Abstract
Background/Aims
We evaluated the usefulness in kidney transplant (KT) candidates of cytomegalovirus (CMV)-specific enzyme-linked immunospot (ELISPOT) assays for predicting the development of post-transplant CMV infections.
Methods
All adult recipients admitted for living-donor KT between March 2014 and March 2015 were prospectively enrolled except donor CMV-seropositive and recipient seronegative (D+/R–) recipients. All the enrolled patients underwent CMV-specific ELISPOT assays before transplant, and a researcher blinded to the results of these assays examined the patients for CMV infection at least 6 months post-transplant.
Results
Of 133 KT recipients, 44 (33%) developed CMV infections. When we used the cut-off determined by receiver operator characteristic curve, 16 of the 34 patients (47%) with negative pp65-specific ELISPOT results (< 11 spots/200,000 cells) developed CMV infections, whereas 28 of the 99 patients (39%) with positive pp65-specific ELISPOT results at baseline (≥ 11 spots/200,000 cells) developed CMV infections after KT (p = 0.02). Based on the multivariable Cox regression model, negative pp65-specific ELISPOT assay results was an independent risk factor for CMV infection (adjusted hazard ratio [AHR], 1.87; 95% confidence interval [CI], 1.01 to 3.46; p = 0.047) as well as age (AHR, 1.05; 95% CI, 1.01 to 1.08; p = 0.007).
Conclusions
Pre-transplant CMV-specific ELISPOT assay appears to predict the development of CMV infections after KT in recipients at moderate risk such as CMV-seropositive recipients (Clinical Trial Registration Number NCT 02025335).
Keywords: Cytomegalovirus, T cell response, Enzyme-linked immunospot assay, Interferon-gamma release tests, Kidney transplantation
INTRODUCTION
Cytomegalovirus (CMV) is considered to be one of the most important pathogens in organ transplant recipients [1,2]. As CMV has a tendency to invade the allograft and has several indirect effects due to its ability to affect the immune system, it is also associated with a variety of syndromes in solid organ transplant (SOT) recipients including allograft rejection and opportunistic infections [3]. Currently, testing for the donor (D) and recipient (R) CMV serostatus before SOT is recommended for assessing the risk of CMV reactivation after SOT [2]. It is well known that seronegative recipients (R–) of CMV-seropositive allografts (D+) have the highest risk of symptomatic CMV infection after SOT, followed by D+/R+, D–/R+, and D–/R– patients [2]. Experts recommend universal ganciclovir prophylaxis for the highest risk group [2]. Either universal prophylaxis or preemptive ganciclovir therapy based on monitoring of early CMV replication is recommended for the moderate risk groups [2] into which more than 95% of Korean SOT candidates fall [4]. Despite these modern advances in preventive strategies, post-transplant CMV infections remains a major problem, and the potential toxicity of the drugs used and the cost of these drugs and of frequent monitoring are also problematic [3]. The question has therefore been raised as to whether the current strategy of evaluating pre-transplant CMV-immunoglobulin G (IgG) serostatus is enough for predicting the risk of CMV infection in all transplant recipients.
In the past 20 years, it has become clear that CMV-specific immunity plays a crucial role in controlling CMV infection [5,6]. Theoretically, a CMV-specific T-cell-based assay before SOT would further categorize these risk groups and might reduce CMV development in conjunction with a customized preventive strategy. We therefore evaluated the usefulness in kidney transplant (KT) candidates of the CMV-specific enzyme-linked immunospot (ELISPOT) assay for predicting the post-transplant CMV infection.
METHODS
Study population
All patients admitted for living-donor KTs to a renal transplant unit between March 2014 and March 2015 in a 2,700-bed, tertiary-care hospital in Seoul, South Korea, were prospectively enrolled. Tests for CMV-IgG were performed on the KT recipients and donors. Exclusion criteria were refusal of informed consent, pediatric renal transplant candidates (< 16 years old), and CMV IgG-negative recipients with CMV IgG-positive donors. We also excluded the pancreas transplant alone recipients and the patients whose ELISPOT assays were performed after induction immunosuppressive therapy due to their weekend admission or emergency transplant surgery due to deceased-donor KT. Universal oral valganciclovir for 3 months was given only to the highest CMV risk group (D+/R–). CMV antigenemia assays were performed weekly during the first month, bi-weekly during the 2nd and 3rd months after KT, and then monthly to 6 months after KT. CMV antigenemia of 50 cells per 200,000 cells was the indication for preemptive therapy. Conventional-dose ganciclovir (5 mg/kg twice daily) as preemptive therapy was given daily for at least 2 weeks and until patients were negative for CMV antigenemia. This investigation was approved by the Institutional Review Board of Asan Medical Center (IRB approval No. 2013-1040) and is registered at Clinicaltrials.gov NCT 02025335 on December 16, 2013. All patients were informed of the nature of the study, and written informed consent was obtained before their inclusion in the study. This study also performed in accordance with the ethical standards laid down in the Declaration of Helsinki and its later amendments. The company of the assay (Lophius, Regensburg, Germany) had no role in study design, data collection, data analysis, data interpretation, or writing of the report, and did not provide any financial support including the cost for the assay.
