Abstract
Primary progressive apraxia of speech (PPAOS) is a term used to describe a neurodegenerative condition in which apraxia of speech (AOS; a planning and/or programming deficit) occurs in the absence of aphasia (a language deficit). PPAOS is strongly associated with 4-repeat tau pathology. Elevated flortaucipir ([18F]AV-1451; FTP) uptake has been observed cross-sectionally in patients with PPAOS and those with aphasia. Here, we evaluated longitudinal changes in previously-identified regions of uptake and their relationship with clinical presentation. Thirteen patients who were diagnosed with PPAOS (5 female) at presentation underwent FTP PET imaging at two visits (mean 1 year interval). Median age was 72, with a median of 4 years disease duration at initial testing. Beta-amyloid status was assessed with Pittsburgh Compound B (PiB), where a global PiB ratio>1.48 was deemed amyloid positive (n=4). FTP uptake was assessed as cortical to cerebellar crus ratios (SUVr) in cortical regions of interest. A single hierarchical linear model (HLM) compared PPAOS patients to 52 cognitively unimpaired controls of similar age and sex. Annualized SUVr change was the outcome, predicted by region, clinical status, and age. Person-specific effects accounted for intra-patient correlations and contralateral regions were included as repeated measures. Changes in clinical measures were assessed using Wilcoxon signed-rank tests; statistically significant changes in the Montreal Cognitive Assessment, MDS-UPDRS, motor section, and PSP Rating Scale were noted between visits. Changes in FTP SUVr were greater for patients than controls. The strongest changes in PPAOS patients were in the precentral gyrus, pallidum, and mid and superior frontal gyri, per the HLM. Qualitatively, larger changes were seen in patients who had developed aphasia by the time of their baseline scan (n=5). While the biological mechanisms of FTP signal in non-AD tauopathies are unknown, this study demonstrates the utility of FTP in tracking disease progression in 4R tauopathies.
Keywords: tauopathy, PET, apraxia of speech, aphasia, longitudinal
1.0. Introduction
Primary progressive apraxia of speech (PPAOS) is a term used to describe a neurodegenerative condition in which apraxia of speech (AOS), a deficit of speech planning and/or programming, occurs in the absence of aphasia, a language deficit. PPAOS is strongly associated with the development of corticobasal syndrome and progressive supranuclear palsy (PSP) as the disease progresses, and underlying 4-repeat tau pathology (Deramecourt et al., 2010; Gorno-Tempini, Murray, Rankin, Weiner, & Miller, 2004; Josephs et al., 2006; Josephs, Duffy, Strand, Machulda, Senjem, Gunter, et al., 2014; Kertesz, McMonagle, Blair, Davidson, & Munoz, 2005; Mochizuki et al., 2003; Tetzloff et al., 2018). Given the strong association with 4R tau, this clinical presentation provides a means for assessing the utility of longitudinal in vivo tau imaging, with a ligand such as flortaucipir ([18F]AV-1451; FTP), in non-Alzheimer’s disease (AD) tauopathies. We previously described cross-sectional patterns of increased FTP uptake in PPAOS, including some who had concomitant, less-prominent aphasia, compared to healthy controls (Utianski, Whitwell, Schwarz, Senjem, et al., 2018). We noted increased uptake in the superior premotor and precentral cortices in patients with PPAOS and additional uptake in Broca’s area in those who had developed aphasia. It is unknown whether FTP tracks disease progression in patients with PPAOS.
Recently, serial assessments of FTP uptake in the normal aging population and in patients with mild cognitive impairment and AD have been completed (Harrison et al., 2018; Jack et al., 2018; Pontecorvo et al., 2019). These studies have demonstrated observable rates of changes across clinical presentations (e.g. cognitively unimpaired to clinically symptomatic) (Jack et al., 2018; Pontecorvo et al., 2019). Cross-sectional differences in tau PET have also been related to hypometabolism on FDG-PET and neurodegeneration on MRI (Das et al., 2018; Sintini et al., 2018; Whitwell, Graff-Radford, et al., 2018). Importantly, findings on FDG-PET and MRI may relate to the presenting symptomology but not the underlying molecular pathology, whereas tau PET imaging may offer a more specific insight into the underlying molecular pathophysiology. Additionally, one study supported the possibility that measurable increases in tau PET signal occur prior to neurodegeneration (Harrison et al., 2018). Of course, there is contradictory evidence on the biological significance of FTP uptake among neurodegenerative disorders (Lowe et al., 2016; Marquie et al., 2017; Marquie et al., 2015; Sander et al., 2016). Overall, these studies demonstrate the viability of longitudinal in vivo assessment of tau accumulation and support its potential as a biological marker to track disease progression.
Currently, the way in which FTP uptake changes longitudinally in patients who present with PPAOS is unknown. Understanding imaging changes over time, and the concordance with clinical progression, is critical before considering tau PET imaging as a biomarker in this patient population and, more broadly, in patients with suspected non-AD tauopathies. Given the lack of strong structural signatures of PPAOS, we were most interested in utilizing clinical characteristics as a means of evaluating tau PET changes. Therefore, the goal of the current study was to assess changes over time in previously-identified cortical regions of FTP uptake and their relationship with clinical presentation, along with the co-occurrence of beta-amyloid deposition, which has also been observed in this population (Josephs et al., 2013; Josephs, Duffy, Strand, Machulda, Senjem, Lowe, et al., 2014; Josephs et al., 2012).
2.0. Materials and methods
2.1. Participants
Between December 2016 and December 2018, thirteen patients who were diagnosed with PPAOS (5 female) at initial presentation underwent FTP PET imaging at two visits approximately one year apart. Additional demographic information is reported in Table 1. Five of the patients had developed a less prominent, mild-moderate aphasia at the time of baseline scan and an additional two patients had unequivocal aphasia by the time of the second scan. As previously reported (Utianski, Whitwell, Schwarz, Senjem, et al., 2018), a diagnosis of aphasia was made based on results from several language tests that assessed naming, grammar, and comprehension. This was a convenience sample and no data were excluded; inclusion/exclusion criteria were established prior to data analysis, and we report all manipulations, and all measures utilized in the study.
