Abstract
Current guidelines recommend that serum C-terminal telopeptide of type I collagen (CTX) and serum procollagen type 1 aminoterminal propeptide (PINP), measured by standardized assays, be used as reference markers in observational and interventional studies. However, there are limited data to determine whether serum CTX and PINP are associated with hip fracture risk among postmenopausal women. We determined the associations of serum CTX and serum PINP with hip fracture risk among postmenopausal women aged 50–79 years at baseline. We performed a prospective case-control study (400 cases, 400 controls) nested in the Women’s Health Initiative Observational Study, which enrolled participants at 40 U.S. clinical centers. Cases were women with incident hip fracture not taking osteoporosis medication; hip fractures were confirmed using medical records. Untreated controls were matched by age, race/ethnicity, and date of blood sampling. Serum CTX and serum PINP were analyzed on 12-hour fasting blood samples. The main outcome measure was incident hip fracture risk (mean follow-up 7.13 years). After adjustment for body mass index, smoking, frequency of falls, history of fracture, calcium and vitamin D intake, and other relevant covariates, neither serum CTX level nor serum PINP level was statistically significantly associated with hip fracture risk (CTX ptrend-value 0.22, PINP ptrend-value 0.53). Our results do not support the utility of serum CTX level or PINP level to predict hip fracture risk in women in this age group. These results will inform future guidelines regarding the potential utility of these markers in fracture prediction.
Keywords: bone turnover, fracture, C-terminal telopeptide of type I collagen, procollagen type 1 aminoterminal propeptide, CTX, PINP
Introduction
The International Osteoporosis Foundation/International Federation of Clinical Chemistry and Laboratory Medicine (IOF/IFCC) Bone Markers Working Group identified one bone resorption marker, C-terminal telopeptide of type I collagen (CTX), and one bone formation marker, procollagen type I aminoterminal propeptide (PINP), as the most promising bone turnover markers.(1,2) The IOF/IFCC recommends that serum CTX and serum PINP, measured by standardized assays, be used as reference markers in observational and interventional studies. Serum PINP is generated during the synthesis of type I collagen.(3) PINP is cleaved from type I pro-collagen during its extracellular processing. Serum CTX is a product of the breakdown of type I collagen containing pyridinium cross-links.(3)
Higher bone turnover marker levels, particularly resorption marker levels, are associated with increased fracture risk in some, but not all, previous studies of older men and women.(4) Most studies have examined bone turnover marker levels in relation to fragility or osteoporotic fractures overall, rather than hip fractures.(5–9) However, because hip fractures are a substantial cause of increased morbidity and mortality, it is of clinical importance to elucidate the ability of serum bone turnover markers to predict hip fracture risk. Studies of bone turnover marker levels in relation to hip fractures often had the disadvantage of measuring the biomarker levels soon after the hip fracture occurred (10–17) (when these biomarkers may be elevated), which does not allow the elucidation of the predictive ability of the bone turnover markers prior to an incident hip fracture. Only 12 studies measured bone turnover marker levels prior to incident hip fracture in women.(18–29) Of those studies, three studies found a significant positive association between bone turnover marker (urinary CTX (28,29), urinary deoxypridinoline(29), urinary N-telopeptide(27)) level and hip fracture risk, one study found positive associations (urinary deoxypyridinoline, urinary deoxypyridinoline) that disappeared after covariate adjustment(26), and seven studies reported a lack of association between bone turnover marker level (serum alkaline phosphatase(20–22,24,25), serum osteocalcin(20–23), urinary hydroxyproline(20), urinary CTX(19), urinary deoxypyridinoline(21), urinary osteocalcin(21,22)) and hip fracture risk. Studies of the two IOF/IFCC-recommended tests, serum PINP and serum CTX, for prediction of hip fracture risk in women are few, and generally find no associations of these markers with hip fracture risk.(18,19,21–23,27) However, none of the studies required sampling in the fasting state. Overnight fasting markedly reduces the circadian variation of serum CTX, leading to the recommendation that morning fasting samples to be used if both CTX and PINP are measured.(3) We have access to serum PINP and CTX assay measurements from samples collected in the fasting state. The objective of this study was to test the hypothesis that increased bone turnover as assessed by serum PINP and CTX is associated with a higher risk of hip fracture in women, independent of other covariates.
Methods
Study participants
We analyzed data from the Women’s Health Initiative (WHI) study, which recruited participants at 40 U.S. clinical centers. At baseline, participants were aged between 50 and 79 years and free of serious medical conditions. The WHI Observational Study examined common causes of morbidity and mortality among postmenopausal women; the WHI Clinical Trials evaluated three distinct interventions: a low-fat eating pattern, menopausal hormone therapy, and calcium plus vitamin D supplementation. Study methods have been described in detail elsewhere.(30,31)
A case-control substudy was performed, nested within the Women’s Health Initiative Observational Study, to examine hormonal predictors of hip fracture in women.(32–34) Information regarding incident hip fractures was collected via self-report of annual questionnaires. All self-reported hip fractures were centrally confirmed by study physicians using medical records.
Inclusion criteria include WHI Observational Study participants who had a first hip fracture between August 1994 and August 2004. Hip fractures were defined as non-pathologic fractures of the proximal femur, including fractures of the femoral neck, intertrochanteric region and greater trochanter. Mean, median, and range of follow-up time of the overall cohort (n = 800) were 7.13 years, 7.13 years, and 0.7–9.3 years. Mean, median, and range of follow-up time of the cases (n = 400) were 7.03 years, 7.04 years, and 0.7–9.3 years. Mean, median, and range of follow-up time of the controls (n = 400) were 7.23 years, 7.30 years, and 2.3–9.2 years. By the end of the follow-up period, 3.7% of the WHI Observational Study participants had withdrawn or were lost to follow-up and 5.3% of participants had died.(35) In the overall WHI Observational Study, annualized hip fractures rate was 0.14%.
The criteria for exclusion were: 1. hip fracture prior to study baseline; 2. use at baseline of medications containing estrogen (up to one year prior to study entry; oral and transdermal forms only), androgens (including anabolic steroids, dehydroepiandrosterone, testosterone), selective estrogen receptor modulators (SERMs), antiestrogens, or medications for bone loss (including bisphosphonates, calcitonin, parathyroid hormone); 3. pathological cause for hip fractures occurring during the study; 4. only local adjudication of hip fractures occurring during the study; 5. unknown ethnicity; and 6. absent baseline serum samples.