The CMV antigenemia assay and CMV ELISPOT assay
The CMV antigenemia assay was performed as previously described [7]. Ethylenediaminetetraacetic acid-treated whole blood samples were fractionated by dextran sedimentation and lysis of erythrocytes. The granulocytes were then centrifuged to prepare cytospin slides. The cells were fixed with formaldehyde, and immunostained sequentially with monoclonal antibodies C10/C11 (Clonab CMV, Biotest, Dreieich, Germany). Counts are expressed as positive cells per 200,000 leukocytes.
A peripheral venous blood sample (approximately 8 mL) was collected from each patient for the CMV ELISPOT assay for T-cells producing interferon γ (IFN-γ; T-track CMV, Lophius Bioscience, Regensburg, Gemany). Briefly, peripheral blood mononuclear cells were immediately (within 30 minutes) separated and collected. The collected cells were resuspended at 2.0 × 106 cells/mL, and placed (2.0 × 105 cells/well) in wells pre-coated with anti-human IFN-γ antibody. The samples were stimulated with phytohemagglutinin (positive control), pp65, immediate-early (IE1), or medium only (negative control) and incubated for 18 hours. The resulting spots were counted with an automated microscope (ELiSpot 04 HR, Autoimmune Diagnostika GmbH, Strassberg, Germany). Backgrounds count, obtained in negative control wells, were subtracted.
Assessment of outcomes
The primary outcome was the development of CMV infection, which was observed by a researcher blinded to the results of the ELISPOT. Post-transplant CMV infection was monitored between March 2014 and September 2015 to obtain at least 6 months of follow-up data on all patients enrolled. Patients with CMV antigenemia or CMV disease were considered to have a CMV infection [2,8]. CMV antigenemia was defined as CMV antigenemia identified by pp65 antigenemia, and CMV disease was defined as CMV syndrome or tissue-invasive CMV disease. CMV syndrome was defined as CMV antigenemia and at least one of the following: fever > 38°C; new onset severe malaise; leukopenia in two successive measurements (white blood cell count of < 3,500 cells/mm3). Tissue-invasive CMV was defined as evidence of localized CMV infection (cells with CMV inclusions, in situ detection of CMV antigen by immunohistochemistry, or DNA) in a biopsy or other appropriate specimen (e.g., bronchoalveolar lavage, cerebrospinal fluid) and symptoms of organ dysfunction. Secondary outcome included significant CMV antigenemia (≥ 50 cells/200,000 cells) which was the target for preemptive therapy in our institution, or tissue-invasive CMV disease.
Statistical analysis
The primary goal of the study was to test the hypothesis that post-KT patients who were ELISPOT-negative developed CMV infection more frequently than those that were ELISPOT-positive patients (comparator). Assuming a 39% negative ELISPOT result in the KT patients, as extrapolated from our previous proof-of-concept study aimed at obtaining the information needed to calculate sample size [9], we calculated statistical power based on an estimated 50% CMV infection rate in the ELISPOT-negative (IE1-specific) patients and 30% CMV infection in the comparator. We concluded that, with type I error of 0.05, a sample size ≥ 192 was needed for 80% statistical power to detect a difference between the ELISPOT-negative group and the comparator.
For each of the tests used to predict CMV infection, namely the pp65- and IE1-specific ELISPOT assays, we examined receiver operator characteristic (ROC) curves plotting sensitivity against the rate of false-positive results over a range of cut-off values [10]. We chose the optimal cut-off value as the point on each ROC curve farthest from the diagonal line that maximized the sum of sensitivity and specificity. Diagnostic performance was expressed in terms of sensitivity, specificity, positive predictive value, and negative predictive value. Categorical variables were compared using Pearson chi-square tests or Fisher exact test, as appropriate. Continuous variables were presented as mean ± SD, and were compared using the Mann-Whitney U test. All tests of significance were two-tailed and a p value of less than 0.05 was considered to indicate statistical significance. The time-to-event analyses for incidence of CMV infection were performed using Kaplan-Meier estimates and the log-rank test. Multivariable analyses for risk factors for CMV infection were performed using Cox proportional hazard regression models. Calculations were performed using SPSS for Windows software package version 21.0 (IBM Co., Armonk, NY, USA), MedCalc software (Med-Calc, Mariakerke, Belgium), and PASS (NCSS Statistical Software S, Kaysville, UT, USA) and figures were created with GraphPad Prism version 5.01 for Windows (Graph-Pad Software, San Diego, CA, USA).
