Abstract
Background
Alcohol‐related liver disease (ALD) represents a major cause of death worldwide, and unfortunately, most patients are diagnosed at an advanced stage of the disease, which is related to poorer outcomes. Liver biopsy has historically been the gold standard for identifying advanced hepatic fibrosis, but this approach has several limitations, including invasiveness, low applicability, sampling variability, and cost.
Main Text
In order to detect earlier features of advanced liver fibrosis, surrogate biomarkers and techniques have been developed. While these were initially developed for chronic liver diseases such as viral hepatitis and nonalcoholic fatty liver disease (NAFLD), their performance in ALD has also been recently studied. Among the noninvasive surrogate markers and techniques used to detect liver fibrosis, the Enhanced Liver Fibrosis test, FibroTest, and Transient Elastography are the most accurate and validated techniques. In this review, we summarize the current status of the noninvasive assessment of liver disease in ALD and provide a synthesis of how these noninvasive tools can be used in clinical practice. Finally, we briefly outline novel biomarkers that are currently being investigated and discuss future directions and new opportunities in the noninvasive diagnosis of ALD.
Keywords: alcohol‐related liver disease, biomarkers, fibrosis, noninvasive diagnosis, transient elastography
1. INTRODUCTION
Liver disease is an important cause of global mortality and morbidity.1, 2 For example, in the United Kingdom, mortality attributable to liver disease rose fourfold between 1980 and 2013,3 and evidence suggests that liver disease will likely overtake ischemic heart disease as the leading cause of years of working life lost.4 Despite the increasing prevalence of nonalcoholic fatty liver disease (NAFLD), 41% of liver deaths are still attributable to alcohol.5 Indeed, alcohol‐related liver disease (ALD) is a major cause of death worldwide.6 Although Europe has the highest levels of reported per capita alcohol consumption,7 there is heterogeneity between countries in terms of liver disease‐related death.8, 9 This is mainly due to discrepancies between effective national public health policies and population level alcohol consumption.8 Unfortunately, despite the fact that liver disease patients die at a younger age, little progress has been made in implementing comprehensive alcohol control strategies.
The spectrum of ALD comprises a variety of clinical, radiological, and histological conditions, from simple steatosis, steatohepatitis, and progressive fibrosis, to cirrhosis and its complications.5, 10 While steatosis is present in almost all heavy drinkers, only 8% to 20% of these patients will ultimately develop cirrhosis.10 However, a recent study reported that 73% of patients admitted to the hospital for the first time with cirrhosis or liver failure were unaware of their condition, suggesting that most patients are diagnosed at a decompensated stage or advanced disease.11 Furthermore, it seems that ALD is rarely detected at early stages compared with liver diseases of other etiologies.12
Since fibrosis is the major predictive factor of long‐term survival in compensated patients,12 its detection is crucial before decompensation (which is associated with a poorer prognosis13, 14) in order to promote the reduction and, ideally, complete withdrawal, of alcohol consumption.
Considering that in its earlier stages, ALD is a silent disease, screening tools to identify individuals with alcohol use disorders (AUDs) and tests to detect liver fibrosis must be implemented, particularly among general practitioners and psychiatric units. Although liver biopsy is still the gold standard for estimating liver fibrosis,15 it cannot be proposed as a screening tool due to the risk of complications and cost.15 Therefore, while initially developed in chronic hepatitis C and NAFLD,16, 17 noninvasive tests have become increasingly used in clinical practice in order to evaluate the severity of liver fibrosis in other etiologies of liver disease. They have proven to have not only an excellent predictive value for diagnosis of advanced fibrosis but also an adequate prognostic value.18
This review article focuses on screening and noninvasive diagnostic tools for the detection of liver fibrosis in patients with ALD and their importance in clinical practice. We will also briefly summarize novel biomarkers currently being investigated as well as future directions and new opportunities in the noninvasive diagnosis of ALD.
2. THE CLINICAL PROBLEM: WHO AND HOW TO EVALUATE AND FOLLOW FOR ALD?
2.1. Who should we screen?
Data are conflicting regarding the definition of a safe alcohol limit, with no clear threshold effect.19 Interestingly, the old statement that moderate alcohol consumption is protective for ischemic heart disease and diabetes in women was recently counterbalanced by a worldwide comprehensive study that assessed estimations of alcohol use, alcohol attributable deaths, and disability‐adjusted life‐years.19 It was reported that the level of consumption that minimizes an individual's risk is 0 g of ethanol per week. This threshold is likely related to the risk of cancer associated with alcohol consumption, which is based on a linear dose relationship,20, 21 whereas for liver diseases, the relationship is exponential.20, 21 A meta‐analysis22 found that the threshold associated with increased risk of mortality from liver cirrhosis among men and women is 12 to 24 g of ethanol per day. Beyond the specific amount of alcohol, drinking patterns are also an issue, with daily and binge drinking also being associated with a higher risk of liver cirrhosis.23, 24, 25 Furthermore, competing risk factors must be taken into account when considering the thresholds of >30 g/d for men and >20 g/d for women used in daily clinical practice.5 Among these, obesity, in addition to being an independent factor associated with ALD progression,26 when associated with a body mass index (BMI) > 30, is not only additive but also synergistic. One study that assessed obese patients with excess drinking (more than 15 drinks per week) compared with lean patients with the same drinking pattern revealed that the adjusted relative rates for liver disease mortality were 18.9 (95% CI, 6.84‐52.4) and 3.16 (95% CI, 1.28‐7.8), respectively.27 Similarly, components of the metabolic syndrome, such as type 2 diabetes and/or insulin resistance, are also independent predictors of liver‐related mortality in ALD.28 Furthermore, a recent study has evaluated the association between early age alcohol consumption and the occurrence of severe liver disease.29 Surprisingly, there was no threshold effect, the risk was dose dependent, and alcohol consumption in early age was associated with an increased risk of severe liver disease. Lastly, lower socioeconomic status has also been associated with a higher risk of mortality from ALD,30, 31 although the underlying explanatory factors for this finding are not yet fully understood. Collectively, as highlighted, the commonly used threshold effect is inaccurate by itself, and we should likely lower the drinking limit in patients who present with comorbid factors.
Finally, screening for harmful alcohol consumption should be done in primary care and other health and community settings in order to deliver effective intervention,5 even though the long‐term effects of screening on abstinence and relapse still need to be determined with real‐life data.
2.2. How do we screen?
Noninvasive methods to detect liver fibrosis rely on two different approaches: the biological approach based on the quantification of biomarkers in serum samples and a physical approach based on the measurement of liver stiffness (LS) using imaging techniques. These two approaches will be described below.
As highlighted previously, considering the fact that liver fibrosis is the major predictor of long‐term survival, we will focus on this aspect in this review, and diagnosis and evaluation of liver steatosis in ALD will not be discussed in this article.
2.2.1. Biological tests
Several nonpatented and patented serum biomarkers (Table 1) are widely used in daily clinical practice, and numerous studies have assessed their performance in liver fibrosis and cirrhosis. Among them, Fibrotest (FT) and Enhanced Liver Fibrosis (ELF), two patented serum biomarkers, demonstrate the highest performance for fibrosis quantification and have comparable diagnostic accuracy.32 The FT score is based on an algorithm calculated from six serum markers, whereas the ELF test integrates three direct serum markers of extracellular matrix remodeling and fibrogenesis, namely, hyaluronic acid, tissue inhibitor of metalloproteinase‐1, and N‐terminal propeptide for collagen type III.35 The latter is considered to be a direct marker of fibrosis, since it provides a direct measurement of the degree of extracellular material deposition.35 The reproducibility and performance of the ELF score was initially evaluated in a large cohort of patients with chronic liver disease with mixed etiologies,35 and a recent Danish study32 has confirmed the high accuracy of the ELF test, showing that it is similar to FT in the assessment of liver fibrosis in ALD (area under the ROC curve [AUROC] of 0.92 and 0.90, respectively). Among other patented biomarkers, Fibrometer and Hepascore show comparable accuracy that does not differ from that of FT in patients with ALD.33 Although the above‐mentioned patented biomarkers and FT showed similar accuracy in the prediction of advanced fibrosis and cirrhosis, in a multivariate analysis, FT alone was the most informative biomarker in terms of diagnostic and prognostic performance. Despite their excellent accuracy, these patented tests lack widespread applicability due to their high costs.
