Abstract
Predictors of treatment effects allow individual tailoring of treatment characteristics, thereby saving resources and optimizing outcomes. Electrical stimulation coupled with language intervention has shown promising results in improving language performance in individuals with Primary Progressive Aphasia (PPA). The current study aimed to identify language and cognitive variables associated with response to therapy consisting of language intervention combined with transcranial direct current stimulation (tDCS). Forty individuals with PPA received written naming/spelling intervention combined with anodal tDCS or Sham, using a between-subjects, randomized design, with intervention delivered over a period of 3 weeks. Participants were assessed using a battery of neuropsychological tests before and after each phase. We measured letter accuracy during spelling of trained and untrained words, before, immediately after, 2 weeks, and 2 months after therapy. We used step-wise regression methods to identify variables amongst the neuropsychological measures and experimental factors that were significantly associated with therapy outcomes at each time-point. For trained words, improvement was related to pre-therapy scores, in RAVLT (5 trials sum), pseudoword spelling, object naming, digit span backward, spatial span backward and years post symptom onset. Regarding generalization to untrained words, improvement in spelling was associated with pseudoword spelling, RAVLT proactive interference, RAVLT immediate recall. Generalization effects were larger under tDCS compared to Sham at the 2-month post training measurement. We conclude that, for trained words, patients who improve the most are those who retain for longer language skills such as sublexical spelling processes (phoneme-to-grapheme correspondences) and word retrieval, and other cognitive functions such as executive functions and working memory, and those who have a better learning capacity. Generalization to untrained words occurs through improvement in knowledge of phoneme-to-grapheme correspondences. Furthermore, tDCS enhances the generalizability and duration of therapy effects.
Keywords: Primary Progressive Aphasia (PPA), Transcranial direct current, stimulation (tDCS), Spelling, Rehabilitation, Predictors
1. Background
There is increasing interest in identifying behavioral and/or neural predictors of response to language therapy. These predictors contribute to our understanding of mechanisms supporting behavioral improvement induced by behavioral and neuromodulatory interventions in aphasia. Knowledge of such mechanisms is crucial for researchers to develop and test novel therapy approaches and to develop predictive models. Ultimately, information drawn from predictive models may allow clinicians to better optimize and individualize treatment protocols, based on patients’ cognitive profiles. This may be particularly important in the case of neurodegenerative disorders such as primary progressive aphasia (PPA), because language deterioration may be rapidly followed by or co-occur with other cognitive deterioration that generates additional emotional and financial burden to the individual and the caregivers (Mesulam, 2013). Therefore, it is fundamental to be able to predict who may benefit from language and neuromodulatory therapies and the combination of the two approaches.
PPA is a progressive loss of language abilities due to neurodegeneration. PPA disproportionately affects language performance, when compared to other cognitive abilities, for at least the first two years of the syndrome (Mesulam, 2001, 2008). Individuals with PPA often show impairments of oral and written language production and comprehension, both at single word and sentence levels (e.g., Grossman, 2012; Weintraub et al., 2009). The patterns of presentation of PPA have been categorized into three clinical variants, varying in behavioral presentation, neuroanatomical distribution of degeneration, and underlying pathology (Gorno-Tempini, Hillis, et al., 2011). However, not all cases fall precisely into these categories (Mesulam et al., 2009; Sajjadi, Patterson, Arnold, Watson, & Nestor, 2012). There are currently no treatments that halt or reverse the progression of the disease. Pharmacological interventions have been tested in clinical trials but have not yielded significant effects (e.g., Boxer et al., 2009). In contrast, language therapy and recent neuromodulatory interventions have shown promising results for improving language performance and staving-off language deterioration (e.g., (Henry et al., 2018; Tsapkini et al., 2018)).
1.1. Therapy approaches in PPA
Many therapy studies have focused on improving lexical retrieval, an impairment observed across PPA variants. Performance in picture naming has been facilitated using semantic, phonemic, orthographic, and gestural cues (Evans, Quimby, Dickey, & Dickerson, 2016; Jokel, Cupit, Rochon, & Leonard, 2009; Macoir et al., 2015; Meyer, Getz, Brennan, Hu, & Friedman, 2015) and using generative naming of items within specific semantic categories (Beeson et al., 2011). Some studies focused on training self-search of orthographic cues (Newhart et al., 2009) or self-generation of semantic, phonological, orthographic cues (Henry et al., 2013) or autobiographic cues (Beales, Cartwright, Whitworth, & Panegyres, 2016). Henry et al. (2013) used a treatment protocol combining different strategies to improve lexical retrieval, including: (1) picture naming with self-generated semantic, phonemic, and orthographic cues, and (2) generation of semantic features, semantic subcategories, and of new items within subcategories. In the study by Henry et al. (2018), script training was used to improve speech production in nonfluent agrammatic variant (nfvPPA). Finally, Meyer, Faria, Tippett, Hillis, and Friedman (2017) trained five participants with svPPA, nine with lvPPA, and seven with nfvPPA using a treatment consisting of reading or listening to a word and copying the word presented with the target picture. Meyer and colleagues reported improvement in naming both the remediation and prophylaxis items at the group level for each treatment type, indicating that preventive therapy may help to maintain language performance.
This body of research has shown that lexical retrieval can be improved for trained words (e.g., Beeson et al., 2011; Evans et al., 2016; Jokel et al., 2009), with maintenance of therapy effects reported for up to 6 months (Heredia, Sage, Ralph, & Berthier, 2009; Jokel et al., 2009). Generalization to untrained words is seldom reported, but Henry et al. (2013) did observe improved naming for a matched set of untrained words and for items in untrained semantic categories. Similar results for untrained words were reported by Beeson et al. (2011). Other studies using self-generated cues also found significant generalization in naming untrained items, albeit with different degrees of improvement across individuals (Beales et al., 2016; (Newhart et al., 2009). In the study by Henry et al. (2018), improvement after script training was reported not only in script accuracy, intelligibility, and grammaticality for trained scripts, but also in intelligibility for untrained scripts.
Spelling impairments occur across all PPA variants, though with different presentations depending on which components of the spelling process are disrupted (Neophytou, Wiley, Rapp, & Tsapkini, 2019; Sepelyak et al., 2011; Shim, Hurley, Rogalski, & Mesulam, 2012). Dual-route (Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001) and connectionist (Brown & Loosemore, 1995; Bullinaria, 1994) models of spelling differ regarding the nature of representations (distributed vs local), the nature of processing (serial vs parallel), and whether learning is included in the model.
According to dual-route models of spelling, in the intact language system, one of two processing routes can achieve accurate spelling. In the lexical route or set of processes, when we hear a word, we can spell it by recognizing the auditory input as a familiar string of phonemes (phonological representation), identifying the meaning (lexical-semantic representation) associated with this string of phonemes, and then activating the knowledge of the spelling associated with the concept (orthographic representations) stored in orthographic long-term memory. Alternatively, we can spell a word using the sublexical process of phoneme-to-grapheme conversion (PGC) in which each phoneme of the auditory stimulus generates a letter or letters that may be used to spell it (Rapp & Fischer-Baum, 2015). However, PGC will only produce correct spellings for regular words with predictable spellings (like ‘cat’) or for pseudowords that do not have an established spelling (e.g., “grint”). In contrast, words with irregular (unpredictable) spellings (e.g., ‘yacht’) require access to orthographic representations that have been stored in orthographic long-term memory. Note that if the spelling of an irregular word is not available due to damage at any stage in the lexical process, then the PGC process can generate a phonologically plausible spelling for the word (e.g., YOT for “yacht”). Regardless of the processing route used to generate the orthographic form, the string of letters (also referred to as graphemes) is stored temporarily in orthographic working memory (the graphemic output buffer; Caramazza, Miceli, Villa, & Romani, 1987), so that the letter representations are available to be produced in different formats, such as in written, oral spelling, or typing.
Each of the aforementioned processes may be selectively impaired in PPA, leading to difficulties with spelling. Individuals with non-fluent agrammatic variant PPA (henceforth nfvPPA) show impairments in phoneme-to-grapheme conversion (PCG) at earlier stages of disease progression. In contrast, those with semantic variant PPA (svPPA) show conversion difficulties only at later stages, and at earlier stages make mostly errors due to poor lexical-semantic knowledge and thus make phonologically plausible errors (Neophytou, Wiley, Rapp, & Tsapkini, 2019; Sepelyak et al., 2011; Shim, Hurley, Rogalski, & Mesulam, 2012). Behavioral (Tsapkini and Hillis, 2013; Rapp & Glucroft, 2009; Beeson and Egnor, 2006) and neuromodulatory approaches to treatment (Tsapkini et al., 2018; Tsapkini, Frangakis, Gomez, Davis, & Hillis, 2014) have yielded positive outcomes in terms of improving behavioral performance in individuals with such impairments.
Several behavioral protocols for language intervention in PPA have been studied. In the spelling domain, Rapp and Glucroft (2009) and Tsapkini and Hillis (2013a) report the results of interventions for two individuals with lvPPA. Both studies report improvement in spelling of trained but not untrained items, after a spell-study-spell training procedure (Rapp & Glucroft, 2009) and spelling therapy focused on PGC (Tsapkini & Hillis, 2013a). These studies show promising effects of orthographic therapies for yielding improvements or decreasing the rate of decline in spelling of trained items, although without significant generalization to untrained items. Neuromodulation research, described next, provides promising results regarding generalization.
1.2. Transcranial direct current stimulation (tDCS) in PPA
Transcranial direct current stimulation (tDCS) is a neuromodulatory technique that can modulate the excitability of the stimulated neural areas. It is non-invasive, with electrodes placed over areas of the scalp with the goal of targeting specific brain areas. A weak electrical current is delivered, and this leads to sub-threshold changes in the resting membrane potentials of neurons in stimulated areas (Kuo, Polanía, & Nitsche, 2016), making them more or less easily excitable depending on stimulation parameters and timing of delivery (Monte-Silva, Kuo, Liebetanz, Paulus, & Nitsche, 2010). To determine effects of tDCS on behavior, stimulation is typically contrasted with sham. Sham is a placebo-like mode where a minimal amount of current is delivered, sufficient to produce cutaneous sensations associated with tDCS, but insufficient to yield effects (Woods et al., 2016). tDCS administered simultaneously with a language task has been shown to enhance task performance in healthy controls, relative to sham stimulation, in post-stroke aphasia and PPA (e.g., Floel, Rosser, Michka, Knecht, & Breitenstein, 2008; see (de Aguiar, Paolazzi, & Miceli, 2015; Holland & Crinion, 2012; Monti et al., 2013); and Tippett, Hillis, & Tsapkini, 2015, for reviews).
Most studies testing the effects of tDCS in individuals with PPA have combined tDCS with language therapy across multiple therapy sessions, providing stimulation typically during the first 20 min of the behavioral treatment. Anodal tDCS over left hemisphere areas has been administered in conjunction with picture naming therapy (Cotelli et al., 2014; Roncero et al., 2017), modified semantic feature analysis (Hung et al., 2017), and narrative therapy (Gervits et al., 2016), all showing statistically significant improvement relative to baseline performance. However, some of these studies did not compare tDCS to a sham condition (Gervits et al., 2016; Hung et al., 2017). Greater improvement in the tDCS phase when compared to a sham phase was reported in two sham-controlled studies evaluating trained items (Cotelli et al., 2014) and also untrained items (Roncero et al., 2017), with maintenance reported up to 12 weeks (Cotelli et al., 2014). Stimulation targets have varied, including the left dorsolateral prefrontal (Cotelli et al., 2014), left fronto-temporal (Gervits et al., 2016), and left temporo-parietal regions (Hung et al., 2017; Roncero et al., 2017).