One control participant was selected for each case from the risk set corresponding to the time of the case’s event. All participants (cases included) were part of the risk set until they have an event or until their last recorded visit (censored if lost to follow-up). Thus, cases could be a potential control for other cases whose events happen earlier, but none of the 400 controls had a hip fracture during the study period. Controls were matched by age at screening (+/− 1 year), race/ethnicity (White, Black, Hispanic, Asian/Pacific Islander, other), and date of serum sample collection (+/− 120 days, which is the same as the date of entry into the WHI-Observational Study). Age and serum sampling date were selected based on a criterion to minimize an overall distance measure. The age and race criteria were weighted 20 times more than the draw date criteria to emphasize their importance. 404 women experienced hip fractures after 7.1 years of follow-up; of these women, we randomly selected 400 women as the incident hip fracture (case) group.(35)
A control meeting the matching criteria was matched to each of the 400 cases. Race/ethnicity was matched exactly for all 400 cases. Age was matched exactly for 395 cases and within a year for everyone. Phlebotomy date was matched exactly for 243 cases, within one week for 387 cases, within 3 weeks for 396 cases, and within 71 days for everyone. No cases were selected as controls for participants with an earlier event, so the final cohort contains 800 participants (400 women with hip fracture and 400 matched controls) (Figure 1).
Figure 1.
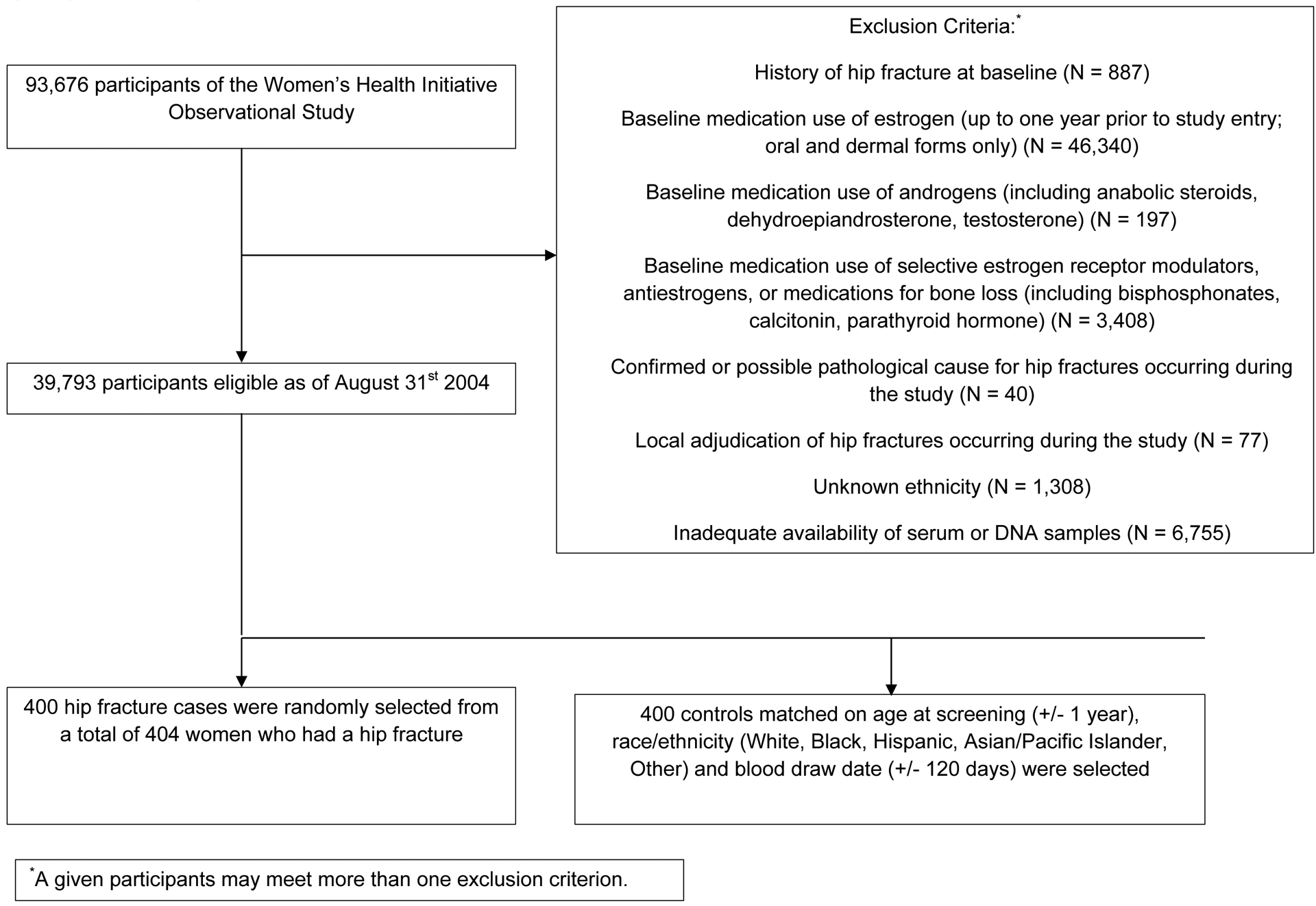
Analytic sample algorithm
Each institution obtained human subjects committee approval. All participants provided written informed consent.
Measurements
Using baseline self-report questionnaires, we collected information regarding age, race/ethnicity, education, living with a partner, parity, smoking, frequency of falls in the past year, fracture prior to baseline, family history of hip fracture, medication use, self-reported health status, dietary and supplemental calcium and vitamin D.
For each participant, a frailty score was calculated from 4 measures: RAND-36 physical function scale score, the RAND-36 vitality scale score, total energy expenditure (metabolic equivalent task hours score from recreational physical activity in the prior week), and whether the participant lost 15 pounds of body weight in the last 6 months without trying. (36–38) For each of the above measures (except unintentional weight loss), a frailty component was assigned if a woman had a score below the 25th percentile of that component. We computed the score by summing the scores of the 4 frailty score measures; physical function component was double-counted because the scale measured both muscle strength and walking ability. Therefore, the range of frailty scores was 0–5. A cut-point of 3 or more was used to define frailty. (36–38)
For each participant, we calculated the Centers for the Epidemiologic Study of Depression short-form score (39,40) and the RAND 36-Item Health Survey (SF-36) score.(41–44)
The predicted 10-year absolute risk of hip fracture was calculated for each participant by the WHO Collaborating Centre for Metabolic Bone Disease using the Fracture Risk Assessment (FRAX) tool without BMD (45), as in prior studies.(46,47)
Weight and height were measured using standard protocols at baseline for calculation of body mass index (BMI, kg/m2).
At the baseline visit, participants of the case-control study provided 12-hour fasting morning serum samples.(35) Serum samples were stored at −80° until they were shipped on dry ice to a central laboratory (Synarc, Lyon, France) for analysis. Laboratory personnel were blinded to case-control status. Serum CTX was measured by a two-site immunoassay using two monoclonal antibodies raised against a specific isomerized 8-amino acid sequence from the C-telopeptide of human type I collagen with an automatic analyzer (Elecsys, Roche Diagnostics); the intra-assay variability was 1–4% and inter-assay variability was 3–6%. Serum intact PINP was measured with a two-site immunoassay based on monoclonal antibodies raised against purified intact human PINP, detecting both mono- and tri-meric forms, but not fragments, using an automated analyzer (Elecsys, Roche Diagnostics). The intra-assay variability was 1–2% and inter-assay variability was 2–4%.
Statistical Analysis
Baseline characteristics of women in the case and control groups were compared using Chi-square tests of association for categorical variables and using t-tests for continuous variables.