RESULTS
Patient characteristics
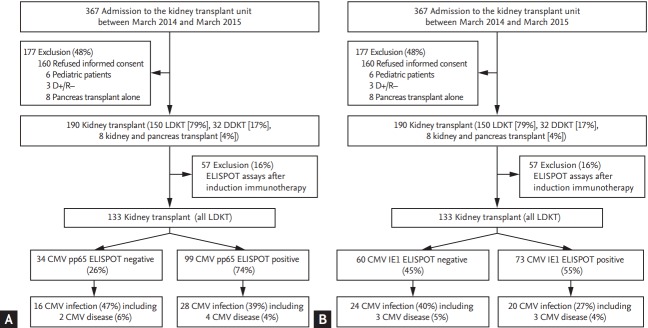
Fig. 1 is a flow chart of the study. Of a total of 367 patients, 177 were excluded: 160 refused informed consent, six were pediatric patients, three were D+/R–, and eight pancreas transplant alone recipients. Of remaining 190 patients, 57 (16%) whose ELISPOT assays were performed after induction immunosuppressive therapy were excluded in the final analysis. The remaining 133 patients were finally analyzed. All these 133 patients received living-donor KT.
Figure 1.
Flow chart of the study according to (A) pp65-specific enzyme-linked immunospot (ELISPOT) result, (B) immediate-early (IE1)-specific ELISPOT results. LDKT, living-donor kidney transplant; DDKT, deceased-donor kidney transplant; CMV, cytomegalovirus.
Development of CMV infections
Of the 133 patients, 44 (33%) developed CMV infections (Table 1). Baseline clinical characteristics between those with CMV infections and those without CMV infections, which were comparable except for age, are shown in Table 1. The development of CMV infection was significantly more common in elderly recipients (51 ± 11 vs. 45 ± 10, p = 0.005) than in the young. Of the 44 patients with CMV infections, nine (20%) had significant levels of CMV antigenemia (> 50 CMV-positive cells/200,000 cells, which was the threshold for ganciclovir preemptive therapy in our hospital). Of these nine patients, four who had tissue-invasive CMV diseases (all had CMV gastritis) and four without tissue-invasive CMV disease received ganciclovir therapy. The remaining one patients with > 50 CMV antigenemia who did not receive ganciclovir preemptive therapy due to their outpatient schedule recovered spontaneously without any complications. On the other hand, two of 35 patients with ≤ 50 CMV antigenemia had tissue-invasive CMV diseases (one gastritis and one retinitis). Among six patients with tissue-invasive CMV diseases, mean age was 49 years old (49 ± 14). Of these six patients, one received ABO-mismatched KT and pre-transplant rituximab therapy. Serostatus of these six patients were D+/R+. Most episodes of CMV infection occurred during the first 3 months after transplant (median 55 days [interquartile range, 27 to 76] after transplant).
Table 1.
Baseline characteristics of the patients included in the study
| Patient characteristic | Total (n = 133) | CMV infection (n = 44) | No CMV infection (n = 89) | p value |
|---|---|---|---|---|
| Age, yr | 47 ± 11 | 51 ± 11 | 45 ± 10 | 0.005 |
| Male sex | 84 (63) | 25 (57) | 59 (66) | 0.34 |
| Primary reason for transplant | 0.33 | |||
| Glomerulonephritis | 40 (30) | 9 (21) | 31 (35) | |
| Hypertension | 11 (8) | 3 (7) | 8 (9) | |
| Diabetes mellitus | 19 (14) | 8 (18) | 11 (12) | |
| Unknown | 49 (37) | 20 (46) | 29 (33) | |
| Polycystic kidney disease | 3 (2) | 0 | 3 (3) | |
| Others | 11 (8) | 4 (9) | 7 (8) | |
| ABO-mismatch transplantation | 41 (31) | 16 (36) | 25 (28) | 0.43 |
| Primary transplant induction therapy at transplant | 0.58 | |||
| Anti-IL-2 receptor antibodies | 125 (94) | 42 (96) | 83 (93) | |
| Cyclosporine including regimena | 2 (2) | 1 (2) | 1 (1) | |
| Tacrolimus including regimena | 6 (5) | 1 (2) | 5 (6) | |
| Pretransplant rituximab | 49 (37) | 20 (46) | 29 (33) | 0.18 |
| CMV serostatus | 0.18 | |||
| Donor (+)/Recipient (+) | 124 (93) | 43 (98) | 81 (91) | |
| Recipient (+) (donor serology unknown) | 3 (2) | 1 (2) | 2 (2) | |
| Donor (–)/Recipient (+) | 5 (4) | 0 | 5 (6) | |
| Donor (–)/Recipient (–) | 1 (1) | 0 | 1 (1) |
Values are presented as mean ± SD or number (%).