Table 1.
Performance of biological tests for the diagnosis of advanced fibrosis and cirrhosis in patients with biopsy‐proven ALD
| Tests | Patients, n | Endpoint | AUC | Se, % | Sp, % | NPV, % | PPV, % |
|---|---|---|---|---|---|---|---|
| ELF ≥10.532 | 289 | F3‐F4 | 0.92‐0.94 | 79 | 91 | 94 | 71 |
| FT ≥0.5832 | 289 | F3‐F4 | 0.88‐0.88 | 67 | 87 | 90 | 60 |
| Fibrometer33, 34 | 218 | F3‐F4 | 0.83‐0.94 | 91.8‐91.8 | 92.3‐92.3 | NA | NA |
| Hepascore33, 34 | 218 | F3‐F4 | 0.83‐0.92 | NA | NA | NA | NA |
| APRI ≥1.032 | 289 | F3‐F4 | 0.80‐0.85 | 38 | 90 | 83 | 52 |
| Fib‐4 ≥ 3.2532 | 289 | F3‐F4 | 0.85‐0.89 | 58 | 91 | 88 | 64 |
Abbreviations: ALD, alcohol‐related liver disease; APRI, aspartate transaminase‐platelet ratio index; AUC, area under the curve; ELF, Enhanced Liver Fibrosis; FT, Fibrotest; Se, sensitivity; Sp, specificity; NPV, negative predictive value; PPV, positive predictive value; NA, not available.
Nonpatented serum biomarkers have also been assessed in ALD. Aspartate transaminase‐platelet ratio index (APRI) includes AST and platelet count as variables and has been assessed in 507 patients with ALD.36 APRI values >1.5 had a sensitivity and specificity of 13.2% and 77.6%, respectively, for the diagnosis of significant fibrosis, whereas a cutoff >2 had a sensitivity and specificity for the diagnosis of cirrhosis of 16.9% and 86.4%, respectively,36 suggesting a lack of clinical utility. This low diagnostic performance was also established in a Danish prospective study that evaluated the accuracy of direct and indirect biomarkers.32 Similarly, the fibrosis‐4 (Fib‐4) score also demonstrated low diagnostic performance, with AUROCs for advanced fibrosis, significant fibrosis, and cirrhosis of 0.85, 0.77, and 0.89, respectively.32 Altogether, despite their higher cost compared with nonpatented and other patented serum biomarkers, the FT and ELF tests provide the best diagnostic and prognostic performance to date in the identification of advanced liver fibrosis. Additionally, these biomarkers (in particular, the ELF test) are highly cost‐effective and should be tested in primary health care settings.37, 38 Lastly, advanced fibrosis can be ruled out in primary health care patients with an ELF value <10.5 or an FT < 0.58.32 Therefore, these tests might be helpful in reducing the need for liver biopsy.
2.2.2. Transient elastography
One‐dimensional ultrasound transient elastography (TE), or Fibroscan (Echosens, Paris, France), is a physical approach aimed at measuring the velocity of a low‐frequency (50 Hz) elastic shear wave spreading through the liver.39 This velocity is directly related to LS, such that the stiffer the tissue, the faster the shear wave spreads. Shear wave velocity is then converted into a liver stiffness measurement (LSM). This technique has numerous advantages, such as a short procedure time (<5 min), immediate results, ability to perform the procedure at the bedside or in an outpatient clinic, well‐defined quality criteria, and good reproducibility.40 Furthermore, it has been demonstrated that the learning curve is reasonable41 and that the minimal training required to be able to perform the test is about 100 exams. Although the methodology has excellent interobserver and intraobserver agreement (intraclass correlation coefficient of 0.98),40, 42 its applicability is lower compared with serum biomarkers. In a French study evaluating the reliability (defined as fewer than 10 valid shots) and failure rate (defined as zero valid shots) of more than 13 369 examinations,43 LSM failure and unreliable results occurred in 3.1% and 15.8% cases, respectively, whereas the mean applicability rate of FT was 99.03%.44 However, despite the failure rate of TE, it still outperformed liver biopsy, which has been associated with a sampling error of nearly 30%.45, 46, 47, 48 Nevertheless, even if TE is an excellent surrogate marker of advanced fibrosis and cirrhosis, it has some limitations, and confounding variables must be addressed to ensure the correct interpretation of results obtained from TE. The main confounders to be taken into consideration are nonfasting,49, 50, 51 inflammation,52, 53 inexperience,41, 43 congestion,54, 55 alcohol,56 obesity,43, 57 cholestasis,58 amyloidosis,59 and alcoholic hepatitis (AH)60 (Table 2). However, for obese patients, an XL probe has been developed,66 which can result in reduced TE failure and improved reliability of LSM, but it must be kept in mind that LS cutoffs are lower with the XL probe. Additionally, these LS cutoffs must also be adjusted to the AST level. This feature was initially observed in viral hepatitis, where LSM correlated positively with transaminase levels,67, 68 and later on, in ALD.56, 69 A German study56 performed sequential LSM before and after normalization of transaminases in patients with ALD admitted for alcohol withdrawal. They demonstrated that an AST level >100 U/L was associated with a lack of reliable diagnosis of fibrosis, whereas levels of AST lower than 100 and 50 U/L were related to high accuracy detection of F3 (only AST level < 50 U/L) and F4 fibrosis, respectively. These results have also been confirmed by other studies,70, 71 in which alcohol withdrawal was associated with a significant decrease in LSM. The influence of AST in LSM might be explained by inflammation, which has been identified as a confounding factor,50, 51 and by the direct relationship (except in the setting of cirrhosis) between AST levels and the amount of alcohol consumed.54 In order to better determine the inflammation‐adapted cutoff values, Mueller et al72 have assessed LS and liver tests in 2086 biopsy‐proven patients with ALD and chronic HCV. They showed that AST has the best correlation with LS, whereas fibrosis cutoff values in patients without elevated transaminases levels were almost comparable between ALD and HCV patients. Lastly, the fibrosis cutoff values increased exponentially as a function of median AST level in ALD patients.
Table 2.
Characteristics of the available elastography techniques for liver fibrosis stratification
| Techniques | Evidence in ALD | Availability | Confounders | Failure Rate (%) | Cost | ||
|---|---|---|---|---|---|---|---|
| Obesity | inflammation | Others | |||||
| TE41, 43, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 | +++ | +++ | ++ | ++ | congestion, alcohol, amyloidosis | 3.1‐15.8 (39) | € |
| ARFI/pSWE61, 62, 63, 64 | + | ++ | + | ++ | ? | 2.1 (66) | €€ |
| 2D‐SWE32, 64 | + | + | ? | ? | ? | 4 | €€ |
| MRE65 | + | + | ++ | ++ | ? | 4.3 | €€€ |
Abbreviations: ?, limited data; ALD, alcoholic liver disease; ARFI, acoustic radiation force imaging; MRE, magnetic resonance elastography; TE, transient elastography; SWE, shear wave elastography.
