Abstract
Although the extra cellular matrix (ECM) comprises a major proportion of the CNS parenchyma, new roles for the ECM in regeneration and repair responses to CNS injury have only recently been appreciated. The ECM undergoes extensive remodeling following injury to the developing or mature CNS in disorders that include perinatal hypoxic-ischemic cerebral injury, multiple sclerosis and age-related vascular dementia. Here we focus on recently described mechanisms involving hyaluronan (HA), which negatively impact myelin repair after cerebral white matter injury. Injury induced depolymerization of hyaluronan (HA) – a component of the neural ECM – can inhibit myelin repair through the actions of specific sizes of HA fragments. These bioactive fragments selectively block the maturation of late oligodendrocyte progenitors via an immune tolerance-like pathway that suppresses pro-myelination signaling. We highlight emerging new pathophysiological roles of the neural ECM, particularly of those played by HA fragments (HAf) after injury and discuss strategies to promoter repair and regeneration of chronic myelination failure.
Introduction
The Extracellular Matrix (ECM) occupies 10-20% of brain volume and contributes to its normal physiology (1–4). The ECM is produced intracellularly and secreted to form a dense network of glycans and interacting proteins, occupying the parenchyma of virtually all cells. These proteins and glycans together provide both structural and chemical support to the developing and adult brain. Structurally, the ECM provides support to anchor neural cells and to facilitate cell-cell and cell-matrix contacts that organize cells in distinct regions of the CNS. Chemically, the components of the ECM both individually and collectively act as sources of molecular cues that guide cellular growth, migration, activity, differentiation and survival (5). During CNS development, dynamic changes in the ECM are regulated in a temporal and spatial manner to influence such diverse processes as maintenance of neural stem cells, neuronal migration, axon path-finding, and synaptogenesis (6). By adulthood, the ECM changes composition and continues to influence a wide range of CNS processes including the maintenance of tissue integrity, myelination and the stabilization of synapses to regulate synaptic plasticity (7). Thus, understanding how the ECM evolves in the developing brain, how it changes in the adult brain, and how it functions following CNS injury is an important goal of molecular and cellular neuroscience research.
Proteoglycans are the main component of the CNS ECM and carry specific sets of carbohydrates, collectively referred to as glycosaminoglycans (GAG). Individual proteoglycans differ in the composition of their core protein and the number and type of attached GAG chains, which may be sulfated at various positions and exist in four main forms: heparan sulfate (HSPG) and heparin; chondroitin sulfate (CSPG) and dermatan sulfate; keratan sulfate and hyaluronan (also known as hyaluronic acid). The first three are protein-bound GAGs in their natural form, and all contain sulfate. Hyaluronic acid (HA), on the other hand, is synthesized as a free GAG and lacks sulfate.
Although numerous studies have implicated proteoglycans in the response to CNS injury, including inflammatory and regenerative responses (8–10), there is now growing evidence that HA itself regulates neural progenitor cell proliferation and differentiation (11, 12). Here, we will highlight how HA synthesis and catabolism are regulated during white matter injury to influence glial progenitor cell responses that mediate remyelination failure. In particular, we highlight the emerging role of HA fragments (HAf) as inhibitors of remyelination during neonatal White Matter Injury (WMI) as a model of how dynamic changes in HA can influence a number of different CNS insults.
HA in the neural ECM
The functional properties of the neural ECM depend on the composition of molecules that are localized in the three main compartments: the basement membrane (basal lamina), the perineuronal nets, and the neural interstitial matrix (a term used to define the ECM molecules distributed in the parenchyma). The basement membrane surrounds the entire pial surface of the CNS and functions as a boundary between endothelial cells and CNS parenchymal tissue. The basement membrane is predominantly made up of collagen, laminin-nidogen complexes, fibronectin, dystroglycan and perlecan (3). Perineuronal nets comprise a condensed layer of meshlike matrix composed of proteoglycans, tenascin R and link proteins that surround the cell bodies and proximal dendrites (13). Besides a probable role in preserving neuronal health, these nets are thought to be critical for maintaining synaptic plasticity (13). They are formed in response to neural activity, largely through potassium and calcium conductance and through activation of NMDA-type glutamate receptors and calcium-permeable AMPA-type glutamate receptors (7). Although it is unclear how perineuronal nets change in response to CNS insults, a number of recent studies have implicated changes in the perineuronal net matrix in the altered neural activity associated with drug addiction (14, 15). By contrast, the neural interstitial matrix consists of ECM components in the parenchyma that are not tightly associated with basement membranes or perineuronal nets. The neural interstitial matrix is comprised of a dense network of proteoglycans, HA, tenascin and link proteins, as well as relatively small amounts of fibrous proteins (collagen and elastin) and adhesive glycoproteins (laminin and fibronectin) (16, 17).
HA is a high molecular weight, linear polysaccharide that consists of repeating disaccharide units of glucuronic acid (GIcA) and N-acetyl glucosamine (GIcNAc) linked by alternate β1-3 and β1-4 linkages (18). As a polymer in solution, HA is best described, on the basis of available data, as a stiff random coil (19). It is critical for the assembly and structure of the ECM at both cellular and tissue levels. The large size of HA provides a rigid scaffold for the assembly of the different constituents of the ECM of the neural parenchyma. Although HA lacks a protein core, it retains the ability to interact with extracellular HA-binding proteins (hyaladherins), such as Tumor necrosis factor stimulated gene 6 (TSG6), and transmembrane HA binding receptors including, for example, cluster of differentiation antigen 44 (CD44), hyaluronan mediated motility receptor (RHAMM/HMMR), HA-receptor for endocytosis (HARE), and lymphatic vessel endothelial receptor-1 (LYVE1). Through interactions with extracellular HA-binding proteins, HA likely acquires different conformations (20).
In peripheral tissues, the hydrophilic groups in HA attract water to hydrate tissues, lubricate joints and eyes, and fill space. An HA enriched ECM influences cellular movement and proliferation to facilitate a wide range of cellular functions including cell migration during early development and regeneration and repair of tissues during wound healing (21). HA can be covalently modified by TSG6 activity that initiates the transfer of Heavy Chain (HC) moieties of inter-alpha-inhibitor (IaI) protein. This HA modification plays a central role during ovulation and fertilization and may also influence inflammatory signaling (22, 23). Importantly, some biological functions of HA are dependent on its size. For example, high molecular weight (MW) and low MW HA have opposing effects on epithelial to mesenchymal transition, inflammation and angiogenesis (24, 25).