Based on the distributions of PINP and CTX levels, PINP and CTX values were natural log-transformed prior to the analysis and back-transformed after analysis for ease of presentation. The primary outcome of the conditional logistic regression models was hip fracture. We created separate models for each of the primary predictors, PINP and CTX. We categorized the bone turnover marker levels in quartiles based on the distribution of bone turnover markers in the controls. We examined non-linear associations of log-transformed serum PINP and CTX with hip fracture risk using generalized additive models. There were no significant non-linear associations, so we present only the results of statistical models using linear bone turnover marker terms.
To determine whether a significant linear trend was present across the quartiles, we calculated ptrend values by entering the bone turnover marker quartile term as a continuous variable. We also calculated p-values from the logistic regression model where the natural log-transformed bone turnover marker level was entered as a continuous term.(48) Finally, to replicate the approach of a previous study (19), we compared the risk of hip fracture in women with turnover marker levels within the highest quartile vs. the lower 3 quartiles.
We adjusted the logistic regression models for potential confounders based on results of prior published studies. In the conditional logistic regression models, we did not adjust for the matching factors (age, race/ethnicity, and date of serum sample collection). The potential confounders, assessed at baseline, included: BMI (<25, 25-<30, ≥30 kg/m2), years of education, whether living with a partner (yes or no), parity, cigarette smoking (never, current, past), frequency of falls in the past year (0, 1, ≥2), history of previous fracture (none, fracture aged ≥55 years, aged <55 years, fracture at unknown age), family history of hip fracture (yes or no). past use of menopausal hormone therapy (yes, no), depressive symptoms (CES-D short-form score ≥0.009 or use of anti-depressant medication, yes vs. no), frailty score (0, 1–2, ≥3), self-reported health status, corticosteroid use (yes or no), RAND SF-36 Health Survey score (continuous), supplemental calcium intake (tertile), supplemental vitamin D intake (tertile), dietary calcium intake (mg/d), and dietary vitamin D intake (IU/d).
We assessed for effect modification by 10-year predicted risk of hip fracture by including the product term “FRAX-predicted 10-year absolute risk of hip fracture * bone turnover marker level” in the regression model. FRAX-predicted hip fracture risk was calculated without BMD information (46,47) and coded as above vs. below the median score. The p-value for the interaction term was calculated with the FRAX score and the bone turnover marker quartiles entered into the model as continuous terms.
In sensitivity analyses, in case associations may be more pronounced earlier in the follow-up period, we examined the subset of participants in whom hip fracture occurred within the first 5 years of follow-up (sample size n = 420). In additional sensitivity analyses, we 2) repeated the primary analyses by categorizing PINP and CTX level as above vs. below the median value, and 2) separately examined associations in the subset of women who were aged 65 years and older. We also separately examined associations between PINP and CTX levels and hip fracture risk according to whether fractures were femoral neck or intertrochanteric fractures.
Subsequent to the ascertainment of cases and their matched controls, which ended in 2004, controls could theoretically have experienced a hip fracture. Therefore, we performed a secondary analysis that excluded control group participants who experienced a medical record-confirmed hip fracture between 2004 and 2010, and/or who did not consent to follow-up during the extension period (2005–2010); 242 control group participants were remained for the secondary analysis. We used unconditional logistic regression models, adjusted for matching factors, for this secondary analysis.
A preplanned statistical power analysis performed prior to the initiation of the case-control study found that we would have 80% power to detect a risk ratio of 1.5; because hip fractures are rare events in WHI, and risk ratios and odds ratios are comparable for rare events, we estimate that we had 80% power to detect an odds ratio of 1.5 for associations between bone turnover markers and hip fracture risk.
Statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/). Statistical tests were two-sided. A p-value <0.05 was considered statistically significant.
Results
Baseline Characteristics of the Analytic Sample
Compared with the women in the control group, women in the case group were more likely to have BMI <25 kg/m2, be current smokers, be frail, be corticosteroid users; cases had higher CES-D scores and were less likely to be living with a partner (Table 1). Baseline mean PINP and CTX levels were similar in the case and control groups (Table 2). The median (interquartile range [IQR]) level of PINP was 45.7 μg/L [35.7–59.2 μg/L] in the control group and 47.1 μg/L [34.7–62.8 μg/L] in the case group; the median (IQR) level of CTX was 390 ng/L [280–510 ng/L] in the control group and 420 ng/L [300–570 ng/L] in the case group. Mean age at the time of hip fracture was 75.4 years. The average length of follow-up was for cases was 7.03 years. Median (range) time to incident hip fracture was 3.83 (0.02–8.33) years.
Table 1.
Baseline Characteristics of Cases and Controls
| Characteristics | P-Value | ||
|---|---|---|---|
| Cases (n = 400) | Control (n = 400) | ||
| Age at Screening, years (Mean ± SD) | 70.78(6.16) | 70.77(6.15) | 0.99 |
| Body mass index, kg/m2 | 0.001 | ||
| <25 | 193(48.61) | 144(36.09) | |
| 25 – <30 | 127(31.99) | 150(37.59) | |
| ≥30 | 77(19.40) | 105(26.32) | |
| Race/Ethnicity | 1.00 | ||
| White | 379(94.99) | 380(95.00) | |
| Black | 10(2.51) | 10(2.50) | |
| Others | 10(2.51) | 10(2.50) | |
| Years of Education | 0.30 | ||
| None to some high school | 18(4.55) | 26(6.55) | |
| High school diploma/GED | 84(21.21) | 67(16.88) | |
| School after high school | 161(40.66) | 171(43.07) | |
| College degree or higher | 133(33.59) | 133(33.50) | |
| Living with Partner | 184(46.12) | 212(53.27) | 0.04 |
| Season of phlebotomy | 1.00 | ||
| Winter | 89(22.25) | 87(21.75) | |
| Spring | 103(25.75) | 104(26.00) | |
| Summer | 130(32.50) | 131(32.75) | |
| Fall | 78(19.50) | 78(19.50) | |
| Parity | 0.06 | ||
| Never pregnant | 65(16.54) | 37(9.34) | |
| Never had term pregnancy | 8(2.04) | 10(2.53) | |
| 1 | 41(10.43) | 32(8.08) | |
| 2 | 86(21.88) | 96(24.24) | |
| 3 | 81(20.61) | 100(25.25) | |