Interestingly, AST levels have an influence not only on LS but also on bilirubin concentration. A recent meta‐analysis60 combining individual data from 1026 patients with ALD has determined LS cutoffs as a function of both AST and bilirubin concentration. Indeed, AST and bilirubin levels higher than 38.7 U/L and 9.0 μmol/L, respectively, were associated with significantly higher LS cutoff values (for F ≥ 1).
Although TE presents some limitations, this technique is characterized by an outstanding performance in the estimation of liver fibrosis. Several biopsy‐proven ALD studies have established LS cutoffs for cirrhosis (Table 3), but substantial interstudy variability exists, which can be explained by the confounders highlighted previously. However, a recent meta‐analysis60 with more than 1000 ALD patients has determined diagnostic cutoffs values for F ≥ 3 and F = 4 of 12.1 and 18.6 kPa, with AUROC values of 0.90 and 0.91, respectively. Lastly, in the Danish study,32 TE showed similar excellent diagnostic accuracy compared with the ELF and FT tests in intention to diagnose but did differ in the per‐protocol analysis, in favor of TE (AUROC for TE was 0.97 versus 0.92 for the ELF test and 0.90 for FT).
Table 3.
Performances of Transient Elastography for the diagnosis of advanced fibrosis and cirrhosis in patients with biopsy‐proven ALD
| Authors | Year | Design | Patients, n | Age, y | Endpoint | Prevalence, % | Cutoff, kPa | AUC | Se, % | Sp, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Nahon et al73 | 2008 | P | 147 | 54.4 ± 8.9 | F3‐F4 | 71‐48 | 12.9‐22.6 | 0.94‐0.87 | 81‐84 | 89‐80 |
| Nguyen‐Khac et al74 | 2008 | P | 103 | 52.6 ± 9.6 | F3‐F4 | 53‐32 | 11‐19.5 | 0.90‐0.92 | 86.7‐85.7 | 80.5‐84.2 |
| Kim et al75 | 2009 | R | 45 | 49 ± 8 | F3‐F4 | 80‐64 | 18.5‐25.8 | 0.98‐0.97 | 89‐90 | 89‐87 |
| Boursier et al76 | 2009 | P | 91 | 56 ± 10 | F3‐F4 | 69‐37 | 11.4‐17.3 | 0.85‐0.91 | 75‐82 | 75‐79 |
| Mueller et al56 | 2010 | P | 101 | 53.2 ± 10.6 | F3‐F4 | 66‐60 | 8.0‐11.5 | 0.91‐0.92 | 91‐100 | 75‐77 |
| Janssens et al77 | 2010 | R | 48 | 55 ± 9 | F3‐F4 | 65‐40 | 17.2‐21.7 | 0.75‐0.89 | 71‐79 | 71‐79 |
| Fernandez et al78 | 2015 | R | 112 | 55 ± 10 | F3‐F4 | 46‐29 | 15.2‐24.3 | 0.84‐0.90 | 79‐81 | 78‐82 |
| Thiele et al79 | 2016 | P | 189 | 49 ± 10 | F3‐F4 | 40‐15 | 8.8‐16.9 | 0.89‐0.94 | 80‐88 | 83‐88 |
| Voican et al80 | 2017 | P | 188 | 55 ± 11 | F3‐F4 | 22‐14 | 13‐20.8 | 0.96‐0.90 | 90‐89 | 90‐90 |
| Nguyen‐Khac et al60 | 2018 | M | 1026 | 54 ± 11 | F3‐F4 | 65‐42 | 12.1‐18.6 | 0.90‐0.91 | 81‐84 | 83‐85 |
Abbreviations: P, prospective; R, retrospective; M, meta‐analysis; AUC, area under the curve; Se, sensitivity; Sp, specificity.
2.2.3. Other imaging techniques
Given the success and the remarkable efficiency of TE in predicting liver fibrosis, other imaging techniques for the assessment of tissue stiffness have been developed and have recently emerged in clinical practice. Acoustic radiation force imaging (ARFI) and 2D‐shear wave elastography (SWE) are increasingly being evaluated in various etiologies of chronic liver disease and have become more commonly used in daily clinical practice. Only a few small trials61, 62 have evaluated ARFI in patients with ALD, and these have shown a diagnostic accuracy of 86% to 88% for advanced fibrosis (F ≥ 3) and 89% for cirrhosis. Nevertheless, ARFI has the advantage of fast implementation on commercial ultrasound machines and lower rates of failure compared with TE, as well as better performance in patients with ascites and obesity81 (Table 2). Similarly to TE, ARFI measurements are influenced by food intake and AST levels.63, 64 A recent meta‐analysis82 based on individual data from 13 centers (mainly related to viral hepatitis and NAFLD) has evaluated the diagnostic accuracy of 2D‐SWE. The study reported AUROC values of 91% and 95% for advanced fibrosis and cirrhosis, with optimal cutoffs of 9.2 and 13.5 kPa, respectively. A smaller clinical trial in ALD patients32 has also assessed the performance of 2D‐SWE compared with patented biomarkers and TE. The authors reported excellent diagnostic accuracy, with AUROC values (in intention to diagnose) of 0.93 for advanced fibrosis and cirrhosis, which is higher than those for TE (AUROC of 0.89 for advanced fibrosis and 0.87 for cirrhosis). Altogether, larger studies are needed in patients with ALD to, first, better characterize the performances of these techniques and, second, perform head‐to‐head comparisons between all the imaging modalities available. Furthermore, quality criteria as well as standardization of units between the different platforms need to be better defined.
Lastly, magnetic resonance elastography (MRE) quantifies elasticity (expressed in kPa) using a formula that characterizes the shear modulus, which is equivalent to one‐third of the Young modulus used with TE.83, 84 It has also been evaluated in a meta‐analysis (mostly viral hepatitis and NAFLD) based on 12 retrospective studies, comprising 697 patients.65 The diagnostic accuracy of any fibrosis, significant fibrosis, advanced fibrosis, and cirrhosis was 0.84, 0.88, 0.93, and 0.92, respectively, with an overall failure rate of 4.3% (Table 2). In a head‐to‐head comparison between 3D‐MRE vs 2D‐MRE, 3D‐MRE was superior to 2D‐MRE, with an AUROC for the detection of advanced fibrosis of 0.98 (3D‐MRE) vs 0.92 (2D‐MRE).85 Unfortunately, its implementation in daily clinical practice is rather difficult due to the higher cost, the time consumed by the procedure, and the low availability of MR machines, ultimately resulting in lower applicability.