In the brain, HA is synthesized by multiple cell types, including cells of the microvessels, which consist of vascular endothelial cells and their associated pericytes and astrocytes (12). HA synthesis on the inner side of the plasma membrane takes place through sequential transfer of uridine diphosphate (UDP)-GlcA and UDP-GlcNAc to the reducing end of the growing polysaccharide chain (21). Three mammalian HA synthase genes have been identified: HAS1, HAS2 and HAS3 (26). HAS1 is the least active of the synthases, requiring very high concentrations of UDP-GlcNAc (27, 28). No developmental abnormalities have been observed in Has1−/− mice but studies focusing on the brain have revealed a mild increase in seizure activity (29). HAS2 is the main synthase that is responsible for the majority of HA synthesis during development (30). Has2−/− mouse embryos die at embryonic day (E) 9.5 due to failure to form the HA rich cardiac cushion and to initiate the epithelial to mesenchymal transition (EMT) required to form the valves and septa (31, 32). A nestin-cre driven deletion of Has2 revealed no defects during brain development but, like the Has1−/− animals, did predispose to increased seizure activity (29). HAS3 is the most active of the HA synthases and produces HA of lower molecular weight (33). Studies focused on the brain in Has3−/− mice have revealed lower levels of HA, crowding of neurons and reduced extracellular space in the hippocampus (29). These mice exhibit a significant increase in electrical activity and a strong predisposition to seizure activity (29).
Insights into the role of HA in CNS injury
CNS Injury leads to dynamic changes in HA levels
While the studies discussed above focused on neuronal phenotypes, less is known about the requirement for HA in gliogenesis and glial cell function, especially in the white matter. Injury to the CNS white matter results in reactive gliosis where glial cells undergo several morphologic and metabolic changes that include hypertrophy and hyperplasia (34). CNS lesions often display persistent diffuse reactive astrogliosis that results in the formation of a glial scar. Glial scar formation is a dynamic process that involves both enhanced HA synthesis as well as HA depolymerization induced primarily by hyaluronidases that generate a variety of HAf(s) (see below).
Enhanced HA synthesis may persist for days to weeks after CNS injury. For example, HA levels have been reported to be elevated for weeks following a photothrombotic lesion and as long as 5 months following dorsal rhizotomy (35, 36). HAS2 expression in the CNS is normally low, but rapidly increased within six hours following middle cerebral artery occlusion in adult rats (37). Consistent with these experimental findings, HAS1 and HAS2 levels are elevated in the infarct and peri-infarct following ischemic stroke in patients (38). Levels of HA rise after acute stroke and can predict 3-month functional outcomes, especially in patients with intracerebral hemorrhage (39). Studies examining neurodegenerative diseases that influence white matter have also documented an increase in HA levels in CNS lesions. HA is elevated in demyelinating white matter lesions from mice with experimental autoimmune encephalomyelitis and in patients with multiple sclerosis (MS). Both are autoimmune conditions where myelin-reactive immune cells induce demyelination (40–42). In demyelinating lesions, including those in multiple sclerosis, partial recovery of function is associated with the recruitment of oligodendrocyte progenitor cells (OPCs) that differentiate into oligodendrocytes (OLs) to remyelinate intact axons (43). However, in chronic white matter lesions, OPCs accumulate and fail to differentiate into OLs (44–46). Studies of post-mortem human brain tissue from MS cases found that HA accumulated within white matter lesions with remyelination failure, suggesting that HA could negatively influence OL biology (40). This response is not limited to chronic white matter lesions arising from autoimmune-mediated demyelination. Similarly, in chronic human ischemic white matter lesions from both preterm neonates and adults with vascular dementia, HA accumulation coincides with a build up of OPCs that fail to differentiate to mature myelinating OLs (47–49).
Indeed, in-vitro studies using primary OPC cultures demonstrated that addition of intact high MW HA (>1.8 MDa) blocked OL maturation (40). Digestion of the intact HA by bacterial hyaluronidase released the block on OL maturation allowing the OPCs to mature into OLs both in vitro and in lysolecithin-induced demyelinating lesions, indicating that accumulation of HA could prevent remyelination and repair (40). However, subsequent studies demonstrated that after addition of megadalton forms of HA to cell cultures or demyelinating lesions, other forms of depolymerized HAf were generated by hyaluronidase activity in OPCs or other cells, which blocked OL maturation (41, 50). Importantly, the active HAf(s) that blocked OL maturation were generated using mammalian but not bacterial hyaluronidases (50). Treatment of demyelinating lesions with a broad-spectrum hyaluronidase inhibitor overcame the inhibitory effects of high molecular weight HA on remyelination and promoted functional recovery, which further supported the hypothesis that it is depolymerization products of HA and not intact high molecular weight HA that block OL maturation and remyelination (50).
Higher levels of HA are also observed in patients with Vanishing White Matter Disease, a genetic leukoencephalopathy caused by mutations in the eukaryotic translation initiation factor 2B (51). HA accumulation was more pronounced in the affected frontal white matter but not in the unaffected cerebellar white matter in these patients. In addition to the findings in adult models of stroke, increased HA levels have also been documented in perinatal hypoxic-ischemic cerebral white matter injury. Neonatal white matter injury is accompanied by disturbances in myelination that contribute to life-long neurobehavioral disabilities and cerebral palsy in survivors of premature birth. HA accumulates in the ECM in human preterm chronic cerebral white matter lesions coincident with extensive reactive gliosis (47). In a pre-clinical fetal sheep model of cerebral hypoxic-ischemic injury, HA levels remain persistently elevated for weeks following injury (52).
CNS injury induces HA-mediated neural progenitor cell migration
In addition to playing a role in OL maturation, HA may also play a role in OPC recruitment to demyelinating lesions. The earliest findings supporting a role for HA in neural progenitor cell migration found that HA potentiated the emigration and subsequent migration of neural crest cells from the dorsal neural tube during early embryonic development (53, 54). Following demyelination, OPCs migrate towards damaged axons before differentiating into myelinating OLs (55). Reduction of CD44 expression in an OPC cell line or treatment of these cells with a CD44 neutralizing antibody reduced OPC migration both in-vitro and following transplantation into inflammatory demyelinating lesions (55). Although elevated levels of HA in the lesions were observed, it remains unknown whether HA, or HA digestion products are directly required for the migratory behavior of these cells.
CNS injury triggers HA depolymerization
While hyaluronidase activity appears to play a role in CNS injury, as discussed above, the first evidence to support that HAf(s) have a role in the CNS came from studies showing high levels of hyaluronidase expression and activity in the developing brain and spinal cord that sharply decline after birth (56). Most vertebrate hyaluronidases function as endo-β-acetyl-hexosaminidases that catalyze HA cleavage at β1,4 glycosidic linkages and generate fragments with varied biological activity (57). Bacterial hyaluronidases are also endo-β-acetyl-hexosaminidases but utilize the lyase mechanism to generate fragments that have limited biological activity (57). Vertebratespossess multiple hyaluronidase genes, including Hyal1 to Hyal5, PH20/Spam1 and the more recently described Tmem2 and KIAA1199/CEMIP, both of which may depolymerize HA through unique mechanisms (24, 58, 59).
