Abstract
Stem/progenitor cells are undifferentiated cells characterized by their exclusive ability for self-renewal and multilineage differentiation potential. In recent years, researchers and investigations explored the prospect of employing stem/progenitor cell therapy in regenerative medicine, especially stem/progenitor cells originating from the oral tissues. In this context, the regeneration of the lost dental tissues including enamel, dentin, and the dental pulp are pivotal targets for stem/progenitor cell therapy. The present review elaborates on the different sources of stem/progenitor cells and their potential clinical applications to regenerate enamel, dentin, and the dental pulpal tissues.
1. Introduction
Dental caries is globally considered among the most prevalent bacterially induced diseases, resulting in enamel and dentin destruction. If untreated, the destruction will mostly lead to irreversible pulpal tissue damage [1]. Currently, the classical line of treatment involves the removal of the affected dental tissues and their subsequent replacement with artificial filling materials, with divergent physical and functional properties [1]. Due to various negative consequences of the restorative techniques and inherent deficiencies of the restoration materials, the ideal solutions to replace defective dental structures could be by biologically restoring/regenerating the lost dental tissues. The development of such new alternative treatment methods is currently considered as an important goal for the dental therapeutic researches.
Mesenchymal stem/progenitor cells (MSCs) are unspecialized plastic-adherent cells with the ability for self-renewal and multilineage differentiation [2] into multiple cell lineages [3]. They have been isolated from a variety of dental tissues, including dental pulp stem cells (DPSCs), stem/progenitor cells isolated from the human pulp of exfoliated deciduous teeth (SHED), periodontal ligament stem/progenitor cells (PDLSCs), stem/progenitor cells from apical papilla (SCAP), alveolar bone-proper-derived stem/progenitor cells (AB-MSCs), gingival mesenchymal stem/progenitor cells (GMSCs), and dental follicle stem/progenitor cells (DFSCs) [4, 5]. The stem/progenitor cells derived from the oral cavity express several mesenchymal markers, including CD29, CD73, CD90, and CD105, as well as embryonic markers such as Sox2, Nanog, and Oct4 [6], but lack the expression of hematopoietic markers, including CD34, CD45, and HLA-DR. Relying on their remarkable proliferative ability and differentiation potential, these stem/progenitor cells are believed to be very promising in the development of future therapeutic approaches to regenerate the enamel, dentin, and pulpal tissues [7].
2. The Tissue Engineering Triad
Tissue engineering is an interdisciplinary field that applies the principles of engineering and life sciences towards the development of biological substitutes that could restore, maintain, or improve tissue and organ functions [8]. The concept of tissue engineering relies on the employment of a triad of stem/progenitor cells, scaffolds, and growth factors [8, 9] to regenerate functional biological tissues. Scaffolds have to be implemented with a suitable choice of cells and signaling molecules to initiate the formation of a new dental tissue that can homogenize with the surrounding tissues [10–12].
Numerous stem cell sources have been identified to play an essential role in tissue regeneration. Stem cells are either embryonic or adult stem cells [13]. Embryonic stem cells are immature, undifferentiated cells derived from the inner cell mass of blastocysts [14, 15], with the ability to undergo continuous self-renewal and differentiation. Adult stem/progenitor cells are undifferentiated cells that are capable of differentiating into certain types of tissues [3]. They maintain the integrity of tissues they reside in such as blood, skin, bone, and dental pulp [16].
Scaffolds could be natural polymers (e.g., collagen, chitosan, alginate, and hyaluronic acid) or synthetic materials (e.g., polyglycolic acid, polylactic acid, and polylactic polyglycolic acid) and bioactive ceramics, with each category having its merits as well as limitations in use [17]. Scaffolds could be utilized as a cell support tool, upon which cells are cultured in vitro, prior to their transplantation together with their produced matrix in vivo. Scaffolds can further be employed as growth factor/drug delivery tools, to attract body cells to the scaffold site in vivo for new tissue formation [18]. In this context, scaffolds are essential to structurally support and transport growth factors, DNA, biologically active proteins, and cells as well as provide physical signals important for biological repair/regeneration processes [19, 20]. Aside from these, the topography, architecture, and composition of scaffolds can interact and affect cell response and subsequent tissue formation [18]. It is important for scaffolds to mimic the natural extracellular matrix of the tissue to be replaced [21, 22]. Optimum design for dental tissue regeneration should be made to achieve mechanical integrity and functionality and to help in cell adhesion and differentiation.
As a third important factor in the tissue engineering triad, growth factors were suggested to be crucial for the regenerative process. They are normally released from cells and are directly presented to cell surface receptors through their interaction with the neighboring extracellular matrix. Binding of growth factors to particular cell-membrane-linked receptors activates various mechanisms and pathways involved in tissue engineering such as cell migration, survival, adhesion, proliferation, growth, and differentiation into the desired cell type [23–27]. Especially, bone morphogenetic protein- (BMP-) 2 was shown to induce the differentiation of dental pulp stem/progenitor cells into odontoblasts [23]. It was also demonstrated that BMP-4 mediates the differentiation of human embryonic stem cells into dental epithelium with the ability for tooth formation [28]. In addition, transforming growth factor-β (TGF-β) promotes the differentiation of odontoblast-like cells and stimulates dental pulp stem cell-mediated mineralization [23]. Broadly speaking, these growth factor-mediated cell responses are crucial for growth, wound healing, and angiogenesis in repair/regeneration processes [26].
3. Enamel Regeneration
3.1. Enamel Structure and Amelogenesis
Enamel, the hardest tissue in the human body, is a highly organized dental tissue, covering the outer layer of the tooth crown. It possesses unique mechanical and structural properties [29–32], relying on its high hydroxyapatite content, the arrangement of apatite crystals into enamel prisms, and finally, the alignment of these prisms in a picket-fence appearance in a tissue of high physical resilience and great hardness [33–37]. Ameloblasts, the enamel-forming cells, are specialized epithelial cells differentiating from the inner cells of the enamel organ [38]. They exhibit polarization and elongation with a pronounced Golgi apparatus and endoplasmic reticulum to form and secrete enamel proteins and influx phosphate and calcium ions into the forming enamel matrix [39, 40]. Enamel proteins are necessary for enamel formation, with amelogenin, ameloblastin, and enamelin being the three major proteins observed in the developing teeth [41]. Recently, this list was amended by amelotin and odontogenic ameloblast-associated protein (ODAM), which were observed in the junctional epithelium and during the maturation stage of amelogenesis [42–45]. Once the enamel matrix is formed, ameloblasts reabsorb water and degrade enamel proteins during the maturation stage of amelogenesis [39, 40]. Finally, they undergo apoptosis and the mature enamel becomes acellular. As a result, once damaged, unlike other biomineralized hard tissues such as dentin and bone, the enamel cannot regenerate by itself [37, 46]. Therefore, a reparative healing of destroyed enamel depends mainly, if at all, on acellular remineralization of superficial demineralized defects [47].
To restore enamel defects either due to caries, trauma, or others, artificial materials were manufactured to resemble its hardness [48]. Unfortunately, most of the current materials do not possess the same mechanical, physical, and esthetic properties of the lost tissues [49]. Despite the urgent need for tooth enamel regeneration, enamel tissue engineering is facing many difficulties [50–53], including the complex posttranslational protein modifications required for crystal growth [54] and the recapitulation of the unique movements of ameloblasts during the organization of hydroxyapatite crystals into the enamel prisms [55]. Despite all these trials and findings, to date, there exists no scheme for viable cell-based in vivo enamel tissue engineering [37]. The main challenge remains to produce an artificial enamel that resembles the prismatic and interprismatic patterns of natural enamel, has the proper anatomy, and can substitute for lost enamel-forming cells [56].
3.2. Cells
As enamel-forming cells are lost following tooth development, alternative cellular sources were needed to bring about a cellular-based regeneration. In this context, nondental epithelium-derived human cells, including gingival epithelial cells [57], induced pluripotent stem cells (iPSCs) [58], and human keratinocyte stem cells (hKSCs) [59, 60] were suggested to differentiate into enamel-forming ameloblasts when combined with mouse or human embryonic dental mesenchyme. Still, only a small percentage of these explants in subrenal cultures demonstrated the formation of dental enamel [60]. Embryonic stem/progenitor cells were similarly demonstrated to differentiate into oral ectoderm and dental epithelium, using variable concentrations of BMP-4 [61]. The formed dental epithelium, when mixed with mouse embryonic dental mesenchyme and transplanted into renal capsules for thirty days, subsequently generated teeth-like structures, including dentin and enamel, with an incisor-like appearance [28]. Similarly, human keratinocyte stem cells when combined with embryonic mouse dental mesenchyme, sonic hedgehog (SHH), and fibroblast growth factor 8- (Fgf8-) soaked agarose beads as reconstructed tooth germs [62] and transplanted into mice renal capsules, demonstrated ameloblastic differentiation with enamel deposition. Mouse induced pluripotent stem cells demonstrated a differentiation into ameloblast-like cells, using epithelial rests of Malassez cell-conditioned medium and gelatin-coated dishes, with high expression of amelogenin, ameloblastin, and keratin 14 [63].