CMV, cytomegalovirus; IL-2, interleukin 2.
Other drugs in regimen include mycophenolate and steroid.
CMV ELISPOT results and development of CMV infection
A total of 133 transplant recipients underwents CMV-specific ELISPOT assays before induction immunosuppressive therapy such as anti-interleukin 2 (IL-2) receptor anti-bodies or anti-thymocyte globulin.
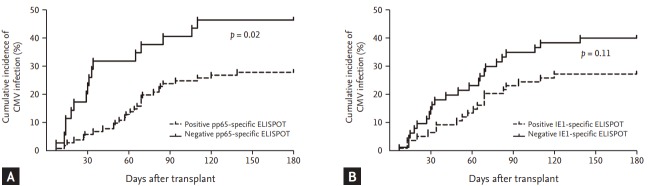
On the basis of the ROC curve obtained for pp65-specific ELISPOT assay, we determined the optimal cut-off for the ELISPOT assay of ≥ 11 spots. The comparison of characteristics between the patients with negative pp65-specific ELISPOT results and with positive pp65-specific ELISPOT results are shown in Table 2. When we used this cut-off for pp65-specific ELISPOT, 28 of the 99 patients (39%) with positive pp65-specific ELISPOT results at baseline (≥ 11 spots/200,000 cells) developed CMV infections, and 16 of the 34 patients (47%) with negative pp65-specific ELISPOT results developed CMV infections (p = 0.02). The sensitivity, specificity, positive predictive value, and negative predictive value of the pp65-specific ELISPOT for predicting CMV infection were 36% (95% confidence interval [CI], 22% to 52%), 80% (95% CI, 70% to 88%), 47% (95% CI, 33% to 61%), and 72% (95% CI, 66% to 76%), respectively. In addition, on the basis of the ROC curve obtained for IE1-specific ELISPOT assay, we determined the optimal cut-off for the ELISPOT assay of ≥ 2 spots. The comparison of characteristics between the patients with negative CMV-specific ELISPOT results and with positive CMV-specific ELISPOT results are also shown in Table 2. When we used this cut-off for IE1-specific ELISPOT, 20 of the 73 patients (27%) with positive IE1-specific ELISPOT results (≥ 2 spots/200,000 cells) at baseline developed CMV infection, and 24 of the 60 patients (40%) with negative IE1-specific ELISPOT results developed CMV infections (p = 0.11). The sensitivity, specificity, positive predictive value, and negative predictive value of the IE1-specific ELISPOT for predicting CMV infection were 55% (95% CI, 39% to 70%), 60% (95% CI, 49% to 70%), 40% (95% CI, 32% to 49%), and 73% (95% CI, 65% to 79%), respectively. Table 3 shows the diagnostic performance of the various cut-off values for the pp65-specific ELISPOT and the IE1-specific ELISPOT. Fig. 2 shows the cumulative incidence of CMV infection after transplant according to CMV-specific ELISPOT results.
Table 2.
