Abstract
Microtubules are essential cellular polymers assembled from tubulin heterodimers. The tubulin dimer consists of a compact folded globular core and intrinsically disordered C-terminal tails. The tubulin tails form a lawn of densely grafted, negatively charged, flexible peptides on the exterior of the microtubule, potentially akin to brush polymers in the field of synthetic materials. These tails are hotspots for conserved, chemically complex posttranslational modifications that have the potential to act in a combinatorial fashion to regulate microtubule polymer dynamics and interactions with microtubule effectors, giving rise to a “tubulin code”. In this review, I summarize our current knowledge of the enzymes that generate the astonishing tubulin chemical diversity observed in cells and describe recent advances in deciphering the roles of tubulin C-terminal tails and their posttranslational modifications in regulating the activity of molecular motors and microtubule associated proteins. Lastly, I outline the promises, challenges and potential pitfalls of deciphering the tubulin code.
Keywords: tubulin, microtubule, post-translational modification, posttranslational modification, tubulin tyrosine ligase, molecular motors, microtubule associated proteins, brush polymer, polyelectrolyte, intrinsically disordered proteins
1. Introduction
Microtubules are hollow cylindrical polymers built through the lateral association of protofilaments composed of longitudinally aligned head-to-tail α-tubulin dimers ([1,2]; Figure 1). Microtubules exhibit “dynamic instability” a property that endows them with stochastic growth and shrinkage through the addition or removal of tubulin dimers at their ends [3,4]. The architecture of the microtubule gives it polarity: the minus and plus ends of the microtubule are capped by α-tubulin and β-tubulin subunits, respectively (Figure 2). The two ends exhibit different behaviors, with the plus end exhibiting higher growth rates and more dynamics. Despite its highly dynamic nature, the microtubule is the most rigid cellular polymer known, exhibiting persistence lengths on the order of a cell’s dimension (ranging from hundreds of microns to as much as millimeters; [5–7]). These unique biophysical properties allow microtubules to perform essential functions in fundamental cellular processes like cell division, differentiation and motility.
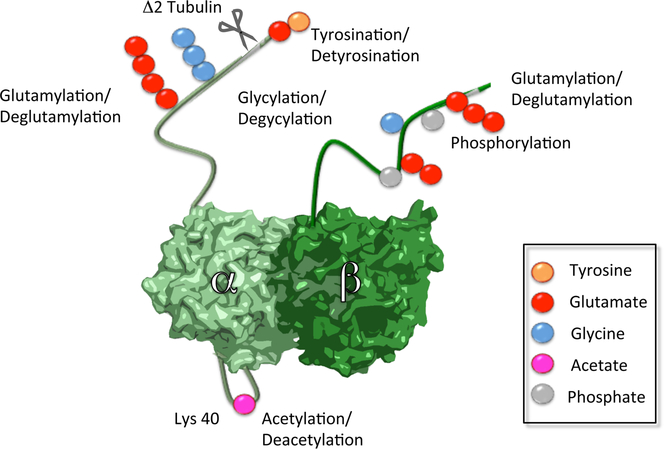
Figure 1. Structure of the αβ-tubulin dimer.
Sites of known posttranslational modifications are indicated by colored spheres (tyrosination, yellow; glutamylation, red; glycylation, cyan; acetylation, magenta; phosphorylation, grey).
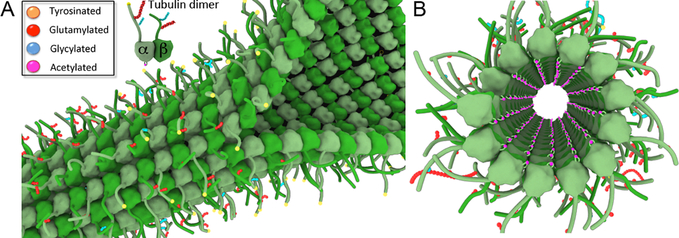
Figure 2. Microtubules are decorated with posttranslational modifications inside and outside.
A. View of the microtubule exterior surface showing the C-terminal tails decorating the microtubule shaft. Posttranslational modifications are denoted by colored spheres (tyrosination, yellow; glutamylation, red; glycylation, cyan).
B. View of the microtubule lumen. Acetylation at Lys40 is denoted by magenta spheres.
The tubulin dimer: a versatile building block for cellular infrastructure
Microtubules give rise to complex cellular structures with diverse morphologies and behaviors: the highly dynamic bipolar spindle, the exquisitely complex neuronal array, the disc-shaped marginal band in platelets, and the nine-fold symmetric arrays in cilia and flagella that can beat as fast as 110Hz [8,9]. All of these structures use the αβ-tubulin heterodimer as their basic building block. The tubulin dimer consists of a compactly folded “body” and disordered, negatively charged α− and β C-terminal tails ([2,10]; Figure 1). The tubulin body is involved in intimate tubulin-tubulin lattice interactions, while the C-terminal tails decorate the microtubule exterior ([1]; Figure 2). Not surprising for an essential polymer, most isoform sequence variations and posttranslational modifications are concentrated on the C-terminal tails (~50% sequence identity between tubulin tails, compared to 80–95% for the tubulin body) where subtle changes can potentially tune the biophysical properties of microtubules and their interactions with cellular effectors without interfering with essential polymerization interfaces [11]. This situation is analogous to histones where protomer interfaces have been stringently conserved and sequence variability and posttranslational modifications are concentrated on the positively charged N-terminal tails and give rise to the now widely accepted “histone code” [12]. By analogy to histones, the genetic and chemical complexity of tubulin has been proposed to form the basis of a “tubulin code” [13]. The tubulin C-terminal tails are in close proximity to the binding sites of many motors and microtubule associated proteins (MAPs) and thus can constitute a code that can be read by cytoskeletal effectors [11,13,14].
Humans have eight α-tubulin and seven β-tubulin isoforms [15]. Some of these isoforms are essential for highly specialized cells like sperm, platelets and neurons, consistent with their cell- or tissue-specific expression [16–21]. Many tubulin isoforms can coexist in a given cell type [22] although our understanding of their distribution and roles is still limited. Several studies support the idea that microtubule dynamics can be tuned in cells by varying the relative levels of tubulin isotypes [23,24]. Mutations in various tubulin isoforms have been associated with a broad spectrum of human pathologies ranging from blood clotting to neurological disorders. For example, mutations in βVI, a tubulin isoform most abundant in platelets, have been identified in patients with congenital macrothrombocytopenia [25]. Platelets from these patients are enlarged and spherical due to defects in the assembly of the microtubule marginal band. Mutations in several tubulin isoforms that co-assemble into neuronal microtubules have also been identified in a range of neurological disorders [26–29] and their locations in the tubulin structure suggests effects both on microtubule dynamics as well as the interaction of motors and MAPs [30].
Intrinsically disordered tubulin tails: lassos for microtubule regulators?
Despite their sequence and length variation, all tubulin C-terminal tails have two common features: they are negatively charged and flexible (Figure 3). The α-tubulin tails have a narrow length distribution, comprising 10–12 residues, while β-tubulin tails show larger length variation (16–22 residues) and more sequence divergence. Thus, the charge and length of the α- and β-tubulin tails is more stringently conserved than their sequences [31]. The C-terminal tails for α- and β-tubulin are disordered in all X-ray and high-resolution electron microscopic reconstructions determined to date, consistent with a high degree of flexibility; however, no biophysical data are currently available on their dynamics in the microtubule context. Molecular dynamics simulation studies suggest that they are extremely flexible, but can adopt transient helical conformations [32].
