Abstract
Objective:
To develop and validate an AKI risk prediction model for hospitalized non–critically ill patients.
Patients and Methods:
We retrospectively identified all Olmsted County, Minnesota, residents admitted to non-ICU wards at Mayo Clinic Hospital, Rochester, Minnesota, in 2013 and 2014. The cohort was divided into development and validation sets by year. The primary outcome was hospital-acquired AKI defined by Kidney Disease: Improving Global Outcomes criteria. Cox regression was used to analyze mortality data. Comorbid risk factors for AKI were identified, and a multivariable model was developed and validated.
Results:
The development and validation cohorts included 3,816 and 3,232 adults, respectively. Approximately 10% of patients in both cohorts had AKI, and patients with AKI had an increased risk of death (hazard ratio, 3.62; 95% CI, 2.97–4.43; P<.001). Significant univariate determinants of AKI were pre-existing kidney disease, diabetes mellitus, hypertension, heart failure, vascular disease, coagulopathy, pulmonary disease, coronary artery disease, cancer, obesity, liver disease, and weight loss (all P<.05). The final multivariable model included increased baseline serum creatinine value, admission to a medical service, pulmonary disease, diabetes mellitus, kidney disease, cancer, hypertension, and vascular disease. The area under the receiver operating characteristic curves for the development and validation cohorts were 0.71 (95% CI, 0.69–0.75) and 0.75 (95% CI, 0.72–0.78), respectively.
Conclusions:
Hospital-acquired AKI is common in non-ICU inpatients and associated with worse outcomes. Patient data at admission can be used to identify increased risk; such patients may benefit from more intensive monitoring and earlier intervention and testing with emerging biomarkers.
Keywords: nephrology, renal disease, renal failure
Summary
Acute kidney injury (AKI) is common in hospitalized non–intensive care unit patients and is associated with adverse outcomes. We developed and validated a simple risk-prediction model using readily available clinical variables, which can be incorporated into clinical use. This tool enables identification of hospitalized patients who are at higher risk for AKI and may benefit from more intensive clinical monitoring and laboratory testing.
Introduction
Acute kidney injury (AKI) is a common complication among hospitalized patients. Patients with AKI are at increased risk for death and short-term and long-term morbidities.1–4 AKI is an independent risk factor for death,5 and mortality rates can be as high as 60%.6–9 AKI also imposes a substantial cost burden on health care systems worldwide.10, 11 Despite prevention efforts, studies suggest a flat,11 if not increasing, incidence.7, 12
Current guidelines define AKI on the basis of changes in serum creatinine value and urine output. Practice guidelines also advocate the use of risk-assessment tools to identify patients at greatest risk for AKI for whom interventions can be initiated early to prevent or mitigate AKI effects.13 Multiple AKI risk-stratification scores have been developed for specific clinical settings (eg, after cardiac surgery, contrast exposure, and high-risk surgery).14–17 Additionally, several AKI risk scores have been developed to predict AKI in the intensive care unit (ICU).15–23 These prediction models are specific for the populations in which they were developed, and comparatively few risk-prediction models have been developed for non-ICU patients.24, 25 Some of these models require data that may not be readily available at the time of admission.
Olmsted County, Minnesota, USA, offers an ideal setting in which to develop risk-stratification tools because accurate and complete medical data are available for all residents through the Rochester Epidemiology Project.26 In the current study, we developed and validated an AKI risk-prediction model for non–critically ill patients admitted to non-ICU hospital units using data existing at the time of admission.