In a large randomized clinical trial of tDCS effects in PPA (N = 36), our group has recently shown that tDCS over the left inferior frontal gyrus (IFG), administered simultaneously to a written naming/spelling intervention was significantly more beneficial than sham for improving spelling of both trained and untrained items: tDCS benefits were maintained over two months and generalized to untrained words (Tsapkini et al., 2018). This study replicated results of a previous PGC intervention study with 6 patients (Tsapkini et al., 2014), where we had administered anodal or sham tDCS to the left IFG concurrently with therapy over 15 sessions in a sham-controlled, crossover design. Importantly, in the more recent study, we found that the three main PPA variants show distinctive tDCS effects for trained and untrained items, with individuals with svPPA showing no generalization. tDCS effects were stronger for untrained than trained words for both nfvPPA and lvPPA, but individuals with svPPA obtained no benefit from stimulation. Therefore, specific patient characteristics may significantly modify the neuromodulatory effects of tDCS, as may the nature of the outcome measure (item-specific vs generalization).
Previous research also highlights that there is wide variability in individual responses to stimulation in healthy individuals (Chew, Ho, & Loo, 2015; Horvath, Carter, & Forte, 2014; López-Alonso, Fernández-del-Olmo, Costantini, Gonzalez-Henriquez, & Cheeran, 2015), in individuals with post-stroke aphasia (Shah-Basak et al., 2015) and individuals with PPA (Tsapkini et al., 2018; Tsapkini, Frangakis, Gomez, Davis, & Hillis, 2014). McConathey et al. (2017) reported that baseline performance was significantly associated with tDCS benefits (compared to sham) such that individuals who performed better at baseline in a composite language measure had greater responses to stimulation. Cotelli et al., 2016 reported that changes in naming for trained nouns were positively correlated with pre-therapy grey matter volume in the left fusiform, left middle temporal gyrus, and right inferior temporal gyrus, while no significant correlations were identified between grey matter volume and improvement in naming untrained nouns. Hence, treatment outcomes are likely to be dependent on a combination of patient and treatment characteristics, including demographic, clinical, neural, and cognitive variables (e.g., de Aguiar, Bastiaanse, & Miceli, 2016).
The present study aimed to identify, in PPA, the variables that are associated with the degree of improvement observed both with language therapy and with tDCS plus language therapy. We included a large set of variables related to the clinical profile (a variety of language and cognitive assessment scores) as well as the characteristics of treatment (including tDCS and spelling therapy type) to identify predictors of improvement. The data reported in this study are from a clinical trial (ClinicalTrials.gov; Identifier: NCT02606422; Tsapkini et al., 2018) that examined the augmentative effects of tDCS over the left IFG coupled with written naming/spelling therapy over sham stimulation combined with the same therapy. Data from 36 out of the 40 participants included here were reported in Tsapkini et al., 2018. In the present analysis we included the larger sample of 40 individuals, given that more data are now available. Effects were evaluated both before and after therapy as well as two weeks and two months post therapy. In the present study we present a data analysis aimed at identifying the baseline patient characteristics (demographic, cognitive and language performance) and therapy characteristics that predict therapy effects at each post-therapy time-point. Given the exploratory nature of the analyses, and that they are secondary analyses of the clinical trial data, the statistical analysis procedures were not pre-registered prior to the research being conducted.
2. Methods
In this section, we report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
2.1. Participants
Data analyzed for this study were from 40 individuals with PPA (22 men), including 15 individuals diagnosed with nfvPPA, 17 with lvPPA, and 8 with svPPA, who were all of the participants who had completed the clinical trial as of July 2018. Inclusion criteria were established at the beginning of the clinical trial, and therefore prior to data analyses. Inclusion criteria were: being native English speakers, with minimum high-school education, proficient spellers before the onset of symptoms, absence of developmental disorders, absence of nondegenerative neurologic disorders (e.g., stroke), presence of progressive language deficits (out of proportion to other cognitive domains), and formal criteria-based diagnosis of PPA (Gorno-Tempini, Hillis, et al., 2011) at a specialized center. On average, participants reported being 4.75 (±2.87) years post-onset of symptoms. At the time of enrollment, the mean severity of language impairment was moderate, as measured by the FTLD-CDR language component (1.96 ± .81). The FTLD-CDR score summed across all items was 7.38 (±4.60). Mean age was 67.68 (±6.76). Detailed patient characteristics per stimulation condition are reported in Table 1. Table 2 shows the characteristics of the baseline assessment, and Table 3 describes the variables in the baseline assessment that were used for diagnostic assessment and served as input in the regression models. Performance for the different assessment time-points in the primary outcome measure per PPA variant and stimulation condition are reported in the supplementary materials.
Table 1 –
Demographics and descriptive statistics of behavioral outcomes. Demographic information and descriptive statistics of change in percentage of accurate letters (mean and SD) for each post-therapy assessment time-point compared to the pre-therapy time point, for both trained and untrained items. Sample size was reduced by 1 participant (lvPPA) for Sham and 2 participants (both lvPPA) for tDCS at two weeks post-therapy and by 4 participants (2 nfvPPA and 2 lvPPA) for tDCS at the 2-month post-therapy time point, due to participant availability for testing.
| Group | Age | Gender | Variant | FTLD-CDR Language | Immediately post-therapy – pre | 2 weeks post therapy – pre | 2 months post-therapy – pre | |
|---|---|---|---|---|---|---|---|---|
| tDCS (n = 21) | 66.1 (7.7) | 12 M, 9 F | 9 lv, 7 nv, 5 sv | 2.0 (.9) | Trained 36.7 (22.8) | 32.8 (22.2) | 27.4 (23.5) | |
| Untrained 13.6 (11.9) | 14.9 (16.2) | 14.4 (18.4) | ||||||
| Sham (n = 19) | 69.4 (5.1) | 10 M, 9 F | 8 lv, 8 nv, 3 sv | 1.9 (.8) | Trained 28.8 (20.8) | 25.2 (17.3) | 12.9 (15.0) | |
| Untrained 9.0 (8.9) | 11.0 (11.7) | 1.2 (12.8) | ||||||
Table 2 –
Variables examined as potential predictors of therapy outcomes. 1Scores derived from the RAVLT test (Rey Auditory Verbal Learning Test, Schmidt, 1996). On trials 1–5 the participant hears one list of 15 words and, each time, tries to recall as many words as possible from that list. On trial 6, the participant hears a new list (the interference list), and then tries to recall as many words from this new list as possible. On trials 7 and 8 the participant tries to recall items from the original list, but trial 8 is given after a 30-min delay (delayed recall).
| Variable | Description | |
|---|---|---|
| Treatment | Therapy type | Written naming/spelling vs Spelling-only therapy (as described in the Method). |
| Stimulation condition | Sham or tDCS | |
| Number of sessions | Number of sessions administered. (varied due to participant availability) | |
| Demographic | Age | At the start of therapy |
| Gender | Male or Female | |
| Clinical/language | Years post onset | Time since symptom onset, derived from discussion with individual and caregiver. |
| PPA variant | Diagnosed by a specialized neurologist, based on behavioral symptoms, imaging and examination as defined in Gorno-Tempini, Hillis, et al., 2011, consensus criteria. | |
| Language FTLD-CDR | Score for the language items only and the total sum of all items in the Frontotemporal Lobal Degeneration version of the Clinical Dementia Rating scale (Knopman et al., 2008) | |
| Total FTLD-CDR | ||
| Language | Pre-therapy spelling scores | Pre-therapy score on the outcome measure (percentage of correctly spelled letters for trained or untrained words separately). |
| Spelling words | Accuracy of spelling to dictation words and pseudowords from the JHU dysgraphia | |
| Spelling pseudowords | Battery (Goodman & Caramazza, 1985). | |
| Spelling high frequency | Accuracy of spelling to dictation items from Hillis, n.d., including high and low frequency items, and items with high and low probability of regular correspondence between phonemes and graphemes. | |
| Spelling low frequency | ||
| Spelling high probability | ||
| Spelling low probability | ||
| Object naming | Accuracy of picture naming of objects in the Boston Naming Test, 30 items (Kaplan, Goodglass, Weintraub, & Goodglass, 1983). | |
| Action naming | Accuracy of picture naming for actions in the Hopkins Assessment of Naming Actions, 34 items (HANA, Breining and Tippett et al., 2015) | |
| Object knowledge | Conceptual knowledge of objects assessed with picture association through the Pyramids and Palm Trees Test, 15 items (PPT, Howard & Patterson, 1992; PPTsf-Breining, Lala, et al., 2015) | |
| Action knowledge | Conceptual knowledge of actions assessed with picture association through the Kissing and Dancing Test, 15 items (KDT, Bak & Hodges, 2003) | |
| Comp. of actives | Auditory comprehension of sentences using a sentence-to-picture matching task with thematic and unrelated distractors, the SOAP test (Subject relatives, Object relatives, Actives, Passives, Love & Oster, 2002). | |
| Comp. of passives | ||
| Comp. of subject relatives | ||
| Comp. of object relatives | ||
| Sentence repetition | Number of words repeated correctly during oral repetition of sentences (Hillis, 2015) | |
| Verbal fluency | Sum of words retrieved in the letter fluency (FAS) and category fluency tasks (animals fruits and vegetables), with one minute given for each of the three letters/categories (Benton, Hamsher, & Sivan, 1994). | |
| Cognitive | Digit span backward | Length of digit span from the Wechsler Adult Intelligence Scale e Revised (WAIS-R, Wechsler, 1981) |
| Digit span forward | ||
| Spatial span backward | Length of spatial span from the Corsi block test (Corsi, 1972). | |
| Spatial span forward | ||
| Visual attention | Response times in part A of the Trail Making Test (Parkington & Leiter, 1949). | |
| Task switching | Response times in part B of the Trail Making Test (Parkington & Leiter, 1949). | |
| Immediate recall | Number of words recalled in RAVLT1 trial 1 (Schmidt, 1996). | |
| Final acquisition | Number of words recalled in RAVLT1 trial 5 (Schmidt, 1996). | |
| Total acquisition | Sum of words recalled in RAVLT1 trials 1 to 5, including each trial (Schmidt, 1996). | |
| Learning of 5 trials | Number of words recalled in RAVLT1 trial 5 minus those recalled in trial 1 (Schmidt, 1996). | |
| Proactive interference | Number of words recalled in RAVLT1 trial 1 minus those recalled in trial 6 (Schmidt, 1996). | |
| Retroactive interference | Number of words recalled in RAVLT1 trial 5 minus those recalled in trial 7 (Schmidt, 1996). | |
| Delayed recall | Number of words recalled in RAVLT1 trial 8 (Schmidt, 1996). |
Table 3 –
Baseline scores in cognitive and language assessment. Means (SD) for each variable. Freq. = frequency. Comp. = Comprehension. Description of each task is provided on Table 2.