2.3. Use in clinical practice
In patients with suspected ALD (presence of AUD, abnormal liver tests with AST/ALT >1, high levels of γ‐glutamyltransferase [although neither specific, nor sensitive, particularly in the cirrhotic stage86], and no other causes of chronic liver disease [HCV, HBV, or NAFLD]), noninvasive tests can be used in clinical practice for the detection of advanced fibrosis. Although physical and biological approaches are complementary, the latter is more suited as a screening tool given the local availability in primary health care. Figure 1 depicts our proposed algorithm for the use of noninvasive methods for risk stratification of patients with ALD in clinical practice. AUD should be screened in primary health care, alcohol rehabilitation centers, and in psychiatric units, since the prevalence of AUD is higher in patients with psychiatric disorders.87 In order to increase the identification of AUD and to better characterize patients' drinking habits, screening tools have been developed, including one of the most validated and widely used, the Alcohol Use Disorders Inventory Test (AUDIT).88 For low prevalence populations, such as patients in the primary health care sector, serum biomarkers with high negative predictive value (>94%), such as the ELF score and FT, should be used as a first‐line method to rule out advanced fibrosis. Although patented biomarkers are considered to have lower applicability compared with nonpatented ones given their higher cost, two recent studies37, 38 have found that the ELF score is cost‐effective in primary health care38 for fibrosis assessment in patients with ALD. Patients with low risk of advanced fibrosis (ELF < 10.5, FT < 0.58) should be offered interventions aiming to reduce, and eventually withdraw, alcohol consumption. For those who reach alcohol abstinence, no further assessments are needed. For those who either resume alcohol consumption or cannot reduce their drinking habits, a follow‐up should be offered in order to detect early advanced fibrosis. Those at high risk of having advanced fibrosis (ELF score ≥ 10.5, FT ≥ 0.58) should be referred to a liver clinic. For high prevalence populations, such as patients in secondary care (alcohol rehabilitation centers and psychiatric units), direct referral to TE is highly effective.38 TE is the most widely available and best evaluated technique in ALD for the measurement of LS, although ARFI and 2D‐SWE are also becoming increasingly available.89 The XL probe should be used for obese patients in order to minimize the expected higher TE failures. In case of TE failure, 2D‐SWE might be an alternative, depending on local availability. Patients at low risk of having advanced fibrosis (LSM < 6 kPa) should be offered the same interventions previously described to reduce and/or withdraw alcohol. In patients with LSM ≥6 kPa, caution should be taken in the interpretation of LS, considering the potential confounders highlighted previously, which could increase LS. An ultrasound should be proposed in order to exclude congestion and mechanical cholestasis. If the laboratory shows elevated AST and bilirubin levels, and if the patient is not abstinent, one may either consider establishing interventions leading to alcohol detoxification and repeat TE afterwards or use AST‐adapted cutoff values if alcohol withdrawal is not feasible. In addition, asymptomatic or symptomatic AH is also associated with increased LS values. Therefore, laboratory features of AH should also be explored in order to exclude this clinical syndrome. So far, in a large ALD meta‐analysis,60 LS cutoffs according to histological fibrosis stage were determined to be 7.0 kPa for F ≥ 1 fibrosis; 9.0 kPa for F ≥ 2 fibrosis; 12.1 kPa for F ≥ 3 fibrosis; and 18.6 kPa for F = 4. Furthermore, if LSM > 20 kPa, an ultrasound should be offered in order to exclude hepatocellular carcinoma (HCC) as well as an upper endoscopy aiming to assess the presence of gastroesophageal varices. Lastly, those with a high risk of having advanced fibrosis (after exclusion of confounders) should be considered for liver biopsy if they present with certain features. These include no indirect signs of liver cirrhosis at imaging (findings of portal hypertension [PHT] or liver dysmorphism) with LSM suggesting F3‐F4 stage, or the presence of diagnostic doubt regarding other causes of chronic liver disease such as viral hepatitis or auto‐immune disease.
Figure 1.
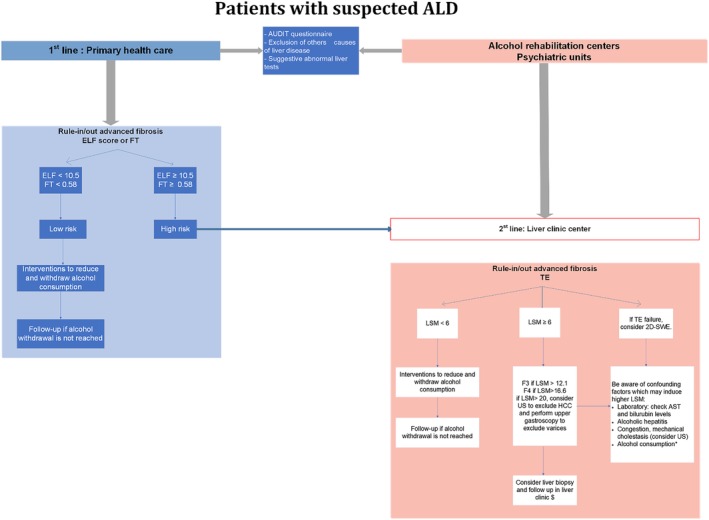
An algorithm for the use of noninvasive tests in clinical practice for predicting advanced fibrosis in patients with alcohol‐related liver disease (ALD). AST, aspartate aminotransferase; ELF, enhance liver fibrosis; FT, Fibrotest; HCC, hepatocellular carcinoma; LSM, liver stiffness measurement; TE, transient elastography; US, ultrasound. *Consider intervention (alcohol withdrawal) or use AST adapted cutoffs if alcohol withdrawal is not feasible. $Consider liver biopsy if the presence of cirrhosis is not clear. Adapted from Castera et al,111 with permission from Elsevier
2.4. Why are noninvasive tests of clinical importance?
In addition to the excellent accuracy of noninvasive tests for the estimation of liver fibrosis, recent studies have shown that TE and serum biomarkers also have the ability to predict clinical decompensation as well as survival in patients with chronic liver disease.90, 91, 92, 93, 94, 95, 96, 97 A meta‐analysis98 based on 17 studies in 7058 patients with chronic liver disease (mainly viral hepatitis) has shown that TE values were significantly associated with risk of hepatic decompensation (six studies; relative risk [RR], 1.07; 95% CI, 1.03‐1.11), HCC (nine studies; RR, 1.11; 95% CI, 1.05‐1.18), death (five studies; RR, 1.22; 95% CI, 1.05‐1.43), or a composite of these outcomes (seven studies; RR, 1.32; 95% CI, 1.16‐1.51). More specifically, it has also been shown in chronic liver disease (mainly viral hepatitis and ALD)99, 100 that there is a positive correlation between LS and hepatic venous pressure gradient (HVPG). Their performance was similar in predicting the occurrence of PHT complications, suggesting that LS is as effective as HPVG in predicting clinical decompensation and PHT‐related complications. These results were confirmed by a meta‐analysis101 based on a total of 18 studies, which included 3644 patients (mainly viral hepatitis). The study found that the diagnostic performance of TE for predicting clinically significant PHT (ie, HVPG ≥10 mmHg) is quite excellent, with a hierarchical summary receiver operating characteristic (HSROC) of 0.93 but has a lower accuracy for the prediction of large esophageal varices (HSROC of 0.78). The 90% specific cutoff in this setting was 21 kPa. However, although widely assessed in chronic liver disease, such as in viral hepatitis and NAFLD, the prognostic value of noninvasive tests in ALD has been less often evaluated. A French study33 has compared the prognostic value of FT with other patented biomarkers, Fibrometer and Hepascore, in a cohort of 218 ALD patients. They found that FT, along with biopsy fibrosis staging, was the most significant independent prognostic factors of overall survival. Fibrometer and Hepascore did not improve either the diagnostic or the prognostic value of FT. More recently, preliminary results on the long‐term prognostic value of TE in ALD patients were presented during the most recent International Liver Congress.102 The authors reported on a prospective study of 675 patients with a mean follow‐up of 3.3 years, which aimed to assess prediction of long‐term survival by LS in heavy drinkers. They showed that (a) LS is the best parameter for predicting survival, (b) LS cutoff >12.5 was associated with 3‐ and 5‐year survival rates of 74% and 64%, respectively, (c) LS remains an independent predictor of survival and liver‐related death (with bilirubin), and, interestingly, (d) LS seems to outperform other prognostic AH scores such as CHILD, MELD, and Maddrey in terms of prediction of overall survival.
3. FUTURE DIRECTIONS
Data in the literature regarding the efficacy and limitations of noninvasive diagnostic modalities of liver fibrosis in ALD are recent and scarce compared with that on other etiologies of chronic liver disease. Furthermore, there are other unmet needs to fulfill: (a) identify novel diagnostic and prognostic biomarkers, ii) determine the ability of available noninvasive modalities to monitor eventual new anti‐fibrotic drugs, and iii) characterize the diagnostic abilities of noninvasive markers in the primary care population.