Comparison of characteristics between the patients with negative CMV-specific ELISPOT results and with positive CMV-specific ELISPOT results
| Patient characteristic | pp65-specific ELISPOT results |
IE1-specific ELISPOT results |
||||
|---|---|---|---|---|---|---|
| Negative (n = 34) | Positive (n = 99) | p value | Negative (n = 60) | Positive (n = 73) | p value | |
| Age, yr | 49 ± 10 | 47 ± 11 | 0.24 | 46 ± 12 | 48 ± 10 | 0.53 |
| Male sex | 23 (68) | 61 (62) | 0.68 | 41 (68) | 43 (59) | 0.96 |
| Primary reason for transplant | 0.88 | 0.88 | ||||
| Glomerulonephritis | 8 (24) | 32 (32) | 20 (33) | 20 (27) | ||
| Hypertension | 3 (9) | 8 (8) | 6 (10) | 5 (7) | ||
| Diabetes mellitus | 5 (15) | 14 (14) | 9 (15) | 10 (14) | ||
| Unknown | 15 (44) | 34 (34) | 19 (32) | 30 (41) | ||
| Polycystic kidney disease | 1 (3) | 2 (2) | 1 (2) | 2 (3) | ||
| Others | 2 (6) | 9 (9) | 5 (8) | 6 (8) | ||
| ABO-mismatch transplant | 5 (15) | 36 (36) | 0.02 | 19 (32) | 22 (30) | 0.85 |
| Primary transplant induction therapy at transplant | 0.65 | 0.83 | ||||
| Anti-IL-2 receptor antibodies | 32 (94) | 93 (94) | 57 (95) | 68 (93) | ||
| Cyclosporine including regimena | 1 (3) | 1 (1) | 1 (2) | 1 (1) | ||
| Tacrolimus including regimena | 1 (3) | 5 (5) | 2 (3) | 4 (6) | ||
| Pretransplant rituximab | 8 (24) | 41 (41) | 0.07 | 21 (35) | 28 (39) | 0.72 |
| CMV serostatus | 0.26 | 0.25 | ||||
| Donor (+)/Recipient (+) | 32 (94) | 92 (93) | 56 (93) | 68 (93) | ||
| Recipient (+) (donor serology unknown) | 0 | 3 (3) | 0 | 3 (4) | ||
| Donor (–)/Recipient (+) | 1 (3) | 4 (4) | 3 (5) | 2 (3) | ||
| Donor (–)/Recipient (–) | 1 (3) | 0 | 1 (2) | 0 | ||
| Significant levels of CMV antigenemia (> 50 CMV-positive cells/200,000cells) | 3 (19) | 6 (21) | 0.99 | 3 (13) | 6 (30) | 0.26 |
Values are presented as mean ± SD or number (%).
CMV, cytomegalovirus; ELISPOT, enzyme-linked immunospot; IE1, immediate-early; IL-2, interleukin 2.
Other drugs in regimen include mycophenolate and steroid.
Table 3.
Diagnostic performance of pp65 and IE1 specific ELISPOT assay for prediction of CMV infection
| Variablea | CMV infection rates |
p value | Sensitivity and specificity, % (95% CI) | |
|---|---|---|---|---|
| No. of patients (%) | No. of CMV infection cases (%) | |||
| KT recipients who underwent ELISPOT before transplant (n = 133) | 133 | 44 (33) | ||
| pp65-specific ELISPOT | ||||
| Negative ELISPOT results (< 11)b | 34 (26) | 16 (47) | 0.06 | Sensitivity 36 (22–52) |
| Positive ELISPOT results (≥ 11)b | 99 (74) | 28 (28) | Specificity 80 (70–88) | |
| IE1-specific ELISPOT | ||||
| Negative ELISPOT results (< 2)b | 60 (45) | 24 (40) | 0.14 | Sensitivity 55 (39–70) |
| Positive ELISPOT results (≥ 2)b | 73 (55) | 20 (27) | Specificity 60 (49–70) | |
| IE1-specific ELISPOT results (≤ 0)c | 43 (32) | 18 (42) | 0.17 | Sensitivity 41 (26–57) |
| IE1-specific ELISPOT results (> 0)c | 90 (68) | 26 (29) | Specificity 72 (61–81) | |
| Combined pp65-specific ELISPOT results with IE1-specific ELISPOT result | ||||
| IE1 < 2b or pp65 < 11b | 73 (55) | 28 (38) | 0.20 | Sensitivity 64 (48–78) |
| IE ≥ 2b and pp65 ≥ 11b | 60 (45) | 16 (27) | Specificity 49 (39–60) | |
| IE1 < 2b and pp65 < 11b | 21 (16) | 12 (57) | 0.02 | Sensitivity 27 (15–43) |
| IE ≥ 2bor pp65 ≥ 11b | 112 (84) | 32 (29) | Specificity 90 (82–95) | |
IE1, immediate-early; ELISPOT, enzyme-linked immunospot; CMV, cytomegalovirus; CI, confidence interval; KT, kidney transplant.
ELISPOT results are expressed as number of spots/2.0 × 105 peripheral blood mononuclear cells.
The optimal cut-off point were determined by estimating the maximum Youden’s index (J). J is the vertical distance between the receiver operating characteristics (ROC) curves and the diagonal or chance line.
This cut-off point was derived from our previous proof-of-concept study; it was also determined by using ROC curves, and the cut-off of pp65 was > 10 in the previous study.
Figure 2.
Cumulative incidence of cytomegalovirus (CMV) infection after transplant, according to (A) pp65-specific enzyme-linked immunospot (ELISPOT) result, (B) immediate-early (IE1)-specific ELISPOT results in total study population (n = 133). The p value comparing two groups were calculated using the log-rank test.