Similarly, the Hertwig epithelial root sheath (HERS) and epithelial rests of Malassez (ERM) cells demonstrate a remarkable ability to produce enamel matrix proteins [64]. HERS cells entrapped in cementum produced amelogenin, ameloblastin, amelotin, and ODAM [45]. It was found that primary cultured HERS/ERM cells possess a primitive stem/progenitor cell population that exhibits embryonic stem cell and epithelial markers [57, 65]. Ex vivo-expanded ERM exhibited both bone marrow mesenchymal stem/progenitor cell- (heat shock protein-90b, CD44 and CD29) and epithelial cell-markers (epithelial membrane protein-1, cytokeratin-8, and E-cadherin) proving their stem/progenitor cell-like properties [66]. An ERM cell line was further successfully generated from human periodontium to be used for future research [67]. ERM cells when cocultured with dental pulp cells were differentiated into ameloblast-like cells and produced enamel-like tissues [68]. Immortalized odontogenic epithelial cells isolated from ERM expressed stem-cell-related genes and generated calcification foci when transplanted into immunocompromised mice [69].
Odontogenic epithelial stem cells (OEpSCs) were first observed in the continuously growing rodent incisors. They are of epithelial origin, interact reciprocally with the mesenchymal stem/progenitor cells of ectomesenchymal origin [70], and possess the ability to generate all the epithelial tissues of the tooth, including the enamel-forming ameloblastic layer [71–73]. In postnatal life, OEpSCs were identified in the epithelial rests of Malassez (ERM) usually present near the incomplete root ends; the junctional epithelium (JE) which surrounds the neck of teeth; the reduced enamel epithelium (REE) covering the newly erupting tooth; the dental lamina (DL) and its remnants, known as cell rests of Serres, in the retromolar area; and the remnants of DL in the gubernaculum cord (GC), found above any erupting tooth [74]. Various genes were recognized in OEpSCs, including Bmi, Sox2, Yap, ABCG2, Lgr5, Oct3/4, Gli1, and Lrig1 [72, 73, 75–78]. It was demonstrated that Sox2+ odontogenic epithelial stem cells are able to produce teeth. The odontogenic epithelial stem cell niche was proved to be regulated by Fgf10 [79, 80].
3.3. Scaffolds and Biodegradable Materials
To successfully culture a patterned enamel organ, it was found that a proper three-dimensional scaffold such as a collagen sponge in combination with feeder cells such as NIH 3T3 mouse fibroblast cells should be present [81–83] to support and compensate for the epithelial-mesenchymal interactions that occur during early tooth formation [84]. Collagen sponge scaffolds and gels were demonstrated to help in cell attachment, proliferation, and differentiation as well as in the formation of calcified tissues [85]. Primary enamel organ cells cultured on feeder cells expressed many enamel proteins as kallikrein 4, ameloblastin, amelogenin, and matrix metalloproteinase (MMP) 20 [83]. Enamel organ epithelial (EOE) cells combined with dental pulp cells in scaffolds produced enamel with amelogenin expression in tall columnar epithelial cells found on enamel and dentin surfaces [48]. A three-dimensional multilayered macroscale biomimetic coculture system, using chitosan and type I collagen was similarly seeded with mesenchymal-derived dental pulp stem cells and HAT-7 dental epithelial cells to simulate epithelial-mesenchymal interactions. This system enabled the coculture of epithelial and mesenchymal cells, and the movement of the two cell types in various directions and Ca deposits were observed [86].
Still, available information is very limited and greatly diverse to distinguish the characteristics of each specific scaffold and its impact on possible stem/progenitor cell-mediated enamel regenerative outcomes [87].
3.4. Signaling Molecules in Amelogenesis
Various signaling molecules were proposed to be involved in the epithelial-mesenchymal interactions that occur during odontogenesis, including fibroblast growth factor (Fgf), sonic hedgehog (SHH), wingless (Wnt), bone morphogenic protein (BMP), and transforming growth factor β (TGF-β) [88, 89]. Activin, BMP, and Fgf signals in the epithelial stem cell niche regulate enamel deposition in mice incisors [90]. Mesenchymal signals involved in ameloblast induction include TGF-β1, BMP-4, and BMP-2 [91, 92]. SHH signaling preserves the stem cell niche present in the molar cervical loops [93]. FAK-YAP-mTOR signaling maintains the equilibrium between stem cell proliferation and differentiation towards ameloblast lineage [94]. BMP signaling was demonstrated to be crucial for ameloblast differentiation [91] and enamel formation [95]. Ectodysplasin (Eda), a signal found in primary and secondary enamel knots and the placodes of all ectodermal appendages, is considered a key regulator of ectodermal organ development, including molecules from vital signaling pathways such as SHH and Fgf20 [96]. In addition, SHH found in epithelial stratum intermedium cells support ameloblastic differentiation and maturation [97, 98]. Other epithelial signaling molecules that regulate ameloblasts are Wnt3, TGF-β1, Follistatin, and Eda. Ameloblasts also express transcription factors such as Msx2 and Sp6 that have important roles in amelogenesis [99]. Regulating these molecules could help to generate ameloblast lineage precursors resembling odontogenic epithelial stem/progenitor cells that could be utilized in enamel regeneration approaches [6] (Table 1 and Figure 1).
Table 1.
Summary of studies on enamel regeneration.
| Enamel regeneration | ||||
|---|---|---|---|---|
| Cells | Carrier/scaffold | Growth factors | Outcome | |
| Angelova et al. 2013 [57] | Adult human gingival epithelial cells | — | — | Tooth-like structures Presence of enamel spaces and ameloblast-like cell populations |
|
| ||||
| Hu et al. 2006 | Mice bone marrow cells+mouse embryonic dental epithelial cells | — | — | Formation of nondividing, polarized, and secretory ameloblast-like cells without cell fusion |
|
| ||||
| Cai et al. 2013 [58] | Integration-free human urine induced pluripotent stem cells | — | — | Tooth-like structures having elastic modulus and hardness similar to human tooth and containing enamel space and enamel organ Presence of ameloblasts with a ruffled border-like structure and papillary layer Expression of ameloblastin |
|
| ||||
| Honda et al. 2005 [48] | Enamel organ epithelial cells | — | — | Production of enamel Expression of amelogenin in tall columnar epithelial cells found on enamel surface |
|
| ||||
| Li et al. 2019 [28] | Human embryonic stem cells | — | Bone morphogenetic protein-4 (BMP-4) | Teeth-like structures Newly generated tooth-like structures contained enamel spaces similar to natural teeth |
|
| ||||
| Shinmura et al. 2008 [68] | Quiescent porcine epithelial cell rests of Malassez from PDL of deciduous incisor teeth | Collagen sponge | — | Enamel-like tissues Positive staining for amelogenin in the enamel-like tissues Presence of well-developed ameloblasts |
|
| ||||
| Wang et al. 2010 [60] | Human keratinocytes | — | Fibroblast growth factor 8 (Fgf8) | Epithelial cells became elongated and deposited enamel Immunohistochemical assays demonstrated the presence of ameloblastin and MMP-20 |
|
| ||||
| Hu et al. 2018 [62] | Human keratinocyte stem cells | — | Fibroblast growth factor 8 and Sonic hedgehog (Fgf8+SHH) |
Tooth-like structures Intact prisms of the regenerated enamel |
|
| ||||
| Liu et al. 2013 | Rat skin epithelial cells | — | — | Enamel-dentin-like tooth germ-like structures |
|
| ||||
| Yoshida et al. 2015 [63] | Mouse induced pluripotent stem cells (Mouse iPS) | — | — | Ameloblast-like cells Expression of high levels of amelogenin and ameloblastin |
Figure 1.

Diagram showing cells, growth factors, and scaffolds examined in the field of enamel regeneration.
4. Dentin Regeneration
4.1. Dentin Structure and Dentinogenesis
The pulp-dentin complex originates embryonically from the neural crest ectomesenchyme [100]. Odontoblasts are differentiated at the late bell stage of tooth development, and their major function is to secrete the extracellular dentin matrix components (ECM), followed by their mineralization, generating the primary dentin, the main bulk of the circumpulpal dentin matrix, and completing root formation. Secondary dentin is laid down as a physiological process throughout life, while tertiary dentin is generated at the pulp-dentin interface in response to environmental stimuli. Tertiary dentin might be reactionary (structurally similar to physiological dentin) or reparative (poorly organized, mainly atubular structure, with cells trapped within the matrix). Each type arises from two different populations of cells, original postmitotic odontoblasts and newly generated cells derived from the pulp (dental pulp stem/progenitor cells (DPSCs)), respectively [101, 102].
4.2. Cells
Aside from the attempts for enamel regeneration, stem/progenitor cell-based tissue engineering remains a promising modality for functional dentin regeneration [103–105]. A lineage-tracing study proved that new odontoblasts generated during reparative dentinogenesis in teeth come up from the perivascular cells identified by α-smooth muscle actin (αSMA) expression. Furthermore, it was demonstrated that the progeny of the αSMA+ population scarcely participated in physiological dentin deposition [106]. Coimplantation of MSCs and ECs accelerated pulp healing with a complete dentin bridge formation [107]. Swine autologous dental pulp stem/progenitor cells (sDPSCs) transferred via hydrogel and transplanted into a mini swine root model showed that vascularized pulp-like tissue and a layer of newly deposited dentin (reparative dentin) were deposited along the canal walls with the creation of a dentin bridge-like structure [108]. A further in vivo study showed that iPSCs generated a pulp-like tissue with functional odontoblasts capable of producing tubular dentin-like structures [109]. In vitro investigation demonstrated that utilizing SHED in combination with different pulp capping materials stimulated proliferation, migration, and odontogenic-like phenotype differentiation of the cells [110].
