Abstract
Background.
Maternal substance use and addiction has been associated with negative consequences for parenting and may increase addiction vulnerability in the developing child. Neuroimaging research suggests that substance use may decrease the reward of caring for infants and heighten stress reactivity to affective infant cues.
Methods.
Thirty-two substance-using mothers and twenty-two non-substance-using mothers were presented with emotional face and cry stimuli generated from their own and a demographically matched unknown infant during fMRI scanning. Between-group differences in neural activity during task performance were assessed using whole-brain, mixed-effects models corrected for multiple comparisons (voxel-level p<0.001, pFWE<0.05).
Results.
Relative to non-substance-using mothers, substance-using mothers exhibited greater activation when viewing their own infant’s face as compared to an unknown infant’s face across multiple brain regions, including superior medial frontal, inferior parietal, and middle temporal regions. Substance-using mothers also had a decreased response to sad infant faces in the ventral striatum relative to the non-substance-using mothers. Neural responses to own vs. unknown infant cries did not significantly differ between substance-using and non-substance-using mothers.
Conclusions.
Findings suggest overlapping cortical and subcortical brain regions implicated in responding to infant faces, with activation differences related to infant familiarity, emotional expression, and maternal substance use. While prior work has focused on attenuated neural responses to infant cues, greater attention is needed toward understanding the increased reactivity to affective infant cues observed in substance-using mothers.
Keywords: maternal substance use, addiction, caregiving, fMRI, infant faces, infant cries
1. Introduction
Maternal substance use remains a significant public health concern, with detrimental consequences extending to both mothers and developing children. In the United States, recent data suggest an increasing trend in prenatal past-month illicit drug, tobacco product, and alcohol use from 2016 to 2017 (Substance Abuse and Mental Health Services Administration, 2018). Even for women able to abstain from substance use during pregnancy, most relapse within two years postpartum (Forray et al., 2015). Substance use during pregnancy may exert teratogenic effects on fetal development and neonatal complications upon delivery (Gutvirtz et al., 2019; Orsolini et al., 2019; Patrick et al., 2015). Postpartum maternal substance use may also detrimentally impact child development. Substance-using mothers evidence less maternal sensitivity during interactions with their children as compared to non-substance-using mothers, interactions which can be marked by maternal disengagement as well as intrusiveness (Gottwald and Thurman, 1994; Hatzis et al., 2017; Johnson et al., 2002). Indeed, parental substance use is positively associated with child maltreatment and child welfare cases (Ghertner et al., 2018; Kepple, 2018). Critically, disrupted maternal care, specifically in the context of addiction, may increase children’s vulnerability for later substance use, abuse, and dependence, laying the foundation for the intergenerational transmission of addiction (Alvarez-Monjaras et al., 2018; Mayes and Suchman, 2006). Therefore it is of critical importance to understand mechanisms underscoring caregiving that may be impaired in mothers with substance use disorders.
One approach to mechanistically probing variability in caregiving is at a neurobiological level through the measurement of neural responses to infant affective cues (e.g., infant faces and cries). This approach provides insight into the neural mechanisms that may mediate optimal caregiving and suggest new directions for interventions to support parents in their caregiving role (Squire and Stein, 2003). In particular, given the typically positively valenced nature of mother-infant interactions, attention has been focused on regions implicated in reward processing at cortical (e.g., prefrontal cortex, PFC) and subcortical (e.g., striatum) levels, particularly when mothers view photographs of their own infant’s emotional facial expressions. For instance, in non-substance-using mothers, viewing their own infant’s face, as compared to an unknown infant’s face, multiple brain regions are activated, including the striatum, substantia nigra, prefrontal cortex (PFC), and insula (Strathearn et al., 2008). Similarly, when listening to infant vocalizations, parents can be distinguished from non-parents by brain activation in the amygdala, ventral PFC, and insula, regions implicated in cognition and emotion (Seifritz et al., 2003). Thus, accumulating research indicates encoding of maternal responsiveness by corticolimbic brain regions (Feldman, 2015; Swain et al., 2007). However, brain regions engaged by these affective infant cues, particularly those implicated in reward and stress reactivity and regulation (e.g., ventral striatum, PFC, amygdala), are also affected by addiction (Rutherford et al., 2013; Rutherford et al., 2011). Therefore, it has been hypothesized that behavioral caregiving difficulties experienced by substance-using mothers may in part relate to dysregulation of these neural circuits in addiction, engendering caregiving to be less pleasurable and more stressful (Rutherford and Mayes, 2017).
Only two fMRI studies to date have specifically examined associations between maternal substance use and the neural correlates of the processing of infant cues to test this proposed reward-stress account of addiction and parenting. Landi et al. (2011) presented recent mothers with affective cues of unknown infants; specifically, happy, sad, and neutral infant faces as well as high- and low-distress infant cries. This investigation studied mothers with poly-substance use, which included use and abuse of licit and illicit drugs. Across all infant facial expressions, relative to non-substance-using mothers, substance-using mothers had decreased activation in the dorsolateral and ventromedial PFC, occipital lobes, and parahippocampus, and amygdala. Maternal substance use was also associated with reduced activation in response to infant cries, again in prefrontal regions, auditory regions, insula, parahippocampus, and amygdala. Taken together, these findings converge with the notion that maternal substance use may be associated with unknown affective infant cues having decreased salience.
Building on this work, Kim et al. (2017a) examined neural responses to emotional infant faces in mothers receiving inpatient treatment for substance-use disorders. Infant face stimuli presented to mothers were either their own infant’s face, or an unknown demographically matched infant’s face. When viewing their own infant’s happy face, relative to the unknown infant’s happy face, decreased activation was observed in the ventromedial PFC, hypothalamus, and ventral striatum. However, increased activation to own, as compared to unknown, happy faces was observed across multiple regions, including in the amygdala, hippocampus, thalamus, cingulate, inferior frontal gyrus, insula, ventral premotor cortex, and cerebellum. Finally, increased activation to own, as compared to unknown, sad infant faces was observed in overlapping regions responsive to happy infant faces, including the thalamus, cingulate, inferior frontal gyrus, and cerebellum. In sum, mothers in treatment for addiction evidenced a decreased response to their own infant’s happy expressions in regions implicated in reward processing; however, increased activation towards own happy and sad expressions were also observed across regions implicated in emotional, motoric, and cognitive processes/functions.