Subjects and Methods
Study Design and Patient Selection
The study was approved by the Mayo Clinic Institutional Review Board. The Rochester Epidemiology Project26 was used to retrospectively identify all adult residents27–29 of Olmsted County, Minnesota, who were admitted to Mayo Clinic Hospital (Methodist and St. Marys campuses) in Rochester, Minnesota, from January 1, 2013, through December 31, 2014. The study was limited to Olmsted County residents to make it population based, and hence, to limit referral bias, make it more generalizable, and allow for maximal follow-up data. Data for patients admitted in 2014 were used for model development (development cohort), and data for patients admitted in 2013 were used for model validation (validation cohort). Patients were included if they were aged 18 years or older at the time of admission and were admitted to a hospital unit other than the ICU. Among readmissions, only the most recent admission in a given calendar year was included in the analysis, and if a patient was admitted in both the development (2014) and validation (2013) years, only the admission during the development year (2014) was included.
We did not include patients who were admitted directly to an ICU or pediatric or psychiatric ward. Patients who had end-stage renal disease, AKI at admission, or a kidney transplant before the index admission or were missing both a baseline and an admission serum creatinine measurement were excluded. We also excluded patients who did not provide research authorization for their medical records.
Setting and Data Source
The electronic health record was screened using an automated search tool (Advanced Cohort Explorer) to obtain the following data for all eligible patients: age, sex, race, admitting service, length of hospital stay, need for renal replacement therapy, in-hospital and posthospitalization death, and comorbid conditions (based on International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes) from 180 to 7 days before the index admission. The Charlson Comorbidity Index27–29 was calculated. Baseline creatinine value, defined as the median of all serum creatinine values obtained during the 180 days before the index admission, was abstracted. If a measured serum creatinine value was not available, the value at admission was used as an imputation value, as described elsewhere.23
Definitions and Outcomes
The primary outcome was hospital-acquired AKI, defined using the serum creatinine level criterion of the Kidney Disease: Improving Global Outcomes definition (serum creatinine value increase by ≥0.3 mg/dL within 48 hours or an increase in serum creatinine value to ≥1.5 times the baseline value during the hospitalization).30 The AKI e-alert system (Sniffer), previously validated,11, 31 was used to adjudicate AKI. Secondary outcomes included the development of moderate or severe AKI by the Acute Kidney Injury Network definition and length of hospital stay.
Data Analysis
Descriptive statistics are reported as counts and percentages or mean (SD) or median (interquartile range), depending on the normality of variable distributions. Patient baseline characteristics were compared by t test, 1-way analysis of variance, Mann-Whitney U test, or χ2 proportion tests, as appropriate. Univariate logistic regression was used to identify comorbid conditions associated with the primary outcome. Multivariable logistic regression with a backward elimination approach was performed to select the final model. Cox proportional hazards regression and log-rank test procedure for mortality data were used to compare mortality rate among patients who achieved the primary outcome versus those who did not. After training, the final model was assessed for discrimination using area under the receiver operating characteristic curve (AUC ROC) in both cohorts and the Hosmer-Lemeshow test for goodness of fit for model calibration in the validation cohort. P values less than .05 were considered significant. Statistical analyses were performed using R 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
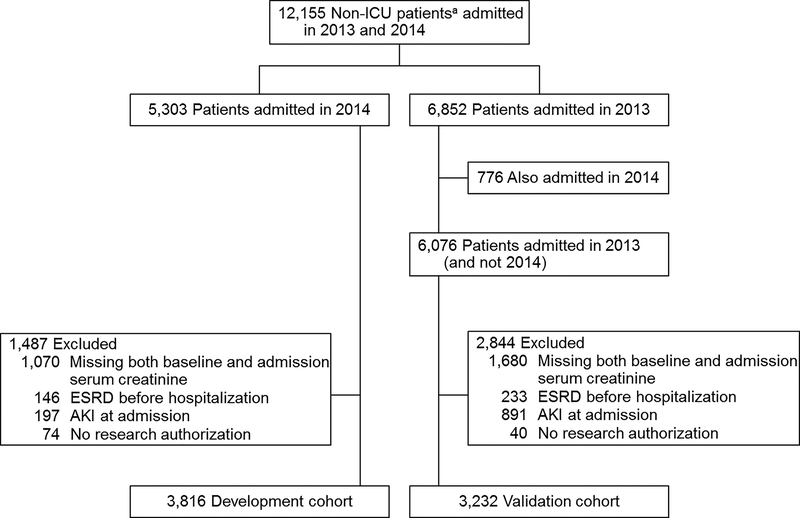
A total of 12,155 adult residents of Olmsted County were admitted to Mayo Clinic Hospital in the 2 years studied. After exclusions, the final development cohort included 3,816 patients, and the validation cohort included 3,232 patients (Figure 1). Most baseline demographics, baseline kidney function, comorbid conditions, and incidence of AKI were similar between the 2 cohorts (Table 1). The development cohort was slightly older and had more patients admitted to medical units (both P<.001). Of note, in 565 patients in the development cohort (14.6%) and 200 (6.1%) of patients in the validation cohort, the baseline serum creatinine value was imputed from their admission serum creatinine value.