4.3. Scaffolds and Biodegradable Materials
Several successful in vitro studies tested variable biomaterials to promote dentin regeneration. A biomembrane composed of a chitosan/collagen matrix embedded with calcium-aluminate microparticles proved to induce the differentiation of HDPCs into odontoblast-like cells, with the deposition of a significant amount of mineralized matrix [111]. Culturing of DPSCs onto human-treated dentin (hTD) regenerated dentin-like tissues [112, 113]. Similarly, fibrin proved to enhance pulp-like tissue generation as well as odontoblast differentiation, with dentin sialoprotein expression [114].
An attempt to utilize a biodegradable collagen sponge as a delivery vehicle for molecules like MTA or other experimental small-molecule GSK3 inhibitors promoted tertiary dentin formation in deep dental lesions, following experimentally induced pulp exposure [115]. Ceramic scaffolds, such as calcium phosphates (Ca/P) and bioactive glasses or glass ceramics, were further tested. Ca/P scaffolds contain tricalcium phosphate (TCP) or hydroxyapatite (HA), which are notably related to the mineralization of the matrix of the tooth [116]. Calcium hydroxide, Mineral Trioxide Aggregate (MTA), and Biodentine were reported to aid in the formation of the tertiary dentin [117]. Another in vitro study tested three capping materials, namely, mineral trioxide aggregate (MTA), calcium hydroxide (CH), and Biodentine (BD), and proved that these materials are biocompatible and could stimulate proliferation, migration, and differentiation of SHED [110]. Nanofibrous spongy microspheres (NF-SMS), nanofibrous microspheres (NF-MS) without a pore structure, and conventional solid microspheres (S-MS) with neither nanofibers nor pore structure were further tested for dentin regeneration. The biodegradable and biocompatible poly(L-lactic acid) block-poly(L-lysine) were fabricated into the NF-SMS with interconnected pores, enhancing the proliferation and odontogenic differentiation of HDPSCs. NF-SMS provided superior dentin-like tissue formation compared to NF-MS or S-MS with a remarkable level of mineralization [118].
The application of biological printing combined with dental stem/progenitor cells employing clinical methods of 3D biofabrication and regeneration of dental tissues are the currently suggested alternative to classical dental restorations. The use of bioinks enabled the synthesis of scaffolds with precise, reproducible microarchitectures. Novel dentin-derived extracellular matrix (ECM) hybrid cell-laden hydrogel bioinks, synthesized from alginate and dentin matrix proteins were characterized and showed high printability and cell survival at different concentrations. Moreover, these hybrid hydrogels demonstrated the ability to be embedded with acid-soluble dentin molecules, enhancing odontogenic differentiation of SCAPs and effectively engineering the pulp-dentin complex [119].
4.4. Signaling Molecules
As previously mentioned, BMP-2 controls odontoblastic differentiation of dental pulp stem cells and transforming growth factor-β (TGF-β) can stimulate odontoblast-like cell differentiation and DPSC-mediated mineralization [23]. Also, platelet-derived growth factor (PDGFBB) and dentin-derived growth factors (eDMP) proved to enhance HDPSC proliferation and odontoblastic differentiation, generating dentin-like mineralized tissues [120, 121]. G-CSF enhanced the proliferation and migration activity of stem/progenitor cells with dentin regeneration [122]. It was reported that the histone demethylation enzyme, lysine demethylase 1A (KDM1A), can regulate the directed differentiation in odontogenic MSCs by forming KDM1A and PLOD2 (procollagene-lysine2, oxoglutarate5-dioxygenase2) protein complex. It was reported that KDM1A in SCAP regulatory mechanisms of dynamic osteo/dentinogenic differentiation showed more diverse outcomes when applied in vitro than in vivo. However, in the final outcome of KDM1A inhibition, it promoted osteo/dentinogenesis in vivo [123]. Moreover, H2S proved to aid in the differentiation of DPSCs and dentin formation in vitro and in vivo via Ca2+ homeostasis and Ca2+ influx/GSK3β/(glycogen synthase kinase-3β) β-catenin cascade response. Also, it was evident that β-catenin signaling plays crucial roles in dentin formation [124].
Simvastatin (SIM), a drug commonly used to treat hyperlipidemia, was further reported to enhance odontogenic differentiation and accelerate mineralized tissue formation and de novo dentin formation [125, 126]. Combining SIM with canine DPSCs enhanced coronal pulp regeneration as well as dentin regeneration effectively and rapidly in beagle dogs. Small molecule inhibitors of glycogen synthase kinase 3 (GSK3) used in clinical trials for the treatment of neurological disorders such as Alzheimer's disease stimulated reparative dentine formation, with naturally generated new dentine at sites of damage [115, 127]. Smaphorin 3A (Sema 3A) and its receptor Nrp1, usually expressed in rat dental pulp tissue and human DPSCs, were thought to be potent factors capable of inducing differentiation of DPSCs into odontoblasts. Sema3A application to dental pulp exposure sites in a rat model induced effective reparative dentin reconstruction and promoted the formation of an odontoblastic layer, dental tubules, and predentin [128] (Table 2 and Figure 2).
Table 2.
Summary of studies on dentin/pulp regeneration.
| Dentin/pulp regeneration | ||||
|---|---|---|---|---|
| Cells | Carrier/scaffold | Growth factors | Outcome | |
| Araujo et al. 2018 [110] | Stem cells from human exfoliated deciduous teeth (SHED) | Mineral trioxide aggregate (MTA) Calcium hydroxide (CH) Biodentine (BD) |
The three tested materials maintained viability and stimulated proliferation, migration, and odontogenic-like phenotype differentiation | |
|
| ||||
| Athirasala et al. 2018 [119] | Human stem cells of apical papilla (human SCAP) | Bioink: printable alginate hydrogels with the soluble and insoluble fractions of dentin matrix | Odontogenic differentiation of SCAPs | |
|
| ||||
| Chen et al. 2017 | Human dental pulp cells (HDPCs) | Human and porcine treated dentin matrix (TDM) | Complete dentin bridge formation Regeneration of reactionary dentin |
|
|
| ||||
| El Ashiry 2018 [141] | Dental pulp stem cells (DPSCs) | Chitosan hydrogel scaffold | Vascular endothelial growth factor (VEGF-2) Basic fibroblast growth factor (bFGF) Platelet-derived growth factor (PDGF) Nerve growth factor (NGF) Bone morphogenetic protein-7 (BMP-7). |
Periapical radiolucency healing Radicular lengthening Radicular thickening Apical closure |
|
| ||||
| Iohara 2013 [148] | Dental pulp stem cells (DPSCs) Allogenic |
Atelocollagen; collagen | G-CSF (granulocyte colony-stimulating factor) | Vascularization and neural regeneration in the DPSC group |
|
| ||||
| Jia et al. 2016 [126] | Dental pulp stem cells (DPSCs) | — | — | Simvastatin stimulates DPSC-induced pulp and dentin regeneration after pulpotomy. |
|
| ||||
| Kuang et al. 2015 [118] | Human dental pulp cells (HDPCs) | Nanofibrous spongy microspheres (NF-SMS) | Dentin-like tissue formation | |
|
| ||||
| Mangione et al. 2017 | Dental pulp stem cells (DPSCs) | Hydrogel | Failure of partial pulp regeneration | |
|
| ||||
| Meza et al. 2019 [137] | Dental pulp stem cells (DPSCs) Autologous |
Leukocyte-platelet-rich fibrin (L-PRF) | Six-month and 3-year follow-ups Periapical index (PAI) score of 1 Cone beam computed tomographic periapical index (CBCT PAI) score of 0 |
|
|
| ||||
| Nakashima 2017 | Isolated human mobilized dental pulp stem cells (MDPSCs) Autologous |
Atelocollagen scaffold | G-CSF (granulocyte colony-stimulating factor) |
CBCT evaluation: Continued thickening of radicular walls (lateral dentin formation) Decrease in the volume of the dental pulp between 16 and 28 weeks MRI evaluation of apical closure: Relative signal intensity of apical part of root canal at 24 weeks EPT (electric pulp tester) for evaluation of the sensibility of teeth: Positive response in 4 cases out of the 5 cases |
|
| ||||
| Sueyama et al. 2017 [107] | MSCs (mesenchymal stem cells) with ECs (endothelial stem cells) | Biodegradable hydrogel-made scaffolds | Pulp tissue regeneration/healing with complete dentin bridge formation | |
|
| ||||
| Tran et al. 2015 [112] | HDPCs | Human-treated dentin (hTD) | Regeneration of dentin-like tissues and expression of specific dentin markers | |
|
| ||||
| Wang et al. 2016 [105] | Human SCAP | NF-MS nanofibrous microspheres | Bone morphogenetic protein-2 (BMP-2) | Newly synthesized matrix and dentin-like tissues were present in BMP-2-treated groups |
|
| ||||
| Xie et al. 2017 | Induced pluripotent stem cells (iPSCs) | Poly-L-lactic acid (Boehringer Ingelheim) scaffold cast | Production of pulp-like tissue with functional odontoblasts capable of generating tubular dentin-like structures in vivo | |
|
| ||||
| Xuan et al. 2018 [136] | HDPSCs (human dental pulp stem cells) Autologous |
— | — | Continued root lengthening and apical closure Increase in vascular formation At 12 months after treatment, laser Doppler flowmetry showed a mean increase in vascular formation |
|
| ||||
| Zheng et al. 2012 [134] | Porcine deciduous pulp stem/progenitor cells (PDPSCs) | beta-Tricalcium phosphate (β-TCP) | Dentin-like structure Completely restored pulp chamber roof defects |
|
|
| ||||
| Zu et al. 2018 | Autologous swine dental pulp stem cells (sDPSCs) | Hydrogel | Dentin bridge formation | |
Figure 2.

