proposed cellular mechanisms that mediate the increase in intrinsic excitability of the mPFC after cocaine withdrawal include enhanced protein kinase A (PKA) activation of voltage-sensitive Ca2+ influx through high-voltage activated L-type channels, reduced potassium inward rectifiers (Kir), and reduced voltage-gated K+ currents (Dong et al., 2005; Nasif et al., 2005b; Hu, 2007; Ford et al., 2009). Other studies suggest that cocaine enhances the intrinsic excitability of PL neurons by potentiating the inhibition of Kv7 ion channels produced by dopamine released from VTA projections in the mPFC (Buchta et al., 2017; Parrilla-Carrero et al., 2018). Also, bath application of dopamine with or without cocaine, optogenetic activation of VTA projections, or bath application of clozapine-N-oxide (CNO) to activate VTA-Gq-DREADDs reduced the sAHP to increase repetitive firing of PL neurons (Buchta et al., 2017). Although most studies did not determine the projections of the PL pyramidal neurons, cocaine does increase the excitability of PL projections to NAc (Hearing et al., 2013).
In addition to enhancing PL intrinsic excitability, cocaine conditioned place preference also potentiates synaptic stimulation of PL pyramidal neurons (Otis et al., 2018). Otis and Mueller showed that retrieval of cocaine-induced memory increases spontaneous excitatory postsynaptic currents and AMPA/NMDA ratio in PL pyramidal neurons. Inhibition of noradrenergic/cAMP-dependent signaling pathway prevents the increase of AMPA/NMDA ratio in PL neurons and reduces cocaine seeking behavior (Otis et al., 2018). Therefore, cocaine-induced learning also increases glutamatergic activation of PL pyramidal neurons.
Cocaine conditioned place preference also increases GABAergic synapses onto fast-spiking inhibitory interneurons leading to reduced GABAergic inhibition of PL pyramidal neurons (Slaker et al., 2015, 2018). These findings suggest that reduced inhibition of PL pyramidal neurons contributes to their increased excitability after cocaine exposure (Hearing et al., 2013; Slaker et al., 2015). In addition, higher concentrations of dopamine in the mPFC activate D2 dopamine receptors which decrease IPSCs (Trantham-Davidson et al., 2004; Xu and Yao, 2010). These studies suggest that cocaine activates dopamine receptors to depress GABAergic interneuron activity and subsequently increase the intrinsic excitability of PL pyramidal neurons, leading to enhanced glutamatergic transmission of PL projections in the NAc to modulate cocaine-seeking behavior (McFarland and Kalivas, 2001; McLaughlin and See, 2003; McFarland et al., 2004; Ma et al., 2013; Suska et al., 2013). In other words, increased glutamate release in the NAc core by cocaine exposure is partially due to increase activation in PL neurons (McFarland et al., 2003, 2004). In support of this, inactivation of PL prevents cue-induced reinstatement, as well as the increase of glutamate release and AMPA/NMDA ratio in the NAc (Gipson et al., 2013).
Overall, the majority of the literature suggests that cocaine-induced learning enhances the intrinsic excitability of PL pyramidal neurons while also increasing synaptic excitation and reducing synaptic inhibition. Figure 2 shows a proposed model for the mechanisms by which cocaine-induced learning alters the intrinsic excitability of mPFC neurons. However, how cocaine induces the cellular changes that regulate the intrinsic excitability in the mPFC, and in turn affect synaptic transmission in other regions of the reward circuitry remains unclear and needs to be further examined.
Figure 2.