Figure 1.
Selection of Development and Validation Cohorts. AKI=acute kidney injury; ESRD=end-stage renal disease; ICU=intensive care unit. a Nonpediatric and nonpsychiatric patients.
Table 1.
Baseline Characteristics and Outcomes of the Development and Validation Cohortsa
| Characteristic | Development Cohort (n=3,816) | Validation Cohort (n=3,232) | P Value |
|---|---|---|---|
| Demographics | |||
| Age, y | 65.4 (19.4) | 63.3 (19.0) | <.001 |
| White | 3,492 (91.5) | 2,957 (91.5) | >.99 |
| Men | 1,705 (44.7) | 1,417 (43.8) | >.99 |
| Admission to medical unit | 2,345 (61.4) | 1,747 (54.1) | <.001 |
| Baseline creatinine, mg/dL | 1.0 (0.4) | 1.1 (0.9) | .73 |
| Charlson Comorbidity Index | 4.8 (3.8) | 4.3 (3.0) | .01 |
| Length of stay, d | 3.9 (4.0) | 3.3 (3.5) | .13 |
| Renal outcomes | |||
| Any AKI | 393 (10.3) | 351 (10.8) | .48 |
| Moderate-severe AKI | 72 (1.9) | 54 (1.7) | .27 |
| Renal replacement | 15 (0.4) | 27 (0.8) | .02 |
Abbreviation: AKI=acute kidney injury.
Values are mean (SD) or No. of patients (%).
Characteristics of the Development Cohort
Among the development cohort, the median age was 68 years (interquartile range, 53–81 years), 44.6% were men, and 91.5% were white. Sixty-one percent were admitted to medical services (as opposed to surgical services), and the mean (SD) hospital stay was 3.9 (4) days. Mean (SD) baseline serum creatinine value was 1.0 (0.4) mg/dL. Mean (SD) follow-up duration after discharge was 399 (154) days; 73 patients (1.9%) had no follow-up after hospital discharge.
Hospital-acquired AKI was observed in 393 patients (10.3%) (Supplemental Table 1): stage 1 in 321 (8.4%), stage 2 in 41 (1.1%), and stage 3 in 31 (0.8%). Moderate-severe AKI occurred in 72 patients (1.9%). The mortality rate was 13.2% (95% CI, 12.1%−14.2%). Compared with those who did not have AKI, affected patients had a significantly higher Charlson Comorbidity Index (mean [SD], 7.0 [4.2] vs 4.8 [3.8]; P<.001) (Supplemental Figure 1) and longer hospital stay (6.4 [6.8] vs 3.4 [3.4] days; P<.001). The severity of AKI correlated with length of hospitalization (linear regression estimate, 2.05; P<.001) (Supplemental Figure 2). Fifteen patients (0.4%) required initiation of hemodialysis during their hospital stay, and 6 of these patients (0.2%) continued to require dialysis after hospital discharge.