Table 1.
Clinical information. Data are presented as first visit (second visit) scores on each clinical instrument. Note: Bolded PiB values deemed amyloid positive; * closest PiB PET performed 2 years after baseline assessment; ** closest PiB PET performed 2 years prior to baseline assessment; MoCA = Montreal Cognitive Assessment; FAB = Frontal Assessment Battery; WAB AQ = Western Aphasia Battery, Revised Aphasia Quotient; BNT = Boston Naming Test, short form; NAT = Northwestern Anagram Test; ASRS-3 = AOS Rating Scale, version 3; MDS-UPDRS 3 = The Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale motor subsection; PSIS = Progressive Supranuclear Palsy (PSP) Saccadic Impairment scale1; PSPRS = PSP Rating Scale; NA = Not administered. Maximum scores noted in column header.
| Sex | Global PiB |
MoCA (/30) |
FAB (/18) |
WAB AQ (/100) |
BNT (/15) |
NAT (/10) |
ASRS - 3 (/52) |
Aphasia Severity (/4) |
Apraxia Severity (/4) |
MDS- UPDRS 3 (/120) |
PSIS (/5) |
PSPRS (/100) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 1.272 | 28 (17) | 16 (12) | 81 (75) | NA (13) | 8 (6) | 38 (35) | 1.5 (2.0) | 4.0 (4.0) | 16(32) | 2(1) | 13(32) |
| 2 | M | 1.327 | 29 (28) | 17 (15) | 98 (92) | 15 (14) | 10 (10) | 9 (12) | 0.0 (0.5) | 1.0 (1.0) | 18(33) | 1(1) | 15(22) |
| 3 | F | 1.494* | 30 (27) | 16 (15) | 97 (NA) | 15 (15) | 7 (NA) | 29 (40) | 0.5 (NA) | 3.0 (4.0) | 13(45) | 1(2) | 9(44) |
| 4 | M | 1.239 | 29 (30) | NA (17) | 100 (100) | 15 (15) | 10 (10) | 17 (21) | 0.0 (0.0) | 1.5 (2.0) | NA(22) | 0(0) | NA(12) |
| 5 | M | 1.407** | 28 (24) | 15 (NA) | 98 (99) | 15 (15) | 10 (10) | 24 (27) | 0.0 (0.5) | 2.5 (3.0) | 8(NA) | 1(NA) | 10(NA) |
| 6 | M | 1.654 | 27 (29) | 14 (15) | 98 (94) | 15 (15) | 10 (10) | 22 (15) | 0.0 (1.0) | 3.0 (3.0) | 45(47) | 2(2) | 32(36) |
| 7 | F | 1.476 | 21 (19) | 15 (15) | 97 (83) | 11 (11) | 7 (4) | 26 (35) | 1.0 (1.5) | 2.0 (3.5) | 10(36) | 1(1) | 20(35) |
| 8 | M | 1.437 | 25 (8) | 15 (7) | 85 (NA) | 15 (NA) | 4 (0) | 28 (27) | 1.0 (2.5) | 3.5 (4.0) | 25(48) | 0(0) | 28(46) |
| 9 | M | 1.216 | 28 (25) | 14 (14) | 99 (99) | 14 (14) | 10 (10) | NA (23) | 0.5 (0.0) | 2.0 (2.5) | 17(30) | 2(3) | 17(29) |
| 10 | F | 1.944 | 26 (23) | 17 (15) | 95 (90) | NA (14) | 10 (9) | 29 (30) | 0.0 (1.0) | 3.0 (3.5) | 16(43) | 0(2) | 21(40) |
| 11 | F | 1.298 | 30 (26) | 17 (17) | 97 (98) | NA (13) | 8 (9) | 20 (39) | 1.0 (0.0) | 2.0 (4.0) | 18(35) | 0(1) | 12(27) |
| 12 | M | 2.999 | 26 (17) | 16 (17) | 92 (93) | NA (13) | 8 (4) | 16 (13) | 1.0 (2.0) | 2.0 (1.0) | 10(11) | 0(0) | 4(7) |
| 13 | F | 1.399 | 29 (25) | 13 (13) | 98 (NA) | 14 (15) | 8 (NA) | 18 (19) | 0.0 (NA) | 1.5 (1.5) | 23(32) | 2(3) | 13(33) |
PSIS score of 1 = mild slowing of vertical saccades; 2 = moderate slowing of vertical saccades; 3 = vertical supranuclear gaze palsy
A cohort of 52 healthy, cognitively unimpaired, amyloid negative controls (31 males) was selected from the Mayo Clinic Study of Aging (MCSA) (Petersen et al., 2010; Roberts et al., 2008), with a similar age and sex distribution to the PPAOS cohort. Median age for the controls was 66.3 years at first visit. Eight patients and 4 controls in the current study were previously included in the cross-sectional study (Utianski, Whitwell, Schwarz, Senjem, et al., 2018). The study was approved by the Mayo Institutional Review Board and all participants consented to research.
2.2. Clinical and Neuroimaging Assessments
Methods are identical to those described in (Utianski, Whitwell, Schwarz, Senjem, et al., 2018), with an additional visit that occurred, on average, one year following the initial visit [(median .98 years (interquartile range: .95-1.09)]. Briefly, patients had neurological, speech and language evaluations at each visit. The Montreal Cognitive Assessment (MoCA), a test of general cognition (Nasreddine et al., 2005), and the Frontal Assessment Battery (FAB) (Dubois, Slachevsky, Litvan, & Pillon, 2000), a test of frontal lobe function, were completed. To assess motor functioning and eye movement abnormalities, the Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale motor subsection (MDS-UPDRS 3), PSP Saccadic Impairment scale (PSIS), and PSP Rating Scale (PSPRS) were completed. No patients met criteria for probable PSP at baseline (Litvan et al., 1996); two patients met criteria for probable PSP at the second visit.