| 4 | 55(13.99) | 60(15.15) | |
| 5+ | 57(14.50) | 61(15.40) | |
| Smoking | <0.001 | ||
| Never | 214(54.31) | 215(54.29) | |
| Past | 144(36.55) | 171(43.18) | |
| Current | 36(9.14) | 10(2.53) | |
| Fall history in the past year | 0.16 | ||
| No falls | 237(60.61) | 260(66.84) | |
| 1 fall | 92(23.53) | 82(21.08) | |
| 2+ falls | 62(15.86) | 47(12.08) | |
| History of Fracture | 0.27 | ||
| Fracture when ≥ 55 years of age | 107(27.51) | 93(24.09) | |
| Fracture when <55 years of age | 44(11.31) | 42(10.88) | |
| No Fracture | 204(52.44) | 227(58.81) | |
| Fracture, unknown age | 34(8.74) | 24(6.22) | |
| Family History of Hip Fracture | 80(22.22) | 64(17.58) | 0.12 |
| Past Use of Menopausal Hormone Therapy | 0.80 | ||
| Never Used | 305(76.25) | 302(75.50) | |
| Past User | 95(23.75) | 98(24.50) | |
| Centers for the Epidemiologic Study of Depression short- form score ≥0.009 or antidepressant medication use | 119(29.75) | 95(23.75) | 0.06 |
| Frailty Index score* | 0.02 | ||
| 0 | 193(51.88) | 229(59.95) | |
| 1–2 | 115(30.91) | 112(29.32) | |
| ≥3 | 64(17.20) | 41(10.73) | |
| Self-reported health status | 0.18 | ||
| Excellent | 62(15.62) | 62(15.78) | |
| Very good | 132(33.25) | 158(40.20) | |
| Good | 142(35.77) | 131(33.33) | |
| Fair | 60(15.11) | 41(10.43) | |
| Poor | 1(0.25) | 1(0.25) | |
| Corticosteroid Use† | 16(4.00) | 4(1.00) | <0.01 |
| RAND 36-Item Health Survey (SF-36) score (Mean ± SD) | 70.99(19.25) | 72.89(17.89) | 0.15 |
| Dietary Calcium intake (mg/d) (Mean ± SD) | 792.5(454.3) | 841.4(455.2) | 0.14 |
| Dietary Vitamin-D intake (IU/d) (Mean ± SD) | 169.5(123.2) | 180.8(132.9) | 0.23 |
| Supplemental Calcium intake (mg/d) | 0.33 | ||
| Not a user | 187(46.75) | 177(44.25) | |
| Lowest tertile | 79(19.75) | 66(16.50) | |
| Middle tertile | 66(16.50) | 81(20.25) | |
| Highest tertile | 68(17.00) | 76(19.00) | |
| Supplemental Vitamin D intake (IU/d) | 0.94 | ||
| Not a user | 205(51.25) | 211(52.75) | |
| Lowest tertile | 30(7.50) | 26(6.50) | |
| Middle tertile | 126(31.50) | 125(31.25) | |
| Highest tertile | 39(9.75) | 38(9.50) | |
Please see text for details regarding frailty score.
Includes use of glucocorticoids, steroid combinations, mineralocorticoids
Table 2.
N-Terminal Propeptide of Type I Procollagen (PINP) and C-Terminal Telopeptide of Type I Collagen (CTX) Levels Overall and by Case-control Status
| N | Mean | Median | Standard Deviation | Minimum | 25th Percentile | 75th Percentile | Maximum | |
|---|---|---|---|---|---|---|---|---|
| PINP (μg/L) | ||||||||
| Overall | 785 | 50.32 | 46.71 | 23.37 | 6.29 | 35.45 | 61.03 | 290.3 |
| Control | 393 | 49.64 | 45.65 | 23.71 | 8.82 | 35.66 | 59.20 | 290.3 |
| Cases | 392 | 51.0 | 47.12 | 23.03 | 6.29 | 34.66 | 62.82 | 144.6 |
| CTX (ng/L) | ||||||||
| Overall | 788 | 430 | 400 | 200 | 20 | 290 | 540 | 1470 |
| Control | 394 | 410 | 390 | 190 | 70 | 280 | 510 | 1400 |
| Cases | 394 | 450 | 420 | 210 | 20 | 300 | 570 | 1470 |
Adjusted Associations between Bone Biomarker Levels and Hip Fracture
In unadjusted logistic regression models, serum CTX level was not significantly associated with hip fracture risk (p for trend = 0.06) (Table 3a). After adjustment for BMI, education, whether living with a partner, parity, smoking, frequency of falls, previous fracture, family history of hip fracture, past use of menopausal hormone therapy, depressive symptoms, frailty index score, self-reported health status, RAND 36-item Health Survey score, corticosteroid use, and dietary and supplemental calcium and vitamin D intake, there were no statistically significant associations between CTX and hip fracture risk. For example, compared to the lowest quartile of serum CTX (<280 ng/L), the odds ratio for hip fracture was 1.25 (95% confidence interval 0.68–2.30) among women in the highest quartile of serum CTX (>510 ng/L). Results were similar for associations between PINP and hip fracture risk, showing no statistically significant associations (p for trend = 0.58) (Table 3b). Associations remained non-significant in the subset of participants who experienced hip fractures during the first five years of follow-up (data not shown). When log-transformed values of the turnover markers were entered into the models as continuous variables instead of as quartiles, the adjusted odds ratios (95% confidence interval) for hip fracture were 1.15 (0.74–1.80) for CTX and 1.00 (0.59–1.71) for PINP. Similarly, when we compared the highest quartile of turnover marker level vs. the lower three quartiles of turnover marker levels, we found no significant associations between serum CTX or PINP level and hip fracture risk (data not shown). In adjusted models, there were no significant associations between CTX or PINP level and hip fracture risk when CTX and PINP level were coded as above vs. below median values, or when we restricted the analyses to women aged ≥65 years. When we separately examined femoral neck and intertrochanteric fractures, there were still no significant associations between CTX or PINP levels and fracture risk (all p-values ≥0.11).
Table 3a.
Adjusted Associations between C-Terminal Telopeptide of Type I Collagen (CTX) Levels and Hip Fracture Risk*
| Model 1 (unadjusted, N = 788) | Model 2* (adjusted, N = 608) | |||
|---|---|---|---|---|
| OR (95% CI) | Ptrend-value | COR (95% CI) | Ptrend-value | |
| CTX Quartiles | 0.06 | 0.22 | ||
| Quartile 1 (0–25th percentile: 0–280 ng/L) | Reference | Reference | ||
| Quartile 2 (25th– 50th percentile: 280–390 ng/L) | 0.86 (0.56, 1.32) | 0.85 (0.43, 1.70) | ||
| Quartile 3 (50th– 75th percentile: 390–510 ng/L) | 1.19 (0.79, 1.78) | 1.53 (0.82, 2.85) | ||
| Quartile 4 (75th– Maximum: ≥510 ng/L) | 1.33 (0.91, 1.96) | 1.25 (0.68, 2.30) | ||
Estimates are based on conditional logistic regression models adjusted for body mass index, years of education, whether living with a partner, parity, smoking, fall history in past year, history of previous fracture, family history of hip fracture, past use of menopausal hormone therapy, Centers for the Epidemiologic Study of Depression score, use of antidepressant medication, frailty index, self-reported health status, RAND 36-item Health Survey score, corticosteroid use, and dietary and supplemental calcium and vitamin D intake. For conditional logistic regression, matching factors (age at screening, race/ethnicity, and season of blood draw) are not included as covariates. The bone turnover marker level was entered into the model as a categorical term in quartiles to obtain odds ratios. The ptrend values were obtained by entering the bone turnover marker quartile term as a continuous variable to determine whether a significant linear trend was present across the quartiles.