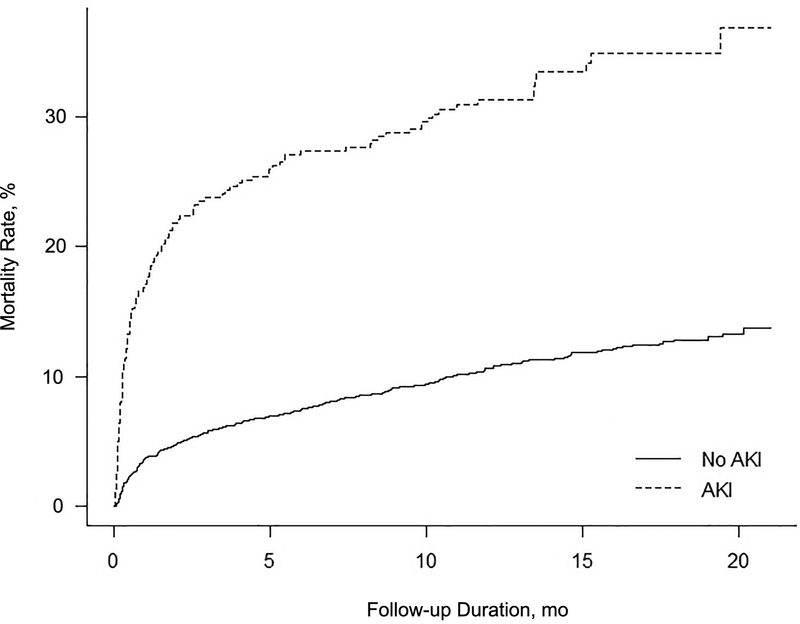
Patients with AKI had a higher mortality rate than those without AKI (33% [95% CI, 28%−37%] vs 11% [95% CI, 10%−12%]; P<.001). Among patients without hospital-acquired AKI, 30-day, 6-month, and 1-year mortality rates were 3.3% (95% CI, 2.8%−4.0%), 7.0% (95% CI, 6.2%−7.9%), and 10.0% (95% CI, 9.0%−11.0%), versus 17.3% (95% CI, 13.5%−21.0%), 27.2% (95% CI, 22.8%−31.6%), and 30.8% (95% CI, 26.2%−35.4%), respectively, among patients with hospital-acquired AKI (Cox proportional hazard ratio, 3.62; 95% CI, 2.97–4.43; P<.001) (Figure 2). In patients with AKI, the severity of AKI was associated with death (Cox proportional hazard ratio, 1.67; 95% CI, 1.34–2.16; P<.001).
Figure 2.
Kaplan-Meier Mortality Curves by Presence or Absence of Acute Kidney Injury (AKI) (Development Cohort).
Characteristics of the Validation Cohort
The main outcomes of the validation cohort were similar to those of the development cohort (Table 1). The incidence of any hospital-acquired AKI was 10.9% (n=351), with a 1.7% incidence (n=54) of moderate-severe AKI. Of these, 27 patients (0.8%) required initiation of hemodialysis during their hospital stay.
Development of a Risk-Prediction Model for Acute Kidney Injury
Supplemental Table 2 lists the ICD-9-CM codes used to identify comorbid conditions among the cohort. For the primary outcome of hospital-acquired AKI, several risk factors were identified by univariate logistic regression as associated with any stage of AKI (Supplemental Table 3) and moderate to severe AKI (Table 2). Results of the final multivariate logistic regression analysis are shown in Table 3. Variables included in the final model were baseline creatinine value, admission to a medical service, and presence of pulmonary disease, diabetes mellitus, kidney disease, cancer, hypertension, and vascular disease.
Table 2.
Comorbid Conditions Associated With Moderate-Severe Hospital-Acquired Acute Kidney Injury in the Development Cohort (Univariate Logistic Regression)
| Feature | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Cancer | 2.79 (1.67–4.54) | <.001 |
| Medical unit | 2.64 (1.51–4.94) | <.001 |
| Coagulopathy | 2.41 (1.30–4.48) | .001 |
| Heart failure | 2.07 (1.16–3.51) | .003 |
| Hypertension | 1.94 (1.14–3.45) | .01 |
| Vascular disease | 1.85 (1.15–2.95) | .02 |
| Renal disease | 1.76 (1.10–2.82) | .01 |
| Charlson score (per 1-point increase) | 1.15 (1.08–1.22) | .02 |
| Age (per 1-y increase) | 1.01 (1.01–1.02) | <.001 |
Table 3.