Recently, “omics” approaches (lipidomics, proteomics, metabolomics, and transcriptomics) have shown promising results with regard to the identification of novel markers in NAFLD,103 and some of these approaches are also currently being assessed in ALD.104 In a mouse model of ALD, proteomic analysis of circulating extracellular vesicles (EVs) has shown a distinct signature of proteins as compared with control‐EVs.105 They have also identified Heat shock protein 90 in ALD‐EVs as a mediator of macrophage activation. On the other hand, among transcriptomic approaches based on circulating small noncoding RNA (miRNA) and long‐noncoding RNAs (lncRNAs), several exosome‐associated miRNAs have been studied as potential biomarkers in preclinical studies.106, 107, 108, 109 Briefly, in mouse models of ALD, serum levels of miR‐155 and miR‐122110 were increased and, interestingly, enriched in circulating exosomes as well as miR‐192 and miR‐30a.108 More importantly, the latter finding has also been confirmed in patients with AH compared with healthy controls.108 Lastly, lncRNAs, such as AK128652 and AK054921, were also increased in the sera of patients with alcoholic cirrhosis and seem to be surrogate markers for survival in these patients.109 Overall, these promising biomarkers are still in the field of translational research, and larger trials to evaluate their accuracy and feasibility are needed.
Finally, the diagnostic abilities of noninvasive markers for ALD must also be assessed in primary care since the prevalence in this population might be different compared with that in secondary and tertiary care settings and could negatively impact the sensitivity and negative predictive value of these surrogate markers of liver fibrosis.
4. CONCLUSION
Significant progress has been made in the noninvasive assessment of liver disease in patients with ALD. Regarding the identification of advanced fibrosis, ELF score, FT, and TE are the most accurate and validated modalities. These patented biomarkers are best suited for first‐line investigation in primary care since they have been shown to be cost‐effective, but additional external validation is needed. TE is well‐suited for second‐line investigation in referral centers in order to select patients who might require liver biopsy or need follow‐up in the liver clinic. The performance of other imaging techniques (ARFI, 2D‐SWE, and MRE), although promising, needs to be better assessed in patients with ALD, with an accurate definition of quality criteria. Initially developed for diagnostic purposes, these noninvasive modalities seem to also have prognostic value in terms of prediction of overall survival, clinical decompensation, and HCC occurrence, but future long‐term studies will help us determine more accurately the role for these markers in the prognosis of patients with ALD. Efforts need to be concentrated on the development of novel biomarkers and, primarily, on the implementation of noninvasive diagnostic modalities in primary care, in order to identify patients earlier, before decompensation, which is associated with poorer outcomes. Finally, considering the growing burden of liver disease worldwide, a great challenge resides in the establishment of efficient public health policies that aim to reduce harmful alcohol consumption as well as to improve accessibility to interventions that allow us to reach this goal.
CONFLICT OF INTEREST
Alia Hadefi, Delphine Degré, Eric Trépo, and Christophe Moreno have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Writing—Original Draft Preparation: Alia Hadefi
Writing—Review & Editing: Alia Hadefi, Delphine Degré, Eric Trépo, and Christophe Moreno
All authors have read and approved the final version of the manuscript. The corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
The lead author, Alia Hadefi, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and if relevant, registered) have been explained.
ACKNOWLEDGMENTS
We acknowledge the contribution of the medical writer, Sandy Field, PhD, for her assistance concerning English‐language editing. Alia Hadefi is supported by a research grant from the “Fonds Erasme pour la recherche médicale”(doctoral research fellow grant). This funding support played no role in study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication.
Hadefi A, Degré D, Trépo E, Moreno C. Noninvasive diagnosis in alcohol‐related liver disease. Health Sci Rep. 2020;3:e146 10.1002/hsr2.146
Data Availability Statement:Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Funding information Fonds Erasme pour la recherche médicale
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England). 2012;380(9859):2095‐2128. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673612617280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJL, Vos T, Lozano R, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England). 2012;380(9859):2197‐2223. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673612616894. [DOI] [PubMed] [Google Scholar]
- 3. Williams R, Ashton K, Aspinall R, et al. Implementation of the Lancet Standing Commission on Liver Disease in the UK. Lancet (London, England). 2015. Nov;21; 386(10008):2098–2111. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673615006807. [DOI] [PubMed] [Google Scholar]
- 4. Williams R, Alexander G, Armstrong I, et al. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet (London, England). 2018. Mar 17;391(10125):1097‐1107. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673617328660. [DOI] [PubMed] [Google Scholar]
- 5. Thursz M, Gual A, Lackner C, et al. EASL clinical practice guidelines: management of alcohol‐related liver disease. J Hepatol. 2018;69(1):154‐181. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29628280. [DOI] [PubMed] [Google Scholar]
- 6. Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013. Jul 1;59(1):160‐168. Available from: https://www.sciencedirect.com/science/article/pii/S0168827813001840. [DOI] [PubMed] [Google Scholar]
- 7. Organisation for Economic Co‐Operation and Development . Tackling Harmful Alcohol use: Economics and Public Health Policy. OECD Publishing; 2015. [Google Scholar]
- 8. Pimpin L, Cortez‐Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018. Sep;69(3):718‐735. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29777749. [DOI] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver . EASL HEPAHEALTH project report 2018: risk factors and the burden of liver disease in Europe and selected Central Asian countries. 2018. Available from: https://ilc?congress.eu/wp?content/uploads/2018/hepahealth/EASL?HEPAHEALTH?Report.pdf (accessed July 25, 2018).