The Western Aphasia Battery- Revised Aphasia Quotient (WAB AQ) (Kertesz, 2007), as a composite measure of global language ability, the Boston Naming Test, short form (BNT) (Lansing, Ivnik, Cullum, & Randolph, 1999), a measure of confrontation naming ability, and the Northwestern Anagram Test (NAT) (Weintraub et al., 2009), a non-speech sentence production task, were also administered. The Apraxia of Speech Rating Scale- v. 3 (ASRS-3) (Strand, Duffy, Clark, & Josephs, 2014; Utianski, Duffy, Clark, Strand, Botha, et al., 2018), an index of the quality and severity of AOS, was scored. Aphasia and AOS severity, on a scale of 0–4 (1 = mild; 4 = severe), were also rated.
Patients and controls underwent a 3.0-Tesla volumetric head magnetic resonance imaging scan and FTP PET scan at each visit. Patients had at least one amyloid-PET scan using Pittsburgh Compound B (PiB) within two years of their baseline FTP PET scan. A global PiB ratio > 1.48, calculated with an updated pipeline in SPM12, was deemed beta-amyloid positive (Jack et al., 2017; Knopman et al., 2019); global PiB ratio is also reported as a continuous variable in Table 1. MRI segmentations were performed using SPM12 (Ashburner & Friston, 2005) with MCALT priors and settings (Schwarz et al., 2017) (https://www.nitrc.org/projects/mcalt/). These were used to create two-compartment partial volume-corrected (Meltzer, Leal, Mayberg, Wagner, & Frost, 1990) FTP PET standard uptake value ratio (SUVr) images using the cerebellar crus gray matter as the reference region. Non partial volume-corrected SUVrs were also calculated.
2.3. Statistical analysis
A single Bayesian hierarchical linear model (HLM) was used to determine whether longitudinal change in FTP uptake in PPAOS differed from longitudinal change in controls. Hierarchical models are well suited to answer this question by employing shrinkage to stabilize estimates while borrowing statistical strength across regions and reducing data artifacts (Gelman & Hill, 2006; Gelman et al., 2013; Greenland, 2000). Here, annualized change in SUVr was predicted across 13 regions of interest (ROIs), adjusting for age at baseline scan. ROIs were selected based on those that demonstrated maximal sensitivity and specificity when comparing these patients (PPAOSall) with controls at their baseline visit in both the statistical and qualitative analyses (e.g. the dentate nucleus of the cerebellum) (Utianski, Whitwell, Schwarz, Senjem, et al., 2018). This parsimonious model utilized measurements from both hemispheres as repeated measures of overall regional change in each individual. Subject specific intercepts were included to account for overall shifts higher or lower in all regions within a subject.
Algebraically, the HLM can be written as where yrdih = β1rd + β2d * Agei + γi + erdih where yrdih denotes the annualized regional change in SUVr in region r, disease group d, individual i, and hemisphere h; β1rd denotes the region and disease specific annualized change; β2d is the age effect in each disease group; γi is the person specific effect; and erdih is the error term for each observation. Of particular interest was whether, for a given r, β1d differs between PPAOS and controls.
The HLM was fit using the software R (Team, 2017) version 3.4.2 and the rjags package (M Plummer, Stukalov, & Denwood, 2016) version 4-6 running JAGS (Martyn Plummer, 2003) version 4.3. Analysis code is included in Supplementary Materials. Markov Chain Monte Carlo simulation was used to estimate the posterior distribution of each parameter of interest by estimating distributions for families of parameters and randomly sampling parameters of interest from these parent distributions. Regional change parameters (β1r) for a given disease group d were assumed to come from a single distribution. The posterior sample was based on eight parallel chains, each of length 100,000, thinned to every tenth value for computational efficiency, with a Gelman-Rubin scale reduction factor of 1.01, indicating no lack of convergence across the posterior chains. Results are reported by summarizing quantiles of the aggregate posterior sample. This is accomplished by performing any calculations in each posterior sample before summarizing the posterior sample to obtain effect estimates and posterior probabilities in each region. All analyses were repeated with no-PVC measures.
Wilcoxon signed-rank tests were used to assess the annualized change in clinical measures in the PPAOS group, with a false discovery rate correction for multiple comparisons.
No part of the study procedures or analyses was pre-registered in a time-stamped, institutional registry prior to the research being conducted.
The conditions of our ethics approval do not permit public archiving of anonymized study data. Readers seeking access to the data should contact the corresponding author. Data will be shared provided certain requisitions for maintaining patient privacy are met, including proper data encryption and restricted data access.
3.0. Results
3.1. Clinical changes
Median age was 72, with a median of 4 years disease duration at initial testing. After correction for multiple comparisons, statistically significant changes in the MoCA, MDS-UPDRS, motor section, and PSPRS were noted between visits. While not statistically significant, there was consistent worsening in AOS (8/13 patients) and aphasia (8/13 patients) severity between visits. Changes in all clinical measures of interest are reported in Table 2.
Table 2.
Raw change [median (Q1, Q3)] in clinical measures in 13 PPAOS cases. We used Wilcoxon signed-rank tests to assess the annualized change in clinical measures in the PPAOS group using a false discovery rate correction for multiple comparisons. Associated p-values, and uncorrected p-values, are reported. Statistical significance was assessed at p < .05.