Consistent with neurobiological models of addiction and parenting, research suggests that maternal substance use and addiction may be associated with neural processing of affective infant cues (Kim et al., 2017a; Landi et al., 2011). However, no study to date has examined responding to own and unknown infant faces and cries in substance-using and non-substance-using mothers. Incorporating own and unknown infant affective stimuli is particularly important to probe the interpersonal importance of maternal brain responding. Therefore, in the current study we presented mothers with happy and sad infant faces and cries from their own and an unknown infant and measured whether regional brain responses to these affective infant cues varied by maternal substance use status (present, absent). Consistent with prior research, we hypothesized that, as compared to non-substance-using mothers, substance-using mothers would evidence: (1) decreased reactivity to affective cues from unknown infants across prefrontal, sensory, and limbic regions (i.e., Landi et al., 2011); and, (2) decreased reactivity to own as compared to unknown infant faces, particularly when happy in expression, in regions implicated in reward processing (i.e., Kim et al., 2017a). We also hypothesized that substance-using mothers, relative to the non-substance-using mothers, would evidence: (3) increased activation in response to own infant face and cry stimuli in regions implicated in emotional and cognitive processes, including the amygdala, cingulate, inferior frontal gyrus, and insula (i.e., Kim et al., 2017a).
2. Materials and Methods
2.1. Participants
Yale School of Medicine’s Human Investigation Committee approved all procedures prior to recruitment. Sixty-two mothers of infants (M=8 months; SD=2 months) were recruited from the local community as part of a larger study on parenting and addiction. All mothers were compensated $80 for completing the MRI visit. Eight participants (4 substance-using; 4 healthy control) were excluded from analyses with excessive motion during the scan, described below, and the final sample consisted of 32 substance-using mothers and 22 healthy control mothers. Demographic data for the final sample are presented in Table 1.
Table 1.
Demographic characteristics of the maternal sample as a function of substance-use status. Maternal age and education are measured in years, means (standard deviation).
| Substance-Using Mothers (n=32) | Non-Substance-Using Mothers (n=22) | |
|---|---|---|
| Maternal Age | 28.17 (5.33) | 28.76 (4.27) |
| Maternal Education* | 12.63 (1.96) | 14.67 (2.71) |
| Maternal Ethnicity | 12 African American | 9 African American |
| 10 Caucasian | 3 Caucasian | |
| 3 Hispanic/Latino | 4 Hispanic/Latino | |
| 3 Other | 2 Other | |
| 1 Prefer not to answer | 3 Asian | |
| 3 Did not report | 1 Did not report | |
| Parity* | 6 Primiparous | 12 Primiparous |
| 24 Multiparous | 9 Multiparous | |
| Hours another person looks after baby | 7 Never | 6 Never |
| 15 Less than 4 hours | 4 Less than 4 hours | |
| 6 5–20 hours | 6 5–20 hours | |
| 2 21–40 hours | 3 21–40 hours | |
| 1 More than 40 hours | 2 More than 40 hours | |
| Breastfeeding Duration* | 3 Still breastfeeding | 11 Still breastfeeding |
| 4 4–6 months | 1 4–6 months | |
| 6 7 weeks to 3 months | 2 7 weeks to 3 months | |
| 3 3–6 weeks | 2 3–6 weeks | |
| 5 2 weeks or less | 1 2 weeks or less | |
| 11 Not at all | 3 Not at all | |
| 2 Did not report |
Note. Data missing from three participants for parity, maternal age, and maternal education and data are missing from two participants from hours another person looks after the baby and breastfeeding duration;
p<.05
Mothers were included in the substance-using group if they met any of the following criteria: current or prior methadone maintenance, current or prior detoxification or treatment programs for substance use, current tobacco smoking with a nicotine dependence score of ≥5 as measured by the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton et al., 1991), positive drug urine toxicology at the time of scan, reported past-year drug abuse and/or meeting drug abuse or dependence criteria on the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). Details of maternal substance use are presented in Table 2. The healthy control mothers did not endorse substance use on any of these measures.
Table 2.
Substance-use information for mothers included in the substance-using group (n=32). Urine toxicology was assessed at the time of the fMRI scan. Mothers may be represented in more than one category given their poly-substance use.
| N | ||
|---|---|---|
| Smoking | Current smoker* | 20 |
| Does not smoke | 11 | |
| Missing | 1 | |
| MINI | Substance use** | 2 |
| No substance use | 17 | |
| Missing data | 13 | |
| FTND | Score above 5 | |
| Score below 5 | 26 | |
| Urine Toxicology | Positive | 15 |
| Negative | 3 | |
| Missing | 14 | |
| Methadone | Current | 4 |
| Past use | 2 | |
| Not using | 25 | |
| Addiction Detoxification or Treatment Program | Current | 7 |
| Past | 7 | |
| None reported | 18 | |
Note.
Only one mother used solely tobacco (and her FTND score was > 5). All other tobacco-smoking mothers reported using additional substances though varied in their FTND scores being above or below 5.
Illicit drugs recorded on the MINI included marijuana, cannabis, cocaine, vicodin, phencyclidine (PCP), and diet pills.