| Variable | Sham | tDCS | ||||
|---|---|---|---|---|---|---|
| lvPPA (n = 8) | nfvPPA (n = 8) | svPPA (n = 3) | lvPPA (n = 9) | nfvPPA (n = 7) | svPPA (n = 5) | |
| Language severity | 1.63 (.74) | 2.38 (.74) | 1.67 (.58) | 1.89 (1.02) | 1.93 (.73) | 2.20 (.84) |
| FTDCDR | 6.00 (4.74) | 9.94 (5.16) | 5.50 (2.65) | 8.94 (4.93) | 5.50 (2.84) | 6.50 (4.90) |
| Spelling words | 84.32 (11.98) | 77.72 (13.83) | 89.08 (12.34) | 78.65 (22.28) | 86.52 (12.29) | 92.71 (3.27) |
| Spelling pseudowords | 79.54 (12.05) | 64.69 (15.07) | 90.25 (12.06) | 74.57 (18.09) | 60.27 (18.65) | 91.32 (4.89) |
| Spelling low probability | 6.13 (4.23) | 4.44 (4.24) | 4.34 (3.77) | 8.50 (3.88) | 7.94 (3.78) | 3.40 (2.61) |
| Spelling high probability | 9.84 (4.87) | 6.80 (5.68) | 8.45 (4.71) | 11.211 (2.41) | 11.99 (1.86) | 12.20 (2.17) |
| Spelling low freq. | 9.96 (4.45) | 6.37 (5.67) | 7.26 (5.42) | 11.24 (3.38) | 11.22 (2.95) | 9.20 (2.59) |
| Spelling high freq. | 8.28 (3.16) | 5.36 (4.46) | 6.28 (3.71) | 9.51 (3.47) | 8.94 (2.37) | 6.40 (1.95) |
| Object naming | 17.75 (7.94) | 13.00 (8.75) | 4.33 (4.51) | 17.32 (11.05) | 18.14 (9.65) | 1.60 (2.07) |
| Action naming | 19.13 (8.29) | 13.63 (8.91) | 12.33 (10.02) | 17.36 (11.21) | 18.20 (11.73) | 4.40 (3.51) |
| Object knowledge | 14.38 (1.06) | 13.00 (3.07) | 13.66 (2.31) | 13.48 (2.65) | 14.64 (.64) | 12.00 (1.58) |
| Action knowledge | 12.38 (2.50) | 11.63 (3.34) | 12.33 (3.06) | 12.62 (2.57) | 13.56 (1.40) | 11.00 (2.24) |
| Comp. actives | 8.50 (1.20) | 8.41 (1.76) | 8.93 (1.01) | 8.01 (1.66) | 8.00 (.77) | 8.40 (.89) |
| Comp. passives | 7.75 (1.58) | 7.23 (2.13) | 7.53 (1.50) | 6.91 (2.24) | 5.74 (2.52) | 6.00 (4.00) |
| Comp. subject relatives | 7.625 (1.92) | 6.68 (2.99) | 9.09 (.87) | 7.38 (2.05) | 7.68 (1.60) | 8.20 (2.17) |
| Comp. object relatives | 6.125 (2.36) | 5.67 (1.49) | 5.96 (3.00) | 4.93 (1.44) | 4.21 (1.99) | 7.00 (2.74) |
| Sentence repetition | 27.63 (3.20) | 21.63 (11.03) | 35.33 (2.08) | 26.28 (7.66) | 23.50 (10.44) | 33.00 (2.35) |
| Verbal fluency | 29.25 (9.79) | 17.80 (10.84) | 31.79 (25.60) | 34.19 (23.39) | 33.43 (13.60) | 26.20 (15.47) |
| Digit span forward | 4.81 (1.16) | 3.13 (1.46) | 6.00 (2.78) | 3.06 (1.69) | 3.79 (1.80) | 6.60 (.74) |
| Digit span backward | 2.69 (.46) | 1.94 (1.05) | 4.83 (2.02) | 2.17 (1.06) | 2.86 (1.84) | 4.50 (1.90) |
| Spatial span forward | 3.69 (1.03) | 2.38 (1.43) | 3.83 (.28) | 3.68 (.86) | 4.36 (1.60) | 5.00 (1.00) |
| Spatial span backward | 3.31 (1.22) | 1.94 (1.45) | 3.90 (.18) | 2.86 (1.31) | 4.07 (.89) | 4.70 (1.52) |
| Visual attention | 88.89 (89.06) | 102.87 (82.06) | 57.00 (26.51) | 133.72 (97.80) | 56.29 (19.72) | 58.20 (41.14) |
| Task switching | 250.89 (82.37) | 264.57 (73.31) | 127.33 (39.31) | 249.99 (69.80) | 254.29 (56.37) | 152.79 (112.58) |
| RAVTL immediate recall | 1.94 (1.26) | 3.06 (2.84) | 1.88 (1.64) | 2.02 (.98) | 3.78 (2.76) | 1.60 (1.52) |
| RAVLT final acquisition | 3.74 (2.37) | 4.52 (2.96) | 4.89 (2.59) | 3.65 (2.44) | 7.74 (3.86) | 4.80 (3.63) |
| RAVLT total acquisition | 15.52 (8.76) | 20.25 (16.43) | 19.32 (10.97) | 15.52 (7.72) | 30.67 (18.49) | 19.60 (14.88) |
| RAVLT learning 5 trials | 1.81 (2.33) | 1.46 (1.00) | 3.02 (1.00) | 1.62 (2.06) | 3.96 (1.44) | 3.20 (2.59) |
| Proactive interference | .75 (2.82) | 1.59 (2.34) | −.84 (1.25) | 1.29 (1.02) | 1.65 (2.40) | −.48 (1.43) |
| Retroactive interference | .77 (1.67) | 1.36 (1.65) | 3.29 (2.35) | .80 (1.27) | 1.32 (.41) | 2.92 (2.06) |
| RAVLT delayed recall | 2.81 (2.97) | 3.43 (4.09) | 2.18 (1.91) | 2.87 (2.76) | 4.83 (2.36) | 2.72 (1.29) |
2.2. Design of treatment protocol
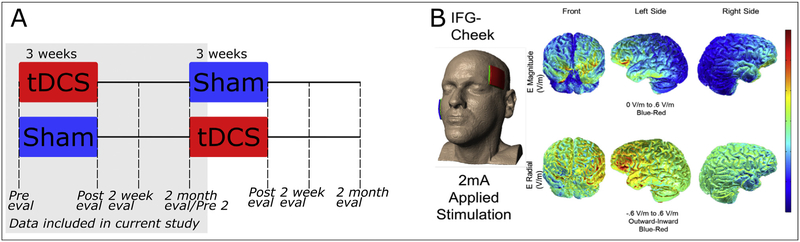
In the randomized clinical trial protocol (NCT0260642), for the first arm of the study, participants received treatment at the same time every day, i.e., fifteen 45-min sessions distributed over three weeks (depending on individual availability; see https://clinicaltrials.gov/ct2/show/NCT02606422 for pre-registered procedures). All changes to the pre-registered procedures are transparently identified. Personal reasons (most commonly other co-morbidities and age-related health issues) introduced some variability in the number of sessions, and therefore this parameter was entered in the regression as well. In Phase 1, participants received an average of 12 (±2) sessions. Participants were randomized to receive either real tDCS first in one arm and sham first in the other arm of the study, using a double-blind procedure. In each arm of the study, participants received the opposite stimulation condition with a two-month interval in between therapy phases (see Fig. 1A). Effects were evaluated before therapy as well as immediately after therapy and at two weeks and two months post therapy. In the present study we concentrated only on the first arm of the study. This was done because in the main trial we found some possible carryover effects for the trained items into the second period (Tsapkini et al., 2018), meaning effects of tDCS received in phase 1 influenced performance on trained items in period 2. According to Jones and Kenward (2004) this does not necessarily indicate carry over effects, especially if the participants could be considered to have started from a different baseline at the second phase (as is sometimes the case in neurodegenerative diseases such as PPA). However, to be conservative we excluded the second phase. Including data only from phase 1 effectively makes this a between-subjects design.
Fig. 1 –
Study design and model of current distribution for stimulation to the IFG (image courtesy: Dr. Marom Bikson).
2.2.1. tDCS
tDCS was administered using two 5 × 5 cm electrodes, with the Soterix 1 × 1 Clinical Trials device, and current intensity was set to 2 mA tDCS or Sham was delivered in the first 20 min and then language therapy continued for another 25 min, as this is the duration of a typical language therapy session. By introducing stimulation at the beginning of the behavioral treatment, we aimed to capitalize on the after-effects of stimulation, which should last approximately the same as the active stimulation period (Nitsche & Paulus, 2000). The anode was placed over the left Inferior Frontal Gyrus (IFG), and the cathode over the right cheek. The F7 co-ordinate of the 10–20 system (Homan, 1988) was used to locate the left IFG. Additionally, the accuracy of this landmark was checked by co-registering this area with MRI data using a fiducial marker, separately for each individual. Fig. 1B shows a model of current distribution for this electrode montage. In the Sham condition, stimulation was delivered only for 30 sec at the beginning and end of the 20-min period, in a ramp-up and ramp-down fashion, respectively (Gandiga, Hummel, & Cohen, 2006).
The IFG is thought to be engaged in multiple spelling processes, including lexical-semantic selection from orthographic long-term memory (Purcell, Turkeltaub, Eden, & Rapp, 2011) and PGC (Rapcsak et al., 2009; see Tsapkini & Hillis, 2013b, for a review). Hence, this area was deemed a suitable stimulation target to enhance brain function associated with therapy tasks emphasizing both lexical retrieval and PGC.
2.2.2. Behavioral therapy
Participants received written naming/spelling or spelling-only therapy (modified from Beeson & Egnor, 2006; Rapp & Glucroft, 2009; Tsapkini & Hillis 2013). Written naming/spelling therapy combined semantic and phonemic cueing (Beeson & Egnor, 2006) with the spell-study-spell procedure (Rapp & Glucroft, 2009), in the following steps.
Participants were asked to orally name a picture.
If needed to facilitate naming, the participant received semantic and/or phonemic cues. If they still could not produce the oral name after these cues were provided, they were given the target word, produced by the therapist.
They completed the spell-study-spell procedure. That is, they were asked to write the target, and if correct, they were asked to study and copy the written word. If the participant did not know the target, the participant was prompted with semantic cues. If the attempt at spelling was incorrect, the participant was provided with the correct spelling of the target word, then read the word, named each of the letters, and copied the word 5 times.
The first eight participants received the spell-study-spell part of the therapy, which means that their treatment only entailed completion of step (3) described above. The therapy was modified after the first eight participants, adding steps (1) and (2), so that patients with greater naming difficulties could be included. In this second therapy type, the written naming/spelling therapy, there was then additional presentation of visual stimuli (picture) and cueing for picture naming (steps 1 and 2). Cueing given for spelling errors in spelling-only therapy (step 3) also included semantic cues, and both versions of the therapy require individuals to retrieve orthographic representations from long-term memory. Agreeingly, the outcome measure (letter accuracy) was based on orthographic representations only. This change in protocol was initially motivated by the inclusion of individuals with svPPA. Such individuals do not exhibit PGC deficits and may perform well in spelling to dictation, as their preserved PGC allows them to produce phonologically plausible spellings, even when they do not recognize the word. By introducing picture naming, participants with svPPA were initially required to access lexical-semantic representations associated with treated words in steps (1) and (2), and then to activate orthographic representations in treatment step (3). In contrast, patients with lvPPA and nfvPPA have relative poorer PGC and can rely further on lexical semantic processing. Therefore, by introducing picture naming, the sequence of processes engaged by the treatment task became more balanced across individuals with different profiles of spelling impairment. We account for differences in therapy type by including this variable in the regression model. Nonetheless, individuals of all variants completed both versions of the protocol, and comparisons of patients receiving the two therapy types indicates that the groups did not differ significantly in any the baseline assessment measures collected.
2.3. Outcome measures
Performance was measured before and immediately after therapy, as well as at 2 weeks and 2 months after the end of therapy (see Fig. 1A). Participants undergoing all three steps of therapy were evaluated with a picture naming task: on each trial they were presented with a picture of a stimulus which they were asked to respond to with oral and then written naming. Participants undergoing only step three of therapy were evaluated with a spelling to dictation task: on each trial the clinician said a stimulus word and they were asked to write the spelling (spelling to dictation). Only written responses were evaluated for the present study. All participants were evaluated on letter accuracy of words that they spelled with or without pictures (‘written naming/spelling’ and ‘spelling-only’ therapy, respectively).