Final Multivariate Logistic Regression Model for Hospital-Acquired Acute Kidney Injury in the Development Cohort
| Feature | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Baseline creatinine (per 1-mg/dL increase) | 2.27 (1.87–2.78) | <.001 |
| Medical unit | 1.71 (1.32–2.25) | <.001 |
| Hypertension | 1.48 (1.10–1.99) | .01 |
| Diabetes mellitus | 1.42 (1.08–1.88) | .01 |
| Pre-existing kidney disease | 1.34 (1.04–1.73) | .02 |
| Cancer | 1.32 (1.00–1.72) | .05 |
| Pulmonary disease | 1.24 (0.97–1.57) | .08 |
| Vascular disease | 1.22 (0.96–1.56) | .10 |
The AUC ROC of the model was 0.72 (Supplemental Figure 3A). To test for calibration, the Hosmer-Lemeshow goodness-of-fit test revealed a χ2 of 36 (P=.58), which indicated proper calibration for the model (Supplemental Figure 3B).
Validation of the Risk-Prediction Model for Acute Kidney Injury
In the validation cohort of 3,232 patients, the AUC ROC of the AKI prediction model was 0.75, which indicated good discrimination (Supplemental Figure 4A). The Hosmer-Lemeshow goodness-of-fit test revealed a χ2 of 51 (P=.07), which indicated that the model was fairly calibrated in the validation cohort (Supplemental Figure 4B). The c-statistic was 0.75 (95% CI, 0.72–0.785). Supplemental Tables 4 and 5 contain the model sensitivity and specificity values in the validation cohort for cutoffs of 80% and 90%, respectively.
Discussion
In this study, we report the development of an AKI risk-prediction model for patients admitted to non-ICU settings which uses only historical data readily available at the time of hospital admission, including pre-existing comorbid conditions and baseline serum creatinine value. The resulting model performed well to identify patients at risk for hospital-acquired AKI, with an AUC ROC of 0.72 and 0.75 in a development and validation dataset, respectively. Thus, this tool could be used to identify patients admitted to a non-ICU ward who are at the greatest risk for AKI and might benefit from more intensive monitoring and/or tests for emerging biomarkers of AKI risk.
Although, as expected, the incidence of hospital-acquired AKI in our study was lower than what has been reported for ICU patients,32 AKI was still a common complication that was associated with a higher mortality rate and a longer hospital stay. AKI in such non–critically ill hospitalized patients presents certain unique challenges. The frequency and intensity of monitoring is often less vigorous in non-ICU settings, with less frequent vital sign checks, incomplete urine output monitoring, and a lower nurse-to-patient ratio. Thus, identification of evolving AKI could be delayed until the serum creatinine concentration is already increasing and after the kidney insult has been established, at which point interventions may have limited effectiveness for mitigating AKI progression and complications. Wilson et al33 studied the use of automated electronic notifications sent to providers to identify patients in the early stages of AKI, but this tool relied on increasing serum creatinine values. In that prospective study, no differences in peak serum creatinine, need for dialysis, or death at 7 days were observed between the alert group and the usual care group. One potential explanation is that reliance on serum creatinine value resulted in late recognition of AKI or AKI risk. This supports the need for a tool that can predict AKI, rather than detect it only after the fact when the serum creatinine starts to increase. Having access to a validated prediction model could enable the care team to focus resources on patients who would benefit most from preventive measures (ie, those with the highest pretest probability of AKI). Examples include more accurate monitoring of urine output, careful optimization of volume status, medication dose adjustments, and strict avoidance of nephrotoxic drugs in this higher-risk group. Such a prediction tool could also help identify patients who would benefit most from further testing with emerging AKI biomarkers.