| First visit (N=13) | Second visit (N=13) | Difference (N=13) | Uncorrected p-value |
FDR corrected p-value |
|
|---|---|---|---|---|---|
| MoCA | 28.0 (26.0, 29.0) | 25.0 (19.0, 27.0) | −3.0 (−4.0, −2.0) | 0.005 | 0.018 |
| FAB | 15.5 (14.8, 16.2) | 15.0 (13.8, 15.5) | 0.0 (−2.0, 0.0) | 0.105 | 0.144 |
| WAB AQ | 97.0 (95.0, 98.0) | 93.5 (90.5, 98.8) | −2.0 (−5.8, 0.8) | 0.105 | 0.144 |
| BNT | 15.0 (14.0, 15.0) | 14.0 (13.0, 15.0) | 0.0 (0.0, 0.0) | 1.000 | 1.000 |
| NAT | 8.0 (8.0, 10.0) | 9.0 (5.0, 10.0) | 0.0 (−2.5, 0.0) | 0.073 | 0.144 |
| ASRS - 3 | 23.0 (18.0, 28.0) | 27.0 (19.0, 35.0) | 1.0 (−1.0, 4.0) | 0.193 | 0.212 |
| Aphasia severity | 0.5 (0.0, 1.0) | 0.8 (0.4, 1.6) | 0.5 (0.0, 1.0) | 0.086 | 0.144 |
| Apraxia severity | 2.0 (2.0, 3.0) | 3.0 (2.0, 4.0) | 0.5 (0.0, 0.5) | 0.061 | 0.144 |
| MDS-UPDRS 3 | 16.5 (12.2, 19.2) | 34.0 (31.5, 43.5) | 16.0 (11.0, 24.5) | < 0.001 | 0.005 |
| PSIS | 1.0 (0.0, 2.0) | 1.0 (0.5, 2.0) | 0.5 (0.0, 1.0) | 0.120 | 0.146 |
| PSPRS | 14.0 (11.5, 20.2) | 32.5 (25.8, 37.0) | 15.0 (9.5, 19.0) | 0.004 | 0.018 |
3.2. Hierarchical Modeling Results
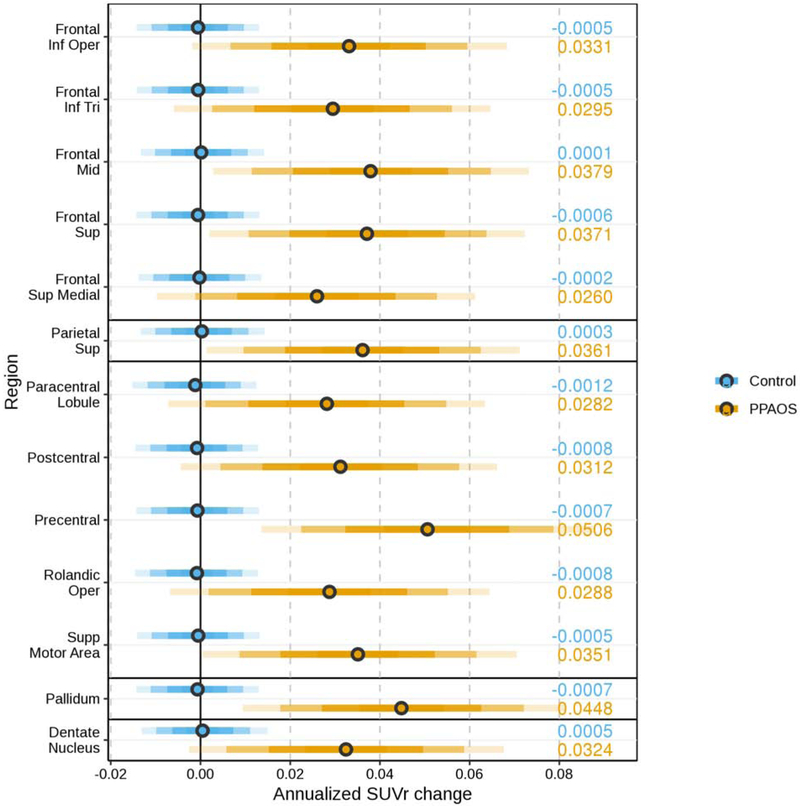
The HLM utilized the ROIs that demonstrated maximal sensitivity and specificity when comparing these patients with controls at their baseline visit (Utianski, Whitwell, Schwarz, Senjem, et al., 2018). The posterior estimated difference between patients and controls from a single HLM predicting SUVr change by region and disease, including age and per-person adjustments are shown in Figure 1. Results derived from measurements without PVC are included in Supplementary Figure 1. Results showed weak evidence in the parietal and frontal superior medial regions, moderate evidence in most other ROIs, and strong evidence in the precentral gyrus, pallidum, and mid and superior frontal gyri that the annualized increase in SUVr was larger in patients with PPAOS than in controls.
Figure 1.