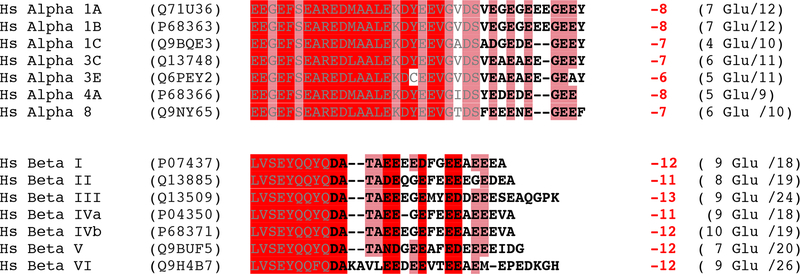
Figure 3. Tubulin C-terminal tails are variable and highly negatively charged.
Tubulin tail sequences from human α- and β-tubulin isoforms. Tubulin tail residues are in bold. Total net charge on the tubulin tails is indicated (including the C-terminal carboxylate).
Negatively charged residues, predominantly glutamates, are overrepresented in both α− and β-tubulin tails (Figure 3). Glutamates have one of the highest propensities for promoting intrinsic disorder in proteins [33]. Aspartate residues are also present, and further enhance the electronegativity of the tails. These anionic residues are interspersed with small nonpolar residues like alanine and valine. Order-promoting amino acids such as isoleucine, tyrosine, leucine and asparagine [33] are poorly represented. Only the C-terminal tails of the neuronal-specific βIII and the leukocyte- and platelet-specific βVI isoforms contain a single positively charged residue. Interestingly, the presence of this lysine decreases kinesin motility compared to the βII isoform [34]. Class III β-tubulin is expressed exclusively in neurons at the onset of differentiation and in certain tumors of non-neuronal origins such as lymphoma, squamous cell carcinoma and malignant melanoma where it is not found before transformation [35], suggesting that this isoform promotes neuronal specific interactions with molecular motors and MAPs.
Complete removal of both C-terminal tails by proteolytic digestion [36,37] lowers the processivity of molecular motors dynein and kinesin-1 [38,39]. More recent studies using S. cerevisiae engineered tubulin have shown that the presence of the β-tail alone is sufficient to rescue this defect in motility both for kinesin-1 and dynein [34]. Interestingly, removal of the α-tubulin C-terminal tail increases both the velocity and processivity of kinesin-2, suggesting that for this motor, the α-tubulin disordered tail functions as a brake [34]. There are currently no high-resolution structures of any motors where the tubulin C-terminal tails are visible, so the nature of the tail engagement with various motors is not well understood. This could be due to the fast kinetics of this interaction or possibly because the interaction is “fuzzy” [40] i.e. the tails adopt distinct, but multiple conformations when bound to the motor and these conformations are averaged out in the crystals or during EM reconstruction. Another possible reason for the lack of clear density of the C-terminal tails is a technical one. Until very recently, it was not possible to produce tubulin recombinantly. Thus, the main source of tubulin for biochemical and structural studies for more than forty years has been brain tissue. Tubulin from this source is highly heterogeneous, containing multiple isoforms of α- and β-tubulin as well as multiple posttranslational modifications [41,42]. Thus, the lack of a clearly defined conformation of the tubulin tails in motor complexes could also be due to the highly heterogeneous nature of the tubulin used to obtain crystals or cryo-electron microscopy (cryo-EM) reconstructions of motor complexes.
In addition to motors, tubulin C-terminal tails also form binding sites for several MAPs such as tau and MAP2 [36,43]. The proposed biophysical mechanism for microtubule severing enzymes spastin and katanin also beautifully illustrates the importance of tubulin tails to microtubule function [44]. Spastin forms a hexameric ring in an ATP-dependent manner and is thought to use its conserved central pore to “tug” on the C-terminal tails of tubulin, generating a mechanical force that destabilizes tubulin-tubulin interactions within the lattice, ultimately breaking the microtubule [45]. Experiments with partially proteolyzed microtubules showed that the severing activity of both spastin and katanin are dependent on the presence of the tubulin C-terminal tails [46,47]. The C-terminal tails also facilitate 1D diffusion of motors and MAPs on the microtubule lattice. For example, kinesin-13/MCAK glides on the microtubule tails to more efficiently find microtubule ends where it can exert its microtubule depolymerase activity [48]. Tau also undergoes bidirectional diffusion on the tubulin tails [49,50] and this mobility is thought to provide a moving roadblock for molecular motors [51–53]. A recent study in S. cerevisiae revealed an extensive network of regulators that interact genetically with the α- and β- tubulin C-terminal tails and uncovered distinct functions for each of the tails in microtubule network regulation [54]. Future work will establish how many of these regulators interact directly with the tubulin tails. Apart from regulating motors and MAPs, tubulin tails also regulate the voltage sensitive closure of the mitochondrial voltage dependent channel (VDAC) possibly by snaking through its pore [31].
Why intrinsically disordered tails? The flexibility and large radius of gyration of the tails have the potential to increase the odds of a productive collision with a variety of orientations of a rigid-body binding partner, thus increasing the rate of association. Disorder can also drastically enhance the off rate by enabling dissociation of part of the interface, analogous to an unzipping mechanism. Consistent with this, electron microscopic studies of kinesin-1 complexes revealed that the absence of the tails leads to a stronger ADP bound state, possibly slowing down the motors [55]. Moreover, the disordered tubulin tails can provide larger interaction surfaces for rigid binding partners. Since an energetic (entropic) penalty must be paid to order these tails concomitant with effector binding, this could give rise to high-specificity coupled with low affinity [56]. Consistent with this, the majority of microtubule regulators bind to microtubules with low- to mid-micromolar dissociation constants.
Disordered tubulin tails: hotspots for posttranslational modifications
In addition to genetic (i.e. isoform) variation, the C-terminal tails of tubulin are subject to abundant and evolutionarily conserved posttranslational modifications such as phosphorylation [57], detyrosination/tyrosination [58–60], glutamylation [61–64] and glycylation [65] (Figure 1). These modifications are chemically diverse and can be informationally complex. Tubulin is subject to additional posttranslational modifications outside the C-terminal tails such as acetylation [66,67], phosphorylation [68] and polyamination [69]. Thus, tubulin modifications give rise in vivo to a staggering number of possible combinations of modifications that are informationally complex and have the potential to tune the recruitment and behavior of microtubule regulators.