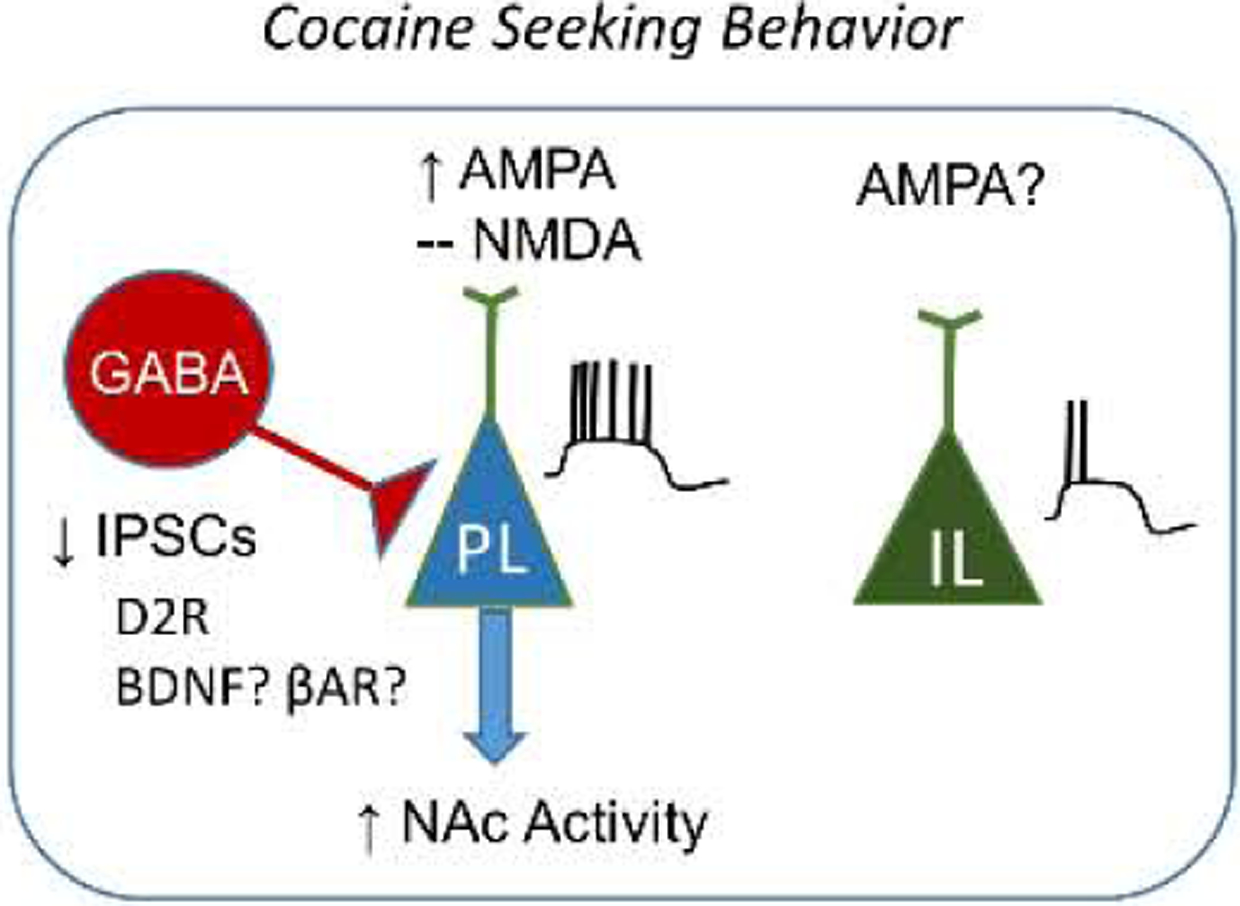
This model depicts the intrinsic and synaptic plasticity induced in PL and IL neurons by moderate exposure to cocaine. No observed changes are indicated by - and undetermined changes are indicted by question marks.
Future Directions
In conclusion, the accumulating evidence summarized in Table 1 suggest that aversive and appetitive learning induces plasticity in mPFC intrinsic excitability often in parallel with synaptic changes. The literature also supports the conclusion that intrinsic and synaptic plasticity in the mPFC contributes to the learned behavioral modifications. However, many unanswered questions remain. First, most if not all studies examined only male rats. Therefore, we do not know whether female rats show similar intrinsic and synaptic plasticity in response to learning. Second, although many receptors modulate the intrinsic excitability of mPFC neurons, very little is known about which receptors mediate associative learning-induced changes in mPFC neuronal excitability. Third, given the evidence of projection-specific changes in intrinsic excitability, it will be critical to evaluate learning-induced changes in the different projections to better understand how learning alters mPFC interactions with other structures. Fourth, to facilitate translation to the genetically diverse human population, we need to consider the contribution of different genetic backgrounds to the identified intrinsic and synaptic changes. For example, since the role of the mPFC in fear extinction depends on the strain of rats (Chang and Maren, 2010; Chang et al., 2010), the corresponding intrinsic and synaptic changes may also vary with the genetic background of the animals. Fifth, we need to understand how the effects of aging on the intrinsic excitability of mPFC neurons contributes to impaired learning in aged animals (Kaczorowski et al., 2012). Finally, it remains to be determined whether deficits in mPFC intrinsic and synaptic changes contribute to human mental disorders.