Hyaluronidase activity and expression are almost undetectable in the adult CNS. However, there is accumulating evidence that hyaluronidase activity and expression change following various insults to the nervous system. For example, in both human ischemic strokes and peri-infarct regions, Hyal1 and Hyal2 were up-regulated in inflammatory cells, microvessels and neurons (38). Hyal1 is an acid active lysosomal enzyme. Recent studies suggest that it can be secreted into the extracellular space to degrade and modify HA (60). Hyal1 mRNA is elevated in rodents following craniotomy or a controlled cortical impact injury (61). However, Hyal1−/− mice do not display any CNS developmental abnormalities. Hyal2 has low activity and is a broadly expressed glycosylphosphatidylinositol-linked (GPI) protein (62). Hyal2 is expressed during embryonic development in the mouse brain but its expression is turned off after birth (63). Hyal3 can enhance Hyal1 activity under certain conditions (64) but overall its biological role has been poorly characterized. Hyal4 is also poorly characterized and its expression and role in the CNS has not been explored. Hyal5 is not expressed in the CNS during development or following injury (50). PH20 has highest expression in the testes but can also be detected in other tissues, including the CNS, but only under certain conditions (50, 61). Overexpression of PH20 can block OL maturation and remyelination (50) but it is unclear to what degree PH20 plays a physiological role in remyelination failure (65, 66).
Two other proteins involved in HA depolymerization have been identified, which are widely expressed in the CNS. KIAA1199/CEMIP is a recently described hyaluronidase that plays a key role in depolymerization of HA in the skin and arthritic synovial fibroblasts (59, 67). It is expressed in the brain and may play role in learning and memory (68, 69). Tmem2 is another recently characterized hyaluronidase, which is expressed in multiple tissues (58). Tmem2 is also expressed in the brain and its expression is up-regulated following neonatal WMI. However, whether it directly contributes to HA depolymerization is not clear (59, 70).
In-vitro studies that sought to determine whether these different Hyals produce various sized HAfs suggest that the final product of any hyaluronidase mediated catalysis is the degradation of intact HA polymer down to the tetrasaccharide level. However, it should be noted that these in vitro assays have employed purified enzymes, substrates and controlled pH. These conditions are far removed from what is encountered in-situ where a wide range of hyaladherins have been identified. The presentation of HA substrates to Hyals may involve interactions with hyaladherins. Characterization of how Hyals are able to recognize, bind and degrade HA when it is bound by interacting hyaladherins is poorly understood. Overall, these observations suggest that at least some Hyals are secreted or elevated at the cell surface in brain lesions to alter the surrounding matrix by depolymerizing HA to bioactive HAfs, which in turn may serve as a paracrine signal to influence regeneration and repair pathways.
Approaches to analyze the roles of HAf in CNS injury
Signaling by HA fragments has been reported in other cell types (71), where HAf size has been broadly defined as either “high” or “low” molecular weight, with no consensus as to what size range defines either category. The HA found in living tissues has a wide polydisperse size distribution (24), and it is unclear how HA signaling systems respond to HAfs within this active range in the presence of larger or smaller sized HAfs. The accumulation of different sizes of HA has been demonstrated in human cases of demyelinating disease e.g., (41) but the precise range of HA sizes generated in these lesions and their specific activities had not previously been determined. We speculate that attempts to quantitatively elucidate the different sizes of HAfs within any tissues have not been successful due to technical limitations.
Most current methods for determination of the MW distribution of HA from tissues and biological fluids have been optimized for high-MW HA (> ~200 kDa). Commonly employed methods include size exclusion chromatography with multiangle laser light scattering (SEC-MALLS) and agarose or polyacrylamide gel electrophoresis (72–78). Detection of low MW HA by light scattering is insensitive and the SEC-MALLS method requires a highly purified HA sample, which is difficult to obtain given the inherent complexity of biological samples. Gel electrophoresis can analyze samples on the microgram scale and can tolerate some impurities in the sample, but nonspecific staining by those impurities can interfere with size distribution analysis of the HA. Blotting of gels to positively charged nylon and detection of HA using a labeled specific binding protein (74) works only for HA with MW greater than approximately 100 kDa (79). Capillary electrophoresis (CE) is limited to pure HA samples (80). A new method that has extremely high sensitivity and works best for low MW HA is GEMMA (gas-phase electrophoretic mobility molecular analysis), but it’s accuracy has not yet been established for impure and polydisperse HA samples (81).
To elucidate the specific actions of HAf in the context of white matter injury, we and others have employed a combination of an in-vivo hypoxic-ischemic injury model, primary glial cultures and chronic forebrain white matter injury slice cultures. To define spatial and temporal changes in HA levels in neonatal hypoxic-ischemic white matter injury, we initially employed a preterm-equivalent rodent model that displays reactive gliosis and OL maturation arrest (82). Both biochemical and immunohistochemical analysis demonstrated that HA was depolymerized in white matter lesions following hypoxic-ischemic injury to the developing brain (70). This decrease in the levels of HA persisted for at least 10 days after injury and was followed by later re-accumulation of intact HA in chronic lesions where evidence of delayed myelination was detected. Protein lysates isolated from neonatal CNS lesions contained apparent endogenous hyaluronidase activity and were able to depolymerize intact HA in an in-vitro assay that yielded HA fragments in the size range of ~200-600 kDa. To determine whether the HAf generated in injured CNS tissues could regulate OL differentiation, commercially available poly-disperse HAf in selected broad size ranges were first screened, which identified a bioactivity less than ~300 kDa that prevented OL differentiation in vitro (70). To more precisely define the bioactive size range, we prepared and screened monodisperse, endotoxin-free HAfs, which identified that a ~200 kDa sized fragment blocked OL differentiation in primary and chronic forebrain white matter injury slice cultures (70). Primary cultures offer the distinct advantage that cell autonomous actions of HAf can be defined. However, two-dimensional (2D) primary cultures can preclude the analysis of more complex ECM signaling. For example, overexpression of Hyal1 in prostate cancer cells in vitro significantly increases rates of cell proliferation. However, when the same Hyal1 expressing cells were injected in vivo, they were moderately tumorigenic (83). Such paradoxical findings underscore the importance to also analyze the role of components of the ECM in three-dimensional (3D) models where the complex interactions among ECM components are preserved.
To examine how HAf negatively impacts OL maturation and myelination in a 3D ECM, we developed a forebrain slice culture model of chronic white matter injury. This model reproduces the major hallmarks of human white matter injury, including: a) reduced numbers of mature Myelin Basic Protein (MBP)-positive OLs; b) reactive gliosis; and c) accumulation of intact HA (84). Screening of mono-disperse HAfs in this chronic slice culture model identified that bioactivity is selectively governed by size, since fragments above or below 200 kDa were inactive (70). Another study, which employed nonquantitative size columns, examined the molecular weight distribution of HA in the mouse cerebral cortex and reported that HA is distributed over a broad MW range with a midpoint of approximately 200 kDa (85). These observations support the notion that 200 kDa sized HAf could be the bioactive size in CNS lesions, but the dynamics of how and when this bioactive fragment is produced following white matter injury remains unclear.
Role of HAf as a paracrine signal during neonatal CNS injury
Contribution of HA receptors to HAf signaling in white matter lesions
There are a number of cell surface receptors containing the HA-binding domain that signal in response to HAf (71). Among these receptors, CD44, RHAMM/Hmmr, HARE/Stabilin-2, and LVYE-1 are expressed in either the normal or diseased CNS (86). Toll-like Receptors 2 and 4 (TLR2 and TLR4) lack the classical HA-binding domains, but have also been demonstrated to play a role in orchestrating downstream signaling in response to HAf (87, 88). TLR2 and TLR4 are expressed by neural progenitors and have been implicated to play a role in neurogenesis (89). TLRs are expressed by OPCs as well as by microglia and astrocytes (87). The roles for most of these receptors in the brain and spinal cord are unclear. However, numerous studies have implicated CD44, TLR2 and TLR4 in nervous system development, homeostasis, repair and injury responses.