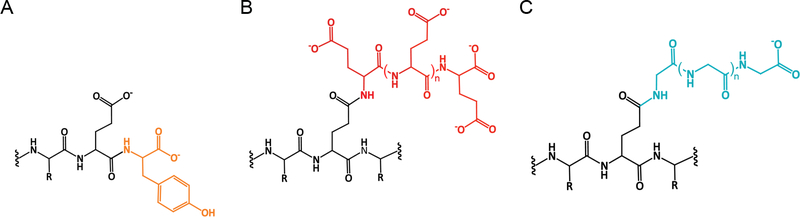
The modifications that are most identified with tubulin are tyrosination, glutamylation and glycylation. All these consist of the addition of an amino acid (tyrosine, glutamate or glycine) to a glutamate in the primary sequence of the α- or β-tubulin C-terminal tails (Figure 4). While tyrosination involves the formation of an α-linked peptide bond to the absolute C-terminal glutamate in α-tubulin, glutamylation and glycylation proceed through the formation of a γ-branched isopeptide bond on internal glutamates in the α-or β-tubulin tails [15,63,70]. After the formation of the branch, glutamates or glycines can be added linearly and chains containing 21 glutamates [15,71–73] and 34 glycines have been detected in cells [65]. While tyrosination and glutamylation are widely distributed in cells, glycylation seems to be a modification of cilia and flagella exclusively. In fact, this modification was first identified on tubulin isolated from the ciliate Paramecium [65]. Consistent with this, loss of tubulin glycylases leads to ciliary disassembly or pronounced defects in cilia and flagella biogenesis [74–77]. Interestingly, humans have lost the ability to elongate glycine chains as the TTLL10 enzyme that is responsible for this activity carries a mutation that renders it inactive [74].
Figure 4. Chemical structures of posttranslational modifications added to the disordered tubulin C-terminal tails.
A. Tyrosination
B. Glutamylation
C. Glycylation
In order to understand the functions of the tubulin tails, we need to understand their conformational ensembles and how these are modified by the presence of posttranslational modifications. Presently, there is no experimental information on the dynamics of modified tubulin tails. Polyglutamylation and polyglycylation with long chains have the potential to dramatically change the radius of gyration and conformations accessed by the tails. Molecular dynamics simulations revealed that polyglycine chains (longer than eight glycines) form ensembles of multiple compact, collapsed conformations in aqueous solutions [78]. Unlike polyglycine chains, peptides rich in negatively charged glutamates favor a swollen coil conformation as long-range electrostatic repulsion between side chains competes with the propensity of the polypeptide backbone to form a collapsed structure [78,79]. Thus, these two modifications, not only change the net charge on the tubulin tails, but also present two distinct types of disorder.
Tubulin tails are grafted longitudinally at 4 nm intervals (and 5 nm laterally) on the microtubule. We do not understand how the conformation of the tails is affected by the underlying negatively charged microtubule body, nor do we understand how they interact with each other at this grafting density and how these interactions are tuned by their varied and abundant posttranslational modifications. At higher density, polyglycine tracks can become more extended because of the available inter-chain contacts that compete with the intra-chain contacts that favor the collapsed structure. Thus, an increase in polyglycylation density on a microtubule can act as a conformational switch for the tubulin tails [80]. Moreover, the co-existence of polyglutamylation on a nearby site on the same tubulin tail or on the tail of a neighboring tubulin protomer in the microtubule can also affect the conformation of the polyglycine chain and bias it to a more extended conformation. Long polyglutamate chains can significantly increase the radii of gyration of the tails, making them larger than the grafting distance between the tails on the microtubule shaft. In this situation, the disordered tubulin tails would be analogous to polymer brushes, dense polymer assemblies tethered at one end to a solid substrate. In the field of synthetic polymers, brush polymers have been shown to have interesting emergent properties that are easily tunable by temperature, ionic strength or solvent interactions because the chains are forced to stretch away from the surface to avoid overlapping and to minimize charged repulsion [81]. Electrostatic repulsion forces could be significant in the case of long polyglutamate chains, consistent with the high sensitivity of brain tubulin polymerization to divalent ion concentrations.
TTL and TTLL proteins: an amino acid ligase superfamily
Tyrosination, glutamylation and glycylation are catalyzed by a family of enzymes whose founding member is tubulin tyrosine ligase (TTL). Thus, the name of the family is tubulin tyrosine ligase-like (TTLL). TTL was the first tubulin modification enzyme identified [60,82]. Most α-tubulin isoforms contain a genomically encoded C-terminal tyrosine that is removed and then added back by TTL as part of a detyrosination/tyrosination cycle conserved in almost all eukaryotes (S. cerevisiae α-tubulin has a terminal phenylalanine and the cycle is missing in this organism). The X-ray structure of TTL revealed an elongated molecule comprised of three domains: an N-domain, a central domain and a C-domain (Figure 5). The active site is at the center of the molecule in a stringently conserved binding pocket, and the ATP, essential for the ligation reaction, is cradled at the interface between the central and C-domains of the enzyme [83].
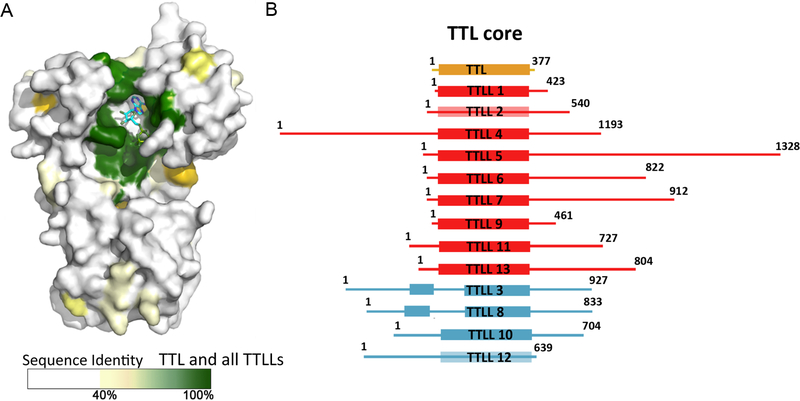
Figure 5. TTL is the structural core of the TTL-like family of tubulin modification enzymes.
A Surface representation of TTL color-coded according to sequence conservation.
B. Schematic domain representation of TTLL family members (residue numbers are for Mus Musculus sequences); tyrosine ligase shown in yellow; glutamylases, in red and glycylases, in cyan.
Despite variation in length among TTLLs from ~400 amino acids (TTLL1) to ~1300 (TTLL5) [84], TTL serves as the structural blueprint for the entire family, with all TTLLs containing a conserved core that is homologous to TTL (Figure 5B). Sequence conservation within the superfamily illustrates how the TTL scaffold supported the evolutionarily expansion of tubulin posttranslational modifications: while active site residues are invariant between all TTLs and TTLLs, consistent with a common reaction mechanism, surface residues that provide the binding surface for the tubulin tails have been reshuffled to accommodate the different substrate specificities of the various enzymes (α- versus. β-tubulin tail, Tyr, Glu or Gly addition; Figure 5). The mechanism behind the preference for α- versus β-tubulin tails of TTLL enzymes is not understood. Moreover, we do not know whether they exhibit any tubulin isoform preference. Experiments performed so far indicate that TTLLs are promiscuous; however, their promiscuity is mostly inferred from overexpression studies and currently we do not know what the physiological concentrations of these enzymes are. One attractive hypothesis is that the large repertoire of TTLLs, especially polyglutamylases, is needed to fine-tune them for the recognition of specific tubulin isoforms.