FTND=Fagerstrom Test for Nicotine Dependence; MINI=Mini-International Neuropsychiatric Interview
2.2. Experimental Task
All infant face and cry stimuli were presented pseudorandomly through E-Prime 1.2 (Schneider et al., 2002). Infant face stimuli consisted of 10 own-happy-, 10 own-sad-, 10 unknown-happy-, and 10 unknown-sad-face exemplars (40 unique faces in total). Infant cry stimuli consisted of 10 own-cry and 10 unknown-cry exemplars (20 unique cries in total). Own-face and own-cry stimuli were collected as part of a prior study visit, where infant expressions were captured through filming and cries were audio recorded. Own and unknown infant face stimuli were matched on race and affect intensity, with the latter coded by research staff trained to reliability (see Kim et al. (2017a) for full details). Own- and unknown-cry stimuli were matched according to cry intensity and cry type. Infant faces were centrally presented in color on a gray background for 2 seconds, and infant cries were presented through headphones for 2 seconds, with a blank visual display. The inter-stimulus interval was jittered from 2–11 seconds. For each condition (own-happy, own-sad, unknown-happy, unknown-sad, own-cry, unknown-cry), six trials were presented within a block, and there were 7 blocks of data collected, leading to a total of 42 trials per condition.
After the fMRI, each own- and unknown-face and -cry stimulus was presented again to participants to rate them in terms of sadness and distress (sad faces and cries) and happiness (happy faces) employing a 1 (least) to 7 (most) likert scale for each measure.
2.3. fMRI Data Acquisition
Data were acquired with a Siemens Trio 3T MRI system employing a standard 12-channel head coil. Functional data were collected with a gradient echo, echoplanar sequence: repetition time (TR) = 2000 ms, echotime (TE) = 30 ms, flip angle = 80°, field of view = 220 × 220 mm, matrix = 64 × 64, slice thickness = 4mm, and 32 slices.
2.4. fMRI Analyses
Data pre-processing, subject-level, and group level statistics were completed in SPM12 (Ashburner et al., 2014). Functional images were aligned prior to normalization to Montreal Neurological Institute space. Functional runs where participant motion was in excess of 3 mm or degrees were excluded. To be included in the analysis, participants needed at least four functional runs to be motion-free (i.e., < 3 mm or degrees). Data were smoothed using a 6mm full-width-half-maximum (FWHM) Gaussian kernel. Face- and cry-stimulus onsets were convolved with the hemodynamic response function and modeled with temporal derivatives. High-pass filtering (128 s) was applied to all models, and motion parameters from realignment were included as regressors of no interest in the final analyses. Group effects were assessed using the full and flexible factorial modules in SPM12. For face and cry functional data, main effects of group (substance-using, non-substance-using) were assessed using a full factorial design. For face data, we examined, using a flexible factorial design, interactions relating to familiarity (own, unknown) by substance-use group and emotional expression (happy, sad) by substance-use group. For these analyses, all face stimuli (own-happy, own-unhappy, unknown-happy, unknown-sad) were included as within-subjects factors. For cries, we similarly examined interactions involving familiarity (own, unknown) by substance-use group. Main effects of group were assessed using full factorial designs. Consistent with current recommendations (Eklund et al., 2016; Flandin and Friston, 2016), whole-brain statistical maps were voxel-level thresholded at p<.001 and family-wise-error-corrected (FWE) at pFWE<.05.
2.5. Post-Scan Infant Face and Cry Ratings
For sad infant faces and cries, repeated-measures ANOVAs specifying familiarity (own, unknown), rated negative emotion (sad, distress), and substance-use group (substance-using, non-substance-using) were conducted. For happy infant faces, a repeated-measures ANOVA specifying familiarity (own, unknown) and substance-use group (substance-using, non-substance-using) was conducted. Correlations between face and cry ratings with activation to infant face and cry regions identified as significant in whole-brain analyses were also conducted.
3. Results
3.1. Ratings Data
Data were missing from one substance-using mother and one non-substance-using mother. For sad-face ratings, while there was no maternal substance-use between-group effect contributing to face ratings for sadness and distress scales, F(1,50)=2.15,p=.15,ηp2 =.041, there was a main effect of infant familiarity, F(1,50)=8.29,p=.006,ηp2 =.142. Mothers rated their own infant (M=7.05; SD=1.54) as feeling more negative emotions than the unknown infant (M=6.57; SD=1.58). The main effect of infant negative emotion (rating sadness or distress) did not reach statistical significance, F(1,50)=3.52,p=.067,ηp2 =.066, and there were no other interactions between these variables. For happy-face ratings, there was no effect of maternal substance-use status, F<1, though all mothers rated their own infant (M=7.74; SD=1.06) as feeling happier than the unknown infant (M=6.98; SD=1.26), F(1,50)=40.10, p<.001,ηp2 =.445.
Maternal substance-use status affected infant cry ratings, F(1,50)=4.34, p=.042,ηp2 =.080, with substance-using mothers rating all infants as less sad (M=5.75; SD=1.71 vs M=6.69; SD=1.15) and less distressed (M=5.83; SD=1.69 vs M=6.65; SD=1.24) than non-substance-using mothers. There was also a main effect of infant familiarity, F(1,50)=9.31, p=.004,ηp2 =.157. Mothers rated their own infants (M=6.38; SD=1.61) as sadder and more distressed than unknown infants (M=5.91; SD=1.68). There were no interactions between these variables.
Taken together, self-reported ratings evidenced (1) maternal substance-use status affected how mothers perceived the feelings of infants when listening to their cries but not when processing their faces; and, (2) infant familiarity increased the strength of emotions mothers reported their children feeling when rating face and cry stimuli.
3.2. Neuroimaging Data
Whole-brain analyses identified a significant infant-familiarity-by-maternal-substance-use-group interaction during infant face processing. Specifically, while substance-using mothers had greater activation to their own infants’ faces as compared to unknown faces, non-substance-using mothers evidenced a greater activation to unknown infants’ faces as compared to their own infants’ faces (Figure 1). Regions identified in the interaction included a superior medial frontal cluster extending into the supplementary motor area, left and right inferior parietal clusters extending into the insula, and a left middle temporal cluster extending into the occipital cortex, cuneus, and lingual gyrus (Table 3). Whole-brain analyses also identified a significant main effect of maternal substance-use status on processing of sad infant faces within the ventral striatum, involving decreased activity among substance-using versus non-substance-using mothers (Table 3; Figure 2). Primary findings were relatively unchanged in follow-up analyses of the extracted cluster values that included demographic factors that differentiated the two maternal groups (i.e., maternal education, parity, and breastfeeding status) as covariates (see supplementary materials).