For prediction of significant CMV antigenemia or tissue-invasive CMV disease, neither pp65-specific nor IE1-specifc ELISPOT was diagnostic (data are shown in Supplementary Table 1). In multivariable analysis, negative pp65-specific ELISPOT assay results was an independent risk factor for CMV infection (adjusted hazard ratio [AHR], 1.87; 95% CI, 1.01 to 3.46; p = 0.047) as well as age (AHR, 1.05; 95% CI, 1.01 to 1.08; p = 0.007) (Supplementary Table 2).
DISCUSSION
Establishing a novel diagnostic test that accurately predicts post-transplant CMV risk in SOT recipients is a critical and unfulfilled need. Prophylactic and preemptive strategies in at-risk SOT recipients based on pre-transplant donor and recipient CMV IgG serostatus are cornerstones of CMV prevention [2]. Despite these measures, CMV viremia and disease are common and problematic. In addition, because of the high frequency of CMV seropositivity (> 95%) of Korean adults [4], most Korean SOT recipients are classified as at moderate risk of CMV infection. In our previous study, post-transplant CMV disease occurred in 5% of such KT recipients at moderate risk of CMV infection, despite of CMV antigenemia-based preemptive therapy [11]. In this clinical situation, a further risk stratification strategy is essential if we wish to reduce CMV development after SOT. We have therefore evaluated the use of the commercial CMV-specific ELISPOT assay for measuring INF-γ release as a marker of protection against CMV infection in KT recipients. We found meaningful association between a negative CMV-specific ELISPOT assay response and the risk of developing post-transplant CMV infection in living-donor KT recipients with moderate risk.
Because CMV specific cell-mediated immunity (CMI) is critical for controlling CMV replication [12], a variety of assays have been developed for measuring the CMI. Several methods rely on measurements of T-cell-secreted cytokines or the T-cell phenotypes (i.e., INF-γ, tumor necrosis factor α, IL-2, CD107a, programmed cell death protein 1) following antigen stimulation. Other methods, such as tetramer assays, are based on direct detection of antigen-specific T-cells or on cell proliferation assays [13-16]. Many studies have used staining of intracellular cytokines (ICS) and flow cytometry to assess CMV-specific CMI because ICS allows T-cell phenotypic characterization via cell surface markers as well as the enumeration of CMV-specific T-cells and has been shown to be useful for predicting post-transplant CMV risk after SOT [17-24]. In contrast, Eid et al. [25] found no significant association between CMV-specific CD4+ and CD8+ T cells and CMV DNAemia. Due to its labor-intensive character and the lack of technical standardization or protective cut-off values, as well as the need for a flow cytometer, ICS has been limited in clinical use to highly specialized laboratories. The number of reports proposing IFN-γ releasing assays (IGRAs) as the diagnostic standard for detecting CMI is growing [6,26-32]. As in the case of IGRAs for tuberculosis, two commercial IGRAs for CMI are currently available in the market on the time of this writing; one is the enzyme-linked immunosorbent assay (ELISA)-based quantiferon-CMV (Cellestis, Valencia, CA, USA) and the other, the ELISPOT-based T-track CMV (Lophius). Numerous studies using Quantiferon-CMV have been reported in SOT recipients [28-30,32-36]. However, the ELISA-based assay is less sensitive than the ELISPOT-based assay [37]. Theoretically, ELISPOT-based assay could be more useful in immunocompromised patients than ELISA-based assays. Despite this, there are a few studies addressing the utility of (in-house) ELISPOT assays for predicting CMV infection after SOT. To date, the data using IGRAs have been inconsistent and mostly limited by the small scale of the studies (Supplementary Table 3) [26-30,32-36,38-43].
To the best of our knowledge, there are no published data on the use of the commercially available ELISPOT assay. Despite failure to include an adequate number of patients based on the results of a proof-of-concept study after exclusion of the patients who received induction immunosuppressive therapy before ELISPOT assay [9], the result revealed that negative CMV-specific ELISPOT was significantly associated with the development of CMV infection. We assumed that induction immunosuppressive therapy might have affected the results of ELISPOT assays and the inclusion of the patients where ELISPOT assays were performed after the transplant could dilute the results by leading to a bias towards the null hypothesis. We hypothesize that we may optimize the ability of pre-transplant ELISPOT assay for predicting CMV infection by including patients where ELISPOT assays were performed before the transplant only, a less biased population. In addition, the multivariate analysis showed that age was the risk factor for development of post-transplant CMV infection, as well as negative pre-transplant pp65-specific ELISPOT assay results. This finding is consistent with an increasing CMV infection in elderly population [44-46]. More studies will be needed to confirm the observation.