Tubulin modifications are reversible: the CCP family of tubulin carboxypeptidases
The action of TTL and TTLLs is reversed by cellular carboxypeptidases. Recent studies have identified several carboxypeptidases (CCPs) that are members of the MC clan and M14D subfamily of metallopeptidases that are capable of removing both long polyglutamate chains as well as branched glutamates from tubulin tails [85–88]. Overexpression studies coupled with the use of antibodies that are specific for long glutamate chains versus glutamate branches show that CCP1, 4 and 6 remove long polyglutamate chains while CCP5 shows a preference for removing branched glutamates [87], however can also hydrolyze α-linked peptide bonds [88]. To the field’s frustration, the enzyme responsible for the removal of the C-terminal tyrosine on α-tubulin has remained a mystery for several decades, despite intense efforts from multiple groups. As in the case of TTLLs, the structure and substrate preferences of these enzymes are poorly understood and will likely attract attention in the following years, especially since loss of CCP enzymes has been implicated in neurodegeneration [87] and defects in axonal regeneration after injury [89]. Moreover, we do not currently know how the activity of these enzymes is affected by the presence of neighboring posttranslational modifications on tubulin tails. For example, could a neighboring polyglycine chain obstruct access of deglutamylating enzymes to a polyglutamylated microtubule and could this be a mechanism to maintain high levels of polyglutamylation in cilia and flagella that are heavily glycylated?
Tyrosination, polyglutamylation and polyglycyation: ON/OFF switches and rheostats for tuning interactions with the microtubule surface?
The C-terminal tyrosine in α-tubulin serves as an ON/OFF signal for the recruitment of microtubule effectors. For example, the microtubule dynamics regulators cytoplasmic linker protein-170, the p150Glued dynactin subunit [90,91] and the mitotic centromere-associated kinesin [92] are recruited to the growing tip of microtubules in a tyrosination-dependent manner. All these regulators recognize the short linear GEEY/F motif in the α-tubulin tail. It is not known how modifications at other sites on the α-tubulin tail affect access and recognition of this motif by TTL and microtubule plus end tracking proteins. The tyrosination mark can also act as an OFF switch [93,94]. For example, in neurons kinesin-1 discriminates against tyrosinated microtubules present in somatodendrites and prefers the detyrosinated microtubules in the axon [94]. A structural element in the motor domain of kinesin is responsible for this discrimination and mutations in this region were shown to abrogate this preference and result in uniform localization of kinesin to both axonal and somatodendritic compartments [94].
TTL forms a 1:1 complex with tubulin and preferentially modifies the unpolymerized tubulin dimer, even preventing it from incorporating into the microtubule lattice [83,95]. Thus, tubulin that is newly incorporated into microtubules is highly enriched in the tyrosination mark. Because the detyrosination reaction occurs primarily on polymerized microtubules [96], a gradient of tyrosination from the older part of the microtubule to the new, growing end of the microtubule is created. By tuning the rates of these two opposing reactions, cells can tune the steepness of the tyrosination gradient on the microtubule and potentially change the density of regulators that are recruited in a tyrosination specific manner to the microtubule [83]. Thus, even though tyrosination serves as a binary switch at the level of the tubulin dimer, it can create a gradient at the mesoscopic scale of the microtubule
In contrast to tyrosination, polyglutamylation and polyglycylation can potentially produce gradated modification of tubulin (by virtue of the variable number of glutamates and glycines added), and therefore subtly tune the biophysical properties of the polymer itself or regulate microtubule interactions with motors and MAPs. The microtubule binding sites of motors and MAPs are enriched in positively charged residues. Thus, the glutamate chains on tubulin tails can act as affinity tuners for these interactions. While an attractive mechanism for modulating microtubule properties in a localized manner, such rheostat-like function of polyglutamylation and polyglycylation remains to be demonstrated biophysically. A wide range in the numbers of polyglutamate residues added to tubulin tails has been observed within cells as well as between different cell types [15,71,72]. Tubulin isolated from human embryonic kidney 293 cells (HEK293) cells has no detectable polyglutamylation [97]; tubulin purified from brain tissue has on average between 3–6 glutamates and as many as eleven [72]. Microtubules in axons are more extensively polyglutamylated than those in the somatodendritic compartment. The longest glutamate chains are observed in ciliary microtubules, where tails with at least twenty-one glutamates have been documented [71,73] and it is likely that longer chains exist but are hard to detect by mass spectrometry because of the low signal of these highly-negatively charged peptides.
Qualitative studies using blot overlay assays have shown that kinesin-1, MAP1B, MAP2 and tau interact preferentially with microtubules that have up to three glutamates on their tails [98–100], while MAP1A shows a preference for microtubules with longer glutamate chains containing up to six glutamates [100]. The microtubule severing enzymes spastin and katanin both show a preference for polyglutamylated microtubules that are especially enriched in neurons [45,101]. Spastin is the most mutated protein in hereditary spastic paraplegias characterized by progressive axonopathy [102]. In animal models, loss of spastin function leads to defects in microtubules dynamics in axons and lower density of microtubules in synaptic boutons [103,104]. An attractive model is that spastin selectively sculpts the glutamylated microtubules in these arrays. Ex vivo studies in which various TTLL enzymes were overexpressed indicate a positive correlation between the degree of polyglutamylation and the overall activity of spastin [101]. The number of added glutamates in these different microtubule preparations as well as the mechanistic basis for this upregulation of severing activity remains to be unraveled.
Tubulin acetylation: functionalizing the microtubule lumen
An intriguing microtubule modification that does not follow the paradigm of all other modifications is acetylation of α-tubulin Lys 40 [67] by tubulin acetyltransferase (TAT) [105,106]. Rather than occurring in the intrinsically disordered tubulin C-terminal tails, this site of acetylation lies in a flexible internal loop of the body of α-tubulin that projects into the microtubule lumen. TAT is conserved in all organisms with cilia or flagella and its loss leads to defective axonal morphology and neurodegeneration in C.elegans [107,108] and sperm abnormalities in mice [109]. Tubulin acetylation is highly enriched in cilia and flagella and, like polyglutamylation, accumulates on long-lived cytoplasmic microtubules [110–114]. Thus, acetylated microtubules have almost become synonymous with stable microtubules in cell biology. This selective marking of stable microtubules is a consequence of the preference of the enzyme for the microtubule over free tubulin and its remarkably low catalytic rate that is on the order of the lifetime of stable cellular microtubules (t1/2~2–16 hours) as opposed to dynamic microtubules (t1/2~2–5 min)[115].
The outer and inner diameters of the microtubule are 24 nm and 15 nm, respectively. Early electron micrographs revealed electron-dense material in the microtubule lumen of insect sperm tails [116,117], platelets [118,119] and neurons [120–122]. Recent electron cryo-tomography also revealed discrete, closely spaced particles decorating the lumen of flagellar [123,124] and neuronal [107,125] microtubules. Intriguingly, they were absent in Potorous tridactylus kidney (PtK) cells that lack detectable tubulin acetylation [125] as well as in neurons from TAT-null C. elegans [107]. These particles disappeared from the lumen upon rapid disassembly and reassembly of microtubules [122] suggesting a slow accumulation with microtubule age and possibly an acetylation-dependent recruitment of luminal proteins. While the existence of luminal microtubule particles has been known for decades, their molecular identities and functions remain uncharacterized and constitute an interesting avenue of future exploration into this confined cytoplasmic compartment.
Effects of tubulin tails and posttranslational modifications on polymer properties
For decades, cell biologists have used antibodies against posttranslationally modified tubulins to distinguish between microtubule populations in cells that display different dynamics. Early work established that dynamic microtubules with short lifetimes (t1/2~ 2–5 min) are enriched in tyrosinated tubulin while stable microtubules (t1/2>1 hr) that are resistant to cold treatment or drug induced depolymerization are enriched in detyrosination and acetylation [113,126]. The hyperstable microtubules found in cilia and flagella are also heavily polyglutamylated [61], polyglycylated [127] and acetylated [111]. However, a long-standing question in microtubule cell biology has been whether these modifications affect the stability of the microtubule directly, whether they are a consequence of increased stability or whether they recruit factors in a modification specific manner that subsequently change their dynamic parameters. It is also possible that the modification is first acquired in an age-dependent manner (i.e., the more stable microtubules are selected for the action of the modification enzymes) and then the modification itself affects polymer stability and also recruits cellular effectors that then can create a positive feedback loop that strengthens the initial stabilization event.