Table 3b.
Adjusted Associations between Procollagen Type I Aminoterminal Propeptide (PINP) and Hip Fracture Risk*
| Model 1 (unadjusted, N = 785) | Model 2 (adjusted, N = 605) | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| PINP Quartiles | 0.58 | 0.53 | ||
| Quartile 1 (0–25th percentile: 0–35.66 μg/L) | Reference | Reference | ||
| Quartile 2 (25th– 50th percentile: 35.66–45.65 μg/L) | 0.80 (0.53, 1.20) | 1.05 (0.54, 2.05) | ||
| Quartile 3 (50th–75th percentile: 45.65–59.2 μg/L) | 0.90 (0.60, 1.34) | 1.07 (0.58, 1.99) | ||
| Quartile 4 (75th – Maximum: ≥ 59.2 μg/L) | 1.09 (0.73, 1.63) | 1.24 (0.65, 2.35) | ||
Estimates are based on conditional logistic regression models adjusted for body mass index, years of education, whether living with a partner, parity, smoking, fall history in past year, history of previous fracture, family history of hip fracture, past use of menopausal hormone therapy, Centers for the Epidemiologic Study of Depression score, use of antidepressant medication, frailty index, self-reported health status, RAND 36-item Health Survey score, corticosteroid use, and dietary and supplemental calcium and vitamin D intake. For conditional logistic regression, matching factors (age at screening, race/ethnicity, and season of blood draw) are not included as covariates. The bone turnover marker level was entered into the model as a categorical term in quartiles. The ptrend values were obtained by entering the bone turnover marker quartile term as a continuous variable to determine whether a significant linear trend was present across the quartiles.
After exclusion of data from control group participants who experienced hip fractures subsequent to case-control enrollment period (after 2004 until 2010), higher serum CTX level was associated with higher risk of hip fracture (p for trend in adjusted model = 0.04)(Table 3c). However, there was no evidence of a consistent threshold. Associations between PINP level and hip fracture risk remained non-significant (Table 3d).
Table 3c.
Adjusted Associations between C-Terminal Telopeptide of Type I Collagen (CTX) Levels and Hip Fracture Risk after Exclusion of Controls who Experienced Hip Fractures during the Extension Study Period*
| Model 1 (age adjusted, N = 632) | Model 2 (fully adjusted, N = 487) | |||
|---|---|---|---|---|
| OR (95% CI) | p-trend* | OR (95% CI) | p-trend* | |
| CTX Quartiles | 0.02 | 0.04 | ||
| Quartile 1 (0–25th percentile: 0–280 ng/L) | Reference | Reference | ||
| Quartile 2 (25th– 50th percentile: 280–390 ng/L) | 1.10 (0.70, 1.75) | 1.28 (0.69, 2.38) | ||
| Quartile 3 (50th– 75th percentile: 390–510 ng/L) | 1.47 (0.92, 2.33) | 1.94 (1.05, 3.59) | ||
| Quartile 4 (75th– Maximum: ≥510 ng/L) | 1.61 (1.04, 2.50) | 1.71 (0.94, 3.11) | ||
Estimates are based on unconditional logistic regression models. The age adjusted model included only age and the bone turnover marker term. The fully adjusted model was adjusted for age, race/ethnicity, body mass index, years of education, season of blood draw, whether living with a partner, parity, smoking, fall history in past year, history of previous fracture, family history of hip fracture, past use of menopausal hormone therapy, Centers for the Epidemiologic Study of Depression score, use of antidepressant medication, frailty index, self-reported health status, RAND 36-item Health Survey score, and dietary and supplemental calcium and vitamin D intake. The bone turnover marker level was entered into the model as a categorical term in quartiles to obtain the odds ratios. The p-trend values were obtained by entering the bone-turnover markers quartile term as a continuous variable to determine if a significant linear trend exists across the quartiles.
Table 3d.
Adjusted Associations between Procollagen Type I Aminoterminal Propeptide (PINP) and Hip Fracture Risk after Exclusion of Controls who Experienced Hip Fractures during the Extension Study Period*
| Model 1* (age adjusted, N = 630) | Model 2* (adjusted, N = 485) | |||
|---|---|---|---|---|
| OR (95% CI) | p-trend* | OR (95% CI) | p-trend* | |
| PINP Quartiles | 0.34 | 0.30 | ||
| Quartile 1 (0–25th percentile: 0–35.66 μg/L) | Reference | Reference | ||
| Quartile 2 (25th– 50th percentile: 35.66–45.65 μg/L) | 0.74 (0.47, 1.17) | 0.72 (0.40, 1.32) | ||
| Quartile 3 (50th–75th percentile: 45.65–59.2 μg/L) | 0.95 (0.60, 1.50) | 0.97 (0.54, 1.77) | ||
| Quartile 4 (75th – Maximum: ≥ 59.2 μg/L) | 1.16 (0.74, 1.82) | 1.27 (0.70, 2.29) | ||
Estimates are based on unconditional logistic regression models. The age adjusted model included only age and the bone turnover marker term. The fully adjusted model was adjusted for age, race/ethnicity, body mass index, years of education, season of blood draw, whether living with a partner, parity, smoking, fall history in past year, history of previous fracture, family history of hip fracture, past use of menopausal hormone therapy, Centers for the Epidemiologic Study of Depression score, use of antidepressant medication, frailty index, self-reported health status, RAND 36-item Health Survey score, and dietary and supplemental calcium and vitamin D intake. The bone turnover marker level was entered into the model as a categorical term in quartiles to obtain the odds ratios. The p-trend values were obtained by entering the bone-turnover markers quartile term as a continuous variable to determine if a significant linear trend exists across the quartiles.
There was no significant effect modification of the associations according to FRAX-predicted 10-year absolute risk of hip fracture (CTX pinteraction 0.24, PINP pinteraction 0.71) (data not shown).
Discussion
In this case-control study of postmenopausal women, of contemporary markers of bone turnover and carefully documented hip fracture, neither CTX nor PINP was significantly associated with hip fracture risk.
Our results are consistent with the five previous studies that have examined associations of serum CTX level with hip fracture risk.(19,21–23,27) With one exception(27), the assay used in four of the five previous studies were similar to that of the current study (Elecsys, Roche Diagnostics). Three out of five of the studies reported that serum CTX levels was not associated with hip fracture risk. The fourth study (EPIDOS) reported a significant association between serum CTX level and hip fracture risk which persisted even after adjustment for baseline hip BMD (Table 4).(19) It is unclear why the EPIDOS study’s results differed from that of all of the other studies, including our current study, but it could be due in part to differences in the cohort and study design. The 95% confidence intervals for the hazard ratios of the adjusted models in the EPIDOS study were not provided, so it is possible that the associations were actually no longer statistically significant in covariate-adjusted models. The EPIDOS cohort had a mean age of 82, older than the mean age of 71 years in our cohort, and we collected blood samples when participants were in the fasted state, while the EPIDOS study did not. In addition, the small numbers of participants who underwent BMD measurement (n = 69) precluded adjustment for BMD in the current study. Finally, although the Singapore Chinese Health Study also showed that serum CTX was positively associated with increased hip fracture risk in a combined analysis of men and women after adjustment for multiple covariates, some associations were not statistically significant in gender-stratified analyses.(27) That study was the only study to use the Orion CTX assay, and only 22% of samples were collected with participants in a fasting state.