Each participant was assessed with one set of trained and one set of untrained words, so that both item-specific training effects and generalization of training effects to untrained items could be determined. The size of the sets depended on the participant’s spelling impairment severity, ranging from 10 to 30 words per set. The number of words and of letters included in treated and untreated sets was matched between the tDCS and Sham groups (number of words in trained set: t(36.68) = .18, p = .86; number of letters in trained set: t(36.32) = .08, p = .94; number of words in untrained set: t(36.73) = .04, p = .97; number of letters in untrained set: t(37.31) = −.34, p = .73).
Trained and untrained sets were matched for frequency and length using norms from the MRC psycholinguistic database (Coltheart, 1981). While trained and untrained sets were not systematically matched for imageability, all words selected were of high imageability. Items were predominantly nouns but could be either nouns or verbs. All items in a set were of the same grammatical category, and patients were treated with items of the same grammatical category across phases. Words with highly unpredictable spellings were not used, so that stimuli were balanced in terms of spelling regularity.
The scoring system evaluated each letter for accuracy taking into account deletions, additions, substitutions, and movements of letters (Goodman & Caramazza, 1985). The percentage of correctly spelled letters, out of the total number of letters across all the words in a set, corresponds to the score for a given individual at a given time-point. Hence, the outcome measures used in the present study corresponded to the absolute percent change in letter accuracy from pre-therapy to each post-therapy time-point. That is, the outcome measures correspond to each Post score minus the Pre score for trained and untrained words. Whole-word accuracy was not considered, because it would represent a coarser measure of improvement, regardless of the type of error produced.
2.4. Variables examined as potential predictors of outcome
We evaluated a total of 39 variables (listed in Table 2). We included variables that reflect the treatment, including Therapy Type, Stimulation (Sham vs tDCS), and number of Therapy Sessions as well as demographic variables of Age and Gender. Clinical/language characteristics included: both the language and overall severity score in the FTLD-CDR (Knopman et al., 2008), years post onset of symptoms (derived from discussion with the participant and family members), and PPA variant (diagnosed by a specialized neurologist, based on behavioral symptoms, imaging and examination as defined in Gorno-Tempini, Hillis, et al., 2011 consensus criteria). Additional predictors of response to treatment consisted of language and other cognitive assessment scores from tests administered before treatment. Baseline scores on all measures are reported on Table 3. The data were extracted from the standard baseline assessment for all participants of the clinical trial. All available language and cognitive data were included.
2.5. Analyses
The goal of the analyses was to evaluate the influence of each experimental variable on improvement in letter accuracy (the outcome measure) at each post-therapy time-point. We also measured the amount of variance in the outcome measure explained by each variable. To do this, the following forward regression model was used:
where Yf is the percent change in correctly written letters obtained by subtracting the pre-therapy letter accuracy (f = immediately after therapy, two weeks and two months after therapy); E(Y | V) is the conditional expectation of Y given V; V contains all the potential predictors including stimulation condition, gender, pre-therapy letter accuracy, variant type, and cognitive and language test scores (see Table 2); and the β′s are model coefficients. After fitting the model, we examined linearity via partial regression plots (see Fig. 3 as an example) and checked residual plots also for validation of the assumption of homogeneity of residual variances.
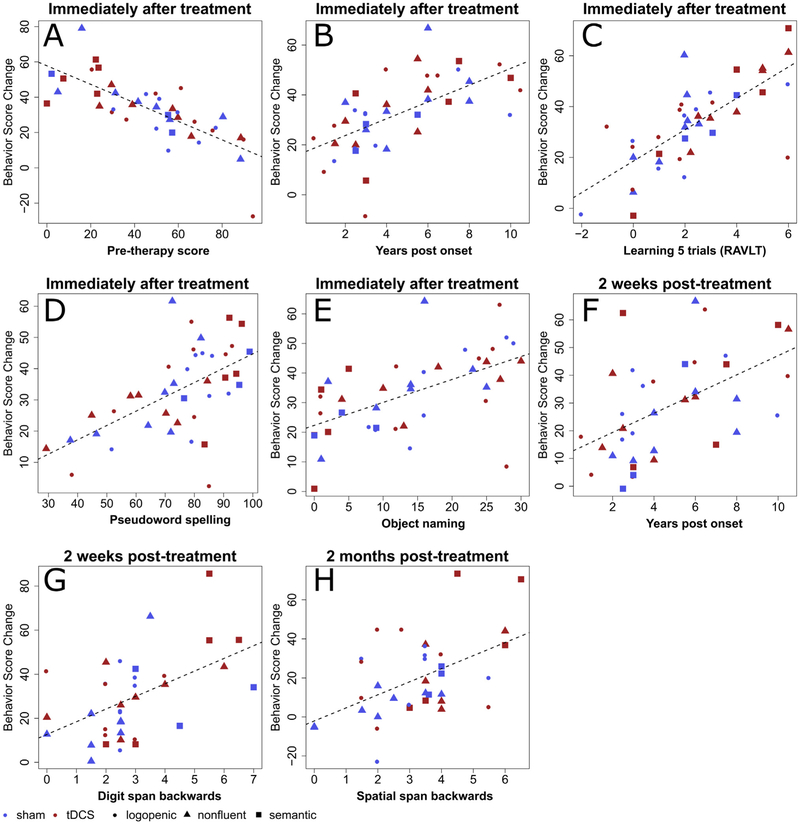
Fig. 3 –
Variables predicting improvement in spelling accuracy for trained words. Panels A–H contain scatter plots of therapy-related letter accuracy change in spelling versus values in predictor variables. The red solid symbols are data points of patients in the tDCS group, and the blue solid symbols represent the sham group. Circles represent individuals with lvPPA; triangles represent those with nfvPPA; and squares represent those with svPPA. Panels A to E show variables selected the first time-point, immediately after training. Panels F and G show variables selected for the second time-point, two weeks after training. Panel H shows the variable selected for the last time-point, two months after training.
In summary, variables were selected using a forward regression model and based on cross-validated R2. At each step of the forward regression, this procedure selects the variable that explains the most variance. Then, accounting (i.e., controlling) for variance explained by the first variable by already including it in the model, the next most informative variable is identified in the next step, until no variable adds a significant amount of variance explained (Stone, 1974) (see Fig. 2). Hence, the criterion for adding a prediction is based on variance explained by each variable. Statistics for each variable reported in Table 4 provide the cumulative variance explained by each variable added to the model after controlling for variables already included in the model. β and p-values were obtained by refitting the model with the chosen variables to add information regarding the directionality and strength of the relation. Hence, p-values provide an additional indication of an accurate selection process but are not the original selection criteria (Stone, 1974). To ensure that findings would be sustained with backward regression (rather than forward regression) we also included a backward removing step after forward selection. The results showed that for all time points no predictors needed to be removed from the model for either trained or untrained words.
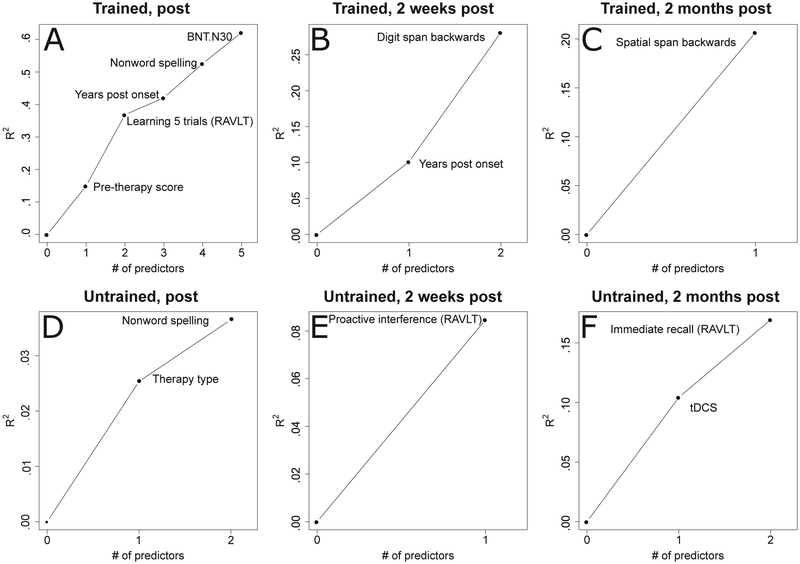
Fig. 2 –
Variables selected in the forward regression models for trained and untrained words. The y axis shows the cumulative R2 of models where variables are added iteratively based on cross-validated R2. Panels A to C show variable selection for trained words and D to F for untrained words, for the three time-points: immediately after training (on the left panel), two weeks (center panel), and two months after training (right panel). The x axis lists the number of predictors selected by the regression, in order of their introduction in the model.
Table 4 –
Predictors of improvement for trained and untrained words. 1Variables were selected based on cross-validated R2. Statistics for each variable provide the cumulative variance explained by each variable added to the model after controlling for variables already included in the model. β and p-values were obtained by refitting the model with the chosen variables to add information regarding the directionality and strength of the relation. Hence, p-values provide additional indication of an accurate selection process but are not the original selection criterion for variable inclusion.
| Trained words | |||
|---|---|---|---|
| Variable | ΔR2 | β | p |
| Immediately After therapy | |||
| Pre-therapy score | 14.9% | −.61 | p < .0001 |
| Learning 5 trials (RAVLT) | 21.9% | .56 | p < .0001 |
| Years post onset | 5.2% | .44 | p < .0001 |
| Spelling pseudowords | 10.5% | .37 | p < .001 |
| Object naming | 9.6% | .36 | p < .01 |
| Two weeks after therapy | |||
| Years post onset | 10.1% | .48 | p < .01 |
| Digit span backward | 18.0% | .49 | p < .01 |
| Two months after therapy | |||
| Spatial span backward | 20.7% | .50 | p < .01 |
| Untrained words | |||
| Variable | ΔR2 | β | p |
| Immediately after therapy | |||
| Therapy type | 25.4% | .57 | p < .0001 |
| Pseudoword spelling | 11.1% | −.38 | p < .01 |
| Two weeks after therapy | |||
| Proactive interference (RAVLT) | 8.5% | .34 | p < .05 |
| Two months after therapy | |||
| tDCS | 10.4% | .38 | p < .05 |
| Immediate recall (RAVLT) | 6.5% | .29 | p = .0561 |
The coefficient of determination (R2) is defined as the proportion of the observed variability in the dependent variable that is explained via the linear association with the independent variables. As the number of predictors increases, R2 increases. Therefore, to avoid potential overfitting, we used a leave-one-out cross-validated R2. For each iteration, we leave one subject out as the testing data, and use the rest (n-1) subjects as the training data to fit the model. Consider the current model as the null model, and the inclusion of a predictor candidate as the full model, the cross-validated R2 is defined as
where is the predicted value of subject i as the testing data under the null model, and is the predicted value under the full model. Both the null and the full models are trained using the rest (n-1) subjects after removing subject i. The cross-validated R2 is robust to the presence of outliers, hence contributing to reduce the occurrence of spurious results (i.e., type-I error) (Picard & Cook, 1984). If a subject is an outlier, the leave-one-out prediction error of that subject is high; and the value of the cross-validated R2 decreases, because outliers will be poorly predicted by the model trained based on the values of the remaining observations.
Missing observations for any of the predictor variables were estimated using Random Forests for data imputation (implemented using the rfImpute R function, Liaw & Wiener, 2015). This machine learning approach initially fills in missing observations with the mode of that column for categorical variables, and the median for numeric variables. Then, values are re-imputed as the weighted average or weighted category of the non-missing observations. The weights are based on a proximity matrix that considers the degree of similarity between a case and other cases in the sample. Similarity is defined based on shared features with other cases in the sample based on all other available variables. This method performs accurately if the rate of missing values does not exceed 50% (Shah, Bartlett, Carpenter, Nicholas, & Hemingway, 2014). Random Forests are a non-parametric statistical method that does not rely on distributional assumptions on covariate relation to the response (Burgette & Reiter, 2010). In our sample, variables had on average 15.3% ± 14.4% missing observations (median = 11.3%, interquartile range = .0%–32.5%, max. = 42.5%). Imputation was necessary for 28 of 39 variables, with differences in the number of observations requiring imputation. Data analysis code is included as supplementary information to this manuscript.