Figure 1.
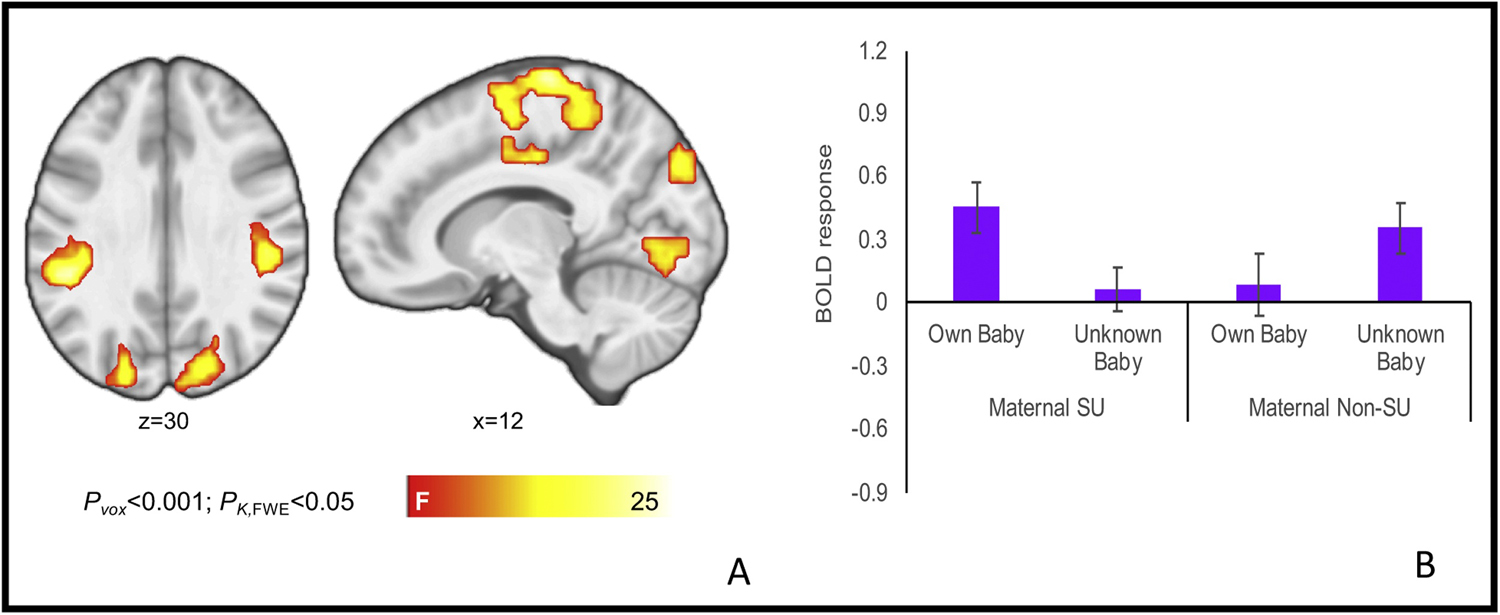
Brain activation in the infant-face-familiarity-by-maternal-substance-use-status interaction (A) with representative interaction term plotted for the cluster incorporating the left and right medial frontal, superior frontal, precentral and postcentral gyri, cingulate gyrus, and supplementary motor area (B). For interaction plots across all clusters, see supplementary materials.
Table 3.
Brain activation in substance-using and non-substance-using mothers viewing infant
| Cluster Description | k | x | y | z | F |
|---|---|---|---|---|---|
| Brain activation in the infant-face-familiarity-by-maternal-substance-use-status interaction | |||||
| Left and right medial frontal, superior frontal, precentral and postcentral gyri, cingulate gyrus, supplementary motor area | 1123 | 6 | −22 | 68 | 26.57 |
| Left inferior parietal lobule, insula, postcentral gyrus | 263 | −48 | −37 | 32 | 26.43 |
| Right inferior parietal lobule, insula, postcentral gyrus | 256 | 51 | −28 | 32 | 20.54 |
| Left middle temporal gyrus, occipital gyrus, cuneus | 231 | −39 | −67 | 8 | 23.37 |
| Right lingual gyrus and bilateral cuneus | 186 | −3 | −91 | 2 | 17.60 |
| Right cuneus, precuneus, superior occipital | 156 | 12 | −82 | 35 | 17.80 |
| Left lingual gyrus | 105 | −21 | −76 | −4 | 16.33 |
| Brain activation for the main effect of substance-use status on sad-infant faces | |||||
| Caudate | 197 | −21 | 29 | 5 | 24.92 |
faces (p<.001, pFWE<.05).
Figure 2.