- 10. Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015. Apr 1;62(1):S38‐S46. Available from: https://www.sciencedirect.com/science/article/pii/S016882781500166X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hazeldine S, Hydes T, Sheron N. Alcoholic liver disease—the extent of the problem and what you can do about it. Clin Med (Northfield Il). 2015. Apr 1;15(2):179‐185. Available from: http://www.clinmed.rcpjournal.org/cgi/doi/10.7861/clinmedicine.15-2-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah ND, Ventura‐Cots M, Abraldes JG, et al. Alcohol‐related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin Gastroenterol Hepatol. 2019;17(11):2320–2329. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Altamirano J, López‐Pelayo H, Michelena J, et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: prediction and impact on long‐term survival. Hepatology. 2017. Dec 1;66(6):1842‐1853. Available from: http://doi.wiley.com/10.1002/hep.29338. [DOI] [PubMed] [Google Scholar]
- 14. Verrill C, Markham H, Templeton A, Carr NJ, Sheron N. Alcohol‐related cirrhosis‐early abstinence is a key factor in prognosis, even in the most severe cases. Addiction. 2009. May 1;104(5):768‐774. Available from: http://doi.wiley.com/10.1111/j.1360-0443.2009.02521.x. [DOI] [PubMed] [Google Scholar]
- 15. European Association for the Study of the Liver . Electronic address: easloffice@easloffice.eu M, European Association for the Study of the Liver A, Lackner C, Mathurin P, Moreno C, Spahr L, et al. EASL clinical practice guidelines: management of alcohol‐related liver disease. J Hepatol. 2018;69(1):154‐181. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29628280. [DOI] [PubMed] [Google Scholar]
- 16. Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005. Feb;128(2):343‐350. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15685546. [DOI] [PubMed] [Google Scholar]
- 17. Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013. Nov 24;10(11):666‐675. Available from: http://www.nature.com/articles/nrgastro.2013.175. [DOI] [PubMed] [Google Scholar]
- 18. Vergniol J, Boursier J, Coutzac C, et al. Evolution of noninvasive tests of liver fibrosis is associated with prognosis in patients with chronic hepatitis C. Hepatology. 2014. Jul;60(1):65‐76. Available from:. http://doi.wiley.com/10.1002/hep.27069. [DOI] [PubMed] [Google Scholar]
- 19. GBD 2016 Alcohol Collaborators MG , Fullman N, Hawley C, et al. Alcohol use and burden for 195 countries and territories, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018. Sep 22;392(10152):1015‐1035. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30146330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burton R, Henn C, Lavoie D, et al. A rapid evidence review of the effectiveness and cost‐effectiveness of alcohol control policies: an English perspective. Lancet. 2017. Apr;389(10078):1558‐1580. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673616324205. [DOI] [PubMed] [Google Scholar]
- 21. Sheron N, Chilcott F, Matthews L, Challoner B, Thomas M. Impact of minimum price per unit of alcohol on patients with liver disease in the UK. Clin Med. 2014. Aug 1;14(4):396‐403. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25099842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. REHM J, TAYLOR B, MOHAPATRA S, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta‐analysis. Drug Alcohol Rev. 2010. Jan 18;29(4):437‐445. Available from: http://doi.wiley.com/10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- 23. Åberg F, Helenius‐Hietala J, Puukka P, Jula A. Binge drinking and the risk of liver events: a population‐based cohort study. Liver Int. 2017. Sep 1;37(9):1373‐1381. Available from: http://doi.wiley.com/10.1111/liv.13408. [DOI] [PubMed] [Google Scholar]
- 24. Askgaard G, Grønbæk M, Kjær MS, Tjønneland A, Tolstrup JS. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol. 2015. May 1;62(5):1061‐1067. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0168827814009234. [DOI] [PubMed] [Google Scholar]
- 25. Simpson RF, Hermon C, Liu B, et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million Women Study. Lancet Public Heal. 2019. Jan;4(1):e41–e48 Available from: http://www.ncbi.nlm.nih.gov/pubmed/30472032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naveau S, Cassard‐Doulcier A‐M, Njiké‐Nakseu M, et al. Harmful effect of adipose tissue on liver lesions in patients with alcoholic liver disease. J Hepatol. 2010. Jun;52(6):895‐902. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168827810001029. [DOI] [PubMed] [Google Scholar]
- 27. Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010. Mar 11;340(mar11 1):c1240 Available from: http://www.bmj.com/cgi/doi/10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population‐based study. Gut. 2010. Oct 1;59(10):1410‐1415. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20660697. [DOI] [PubMed] [Google Scholar]
- 29. Hagström H, Hemmingsson T, Discacciati A, Andreasson A. Alcohol consumption in late adolescence is associated with an increased risk of severe liver disease later in life. J Hepatol. 2018. Mar;68(3):505‐510. Available from:. http://www.ncbi.nlm.nih.gov/pubmed/29395457. [DOI] [PubMed] [Google Scholar]
- 30. Mackenbach JP, Kulhánová I, Bopp M, Borrell C, Deboosere P, Kovács K, et al. Inequalities in alcohol‐related mortality in 17 European countries: a retrospective analysis of mortality registers. Rehm J, editor. PLOS Med. 2015. Dec 1; 12(12):e1001909. Available from: https://dx.plos.org/10.1371/journal.pmed.1001909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siegler V, Al‐Hamad A, Johnson B, Wells C, Sheron N. Social inequalities in alcohol‐related adult mortality by National Statistics Socio‐economic Classification, England and Wales, 2001‐03. Heal Stat Q. 2011. May 24;50(50):4‐39. Available from: http://link.springer.com/10.1057/hsq.2011.7. [DOI] [PubMed] [Google Scholar]
- 32. Thiele M, Madsen BS, Hansen JF, Detlefsen S, Antonsen S, Krag A. Accuracy of the enhanced liver Fibrosis Test vs FibroTest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology. 2018;154(5):1369‐1379. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29317276. [DOI] [PubMed] [Google Scholar]
- 33. Naveau S, Gaudé G, Asnacios A, et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology. 2009. Jan;49(1):97‐105. Available from: http://doi.wiley.com/10.1002/hep.22576. [DOI] [PubMed] [Google Scholar]
- 34. Lombardi R, Buzzetti E, Roccarina D, Tsochatzis EA. Non‐invasive assessment of liver fibrosis in patients with alcoholic liver disease. World J Gastroenterol. 2015. Oct 21;21(39):11044‐52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26494961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rosenberg WMC, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004. Dec 1;127(6):1704‐1713. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0016508504015537. [DOI] [PubMed] [Google Scholar]
- 36. Lieber CS, Weiss DG, Morgan TR, Paronetto F. Aspartate aminotransferase to platelet ratio index in patients with alcoholic liver fibrosis. Am J Gastroenterol. 2006. Jul;101(7):1500‐1508. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16863553. [DOI] [PubMed] [Google Scholar]
- 37. Soto M, Sampietro‐Colom L, Lasalvia L, Mira A, Jiménez W, Navasa M. Cost‐effectiveness of enhanced liver fibrosis test to assess liver fibrosis in chronic hepatitis C virus and alcoholic liver disease patients. World J Gastroenterol. 2017;23(17):3163 Available from: http://www.wjgnet.com/1007-9327/full/v23/i17/3163.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Asphaug L, Thiele M, Krag A, et al. Cost‐effectiveness of non‐invasive screening for alcohol‐related liver fibrosis. Hepatology. 2019. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31595545. [DOI] [PubMed] [Google Scholar]
- 39. Sandrin L, Fourquet B, Hasquenoph J‐M, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003. Dec;29(12):1705‐1713. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14698338. [DOI] [PubMed] [Google Scholar]
- 40. Boursier J, Konaté A, Gorea G, et al. Reproducibility of liver stiffness measurement by ultrasonographic elastometry. Clin Gastroenterol Hepatol. 2008. Nov;6(11):1263‐1269. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18995217. [DOI] [PubMed] [Google Scholar]
- 41. Boursier J, Konate A, Guilluy M, et al. Learning curve and interobserver reproducibility evaluation of liver stiffness measurement by transient elastography. Eur J Gastroenterol Hepatol. 2008. Jul;20(7):693‐701. Available from: https://insights.ovid.com/crossref?an=00042737-200807000-00017. [DOI] [PubMed] [Google Scholar]