The posterior estimate of annualized change in SUVr in patients (orange) and controls (blue) estimated using a single hierarchical linear model predicting SUVr change by region and disease, including age and per-person adjustments. The bars, moving out from the median (circle) in each row, cover 50%, 80%, 95%, and 99% of the posterior samples. The median estimate is displayed on the right side of the plot.
3.3. Flortaucipir Changes
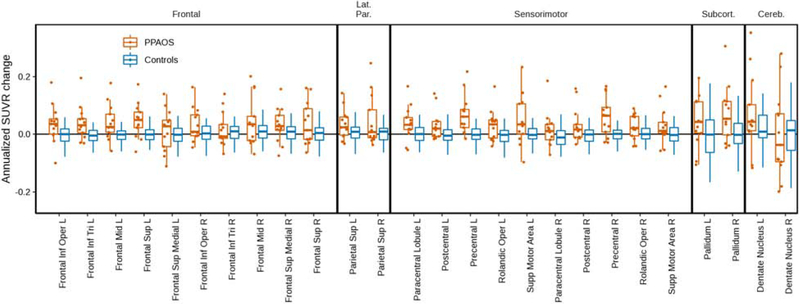
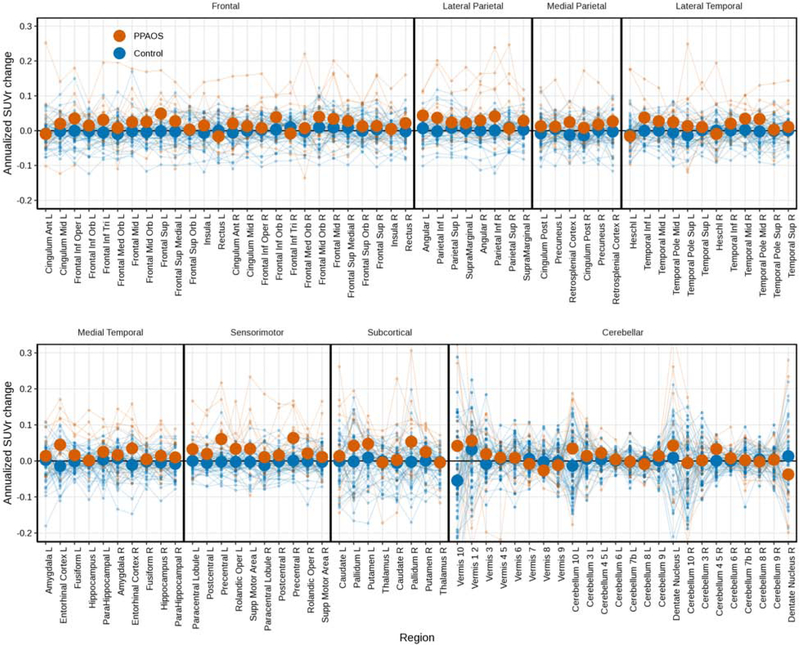
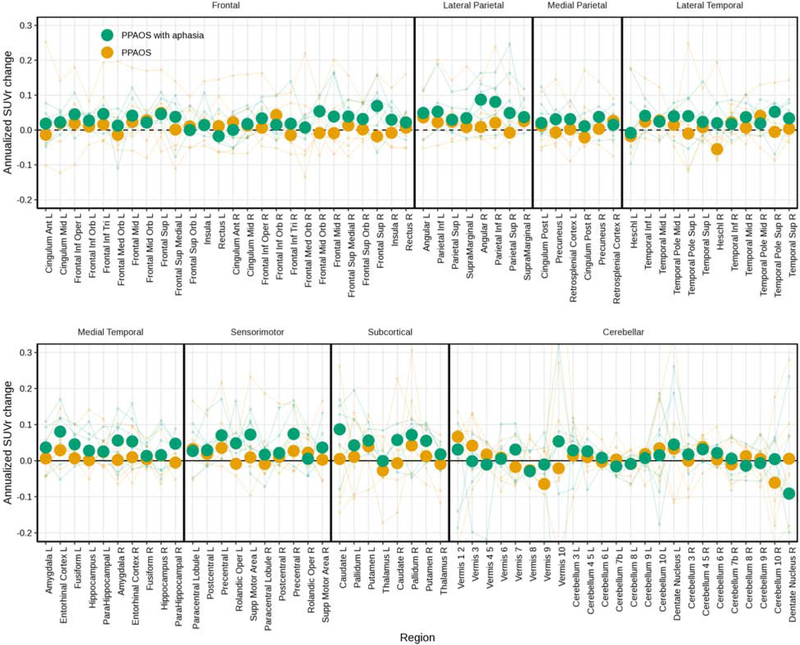
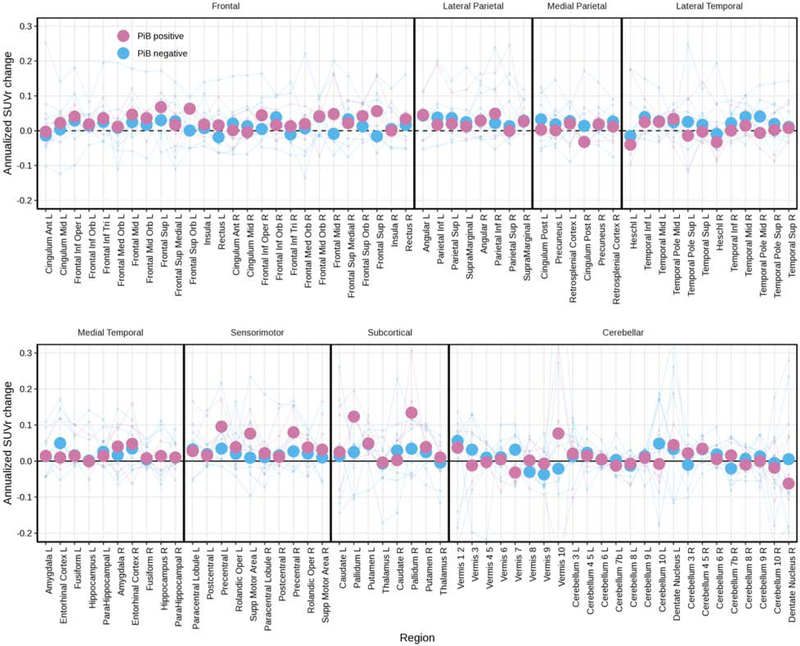
The raw data used in the HLM are shown in box plots of annualized change in FTP PET SUVrs for patients and controls for ROIs (Figure 2). Changes in SUVr were greater for patients than controls (visualized further in Figure 3), per the HLM above. Larger changes were seen in the patients who had aphasia than those without (Figure 4). Changes in FTP uptake were noted bilaterally, with greater changes noted in the left hemisphere. Qualitatively, greater uptake was observed in the frontal and parietal regions in patients with aphasia, but the small sample size precluded formal statistical analysis. Also qualitatively, it does not appear that global amyloid influences the degree of clinical change over time (see Figure 5). Results derived from measurements without PVC are included in Supplementary Figures 2-5.
Figure 2.
Box plots of individual change in SUVrs (annualized) for patients and controls for ROIs utilized in the hierarchical linear model.
Figure 3.
Spaghetti plot of individual annualized change in FTP PET SUVrs for patients and controls for all available ROIs, with median values overlaid as heavy dots in each group.
Figure 4.
Spaghetti plot of individual annualized change in SUVrs for patients, noting the presence of aphasia, for all available ROIs, with median values overlaid as heavy dots in each group.
Figure 5.
Spaghetti plot of individual annualized change in FTP PET SUVrs for patients, noting PiB (amyloid) status, for all available ROIs, with median values overlaid as heavy dots in each group.