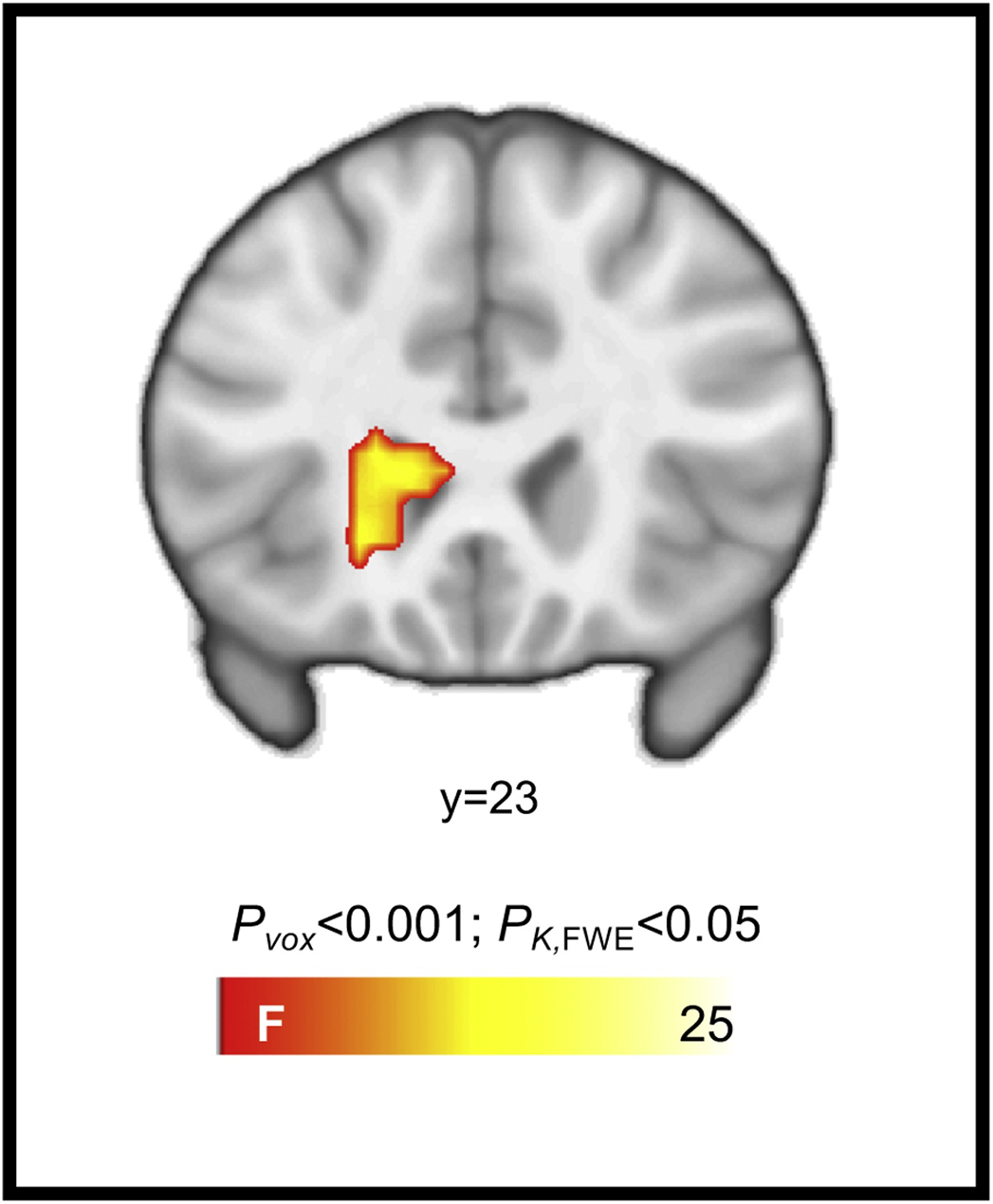
Brain activation for the main effect of substance-use status when viewing sad infant faces, incorporating the caudate.
Maternal substance-use status did not interact with infant facial emotional expression or infant familiarity for cry trials. There was also no main effect of maternal substance-use status across infant face and cry data.
3.3. Neuroimaging and Ratings Data
Substance-using mothers’ responses to own-infant faces in the right lingual gyrus and bilateral cuneus cluster were positively correlated with rating their own infant’s face with respect to distress, r=.41, p=.021, and ratings of sadness, r=.44, p=.013. Similarly, substance-using mothers’ ratings were positively correlated with BOLD responses to unknown-infant faces in this cluster when rating an unknown infant’s feelings of distress, r=.41, p=.020, sadness, r=.37, p=.040, and happiness, r=.36, p=.046. There were no statistically significant associations between brain activations and ratings in the non-substance-using maternal group. Activations in the ventral striatum cluster to sad infant faces were also not associated with maternal ratings of these faces.
4. Discussion
In the current study, we examined relationships between maternal substance-use status and the processing of affective cues from own and unknown infants. We found that relative to non-substance-using mothers, substance-using mothers evidenced greater activation to own, as compared to unknown, infant faces in superior medial frontal, inferior parietal, and middle temporal regions, extending into the occipital cortex. In contrast, non-substance-using mothers evidenced greater activation to an unknown, as compared to their own, infant’s face across the same brain areas. Furthermore, we also found that, as compared to the substance-using mothers, non-substance-using mothers had a heightened response to infant sad faces (own and unknown) in the ventral striatum. Importantly, there were no group differences in processing own or unknown infant cries. Taken together, these findings suggest that maternal substance use may be associated with altered reactivity to infant affective cues, specifically processing of infant faces.
Neurobiological models of addiction and parenting propose that neural circuits implicated in parenting are compromised by addiction (Rutherford et al., 2013; Rutherford et al., 2011). Specifically, dysregulation of stress and reward neural circuits are hypothesized to lead to decreased reward and motivation for caregiving, with infant affective cues instead being more stress-provoking in mothers with substance-use disorders as compared to non-substance-using mothers (Rutherford and Mayes, 2017). Consistent with this notion, non-substance-using mothers had a greater response to sad infant faces in the ventral striatum relative to the substance-using mothers. The ventral striatum has previously been implicated in maternal neural responses to infant faces (Strathearn et al., 2009; Strathearn et al., 2008), and is a region more generally implicated in reward and motivation (Haber and Knutson, 2009; Knutson and Cooper, 2005; McClure et al., 2004). Notably, dopaminergic projections connect multiple brain regions underscoring brain responses to rewards with activity in the ventral striatum proposed to drive responding toward rewards (Koob and Volkow, 2009). Thus, the increased activation to sad infant faces observed in the non-substance-using mothers in the current study may suggest an increased motivation towards the sad infant faces to provide care and the relief of the infant’s distress. Indeed, similar reactivity has also been observed in the ventral striatum when mothers listened to infant cry recordings (Laurent and Ablow, 2011). The attenuated response in the substance-using mothers may reflect the decreased motivational salience of infant affective cues secondary to addiction (Rutherford et al., 2013; Rutherford et al., 2011), although longitudinal research is needed to fully explore this notion. Notably, decreased responses to sad infant faces in the ventral striatum have also been observed in mothers with insecure patterns of attachment as compared to mothers with secure patterns of attachment (Strathearn et al., 2009). Given that attachment insecurity has been implicated in developmental models of addiction (e.g., Alvarez-Monjaras et al., 2018), future research incorporating measures of attachment patterns could provide mechanistic insight into our understanding of aberrant processing of infant cues in substance-using mothers.