- 42. Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007. Jul 1;56(7):968‐973. Available from: http://gut.bmj.com/cgi/doi/10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Castéra L, Foucher J, Bernard P‐H, et al. Pitfalls of liver stiffness measurement: a 5‐year prospective study of 13,369 examinations. Hepatology. 2010. Mar;51(3):828‐835. Available from: http://doi.wiley.com/10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 44. Poynard T, Munteanu M, Deckmyn O, et al. Applicability and precautions of use of liver injury biomarker FibroTest. A reappraisal at 7 years of age. BMC Gastroenterol. 2011. Apr;14(11(1):39. Available from: https://bmcgastroenterol.biomedcentral.com/articles/10.1186/1471-230X-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maharaj B, Leary WP, Naran AD, et al. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986. Mar;327(8480):523‐525. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673686908834. [DOI] [PubMed] [Google Scholar]
- 46. Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002. Oct;97(10):2614‐2618. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12385448. [DOI] [PubMed] [Google Scholar]
- 47. Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000. Sep;32(3):477‐481. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10960438. [DOI] [PubMed] [Google Scholar]
- 48. Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003. Dec;38(6):1449‐57.ajhep09022. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14647056. [DOI] [PubMed] [Google Scholar]
- 49. Berzigotti A, De Gottardi A, Vukotic R, Siramolpiwat S, Abraldes JG, García‐Pagan JC, et al. Effect of meal ingestion on liver stiffness in patients with cirrhosis and portal hypertension. Rodriguez‐Ortigosa CM, editor. PLoS One. 2013. Mar 8; 8(3):e58742 Available from: http://dx.plos.org/10.1371/journal.pone.0058742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mederacke I, Wursthorn K, Kirschner J, et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009. Nov 1;29(10):1500‐1506. Available from: http://doi.wiley.com/10.1111/j.1478-3231.2009.02100.x. [DOI] [PubMed] [Google Scholar]
- 51. Arena U, Lupsor Platon M, Stasi C, et al. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology. 2013. Jul 1;58(1):65‐72. Available from: http://doi.wiley.com/10.1002/hep.26343. [DOI] [PubMed] [Google Scholar]
- 52. Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008. Feb 20;47(2):592‐595. Available from: http://doi.wiley.com/10.1002/hep.22056. [DOI] [PubMed] [Google Scholar]
- 53. Dechêne A, Sowa J‐P, Gieseler RK, et al. Acute liver failure is associated with elevated liver stiffness and hepatic stellate cell activation. Hepatology. 2010. Sep;52(3):1008‐1016. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20684020. [DOI] [PubMed] [Google Scholar]
- 54. Millonig G, Friedrich S, Adolf S, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010. Feb 1;52(2):206‐210. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0168827809007946. [DOI] [PubMed] [Google Scholar]
- 55. Lebray P, Varnous S, Charlotte F, Varaut A, Poynard T, Ratziu V. Liver stiffness is an unreliable marker of liver fibrosis in patients with cardiac insufficiency. Hepatology. 2008. Dec 1;48(6):2089‐2089. Available from: http://doi.wiley.com/10.1002/hep.22594. [DOI] [PubMed] [Google Scholar]
- 56. Mueller S, Millonig G, Sarovska L, et al. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010. Feb 28;16(8):966‐972. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roulot D, Czernichow S, Le Clésiau H, Costes J‐L, Vergnaud A‐C, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008. Apr 1;48(4):606‐613. Available from: https://www.sciencedirect.com/science/article/abs/pii/S016882780700685X. [DOI] [PubMed] [Google Scholar]
- 58. Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008. Nov 1;48(5):1718‐1723. Available from: http://doi.wiley.com/10.1002/hep.22577. [DOI] [PubMed] [Google Scholar]
- 59. Lanzi A, Gianstefani A, Mirarchi MG, Pini P, Conti F, Bolondi L. Liver AL amyloidosis as a possible cause of high liver stiffness values. Eur J Gastroenterol Hepatol. 2010. Jul;22(7):895‐897. Available from: https://insights.ovid.com/crossref?an=00042737-201007000-00022. [DOI] [PubMed] [Google Scholar]
- 60. Nguyen‐Khac E, Thiele M, Voican C, et al. Non‐invasive diagnosis of liver fibrosis in patients with alcohol‐related liver disease by transient elastography: an individual patient data meta‐analysis. Lancet Gastroenterol Hepatol. 2018;3(9):614‐625. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2468125183013249. [DOI] [PubMed] [Google Scholar]
- 61. Kiani A, Brun V, Lainé F, et al. Acoustic radiation force impulse imaging for assessing liver fibrosis in alcoholic liver disease. World J Gastroenterol. 2016. May 28;22(20):4926‐35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27239119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang D, Li P, Chen M, et al. Non‐invasive assessment of liver fibrosis in patients with alcoholic liver disease using acoustic radiation force impulse elastography. Abdom Imaging. 2015. Apr 9;40(4):723‐729. Available from: http://link.springer.com/10.1007/s00261-014-0154-5. [DOI] [PubMed] [Google Scholar]
- 63. Bota S, Sporea I, Peck‐Radosavljevic M, et al. The influence of aminotransferase levels on liver stiffness assessed by acoustic radiation force impulse elastography: a retrospective multicentre study. Dig Liver Dis. 2013. Sep;45(9):762‐768. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1590865813000613. [DOI] [PubMed] [Google Scholar]
- 64. Popescu A, Bota S, Sporea I, et al. The influence of food intake on liver stiffness values assessed by acoustic radiation force impulse elastography‐preliminary results. Ultrasound Med Biol. 2013. Apr;39(4):579‐584. Available from: https://linkinghub.elsevier.com/retrieve/pii/S030156291200703X. [DOI] [PubMed] [Google Scholar]
- 65. Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta‐analysis of individual participant data. Clin Gastroenterol Hepatol. 2015. Mar;13(3):440‐451.e6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25305349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Myers RP, Pomier‐Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012. Jan 1;55(1):199‐208. Available from: http://doi.wiley.com/10.1002/hep.24624. [DOI] [PubMed] [Google Scholar]
- 67. Coco B, Oliveri F, Maina AM, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007. May;14(5):360‐369. Available from: http://doi.wiley.com/10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 68. Chan HL‐Y, Wong GL‐H, Choi PC‐L, et al. Alanine aminotransferase‐based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009. Jan;16(1):36‐44. Available from: http://doi.wiley.com/10.1111/j.1365-2893.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 69. Mueller S, Sandrin L. Liver stiffness: a novel parameter for the diagnosis of liver disease. Hepat Med. 2010. May 25;2:49‐67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24367208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Trabut J‐B, Thépot V, Nalpas B, et al. Rapid decline of liver stiffness following alcohol withdrawal in heavy drinkers. Alcohol Clin Exp Res. 2012. Aug;36(8):1407‐1411. Available from: http://doi.wiley.com/10.1111/j.1530-0277.2012.01737.x. [DOI] [PubMed] [Google Scholar]
- 71. Gelsi E, Dainese R, Truchi R, et al. Effect of detoxification on liver stiffness assessed by Fibroscan® in alcoholic patients. Alcohol Clin Exp Res. 2011. Mar;35(3):566‐570. Available from: http://doi.wiley.com/10.1111/j.1530-0277.2010.01374.x. [DOI] [PubMed] [Google Scholar]
- 72. Mueller S, Englert S, Seitz HK, et al. Inflammation‐adapted liver stiffness values for improved fibrosis staging in patients with hepatitis C virus and alcoholic liver disease. Liver Int. 2015. Dec;35(12):2514‐2521. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26121926. [DOI] [PubMed] [Google Scholar]
- 73. Nahon P, Kettaneh A, Tengher‐Barna I, et al. Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol. 2008. Dec;49(6):1062‐1068. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168827808006089. [DOI] [PubMed] [Google Scholar]
- 74. Nguyen‐Khac E, Chatelain D, Tramier B, et al. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non‐invasive laboratory tests. Aliment Pharmacol Ther. 2008. Nov 15;28(10):1188‐1198. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18705692. [DOI] [PubMed] [Google Scholar]
- 75. Kim SG, Kim YS, Jung SW, et al. The usefulness of transient elastography to diagnose cirrhosis in patients with alcoholic liver disease. Korean J Hepatol. 2009. Mar;15(1):42‐51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19346784. [DOI] [PubMed] [Google Scholar]
- 76. Boursier J, Vergniol J, Sawadogo A, et al. The combination of a blood test and Fibroscan improves the non‐invasive diagnosis of liver fibrosis. Liver Int. 2009. Nov;29(10):1507‐1515. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19725892. [DOI] [PubMed] [Google Scholar]