3. Results
3.1. Variables associated with improvement in letter accuracy in trained words
Immediately after therapy, improvement in letter accuracy (post-pre scores) for trained words was associated with pre-therapy spelling scores on these words (R2 = 14.9%, β = −.61 p < .0001), with greater change observed in individuals with lower pre-therapy scores (see Table 4). Conversely, the following variables all showed positive relations with the amount of improvement, such that higher scores on each task predicted greater improvement. After controlling for pre-therapy spelling scores on trained words, improvement was associated with RAVLT learning across 5 trials (that is, the number of words recalled in RAVLT trial 5 minus those recalled in trial 1; Schmidt, 1996) (ΔR2 = 21.9%, β = .56 p < .0001). Additionally, the amount of improvement was associated with the number of years after the onset of symptoms (ΔR2 = 5.2%, β = .44p < .0001), scores in pseudoword spelling (ΔR2 = 10.5%, β = .37 p < .001), and object naming scores (ΔR2 = 9.6%, β = .36 p < .01).
Two weeks after therapy, improvement was associated with the years post onset of symptoms (ΔR2 = 10.1%, β = .48 p < .01) with better outcome for individuals with longer symptom duration. After controlling for this variable, improvement was also associated with backward digit span (ΔR2 = 18.0%, β = .49 p < .01), with greater span associated with greater improvement in spelling trained words.
Two months after therapy, improvement in spelling trained words was associated with backward spatial span (ΔR2 = 20.7%, β = .50 p < .01), with greater improvement for individuals with longer span. These findings are summarized in Table 4 and Figs. 2 and 3.
3.2. Variables associated with improvement in letter accuracy in untrained words
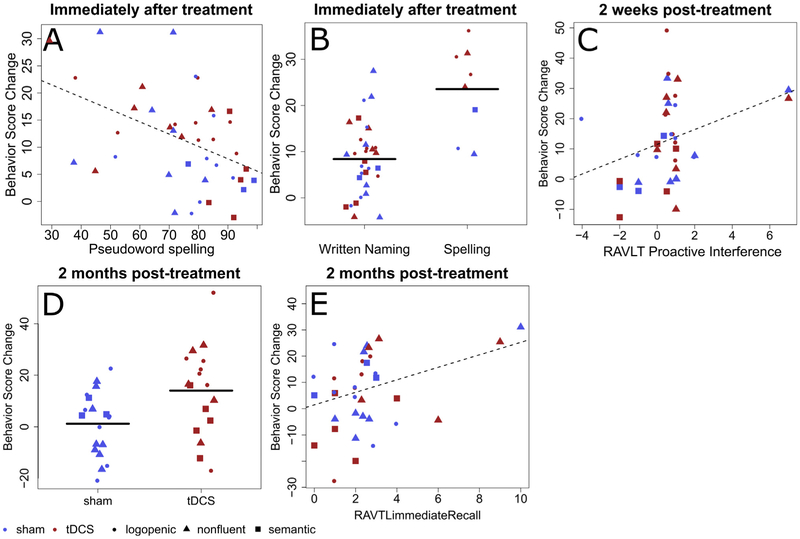
For the first timepoint immediately after therapy, improvement for untrained words was significantly associated only with therapy type, with greater improvement for untrained words in spelling-only therapy, compared to written naming/spelling therapy. Furthermore, outcome was associated with baseline accuracy in spelling pseudowords (ΔR2 = 5.4%, β = −.33 p < .05), with greater improvement for individuals with lower baseline pseudoword spelling scores.
Two weeks after therapy, only proactive interference (RAVLT) was significantly associated with improvement (ΔR2 = 8.5%, β = .34 p < .05). Greater improvement was observed for individuals with greater proactive interference.
Two months after therapy, improvement on untrained words was predicted by stimulation condition (ΔR2 = 10.4%, β = .38 p < .05). Greater generalization was also observed for individuals in the tDCS condition, compared to Sham. Furthermore, when controlling for stimulation condition, improvement was also associated with the RAVLT immediate recall measure (ΔR2 = 6.5%, β = .29 p = .056). Here, greater improvement was present for individuals able to recall a larger number of items at baseline. These findings are summarized in Table 4 (see Fig. 4).
Fig. 4 –
Variables predicting improvement in spelling accuracy for untrained words. Panels A–D contain scatter plots of therapy-related letter accuracy change in spelling versus values in predictor variables. The red solid symbols are data points of patients in the tDCS group, and the blue solid symbols represent the sham group. Circles represent individuals with lvPPA; triangles represent those with nfvPPA; and square represent individuals with svPPA. Panels A and B show the variable selected for the first time-point, immediately after training. Panel C shows variables selected for the second time-point, two weeks after training. Panels D and E show variables selected for the last time-point, two months after training.
4. Discussion
In the present study we addressed the question of which treatment (written naming/spelling or spelling-only as well as their combination with tDCS over the left IFG) and patient characteristics (in terms of language and cognitive performance) may predict the degree of improvement measured in letter accuracy. We have identified several variables showing a relationship with therapy outcomes, for trained and/or untrained words, immediately post, two-weeks post, and two-months post intervention in a between subjects design.
In summary, lower pre-therapy spelling of trained items, better learning ability (RAVLT cumulative learning of 5 trials) and better-preserved naming and pseudoword spelling abilities predicted the degree of improvement in trained words immediately post-therapy. Improvement in trained words at two weeks post-therapy was associated with better preserved working memory (digit span backward) and more years post onset. Two months post-therapy, improvement in trained words was associated with better working memory (spatial span backwards).
Two factors predicted generalization of treatment effects immediately after therapy: receiving spelling-only therapy and having lower pre-therapy pseudoword spelling performance. Higher proactive interference (at two weeks post-therapy) predicted improvement in untrained words. Importantly, two other factors - receiving tDCS and higher immediate recall capacity (RAVLT immediate recall) at baseline – predicted generalization of treatment to untrained words at two months post-therapy. We will interpret and discuss these findings in the following sections.
4.1. Variables associated with improvement in spelling of trained words
Several variables were identified as predictors of therapy outcome for trained words, immediately after therapy. Pre-therapy scores for the trained words were significantly associated with improvement for these words, immediately after therapy. Participants with lower baseline scores in spelling words improved more. This finding is expected given that these individuals start with a greater potential to show change along the measurement scale. Pre-therapy scores also reflect severity in spelling impairments relevant to the therapy task. In the post-stroke aphasia literature, severity of language impairment was shown to determine the recovery path. That is, a plateau in recovery occurs earlier for individuals with less severe impairments, while those with more severe impairments continue to improve for a longer period (Pedersen, Jørgensen, Nakayama, Raaschou, & Olsen, 1995). This is also in line with another predictor of improvement in trained words in the present study, i.e., years post-onset. More years post-onset from the disease predicted greater improvement in spelling performance of trained words, both immediately after and at two weeks post-therapy. The variable ‘Years post-onset’ was selected after pre-therapy scores were already taken into account, and therefore provides an independent contribution to predicting improvement, even if the two variables are generally correlated. It is possible that those participants who were enrolled at higher years post-onset generally had a slower decline in language (more gradual disease course), which was associated with better response to therapy.
In addition to years post onset, two other characteristics of patients’ language performance and one learning characteristic were identified as predictors of improvement in trained words. Better PGC (pseudoword spelling) and baseline lexical retrieval (object picture naming) predicted larger benefits from treatment for trained words. In addition to these parameters, improvement in therapy outcomes was predicted by verbal learning abilities of the patients: those with higher verbal learning ability (indicated by the amount of learning from the first to the fifth trial in RAVLT) benefited more from therapy. Therefore, patients able to maintain their phoneme-to-grapheme (sublexical) conversion ability and lexical retrieval abilities and who have better verbal learning ability may benefit more from therapy. These patient characteristics are discussed in detail below.
Pseudoword spelling scores reflect the ability to convert sounds to letters, using PGC rules. Sublexical conversion, access to phonological word forms, and to orthographic word forms are all functionally independent (Coltheart et al., 2001). Nonetheless, sublexical conversion interacts with lexical selection, and adequate knowledge of phoneme-to-grapheme correspondences may prevent spelling errors (Miceli, Capasso, & Caramazza, 1994). This way, good baseline PGC skills may provide participants with additional resources to use in therapy. In addition to memorizing orthographic labels for trained words, individuals with better baseline knowledge of PCG rules may be able to start identifying regularities and to use those to facilitate their spelling. While not the main focus of therapy, these spelling relationships were discussed during training. These mechanisms are discussed further in the next section.
Greater improvement was also observed for individuals with higher baseline object naming scores. Individuals with higher baseline scores in object (picture) naming may have strengths in any of the lexical-semantic levels and processes that support naming. For all but 8 participants, therapy entailed picture naming. These individuals were presented with a picture representative of the item to spell and were asked to name the item and then spell the word. This was the premise of Beeson and Egnor (2006) as they designed the treatment approach combining oral and written naming/spelling therapy. It is possible that patients with better residual ability to process the lexical-semantic demands of the therapy task could derive greater benefit from training. Thus, support from a more functional lexical-semantic route for spelling during treatment may enhance treatment-related learning.
Greater improvement was observed in individuals with higher RAVLT learning across 5 trials, that is, the number of words recalled in trial 5 minus those recalled in trial 1. The RAVLT is a test that is widely used for the assessment of short-term auditory verbal learning memory, among other cognitive functions (Macartney-Filgate & Vriezen, 1988). The RAVLT learning across 5 trials was identified as a predictor of improvement on trained words. This particular RAVLT measure reflects the learning rate achieved by providing additional repetitions of items from the same list (Lezak, Howieson, & Loring, 2012). In other words, it reflects an individual’s ability to derive learning of words from repeated exposure to those same items. This is consistent with predicting treatment effects for trained words, which were seen repeatedly in therapy sessions.
Working memory was identified as important for maintenance of treatment effects at two weeks and two months post intervention. Digit span backwards and spatial span backwards were identified as predictors of improvement at two weeks and two months post-therapy, respectively. In both cases, longer spans were associated with greater improvement on trained words. Similarly to forward spans, backward spans require maintenance of information in the working memory slave systems (the phonological loop and the visuo-spatial sketchpad, measured by digit and spatial span, respectively) (Baddeley, 1992). In addition, backward spans require manipulation of information, engaging working memory’s central executive. The central executive is thought to coordinate information between two or more systems, managing attentional resources, task switching, and the interface with long-term memory (Baddeley, 2012). Performance deteriorates with aging both in forward and backward digit span, to a similar degree (Hester, Kinsella, & Ong, 2004). The finding that the two backward span tasks predict improvement at two weeks and two months after therapy supports that working memory is important for long-term learning (Burgess & Hitch, 2006). We find that scores on digit and spatial backward span tasks are significantly correlated (R = .58, p < .001), further supporting a common mechanism for the role of these two variables.
Across the three post-therapy time points, different variables were identified as predictors of improvement for trained words. In terms of the nature of the variables, there seems to be a tendency for variables to be more language-specific closer to the end of therapy than at the 2-month post-therapy time point. Other cognitive functions seem to become more relevant in later assessments. The greater distance from the baseline (where all scores for predictors were collected) may make very specific language measures less representative of skills at later timepoints. Hence, baseline performance becomes less representative of current skills as the participants move forward in the protocol. This may be the reason why the amount of variance explained also seems to decrease over time. Furthermore, impairments in other cognitive functions may also increase over-time, as the disease progresses (Mesulam, 2007). This progression increases the range of performance observed across the sample in measures representative of those non-linguistic cognitive functions (e.g., spatial span) and makes it possible to detect effects of individual variability in response to therapy.