Unlike prior work, we did not observe a decreased neural response to own, as compared to unknown, happy infant faces in regions implicated in reward processing, including the ventromedial PFC and ventral striatum when specified as regions of interest (i.e., Kim et al., 2017a). Further, we did not observe an overall between-group effect wherein substance-using mothers had an attenuated response relative to non-substance-using mothers to infant face stimuli (i.e., Landi et al., 2011). Instead, we found that substance-using mothers had increased reactivity to own, as compared to unknown, infant faces across multiple cortical regions. Consistent with our finding, Kim et al. (2017a) also reported increased activation to own, as compared to unknown, infant faces in mothers receiving inpatient treatment for substance-use disorders in the cingulate, insula, precuneus, and precentral and postcentral gyrus – regions overlapping with those reported here. In contrast, mothers not currently using substances evidenced the opposite pattern of responding: greater reactivity to unknown, as compared to own, infant faces. Nevertheless, there was convergence in findings between the current and prior work. Specifically, Landi et al. (2011) evidenced a decreased response to unknown infant faces in substance-using mothers as compared to non-substance-using mothers. When viewing the comparable unknown infant face findings in Figure 1, we also observed a descriptively decreased response to unknown infant faces in the maternal substance-using group as compared to the non-substance-using group.
Why might maternal substance use be associated with increased reactivity to own, as compared to unknown, emotional infant faces? Consistent with neurobiological models of parenting and addiction (Rutherford et al., 2013; Rutherford et al., 2011), this increased responsiveness to own-infant cues may relate to heightened arousal or reactivity to infant facial cues of affect in substance-using mothers. Prior research in non-parents has also evidenced increased activation in the occipital gyrus, and right inferior and superior parietal lobule, to emotional as compared to neutral pictures, with more arousing pictures (positive and negative) being associated with more widespread activity in these areas (Lang et al., 1998). Furthermore, ratings collected from substance-using mothers after the scanning protocol indicate that, in the cluster incorporating the right lingual gyrus and bilateral cuneus, increased activities to infant face stimuli were associated with increased ratings of affective intensities of the faces. Finally, increased activation to own-infant sad faces as compared to unknown-infant sad faces has previously been shown in the insula in mothers with attachment insecurity (Strathearn et al., 2009). The insula has been associated with emotional experiences and may be involved in higher-level representation of emotional states (Singer et al., 2009). Furthermore, insula activity elicited by negatively valenced stimuli can be down-regulated by cognitive reappraisal (Goldin et al., 2008). Therefore, one explanation of the current findings may be that substance-using mothers evidence increased reactivity to own-infant cues akin to an arousal or an affective/stress response.
Consideration is also warranted for why non-substance-using mothers may have a greater neural response to unknown, as compared to own, infant faces across these same cortical regions. An attenuated response may suggest potential habituation to these presumably salient infant affective stimuli (Wright et al., 2001), possibly related to non-substance-using mothers spending more time with their infants. However, contrary to this, substance-using and non-substance-using mothers in this study were fairly comparable in the durations spent away from their child. However, substance-using mothers had shorter durations of breastfeeding with more women reporting not breastfeeding at all, relative to the non-substance-using mothers. Although we did not measure maternal oxytocin levels in the current study, oxytocin is implicated in breastfeeding and mothers with insecure patterns of attachment have evidenced a decreased oxytocin response during interactions with their infants (Strathearn et al., 2009), perhaps implicating a role for the oxytocinergic and attachment systems in our current findings. Alternatively, reactivity to own-infant cues may be tempered in non-substance-using mothers, given greater tendencies to understand and interpret their infant’s affective bids, and thus not become overwhelmed by these emotional cues, a capacity that is often compromised in mothers with addictions (Suchman et al., 2010; Suchman et al., 2017). Thus, future studies developing this line of research should incorporate more precise measures of the quality and quantity of mother-infant interactions, including levels of oxytocin and attachment security, alongside measures that provide an indication of the capacity to respond and interpret affective signals from infants to inform our understanding of maternal brain findings.
We found no evidence for alterations in neural processing of infant cries as a function of maternal substance use. This contrasts with prior work, employing unknown infant cries, that evidenced a decreased response to infant cries in substance-using, as compared to non-substance-using, mothers (Landi et al., 2011). Other studies have shown differential sensitivity to infant cries in clinical groups, including mothers with and without depression (Laurent and Ablow, 2011). Furthermore, experimental instructions relating to cry perception and parenting characteristics are associated with changing responses to cries at neurobiological levels (Swain et al., 2017; Swain et al., 2008). These latter studies have employed cry recordings longer in duration than the two-second cries employed here, perhaps explaining this discrepancy. Thus, our null findings of maternal substance-use status and familiarity may in part be attributable to these differences in cry parameters. That being said, it is worth noting differences in self-reported measures of cries that occurred after the scanning protocol wherein substance-using mothers rated the cry stimuli (own and unknown) as less sad and distressed than non-substance-using mothers. Furthermore, across the sample, mothers rated their own infant’s cries as sadder and more distressed than the unknown infant’s cries. Therefore, at a self-report level, maternal substance-use and familiarity effects emerge, but we do not see this same distinction at the level of neural function. This divergence in brain and behavioral measures might reflect a response bias, a limitation of subjective measures, or a decrease in salience in response to repeated exposure to the cries across the scanning protocol.