Diagram showing cells, growth factors, and scaffolds examined in the field of dentin regeneration.
5. Pulpal Tissue Regeneration
5.1. Pulpal Tissue Structure
Dental pulp is the soft tissue located in the center of the tooth, and it is surrounded by dentin. The primary function of the pulp is formative; it gives rise to odontoblasts that form dentin. Odontoblasts are the most distinctive cells of the pulp. They form a single layer at the periphery and synthesize the matrix, which becomes mineralized and form dentin. As previously discussed, the pulp-dentin complex originates embryonically from the neural crest ectomesenchyme and constitutes physiologically and functionally a single unit, providing vital functions for tooth homeostasis [100].
The dental pulp is a richly vascularized and innervated connective tissue comprising heterogeneous cell populations, among which stem/progenitor cells are anticipated to constantly replenish odontoblasts to form secondary and tertiary/reparative dentin throughout adult life [101, 102, 129, 130]. Mesenchymal stem/progenitor cell transplantation into endodontically treated root canals was attempted to regenerate the damaged dental pulp-dentin complex [131]. Although most of the research conducted on stem/progenitor cell-mediated reparative/regenerative endodontics used animal models, initial human clinical data are available now.
5.2. Cells
Coimplementation of endothelial cells with MSCs induced the acceleration of pulp tissue regeneration/healing and dentin bridge formation together with the upregulation of proangiogenic factors and the formation of a more organized dental pulp-like tissue and a thicker dentin bridge [107, 131–133]. Porcine deciduous pulp stem/progenitor cells (PDPSCs) transplanted to repair pulp chamber roof defects in the premolars of swine showed that after 16 weeks they regenerated dentin-like structures and nearly completely restored pulp chamber roof defects [134]. MDPSC (mobilized dental pulp stem cell) transplantation into pulpectomized teeth with G-CSF resulted in pulp/dentin regeneration as was evident by electric pulp testing, magnetic resonance imaging, and cone beam computed tomography [135]. Transplanted autogenous HDPSCs (human dental pulp stem cells), regenerated, innervated, and vascularized dental pulpal tissue in 26 patients with root length completion and apical foramen closure. Following up patients with the implanted HDPSCs for 24 months did not demonstrate any adverse events [136]. DPSCs (dental pulp stem cells) isolated from an inflamed third molar after being extracted and cultured then inoculated in another tooth of the same patient showed a normal periapical area after 3-year follow-up using cone beam computed tomography [137]. Hence stem/progenitor cell transplantation holds a promising potential for dentin/pulp complex regeneration.
5.3. Scaffolds and Biodegradable Materials
Scaffolds harboring the appropriate growth/differentiation factors are very important for the success of pulpal tissue regeneration [132]. These scaffolds should mimic the natural pulpal microenvironment, providing the necessary structural signals, adhesion molecules, and pore sizes for homing, differentiation, and cellular phenotypic conversion, through permitting cell-matrix and cell-cell interactions [138]. Different scaffolds were used in different studies such as mineralized β-tricalcium phosphate carrier/scaffolds [134], injectable collagen [122, 139, 140] and hydrogel-chitosan carriers [141], and gelatin sponge [126]. Platelet-rich fibrin (PRF), centrifuged from the patient's own blood samples, was suggested as a natural scaffold for pulpal tissue regeneration. PRF introduced inside the root canal allows cellular migration, cytokine enmeshment, and slow continuous release of cytokines such as platelet-derived growth factor, transforming growth factor beta 1, fibroblast growth factor, and vascular endothelial growth factor from 7 to 28 days, achieving the peak level on day 14 [142]. In addition, it provides a strong firm architecture and a specific 3-dimensional distribution of platelets and leukocytes [137]. A novel transplant consisting of cell-sheet fragments of DPSCs and PRF granules proved to regenerate pulp-dentin-like tissues in the root canal, both subcutaneously in nude mice and in the roots of canines. It induced a favorable regeneration of compact pulp-like tissues, and a remarkable deposition of regenerated dentin along the intracanal walls at 8 weeks postoperation was observed [143]. Still, the impact of the characteristics of carrier/scaffolds on the transplanted stem/progenitor cell-mediated regenerative outcomes are currently only partly elucidated [87].
5.4. Signaling Molecules
Similar to enamel and dentin formation, cytokines or signaling molecules participate in pulp regeneration through their ability to mobilize endogenous cells and to regulate the proliferation and differentiation of the stem/progenitor cells [144]. Signaling molecules have been used and added to the scaffolds for proliferation, differentiation, and survival of stem/progenitor cells, with potentially important roles in signaling during pulp regeneration. Several studies suggested that many cytokines and growth factors were involved in promoting chemotaxis, proliferation, and differentiation of the stem/progenitor cells inside the root canal which led to generation of new tissues [145–147]. Transplantation of processed autologous dental pulp with growth factors (vascular endothelial growth factor-2 (VEGF-2), basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), nerve growth factor (NGF), and bone morphogenetic protein-7 (BMP-7)) embedded in a chitosan hydrogel scaffold was useful in regenerating pulp and dentin-like tissues in necrotic immature permanent teeth with apical periodontitis in dogs [141] with promising results. G-CSF in combination with DPSCs demonstrated pulpal tissue regeneration, vascularization, and nerve regeneration [148]. Basic fibroblast growth factor (bFGF) was demonstrated to be a potent homing/migration factor in pulp regeneration therapy similar to the influence of G-CSF [138]. Mobilized dental pulp stem cells and granulocyte colony-stimulating factor (G-CSF) with collagen transplanted into mature pulpectomized dogs' teeth completely filled the root canal with pulp-like tissue with large blood vessels and secondary dentin formation. However, MRI examination implied that the regenerated dentin might be undermineralized [149]. Another study revealed that stem cell factor (SCF) can accelerate cell homing and the maturation of the pulp-dentin complex in human immature teeth, as well as proliferation and odonto/osteogenic differentiation [150]. Still, the ideal constellation of growth/differentiation factors for functional pulpal regeneration remains largely unknown (Table 2 and Figure 3).
Figure 3.
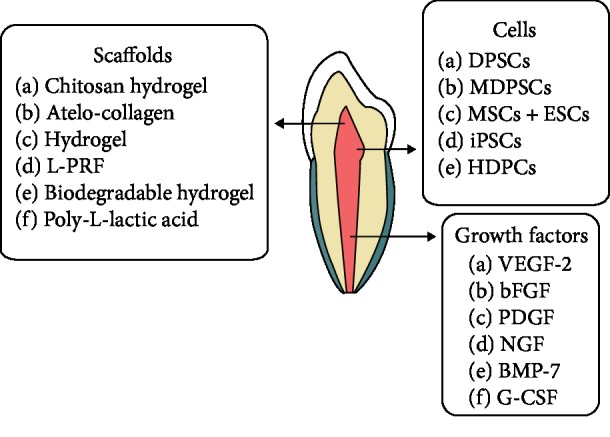
Diagram showing cells, growth factors, and scaffolds examined in the field of pulp regeneration.
6. Conclusion
Stem/progenitor cell-based tissue engineering and bioprinting are promising approaches to protect the vitality and restore the integrity of dental tissues. Many attempts proved to be very promising, as reported in various in vitro studies, animal studies, and very few human trials. Despite the fact that the proposed biomaterials and techniques could be promising for future dental tissues' regeneration, still the complexity and the multicellular interactions naturally existing in dental structures represent great currently unsolved challenges. A clear set of universally accepted markers for the isolation and characterization of stem/progenitor cells and the development of serum and animal product-free culturing media for cell expansion are further major hurdles prior to considering stem/progenitor cell-based transplantation therapies for routine clinical application. Finally, the side effects of stem/progenitor transplantation should be clearly investigated, prior to becoming a clinical therapeutic reality in restorative dentistry.