Table 1.
Intrinsic plasticity of mPFC neurons related to conditioned fear and cocaine seeking behavior.
| Behavior | Intrinsic Plasticity of Pyramidal Neurons | Mechanisms | References |
|---|---|---|---|
Fear Conditioning
|
|
|
|
Fear Extinction
|
|
|
|
Cocaine
|
|
|
Highlights.
We compiled the evidence that associative learning induces plasticity in both the intrinsic and synaptic excitability of mPFC neurons to modulate conditioned fear and cocaine seeking behavior.
Plasticity in the intrinsic excitability of mPFC neurons represents a major cellular mechanism that interacts with synaptic changes to alter the impact of the mPFC on fear and reward circuits.
Acknowledgements
This work was supported by the RCMI BRAIN and MAGIC Cores (NIMHHD G12 MD007579-27) and R15 MH116345 from the National Institutes of Health and the Catalyzer Research Grant Program (CRG-2020-00114) from the Puerto Rico Science, Technology and Research Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel HJ, Lee JC, Callaway JC, Foehring RC (2004) Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol 91:324–335. [DOI] [PubMed] [Google Scholar]
- Adhikari A, Lerner TN, Finkelstein J, Pak S, Jennings JH, Davidson TJ, Ferenczi E, Gunaydin LA, Mirzabekov JJ, Ye L, Kim S-Y, Lei A, Deisseroth K (2015) Basomedial amygdala mediates top-down control of anxiety and fear. Nature 527:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Paré D (2010) Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13:489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Clem RL (2014) Pathway-Selective Adjustment of Prefrontal-Amygdala Transmission during Fear Encoding. J Neurosci 34:15601–15609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood DW, Sugam JA, Holmes A, Kash TL (2018) Fear extinction requires infralimbic cortex projections to the basolateral amygdala. Transl Psychiatry 8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchta WC, Mahler SV, Harlan B, Aston-Jones GS, Riegel AC (2017) Dopamine terminals from the ventral tegmental area gate intrinsic inhibition in the prefrontal cortex. Physiol Rep 5:e13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ (2007) Consolidation of Fear Extinction Requires NMDA Receptor-Dependent Bursting in the Ventromedial Prefrontal Cortex. [DOI] [PubMed] [Google Scholar]
- Carr DB, Andrews GD, Glen WB, Lavin A (2007) α 2 -Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J Physiol 584:437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR (1996) Hippocampal afferents to the rat prefrontal cortex: Synaptic targets and relation to dopamine terminals. J Comp Neurol 369:1–15. [DOI] [PubMed] [Google Scholar]
- Chang C, Berke JD, Maren S (2010) Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS One 5:e11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Maren S (2010) Strain difference in the effect of infralimbic cortex lesions on fear extinction in rats. Behav Neurosci 124:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau H-J, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A (2013) Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496:359–362. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW (2000) Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20:RC89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW (2001) Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J Addict Dis 20:43–54. [DOI] [PubMed] [Google Scholar]
- Criado-Marrero M, Santini E, Porter JT (2014) Modulating fear extinction memory by manipulating SK potassium channels in the infralimbic cortex. Front Behav Neurosci 8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz E, López AV, Porter JT (2014) Spontaneous Recovery of Fear Reverses Extinction-Induced Excitability of Infralimbic Neurons. PLoS One 9:e103596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM (2006) Learning, aging and intrinsic neuronal plasticity. Trends Neurosci 29:587–599. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nasif FJ, Tsui JJ, Ju WY, Cooper DC, Hu XT, Malenka RC, White FJ (2005) Cocaine-induced plasticity of intrinsic membrane properties in prefrontal cortex pyramidal neurons: adaptations in potassium currents. J Neurosci 25:936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT (2011) Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex 21:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KA, Wolf ME, Hu X-T (2009) Plasticity of L-type Ca 2+ channels after cocaine withdrawal. Synapse 63:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ (2005) Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 492:145–177. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ (2010) Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem 17:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW (2013) Relapse Induced by Cues Predicting Cocaine Depends on Rapid, Transient Synaptic Potentiation. Neuron 77:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Fitzgerald PJ, Maren S (2016) Fear Expression Suppresses Medial Prefrontal Cortical Firing in Rats Dravid SM, ed. PLoS One 11:e0165256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Maren S (2015) The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Front Behav Neurosci 9:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M (1989) Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci U S A 86:9015–9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing M, Kotecki L, Marron Fernandez de Velasco E, Fajardo-Serrano A, Chung HJ, Luján R, Wickman K (2013) Repeated Cocaine Weakens GABAB-Girk Signaling in Layer 5/6 Pyramidal Neurons in the Prelimbic Cortex. Neuron 80:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X-T (2007) Cocaine withdrawal and neuro-adaptations in ion channel function. Mol Neurobiol 35:95–112. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146:373–390. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Davis SJ, Moyer JR (2012) Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiol Aging 33:1744–1757. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft J, Spruston N (2007) Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. J Physiol 578:799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, LaLumiere RT, Knackstedt L, Shen H (2009) Glutamate transmission in addiction. Neuropharmacology 56:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K, Bowers S, Szumlinski K, Xi Z-X, Baker D (2003) Glutamate transmission and addiction to cocaine. Ann N Y Acad Sci 1003:169–175. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8:844–858. [DOI] [PubMed] [Google Scholar]
- Koppensteiner P, Galvin C, Ninan I (2019) Lack of experience-dependent intrinsic plasticity in the adolescent infralimbic medial prefrontal cortex. Synapse 73:e22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, Helmstetter FJ (2014) Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiol Learn Mem 113:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C, Gaspar P (1998) Subpopulations of cortical GABAergic interneurons differ by their expression of D1 and D2 dopamine receptor subtypes. Brain Res Mol Brain Res 58:231–236. [DOI] [PubMed] [Google Scholar]
- Ma Y-Y, Henley SM, Toll J, Jentsch JD, Evans CJ, Levine MS, Cepeda C (2013) Drug-Primed Reinstatement of Cocaine Seeking in Mice: Increased Excitability of Medium-Sized Spiny Neurons in the Nucleus Accumbens. ASN Neuro 5:AN20130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Jin J, Goode TD, Giustino TF, Wang Q, Acca GM, Holehonnur R, Ploski JE, Fitzgerald PJ, Lynagh T, Lynch JW, Maren S, Sah P (2018) Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24:1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW (2001) The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 21:8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW (2003) Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23:3531–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, Disterhoft JF (2009) Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J Neurophysiol 102:2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE (2003) Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 168:57–65. [DOI] [PubMed] [Google Scholar]
- Medina JF, Christopher Repa J, Mauk MD, LeDoux JE (2002) Parallels between cerebellum- and amygdala-dependent conditioning. Nat Rev Neurosci 3:122–131. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420:70–74. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ (2012) Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 63:129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milella MS, Fotros A, Gravel P, Casey KF, Larcher K, Verhaeghe JAJ, Cox SML, Reader AJ, Dagher A, Benkelfat C, Leyton M (2016) Cocaine cue–induced dopamine release in the human prefrontal cortex. J Psychiatry Neurosci 41:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR Jr., Thompson LT, Disterhoft JF (1996) Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci 16:5536–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ (2008) Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci 28:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, Wickersham IR, Gray JM, Tye KM (2015) A circuit mechanism for differentiating positive and negative associations. Nature 520:675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Hu X-T, White FJ (2005a) Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. J Neurosci 25:3674–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasif FJ, Sidiropoulou K, Hu X-T, White FJ (2005b) Repeated Cocaine Administration Increases Membrane Excitability of Pyramidal Neurons in the Rat Medial Prefrontal Cortex. J Pharmacol Exp Ther 312:1305–1313. [DOI] [PubMed] [Google Scholar]