The strengths of our study included the well-characterized sample of substance-using and non-substance-using mothers and our simultaneous assessment of infant face and infant cry familiarity effects. However, important limitations and directions for future research should be noted. Importantly, this was a cross-sectional design, preventing causal inference regarding the potential impact of maternal substance use on neural responses to infant affective cues. Instead these findings are best viewed as associations between mothers with and without current substance use and their neural response to infant cues, rather than as evidence of substance use leading to changing neural responses to infant cues. Furthermore, there may be other factors that co-occur with substance use that might be associated with neuroanatomical and functional differences in neural responses to infant affective cues, such as differences in attachment or unresolved trauma (Kim et al., 2017b). This warrants further investigation in future research – both within the substance-using group and in comparison with the non-substance-using group.
In contrast to our hypothesis, our findings were not consistent with prior work which evidenced that when substance-using mothers viewed their own infant’s happy face, relative to an unknown infant, there was decreased activation in the ventromedial PFC, hypothalamus, and ventral striatum (Kim et al., 2017a). There may be two reasons for these differences. The first reflects differences between the maternal samples; the current study employed poly-substance-using mothers living in the community with only 7 women reporting receiving current substance-use treatment. In contrast, all mothers were receiving inpatient treatment for substance-use disorders in the study by Kim and colleagues (2017a). Secondly, we examined data collected across at least four functional runs in our maternal sample, whereas prior work examined functional data from only two runs (Kim et al., 2017a). Future work should examine the time course of maternal neural responses to infant faces in the presence (and absence) of addictive behaviors.
Although we examined a poly-substance-using sample of mothers, we were not sufficiently powered to disentangle contributions of individual substances to our findings. Despite this limitation, it is important to note that, among substance-using mothers, polysubstance use (as opposed to limited use of only one substance) is common (e.g., Eiden et al., 2006; Metz et al., 2018; Reitan, 2017). In addition, while inconsistencies across studies of maternal substance use may relate to differences in substance use across samples, they may also reflect the absence of stability in responding to infant affective cues, which may vary at a within-subjects level, as well as at a group level, when considering the maternal substance-using group. Such variability may present as decreased reactivity (consistent with Kim et al., 2017a, and Landi et al., 2011) as well as the increased reactivity observed here. This interpretation is consistent with behavioral studies demonstrating associations between substance use and mother-child interactions. Namely, past research has demonstrated that, while substance-using mothers may be passive and disengaged during their interactions with their child (e.g., Gottwald and Thurman, 1994), they may also be hostile and intrusive (e.g., Johnson et al., 2002). Therefore, it is important to acknowledge that variability in reactivity to infant affective cues (with respect to brain function or behavior) associated with maternal substance use may reflect differing points in the cycle of addiction, which warrant concurrent measurement and/or explicit manipulation (e.g., through completing experimental protocols during abstinent and satiated states) in future research. This work will be advanced by examining maternal brain function and behavior within the same session to more adequately inform whether changing neural responses to infant cues may translate to maternal behavior and child outcomes. Furthermore, as this work continues, it will also be important to address whether differential patterns of maternal brain activity (and behavior) vary as a function of the experimental context and/or task demands.
In conclusion, this is one of the first studies to examine how mothers with and without substance use respond to cues from own and unknown infants and of varying affective states. Our findings suggest overlapping cortical and subcortical (e.g., striatal) brain regions are involved in responding to infant faces, and responses are influenced by infant familiarity, emotional expression, and maternal substance-use status. These findings advance our understanding of the neurobiology of parenting and addiction and highlight the need to more explicitly include in theoretical models and in empirical research increased reactivity to salient infant affective cues, in addition to attenuated neural responses, which are often the focus of this work. Furthermore, future research should include multiple levels of assessment, including brain, self-report, and behavioral indices of parenting.
Supplementary Material
Highlights.
Maternal substance use (SU) can impact caregiving and child development
SU may also shape maternal brain activation to salient infant cues
Greater activity to own vs. unknown infant faces was observed in SU mothers
Decreased activity to sad infant faces was present in SU mothers vs. non-SU mothers
No effect of maternal SU on listening to own vs. unknown infant cries
Role of Funding Source
This work was supported by grants from the National Institutes of Health P01 DA022446, R01 DA026437, K01DA039299, and R03 DA045289. The views presented in this manuscript are those of the authors and do not necessarily reflect those of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest with respect to the content of this manuscript. Dr. Potenza has: consulted for and advised Game Day Data, the Addiction Policy Forum, Rivermend Health, Opiant/Lightlake Therapeutics and Jazz Pharmaceuticals; received research support from the Mohegan Sun Casino and the National Center for Responsible Gaming; and consulted for legal and gambling entities on issues related to impulse-control disorders and addictions. The other authors report no disclosures.