Table 4.
Summary of Studies Regarding Serum C-terminal Telopeptide of Type I Collagen in Relation to Hip Fracture Risk (Hazard Ratio [HR] or Odds Ratio [OR] (95% Confidence Interval [CI]))
| Study authors (reference) | Unadjusted HR or OR (95% CI) | Adjusted HR or OR (95% CI if available)-covariates |
|---|---|---|
| Chapurlat et al (19) | HR 1.9 (1.05–3.4) for serum CTX in highest quartile | HR 1.75-adjusted for body weight, HR 1.48-adjusted for gait speed HR 1.57-adjusted for femoral neck BMD |
| Dai et al (27) | OR 1.43 (1.06–1.94) Per SD increase in CTX | OR 1.78 (1.24–2.56) per SD increase in CTX-adjusted for age, sex, body mass index, education level, smoking status, physical activity level, diabetes mellitus-men and women were analyzed together because some associations were not statistically significant in gender-stratified analyses. |
| Dobnig et al (23) | Not described | HR 1.27 (0.45–3.60) per increment of 1 ng/ml-adjusted for age, body mass index, mobility score, past fractures, creatinine clearance rate, and calcaneal stiffness |
| Gerdhem et al (21) | OR 1.01 (0.48–2.11) for serum CTX in highest quartile | OR 1.53 (0.79–2.97)-adjusted for femoral neck BMD |
| Ivaska et al (22) | Not described | Not described |
In our study, we found no clear evidence of a consistent threshold of CTX level that was associated with increased hip fracture risk; the only significant trend we found was in a sensitivity analysis after excluding additional hip fractures that occurred in the control group after the initial study follow-up period. Although the result of our sensitivity analysis may be consistent with the previous EPIDOS study results, in aggregate, the lack of apparent threshold above which risk was statistically elevated, the weak magnitude of the association that was only apparent in one sensitivity analysis, and the likelihood that the association would be dampened after further adjustment for BMD, likely decrease the clinical relevance of the finding. The bone turnover marker levels that we observed in this study are similar to those of other postmenopausal population-based cohorts in North America (49), Germany(50), Australia(51), and Denmark(52), but lower levels reported in an Australian cohort of frail elderly women(53) and a cohort of similarly-aged French women.(54)
To our knowledge, only two published studies have examined associations of serum PINP level with hip fracture risk in women; one of them (18) reported no association, while the other found an association that persisted after adjustment for covariates.(27) However, the latter study jointly reported results from men and women, and some of the associations did not persist after gender-stratification. Both of the prior studies used an Orion assay, and specimens were either nonfasting (18) or had a majority (78%) of participants in the nonfasting state.(27)
One reason for the absence of an association between serum CTX or PINP and hip fracture risk may be that associations between bone turnover markers and fracture risk dampen with increased follow-up time. In a prior study, associations between elevated bone turnover markers and risk of experiencing any clinical fracture were strongest within the first few years of follow-up than after longer follow-up.(22) However, in our sensitivity analysis, associations remained non-significant in the subset of participants who experienced hip fractures during the first five years of follow-up. We also considered the possibility that falls could outweigh bone turnover markers in their influence on hip fracture risk, especially in the age range of our study cohort. Some researchers have suggested that the increasing frequency of falls, a strong fracture risk factor, with increasing age may overwhelm any potential contributions of increased bone turnover marker levels to hip fracture risk.(22) For example, in a previous study, the association between urinary free pyridinoline level (a bone resorption marker) and hip fracture risk disappeared after adjustment for disability.(26) However, in the current study, we found no significant associations even prior to adjustment for any covariates, including frequency of falls and frailty, and no significant interaction by FRAX score.
Our study examined serum PINP and serum CTX in accordance with the IOF/IFCC recommendations. Three previous case-control studies examined other bone turnover marker levels in relation to hip fracture risk are few, and with conflicting results. Two analyses from the EPIDOS cohort of elderly women found associations of urinary CTX, but no associations of urinary NTX, with increased hip fracture risk after adjustment for history of fracture.(28,29) It appears that the EPIDOS analyses were not adjusted for additional covariates other than history of fracture. The third paper from the Rotterdam study (women of mean age 81 years) found associations between urinary free deoxypyridinoline level and hip fracture risk before, but not after, adjustment for disability.(26)
These results are clinically relevant. The American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis stated based on Grade B evidence that clinicians should “consider using bone turnover markers in the initial evaluation of osteoporosis patients. Elevated levels can predict higher fracture risk”.(55) The results of the current study of community-dwelling postmenopausal U.S. women, along with results from the Malmo OPRA and Austrian nursing home cohorts, will help to inform future iterations of guidelines regarding the utility of bone turnover markers for hip fracture prediction in clinical practice among individuals not taking osteoporosis pharmacotherapy. At least over the mean 7.1-year follow-up, we found that serum CTX and serum NTX were not significantly associated with hip fracture risk. The current results do not apply to studies involving other time frames, bone turnover markers, or fracture types.
This study has several potential limitations. Because BMD measurements were measured only a subset of WHI participants, we could not adjust for BMD. However, we did perform analyses adjusted for underlying probability of hip fracture using FRAX scores (without BMD information). It is possible that there are meaningful associations between the biomarker levels examined and risk of fractures other than hip fracture, at bone areas that are more predominantly cancellous in composition than the hip. However, we could not examine this question in the present study. We also did not have sample stability data regarding the stored serum samples. It is also possible that that the availability of more than one control per case would have increased our statistical power, but funding precluded inclusion of additional controls. Our study was powered to detect a clinically important association (a HR of 1.5 between bone turnover markers and hip fractures). This study also has several strengths, including the prospective design, the long-term follow-up, the ability to adjust for multiple relevant covariates in a well-characterized cohort, the medical record verification of incident hip fractures, and the use of fasting serum samples. It is recommended that serum samples for CTX be collected in the morning hours in the fasting state because food intake is known to affect bone turnover marker levels.(3) The previous six studies focused on associations between serum CTX and PINP and hip fracture risk did not specify a fasting state for collection of serum samples. Finally, because a fracture is associated with an acute increase in bone turnover biomarker levels, these samples were not collected at the time of a fracture, but at the time of randomization in age- and race-matched cases and controls.
In summary, in this prospective nested case-control study of postmenopausal women of mean age 71, serum CTX and PINP levels were not associated with hip fracture risk. Our results do not support the utility of these markers to predict hip fracture risk in women in this age group.
Acknowledgements:
The authors thank the participants of the Women’s Health Initiative Study for their time and effort.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. Additional support for these analyses was provided by US Public Health Service Research grants: AR053105 and AR048919.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A.Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis
Footnotes
Disclosures
The following authors have no disclosures relevant to this manuscript: CJC, SV, ALC, MSL, JAC, JAR, RDJ, DCB.