Early studies established the importance of the C-terminal tails for tubulin polymerization. Subtilisin-treated tubulin that lacks the C-terminal tails polymerizes into microtubules at significantly lower concentrations than tubulin purified from brain tissue that contains multiple tubulin isoforms carrying a large number of posttranslational modifications (the critical concentration, the minimal concentration at which polymer will form, is ~ 50 fold lower after removal of both α- and β-tubulin tails by subtilisin treatment). Moreover, tubulin lacking C-terminal tails also polymerizes in sheets, rings and aggregates [37], indicating that even though the tubulin tails are not directly engaged in the polymerization interfaces, long-range interactions between the tails affect polymerization. Tubulin with low levels of posttranslational modifications purified from HeLa cells also has a lower critical concentration than tubulin purified from brain [128], suggesting that the posttranslational modifications enriched in brain tubulin affect microtubule nucleation. However, tubulin isolated from HeLa cells is also enriched in different isoforms than those found in brain tubulin preparations, so the observed differences could also be due to isoform differences. In vitro microtubule dynamics experiments with well-defined tubulin isoform compositions and posttranslational modifications will be needed to understand the contribution of the C-terminal tail sequences and modifications to tubulin polymerization dynamics and microtubule thermal and mechanical stability.
Concluding Remarks
The properties of the microtubule polymer are fascinating and despite intense, multifaceted investigations into their nature, we still understand very little about the basic physical principles that govern their dynamic behavior and mechanical properties and how these properties are modulated by isoform-specific sequence variations and posttranslational modifications. Quantitative biophysical approaches will be key to future studies because cells have likely devised combinatorial, complex means of compensating for changes in microtubule behaviors through these modifications.
Technical difficulties in obtaining unmodified tubulin and tubulin that is marked uniformly with only one type of modification (and where the poly-amino acid chain length can be varied) have hindered quantitative biophysical studies into the effects of posttranslational modifications. At a most basic level, it is not yet clear how any of these modifications affect the conformational space sampled by the tubulin tails. One would expect that adding a chain of negatively charged glutamates will likely have a strong effect on the electrostatic interaction with molecular effectors and affect the polymerization and depolymerization kinetics of the microtubule; or that adding a long chain of glycines will increase the exclusion volume around the microtubule and act as a barrier for potential binding partners. Recent advances in obtaining biochemical amounts of tubulin carrying only one type of posttranslational modification by purifying unmodified tubulin from cells that have naturally low levels of posttranslational modifications [129] and then modifying it in vitro with homogenous active preparations of tubulin modification enzymes [97] finally open the possibility of quantitative biophysical investigations into the effects of posttranslational modifications on microtubule dynamics, mechanical properties as well as interactions with motors and MAPs. S. cerevisiae has also proven to be a useful system to express tubulin chimeras composed of the yeast tubulin body upon which human tubulin tails were grafted that carry a cysteine to which a polyglutamate chain can be linked [34]. However, in this system, the glutamate chain is not linked through the glutamate branch that the native glutamylation reaction creates. The more recent success of expressing α1ΑβIII tubulin using a baculovirus system [130] finally opens the possibility of producing different tubulin isoforms as well as mutant human tubulins in biochemical amounts for biophysical studies.
Like many epigenetic biochemical phenomena, the effects of tubulin posttranslational modifications can potentially be quantitative rather than qualitative. That is, a given modification (in a particular location of a specific cell type) could result in fine-tuning of fitness or developmental or behavioral pattern, rather than overt organismal life and death. With the advent of methodology to make biochemical and biophysical quantities of well-characterized modified tubulin and microtubules, the next critical challenge for this field will therefore be to devise quantitative assays that can characterize the effects of these modifications in vivo and in vitro even when they are complex, subtle and combinatorial.
Highlights.
Tubulin is composed of a folded core and intrinsically disordered C-terminal tails
Disordered, negatively charged tubulin tails decorate the microtubule exterior
Tubulin tails are hotspots for genetic variation and posttranslational modifications
Tubulin posttranslational modifications can function as complex tuners of microtubule regulators and polymer dynamics
Acknowledgements
I apologize to the many colleagues whose work I was unable to cover because of space limitations. I also thank the anonymous reviewers for their insights and suggestions. A. R-M. is a Searle Scholar and is supported by the intramural programs of the National Institute of Neurological Disorders and Stroke (NINDS) and the National Heart, Lung and Blood Institute (NHLBI).
References
- 1.Nogales E, Whittaker M, Milligan RA & Downing KH High-resolution model of the microtubule. Cell 96, 79–88 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Nogales E, Wolf SG & Downing KH Structure of the alpha beta tubulin dimer by electron crystallography. Nature 391, 199–203, doi: 10.1038/34465 (1998). [DOI] [PubMed] [Google Scholar]
- 3.Mitchison T & Kirschner M Microtubule assembly nucleated by isolated centrosomes. Nature 312, 232–237 (1984). [DOI] [PubMed] [Google Scholar]
- 4.Mitchison T & Kirschner M Dynamic instability of microtubule growth. Nature 312, 237–242 (1984). [DOI] [PubMed] [Google Scholar]
- 5.Gittes F, Mickey B, Nettleton J & Howard J Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biol 120, 923–934 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Mameren J, Vermeulen KC, Gittes F & Schmidt CF Leveraging single protein polymers to measure flexural rigidity. The journal of physical chemistry. B 113, 3837–3844 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Felgner H, Frank R & Schliwa M Flexural rigidity of microtubules measured with the use of optical tweezers. J Cell Sci 109 (Pt 2), 509–516 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Lodish H et al. Molecuar Cell Biology. (Freeman WH, 2000).