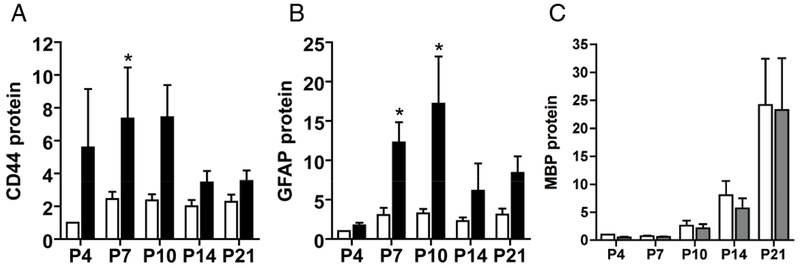
CD44 is expressed throughout both the central and peripheral nervous systems predominantly by glial cells, although some neuronal populations are also transiently CD44 positive (90). Observations from traumatic brain injuries indicate that CD44 expression is chronically elevated within areas of reactive gliosis (91, 92). CD44 is similarly elevated in lesions from post-mortem brain tissue of human infants (47) and sheep (52) with perinatal hypoxic-ischemic cerebral white matter injury, in the gray matter of aged macaques (93) and in human vascular white matter injury associated with age-related cognitive decline (48). CD44 expression has also been reported to increase in a mouse model of amyotrophic lateral sclerosis (ALS) (94). In the context of CNS demyelinating lesions seen in post-mortem brain tissue, elevated CD44 expression in astrocytes and oligodendrocytes has been observed in conjunction with accumulation of HA (40, 95, 96). Elevation of CD44 expression in OPCs by transgenic means leads to HA accumulation and the persistence of OPCs that fail to mature into OLs (97). We examined changes in CD44 levels during neonatal hypoxic-ischemic injury. Analysis by quantitative PCR revealed significant increases in CD44 mRNA in white matter lesions compared to uninjured controls (Figure 1A). Changes in mRNA levels also lead to significant increases in CD44 protein levels compared to uninjured controls (Figure 1B). These results highlight that neonatal hypoxic-ischemic injury also leads to an increase in CD44 levels in lesions while inducing depolymerization of the primary CD44 ligand, HA.
Figure 1. CD44 protein levels rapidly increase following neonatal hypoxic-ischemic injury.
To define how reactive gliosis, myelination and ECM remodeling are temporally related in evolving WMI, expression of GFAP (as a marker of reactive gliosis and injury), Myelin Basic Protein (MBP, as a marker of myelination) and the HA receptor CD44 was analyzed by western blot at five successive time points (n=18 animals/time point) after H-I between P4 and P21. H-I was induced as previously described (82) and generated unilateral WMI with the contralateral hemisphere serving as control. The data is presented as the mean fold-change for the 18 animals relative to the controls. (A, B) Chronic WMI led to a rapid increase in CD44 protein levels (panel A) in the lesions (black bars) compared to controls (unfilled bars). Note that increase in CD44 levels precede that of GFAP (panel B) suggesting that it could be used as a more sensitive indicator of WMI. C) The protein levels of the myelination marker, MBP, were lower in lesions relative to controls through P10. Additional studies employed area fraction analysis, which revealed significantly lower levels of MBP at P14 and P21 (70) in the WM lesions. Western blots did not detect such changes, which may have been related to the reduced sensitivity of this approach in anatomically complex white matter lesions.
To determine which signaling receptor functions downstream of HAf to regulate OPC maturation in the context of neonatal white matter injury, we screened chronic forebrain slice cultures derived from CD44−/−, TLR2−/− and TLR4−/−. Surprisingly, we identified distinct roles for CD44, TLR2 and TLR4 in regulation of OPC maturation after neonatal white matter injury. When either CD44 or TLR2 was deleted, the 200 kDa HAf retained activity to block OPC maturation, which excluded a role for these receptors to mediate arrested OPC maturation (70). However, slices derived from TLR4−/− mice were insensitive to the 200 kDa HAf, which supported a primary role for 200 kDa HAf to mediate OPC maturation arrest via TLR4 and independently of CD44 and TLR2. Although we defined a requirement for depolymerization of high MW in order for it to block OPC maturation, we unexpectedly, identified a role for CD44, TLR2 and TLR4 to mediate this effect. We found that deletion of CD44, TLR2 or TLR4 alone abrogated the effect of high MW HA on OL maturation, which suggested that an interaction among these receptors preceded the generation of bioactive 200 kDa HAf. These findings are reminiscent of observations where endogenous polydisperse high MW HA signals through both CD44 and TLR4 to regulate small intestine and colon growth (98) as well as the biological actions of human milk in the gut (99), whereas a smaller 35 kDa HAf mediates the same biological effects as human milk and signals through TLR4, independently of CD44 (99, 100). We speculate that CD44 either alone or in complex with TLR2 may be involved in regulating hyaluronidase activity that ultimately leads to depolymerization of HA as previously reported (101). Thus, targeting CD44 and/or TLR2 to prevent HA depolymerization or TLR4 to prevent 200 kDa HAf signaling maybe an attractive strategy to promote regeneration and repair of neonatal white matter injury.
Desensitization of signaling pathways in response to HAf as a potential roadblock to repair of white matter lesions
TLR-mediated downstream activity classically leads to chemokine and cytokine immune responses (102). Since excessive cytokine actions in response to persistent presence of signaling ligand can also damage the host cell, cellular check points have evolved that turn off or attenuate downstream TLR signaling, which leads to induction of Immune Tolerance (IT) (103). IT is a transient state in cells of the innate immune system where repeated exposure to pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) renders cells unresponsive to further PAMP/DAMP exposure (104, 105). Removal of the PAMP/DAMP recalibrates the system with restoration of immune responses. Inefficient repair of CNS lesions may not involve an overall lack of repair capacity, but rather insufficient regulation of dysfunctional reparative events that must be terminated prior to tissue regeneration and repair (106). IκK-NFκB is the dominant signaling pathway downstream of TLR4 but it does not play a role during developmental myelination or remyelination (107) suggesting involvement of other downstream signaling effectors. AKT and MAPK are the two other signaling pathways downstream of TLR4 (102). They play critical roles in OL maturation and myelination (108) and have also been implicated in signaling in response to HAf (109). Although we found no apparent activation of MAPK in response to 200 kDa HAf treatment, robust AKT activation was observed by phosphorylation analysis (65). Unexpectedly, this AKT activation was not sustained. In fact, as long as neonatal brain slices were cultured in the presence of 200 kDa HAf, AKT phosphorylation was always found below the levels seen in untreated slices. This was not observed in slices derived from TLR4−/− animals indicating that AKT activation was downstream of TLR4 (70). This was also in sharp contrast to Brain Derived Neurotrophic Factor (BDNF), which caused a sustained activation of AKT. Notably, as long as slice cultures were maintained in the presence of BDNF, AKT was hyper-phosphorylated compared to untreated slices (70). In our studies, the forebrain slice cultures that were pre-exposed to 200 kDa HAf were unable to restore AKT phosphorylation even after subsequent exposure to BDNF, consistent with an immune-tolerant state (70). Reduced AKT phosphorylation was also observed in-vivo in chronic neonatal hypoxic-ischemic white matter lesions that displayed HA depolymerization and delayed myelination. Notably, as HA levels normalized in advanced lesions and returned to baseline so did AKT phosphorylation levels in comparison to un-injured white matter. These observations suggest that HA depolymerization results in generation of HAf in vivo that may act in a paracrine manner via TLR4 to induce an immune-tolerance like state to desensitize AKT activity in maturing OLs to negatively impact myelination and white matter regeneration and repair.