Our study has some limitations. First, it was performed in a region with a high level of CMV seropositivity. Therefore, the findings may not be generalizable to other settings. However, the study population like our setting has a relatively homogenous risk for post-transplant CMV infection based on CMV serology, in which the further risk prediction strategy is most required. Second, although both pp65 and IE1 are considered dominant T-cell targets for CMV infection [9], it is not clear which measurements of the various target antigens optimally reflect the protective T-cell response in CMV infections. Furthermore, the ELISPOT focused on a single cytokine output (IFN-γ) may not fully reflect the protective CMV CMI. Recently, Snyder et al. [24] demonstrated that a specific T-cell polyfunctional response to CMV antigen stimulation provided a more reliable prediction of subsequent CMV risk than an assay that measured a single cytokine response. Hence, studies are needed of other target antigens such as IE2, pp50 or pp150, other platform technologies for producing the antigens, such as overlapping peptides or CMV cell lysates, and other diagnostic methods, such as fluorescence-activated cell sorting-based ICS staining are needed. Third, we measured CMI at a single time point in the pre-transplant period. CMI is not static, and the abrupt change (i.e., steep decline) of CMV CMI following intense immunosuppression immediately after transplant may have more impact on the development of CMV infection than any pre-transplant CMV CMI value at a single time point [35], as indeed has been described in studies of BK virus CMI [47] and varicella-zoster virus CMI [48]. Future studies monitoring CMI dynamic longitudinally in various populations are warranted. Lastly, we did not perform quantitative real-time blood CMV polymerase chain reaction (PCR), which is considered more sensitive than the CMV antigenemia assay [49], and has been the gold standard test for CMV infection [50]. Some argue that CMV PCR would be more sensitive than CMV antigenemia assay. However, in the light of previous studies [11,51], it is likely that the use of PCR as a surveillance tool would not have yielded very different results.
In conclusion, pre-transplant CMV-specific ELISPOT assay in the living-donor KT appears to predict the development of CMV infections after KT in recipients at moderate risk. Further studies are necessary to confirm our findings and future studies of various other target antigens or antigen producing technologies, and other assays to measure CMV-specific CMI are also needed.
KEY MESSAGE
1. Despite the current strategies of post-transplant cytomegalovirus (CMV) prevention, based on pre-transplant donor and recipient CMV immunoglobulin G serostatus, post-transplant CMV infections remain a major problem.
2. Establishing a novel diagnostic test that accurately predicts post-transplant CMV risk in solid organ transplant recipients is a critical and unfulf illed need; therefore, we evaluated the usefulness of the commercial CMV-specific enzyme-linked immunospot (ELISPOT) assay in kidney transplant (KT) candidates for predicting the post-transplant CMV infection.
3. We found meaningful association between a negative CMV-specific ELISPOT assay response and the risk of developing post-transplant CMV infection in KT recipients with moderate risk.
Acknowledgments
This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare (grant no. HI15C1763), from the National Research Foundation of Korea, funded by the Ministry of Education (NRF-2015R1D1A1A01059315) and from the Asan Institute for Life Sciences, Asan Medical Centre (2015-462).
Footnotes
No potential conflict of interest relevant to this article was reported.
Supplementary Materials
Diagnostic performance of pp65 and IE1 specific ELISPOT assay for prediction of significant CMV antigenemia or tissue-invasive CMV disease
Multivariable Cox proportional hazard regression model for risk factors of cytomegalovirus infection
Previous studies of CMV-specific immune monitoring using ELISPOT or Quantiferon-CMV in organ transplant patients to predict cytomegalovirus infection
REFERENCES
- 1.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 2.Razonable RR, Humar A; AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:93–106. doi: 10.1111/ajt.12103. [DOI] [PubMed] [Google Scholar]
- 3.Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation: challenging the status quo. Clin Transplant. 2007;21:149–158. doi: 10.1111/j.1399-0012.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 4.Hahn JS, Lee SJ, Park WK, Ko YW, Kim HO, Lee SY. A survey on the cytomegalovirus antibodies in blood donors and the diseased. Korean J Blood Transfus. 1990;1:21–33. [Google Scholar]
- 5.Barron MA, Gao D, Springer KL, et al. Relationship of reconstituted adaptive and innate cytomegalovirus (CMV)-specific immune responses with CMV viremia in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:1777–1783. doi: 10.1086/648423. [DOI] [PubMed] [Google Scholar]
- 6.Egli A, Humar A, Kumar D. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin Infect Dis. 2012;55:1678–1689. doi: 10.1093/cid/cis818. [DOI] [PubMed] [Google Scholar]