- 9.Alberts B et al. Molecular Biology of the Cell. (Garland Science, 2002). [Google Scholar]
- 10.Lowe J, Li H, Downing KH & Nogales E Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol 313, 1045–1057, doi: 10.1006/jmbi.2001.5077 S0022-2836(01)95077-6 [pii] (2001). [DOI] [PubMed] [Google Scholar]
- 11.Garnham CP & Roll-Mecak A The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton 69, 442–463, doi: 10.1002/cm.21027 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenuwein T & Allis CD Translating the histone code. Science 293, 1074–1080, doi: 10.1126/science.1063127 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Verhey KJ & Gaertig J The tubulin code. Cell Cycle 6, 2152–2160 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Fulton C & Simpson PA Selective synthesis and utilization of flagellar tubulin. The multitubulin hypothesis. Cell. Motil. 3, 8 (1976). [Google Scholar]
- 15.Redeker V Mass spectrometry analysis of C-terminal posttranslational modifications of tubulins. Methods Cell Biol 95, 77–103, doi: 10.1016/S0091-679X(10)95006-1 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Villasante A, Lewis SA & Cowan NJ The mammalian beta-tubulin repertoire: hematopoietic expression of a novel, heterologous beta-tubulin isotype. J Cell Biol 103, 1903–1910 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villasante A et al. Six mouse alpha-tubulin mRNAs encode five distinct isotypes: testis-specific expression of two sister genes. Mol Cell Biol 6, 2409–2419 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denoulet P, Edde B & Gros F Differential expression of several neurospecific beta-tubulin mRNAs in the mouse brain during development. Gene 50, 289–297 (1986). [DOI] [PubMed] [Google Scholar]
- 19.Lewis SA, Lee MG & Cowan NJ Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol 101, 852–861 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis SA, Gilmartin ME, Hall JL & Cowan NJ Three expressed sequences within the human beta-tubulin multigene family each define a distinct isotype. J Mol Biol 182, 11–20 (1985). [DOI] [PubMed] [Google Scholar]
- 21.Cleveland DW, Kirschner MW & Cowan NJ Isolation of separate mRNAs for alpha- and beta-tubulin and characterization of the corresponding in vitro translation products. Cell 15, 1021–1031 (1978). [DOI] [PubMed] [Google Scholar]
- 22.Lewis SA, Gu W & Cowan NJ Free intermingling of mammalian beta-tubulin isotypes among functionally distinct microtubules. Cell 49, 539–548 (1987). [DOI] [PubMed] [Google Scholar]
- 23.Lu Q & Luduena RF In vitro analysis of microtubule assembly of isotypically pure tubulin dimers. Intrinsic differences in the assembly properties of alpha beta II, alpha beta III, and alpha beta IV tubulin dimers in the absence of microtubule-associated proteins. J Biol Chem 269, 2041–2047 (1994). [PubMed] [Google Scholar]
- 24.Panda D, Miller HP, Banerjee A, Luduena RF & Wilson L Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc Natl Acad Sci U S A 91, 11358–11362 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunishima S, Kobayashi R, Itoh TJ, Hamaguchi M & Saito H Mutation of the beta1-tubulin gene associated with congenital macrothrombocytopenia affecting microtubule assembly. Blood 113, 458–461, doi: 10.1182/blood-2008-06-162610 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Tischfield MA et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 140, 74–87, doi: 10.1016/j.cell.2009.12.011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niwa S, Takahashi H & Hirokawa N beta-Tubulin mutations that cause severe neuropathies disrupt axonal transport. EMBO J 32, 1352–1364, doi: 10.1038/emboj.2013.59 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keays DA et al. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell 128, 45–57, doi: 10.1016/j.cell.2006.12.017 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cederquist GY et al. An inherited TUBB2B mutation alters a kinesin-binding site and causes polymicrogyria, CFEOM and axon dysinnervation. Hum Mol Genet 21, 5484–5499, doi: 10.1093/hmg/dds393 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tischfield MA, Cederquist GY, Gupta ML Jr. & Engle EC Phenotypic spectrum of the tubulin-related disorders and functional implications of disease-causing mutations. Curr Opin Genet Dev 21, 286–294, doi: 10.1016/j.gde.2011.01.003 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rostovtseva TK et al. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci U S A 105, 18746–18751, doi: 10.1073/pnas.0806303105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luchko T, Huzil JT, Stepanova M & Tuszynski J Conformational analysis of the carboxy-terminal tails of human beta-tubulin isotypes. Biophys J 94, 1971–1982, doi: 10.1529/biophysj.107.115113 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campen A et al. TOP-IDP-scale: a new amino acid scale measuring propensity for intrinsic disorder. Protein and peptide letters 15, 956–963 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sirajuddin M, Rice LM & Vale RD Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol 16, 335–344, doi: 10.1038/ncb2920 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgoyne RD, Cambray-Deakin MA, Lewis SA, Sarkar S & Cowan NJ Differential distribution of beta-tubulin isotypes in cerebellum. EMBO J 7, 2311–2319 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serrano L, Avila J & Maccioni RB Controlled proteolysis of tubulin by subtilisin: localization of the site for MAP2 interaction. Biochemistry 23, 4675–4681 (1984). [DOI] [PubMed] [Google Scholar]
- 37.Bhattacharyya B, Sackett DL & Wolff J Tubulin, hybrid dimers, and tubulin S. Stepwise charge reduction and polymerization. J Biol Chem 260, 10208–10216 (1985). [PubMed] [Google Scholar]
- 38.Wang Z & Sheetz MP The C-terminus of tubulin increases cytoplasmic dynein and kinesin processivity. Biophys J 78, 1955–1964, doi: 10.1016/S0006-3495(00)76743-9 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorn KS, Ubersax JA & Vale RD Engineering the processive run length of the kinesin motor. J Cell Biol 151, 1093–1100 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tompa P & Fuxreiter M Fuzzy complexes: polymorphism and structural disorder in protein-protein interactions. Trends Biochem Sci 33, 2–8, doi: 10.1016/j.tibs.2007.10.003 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Wolff A, Denoulet P & Jeantet C High level of tubulin microheterogeneity in the mouse brain. Neurosci Lett 31, 323–328 (1982). [DOI] [PubMed] [Google Scholar]
- 42.Zambito AM, Knipling L & Wolff J Charge variants of tubulin, tubulin S, membrane-bound and palmitoylated tubulin from brain and pheochromocytoma cells. Biochim Biophys Acta 1601, 200–207 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Marya PK, Syed Z, Fraylich PE & Eagles PA Kinesin and tau bind to distinct sites on microtubules. J Cell Sci 107 (Pt 1), 339–344 (1994). [DOI] [PubMed] [Google Scholar]
- 44.Roll-Mecak A & McNally FJ Microtubule-severing enzymes. Curr Opin Cell Biol 22, 96–103, doi: 10.1016/j.ceb.2009.11.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roll-Mecak A & Vale RD Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature 451, 363–367, doi: 10.1038/nature06482 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartman JJ & Vale RD Microtubule disassembly by ATP-dependent oligomerization of the AAA enzyme katanin. Science 286, 782–785 (1999). [DOI] [PubMed] [Google Scholar]