- Oh MM, Disterhoft JF (2015) Increased Excitability of Both Principal Neurons and Interneurons during Associative Learning. Neurosci 21:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, Oliveira FA, Disterhoft JF (2010) Learning and aging related changes in intrinsic neuronal excitability. Front Aging Neurosci 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis JM, Fitzgerald MK, Yousuf H, Burkard JL, Drake M, Mueller D (2018) Prefrontal Neuronal Excitability Maintains Cocaine-Associated Memory During Retrieval. Front Behav Neurosci 12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14:383–400. [DOI] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, Ledoux JE (2004) New vistas on amygdala networks in conditioned fear. J Neurophysiol 92:1–9. [DOI] [PubMed] [Google Scholar]
- Parrilla-Carrero J, Buchta WC, Goswamee P, Culver O, McKendrick G, Harlan B, Moutal A, Penrod R, Lauer A, Ramakrishnan V, Khanna R, Kalivas P, Riegel AC (2018) Restoration of Kv7 Channel-Mediated Inhibition Reduces Cued-Reinstatement of Cocaine Seeking. J Neurosci 38:4212–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS (2012) Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A 109:16318–16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F (2006) Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60:337–343. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D (2003) Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23:8800–8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM (2011) Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning Tsien JZ, ed PLoS One 6:e15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM (2014) Bridging the interval: Theory and neurobiology of trace conditioning. Behav Processes 101:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES (2002) Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66:345–353. [DOI] [PubMed] [Google Scholar]
- Santini E, Porter JT (2010) M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci 30:12379–12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT (2008) Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci 28:4028–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Sepulveda-Orengo M, Porter JT (2012) Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology 37:2047–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Healey KL, Kim R, Auriemma AC, Rojas J, Woronoff N, Hyppolite R, Reissner KJ (2018) Riluzole Impairs Cocaine Reinstatement and Restores Adaptations in Intrinsic Excitability and GLT-1 Expression. Neuropsychopharmacology 43:1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda-Orengo MT, Lopez AV, Soler-Cedeno O, Porter JT (2013) Fear Extinction Induces mGluR5-Mediated Synaptic and Intrinsic Plasticity in Infralimbic Neurons. J Neurosci 33:7184–7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, Darling RA, Brown TE, Sorg BA (2015) Removal of Perineuronal Nets in the Medial Prefrontal Cortex Impairs the Acquisition and Reconsolidation of a Cocaine-Induced Conditioned Place Preference Memory. J Neurosci 35:4190–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaker ML, Jorgensen ET, Hegarty DM, Liu X, Kong Y, Zhang F, Linhardt RJ, Brown TE, Aicher SA, Sorg BA (2018) Cocaine Exposure Modulates Perineuronal Nets and Synaptic Excitability of Fast-Spiking Interneurons in the Medial Prefrontal Cortex. eneuro 5:ENEURO.0221–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Cedeño O, Cruz E, Criado-Marrero M, Porter JT (2016) Contextual fear conditioning depresses infralimbic excitability. Neurobiol Learn Mem 130:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Cedeño O, Torres-Rodríguez O, Bernard F, Maldonado L, Hernández A, Porter JT (2019) Plasticity of NMDA Receptors at Ventral Hippocampal Synapses in the Infralimbic Cortex Regulates Cued Fear. eneuro 6:ENEURO.0354–18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Ehlers VL, Moyer JR (2015) Trace Fear Conditioning Differentially Modulates Intrinsic Excitability of Medial Prefrontal Cortex-Basolateral Complex of Amygdala Projection Neurons in Infralimbic and Prelimbic Cortices. J Neurosci 35:13511–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y-Y, Cai W, Yu J, Liu S-S, Zhuo M, Li B-M, Zhang X-H (2016) Surface expression of hippocampal NMDA GluN2B receptors regulated by fear conditioning determines its contribution to memory consolidation in adult rats. Sci Rep 6:30743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suska A, Lee BR, Huang YH, Dong Y, Schlüter OM (2013) Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proc Natl Acad Sci U S A 110:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR Jr., Disterhoft JF (1996) Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol 76:1836–1849. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM (2005) The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci 25:2609–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK (2004) Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci 24:10652–10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. [DOI] [PubMed] [Google Scholar]
- Xu T-X, Yao W-D (2010) D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc Natl Acad Sci U S A 107:16366–16371. [DOI] [PMC free article] [PubMed] [Google Scholar]