4.2. Variables associated with improvement in spelling untrained words (generalization)
Several accounts have been offered to explain the occurrence and mechanisms of generalization. In post-stroke aphasia, generalization in spelling has been found in treatment studies targeting PGC (Luzzatti, Colombo, Frustaci, & Vitolo, 2000; Partz, Seron, & Linden, 1992). However, PGC therapy does not necessarily result in generalization in PPA (Tsapkini & Hillis, 2013a) since it may depend on factors such as progression of disease and severity of symptoms. In the oral naming modality, generalization has more frequently been attributed to activation of semantic features shared between trained and untrained items after semantic therapy (e.g., Semantic Feature Analysis, SFA; Boyle & Coelho, 1995). It is possible that a similar mechanism would facilitate the functioning of the lexical-semantic spelling route (Coltheart et al., 2001). Additionally, it has been suggested that generalization may occur due to therapy-related improvements in the functioning of the graphemic buffer (Aliminosa, McCloskey, Goodman-schulman, & Sokol, 1993).
Two of our findings may, at first, seem contradicting. However, they reveal critical differences in mechanisms of item-specific improvement and generalization. On the one hand, higher baseline pseudoword spelling predicts greater improvement for trained words. On the other hand, lower baseline pseudoword spelling predicts greater generalization to untrained words. First, the finding for trained words indicates that better residual PGC may facilitate learning during therapy sessions. PCG interacts with lexical selection (Miceli, Capasso, & Caramazza, 1994), meaning that a partially correct spelling based on PCG may narrow down the lexical search to items in the lexicon that contain the correctly spelled letters. An individual with poor PGC may then receive less facilitation of lexical selection from partially correct spellings than an individual with better PCG, because s/he will produce fewer correct letters in the initial attempt to spell a word. In therapy, after an incorrect or partially correct spelling the patient is eventually presented and asked to repeatedly write the correct lexical form. Patients with better baseline PGC essentially have less information to learn during training and greater ability to use their partial spellings to select the correct lexical items. In addition to memorizing orthographic labels for trained words, individuals with better baseline knowledge of PCG rules may be able to start identifying regularities and to use those to facilitate their spelling. In either case, given the repeated exposure entailed by treatment, graphemic representations of trained words in the graphemic output lexicon may be strengthened (Beeson, 1999). Once spellings for trained words have been learned they can be accessed using the lexical -semantic or direct lexical routes of spelling (Coltheart et al., 2001), which means that they do not have to be spelled using PGC. This way, while a strong baseline PGC knowledge can facilitate improvement, PGC itself does not need to improve to yield change in spelling if therapy strengthens lexical representations or access to lexical units. We believe that this finding relates to a learning mechanism that facilitates access to strengthened lexical units (and not to learning of generalizable rules) because this effect is present only for trained words.
A very different learning process seems to enable generalization. Here, the individuals showing greater generalization are those with lower pseudoword spelling, and hence lower baseline PGC knowledge. In practice, patients with poorer baseline knowledge of PGC rules have more room to improve in PGC knowledge during treatment, compared to participants who already start with a high level of PGC knowledge. Importantly, there was no exposure to untrained words during treatment, and therefore improvement on untrained words cannot be linked to explicit memorization of lexical forms. Instead, improvement on untrained words may be occurring through learning of PGC rules. This knowledge becomes available through training and can be used to spell untrained words with greater accuracy after therapy compared to before therapy, because this knowledge was less available before therapy. If it is true that sublexical processing improves with therapy, participants should show improvement in other measures of PCG knowledge, such as pseudoword spelling. These data were available for 25/40 participants, and we conducted an additional comparison to test this prediction. Indeed, a significant increase in accuracy in pseudoword spelling is observed in our sample from pre to post-training (Z = 85, p < .05). These observations are then well aligned with prior literature in post-stroke aphasia showing that generalization occurs when phoneme-to-grapheme correspondences are trained (Luzzatti et al., 2000; Partz et al., 1992).
While PPA variant was not selected as a predictor of generalization, we note that individuals with svPPA tend to have relatively spared knowledge of conversion rules, as well as buffer functioning, while those with nfvPPA are particularly poor in this knowledge (Sepelyak et al., 2011; Shim et al., 2012; Neophytou et al., in press). Therefore, in more severe lexical-semantic impairments, such as in svPPA, where there is little room for improvement in the PGC process, the lack of generalization is not surprising (Tsapkini et al., 2018). In line with that, Tsapkini et al., 2018 also found that those with more severe impairments in PGC (i.e., individuals with nfvPPA), showed the greatest generalization in untrained words. We also note that individuals with nfvPPA in our sample showed greater change in pseudoword spelling than those with svPPA (U = 7, p < .05) and marginal differences in change in pseudoword spelling compared to those with lvPPA (U = 68, p = .65).
In addition to pseudoword spelling, therapy type had an effect on improvement for untrained words immediately after therapy. Specifically, generalization was greater for individuals receiving spelling-only therapy, compared to written naming/spelling therapy. In written naming/spelling therapy, patients saw a picture, were asked to name it and were given semantic and phonemic cues as needed. In both therapies, participants heard the target word (either produced by the therapist or named by themselves), and then spelled the word using the spell-study-spell procedure that emphasizes phoneme-to-grapheme correspondences for each letter of the word. Hence, the main differences between the therapies are the additional picture naming step, with the corresponding cueing for written naming/spelling therapy and additional attention to PGC in spelling-only therapy. It is, thus, possible that spelling-only therapy was more effective because a greater proportion of therapy time was dedicated to practicing spelling.
At two weeks after training, proactive interference measured with RAVLT was also identified as a predictor of improvement for untrained words. Proactive interference was generated by subtracting the number of words retrieved immediately in the interference list of RAVLT (list B, after exposure to the first list 5 times) from those retrieved immediately for list A (trial 1). Larger values of proactive interference denote greater interference of prior learning (with list A) on new learning (with list B). Larger proactive interference has been linked to executive dysfunction (Gershberg & Shimamura, 1995), and working memory impairments (Nelson, Reuter-Lorenz, Sylvester, Jonides, & Smith, 2003). Furthermore, proactive interference occurs in patients with frontal lesions (McDonald, Bauer, Grande, Gilmore, & Roper, 2001), who tend to be more impaired in knowledge of PGC. We would then expect RAVLT proactive interference to correlate with pseudoword spelling scores. This correlation was in the expected direction (R = −.37, p = .07), but only marginally significant. Therefore, our current findings only allow us to provide a speculative explanation for the role of proactive interference. Proactive interference corresponds to ‘failure to inhibit’ (Fischer-Baum & Rapp, 2012). According to this finding, the more a participant fails to inhibit the less generalization occurs. What is left unclear is the exact nature of the processes or information that need to be inhibited so that better generalization can happen. We may speculate that, just like in the RAVLT prior learning of studied words can interfere with new words, in our treatment prior learning of spelling trained words interferes with spelling of untrained words. Future research should further characterize the effect of proactive interference in how it relates to language processing and specific spelling impairments.
Two months after training another RAVLT measure was identified as a predictor of improvement for untrained words. This score corresponds to the immediate recall of items presented in the first RAVLT trial. We have already argued in our discussion of findings for trained words that the RAVLT recall measures are markers of short-term auditory verbal learning memory (Macartney-Filgate & Vriezen, 1988). Here we add that short-term memory is not only relevant for learning trained words, for which there was direct exposure, but also relates to learning of untrained words. If, as argued above, generalization occurs through learning of regularities in PGC, then short-term memory supports rule-based learning. Previous research has found correlations between short-term memory and morphological rule learning (Williams & Lovatt, 2003). Our data suggest that this relation with short-term memory may extend to learning orthographic rules.
Greater generalization was observed when participants received anodal tDCS over the left IFG rather than Sham. The IFG was chosen for stimulation based on its general role in language and specific engagement in multiple spelling processes: it was reported to be crucially engaged in semantic selection (Thompson-Schill, D’Esposito, & Kan, 1999), orthographic long-term memory (Purcell et al., 2011), and phoneme-to-grapheme conversion (Rapcsak et al., 2009; see Tsapkini & Hillis, 2013b, for a review). Interestingly, the advantage for participants receiving active stimulation was observed only in the 2-month post training measurement. Furthermore, this enhancement was specific to untrained words. As reported by Tsapkini et al., 2018 these results suggest that patients in the Sham group have a faster decay of treatment-related generalization, compared to those individuals in the tDCS group. tDCS modulates functional connectivity between the stimulated area and areas of the temporal lobe (Ficek et al., 2018), and thus may induce a more stable change in the functions subserved by these areas, namely lexical retrieval of stored representations. Thus, tDCS may increase the durability of the results. The differences between tDCS and Sham may be more evident for the therapy effects that are smaller and less stable over time (generalization and maintenance; e.g., de Aguiar et al., 2015). Importantly, tDCS seems to have increased therapy efficiency, that is, getting better results (more sustained and more generalized) with the same intervention time, compared to the sham phase.
4.3. Limitations and future directions
In this study we aimed to identify variables that predict response to written naming/spelling intervention combined with tDCS applied to the left IFG, in order to increase understanding of the cognitive mechanisms that underly improvement and allow prediction of therapy effects. Replication of these findings is crucial, and so is expanding the search space to other potentially informative measures. It is important to acknowledge that we relied on a set of language and other cognitive variables for which we had readily accessible information. PPA variant was diagnosed following international consensus criteria (Gorno-Tempini, Hillis, et al., 2011), but there is still significant variability in the profiles of individuals within each variant. The multidimensional nature of the PPA Variant predictor is probably the reason why this predictor may not have explained enough variance by itself to be selected in any of the time-points, for trained or untrained words. When we looked at the tDCS effects per se in the larger trial, we did find PPA variant-specific patterns (Tsapkini et al., 2018). In addition, we study predictors associated with a specific treatment protocol. It is possible, and very likely, that effects of other behavioral and/or neuromodulatory treatment approaches rely on at least partially distinct learning processes. This way, effectiveness of different therapies is expected to be predicted by different variables and the identification of such predictors should be the focus of future research (e.g., de Aguiar et al., 2016).
We must also acknowledge that this study is largely exploratory and that the sample size, despite being large for this population, is still small. An exploratory study allows for substantial hypothesis generation. Nonetheless, this also means that the results will need to be confirmed with additional studies designed to test the hypotheses generated. Specifically, future research should confirm that generalization after spelling-only therapy occurs due to learning of regularities in PGC. Regarding tDCS, future studies may address whether there are different predictors for the tDCS vs Sham conditions or groups, whether stimulation is more effective when targeting healthy tissue or brain tissue with degeneration, and whether localized cognitive functions are more susceptible to enhancement with anodal tDCS compared to cognitive functions with a more distributed representation.
We should also consider a characteristic of our scoring system, which measured accuracy for each letter. While this is an unlikely scenario, our approach allows a participant who scored 5 incorrect letters in a single word to have the same score as another participant who scored 1 letter incorrect across 5 words. We understand that improvement of these two types of individuals may not be identical. However, we think that scoring letter accuracy (rather than whole word accuracy) has two important advantages. First, whole-word accuracy would represent only a coarse measure of improvement. Second, this more fine-grained measure may be more functionally relevant than whole-word accuracy, as improvement in partial spellings of words may allow communication partners to better guess what a patient is attempting to write.
In addition, studying the relation between severity of impairments (in various language and other cognitive processes) and response to interventions will be crucial for understanding if there is a ‘tipping point’ in PPA after which direct, impairment-oriented intervention is no longer effective. This will effectively help to guide clinicians to know when to focus intervention in compensatory strategies such as communication partner training, but it will also require much larger sample sizes and analyses that can identify interactions between predictors.