Many AKI prediction models have been reported in the literature.24, 31–35 However, most of them have been developed for specific patient populations (eg, post cardiac surgery or critically ill patients) and cannot be reliably applied to other patient groups. Unlike many surgical patients, general ward patients often have multifactorial insults to the kidney with an unclear timing of onset. Thus, models developed in a cardiac surgery cohort may not work well in general ward patients.36, 37 Critically ill patients have higher AKI event rates than do non-ICU patients and also have unique risk factors. These factors can all affect the performance of any model developed in an ICU setting if applied to general ward patients.22, 23 For example, Malhotra et al32 reported a model that performed well (AUC ROC of 0.81) that required chronic comorbid conditions (as in our study) but also ICU-specific variables (such as the need for mechanical ventilation). Furthermore, the incidence of AKI in their high-risk ICU cohort was 37%, much higher than was observed in our study.
Thus, despite the many AKI prediction tools in the literature, parallel tools to detect AKI risk in the non-ICU setting are lacking. One notable exception is a recent study by Koyner and colleagues24 that, similar to our current study, developed an AKI risk-prediction algorithm using electronic health record data in a cohort of non-ICU patients. The incidence of AKI in that cohort was 8.6%, which is slightly lower than the incidence in our cohort (10%). Their model performed well, with an AUC ROC of 0.74, but it incorporated additional data including vital signs and laboratory tests that are sometimes not available at the time of admission. In a separate project, Koyner et al38 used a machine learning technique to predict AKI and developed a model that performed very well (AUC ROC, 0.96). However, that tool requires complex computer-based calculations, whereas our current model is intended for calculation at the bedside using routine and readily available variables.
Our study has important strengths. The risk factors identified in our study are consistent with the current literature.22, 24, 32, 39 Pre-existing kidney disease is a strong predictor of AKI in many existing models. Other risk factors in our model that were previously identified include heart failure, hypertension, and liver disease.25, 40–47 Our development and validation cohorts each had a large sample size. In addition, we studied a population-based cohort to avoid potential referral bias and maximize follow-up, and the definitions of the comorbid conditions were standardized on the basis of ICD-9-CM codes. The score validates far better for specificity than sensitivity, which suggests that this model could be most effectively used to rule out AKI. The higher-risk group might require further testing (eg, AKI biomarkers) to further stratify their AKI risk. The model is also relatively simple so that it can be easily incorporated into clinical practice as a screening tool for AKI in a non-ICU ward setting.
Our study also has several limitations. First, urine output was not used to diagnose AKI because it is not often accurately measured in hospitalized patients outside of the ICU. This most likely decreased the overall incidence of patients receiving an AKI diagnosis.48 However, more advanced AKI stages still were detected by the serum creatinine criteria. In addition, predictors of AKI were based on pre-existing comorbid conditions and did not include any acute risk factors specific to the hospitalization (such as hypotension, hypovolemia, or sepsis). These factors were intentionally omitted because our aim was to develop a model ready to be used on patient arrival to the hospital and guide their subsequent care. Furthermore, patients in this cohort were >90% white, relatively highly educated, and wealthy compared with the overall United States population. However, residents of Olmsted County have similar age- and sex-specific mortality rates to the United States population overall.49, 50 Thus, although the model will require further validation in other settings, there is no clear indication that it would not perform well in other cohorts. Finally, 15% of patients in the development cohort and 6% of patients in the validation cohort did not have a baseline serum creatinine value, which required the use of the serum creatinine at admission to impute a value. However, this approach has been previously validated.23
Conclusion
In this study, AKI occurred in 1 in 10 patients admitted to a non-ICU ward and was associated with a more than 3-fold increased risk of death and 2-fold longer hospital stay. A simple risk-prediction model was developed and validated which uses readily available clinical variables and can, thus, be easily incorporated into clinical care. This tool could enable identification of hospitalized patients at higher risk for AKI who might benefit from more intensive clinical monitoring and laboratory testing. Further research is needed to determine whether the use of such a tool to guide the use of AKI biomarkers and earlier interventions could decrease the incidence of AKI and related morbidities outside of the ICU.