Abbreviations
- BMP-4/2/7:
Bone morphogenic protein-4/2/7
- Fgf8:
Fibroblast growth factor-8
- SHH:
Sonic hedgehog
- HGECs:
Human gingival epithelial cells
- BMCs:
Bone marrow cells
- EDECs:
Embryonic dental epithelial cells
- iPSCs:
Induced pluripotent stem cells
- HESCs:
Human embryonic stem cells
- ERM:
Epithelial rests of Malassez
- KSCs:
Keratinocyte stem cells
- MTA:
Mineral trioxide aggregate
- CH:
Calcium hydroxide
- BD:
Biodentine
- TDM:
Treated dentin matrix
- NF-SMS:
Nanofibrous spongy microspheres
- hTD:
Human-treated dentin
- NF-MS:
Nanofibrous microspheres
- β-TCP:
β-Tricalcium phosphate
- SHED:
Stem cells from human exfoliated deciduous teeth
- SCAP:
Stem cells from apical papilla
- HDPSCs:
Human dental pulp stem cells
- DPSCs:
Dental pulp stem cells
- MDPSCs:
Mobilized dental pulp stem cells
- MSCs:
Mesenchymal stem cells
- ESCs:
Endothelial stem cells
- PDPSCs:
Pig dental pulp stem cells
- sDPSCs:
Swine dental pulp stem cells
- G-CSF:
Granulocyte colony-stimulating factor
- L-PRF:
Leucocyte-platelet-rich fibrin
- VEGF-2:
Vascular endothelial growth factor-2
- bFGF:
Basic fibroblast growth factor
- PDGF:
Platelet-derived growth factor
- NGF:
Nerve growth factor.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Setzer F. C., Kim S. Comparison of long-term survival of implants and endodontically treated teeth. Journal of Dental Research. 2014;93(1):19–26. doi: 10.1177/0022034513504782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu H., Guo Z. K., Jiang X. X., et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nature Protocols. 2010;5(3):550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 3.Lv F.-J., Tuan R. S., Cheung K. M. C., Leung V. Y. L. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–1419. doi: 10.1002/stem.1681. [DOI] [PubMed] [Google Scholar]
- 4.Fawzy El-Sayed K. M., Dorfer C., Fandrich F., Gieseler F., Moustafa M. H., Ungefroren H. Mesenchymal Stem Cells - Basics and Clinical Application II. Vol. 130. Springer; 2013. Erratum to: Adult mesenchymal stem cells explored in the dental field; pp. 301–302. (Advances in Biochemical Engineering/Biotechnology). [DOI] [PubMed] [Google Scholar]
- 5.Fawzy El-Sayed K. M., Dorfer C., Fandrich F., Gieseler F., Moustafa M. H., Ungefroren H. Mesenchymal Stem Cells - Basics and Clinical Application II. Advances in Biochemical Engineering/Biotechnology, vol 130. Berlin, Heidelberg: Springer; 2013. Adult mesenchymal stem cells explored in the dental field; pp. 89–103. [DOI] [PubMed] [Google Scholar]
- 6.Balic A. Biology explaining tooth repair and regeneration: a mini-review. Gerontology. 2018;64(4):382–388. doi: 10.1159/000486592. [DOI] [PubMed] [Google Scholar]
- 7.Zhai Q., Dong Z., Wang W., Li B., Jin Y. Dental stem cell and dental tissue regeneration. Frontiers of Medicine. 2019;13(2):152–159. doi: 10.1007/s11684-018-0628-x. [DOI] [PubMed] [Google Scholar]
- 8.Langer R., Vacanti J. P. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 9.Howard D., Buttery L. D., Shakesheff K. M., Roberts S. J. Tissue engineering: strategies, stem cells and scaffolds. Journal of Anatomy. 2008;213(1):66–72. doi: 10.1111/j.1469-7580.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slaughter A., Evans L. K. Culturally sensitive oral health educational materials for older African Americans. Journal of Health Care for the Poor and Underserved. 2007;18(4):868–886. doi: 10.1353/hpu.2007.0108. [DOI] [PubMed] [Google Scholar]
- 11.Yen A. H., Sharpe P. T. Stem cells and tooth tissue engineering. Cell and Tissue Research. 2008;331(1):359–372. doi: 10.1007/s00441-007-0467-6. [DOI] [PubMed] [Google Scholar]
- 12.Taylor P. M. Biological matrices and bionanotechnology. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1484):1313–1320. doi: 10.1098/rstb.2007.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stock U. A., Vacanti J. P. Tissue engineering: current state and prospects. Annual Review of Medicine. 2001;52(1):443–451. doi: 10.1146/annurev.med.52.1.443. [DOI] [PubMed] [Google Scholar]
- 14.Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 15.McKay R. Stem cells—hype and hope. Nature. 2000;406(6794):361–364. doi: 10.1038/35019186. [DOI] [PubMed] [Google Scholar]
- 16.Vats A., Tolley N. S., Polak J. M., Buttery L. D. K. Stem cells: sources and applications. Clinical Otolaryngology and Allied Sciences. 2002;27(4):227–232. doi: 10.1046/j.1365-2273.2002.00579.x. [DOI] [PubMed] [Google Scholar]
- 17.Hashemi-Beni B., Khoroushi M., Foroughi M. R., Karbasi S., Khademi A. A. Tissue engineering: Dentin – pulp complex regeneration approaches (A review) Tissue and Cell. 2017;49(5):552–564. doi: 10.1016/j.tice.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Ripamonti U. Soluble, insoluble and geometric signals sculpt the architecture of mineralized tissues. Journal of Cellular and Molecular Medicine. 2004;8(2):169–180. doi: 10.1111/j.1582-4934.2004.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. The New England Journal of Medicine. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 20.Richardson J. B., Caterson B., Evans E. H., Ashton B. A., Roberts S. Repair of human articular cartilage after implantation of autologous chondrocytes. The Journal of Bone and Joint Surgery. British Volume. 1999;81-B(6):1064–1068. doi: 10.1302/0301-620X.81B6.0811064. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg M., Smith A. J. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Critical Reviews in Oral Biology & Medicine. 2004;15(1):13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 22.Du C., Moradian-Oldak J. Tooth regeneration: challenges and opportunities for biomedical material research. Biomedical Materials. 2006;1(1):R10–R17. doi: 10.1088/1748-6041/1/1/R02. [DOI] [PubMed] [Google Scholar]
- 23.Oshima M., Tsuji T. Functional tooth regenerative therapy: tooth tissue regeneration and whole-tooth replacement. Odontology. 2014;102(2):123–136. doi: 10.1007/s10266-014-0168-z. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura C., Nishihara T., Terashita M., Tabata Y., Washio A. Local regeneration of dentin-pulp complex using controlled release of fgf-2 and naturally derived sponge-like scaffolds. International Journal of Dentistry. 2012;2012:8. doi: 10.1155/2012/190561.190561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman R. Growth factors and chronic wound healing: past, present, and future. Advances in Skin & Wound Care. 2004;17(1):24–35. doi: 10.1097/00129334-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Grazul-Bilska A. T., Johnson M. L., Bilski J. J., et al. Wound healing: the role of growth factors. Drugs of Today. 2003;39(10):787–800. doi: 10.1358/dot.2003.39.10.799472. [DOI] [PubMed] [Google Scholar]
- 27.Atanasova M., Whitty A. Understanding cytokine and growth factor receptor activation mechanisms. Critical Reviews in Biochemistry and Molecular Biology. 2012;47(6):502–530. doi: 10.3109/10409238.2012.729561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q., Zhang S., Sui Y., Fu X., Li Y., Wei S. Sequential stimulation with different concentrations of BMP4 promotes the differentiation of human embryonic stem cells into dental epithelium with potential for tooth formation. Stem Cell Research & Therapy. 2019;10(1):p. 276. doi: 10.1186/s13287-019-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson R. E. The organic constituent of enamel. Tufts Dental Outlook. 1945;19(4):5–10. [PubMed] [Google Scholar]
- 30.Jansen M. T. Serial sections of undecalcified enamel. Journal of Dental Research. 1946;25(5):355–365. doi: 10.1177/00220345460250050701. [DOI] [PubMed] [Google Scholar]
- 31.Reed B. P., Reed C. I. Electron microscopy of erythrocytes from dog blood. Federation Proceedings. 1947;6, 1, Part 2:p. 185. [PubMed] [Google Scholar]
- 32.Agnew R. G. Observations on enamel formation. Journal of Dental Research. 1947;26(6):p. 462. [PubMed] [Google Scholar]
- 33.Szczes A., Holysz L., Chibowski E. Synthesis of hydroxyapatite for biomedical applications. Advances in Colloid and Interface Science. 2017;249:321–330. doi: 10.1016/j.cis.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Loo S. C., Moore T., Banik B., Alexis F. Biomedical applications of hydroxyapatite nanoparticles. Current Pharmaceutical Biotechnology. 2010;11(4):333–342. doi: 10.2174/138920110791233343. [DOI] [PubMed] [Google Scholar]
- 35.Habraken W., Habibovic P., Epple M., Bohner M. Calcium phosphates in biomedical applications: materials for the future? Materials Today. 2016;19(2):69–87. doi: 10.1016/j.mattod.2015.10.008. [DOI] [Google Scholar]
- 36.Nasr-Esfahani M., Fekri S. Alumina/TiO2/hydroxyapatite interface nanostructure composite filters as efficient photocatalysts for the purification of air. Reaction Kinetics, Mechanisms and Catalysis. 2012;107(1):89–103. doi: 10.1007/s11144-012-0457-x. [DOI] [Google Scholar]
- 37.Pandya M., Diekwisch T. G. H. Enamel biomimetics—fiction or future of dentistry. International Journal of Oral Science. 2019;11(1, article 38):p. 8. doi: 10.1038/s41368-018-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsiadis T. A., Graf D. Cell fate determination during tooth development and regeneration. Birth Defects Research Part C: Embryo Today: Reviews. 2009;87(3):199–211. doi: 10.1002/bdrc.20160. [DOI] [PubMed] [Google Scholar]