References
- Alvarez-Monjaras M, Mayes LC, Potenza MN, Rutherford HJ, 2018. A developmental model of addictions: integrating neurobiological and psychodynamic theories through the lens of attachment. Attachment & Human Development, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Barnes G, Chen C, Daunizeau J, Flandin G, Friston K, Kiebel S, Kilner J, Litvak V, Moran R, 2014. SPM12 manual Wellcome Trust Centre for Neuroimaging, London, UK. [Google Scholar]
- Eiden RD, Stevens A, Schuetze P, Dombkowski LE, 2006. A conceptual model for maternal behavior among polydrug cocaine-using mothers: The role of postnatal cocaine use and maternal depression. Psychology of Addictive Behaviors, 20, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci, 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, 2015. The adaptive human parental brain: implications for children’s social development. TINS, 38, 387–399. [DOI] [PubMed] [Google Scholar]
- Flandin G, Friston KJ, 2016. Analysis of family-wise error rates in statistical parametric mapping using random field theory Wellcome Trust Centre for Neuroimaging, University College London. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray A, Merry B, Lin H, Ruger JP, Yonkers KA, 2015. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug. Alcohol. Depend, 150, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghertner R, Waters A, Radel L, Crouse G, 2018. The role of substance use in child welfare caseloads. Children and Youth Services Review, 90, 83–93. [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ, 2008. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry, 63, 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald SR, Thurman SK, 1994. The effects of prenatal cocaine exposure on mother--infant interaction and infant arousal in the newborn period. Topics in Early Childhood Special Education, 14, 217–231. [Google Scholar]
- Gutvirtz G, Wainstock T, Landau D, Sheiner E, 2019. Maternal smoking during pregnancy and long-term neurological morbidity of the offspring. Addict. Behav, 88, 86–91. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2009. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzis D, Dawe S, Harnett P, Barlow J, 2017. Quality of caregiving in mothers with illicit substance use: A systematic review and meta-analysis. Substance Abuse: Research And Treatment 11, 1178221817694038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O, 1991. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Morrow CE, Accornero VH, Xue L, Anthony JC, Bandstra ES, 2002. Maternal Cocaine Use: Estimated Effects on Mother-Child Play Interactions in the Preschool Period. J. Dev. Behav. Pediatr, 23, 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepple NJ, 2018. Does parental substance use always engender risk for children? Comparing incidence rate ratios of abusive and neglectful behaviors across substance use behavior patterns. Child. Abuse. Negl, 76, 44–55. [DOI] [PubMed] [Google Scholar]
- Kim S, Iyengar U, Mayes LC, Potenza MN, Rutherford HJ, Strathearn L, 2017a. Mothers with substance addictions show reduced reward responses when viewing their own infant’s face. Human Brain Mapping, 38, 5421–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kwok S, Mayes LC, Potenza MN, Rutherford HJ, Strathearn L, 2017b. Early adverse experience and substance addiction: dopamine, oxytocin, and glucocorticoid pathways. Ann. N.Y. Acad. Sci, 1394, 74–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC, 2005. Functional magnetic resonance imaging of reward prediction. Curr. Opin. Neurol, 18, 411–417. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2009. Neurocircuitry of Addiction. Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford HJV, Mencl E, Worhunsky P, Potenza MN, Mayes LC, 2011. Maternal neural responses to infant cries and faces: Relationships with substance use. Frontiers in Psychiatry, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, Nangia V, 1998. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology, 35, 199–210. [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, 2011. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience, 7, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes L, Suchman NE, 2006. Developmental pathways to substance abuse In: Cicchetti D, Cohen DJ (Eds.), Developmental Psychopathology. John Wiley & Sons, Hoboken, NJ: pp. 599–619. [Google Scholar]
- McClure SM, York MK, Montague PR, 2004. The neural substrates of reward processing in humans: the modern role of FMRI. The Neuroscientist, 10, 260–268. [DOI] [PubMed] [Google Scholar]
- Metz VE, Brown QL, Martins SS, Palamar JJ, 2018. Characteristics of drug use among pregnant women in the United States: Opioid and non-opioid illegal drug use. Drug. Alcohol. Depend, 183, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsolini L, Bellantuono C, De Berardis D, Schifano F, 2019. Alcohol Use Disorders In: Uguz F and Orsolini L (Eds.) Perinatal Psychopharmacology. Springer Nature, Switzerland. [Google Scholar]
- Patrick SW, Davis MM, Lehmann C, Cooper WO, 2015. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J. Perinatol, 35, 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan T, 2017. Patterns of polydrug use among pregnant substance abusers. Nordic Studies on Alcohol and Drugs, 34, 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJV, Mayes L, 2017. Parenting and Addiction: Neurobiological Insights. Current Opinion in Psychology, 15, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJV, Potenza MN, Mayes LC, 2013. The neurobiology of addiction and attachment In: Suchman N, Pajulo M, Mayes LC (Eds.), Parents and Substance Addiction: Developmental Approaches to Intervention. Oxford University Press, New York. [Google Scholar]
- Rutherford HJV, Williams SK, Moy S, Mayes LC, Johns JM, 2011. Disruption of maternal parenting circuitry by addictive process: rewiring of reward and stress systems. Frontiers in Psychiatry, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A, 2002. E-Prime user’s guide. Psychology Software Tools Inc, Pittsburgh. [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, 2003. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol. Psychiatry, 54, 1367–1375. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K, 2009. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences, 13, 334–340. [DOI] [PubMed] [Google Scholar]
- Squire S, Stein A, 2003. Functional MRI and parental responsiveness: a new avenue into parental psychopathology and early parent-child interactions? Br. J. Psychiatry, 183, 481–483. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR, 2009. Adult Attachment Predicts Maternal Brain and Oxytocin Response to Infant Cues. Neuropsychopharmacology 34, 2655–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR, 2008. What’s in a Smile? Maternal Brain Responses to Infant Facial Cues. Pediatrics 122, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2018. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18–5068, NSDUH Series H-53). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Suchman NE, DeCoste C, Leigh D, Borelli J, 2010. Reflective functioning in mothers with drug use disorders: Implications for dyadic interactions with infants and toddlers. Attachment & Human Development, 12, 567–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchman NE, DeCoste CL, McMahon TJ, Dalton R, Mayes LC, Borelli J, 2017. Mothering From the Inside Out: Results of a second randomized clinical trial testing a mentalization-based intervention for mothers in addiction treatment. Dev. Psychopathol, 29, 617–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Ho SS, Rosenblum KL, Morelen D, Dayton CJ, Muzik M, 2017. Parent–child intervention decreases stress and increases maternal brain activity and connectivity during own baby-cry: An exploratory study. Dev. Psychopathol, 29, 535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L, 2007. Brain basis of early parent–infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J. Child Psychol. Psychiatry, 48, 262–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Todd Constable R, Leckman JF, 2008. Maternal brain response to own baby-cry is affected by cesarean section delivery. J. Child Psychol. Psychiatry, 49, 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL, 2001. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport, 12, 379–383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