We explored several potential pathways downstream of AKT that could disrupt OPC maturation (65). Prior reports suggested that AKT inactivation results in GSK3β activation and that GSK3β inhibition can enhance OPC maturation (110). Based on these observations, we hypothesized that treatment with a GSK3β inhibitor would enhance OPC maturation if 200 kDa HAf up-regulates GSK3β activation by inactivating AKT. Although we observed that 200 kDa HAf lead to GSK3β activation, treatment with GSK3β pharmacological inhibitors failed to promote OPC maturation, which supported that GSK3β is not part of the signaling pathway (70). AKT also regulates mTOR activity, another downstream signaling effector that plays a role in OPC maturation and myelination (108, 110). However, even though AKT activation was down regulated by 200 kDa HAf, the status of mTOR phosphorylation remained un-changed indicating that mTOR is not a downstream target of the HA-TLR signaling pathway in the context of white matter injury (70).
Molecular Mechanisms Relevant to Potential Strategies for repair and regeneration in CNS lesions
Since hyaluronidase activity is undetectable in uninjured brain tissue and only selectively upregulated in CNS lesions, targeting hyaluronidase activity could be an attractive strategy to promote regeneration and repair in the CNS. Treatment of OPCs and cultured forebrain slices with a broad-spectrum hyaluronidase inhibitor (ascorbate 6-hexadecanoate/Vcpal) promotes OL differentiation in the presence of high MW HA (41, 50, 70). In addition, treatment of demyelinating lesions with this inhibitor promotes functional recovery as it overcomes the negative effects of high MW HA on remyelination (50). However, the use of broad-spectrum hyaluronidase inhibitors to promote CNS repair, may have significant deleterious effects given the important roles of hyaluronidases in peripheral tissue homeostasis. In the CNS, for example, HA present in perineuronal nets can influence the efficacy and architecture of synapses. Disruption of perineuronal nets by hyaluronidase treatment of neonatal rodent hippocampal slices lead to width reduction of the synaptic cleft and an increase in the amplitude of excitatory postsynaptic potentials (EPSPs) in CA1 neurons (111). Hyaluronidase treatment has also been reported to regulate AMPA receptor trafficking in and out of the synapse (112). Thus, using hyaluronidase inhibitors as a therapeutic strategy will require the identification of the specific hyaluronidases that are upregulated in CNS injury and the development of agents that selectively target these hyaluronidases.
A second approach to develop pro-myelinating agents involves the use of small molecule inhibitors that influence the TLR4-driven pathway induced by HA digestion products. We recently identified FoxO3 as a downstream target of 200 kDa HAf signaling and demonstrated that FoXO3 could block OL maturation only when the 200 kDa HAf was present (70). FoxO transcription factors maintain tissue homeostasis and are sensitive to cellular stress. Studies utilizing FoxO3−/− mice suggest that it induces a program that prevents premature differentiation of oligodendrocytes by repressing myelination genes (113). Even though our studies support a role for FoxO3 in neonatal white matter injury, the role of other FoxO transcription factors is unclear. In macrophages, in the context of obesity and insulin resistance, FoxO1 has been identified as transcriptional regulator of the TLR4 gene and its inflammatory pathway (114).
Interestingly, myelin gene transcription and OL lineage specification and differentiation are regulated by the SWI/SNF chromatin remodeling factors (115, 116). We found that FOXO3 can disrupt myelin gene transcription by interfering with the interactions between SWI/SNF subunits and myelin gene promoters (70). FoxO3 activation induces a positive feedback loop that promotes transcription of FoxO1 and FoxO4 transcription factors (117). FoxO1, in a mouse model of chronic neonatal hypoxia was shown to regulate OPC proliferation (118). FoxO3 acetylation was increased in this chronic neonatal hypoxia model in the absence of Sirtl and knockdown of FoxO3 by siRNA was shown to negatively impact the percentage of Olig2+ OPCs (119). TGFβ signaling regulates the timing of myelination by regulating the interaction of FoxO1 and the transcription factor Sp1 (120), but TGFβ signaling does not regulate AKT or FoxO3 activation (121). These findings suggest that FoxO1 and FoxO3 may be regulated by different signaling pathways and may play distinct or redundant roles during different stages of OPC lineage maturation. Identification of these particular signaling pathways and definition of the epigenetic relationships between different FoxO transcription factors during chronic white matter injury could provide new targets for promoting regeneration and repair.
Conclusions
During neonatal or adult white matter injury, the range of biological responses induced by either HA accumulation or HA depolymerization is only partially understood. CNS injury induces depolymerization of intact megadalton forms of HA, which function as a scaffold for interacting proteoglycans to organize the ECM. HA depolymerization generates bioactive HA fragments, which exert a sustained negative block on white matter repair and regeneration pathways via persistent desensitization of AKT-mediated pro-myelination signals (Figure 2). Definition of the hyaluronidase activities that promote HA depolymerization in CNS lesions may lead to novel therapeutic agents to block hyaluronidase-mediated generation of HA fragments that signal to desensitize repair and regeneration pathways. Currently under investigation are various classes of antagonists of TLR4 and its downstream signaling components (122, 123). Finally, it is currently unclear whether the 200 kDa HAf or other sizes of HA have additional actions that may influence resident glial cells during normal development or in CNS lesions. Identification of other potential actions of HA fragments is an important future direction to fully understand how these fragments influence regeneration and repair of CNS lesions.