Contributor Information
Carolyn J. Crandall, Division of General Internal Medicine and Health Services Research, Dept. of Medicine, David Geffen School of Medicine at the University of California, Los Angeles, Los Angeles, CA, 90049,.
Sowmya Vasan, Women’s Health Initiative, Fred Hutchinson Cancer Research Center, Seattle, WA,.
Andrea LaCroix, Family and Preventive Medicine, University of California, San Diego, La Jolla, CA,.
Meryl S. LeBoff, Endocrine, Diabetes and Hypertension Division, Dept. of Medicine, Brigham and Women’s Hospital, Boston, Massachusetts, 02115,.
Jane A. Cauley, Dept. of Epidemiology, University of Pittsburgh, Pittsburgh, PA, 15213,.
John A. Robbins, Dept. of Medicine, UC Davis Medical Center, Sacramento, CA 95817,.
Rebecca D. Jackson, Division of Endocrinology, Diabetes and Metabolism, Dept. of Medicine, The Ohio State University, Columbus, OH, 43210,.
Douglas C. Bauer, Depts. of Medicine and Epidemiology & Biostatistics, University of California, San Francisco, San Francisco, CA, 94107.
References
- 1.Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int February 2011;22(2):391–420. Epub 2010/12/25. [DOI] [PubMed] [Google Scholar]
- 2.Bauer D, Krege J, Lane N, Leary E, Libanati C, Miller P, et al. National Bone Health Alliance Bone Turnover Marker Project: current practices and the need for US harmonization, standardization, and common reference ranges. Osteoporos Int. October 2012;23(10):2425–33. Epub 2012/07/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET, National Bone Health Alliance Bone Turnover Marker P. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. June 19 2017. Epub 2017/06/21. [DOI] [PubMed] [Google Scholar]
- 4.Johansson H, Oden A, Kanis JA, McCloskey EV, Morris HA, Cooper C, et al. A meta-analysis of reference markers of bone turnover for prediction of fracture. Calcif Tissue Int. May 2014;94(5):560–7. Epub 2014/03/05. [DOI] [PubMed] [Google Scholar]
- 5.Chen JS, Seibel MJ, Zochling J, March L, Cameron ID, Cumming RG, et al. Calcaneal ultrasound but not bone turnover predicts fractures in vitamin D deficient frail elderly at high risk of falls. Calcif Tissue Int. July 2006;79(1):37–42. [DOI] [PubMed] [Google Scholar]
- 6.Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. August 2000;15(8):1526–36. [DOI] [PubMed] [Google Scholar]
- 7.Shigdel R, Osima M, Ahmed LA, Joakimsen RM, Eriksen EF, Zebaze R, et al. Bone turnover markers are associated with higher cortical porosity, thinner cortices, and larger size of the proximal femur and non-vertebral fractures. Bone. December 2015;81:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY study. J Bone Miner Res. October 2005;20(10):1813–9. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T. Biochemical markers of bone turnover as predictors of osteoporosis and osteoporotic fractures in men and women: 10-year follow-up of the Taiji cohort. Mod Rheumatol. December 2011;21(6):608–20. Epub 2011/04/23. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M, Kushida K, Hoshino H, Ohishi T, Inoue T. Evaluation of bone turnover in postmenopause, vertebral fracture, and hip fracture using biochemical markers for bone formation and resorption. Journal of endocrinological investigation. March 1997;20(3):112–7. Epub 1997/03/01. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino H, Takahashi M, Kushida K, Ohishi T, Inoue T. Urinary excretion of type I collagen degradation products in healthy women and osteoporotic patients with vertebral and hip fractures. Calcif Tissue Int. January 1998;62(1):36–9. Epub 1998/03/14. [DOI] [PubMed] [Google Scholar]
- 12.Wanby P, Nobin R, Von SP, Brudin L, Carlsson M. Serum levels of the bone turnover markers dickkopf-1, sclerostin, osteoprotegerin, osteopontin, osteocalcin and 25-hydroxyvitamin D in Swedish geriatric patients aged 75 years or older with a fresh hip fracture and in healthy controls. Journal of endocrinological investigation. August 2016;39(8):855–63. Epub 2016/02/07. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, Naitou K, Ohishi T, Nagano A. Comparison of biochemical markers of bone turnover and bone mineral density between hip fracture and vertebral fracture. J Clin Densitom. Fall 2003;6(3):211–8. Epub 2003/09/30. [DOI] [PubMed] [Google Scholar]
- 14.Cheung CK, Panesar NS, Lau E, Woo J, Swaminathan R. Increased bone resorption and decreased bone formation in Chinese patients with hip fracture. Calcif Tissue Int. May 1995;56(5):347–9. Epub 1995/05/01. [DOI] [PubMed] [Google Scholar]
- 15.Akesson K, Vergnaud P, Gineyts E, Delmas PD, Obrant KJ. Impairment of bone turnover in elderly women with hip fracture. Calcif Tissue Int. September 1993;53(3):162–9. Epub 1993/09/01. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Li N, Gong X, He L. Serum 25-hydroxyvitamin D, bone turnover markers and bone mineral density in postmenopausal women with hip fractures. Clin Chim Acta. February 2018;477:135–40. Epub 2017/12/17. [DOI] [PubMed] [Google Scholar]
- 17.Erem C, Tanakol R, Alagol F, Omer B, Cetin O. Relationship of bone turnover parameters, endogenous hormones and vit D deficiency to hip fracture in elderly postmenopausal women. Int J Clin Pract. June 2002;56(5):333–7. [PubMed] [Google Scholar]
- 18.Finnes TE, Lofthus CM, Meyer HE, Eriksen EF, Apalset EM, Tell GS, et al. Procollagen type 1 amino-terminal propeptide (P1NP) and risk of hip fractures in elderly Norwegian men and women. A NOREPOS study. Bone. July 2014;64:1–7. Epub 2014/03/29. [DOI] [PubMed] [Google Scholar]
- 19.Chapurlat RD, Garnero P, Breart G, Meunier PJ, Delmas PD. Serum type I collagen breakdown product (serum CTX) predicts hip fracture risk in elderly women: the EPIDOS study. Bone. August 2000;27(2):283–6. Epub 2000/07/29. [DOI] [PubMed] [Google Scholar]
- 20.Melton LJ 3rd, Crowson CS, O’Fallon WM, Wahner HW, Riggs BL. Relative contributions of bone density, bone turnover, and clinical risk factors to long-term fracture prediction. J Bone Miner Res. February 2003;18(2):312–8. [DOI] [PubMed] [Google Scholar]
- 21.Gerdhem P, Ivaska KK, Alatalo SL, Halleen JM, Hellman J, Isaksson A, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. March 2004;19(3):386–93. Epub 2004/03/26. [DOI] [PubMed] [Google Scholar]
- 22.Ivaska KK, Gerdhem P, Vaananen HK, Akesson K, Obrant KJ. Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. J Bone Miner Res. February 2010;25(2):393–403. [DOI] [PubMed] [Google Scholar]
- 23.Dobnig H, Piswanger-Solkner JC, Obermayer-Pietsch B, Tiran A, Strele A, Maier E, et al. Hip and nonvertebral fracture prediction in nursing home patients: role of bone ultrasound and bone marker measurements. J Clin Endocrinol Metab. May 2007;92(5):1678–86. Epub 2007/02/22. [DOI] [PubMed] [Google Scholar]