4.0. Discussion
This study demonstrates the potential utility of FTP in tracking disease in presumed 4R tauopathies. While the biological mechanisms of FTP signal in non-AD tauopathies are unknown, this study demonstrates clinically meaningful signal changes in patients who present with PPAOS and likely have an underlying 4R tauopathy. The longitudinal changes appear less prominent in the medial temporal lobe than in parietal and frontal lobes, demonstrating topographical changes fitting of the clinical presentation. Importantly, it is possible there is greater FTP uptake associated with the development and worsening of aphasia in patients who initially presented with PPAOS. The simultaneous presence of aphasia and AOS is common in the nonfluent/agrammatic variant of Primary Progressive Aphasia (PPA) (Gorno-Tempini et al., 2011) and develops later in those who present with PPAOS (Utianski, Duffy, Clark, Strand, Boland, et al., 2018; Whitwell et al., 2017). We do not yet understand if the order in which aphasia or AOS develops or worsens relates to overall clinical progression or underlying pathology. This and other studies (Josephs et al., 2013; Utianski, Whitwell, Schwarz, Duffy, et al., 2018) are building evidence to better understand the underlying and evolving disease processes that manifest as combinations of language and motor speech deficits.
In the current sample of patients, there were statistically significant changes in clinical measures of motor (MDS-UPDRS 3 and PSPRS) and cognitive (MoCA) functioning between the two visits. There was also worsening noted in AOS and aphasia severity scores between visits in the majority of patients that failed to reach statistical significance. This is important in the context of relating biological and behavioral outcome measures. While FTP uptake may be associated with the development and worsening of aphasia, discussed further below, there is also a possible effect of the overall disease progression, as reflected in the changes in motor and cognitive measures. In fact, it may be the case that the global deterioration accounts for the focal language and motor speech changes. Further investigation in a larger sample is necessary to fully understand these findings.
Overall, changes in SUVr were greater for patients than controls between the two visits. There was evidence for individual variability, but most patients demonstrated greater FTP uptake at the second visit than at first visit in most ROIs (see Figure 2). While the HLM statistically assessed changes between only a subset of ROIs, visualizing all available regions demonstrates increased FTP uptake across the cortex (see Figure 3), not just in the areas where FTP uptake was noted at baseline; this is particularly true for the patients with aphasia at the first scan. The overall, general increase in tau PET uptake is similar to findings seen when evaluating meta-ROIs, described in a recent study of normal aging and Alzheimer’s disease dementia (Jack et al., 2018). Additionally, even though some of the FTP uptake, and possibly tau accumulation, is accounted for by age (Harrison et al., 2018), the comparison with similarly aged cognitively unimpaired controls in the current study argues against age being the only driver of uptake.
The HLM results showed weak evidence in the parietal and superior medial frontal regions, moderate evidence in most other ROIs, and strong evidence in the pallidum, dentate nucleus of the cerebellum, and precentral regions for larger annualized increase in SUVr in patients with PPAOS than in controls (see Figure 1). These results are compatible with those of a recent longitudinal study of FTP uptake in PSP (Whitwell, Tosakulwong, et al., 2019), a closely related clinical syndrome that is also associated with underlying 4R tau. Specifically, there was also strong evidence for increased uptake over time in the pallidum and in the dentate nucleus of the cerebellum in patients with PSP. The current study provides additional converging evidence of overlapping pathophysiology between these clinical presentations (Whitwell et al., 2013).
Larger changes were seen in the patients with aphasia at first visit than those without (see Figure 4). This provides support for a possible relationship between aphasia and trajectory of SUVr. Not surprisingly, greater uptake is observed in the frontal and parietal regions in patients with aphasia, but both hemispheres appear involved. Again, we do not yet understand if the order in which aphasia or AOS develops or worsens is indicative of clinical progression or distribution of the underlying pathology. Comparison of these patients with those who present first with the nonfluent/ agrammatic variant of PPA and then develop apraxia of speech will be critical in developing our understanding of disease trajectory. Even more broadly, this will help inform whether different diagnostic categories are necessary or meaningful for prognostication.
Overall, we cannot determine whether the presence of beta-amyloid influences changes in speech, language, or neurologic measures in patients with PPAOS. While there are not enough cases to statistically analyze them separately, it does not appear that global amyloid influences the degree of clinical change over time (Utianski, Duffy, Clark, Martin, et al., 2018) (see Figure 5). This is consistent with another study of amyloid in patients with PSP (Whitwell, Ahlskog, et al., 2018). However, another recent study has suggested that amyloid positive presumed “non-AD” clinical cases have more FTP uptake, depending on the clinical presentation (Ali et al., 2018; Whitwell, Martin, et al., 2019). Whether these findings are due to co-pathology, or FTP binding to neuritic tau, genetic factors (Josephs, Duffy, Strand, Machulda, Senjem, Lowe, et al., 2014), or something else, is unclear. These possible causes need to be further assessed in a larger sample over longer time intervals, ideally with pathological confirmation.
There are many strengths of the current study, including that this is the first ever study to evaluate FTP uptake longitudinally in patients who present with PPAOS. Of course, there are also limitations. Methodology to assess FTP uptake, especially in non-AD tauopathies, is not straightforward. We attempted to overcome this by providing both partial volume corrected and uncorrected measurements. Additionally, many patients demonstrate increased uptake in the cerebellum; this may pose instability of the reference region in this group of patients. Exploration of findings with alternative reference regions is warranted. We acknowledge that in a sample this size the power to detect significant annualized change in clinical measures after correction is modest, but think it is important to report those that are statistically significantly different along with the effect estimates. Further work is necessary to evaluate the relationship between clinical and neuroimaging changes in a larger sample over longer courses of the disease, including additional cognitive measures that may be more sensitive and robust to co-existing language impairment. Examining this relationship among cognitively unimpaired controls is also of interest. Given the relationship between the clinical presentation of PPAOS and the development of PSP and CBS, we will track the evolution of these patients over time and assess the sensitivity and specificity of FTP uptake compared to other imaging biomarkers. Specifically, future studies could incorporate comparisons with other imaging modalities, including FDG-PET and diffusion tensor imaging. Finally, future explorations in larger samples could expand upon our model to additionally investigate relationships with either global or regional amyloid measures as well as whether the discovered effects vary between hemispheres (lateralized vs. bilateral) within region. In particular, it will be interesting to determine whether the hemispheric differences that were seen cross-sectionally (Utianski, Whitwell, Schwarz, Senjem, et al., 2018) are noted longitudinally within the patient population.