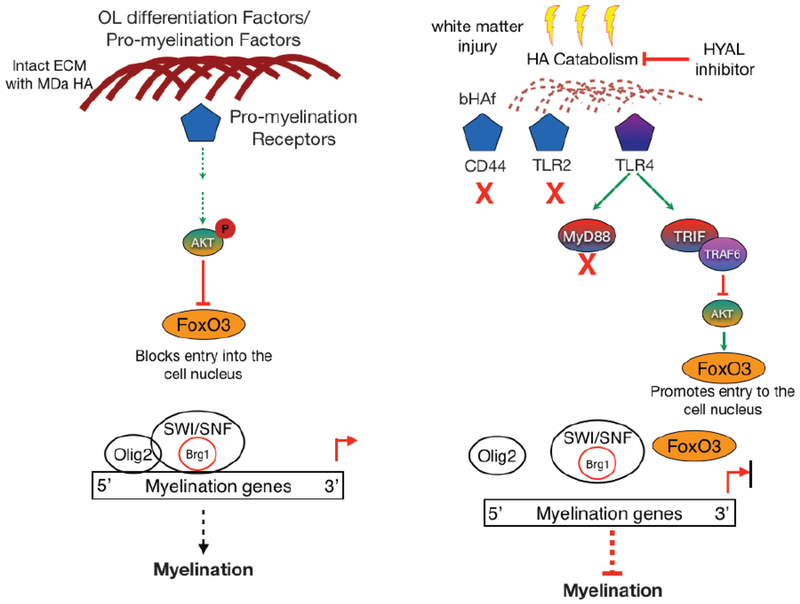
Figure 2. Generation of bioactive HA fragment via hyaluronidase activity induces a tolerant-like state that attenuates AKT activity and promotes myelination failure.
Intact ECM allows for signaling via pro-myelination receptors to restrain FoxO3 in the cytoplasm and promote myelination via the Brg1-SWI/SNF complex, which interacts with myelin gene promoters. A bioactive HA fragment inactivates AKT downstream of TLR4 and drives FoxO3 nuclear localization. Recruitment of SWI/SNF and Olig 2 to myelination gene promoters is disrupted, which blocks transcription of myelination genes to negatively impact repair and regeneration of CNS lesions.
The actions of HA fragments are a function of their size. Given that hyaluronidase digestion in tissues generates an apparently random collection of HA fragment sizes at different times post-injury, bioactivity may be conferred when a particular HA fragment assumes a novel conformation that may be recognized by specific hyaladherins. Identification of the hyaladherins that promote bioactivity of some HA fragments in CNS lesions, may be another therapeutic target to promote white matter repair. Study of ECM remodeling thus represents an emerging field that may be central to define the regulation and treatment of both acute and chronic CNS injury.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke (NS054044; NS045737 to SAB), the National Institute on Aging (AG-31892 to SAB and LSS), an NIH Directors award for the operation of the Oregon National Primate Research Center (P51OD01109), the American Stroke Association (Grant-in-Aid 11GRNT7510072 to SAB), the Congressionally Directed Medical Research Programs (MS160144) and the National Multiple Sclerosis Society (RG 4843A5/1). TS is supported by a Huebner Family Postdoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
The authors declare no conflicts of interests.
References:
- 1.Bignami A, Hosley M, Dahl D, Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat Embryol (Berl) 188, 419–433 (1993). [DOI] [PubMed] [Google Scholar]
- 2.Cragg B, Brain extracellular space fixed for electron microscopy. Neurosci Lett 15, 301–306 (1979). [DOI] [PubMed] [Google Scholar]
- 3.Lau LW, Cua R, Keough MB, Haylock-Jacobs S, Yong VW, Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci 14, 722–729 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Nicholson C, Hrabetova S, Brain Extracellular Space: The Final Frontier of Neuroscience. Biophys J 113, 2133–2142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandtlow CE, Zimmermann DR, Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev 80, 1267–1290 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Barros CS, Franco SJ, Muller U, Extracellular matrix: functions in the nervous system. Cold Spring Harb Perspect Biol 3, a005108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dityatev A, Schachner M, Sonderegger P, The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11, 735–746 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Cregg JM et al. , Functional regeneration beyond the glial scar. Exp Neurol 253, 197–207 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudet AD, Popovich PG, Extracellular matrix regulation of inflammation in the healthy and injured spinal cord. Exp Neurol 258, 24–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnside ER, Bradbury EJ, Manipulating the extracellular matrix and its role in brain and spinal cord plasticity and repair. Neuropathol Appl Neurobiol 40, 26–59 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Sherman LS, Back SA, A ‘GAG’ reflex prevents repair of the damaged CNS. Trends Neurosci 31, 44–52 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Sherman LS, Matsumoto S, Su W, Srivastava T, Back SA, Hyaluronan Synthesis, Catabolism, and Signaling in Neurodegenerative Diseases. Int J Cell Biol 2015, 368584 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok JC, Dick G, Wang D, Fawcett JW, Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71, 1073–1089 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Slaker M, Blacktop JM, Sorg BA, Caught in the Net: Perineuronal Nets and Addiction. Neural Plast 2016, 7538208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su W, Matsumoto S, Sorg B, Sherman LS, Distinct roles for hyaluronan in neural stem cell niches and perineuronal nets. Matrix Biol, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch U, Extracellular matrix components associated with remodeling processes in brain. Cell Mol Life Sci 61, 2031–2045 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauch U, Brain matrix: structure, turnover and necessity. Biochem Soc Trans 35, 656–660 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Day AJ, Sheehan JK, Hyaluronan: polysaccharide chaos to protein organisation. Curr Opin Struct Biol 11, 617–622 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Scott JE, Cummings C, Brass A, Chen Y, Secondary and tertiary structures of hyaluronan in aqueous solution, investigated by rotary shadowing-electron microscopy and computer simulation. Hyaluronan is a very efficient network forming polymer. Biochem J 274 ( Pt 3), 699–705 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day AJ, Prestwich GD, Hyaluronan-binding proteins: tying up the giant. J Biol Chem 277, 4585–4588 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Toole BP, Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4, 528–539 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Petrey AC, de la Motte CA, Hyaluronan, a crucial regulator of inflammation. Front Immunol 5, 101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salustri A, Camaioni A, Di Giacomo M, Fulop C, Hascall VC, Hyaluronan and proteoglycans in ovarian follicles. Hum Reprod Update 5, 293–301 (1999). [DOI] [PubMed] [Google Scholar]
- 24.Stern R, Kogan G, Jedrzejas MJ, Soltes L, The many ways to cleave hyaluronan. Biotechnol Adv 25, 537–557 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Stern R, Asari AA, Sugahara KN, Hyaluronan fragments: an information-rich system. Eur J Cell Biol 85, 699–715 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Weigel PH, DeAngelis PL, Hyaluronan synthases: a decade-plus of novel glycosyltransferases. J Biol Chem 282, 36777–36781 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Itano N et al. , Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem 274, 25085–25092 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Rilla K et al. , Hyaluronan synthase 1 (HAS1) requires higher cellular UDP-GlcNAc concentration than HAS2 and HAS3. J Biol Chem 288, 5973–5983 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arranz AM et al. , Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci 34, 6164–6176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spicer AP, Tien JL, Joo A, Bowling RA Jr., Investigation of hyaluronan function in the mouse through targeted mutagenesis. Glycoconj J 19, 341–345 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Camenisch TD et al. , Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest 106, 349–360 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA, Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med 8, 850–855 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Baggenstoss BA et al. , Hyaluronan synthase control of synthesis rate and hyaluronan product size are independent functions differentially affected by mutations in a conserved tandem B-X7-B motif. Glycobiology 27, 154–164 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Back SA, Rosenberg PA, Pathophysiology of glia in perinatal white matter injury. Glia 62, 1790–1815 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindwall C, Olsson M, Osman AM, Kuhn HG, Curtis MA, Selective expression of hyaluronan and receptor for hyaluronan mediated motility (Rhamm) in the adult mouse subventricular zone and rostral migratory stream and in ischemic cortex. Brain Res 1503, 62–77 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Mansour H et al. , Permissive and non-permissive reactive astrocytes: immunofluorescence study with antibodies to