- 39.Kirkham J., Brookes S. J., Diekwisch T. G. H., Margolis H. C., Berdal A., Hubbard M. J. Enamel research: priorities and future directions. Frontiers in Physiology. 2017;8:p. 513. doi: 10.3389/fphys.2017.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein O. D., Duverger O., Shaw W., et al. Meeting report: a hard look at the state of enamel research. International Journal of Oral Science. 2017;9(11, article e3) doi: 10.1038/ijos.2017.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fincham A. G., Moradian-Oldak J., Simmer J. P. The structural biology of the developing dental enamel matrix. Journal of Structural Biology. 1999;126(3):270–299. doi: 10.1006/jsbi.1999.4130. [DOI] [PubMed] [Google Scholar]
- 42.Moffatt P., Smith C. E., St-Arnaud R., Simmons D., Wright J. T., Nanci A. Cloning of rat amelotin and localization of the protein to the basal lamina of maturation stage ameloblasts and junctional epithelium. Biochemical Journal. 2006;399(1):37–46. doi: 10.1042/BJ20060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moffatt P., Smith C. E., St-Arnaud R., Nanci A. Characterization of apin, a secreted protein highly expressed in tooth-associated epithelia. Journal of Cellular Biochemistry. 2008;103(3):941–956. doi: 10.1002/jcb.21465. [DOI] [PubMed] [Google Scholar]
- 44.Nishio C., Wazen R., Moffatt P., Nanci A. Expression of odontogenic ameloblast-associated and amelotin proteins in the junctional epithelium. Periodontol 2000. 2013;63(1):59–66. doi: 10.1111/prd.12031. [DOI] [PubMed] [Google Scholar]
- 45.Iwasaki K., Bajenova E., Somogyi-Ganss E., et al. Amelotin—a novel secreted, ameloblast-specific protein. Journal of Dental Research. 2005;84(12):1127–1132. doi: 10.1177/154405910508401207. [DOI] [PubMed] [Google Scholar]
- 46.Moradian-Oldak J. Protein-mediated enamel mineralization. Frontiers in Bioscience. 2012;17(7):1996–2023. doi: 10.2741/4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ten Cate J. M. Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. European Journal of Oral Sciences. 1997;105, 5, Part 2:461–465. doi: 10.1111/j.1600-0722.1997.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 48.Honda M. J., Sumita Y., Kagami H., Ueda M. Histological and immunohistochemical studies of tissue engineered odontogenesis. Archives of Histology and Cytology. 2005;68(2):89–101. doi: 10.1679/aohc.68.89. [DOI] [PubMed] [Google Scholar]
- 49.Chatzistavrou X., Papagerakis S., Ma P. X., Papagerakis P. Innovative approaches to regenerate enamel and dentin. International Journal of Dentistry. 2012;2012:5. doi: 10.1155/2012/856470.856470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simmer J. P., Richardson A. S., Hu Y. Y., Smith C. E., Ching-Chun Hu J. A post-classical theory of enamel biomineralization… and why we need one. International Journal of Oral Science. 2012;4(3):129–134. doi: 10.1038/ijos.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uskokovic V. Prospects and pits on the path of biomimetics: the case of tooth enamel. Journal of Biomimetics, Biomaterials and Tissue Engineering. 2010;8:45–78. doi: 10.4028/www.scientific.net/JBBTE.8.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruan Q., Moradian-Oldak J. Amelogenin and enamel biomimetics. Journal of Materials Chemistry B. 2015;3:3112–3129. doi: 10.1039/C5TB00163C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mann S. Ciba Foundation Symposium 205 - Dental Enamel Ciba Foundation Symposium 205. John Wiley & Sons Ltd.; 1997. The biomimetics of enamel: a paradigm for organized biomaterials synthesis; pp. 261–269. [DOI] [PubMed] [Google Scholar]
- 54.Pandya M., Lin T., Li L., et al. Posttranslational amelogenin processing and changes in matrix assembly during enamel development. Frontiers in Physiology. 2017;8:p. 790. doi: 10.3389/fphys.2017.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pugach M. K., Suggs C., Li Y., et al. M180 amelogenin processed by MMP20 is sufficient for decussating murine enamel. Journal of Dental Research. 2013;92(12):1118–1122. doi: 10.1177/0022034513506444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jayasudha, Baswaraj, Navin H. K., Prasanna K. B. Enamel regeneration—current progress and challenges. Journal of Clinical and Diagnostic Research. 2014;8(9):Ze06–Ze09. doi: 10.7860/jcdr/2014/10231.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angelova Volponi A., Kawasaki M., Sharpe P. T. Adult human gingival epithelial cells as a source for whole-tooth bioengineering. Journal of Dental Research. 2013;92(4):329–334. doi: 10.1177/0022034513481041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai J., Zhang Y., Liu P., et al. Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regeneration. 2013;2(1) doi: 10.1186/2045-9769-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu X., Lin C., Shen B., et al. Conserved odontogenic potential in embryonic dental tissues. Journal of Dental Research. 2014;93(5):490–495. doi: 10.1177/0022034514523988. [DOI] [PubMed] [Google Scholar]
- 60.Wang B., Li L., du S., et al. Induction of human keratinocytes into enamel-secreting ameloblasts. Developmental Biology. 2010;344(2):795–799. doi: 10.1016/j.ydbio.2010.05.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng L. W., Linthicum L., DenBesten P. K., Zhang Y. The similarity between human embryonic stem cell-derived epithelial cells and ameloblast-lineage cells. International Journal of Oral Science. 2013;5(1):1–6. doi: 10.1038/ijos.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu X., Lee J. W., Zheng X., et al. Efficient induction of functional ameloblasts from human keratinocyte stem cells. Stem Cell Research & Therapy. 2018;9(1):p. 126. doi: 10.1186/s13287-018-0822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida K., Sato J., Takai R., et al. Differentiation of mouse iPS cells into ameloblast-like cells in cultures using medium conditioned by epithelial cell rests of Malassez and gelatin-coated dishes. Medical Molecular Morphology. 2015;48(3):138–145. doi: 10.1007/s00795-014-0088-6. [DOI] [PubMed] [Google Scholar]
- 64.Fong C. D., Hammarstrom L. Expression of amelin and amelogenin in epithelial root sheath remnants of fully formed rat molars. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics. 2000;90(2):218–223. doi: 10.1067/moe.2000.107052. [DOI] [PubMed] [Google Scholar]
- 65.Nam H., Kim J., Park J., et al. Expression profile of the stem cell markers in human Hertwig’s epithelial root sheath/epithelial rests of Malassez cells. Molecules and Cells. 2011;31(4):355–360. doi: 10.1007/s10059-011-0045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong J., Mrozik K., Gronthos S., Bartold P. M. Epithelial cell rests of Malassez contain unique stem cell populations capable of undergoing epithelial–mesenchymal transition. Stem Cells and Development. 2012;21(11):2012–2025. doi: 10.1089/scd.2011.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nam H., Kim J. H., Kim J. W., et al. Establishment of Hertwig’s epithelial root sheath/epithelial rests of Malassez cell line from human periodontium. Molecules and Cells. 2014;37(7):562–567. doi: 10.14348/molcells.2014.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shinmura Y., Tsuchiya S., Hata K., Honda M. J. Quiescent epithelial cell rests of Malassez can differentiate into ameloblast-like cells. Journal of Cellular Physiology. 2008;217(3):728–738. doi: 10.1002/jcp.21546. [DOI] [PubMed] [Google Scholar]
- 69.Tsunematsu T., Fujiwara N., Yoshida M., et al. Human odontogenic epithelial cells derived from epithelial rests of Malassez possess stem cell properties. Laboratory Investigation. 2016;96(10):1063–1075. doi: 10.1038/labinvest.2016.85. [DOI] [PubMed] [Google Scholar]
- 70.Smith C. E., Warshawsky H. Cellular renewal in the enamel organ and the odontoblast layer of the rat incisor as followed by radioautography using 3H-thymidine. The Anatomical Record. 1975;183(4):523–561. doi: 10.1002/ar.1091830405. [DOI] [PubMed] [Google Scholar]
- 71.Harada H., Kettunen P., Jung H. S., Mustonen T., Wang Y. A., Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. Journal of Cell Biology. 1999;147(1):105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Juuri E., Saito K., Ahtiainen L., et al. Sox2+ stem cells contribute to all epithelial lineages of the tooth via Sfrp5+ progenitors. Developmental Cell. 2012;23(2):317–328. doi: 10.1016/j.devcel.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biehs B., Hu J. K., Strauli N. B., et al. BMI1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nature Cell Biology. 2013;15(7):846–852. doi: 10.1038/ncb2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Padma Priya S., Higuchi A., Abu Fanas S., et al. Odontogenic epithelial stem cells: hidden sources. Laboratory Investigation. 2015;95(12):1344–1352. doi: 10.1038/labinvest.2015.108. [DOI] [PubMed] [Google Scholar]
- 75.Li L., Kwon H. J., Harada H., Ohshima H., Cho S. W., Jung H. S. Expression patterns of ABCG2, Bmi-1, Oct-3/4, and Yap in the developing mouse incisor. Gene Expression Patterns. 2011;11(3-4):163–170. doi: 10.1016/j.gep.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Seidel K., Ahn C. P., Lyons D., et al. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 2010;137(22):3753–3761. doi: 10.1242/dev.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suomalainen M., Thesleff I. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/β-catenin signaling in the epithelial stem cells. Developmental Dynamics. 2010;239(1):364–372. doi: 10.1002/dvdy.22106. [DOI] [PubMed] [Google Scholar]