- 47.Roll-Mecak A & Vale RD The Drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Curr Biol 15, 650–655 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Helenius J, Brouhard G, Kalaidzidis Y, Diez S & Howard J The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature 441, 115–119, doi: 10.1038/nature04736 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Hinrichs MH et al. Tau protein diffuses along the microtubule lattice. J Biol Chem 287, 38559–38568, doi: 10.1074/jbc.M112.369785 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McVicker DP, Hoeprich GJ, Thompson AR & Berger CL Tau interconverts between diffusive and stable populations on the microtubule surface in an isoform and lattice specific manner. Cytoskeleton 71, 184–194, doi: 10.1002/cm.21163 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dixit R, Ross JL, Goldman YE & Holzbaur EL Differential regulation of dynein and kinesin motor proteins by tau. Science 319, 1086–1089, doi: 10.1126/science.1152993 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoeprich GJ, Thompson AR, McVicker DP, Hancock WO & Berger CL Kinesin’s neck-linker determines its ability to navigate obstacles on the microtubule surface. Biophys J 106, 1691–1700, doi: 10.1016/j.bpj.2014.02.034 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scholz T & Mandelkow E Transport and diffusion of Tau protein in neurons. Cellular and molecular life sciences : CMLS 71, 3139–3150, doi: 10.1007/s00018-014-1610-7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aiken J et al. Genome-wide analysis reveals novel and discrete functions for tubulin carboxy-terminal tails. Curr Biol 24, 1295–1303, doi: 10.1016/j.cub.2014.03.078 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skiniotis G et al. Modulation of kinesin binding by the C-termini of tubulin. EMBO J 23, 989–999, doi: 10.1038/sj.emboj.7600118 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dyson HJ & Wright PE Coupling of folding and binding for unstructured proteins. Curr Opin Struct Biol 12, 54–60 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Eipper BA Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci U S A 69, 2283–2287 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barra HS, Rodriguez JA, Arce CA & Caputto R A soluble preparation from rat brain that incorporates into its own proteins ( 14 C)arginine by a ribonuclease-sensitive system and ( 14 C)tyrosine by a ribonuclease-insensitive system. J Neurochem 20, 97–108 (1973). [DOI] [PubMed] [Google Scholar]
- 59.Arce CA, Rodriguez JA, Barra HS & Caputo R Incorporation of L-tyrosine, L-phenylalanine and L-3,4-dihydroxyphenylalanine as single units into rat brain tubulin. Eur J Biochem 59, 145–149 (1975). [DOI] [PubMed] [Google Scholar]
- 60.Raybin D & Flavin M An enzyme tyrosylating alpha-tubulin and its role in microtubule assembly. Biochem Biophys Res Commun 65, 1088–1095 (1975). [DOI] [PubMed] [Google Scholar]
- 61.Eddé B et al. Posttranslational glutamylation of alpha-tubulin. Science 247, 83–85 (1990). [DOI] [PubMed] [Google Scholar]
- 62.Alexander JE et al. Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc Natl Acad Sci U S A 88, 4685–4689 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Redeker V, Le Caer JP, Rossier J & Prome JC Structure of the polyglutamyl side chain posttranslationally added to alpha-tubulin. J Biol Chem 266, 23461–23466 (1991). [PubMed] [Google Scholar]
- 64.Redeker V, Melki R, Prome D, Le Caer JP & Rossier J Structure of tubulin C-terminal domain obtained by subtilisin treatment. The major alpha and beta tubulin isotypes from pig brain are glutamylated. FEBS Lett 313, 185–192 (1992). [DOI] [PubMed] [Google Scholar]
- 65.Redeker V et al. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science 266, 1688–1691 (1994). [DOI] [PubMed] [Google Scholar]
- 66.Greer K, Maruta H, L’Hernault SW & Rosenbaum JL Alpha-tubulin acetylase activity in isolated Chlamydomonas flagella. J Cell Biol 101, 2081–2084 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.L’Hernault SW & Rosenbaum JL Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 24, 473–478 (1985). [DOI] [PubMed] [Google Scholar]
- 68.Fourest-Lieuvin A et al. Microtubule regulation in mitosis: tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Mol Biol Cell 17, 1041–1050, doi: 10.1091/mbc.E05-07-0621 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song Y et al. Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules. Neuron 78, 109–123, doi: 10.1016/j.neuron.2013.01.036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Redeker V, Rusconi F, Mary J, Prome D & Rossier J Structure of the C-terminal tail of alpha-tubulin: increase of heterogeneity from newborn to adult. J Neurochem 67, 2104–2114 (1996). [DOI] [PubMed] [Google Scholar]
- 71.Geimer S, Teltenkotter A, Plessmann U, Weber K & Lechtreck KF Purification and characterization of basal apparatuses from a flagellate green alga. Cell Motil Cytoskeleton 37, 72–85, doi: (1997). [DOI] [PubMed] [Google Scholar]
- 72.Redeker V, Rossier J & Frankfurter A Posttranslational modifications of the C-terminus of alpha-tubulin in adult rat brain: alpha 4 is glutamylated at two residues. Biochemistry 37, 14838–14844, doi: 10.1021/bi981335k (1998). [DOI] [PubMed] [Google Scholar]
- 73.Schneider A, Plessmann U, Felleisen R & Weber K Posttranslational modifications of trichomonad tubulins; identification of multiple glutamylation sites. FEBS Lett 429, 399–402 (1998). [DOI] [PubMed] [Google Scholar]
- 74.Rogowski K et al. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 137, 1076–1087, doi:S0092–8674(09)00577–7 [pii] 10.1016/j.cell.2009.05.020 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Wloga D et al. TTLL3 Is a tubulin glycine ligase that regulates the assembly of cilia. Dev Cell 16, 867–876, doi:S1534–5807(09)00170–1 [pii] 10.1016/j.devcel.2009.04.008 (2009). [DOI] [PubMed] [Google Scholar]
- 76.Pathak N, Austin CA & Drummond IA Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility. J Biol Chem 286, 11685–11695, doi: 10.1074/jbc.M110.209817 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bosch Grau M et al. Tubulin glycylases and glutamylases have distinct functions in stabilization and motility of ependymal cilia. J Cell Biol 202, 441–451, doi: 10.1083/jcb.201305041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran HT, Mao A & Pappu RV Role of backbone-solvent interactions in determining conformational equilibria of intrinsically disordered proteins. Journal of the American Chemical Society 130, 7380–7392, doi: 10.1021/ja710446s (2008). [DOI] [PubMed] [Google Scholar]
- 79.van der Lee R et al. Classification of intrinsically disordered regions and proteins. Chemical reviews 114, 6589–6631, doi: 10.1021/cr400525m (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pappu RV, Wang X, Vitalis A & Crick SL A polymer physics perspective on driving forces and mechanisms for protein aggregation. Archives of biochemistry and biophysics 469, 132–141, doi: 10.1016/j.abb.2007.08.033 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao B & Brittain WJ Polymer brushes: surface-immobilized macromolecules. Progress Polym. Sci. 25, 14 (2000). [Google Scholar]
- 82.Raybin D & Flavin M Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry 16, 2189–2194 (1977). [DOI] [PubMed] [Google Scholar]
- 83.Szyk A, Deaconescu AM, Piszczek G & Roll-Mecak A Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nat Struct Mol Biol 18, 1250–1258, doi: 10.1038/nsmb.2148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Dijk J et al. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell 26, 437–448, doi:S1097–2765(07)00248–1 [pii] 10.1016/j.molcel.2007.04.012 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Kalinina E et al. A novel subfamily of mouse cytosolic carboxypeptidases. FASEB J 21, 836–850, doi: 10.1096/fj.06-7329com (2007). [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez de la Vega M. et al. Nna1-like proteins are active metallocarboxypeptidases of a new and diverse M14 subfamily. FASEB J 21, 851–865, doi: 10.1096/fj.06-7330com (2007). [DOI] [PubMed] [Google Scholar]
- 87.Rogowski K et al. A family of protein-deglutamylating enzymes associated with neurodegeneration. Cell 143, 564–578, doi: 10.1016/j.cell.2010.10.014 (2010). [DOI] [PubMed] [Google Scholar]