5. Conclusions
The current study identifies variables associated with response to a large written naming/spelling intervention study in PPA and highlights some of the potential mechanisms by which individuals with PPA may improve their spelling abilities, in response to treatment with and without tDCS. For trained words, greater response to treatment was associated to initial severity of the spelling impairment, overall progression of the disease, baseline phoneme-to-grapheme conversion skills, and learning rate, as well as working memory abilities. We showed that the patients who may improve in learning and retaining the benefits of therapy are those who have better residual language abilities such as sublexical spelling mechanisms and word retrieval, as well as certain cognitive functions such as executive functions/working memory and have better learning capacity. For untrained words, sublexical spelling (that is, learning phoneme-to-grapheme correspondences) may be an important source of generalization. We also showed that tDCS improves generalization and maintenance of therapy effects for untrained words.
Identifying which patients may benefit from interventions is important because it can offer significant insights on how to improve our interventions, how to select intervention approaches suitable for specific patients, and to save resources. For example, knowing that baseline PGC skills are positively correlated with improvement for trained words may prompt clinicians to use this strength by reviewing these phoneme-grapheme correspondences explicitly in therapy sessions, even if the patient does not have impairment affecting this type of knowledge. Furthermore, if generalization occurs via learning of these phoneme-to-grapheme correspondences, directly targeting this impairment may be beneficial, even in the presence of severe spelling deficits. Future multi-center studies with larger samples and using tools to identify interactions between predictors will be crucial for providing more specific support for clinical decision-making.
Supplementary Material
Acknowledgements
This work was supported by grants from the Science of Learning Institute at Johns Hopkins University and by the National Institutes of Health (National Institute of Deafness and Communication Disorders) through award R01 DC014475 to KT. AH was supported by NIH (NIDCD) through awards R01 DC05375, R01 DC011317 and P50 DC014664. BR was supported by NIH (NIDCD) through award P50 DC012283 We are grateful to our participants for their unfailing commitment and interest in our study. We also thank referring physicians.
Footnotes
Data availability
Legal restrictions prevent us from publicly archiving the study data at this time. The data from this study are under a Data Sharing agreement with NIH. According to this agreement, these data are part of the official clinical trial (ClinicalTrials.gov Identifier: NCT02606422). The full set will be available to the public after the official and legal completion of the study in April 2020. We are legally bound to disclose the data from the study after its official completion. Before that date, specific requests by individual researchers should be addressed to the PI of the study, Dr. Kyrana Tsapkini (tsapkini@jhmi.edu). Data sharing agreement: We will make the data and associated documentation available to users only under a data-sharing agreement that provides for: (1) a commitment to using the data only for research purposes and not to identify any individual participant; (2) a commitment to securing the data using appropriate computer technology; (3) proper acknowledgment of data source; and (4) a commitment to destroying or returning the data after analyses are completed.
Open practices
The study in this article earned a Preregistered badge for transparent practices. Pre-registered procedures for the study are available at https://clinicaltrials.gov/ct2/show/NCT02606422.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cortex.2019.11.001.
REFERENCES
- Aliminosa D, McCloskey M, Goodman-schulman R, & Sokol SM (1993). Remediation of acquired dysgraphia as a technique for testing interpretations of deficits. Aphasiology, 7(1), 55–69. 10.1080/02687039308249499. [DOI] [Google Scholar]
- Baddeley A (1992). Working memory. Science, 255(5044), 556–559. 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A (2012). Working memory: Theories, models, and controversies. Annual Review of Psychology, 63, 1–29. [DOI] [PubMed] [Google Scholar]
- Bak TH, & Hodges JR (2003). Kissing and dancing—a test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. Journal of Neurolinguistics, 16(2), 169–181. 10.1016/S0911-6044(02)00011-8. [DOI] [Google Scholar]
- Beales A, Cartwright J, Whitworth A, & Panegyres PK (2016). Exploring generalisation processes following lexical retrieval intervention in primary progressive aphasia. International Journal of Speech-Language Pathology, 18(3), 299–314. 10.3109/17549507.2016.1151936. [DOI] [PubMed] [Google Scholar]
- Beeson PM (1999). Treating acquired writing impairment: Strengthening graphemic representations. Aphasiology, 13(9–11), 767–785. [Google Scholar]
- Beeson PM, & Egnor H (2006). Combining treatment for written and spoken naming. Journal of the International Neuropsychological Society: JINS, 12(6), 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson PM, King RM, Bonakdarpour B, Henry ML, Cho H, & Rapcsak SZ (2011). Positive effects of language treatment for the logopenic variant of primary progressive aphasia. Journal of Molecular Neuroscience : MN, 45(3), 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Hamsher KS, & Sivan AB (1994). Multilingual aphasia examination (3rd ed.). Lutz, FL: Psychological Assessment Resources (PAR). [Google Scholar]
- Boxer AL, Lipton AM, Womack K, Merrilees J, Neuhaus J, Pavlic D, et al. (2009). An open-label study of memantine treatment in 3 subtypes of frontotemporal lobar degeneration. Alzheimer Disease and Associated Disorders, 23(3), 211–217. 10.1097/WAD.0b013e318197852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M, & Coelho CA (1995). Application of semantic feature analysis as a treatment for aphasic dysnomia. American Journal of Speech Language Pathology, 4(4), 94–98. 10.1044/1058-0360.0404.94. [DOI] [Google Scholar]
- Breining BL, Lala T, Cuitño MM, Manes F, Peristeri E, Tsapkini K, et al. (2015). A brief assessment of object semantics in primary progressive aphasia. Aphasiology, 29(4), 488–505. [Google Scholar]
- Breining BL, Tippett DC, Davis C, Posner J, Sebastian R Oishie K, … (2015). Assessing dissociations of object and action naming in acute stroke In Paper presented at the clinical Aphasiology Conference, Monterey, CA. [Google Scholar]
- Brown GDA, & Loosemore RPW (1995). A computational approach to dyslexic reading and spelling In Joshi RM, & Leong CK (Eds.), Developmental and acquired dyslexia: Neuropsychological and neurolinguistic perspectives. Dordrecht: Kluwer Press. [Google Scholar]
- Bullinaria JA (1994). Connectionist modelling of spelling. In Proceedings of the 16th Annual Conference of the Cognitive Science Society (pp. 78–83). Hillsdale, NJ: Erlbaum; Retrieved from: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.63.6212&rep=rep1&type=pdf. [Google Scholar]
- Burgess N, & Hitch GJ (2006). A revised model of short-term memory and long-term learning of verbal sequences. Journal of Mee, 55(4), 627–652. 10.1016/jjml.2006.08.005. [DOI] [Google Scholar]
- Burgette LF, & Reiter JP (2010). Multiple imputation for missing data via sequential regression trees. American Journal of Epidemiology, 172(9), 1070–1076. 10.1093/aje/kwq260. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Miceli G, Villa G, & Romani C (1987). The role of the graphemic buffer in spelling: Evidence from a case of acquired dysgraphia. Cognition, 26(1), 59–85. [DOI] [PubMed] [Google Scholar]
- Chew T, Ho K-A, & Loo CK (2015). Inter- and intra-individual variability in response to transcranial direct current stimulation (tDCS) at varying current intensities. Brain Stimulation, 8(6), 1130–1137. 10.1016/j.brs.2015.07.031. [DOI] [PubMed] [Google Scholar]
- Coltheart M (1981). The MRC psycholinguistic database. The Quarterly Journal of Experimental Psychology, 33(4), 497–505. [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, & Ziegler J (2001). DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review, 108(1), 204. [DOI] [PubMed] [Google Scholar]
- Corsi PM (1972). Human memory and the medial region of the brain. Dissertation Abstracts International, 34, 819B. [Google Scholar]
- Cotelli M, Manenti R, Paternicò D, Cosseddu M, Brambilla M, Petesi M, et al. (2016). Grey matter density predicts the improvement of naming abilities after tDCS intervention in agrammatic variant of primary progressive aphasia. Brain Topography, 29(5), 738–751. 10.1007/s10548-016-0494-2. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Petesi M, Brambilla M, Cosseddu M, Zanetti O, et al. (2014). Treatment of primary progressive aphasias by transcranial direct current stimulation combined with language training. Journal of Alzheimer’s Disease : JAD, 39(4), 799–808. [DOI] [PubMed] [Google Scholar]
- de Aguiar V, Bastiaanse R, & Miceli G (2016). Improving production of treated and untreated verbs in aphasia: A meta-analysis. Frontiers in Human Neuroscience, 10 10.3389/fnhum.2016.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aguiar V, Paolazzi CL, & Miceli G (2015). tDCS in post-stroke aphasia: the role of stimulation parameters, behavioral treatment and patient characteristics. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 63, 296–316. 10.1016/j.cortex.2014.08.015. [DOI] [PubMed] [Google Scholar]
- de Partz M-P, Seron X, & der Linden MV (1992). Re-education of a surface dysgraphia with a visual imagery strategy. Cognitive Neuropsychology, 9(5), 369–401. 10.1080/02643299208252065. [DOI] [Google Scholar]
- Evans WS, Quimby M, Dickey MW, & Dickerson BC (2016). Relearning and retaining personally-relevant words using computer-based flashcard software in primary progressive aphasia. Frontiers in Human Neuroscience, 10 10.3389/fnhum.2016.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficek BN, Wang Z, Zhao Y, Webster KT, Desmond JE, Hillis AE, & Tsapkini K (2018). The effect of tDCS on functional connectivity in primary progressive aphasia. NeuroImage: Clinical, 19, 703–715. 10.1016/j.nicl.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Baum S, & Rapp B (2012). Underlying cause (s) of letter perseveration errors. Neuropsychologia, 50(2), 305–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floel A, Rosser N, Michka O, Knecht S, & Breitenstein C (2008). Noninvasive brain stimulation improves language learning. Journal of Cognitive Neuroscience, 20(8), 1415–1422. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, & Cohen LG (2006). Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology, 117(4), 845–850. [DOI] [PubMed] [Google Scholar]
- Gershberg FB, & Shimamura AP (1995). Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia, 33(10), 1305–1333. 10.1016/0028-3932(95)00103-A. [DOI] [PubMed] [Google Scholar]
- Gervits F, Ash S, Coslett HB, Rascovsky K, Grossman M, & Hamilton R (2016). Transcranial direct current stimulation for the treatment of primary progressive aphasia: An open-label pilot study. Brain and Language, 162, 35–41. 10.1016/j.bandl.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RA, & Caramazza A (1985). The Johns Hopkins University Dysgraphia Battery. Baltimore, MD: Johns Hopkins University. [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M (2012). The non-fluent/agrammatic variant of primary progressive aphasia. The Lancet Neurology, 11(6), 545–555. 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Hubbard HI, Grasso SM, Mandelli ML, Wilson SM, Sathishkumar MT, et al. (2018). Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia. Brain: a Journal of Neurology, 141(6), 1799–1814. 10.1093/brain/awy101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry ML, Rising K, DeMarco AT, Miller BL, Gorno-Tempini ML, & Beeson PM (2013). Examining the value of lexical retrieval treatment in primary progressive aphasia: Two positive cases. Brain and Language, 127(2), 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia CG, Sage K, Ralph MAL, & Berthier ML (2009). Relearning and retention of verbal labels in a case of semantic dementia. Aphasiology, 23(2), 192–209. [Google Scholar]
- Hester RL, Kinsella GJ, & Ong B (2004). Effect of age on forward and backward span tasks. Journal of the International Neuropsychological Society, 10(4), 475–481. 10.1017/S1355617704104037. [DOI] [PubMed] [Google Scholar]