5.0. Conclusion
Our study provides evidence that FTP uptake changes over time in patients with PPAOS, in patterns similar to those seen in patients with PSP. There is some evidence that the presence of aphasia may relate to higher uptake and possible tau accumulation. Autoradiography evaluation and autopsy confirmation in larger cohorts will be critical to validate and determine the biological significance of these findings.
Supplementary Material
6.0. Acknowledgments
We extend gratitude to these participants, patients, and their families for their commitment to our research program. We thank AVID Radiopharmaceuticals, Inc., for their support in supplying [18F]AV-1451 precursor, chemistry production advice and oversight, and FDA regulatory cross-filing permission and documentation needed for this work.
Funding
The study was funded by National Institutes of Health grants [R01 DC010367 (Josephs), R21 NS94684 (Josephs), R01 DC12519 (Whitwell), R01 NS89757 (Josephs and Whitwell), U01 AG006786 (Petersen), R01 NS89757 (Whitwell), R01 AG034676 (Rocca), P50 AG016574 (Petersen), R01 AG011378 (Jack), R01 AG041851 (Jack and Knopman)]; a grant from the Department of Radiology, Mayo Clinic; the Gerald and Henrietta Rauenhorst Foundation; Elsie and Marvin Dekelboum Family Foundation; Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic; and the Schuler Foundation.
Footnotes
Declaration of interests: All authors receive research support from the National Institutes of Health. Dr. Jack consults for Lily and serves on an independent data monitoring board for Roche but he receives no personal compensation from any commercial entity; he receives research support from the NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali F, Whitwell JL, Martin PR, Senjem ML, Knopman DS, Jack CR, … Josephs KA (2018). [(18)F] AV-1451 uptake in corticobasal syndrome: the influence of beta-amyloid and clinical presentation. J Neurol, 265(5), 1079–1088. doi: 10.1007/s00415-018-8815-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, & Friston KJ (2005). Unified segmentation. Neuroimage, 26(3), 839–851. [DOI] [PubMed] [Google Scholar]
- Das SR, Xie L, Wisse LEM, Ittyerah R, Tustison NJ, Dickerson BC, … Wolk DA (2018). Longitudinal and cross-sectional structural magnetic resonance imaging correlates of AV-1451 uptake. Neurobiol Aging, 66, 49–58. doi: 10.1016/j.neurobiolaging.2018.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, … Pasquier F (2010). Prediction of pathology in primary progressive language and speech disorders. Neurology, 74(1), 42–49. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, & Pillon B (2000). The FAB: a Frontal Assessment Battery at bedside. Neurology, 55, 1621–1626. [DOI] [PubMed] [Google Scholar]
- Gelman A, & Hill J (2006). Data analysis using regression and multilevel/hierarchical models: Cambridge university press. [Google Scholar]
- Gelman A, Stern HS, Carlin JB, Dunson DB, Vehtari A, & Rubin DB (2013). Bayesian data analysis: Chapman and Hall/CRC. [Google Scholar]
- Gorno-Tempini M, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SE, & Manes F (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M, Murray R, Rankin K, Weiner M, & Miller B (2004). Clinical, cognitive and anatomical evolution from nonfluent progressive aphasia to corticobasal syndrome: a case report. Neurocase, 10, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S (2000). Principles of multilevel modelling. Int J Epidemiol, 29(1), 158–167. [DOI] [PubMed] [Google Scholar]
- Harrison TM, La Joie R, Maass A, Baker SL, Swinnerton K, Fenton L, … Jagust WJ (2018). Longitudinal tau accumulation and atrophy in aging and Alzheimer's disease. Ann Neurol. doi: 10.1002/ana.25406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Schwarz CG, Lowe VJ, Senjem ML, Vemuri P, … Petersen RC (2018). Longitudinal tau PET in ageing and Alzheimer's disease. Brain, 141(5), 1517–1528. doi: 10.1093/brain/awy059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, … Petersen RC (2017). Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimer's & Dementia, 13(3), 205–216. doi: 10.1016/j.jalz.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand E, Whitwell J, Layton K, Parisi J, … Petersen RC (2006). Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain, 129, 1385–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, … Whitwell JL (2014). The evolution of primary progressive apraxia of speech. Brain, 137(10), 2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, … Whitwell JL (2013). Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology, 81(4), 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, … Whitwell JL (2014). APOE ε4 influences β-amyloid deposition in primary progressive aphasia and speech apraxia. Alzheimer's & Dementia, 10(6), 630–636. doi: 10.1016/j.jalz.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, … Whitwell JL (2012). Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain, 135(Pt 5), 1522–1536. doi: 10.1093/brain/aws032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A (2007). Western Aphasia Battery (Revised). San Antonio, TX: PsychCorp. [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, & Munoz DG (2005). The evolution and pathology of frontotemporal dementia. Brain, 128(9), 1996–2005. doi: 10.1093/brain/awh598 [DOI] [PubMed] [Google Scholar]
- Knopman DS, Lundt ES, Therneau TM, Vemuri P, Lowe VJ, Kantarci K, … Jack CR (2019). Entorhinal cortex tau, amyloid-beta, cortical thickness and memory performance in non-demented subjects. Brain, 142(4), 1148–1160. doi: 10.1093/brain/awz025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, & Randolph C (1999). An empirically derived short form of the Boston Naming Test. Archives of Clinical Neuropsychology, 14, 481–487. [PubMed] [Google Scholar]
- Litvan I, Agin Y, Calne D, Campbell G, Dubois B, Duvoisin RC, & al, e. (1996). Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology, 47, 1–9. [DOI] [PubMed] [Google Scholar]
- Lowe V, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, … Murray ME (2016). An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun, 4(1), 58. doi: 10.1186/s40478-016-0315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquie M, Normandin MD, Meltzer AC, Siao Tick Chong M, Andrea NV, Anton-Fernandez A, … Gomez-Isla T (2017). Pathological correlations of [F-18]-AV-1451 imaging in non-alzheimer tauopathies. Ann Neurol, 81(1), 117–128. doi: 10.1002/ana.24844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquie M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, … Gomez-Isla T (2015). Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol, 78(5), 787–800. doi: 10.1002/ana.24517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Leal JP, Mayberg HS, Wagner HN, & Frost JJ (1990). Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. Journal of computer assisted tomography, 14(4), 561–570. [DOI] [PubMed] [Google Scholar]