- 78.Seidel K., Marangoni P., Tang C., et al. Resolving stem and progenitor cells in the adult mouse incisor through gene co-expression analysis. eLife. 2017;6 doi: 10.7554/eLife.24712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yokohama-Tamaki T., Ohshima H., Fujiwara N., et al. Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development. 2006;133(7):1359–1366. doi: 10.1242/dev.02307. [DOI] [PubMed] [Google Scholar]
- 80.Harada H., Toyono T., Toyoshima K., et al. FGF10 maintains stem cell compartment in developing mouse incisors. Development. 2002;129(6):1533–1541. doi: 10.1242/dev.129.6.1533. [DOI] [PubMed] [Google Scholar]
- 81.Honda M. J., Shimodaira T., Ogaeri T., Shinohara Y., Hata K., Ueda M. A novel culture system for porcine odontogenic epithelial cells using a feeder layer. Archives of Oral Biology. 2006;51(4):282–290. doi: 10.1016/j.archoralbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 82.Honda M. J., Shinmura Y., Shinohara Y. Enamel tissue engineering using subcultured enamel organ epithelial cells in combination with dental pulp cells. Cells Tissues Organs. 2009;189(1-4):261–267. doi: 10.1159/000151743. [DOI] [PubMed] [Google Scholar]
- 83.Matsumoto A., Harada H., Saito M., Taniguchi A. Induction of enamel matrix protein expression in an ameloblast cell line co-cultured with a mesenchymal cell line in vitro. In Vitro Cellular & Developmental Biology - Animal. 2011;47(1):39–44. doi: 10.1007/s11626-010-9362-7. [DOI] [PubMed] [Google Scholar]
- 84.Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. Journal of Cell Science. 2003;116, Part 9:1647–1648. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 85.Sumita Y., Honda M. J., Ohara T., et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials. 2006;27(17):3238–3248. doi: 10.1016/j.biomaterials.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 86.Ravindran S., Song Y., George A. Development of three-dimensional biomimetic scaffold to study epithelial–mesenchymal interactions. Tissue Engineering Part A. 2010;16(1):327–342. doi: 10.1089/ten.TEA.2009.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukushima K. A., Marques M. M., Tedesco T. K., et al. Screening of hydrogel-based scaffolds for dental pulp regeneration—a systematic review. Archives of Oral Biology. 2019;98:182–194. doi: 10.1016/j.archoralbio.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 88.Bei M. Molecular genetics of tooth development. Current Opinion in Genetics & Development. 2009;19(5):504–510. doi: 10.1016/j.gde.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tummers M., Thesleff I. The importance of signal pathway modulation in all aspects of tooth development. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2009;312B(4):309–319. doi: 10.1002/jez.b.21280. [DOI] [PubMed] [Google Scholar]
- 90.Wang X. P., Suomalainen M., Felszeghy S., et al. An integrated gene regulatory network controls stem cell proliferation in teeth. PLoS Biology. 2007;5(6, article e159) doi: 10.1371/journal.pbio.0050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang X. P., Suomalainen M., Jorgez C. J., Matzuk M. M., Werner S., Thesleff I. Follistatin regulates enamel patterning in mouse incisors by asymmetrically inhibiting BMP signaling and ameloblast differentiation. Developmental Cell. 2004;7(5):719–730. doi: 10.1016/j.devcel.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 92.Coin R., Haikel Y., Ruch J. V. Effects of apatite, transforming growth factor β-1, bone morphogenetic protein-2 and interleukin-7 on ameloblast differentiation in vitro. European Journal of Oral Sciences. 1999;107(6):487–495. doi: 10.1046/j.0909-8836.1999.eos107611.x. [DOI] [PubMed] [Google Scholar]
- 93.Li J., Feng J., Liu Y., et al. BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Developmental Cell. 2015;33(2):125–135. doi: 10.1016/j.devcel.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu J. K.-H., du W., Shelton S. J., Oldham M. C., DiPersio C. M., Klein O. D. An FAK-YAP-mTOR signaling axis regulates stem cell-based tissue renewal in mice. Cell Stem Cell. 2017;21(1):91–106.e6. doi: 10.1016/j.stem.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plikus M. V., Zeichner-David M., Mayer J. A., et al. Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evolution Development. 2005;7(5):440–457. doi: 10.1111/j.1525-142X.2005.05048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mikkola M. L. Molecular aspects of hypohidrotic ectodermal dysplasia. American journal of medical genetics Part A. 2009;149A(9):2031–2036. doi: 10.1002/ajmg.a.32855. [DOI] [PubMed] [Google Scholar]
- 97.Dassule H. R., Lewis P., Bei M., Maas R., McMahon A. P. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127(22):4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- 98.Gritli-Linde A., Bei M., Maas R., Zhang X. M., Linde A., McMahon A. P. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002;129(23):5323–5337. doi: 10.1242/dev.00100. [DOI] [PubMed] [Google Scholar]
- 99.Bei M. Molecular genetics of ameloblast cell lineage. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2009;312B(5):437–444. doi: 10.1002/jez.b.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mao J. J., Prockop D. J. Stem cells in the face: tooth regeneration and beyond. Cell Stem Cell. 2012;11(3):291–301. doi: 10.1016/j.stem.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith A. J., Cassidy N., Perry H., Begue-Kirn C., Ruch J. V., Lesot H. Reactionary dentinogenesis. International Journal of Developmental Biology. 2003;39(1):273–280. [PubMed] [Google Scholar]
- 102.Sloan A. J., Waddington R. J. Dental pulp stem cells: what, where, how? International Journal of Paediatric Dentistry. 2009;19(1):61–70. doi: 10.1111/j.1365-263X.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 103.Guo T., Li Y., Cao G., et al. Fluorapatite-modified scaffold on dental pulp stem cell mineralization. Journal of Dental Research. 2014;93(12):1290–1295. doi: 10.1177/0022034514547914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murakami M., Hayashi Y., Iohara K., Osako Y., Hirose Y., Nakashima M. Trophic effects and regenerative potential of mobilized mesenchymal stem cells from bone marrow and adipose tissue as alternative cell sources for pulp/dentin regeneration. Cell Transplantation. 2015;24(9):1753–1765. doi: 10.3727/096368914X683502. [DOI] [PubMed] [Google Scholar]
- 105.Wang W., Dang M., Zhang Z., et al. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomaterialia. 2016;36:63–72. doi: 10.1016/j.actbio.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vidovic I., Banerjee A., Fatahi R., et al. αSMA-expressing perivascular cells represent dental pulp progenitors in vivo. Journal of Dental Research. 2017;96(3):323–330. doi: 10.1177/0022034516678208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sueyama Y., Kaneko T., Ito T., Kaneko R., Okiji T. Implantation of endothelial cells with mesenchymal stem cells accelerates dental pulp tissue regeneration/healing in pulpotomized rat molars. Journal of Endodontics. 2017;43(6):943–948. doi: 10.1016/j.joen.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 108.Zhu X., Liu J., Yu Z., et al. A miniature swine model for stem cell-based de novo regeneration of dental pulp and dentin-like tissue. Tissue Engineering Part C: Methods. 2018;24(2):108–120. doi: 10.1089/ten.tec.2017.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xie H., Dubey N., Shim W., et al. Functional odontoblastic-like cells derived from human iPSCs. Journal of Dental Research. 2018;97(1):77–83. doi: 10.1177/0022034517730026. [DOI] [PubMed] [Google Scholar]
- 110.Araújo L. B., Cosme-Silva L., Fernandes A. P., et al. Effects of mineral trioxide aggregate, Biodentine™ and calcium hydroxide on viability, proliferation, migration and differentiation of stem cells from human exfoliated deciduous teeth. Journal of Applied Oral Science. 2018;26 doi: 10.1590/1678-7757-2016-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soares D. G., Rosseto H. L., Basso F. G., Scheffel D. S., Hebling J., de Souza Costa C. A. Chitosan-collagen biomembrane embedded with calcium-aluminate enhances dentinogenic potential of pulp cells. Brazilian Oral Research. 2016;30(1) doi: 10.1590/1807-3107bor-2016.vol30.0054. [DOI] [PubMed] [Google Scholar]
- 112.Tran H. L. B., Doan V. N. Human dental pulp stem cells cultured onto dentin derived scaffold can regenerate dentin-like tissue in vivo. Cell and Tissue Banking. 2015;16(4):559–568. doi: 10.1007/s10561-015-9503-z. [DOI] [PubMed] [Google Scholar]
- 113.Aliasghari A., Rabbani Khorasgani M., Vaezifar S., Rahimi F., Younesi H., Khoroushi M. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: an in vitro study. Iranian Journal of Microbiology. 2016;8(2):93–100. [PMC free article] [PubMed] [Google Scholar]
- 114.Galler K. M., Brandl F. P., Kirchhof S., et al. Suitability of different natural and synthetic biomaterials for dental pulp tissue engineering. Tissue Engineering Part A. 2018;24(3-4):234–244. doi: 10.1089/ten.TEA.2016.0555. [DOI] [PubMed] [Google Scholar]