the glial hyaluronate-binding protein. J Neurosci Res 25, 300–311 (1990). [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Zhan Y, Xu L, Feuerstein GZ, Wang X, Use of suppression subtractive hybridization for differential gene expression in stroke: discovery of CD44 gene expression and localization in permanent focal stroke in rats. Stroke 32, 1020–1027 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Al’Qteishat A et al. , Changes in hyaluronan production and metabolism following ischaemic stroke in man. Brain 129, 2158–2176 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Tang SC et al. , Association between plasma levels of hyaluronic acid and functional outcome in acute stroke patients. J Neuroinflammation 11, 101 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Back SA et al. , Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med 11, 966–972 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Sloane JA et al. , Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci U S A 107, 11555–11560 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang A et al. , Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol 72, 918–926 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franklin RJ, Gallo V, The translational biology of remyelination: past, present, and future. Glia 62, 1905–1915 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Wolswijk G, Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci 18, 601–609 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD, NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci 20, 6404–6412 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolswijk G, Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain 125, 338–349 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Buser JR et al. , Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 71, 93–109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Back SA et al. , White matter lesions defined by diffusion tensor imaging in older adults. Ann Neurol 70, 465–476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bagi Z et al. , Vasodilator dysfunction and oligodendrocyte dysmaturation in aging white matter. Ann Neurol 83, 142–152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preston M et al. , Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol 73, 266–280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bugiani M et al. , Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain 136, 209–222 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Hagen MW et al. , Role of recurrent hypoxia-ischemia in preterm white matter injury severity. PLoS One 9, e112800 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perris R et al. , Spatial and temporal changes in the distribution of proteoglycans during avian neural crest development. Development 111, 583–599 (1991). [DOI] [PubMed] [Google Scholar]
- 54.Casini P, Nardi I, Ori M, Hyaluronan is required for cranial neural crest cells migration and craniofacial development. Dev Dyn 241, 294–302 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Piao JH, Wang Y, Duncan ID, CD44 is required for the migration of transplanted oligodendrocyte progenitor cells to focal inflammatory demyelinating lesions in the spinal cord. Glia 61, 361–367 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Polansky JR, Toole BP, Gross J, Brain hyaluronidase: changes in activity during chick development. Science 183, 862–864 (1974). [DOI] [PubMed] [Google Scholar]
- 57.Stern R, Jedrzejas MJ, Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev 106, 818–839 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto H et al. , A mammalian homolog of the zebrafish transmembrane protein 2 (TMEM2) is the long-sought-after cell-surface hyaluronidase. J Biol Chem 292, 7304–7313 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshino Y, Goto M, Hara H, Inoue S, The role and regulation of TMEM2 (transmembrane protein 2) in HYBID (hyaluronan (HA)-binding protein involved in HA depolymerization/ KIAA1199/CEMIP)-mediated HA depolymerization in human skin fibroblasts. Biochem Biophys Res Commun 505, 74–80 (2018). [DOI] [PubMed] [Google Scholar]
- 60.McAtee CO et al. , Hyaluronidase Hyal1 Increases Tumor Cell Proliferation and Motility through Accelerated Vesicle Trafficking. J Biol Chem 290, 13144–13156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xing G, Ren M, Verma A, Divergent Temporal Expression of Hyaluronan Metabolizing Enzymes and Receptors with Craniotomy vs. Controlled-Cortical Impact Injury in Rat Brain: A Pilot Study. Front Neurol 5, 173 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lepperdinger G, Strobl B, Kreil G, HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem 273, 22466–22470 (1998). [DOI] [PubMed] [Google Scholar]
- 63.Lepperdinger G, Mullegger J, Kreil G, Hyal2--less active, but more versatile? Matrix Biol 20, 509–514 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Hemming R et al. , Mouse Hyal3 encodes a 45- to 56-kDa glycoprotein whose overexpression increases hyaluronidase 1 activity in cultured cells. Glycobiology 18, 280–289 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Marella M et al. , PH20 is not expressed in murine CNS and oligodendrocyte precursor cells. Ann Clin Transl Neurol 4, 191–211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherman LS, Back SA, Comment on: PH20 is not expressed in murine CNS and oligodendrocyte precursor cells. Ann Clin Transl Neurol 4, 608–609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimizu H et al. , Hyaluronan-Binding Protein Involved in Hyaluronan Depolymerization Is Up-Regulated and Involved in Hyaluronan Degradation in Human Osteoarthritic Cartilage. Am J Pathol 188, 2109–2119 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Yoshino Y et al. , Distribution and function of hyaluronan binding protein involved in hyaluronan depolymerization (HYBID, KIAA1199) in the mouse central nervous system. Neuroscience 347, 1–10 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Yoshino Y et al. , Targeted deletion of HYBID (hyaluronan binding protein involved in hyaluronan depolymerization/ KIAA1199/CEMIP) decreases dendritic spine density in the dentate gyrus through hyaluronan accumulation. Biochem Biophys Res Commun 503, 1934–1940 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Srivastava T et al. , A TLR/AKT/FoxO3 immune tolerance-like pathway disrupts the repair capacity of oligodendrocyte progenitors. J Clin Invest 128, 2025–2041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang D, Liang J, Noble PW, Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 23, 435–461 (2007). [DOI] [PubMed] [Google Scholar]
- 72.Min H, Cowman MK, Combined alcian blue and silver staining of glycosaminoglycans in polyacrylamide gels: application to electrophoretic analysis of molecular weight distribution. Anal Biochem 155, 275–285 (1986). [DOI] [PubMed] [Google Scholar]
- 73.Kvam C, Granese D, Flaibani A, Zanetti F, Paoletti S, Purification and characterization of hyaluronan from synovial fluid. Anal Biochem 211, 44–49 (1993). [DOI] [PubMed] [Google Scholar]
- 74.Lee HG, Cowman MK, An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem 219, 278–287 (1994). [DOI] [PubMed] [Google Scholar]
- 75.Adam N, Ghosh P, Hyaluronan molecular weight and polydispersity in some commercial intra-articular injectable preparations and in synovial fluid. Inflamm Res 50, 294–299 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Baggenstoss BA, Weigel PH, Size exclusion chromatography-multiangle laser light scattering analysis of hyaluronan size distributions made by membrane-bound hyaluronan synthase. Anal Biochem 352, 243–251 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cowman MK et al. , Improved agarose gel electrophoresis method and molecular mass calculation for high molecular mass hyaluronan. Anal Biochem 417, 50–56 (2011). [DOI] [PubMed] [Google Scholar]