- Mochizuki A, Ueda Y, Komatsuzaki Y, Tsuchiya K, Arai T, & Shoji S (2003). Progressive supranuclear palsy presenting with primary progressive aphasia—clinicopathological report of an autopsy case. Acta Neuropathol, 105(6), 610–614. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z, Phillips N, Bedirian V, Charbonneau S, Whitehead V, Collin I, … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–669. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, … Petersen RC (2010). The Mayo Clinic Study of Aging: prevalence of mild cognitive impairment is higher in men. Neurology, 75, 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer M (2003). JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. Paper presented at the Proceedings of the 3rd international workshop on distributed statistical computing. [Google Scholar]
- Plummer M, Stukalov A, & Denwood M (2016). rjags: Bayesian graphical models using MCMC (R package version 4-6)[Computer software manual]. In. [Google Scholar]
- Pontecorvo MJ, investigators, f. t. F.-A.-.-A., Devous MD, investigators, f. t. F.-A.-.-A., Kennedy I, investigators, f. t. F.-A.-.-A., … investigators, f. t. F.-A.-.-A. (2019). A multicentre longitudinal study of flortaucipir (18F) in normal ageing, mild cognitive impairment and Alzheimer’s disease dementia. Brain, 142(6), 1723–1735. doi: 10.1093/brain/awz090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, … Rocca WA (2008). The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology, 30(1), 58–69. doi: 10.1159/000115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander K, Lashley T, Gami P, Gendron T, Lythgoe MF, Rohrer JD, … Arstad E (2016). Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer's disease, primary tauopathies, and other dementias. Alzheimers Dement, 12(11), 1116–1124. doi: 10.1016/j.jalz.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Ward CP, Vemuri P, Senjem ML, Wiste HJ, … Jack CR (2017). The Mayo Clinic Adult Lifespan Template (MCALT): Better Quantification across the Lifespan. Paper presented at the Alzheimer's Association International Conference. [Google Scholar]
- Sintini I, Schwarz CG, Martin PR, Graff-Radford J, Machulda MM, Senjem ML, … Lowe VJ (2018). Regional multimodal relationships between tau, hypometabolism, atrophy, and fractional anisotropy in atypical Alzheimer's disease. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand EA, Duffy JR, Clark HM, & Josephs KA (2014). The Apraxia of Speech Rating Scale: A new tool for diagnosis and description of AOS. Journal of Communication Disorders, 51, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC (2017). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. In: ISBN3-900051-07-0 https://www.R-project.org. [Google Scholar]
- Tetzloff KA, Duffy JR, Strand EA, Machulda MM, Boland SM, Utianski RL, … Whitwell JL (2018). Clinical and imaging progression over 10 years in a patient with primary progressive apraxia of speech and autopsy-confirmed corticobasal degeneration. Neurocase, 24(2), 111–120. doi: 10.1080/13554794.2018.1477963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Duffy JR, Clark HM, Martin PR, Senjem ML, Schwarz CG, … Whitwell JL (2018). The Influence of Beta-Amyloid on the Progression of Progressive Apraxia of Speech. Alzheimer's & Dementia: The Journal of the Alzheimer's Association, 14(7), P810–P811. doi: 10.1016/j.jalz.2018.06.1024 [DOI] [Google Scholar]
- Utianski RL, Duffy JR, Clark HM, Strand E, Botha H, Schwarz CG, … Josephs KA (2018). Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain and Language, 184, 54–65. doi: 10.1016/j.bandl.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Duffy JR, Clark HM, Strand EA, Boland SM, Machulda MM, … Josephs KA (2018). Clinical Progression in Four Cases of Primary Progressive Apraxia of Speech. American journal of speech-language pathology, 1–16. doi: 10.1044/2018_AJSLP-17-0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Whitwell JL, Schwarz CG, Duffy JR, Botha H, Clark HM, … Josephs KA (2018). Tau Uptake in Agrammatic Primary Progressive Aphasia with and without Apraxia of Speech. European Journal of Neurology. doi: 10.1111/ene.13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utianski RL, Whitwell JL, Schwarz CG, Senjem ML, Tosakulwong N, Duffy JR, … Josephs KA (2018). Tau-PET imaging with [18F]AV-1451 in Primary Progressive Apraxia of Speech. Cortex, 99, 358–374. doi: 10.1016/j.cortex.2017.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, & Thompson CK (2009). The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen, 24(5), 408–416. doi: 10.1177/1533317509343104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Ahlskog JE, Tosakulwong N, Senjem ML, Spychalla AJ, Petersen RC, … Josephs KA (2018). Pittsburgh Compound B and AV-1451 positron emission tomography assessment of molecular pathologies of Alzheimer's disease in progressive supranuclear palsy. Parkinsonism Relat Disord, 48, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, … Josephs KA (2013). Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. European Journal of Neurology, 20(4), 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Graff-Radford J, Tosakulwong N, Weigand SD, Machulda MM, Senjem ML, … Drubach DA (2018). Imaging correlations of tau, amyloid, metabolism, and atrophy in typical and atypical Alzheimer's disease. Alzheimer's & Dementia, 14(8), 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Martin PR, Duffy JR, Clark HM, Machulda MM, Schwarz CG, … Josephs KA (2019). The influence of beta-amyloid on [(18)F]AV-1451 in semantic variant of primary progressive aphasia. Neurology. doi: 10.1212/wnl.0000000000006913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Tosakulwong N, Schwarz CG, Botha H, Senjem ML, Spychalla AJ, … Josephs KA (2019). MRI Outperforms [18F]AV-1451 PET as a Longitudinal Biomarker in Progressive Supranuclear Palsy. Movement Disorders, 34(1), 105–113. doi:doi: 10.1002/mds.27546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Weigand S, Duffy J, Clark H, Strand E, Machulda M, … Josephs KA (2017). Predicting clinical decline in progressive agrammatic aphasia and apraxia of speech. Neurology, 89, 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.