Supplementary Material
Financial Support:
The study was supported by the Mayo Foundation and a grant from the Mayo Clinic Center for Clinical and Translational Science (UL1TR000135). This study was also made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder had no role in any aspect of the project.
Abbreviations
- AKI
acute kidney injury
- AUC ROC
area under the receiver operating characteristic curve
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICU
intensive care unit
Footnotes
Presented at the National Kidney Foundation 2016 Spring Clinical Meetings, Boston, Massachusetts, April 27-May 1, 2016.
Conflict of interest: None.
References
- 1.Kellum JA, Bellomo R, Ronco C. Kidney attack. JAMA. 2012;307(21):2265–2266. [DOI] [PubMed] [Google Scholar]
- 2.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77(6):527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453. [DOI] [PubMed] [Google Scholar]
- 4.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann. Surg 2009;249(5):851–858. [DOI] [PubMed] [Google Scholar]
- 5.Kellum JA, Angus DC. Patients are dying of acute renal failure. Crit. Care Med 2002;30(9):2156–2157. [DOI] [PubMed] [Google Scholar]
- 6.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J. Am. Soc. Nephrol 2007;18(4):1292–1298. [DOI] [PubMed] [Google Scholar]
- 7.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50(3):811–818. [DOI] [PubMed] [Google Scholar]
- 8.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. [DOI] [PubMed] [Google Scholar]
- 9.Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol 2006;17(4):1135–1142. [DOI] [PubMed] [Google Scholar]
- 10.Lewington AJ, Cerda J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84(3):457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashani K, Shao M, Li G, et al. No increase in the incidence of acute kidney injury in a population-based annual temporal trends epidemiology study. Kidney Int. 2017;92(3):721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ. 1993;306(6876):481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidney Disease: Improving Global Outcomes (KDIGO), Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int. 2012;2(S):1–138. [Google Scholar]
- 14.Fortescue EB, Bates DW, Chertow GM. Predicting acute renal failure after coronary bypass surgery: cross-validation of two risk-stratification algorithms. Kidney Int. 2000;57(6):2594–2602. [DOI] [PubMed] [Google Scholar]
- 15.Coritsidis GN, Guru K, Ward L, Bashir R, Feinfeld DA, Carvounis CP. Prediction of acute renal failure by “bedside formula” in medical and surgical intensive care patients. Ren. Fail 2000;22(2):235–244. [DOI] [PubMed] [Google Scholar]
- 16.Fan PC, Chang CH, Tsai MH, et al. Predictive value of acute kidney injury in medical intensive care patients with sepsis originating from different infection sites. Am. J. Med. Sci 2012;344(2):83–89. [DOI] [PubMed] [Google Scholar]
- 17.Plataki M, Kashani K, Cabello-Garza J, et al. Predictors of acute kidney injury in septic shock patients: an observational cohort study. Clin. J. Am. Soc. Nephrol 2011;6(7):1744–1751. [DOI] [PubMed] [Google Scholar]
- 18.Peres LA, Wandeur V, Matsuo T. Predictors of acute kidney injury and mortality in an Intensive Care Unit. J Bras Nefrol. 2015;37(1):38–46. [DOI] [PubMed] [Google Scholar]
- 19.Chawla LS, Abell L, Mazhari R, et al. Identifying critically ill patients at high risk for developing acute renal failure: a pilot study. Kidney Int. 2005;68(5):2274–2280. [DOI] [PubMed] [Google Scholar]
- 20.Cruz DN, Ferrer-Nadal A, Piccinni P, et al. Utilization of small changes in serum creatinine with clinical risk factors to assess the risk of AKI in critically lll adults. Clin. J. Am. Soc. Nephrol 2014;9(4):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu RK, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J. Am. Soc. Nephrol 2003;14(4):1022–1030. [DOI] [PubMed] [Google Scholar]