- 78.Bhilocha S et al. , Agarose and polyacrylamide gel electrophoresis methods for molecular mass analysis of 5- to 500-kDa hyaluronan. Anal Biochem 417, 41–49 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan H et al. , Molecular mass dependence of hyaluronan detection by sandwich ELISA-like assay and membrane blotting using biotinylated hyaluronan binding protein. Glycobiology 23, 1270–1280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hayase S, Oda Y, Honda S, Kakehi K, High-performance capillary electrophoresis of hyaluronic acid: determination of its amount and molecular mass. J Chromatogr A 768, 295–305 (1997). [DOI] [PubMed] [Google Scholar]
- 81.Malm L, Hellman U, Larsson G, Size determination of hyaluronan using a gas-phase electrophoretic mobility molecular analysis. Glycobiology 22, 7–11 (2012). [DOI] [PubMed] [Google Scholar]
- 82.Segovia KN et al. , Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol 63, 520–530 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simpson MA, Concurrent expression of hyaluronan biosynthetic and processing enzymes promotes growth and vascularization of prostate tumors in mice. Am J Pathol 169, 247–257 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dean JM et al. , An organotypic slice culture model of chronic white matter injury with maturation arrest of oligodendrocyte progenitors. Mol Neurodegener 6, 46 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reed MJ et al. , Microvasculature of the Mouse Cerebral Cortex Exhibits Increased Accumulation and Synthesis of Hyaluronan With Aging. J Gerontol A Biol Sci Med Sci 72, 740–746 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vigetti D et al. , Hyaluronan: biosynthesis and signaling. Biochim Biophys Acta 1840, 2452–2459 (2014). [DOI] [PubMed] [Google Scholar]
- 87.Sloane JA, Blitz D, Margolin Z, Vartanian T, A clear and present danger: endogenous ligands of Toll-like receptors. Neuromolecular Med 12, 149–163 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Okun E et al. , TLR2 activation inhibits embryonic neural progenitor cell proliferation. J Neurochem 114, 462–474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rolls A et al. , Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol 9, 1081–1088 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Preston M, Sherman LS, Neural stem cell niches: roles for the hyaluronan-based extracellular matrix. Front Biosci (Schol Ed) 3, 1165–1179 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stylli SS, Kaye AH, Novak U, Induction of CD44 expression in stab wounds of the brain: long term persistence of CD44 expression. J Clin Neurosci 7, 137–140 (2000). [DOI] [PubMed] [Google Scholar]
- 92.Jones LL et al. , Regulation of the cell adhesion molecule CD44 after nerve transection and direct trauma to the mouse brain. J Comp Neurol 426, 468–492 (2000). [DOI] [PubMed] [Google Scholar]
- 93.Cargill R et al. , Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiol Aging 33, 830 e813–824 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsumoto T et al. , CD44 expression in astrocytes and microglia is associated with ALS progression in a mouse model. Neurosci Lett 520, 115–120 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Vogel H, Butcher EC, Picker LJ, H-CAM expression in the human nervous system: evidence for a role in diverse glial interactions. J Neurocytol 21, 363–373 (1992). [DOI] [PubMed] [Google Scholar]
- 96.Alldinger S, Fonfara S, Kremmer E, Baumgartner W, Up-regulation of the hyaluronate receptor CD44 in canine distemper demyelinated plaques. Acta Neuropathol 99, 138–146 (2000). [DOI] [PubMed] [Google Scholar]
- 97.Tuohy TM et al. , CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation-independent demyelination and dysmyelination. Glia 47, 335–345 (2004). [DOI] [PubMed] [Google Scholar]
- 98.Riehl TE, Santhanam S, Foster L, Ciorba M, Stenson WF, CD44 and TLR4 mediate hyaluronic acid regulation of Lgr5+ stem cell proliferation, crypt fission, and intestinal growth in postnatal and adult mice. Am J Physiol Gastrointest Liver Physiol 309, G874–887 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hill DR et al. , Human milk hyaluronan enhances innate defense of the intestinal epithelium. J Biol Chem 288, 29090–29104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hill DR, Kessler SP, Rho HK, Cowman MK, de la Motte CA, Specificsized hyaluronan fragments promote expression of human beta-defensin 2 in intestinal epithelium. J Biol Chem 287, 30610–30624 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harada H, Takahashi M, CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and −2. J Biol Chem 282, 5597–5607 (2007). [DOI] [PubMed] [Google Scholar]
- 102.Kawai T, Akira S, TLR signaling. Semin Immunol 19, 24–32 (2007). [DOI] [PubMed] [Google Scholar]
- 103.Kondo T, Kawai T, Akira S, Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol 33, 449–458 (2012). [DOI] [PubMed] [Google Scholar]
- 104.Biswas SK, Lopez-Collazo E, Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 30, 475–487 (2009). [DOI] [PubMed] [Google Scholar]
- 105.Morris MC, Gilliam EA, Li L, Innate immune programing by endotoxin and its pathological consequences. Front Immunol 5, 680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shechter R, Schwartz M, CNS sterile injury: just another wound healing? Trends Mol Med 19, 135–143 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Raasch J et al. , IkappaB kinase 2 determines oligodendrocyte loss by non-cell-autonomous activation of NF-kappaB in the central nervous system. Brain 134, 1184–1198 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bercury KK, Macklin WB, Dynamics and mechanisms of CNS myelination. Dev Cell 32, 447–458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang D, Liang J, Noble PW, Hyaluronan as an immune regulator in human diseases. Physiol Rev 91, 221–264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Azim K, Butt AM, GSK3beta negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 59, 540–553 (2011). [DOI] [PubMed] [Google Scholar]
- 111.Kul’chitskii SV et al. , Changes in neuropil ultrastructure in hippocampal field CA1 in rat pups after application of hyaluronidase. Neurosci Behav Physiol 39, 517–521 (2009). [DOI] [PubMed] [Google Scholar]
- 112.Frischknecht R et al. , Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 12, 897–904 (2009). [DOI] [PubMed] [Google Scholar]
- 113.Renault VM et al. , FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 5, 527–539 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fan W et al. , FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J 29, 4223–4236 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Banine F et al. , SWI/SNF chromatin-remodeling factors induce changes in DNA methylation to promote transcriptional activation. Cancer Res 65, 3542–3547 (2005). [DOI] [PubMed] [Google Scholar]
- 116.Matsumoto S et al. , Brg1 directly regulates Olig2 transcription and is required for oligodendrocyte progenitor cell specification. Dev Biol 413, 173–187 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB, The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem 284, 10334–10342 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jablonska B et al. , Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J Neurosci 32, 14775–14793 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jablonska B et al. , Sirt1 regulates glial progenitor proliferation and regeneration in white matter after neonatal brain injury. Nat Commun 7, 13866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Palazuelos J, Klingener M, Aguirre A, TGFbeta signaling regulates the timing of CNS myelination by modulating oligodendrocyte progenitor cell cycle exit through SMAD3/4/FoxO1/Sp1. J Neurosci 34, 7917–7930 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seoane J, Le HV, Shen L, Anderson SA, Massague J, Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117, 211–223 (2004). [DOI] [PubMed] [Google Scholar]
- 122.Molteni M, Bosi A, Rossetti C, Natural Products with Toll-Like Receptor 4 Antagonist Activity. Int J Inflam 2018, 2859135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao W, Xiong Y, Li Q, Yang H, Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front Physiol 8, 508 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]