- 88.Berezniuk I et al. Cytosolic carboxypeptidase 5 removes alpha- and gamma-linked glutamates from tubulin. J Biol Chem 288, 30445–30453, doi: 10.1074/jbc.M113.497917 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghosh-Roy A, Goncharov A, Jin Y & Chisholm AD Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Dev Cell 23, 716–728, doi: 10.1016/j.devcel.2012.08.010 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bieling P et al. CLIP-170 tracks growing microtubule ends by dynamically recognizing composite EB1/tubulin-binding sites. J Cell Biol 183, 1223–1233, doi: 10.1083/jcb.200809190 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peris L et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J Cell Biol 174, 839–849, doi: 10.1083/jcb.200512058 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peris L et al. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol 185, 1159–1166, doi: 10.1083/jcb.200902142 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liao G & Gundersen GG Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J Biol Chem 273, 9797–9803 (1998). [DOI] [PubMed] [Google Scholar]
- 94.Konishi Y & Setou M Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci 12, 559–567, doi: 10.1038/nn.2314 (2009). [DOI] [PubMed] [Google Scholar]
- 95.Wehland J & Weber K Tubulin-tyrosine ligase has a binding site on beta-tubulin: a two-domain structure of the enzyme. J Cell Biol 104, 1059–1067 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kumar N & Flavin M Preferential action of a brain detyrosinolating carboxypeptidase on polymerized tubulin. J Biol Chem 256, 7678–7686 (1981). [PubMed] [Google Scholar]
- 97.Vemu A, Garnham CP, Lee DY & Roll-Mecak A Generation of differentially modified microtubules using in vitro enzymatic approaches. Methods Enzymol 540, 149–166, doi: 10.1016/B978-0-12-397924-7.00009-1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larcher JC, Boucher D, Lazereg S, Gros F & Denoulet P Interaction of kinesin motor domains with alpha- and beta-tubulin subunits at a tau-independent binding site. Regulation by polyglutamylation. J Biol Chem 271, 22117–22124 (1996). [DOI] [PubMed] [Google Scholar]
- 99.Boucher D, Larcher JC, Gros F & Denoulet P Polyglutamylation of tubulin as a progressive regulator of in vitro interactions between the microtubule-associated protein Tau and tubulin. Biochemistry 33, 12471–12477 (1994). [DOI] [PubMed] [Google Scholar]
- 100.Bonnet C et al. Differential binding regulation of microtubule-associated proteins MAP1A, MAP1B, and MAP2 by tubulin polyglutamylation. J Biol Chem 276, 12839–12848, doi: 10.1074/jbc.M011380200 (2001). [DOI] [PubMed] [Google Scholar]
- 101.Lacroix B et al. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol 189, 945–954, doi: 10.1083/jcb.201001024 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fink JK Hereditary spastic paraplegia. Neurol Clin 20, 711–726 (2002). [DOI] [PubMed] [Google Scholar]
- 103.Sherwood NT, Sun Q, Xue M, Zhang B & Zinn K Drosophila Spastin Regulates Synaptic Microtubule Networks and Is Required for Normal Motor Function. PLoS Biol 2, e429 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Orso G et al. Disease-related phenotypes in a Drosophila model of hereditary spastic paraplegia are ameliorated by treatment with vinblastine. J Clin Invest 115, 3026–3034 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Akella JS et al. MEC-17 is an alpha-tubulin acetyltransferase. Nature 467, 218–222, doi: 10.1038/nature09324 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shida T, Cueva JG, Xu Z, Goodman MB & Nachury MV The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A 107, 21517–21522, doi: 10.1073/pnas.1013728107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Topalidou I et al. Genetically separable functions of the MEC-17 tubulin acetyltransferase affect microtubule organization. Curr Biol 22, 1057–1065, doi: 10.1016/j.cub.2012.03.066 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neumann B & Hilliard MA Loss of MEC-17 Leads to Microtubule Instability and Axonal Degeneration. Cell reports 6, 93–103, doi: 10.1016/j.celrep.2013.12.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim GW, Li L, Gorbani M, You L & Yang XJ Mice Lacking alpha-Tubulin Acetyltransferase 1 Are Viable but Display alpha-Tubulin Acetylation Deficiency and Dentate Gyrus Distortion. J Biol Chem 288, 20334–20350, doi: 10.1074/jbc.M113.464792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Black MM, Baas PW & Humphries S Dynamics of alpha-tubulin deacetylation in intact neurons. J Neurosci 9, 358–368 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Piperno G & Fuller MT Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol 101, 2085–2094 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Piperno G, LeDizet M & Chang XJ Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol 104, 289–302 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schulze E, Asai DJ, Bulinski JC & Kirschner M Posttranslational modification and microtubule stability. J Cell Biol 105, 2167–2177 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Webster DR & Borisy GG Microtubules are acetylated in domains that turn over slowly. J Cell Sci 92 (Pt 1), 57–65 (1989). [DOI] [PubMed] [Google Scholar]
- 115.Szyk A et al. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell 157, 1405–1415, doi: 10.1016/j.cell.2014.03.061 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bassot JM & Martoja R [Histological and ultrastructural data on the cytoplasmic microtubules of the ejaculatory canal of orthopteral insects]. Zeitschrift fur Zellforschung und mikroskopische Anatomie 74, 145–181 (1966). [PubMed] [Google Scholar]
- 117.Afzelius BA Microtubules in the spermatids of stick insects. Journal of ultrastructure and molecular structure research 98, 94–102 (1988). [DOI] [PubMed] [Google Scholar]
- 118.Behnke O Incomplete microtubules observed in mammalian blood platelets during microtubule polymerization. J Cell Biol 34, 697–701 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu Z & Afzelius BA The substructure of marginal bundles in human blood platelets. Journal of ultrastructure and molecular structure research 99, 244–253 (1988). [DOI] [PubMed] [Google Scholar]
- 120.Peters A, Proskauer CC & Kaiserman-Abramof IR The small pyramidal neuron of the rat cerebral cortex. The axon hillock and initial segment. J Cell Biol 39, 604–619 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodriguez Echandia EL, Piezzi RS & Rodriguez EM Dense-core microtubules in neurons and gliocytes of the toad Bufo arenarum Hensel. The American journal of anatomy 122, 157–166, doi: 10.1002/aja.1001220110 (1968). [DOI] [PubMed] [Google Scholar]
- 122.Burton PR Luminal material in microtubules of frog olfactory axons: structure and distribution. J Cell Biol 99, 520–528 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sui H & Downing KH Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature 442, 475–478, doi: 10.1038/nature04816 (2006). [DOI] [PubMed] [Google Scholar]
- 124.Nicastro D et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313, 944–948, doi: 10.1126/science.1128618 (2006). [DOI] [PubMed] [Google Scholar]
- 125.Garvalov BK et al. Luminal particles within cellular microtubules. J Cell Biol 174, 759–765, doi: 10.1083/jcb.200606074 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schulze E & Kirschner M Dynamic and stable populations of microtubules in cells. J Cell Biol 104, 277–288 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Levilliers N, Fleury A & Hill AM Monoclonal and polyclonal antibodies detect a new type of post-translational modification of axonemal tubulin. J Cell Sci 108 (Pt 9), 3013–3028 (1995). [DOI] [PubMed] [Google Scholar]
- 128.Newton CN et al. Intrinsically slow dynamic instability of HeLa cell microtubules in vitro. J Biol Chem 277, 42456–42462, doi: 10.1074/jbc.M207134200 (2002). [DOI] [PubMed] [Google Scholar]
- 129.Widlund PO et al. One-step purification of assembly-competent tubulin from diverse eukaryotic sources. Mol Biol Cell 23, 4393–4401, doi: 10.1091/mbc.E12-06-0444 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Minoura I et al. Overexpression, purification, and functional analysis of recombinant human tubulin dimer. FEBS Lett 587, 3450–3455, doi: 10.1016/j.febslet.2013.08.032 (2013). [DOI] [PubMed] [Google Scholar]