- 23.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013;17(1):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyner JL, Adhikari R, Edelson DP, Churpek MM. Development of a Multicenter Ward-Based AKI Prediction Model. Clin. J. Am. Soc. Nephrol 2016;11(11):1935–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matheny ME, Miller RA, Ikizler TA, et al. Development of inpatient risk stratification models of acute kidney injury for use in electronic health records. Med. Decis. Making 2010;30(6):639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ, 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin. Proc 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 30.Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract 2012;120(4):c179–184. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed A, Vairavan S, Akhoundi A, et al. Development and validation of electronic surveillance tool for acute kidney injury: A retrospective analysis. J. Crit. Care 2015;30(5):988–993. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra R, Kashani KB, Macedo E, et al. A risk prediction score for acute kidney injury in the intensive care unit. Nephrol. Dial. Transplant 2017;32(5):814–822. [DOI] [PubMed] [Google Scholar]
- 33.Wilson FP, Shashaty M, Testani J, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet. 2015;385(9981):1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kashani K, Herasevich V. Sniffing out acute kidney injury in the ICU: do we have the tools? Curr. Opin. Crit. Care 2013;19(6):531–536. [DOI] [PubMed] [Google Scholar]
- 35.Kashani K, Herasevich V. Utilities of Electronic Medical Records to Improve Quality of Care for Acute Kidney Injury: Past, Present, Future. Nephron. 2015;131(2):92–96. [DOI] [PubMed] [Google Scholar]
- 36.Sun S, Ma F, Li Q, et al. Risk model for deaths and renal replacement therapy dependence in patients with acute kidney injury after cardiac surgery. Interact. Cardiovasc. Thorac. Surg 2017;25(4):548–554. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Zou Z, Jin J, et al. Urinary TIMP-2 and IGFBP7 for the prediction of acute kidney injury following cardiac surgery. BMC Nephrol. 2017;18(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koyner JL, Carey KA, Edelson DP, Churpek MM. The Development of a Machine Learning Inpatient Acute Kidney Injury Prediction Model. Crit. Care Med 2018;46(7):1070–1077. [DOI] [PubMed] [Google Scholar]
- 39.Vivino G, Antonelli M, Moro ML, et al. Risk factors for acute renal failure in trauma patients. Intensive Care Med 1998;24(8):808–814. [DOI] [PubMed] [Google Scholar]
- 40.Palomba H, de Castro I, Neto AL, Lage S, Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS Score. Kidney Int. 2007;72(5):624–631. [DOI] [PubMed] [Google Scholar]
- 41.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J. Am. Soc. Nephrol 2005;16(1):162–168. [DOI] [PubMed] [Google Scholar]
- 42.Kristovic D, Horvatic I, Husedzinovic I, et al. Cardiac surgery-associated acute kidney injury: risk factors analysis and comparison of prediction models. Interact. Cardiovasc. Thorac. Surg 2015;21(3):366–373. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13(11):697–711. [DOI] [PubMed] [Google Scholar]
- 44.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J. Am. Coll. Cardiol 2004;44(7):1393–1399. [DOI] [PubMed] [Google Scholar]
- 45.Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology. 2009;110(3):505–515. [DOI] [PubMed] [Google Scholar]
- 46.Rueggeberg A, Boehm S, Napieralski F, et al. Development of a risk stratification model for predicting acute renal failure in orthotopic liver transplantation recipients. Anaesthesia. 2008;63(11):1174–1180. [DOI] [PubMed] [Google Scholar]
- 47.Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107(6):892–902. [DOI] [PubMed] [Google Scholar]
- 48.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by Urine Output versus Serum Creatinine Level. J. Am. Soc. Nephrol 2015;26(9):2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin. Proc 2012;87(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int. J. Epidemiol 2012;41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.