- Hillis A (2015). Sentence repetition test. In NACC UDS-FTLD Neuropsychological battery instructions (form C1-F) (3.0). Washington: University of Washington. [Google Scholar]
- Holland R, & Crinion J (2012). Can tDCS enhance treatment of aphasia after stroke? Aphasiology, 26(9), 1169–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan RW (1988). The 10–20 electrode system and cerebral location. American Journal of EEG Technology, 28(4), 269–279. [Google Scholar]
- Horvath JC, Carter O, & Forte JD (2014). Transcranial direct current stimulation: Five important issues we aren’t discussing (but probably should be). Frontiers in Systems Neuroscience, 8 10.3389/fnsys.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, & Patterson KE (1992). The Pyramids and Palm Trees Test: a test of semantic access from words and pictures. Thames Valley Test Company. [Google Scholar]
- Hung J, Bauer A, Grossman M, Hamilton RH, Coslett HB, & Reilly J (2017). Semantic feature training in combination with transcranial direct current stimulation (tDCS) for progressive anomia. Frontiers in Human Neuroscience, 11 10.3389/fnhum.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokel R, Cupit J, Rochon E, & Leonard C (2009). Relearning lost vocabulary in nonfluent progressive aphasia with MossTalk Words®. Aphasiology, 23(2), 175–191. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S, & Goodglass H (1983). Boston naming test. Philadelphia: Lea & Febiger. [Google Scholar]
- Knopman DS, Kramer JH, Boeve BF, Caselli RJ, Graff-Radford NR, Mendez MF, et al. (2008). Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain, 131(11), 2957–2968. 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M-F, Polanía R, & Nitsche M (2016). Physiology of transcranial direct and alternating current stimulation. In Transcranial direct current stimulation in Neuropsychiatric disorders (pp. 29–46). Cham: Springer. 10.1007/978-3-319-33967-2_3. [DOI] [Google Scholar]
- Lezak M, Howieson D, & Loring D (2012). Neuropsychological assessment (5th ed.). Oxford, New York: Oxford University Press. [Google Scholar]
- López-Alonso V, Fernández-del-Olmo M, Costantini A, Gonzalez-Henriquez JJ, & Cheeran B (2015). Intra-individual variability in the response to anodal transcranial direct current stimulation. Clinical Neurophysiology, 126(12), 2342–2347. 10.1016/j.clinph.2015.03.022. [DOI] [PubMed] [Google Scholar]
- Love T, & Oster E (2002). On the categorization of aphasic typologies: The SOAP (A test of syntactic complexity). Journal of Psycholinguistic Research, 31(5), 503–529. 10.1023/A:1021208903394. [DOI] [PubMed] [Google Scholar]
- Luzzatti C, Colombo C, Frustaci M, & Vitolo F (2000). Rehabilitation of spelling along the sub-word-level routine. Neuropsychological Rehabilitation, 10(3), 249–278. [Google Scholar]
- Macartney-Filgate MS, & Vriezen ER (1988). Intercorrelation of clinical tests of verbal memory. Archives of Clinical Neuropsychology, 3(2), 121–126. 10.1093/arclin/3.2.121. [DOI] [PubMed] [Google Scholar]
- Macoir J, Leroy M, Routhier S, Auclair-Ouellet N, Houde M, & Jr RL (2015). Improving verb anomia in the semantic variant of primary progressive aphasia: The effectiveness of a semantic-phonological cueing treatment. Neurocase, 21(4), 448–456. 10.1080/13554794.2014.917683. [DOI] [PubMed] [Google Scholar]
- McConathey EM, White NC, Gervits F, Ash S, Coslett HB, Grossman M, et al. (2017). Baseline performance predicts tDCS-mediated improvements in language symptoms in primary progressive aphasia. Frontiers in Human Neuroscience, 11, 347 10.3389/fnhum.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Bauer RM, Grande L, Gilmore R, & Roper S (2001). The role of the frontal lobes in memory: Evidence from unilateral frontal resections for relief of intractable epilepsy. Archives of Clinical Neuropsychology, 16(6), 571–585. 10.1016/S0887-6177(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (2001). Primary progressive aphasia. Annals of Neurology, 49(4), 425–432. [PubMed] [Google Scholar]
- Mesulam MM (2007). Primary progressive aphasia: A 25-year retrospective. Alzheimer Disease and Associated Disorders, 21(4), S8–S11. [DOI] [PubMed] [Google Scholar]
- Mesulam M (2008). Primary progressive aphasia pathology. Annals of Neurology, 63(1), 124–125. [DOI] [PubMed] [Google Scholar]
- Mesulam M (2013). Primary progressive aphasia: A dementia of the language network. Dementia & Neuropsychologia, 7(1), 2–9. 10.1590/S1980-57642013DN70100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, & Weintraub S (2009). Quantitative template for subtyping primary progressive aphasia. Archives of Neurology, 66(12), 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Faria AV, Tippett DC, Hillis AE, & Friedman RB (2017). The relationship between baseline volume in temporal areas and post-treatment naming accuracy in primary progressive aphasia. Aphasiology, 31(9), 1059–1077. 10.1080/02687038.2017.1296557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Getz HR, Brennan DM, Hu TM, & Friedman RB (2015). Telerehabilitation of anomia in primary progressive aphasia. Aphasiology. Retrieved from http://www.tandfonline.com/doi/abs/10.1080/02687038.2015.1081142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli G, Capasso R, & Caramazza A (1994). The interaction of lexical and sublexical processes in reading, writing and repetition. Neuropsychologia, 32(3), 317–333. 10.1016/0028-3932(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Liebetanz D, Paulus W, & Nitsche MA (2010). Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). Journal of Neurophysiology, 103(4), 1735–1740. [DOI] [PubMed] [Google Scholar]
- Monti A, Ferrucci R, Fumagalli M, Mameli F, Cogiamanian F, Ardolino G, et al. (2013). Transcranial direct current stimulation (tDCS) and language. Journal of Neurology, Neurosurgery, and Psychiatry, 84(8), 832–842. 10.1136/jnnp-2012-302825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Sylvester C-YC, Jonides J, & Smith EE (2003). Dissociable neural mechanisms underlying response-based and familiarity-based conflict in working memory. Proceedings of the National Academy of Sciences, 100(19), 11171–11175. 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neophytou K, Wiley R, Rapp B, & Tsapkini K (2019). The use of spelling for variant classification in primary progressive aphasia: Theoretical and practical implications. Neuropsychologia, 133, 1–15. 10.1016/j.neuropsychologia.2019.107157, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhart M, Davis C, Kannan V, Heidler-Gary J, Cloutman L, & Hillis AE (2009). Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology, 23(7–8), 823–834. 10.1080/02687030802661762. [DOI] [Google Scholar]
- Nitsche MA, & Paulus W (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of physiology, 527(3), 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkington JE, & Leiter RG (1949). Parkington’s pathway test. The Psychological Service Center Bulletin, 1, 9–20. [Google Scholar]
- Pedersen PM, Jørgensen HS, Nakayama H, Raaschou HO, & Olsen TS (1995). Aphasia in acute stroke: Incidence, determinants, and recovery. Annals of Neurology, 38(4), 659–666. 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- Picard RR, & Cook RD (1984). Cross-validation of regression models. Journal of the American Statistical Association, 79(387), 575–583. 10.1080/01621459.1984.10478083. [DOI] [Google Scholar]
- Purcell JJ, Turkeltaub PE, Eden GF, & Rapp B (2011). Examining the central and peripheral processes of written word production through meta-analysis. Frontiers in Psychology, 2, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM, Henry ML, Leyden A, Kim E, Rising K, et al. (2009). Phonological dyslexia and dysgraphia: Cognitive mechanisms and neural substrates. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 45(5), 575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, & Fischer-Baum S (2015). Uncovering the cognitive architecture of spelling In The handbook of Adult language disorders (2nd ed., pp. 59–86). New York: Psychology Press. [Google Scholar]
- Rapp B, & Glucroft B (2009). The benefits and protective effects of behavioural treatment for dysgraphia in a case of primary progressive aphasia. Aphasiology, 23(2), 236–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero C, Kniefel H, Service E, Thiel A, Probst S, & Chertkow H (2017). Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimer’s & Dementia: Translational Research & Clinical Interuentions, 3(2), 247–253. 10.1016/j.trci.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi SA, Patterson K, Arnold RJ, Watson PC, & Nestor PJ (2012). Primary progressive aphasia. Neurology, 78(21), 1670–1677. 10.1212/WNL.0b013e3182574f79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M (1996). Rey auditory verbal learning test: A handbook. Los Angeles, CA: Western Psychological Services; Retrieved from https://www.wpspublish.com/store/p/2933/ravlt-rey-auditory-verbal-learning-test. [Google Scholar]
- Sepelyak K, Crinion J, Molitoris J, Epstein-Peterson Z, Bann M, Davis C, et al. (2011). Patterns of breakdown in spelling in primary progressive aphasia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 47(3), 342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah-Basak PP, Norise C, Garcia G, Torres J, Faseyitan O, & Hamilton RH (2015). Individualized treatment with transcranial direct current stimulation in patients with chronic non-fluent aphasia due to stroke. Frontiers in Human Neuroscience, 9 10.3389/fnhum.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AD, Bartlett JW, Carpenter J, Nicholas O, & Hemingway H (2014). Comparison of random forest and parametric imputation models for imputing missing data using MICE: A CALIBER study. American Journal of Epidemiology, 179(6), 764–774. 10.1093/aje/kwt312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H, Hurley RS, Rogalski E, & Mesulam M (2012). Anatomic, clinical, and neuropsychological correlates of spelling errors in primary progressive aphasia. Neuropsychologia, 50(8), 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M (1974). Cross-validatory choice and assessment of statistical predictions. Journal of the Royal Statistical Society. Series B (Methodological), 36(2), 111–147. [Google Scholar]
- Thompson-Schill SL, D’Esposito M, & Kan IP (1999). Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron, 23(3), 513–522. 10.1016/S0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Tippett DC, Hillis AE, & Tsapkini K (2015). Treatment of primary progressive aphasia. Current Treatment Options in Neurology, 17(8), 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis C, Gomez Y, Davis C, & Hillis AE (2014). Augmentation of spelling therapy with transcranial direct current stimulation in primary progressive aphasia: Preliminary results and challenges. Aphasiology, 28(8–9), 1112–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, & Hillis AE (2013a). Spelling intervention in post-stroke aphasia and primary progressive aphasia. Behavioural Neurology, 26(1–2), 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K, & Hillis A (2013b). Cognitive neuroscience of Written Language: Neural substrates of reading and writing In Ochsner KN, & Kosslyn Stephen (Eds.), The Oxford Handbook of Cognitive Neuroscience, 1 Oxford: Oxford University Press; 10.1093/oxfordhb/9780199988693.013.0024. [DOI] [Google Scholar]
- Tsapkini K, Webster KT, Ficek BN, Desmond JE, Onyike CU, Rapp B, et al. (2018). Electrical brain stimulation in different variants of primary progressive aphasia: A randomized clinical trial. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 5(4), 461–472. 10.1016/j.trci.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1981). Wechsler Adult Intelligence Scale-Revised. San Antonio: Psychological Corporation. [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, & Thompson CK (2009). The northwestern anagram test: Measuring sentence production in primary progressive aphasia. American Journal of Alzheimer’s Disease and Other Dementias, 24(5), 408–416. 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JN, & Lovatt P (2003). Phonological memory and rule learning. Language Learning, 53(1), 67–121. [Google Scholar]
- Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. Nitsche MA (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology, 127(2), 1031–1048. 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw A, & Wiener M (2015). RandomForest: Breiman and Cutler’s random forests for classification and regression (Version 46–12). Retrieved from https://cran.r-project.org/web/packages/randomForest/randomForest.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.