- 77. Janssens F, de Suray N, Piessevaux H, Horsmans Y, de Timary P, Stärkel P. Can transient elastography replace liver histology for determination of advanced fibrosis in alcoholic patients: a real‐life study. J Clin Gastroenterol. 2010. Sep;44(8):575‐582. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00004836-900000000-99434. [DOI] [PubMed] [Google Scholar]
- 78. Fernandez M, Trépo E, Degré D, et al. Transient elastography using Fibroscan is the most reliable noninvasive method for the diagnosis of advanced fibrosis and cirrhosis in alcoholic liver disease. Eur J Gastroenterol Hepatol. 2015. Sep;27(9):1074‐1079. Available from: http://insights.ovid.com/crossref?an=00042737-201509000-00014. [DOI] [PubMed] [Google Scholar]
- 79. Thiele M, Detlefsen S, Sevelsted Møller L, et al. Transient and 2‐dimensional shear‐wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology. 2016. Jan;150(1):123‐133. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0016508515014262. [DOI] [PubMed] [Google Scholar]
- 80. Voican CS, Louvet A, Trabut J‐B, et al. Transient elastography alone and in combination with FibroTest® for the diagnosis of hepatic fibrosis in alcoholic liver disease. Liver Int. 2017. Nov;37(11):1697‐1705. Available from: http://doi.wiley.com/10.1111/liv.13440. [DOI] [PubMed] [Google Scholar]
- 81. Bota S, Herkner H, Sporea I, et al. Meta‐analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013. Sep;33(8):1138‐1147. Available from: http://doi.wiley.com/10.1111/liv.12240. [DOI] [PubMed] [Google Scholar]
- 82. Herrmann E, de Lédinghen V, Cassinotto C, et al. Assessment of biopsy‐proven liver fibrosis by two‐dimensional shear wave elastography: an individual patient data‐based meta‐analysis. Hepatology. 2018;67(1):260‐272. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28370257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008. Jul;135(1):32‐40. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0016508508005726. [DOI] [PubMed] [Google Scholar]
- 84. Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995. Sep 29;269(5232):1854‐1857. Available from: http://www.sciencemag.org/cgi/doi/10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 85. Loomba R, Cui J, Wolfson T, et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. Am J Gastroenterol. 2016. Jul;111(7):986‐994. Available from: http://insights.ovid.com/crossref?an=00000434-201607000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nyblom H, Berggren U, Balldin J, Olsson R. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol. 2004. Jul 1;39(4):336‐339. Available from: https://academic.oup.com/alcalc/article-lookup/doi/10.1093/alcalc/agh074. [DOI] [PubMed] [Google Scholar]
- 87. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM‐5 Alcohol Use Disorder. JAMA Psychiatry. 2015. Aug 1;72(8):757‐66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26039070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction. 1993. Jun;88(6):791‐804. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8329970. [DOI] [PubMed] [Google Scholar]
- 89. European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado . EASL‐ALEH clinical practice guidelines: non‐invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015. Jul;63(1):237‐264. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25911335. [DOI] [PubMed] [Google Scholar]
- 90. Boursier J, Vergniol J, Guillet A, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non‐alcoholic fatty liver disease. J Hepatol. 2016. Sep;65(3):570‐578. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168827816301672. [DOI] [PubMed] [Google Scholar]
- 91. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013. Apr 1;57(4):1357‐1365. Available from: http://doi.wiley.com/10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mayo MJ, Parkes J, Adams‐Huet B, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology. 2008. Nov;48(5):1549‐1557. Available from: http://doi.wiley.com/10.1002/hep.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006. Oct 1;52(10):1887‐1896. Available from: http://www.clinchem.org/cgi/doi/10.1373/clinchem.2006.070961. [DOI] [PubMed] [Google Scholar]
- 94. Munteanu M, Pais R, Peta V, et al. Long‐term prognostic value of the FibroTest in patients with non‐alcoholic fatty liver disease, compared to chronic hepatitis C, B, and alcoholic liver disease. Aliment Pharmacol Ther. 2018. Nov;48(10):1117‐1127. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30334263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. de Lédinghen V, Vergniol J, Barthe C, et al. Non‐invasive tests for fibrosis and liver stiffness predict 5‐year survival of patients chronically infected with hepatitis B virus. Aliment Pharmacol Ther. 2013. May;37(10):979‐988. Available from: http://doi.wiley.com/10.1111/apt.12307. [DOI] [PubMed] [Google Scholar]
- 96. Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5‐year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011. Jun;140(7):1970‐1979, 1979.e1‐3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0016508511002708. [DOI] [PubMed] [Google Scholar]
- 97. Pang JXQ, Zimmer S, Niu S, Crotty P, Tracey J, Pradhan F, et al. Liver stiffness by transient elastography predicts liver‐related complications and mortality in patients with chronic liver disease. Kim SU, editor. PLoS One. 2014. Apr 22; 9(4):e95776. Available from: https://dx.plos.org/10.1371/journal.pone.0095776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2013. Dec;11(12):1573‐1584.e1‐2; quiz e88‐9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1542356513011671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. LEMOINE M, KATSAHIAN S, ZIOL M, et al. Liver stiffness measurement as a predictive tool of clinically significant portal hypertension in patients with compensated hepatitis C virus or alcohol‐related cirrhosis. Aliment Pharmacol Ther. 2008. Nov 1;28(9):1102‐1110. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18691352. [DOI] [PubMed] [Google Scholar]
- 100. Robic MA, Procopet B, Métivier S, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011. Nov;55(5):1017‐1024. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168827811002017. [DOI] [PubMed] [Google Scholar]
- 101. Shi K‐Q, Fan Y‐C, Pan Z‐Z, et al. Transient elastography: a meta‐analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int. 2013. Jan;33(1):62‐71. Available from: http://doi.wiley.com/10.1111/liv.12003. [DOI] [PubMed] [Google Scholar]
- 102. Mueller J et al. Survival in a 10 year prospective cohort of heavy drinkers: Liver stiffness is the best long‐term prognostic parameter. EASL. 2019. Available from: https://easl.meta-dcr.com/ilc2019/crs/survival-in-a-10-year-prospective-cohort-of-heavy-drinkers-liver-stiffness-is-the-best-long-term-prognostic-parameter. [Google Scholar]
- 103. Pirola CJ, Sookoian S. Multiomics biomarkers for the prediction of nonalcoholic fatty liver disease severity. World J Gastroenterol. 2018. Apr 21;24(15):1601‐1615. Available from: http://www.wjgnet.com/1007-9327/full/v24/i15/1601.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Moreno C, Mueller S, Szabo G. Non‐invasive diagnosis and biomarkers in alcohol‐related liver disease. J Hepatol. 2019. Feb; 70(2):273–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30658728 [DOI] [PubMed] [Google Scholar]
- 105. Saha B, Momen‐Heravi F, Furi I, et al. Extracellular vesicles from mice with alcoholic liver disease carry a distinct protein cargo and induce macrophage activation through heat shock protein 90. Hepatology. 2018. May;67(5):1986‐2000. Available from: http://doi.wiley.com/10.1002/hep.29732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bala S, Szabo G. MicroRNA signature in alcoholic liver disease. Int J Hepatol. 2012;2012:1‐6. Available from: http://www.hindawi.com/journals/ijh/2012/498232/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013. Sep 21;10(9):542‐552. Available from: http://www.nature.com/articles/nrgastro.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Momen‐Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015. Aug 12;13(1):261 Available from: http://www.translational-medicine.com/content/13/1/261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Yang Z, Ross RA, Zhao S, Tu W, Liangpunsakul S, Wang L. LncRNA AK054921 and AK128652 are potential serum biomarkers and predictors of patient survival with alcoholic cirrhosis. Hepatol Commun. 2017. Aug;1(6):513‐523. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29104954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Szabo G, Bala S. Reply: hepatology. 2013. Jun;57(6):2547‐2547. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23112144. [DOI] [PubMed] [Google Scholar]
- 111. Castera L, Friedrich‐Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology [Internet]. 2019. Apr [cited 2019 Aug16];156(5):1264‐1281.e4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30660725. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