- 115.Neves V. C. M., Babb R., Chandrasekaran D., Sharpe P. T. Promotion of natural tooth repair by small molecule GSK3 antagonists. Scientific Reports. 2017;7(1, article 39654) doi: 10.1038/srep39654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Montazeri M., Karbasi S., Foroughi M. R., Monshi A., Ebrahimi-Kahrizsangi R. Evaluation of mechanical property and bioactivity of nano-bioglass 45S5 scaffold coated with poly-3-hydroxybutyrate. Journal of Materials Science: Materials in Medicine. 2015;26(2):p. 62. doi: 10.1007/s10856-014-5369-z. [DOI] [PubMed] [Google Scholar]
- 117.Nowicka A., Lipski M., Parafiniuk M., et al. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. Journal of Endodontics. 2013;39(6):743–747. doi: 10.1016/j.joen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 118.Kuang R., Zhang Z., Jin X., et al. Nanofibrous spongy microspheres enhance odontogenic differentiation of human dental pulp stem cells. Advanced Healthcare Materials. 2015;4(13):1993–2000. doi: 10.1002/adhm.201500308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Athirasala A., Tahayeri A., Thrivikraman G., et al. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication. 2018;10(2, article 024101) doi: 10.1088/1758-5090/aa9b4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang M., Jiang F., Zhang X., et al. The effects of platelet-derived growth factor-BB on human dental pulp stem cells mediated dentin-pulp complex regeneration. Stem Cells Translational Medicine. 2017;6(12):2126–2134. doi: 10.1002/sctm.17-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Widbiller M., Driesen R. B., Eidt A., et al. Cell homing for pulp tissue engineering with endogenous dentin matrix proteins. Journal of Endodontics. 2018;44(6):956–962.e2. doi: 10.1016/j.joen.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 122.Iohara K., Murakami M., Nakata K., Nakashima M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Experimental Gerontology. 2014;52:39–45. doi: 10.1016/j.exger.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 123.Wang L., Yang H., Lin X., et al. KDM1A regulated the osteo/dentinogenic differentiation process of the stem cells of the apical papilla via binding with PLOD2. Cell Proliferation. 2018;51(4, article e12459) doi: 10.1111/cpr.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang R., Liu Y., Yu T., et al. Hydrogen sulfide maintains dental pulp stem cell function via TRPV1-mediated calcium influx. Cell Death Discovery. 2018;4(1):p. 69. doi: 10.1038/s41420-018-0071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Okamoto Y., Sonoyama W., Ono M., et al. Simvastatin induces the odontogenic differentiation of human dental pulp stem cells in vitro and in vivo. Journal of Endodontics. 2009;35(3):367–372. doi: 10.1016/j.joen.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 126.Jia W., Zhao Y., Yang J., et al. Simvastatin promotes dental pulp stem cell–induced coronal pulp regeneration in pulpotomized teeth. Journal of Endodontics. 2016;42(7):1049–1054. doi: 10.1016/j.joen.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 127.del Ser T., Steinwachs K. C., Gertz H. J., et al. Treatment of Alzheimer’s disease with the GSK-3 inhibitor tideglusib: a pilot study. Journal of Alzheimer's Disease. 2012;33(1):205–215. doi: 10.3233/JAD-2012-120805. [DOI] [PubMed] [Google Scholar]
- 128.Yoshida S., Wada N., Hasegawa D., et al. Semaphorin 3A induces odontoblastic phenotype in dental pulp stem cells. Journal of Dental Research. 2016;95(11):1282–1290. doi: 10.1177/0022034516653085. [DOI] [PubMed] [Google Scholar]
- 129.Kim S. G., Zheng Y., Zhou J., et al. Dentin and dental pulp regeneration by the patient’s endogenous cells. Endodontic Topics. 2013;28(1):106–117. doi: 10.1111/etp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fawzy El-Sayed K. M., Klingebiel P., Dorfer C. E. Toll-like receptor expression profile of human dental pulp stem/progenitor cells. Journal of Endodontics. 2016;42(3):413–417. doi: 10.1016/j.joen.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 131.Fawzy El-Sayed K. M., Jakusz K., Jochens A., Dorfer C., Schwendicke F. Stem cell transplantation for pulpal regeneration: a systematic review. Tissue Engineering Part B: Reviews. 2015;21(5):451–460. doi: 10.1089/ten.teb.2014.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fawzy El-Sayed K. M., Ahmed G. M., Abouauf E. A., Schwendicke F. Stem/progenitor cell-mediated pulpal tissue regeneration: a systematic review and meta-analysis. International Endodontic Journal. 2019;52(11):1573–1585. doi: 10.1111/iej.13177. [DOI] [PubMed] [Google Scholar]
- 133.Fawzy El-Sayed K. M., Elsalawy R., Ibrahim N., et al. The dental pulp stem/progenitor cells-mediated inflammatory-regenerative axis. Tissue Engineering Part B: Reviews. 2019;25(5):445–460. doi: 10.1089/ten.TEB.2019.0106. [DOI] [PubMed] [Google Scholar]
- 134.Zheng Y., Wang X. Y., Wang Y. M., et al. Dentin regeneration using deciduous pulp stem/progenitor cells. Journal of Dental Research. 2012;91(7):676–682. doi: 10.1177/0022034512449834. [DOI] [PubMed] [Google Scholar]
- 135.Nakashima M., Iohara K., Murakami M., et al. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Research & Therapy. 2017;8(1):p. 61. doi: 10.1186/s13287-017-0506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xuan K., Li B., Guo H., et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Science Translational Medicine. 2018;10(455, article eaaf3227) doi: 10.1126/scitranslmed.aaf3227. [DOI] [PubMed] [Google Scholar]
- 137.Meza G., Urrejola D., Saint Jean N., et al. Personalized cell therapy for pulpitis using autologous dental pulp stem cells and leukocyte platelet-rich fibrin: a case report. Journal of Endodontics. 2019;45(2):144–149. doi: 10.1016/j.joen.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 138.Eramo S., Natali A., Pinna R., Milia E. Dental pulp regeneration via cell homing. International Endodontic Journal. 2018;51(4):405–419. doi: 10.1111/iej.12868. [DOI] [PubMed] [Google Scholar]
- 139.Iohara K., Zheng L., Ito M., et al. Regeneration of dental pulp after pulpotomy by transplantation of CD31−/CD146− side population cells from a canine tooth. Regenerative Medicine. 2009;4(3):377–385. doi: 10.2217/rme.09.5. [DOI] [PubMed] [Google Scholar]
- 140.Iohara K., Imabayashi K., Ishizaka R., et al. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Engineering Part A. 2011;17(15-16):1911–1920. doi: 10.1089/ten.TEA.2010.0615. [DOI] [PubMed] [Google Scholar]
- 141.El Ashiry E. A., Alamoudi N. M., El Ashiry M. K., Bastawy H. A., El Derwi D. A., Atta H. M. Tissue engineering of necrotic dental pulp of immature teeth with apical periodontitis in dogs: radiographic and histological evaluation. Journal of Clinical Pediatric Dentistry. 2018;42(5):373–382. doi: 10.17796/1053-4625-42.5.9. [DOI] [PubMed] [Google Scholar]
- 142.Dohan Ehrenfest D. M., Del Corso M., Diss A., Mouhyi J., Charrier J. B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. Journal of Periodontology. 2010;81(4):546–555. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 143.Chen Y. J., Zhao Y. H., Zhao Y. J., et al. Potential dental pulp revascularization and odonto-/osteogenic capacity of a novel transplant combined with dental pulp stem cells and platelet-rich fibrin. Cell and Tissue Research. 2015;361(2):439–455. doi: 10.1007/s00441-015-2125-8. [DOI] [PubMed] [Google Scholar]
- 144.Wang F., Jiang Y., Huang X., et al. Pro-inflammatory cytokine TNF-α attenuates BMP9-induced osteo/odontoblastic differentiation of the stem cells of dental apical papilla (SCAPs) Cellular Physiology and Biochemistry. 2017;41(5):1725–1735. doi: 10.1159/000471865. [DOI] [PubMed] [Google Scholar]
- 145.Paryani K., Kim S. G. Regenerative endodontic treatment of permanent teeth after completion of root development: a report of 2 cases. Journal of Endodontics. 2013;39(7):929–934. doi: 10.1016/j.joen.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 146.Arany P. R., Cho A., Hunt T. D., et al. Photoactivation of endogenous latent transforming growth factor–β1 directs dental stem cell differentiation for regeneration. Science Translational Medicine. 2014;6(238, article 238ra69) doi: 10.1126/scitranslmed.3008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li M., Sun X., Ma L., et al. SDF-1/CXCR4 axis induces human dental pulp stem cell migration through FAK/PI3K/Akt and GSK3β/β-catenin pathways. Scientific Reports. 2017;7(1, article 40161) doi: 10.1038/srep40161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iohara K., Murakami M., Takeuchi N., et al. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Translational Medicine. 2013;2(10):p. 818. doi: 10.5966/sctm.2012-0132erratum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Iohara K., Fujita M., Ariji Y., et al. Assessment of pulp regeneration induced by stem cell therapy by magnetic resonance imaging. Journal of Endodontics. 2016;42(3):397–401. doi: 10.1016/j.joen.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 150.Ruangsawasdi N., Zehnder M., Patcas R., et al. Effects of stem cell factor on cell homing during functional pulp regeneration in human immature teeth. Tissue Engineering Part A. 2017;23(3-4):115–123. doi: 10.1089/ten.TEA.2016.0227. [DOI] [PubMed] [Google Scholar]