- 24.Robinson-Cohen C, Katz R, Hoofnagle AN, Cauley JA, Furberg CD, Robbins JA, et al. Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab. July 2011;96(7):2186–93. Epub 2011/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tromp AM, Ooms ME, Popp-Snijders C, Roos JC, Lips P. Predictors of fractures in elderly women. Osteoporos Int. 2000;11(2):134–40. Epub 2000/05/04. [DOI] [PubMed] [Google Scholar]
- 26.van Daele PL, Seibel MJ, Burger H, Hofman A, Grobbee DE, van Leeuwen JP, et al. Case-control analysis of bone resorption markers, disability, and hip fracture risk: the Rotterdam study. BMJ. February 24 1996;312(7029):482–3. Epub 1996/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai Z, Wang R, Ang LW, Yuan JM, Koh WP. Bone turnover biomarkers and risk of osteoporotic hip fracture in an Asian population. Bone. February 2016;83:171–7. Epub 2015/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garnero P, Dargent-Molina P, Hans D, Schott AM, Breart G, Meunier PJ, et al. Do markers of bone resorption add to bone mineral density and ultrasonographic heel measurement for the prediction of hip fracture in elderly women? The EPIDOS prospective study. Osteoporos Int. 1998;8(6):563–9. Epub 1999/05/18. [DOI] [PubMed] [Google Scholar]
- 29.Garnero P, Hausherr E, Chapuy MC, Marcelli C, Grandjean H, Muller C, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. October 1996;11(10):1531–8. [DOI] [PubMed] [Google Scholar]
- 30.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Controlled clinical trials. February 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 31.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. October 2003;13(9 Suppl):S107–21. [DOI] [PubMed] [Google Scholar]
- 32.Leboff MS, Narweker R, LaCroix A, Wu L, Jackson R, Lee J, et al. Homocysteine levels and risk of hip fracture in postmenopausal women. J Clin Endocrinol Metab. April 2009;94(4):1207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JS, LaCroix AZ, Wu L, Cauley JA, Jackson RD, Kooperberg C, et al. Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. J Clin Endocrinol Metab. May 2008;93(5):1796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaCroix AZ, Lee JS, Wu L, Cauley JA, Shlipak MG, Ott SM, et al. Cystatin-C, renal function, and incidence of hip fracture in postmenopausal women. J Am Geriatr Soc. August 2008;56(8):1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. August 19 2008;149(4):242–50. Epub 2008/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. August 2005;53(8):1321–30. [DOI] [PubMed] [Google Scholar]
- 37.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. March 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 38.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. March 2001;56(3):M158–66. [DOI] [PubMed] [Google Scholar]
- 39.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. Mar-Apr 1994;10(2):77–84. [PubMed] [Google Scholar]
- 40.Cauley JA, Wu L, Wampler NS, Barnhart JM, Allison M, Chen Z, et al. Clinical risk factors for fractures in multi-ethnic women: the Women’s Health Initiative. J Bone Miner Res. November 2007;22(11):1816–26. [DOI] [PubMed] [Google Scholar]
- 41.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. June 1992;30(6):473–83. [PubMed] [Google Scholar]
- 42.Willund I, Gorkin L, Pawitan Y, Schron E, Schoenberger J, Jared LL, et al. Methods for assessing quality of life in the cardiac arrhythmia suppression trial (CAST). Qual Life Res. June 1992;1(3):187–201. [DOI] [PubMed] [Google Scholar]
- 43.Stewart AL, Ware JE. Measuring functioning and well-being : the medical outcomes study approach. Durham: Duke University Press; 1992. xxiii, 449 p. p. [Google Scholar]
- 44.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. October 1993;2(3):217–27. [DOI] [PubMed] [Google Scholar]
- 45.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. April 2008;19(4):385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crandall CJ, Larson J, Gourlay ML, Donaldson MG, LaCroix A, Cauley JA, et al. Osteoporosis screening in postmenopausal women 50 to 64 years old: comparison of US Preventive Services Task Force strategy and two traditional strategies in the Women’s Health Initiative. J Bone Miner Res. July 2014;29(7):1661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crandall CJ, Larson JC, Watts NB, Gourlay ML, Donaldson MG, LaCroix A, et al. Comparison of fracture risk prediction by the US Preventive Services Task Force strategy and two alternative strategies in women 50–64 years old in the Women’s Health Initiative. J Clin Endocrinol Metab. December 2014;99(12):4514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kooperberg C, Cushman M, Hsia J, Robinson JG, Aragaki AK, Lynch JK, et al. Can biomarkers identify women at increased stroke risk? The Women’s Health Initiative Hormone Trials. PLoS Clin Trials. June 15 2007;2(6):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langsetmo L, Barr SI, Dasgupta K, Berger C, Kovacs CS, Josse RG, et al. Dietary patterns in men and women are simultaneously determinants of altered glucose metabolism and bone metabolism. Nutr Res. April 2016;36(4):328–36. Epub 2016/03/24. [DOI] [PubMed] [Google Scholar]
- 50.Michelsen J, Wallaschofski H, Friedrich N, Spielhagen C, Rettig R, Ittermann T, et al. Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone. December 2013;57(2):399–404. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins N, Black M, Paul E, Pasco JA, Kotowicz MA, Schneider HG. Age-related reference intervals for bone turnover markers from an Australian reference population. Bone. August 2013;55(2):271–6. Epub 2013/04/23. [DOI] [PubMed] [Google Scholar]
- 52.Jorgensen NR, Mollehave LT, Hansen YBL, Quardon N, Lylloff L, Linneberg A. Comparison of two automated assays of BTM (CTX and P1NP) and reference intervals in a Danish population. Osteoporos Int. July 2017;28(7):2103–13. Epub 2017/04/30. [DOI] [PubMed] [Google Scholar]
- 53.Chen JS, Cameron ID, Cumming RG, Lord SR, March LM, Sambrook PN, et al. Effect of age-related chronic immobility on markers of bone turnover. J Bone Miner Res. February 2006;21(2):324–31. Epub 2006/01/19. [DOI] [PubMed] [Google Scholar]
- 54.Chavassieux P, Portero-Muzy N, Roux JP, Garnero P, Chapurlat R. Are Biochemical Markers of Bone Turnover Representative of Bone Histomorphometry in 370 Postmenopausal Women? J Clin Endocrinol Metab. December 2015;100(12):4662–8. Epub 2015/10/28. [DOI] [PubMed] [Google Scholar]
- 55.Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis - 2016. Endocr Pract. September 02 2016;22(Suppl 4):1–42. [DOI] [PubMed] [Google Scholar]