- 7.Mori T, Okamoto S, Matsuoka S, et al. Risk-adapted pre-emptive therapy for cytomegalovirus disease in patients undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant. 2000;25:765–769. doi: 10.1038/sj.bmt.1702227. [DOI] [PubMed] [Google Scholar]
- 8.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Lee HJ, Kim SM, et al. Diagnostic usefulness of cytomegalovirus (CMV)-specific T cell immunity in predicting CMV infection after kidney transplantation: a pilot proof-of-concept study. Infect Chemother. 2015;47:105–110. doi: 10.3947/ic.2015.47.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Cho OH, Park SJ, et al. Rapid diagnosis of tuberculous meningitis by T cell-based assays on peripheral blood and cerebrospinal fluid mononuclear cells. Clin Infect Dis. 2010;50:1349–1358. doi: 10.1086/652142. [DOI] [PubMed] [Google Scholar]
- 11.Kim T, Lee YM, Lee SO, et al. Differences of cytomegalovirus diseases between kidney and hematopoietic stem cell transplant recipients during preemptive therapy. Korean J Intern Med. 2016;31:961–970. doi: 10.3904/kjim.2015.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22:76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sund F, Lidehall AK, Claesson K, et al. CMV-specific T-cell immunity, viral load, and clinical outcome in seropositive renal transplant recipients: a pilot study. Clin Transplant. 2010;24:401–409. doi: 10.1111/j.1399-0012.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- 14.Ravkov EV, Pavlov IY, Hanson KE, Delgado JC. Validation of cytomegalovirus immune competence assays for the characterization of CD8(+) T cell responses posttransplant. Clin Dev Immunol. 2012;2012:451059. doi: 10.1155/2012/451059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritter M, Schmidt T, Dirks J, et al. Cytomegalovirus-specific T cells are detectable in early childhood and allow assignment of the infection status in children with passive maternal antibodies. Eur J Immunol. 2013;43:1099–1108. doi: 10.1002/eji.201243100. [DOI] [PubMed] [Google Scholar]
- 16.Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus-specific T cells correlates with viremia and reversible functional anergy. Am J Transplant. 2008;8:1486–1497. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 17.Bunde T, Kirchner A, Hoffmeister B, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sester U, Gartner BC, Wilkens H, et al. Differences in CMV-specific T-cell levels and long-term susceptibility to CMV infection after kidney, heart and lung transplantation. Am J Transplant. 2005;5:1483–1489. doi: 10.1111/j.1600-6143.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- 19.Sester M, Sester U, Gartner B, et al. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation. 2001;71:1287–1294. doi: 10.1097/00007890-200105150-00018. [DOI] [PubMed] [Google Scholar]
- 20.Egli A, Binet I, Binggeli S, et al. Cytomegalovirus-specific T-cell responses and viral replication in kidney transplant recipients. J Transl Med. 2008;6:29. doi: 10.1186/1479-5876-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerna G, Lilleri D, Chiesa A, et al. Virologic and immunologic monitoring of cytomegalovirus to guide preemptive therapy in solid-organ transplantation. Am J Transplant. 2011;11:2463–2471. doi: 10.1111/j.1600-6143.2011.03636.x. [DOI] [PubMed] [Google Scholar]
- 22.Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. Primary cytomegalovirus phosphoprotein 65-specific CD8+ T-cell responses and T-bet levels predict immune control during early chronic infection in lung transplant recipients. J Infect Dis. 2011;204:1663–1671. doi: 10.1093/infdis/jir624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabanti E, Bruno F, Lilleri D, et al. Human cytomegalovirus (HCMV)-specific CD4+ and CD8+ T cells are both required for prevention of HCMV disease in seropositive solid-organ transplant recipients. PLoS One. 2014;9:e106044. doi: 10.1371/journal.pone.0106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder LD, Chan C, Kwon D, et al. Polyfunctional T-cell signatures to predict protection from cytomegalovirus after lung transplantation. Am J Respir Crit Care Med. 2016;193:78–85. doi: 10.1164/rccm.201504-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eid AJ, Brown RA, Arthurs SK, et al. A prospective longitudinal analysis of cytomegalovirus (CMV)-specific CD4+ and CD8+ T cells in kidney allograft recipients at risk of CMV infection. Transpl Int. 2010;23:506–513. doi: 10.1111/j.1432-2277.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 26.Abate D, Saldan A, Fiscon M, et al. Evaluation of cytomegalovirus (CMV)-specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV-specific T cell reconstitution in kidney transplant recipients. J Infect Dis. 2010;202:585–594. doi: 10.1086/654931. [DOI] [PubMed] [Google Scholar]
- 27.Abate D, Fiscon M, Saldan A, et al. Human cytomegalovirus-specific T-cell immune reconstitution in preemptively treated heart transplant recipients identifies subjects at critical risk for infection. J Clin Microbiol. 2012;50:1974–1980. doi: 10.1128/JCM.06406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abate D, Saldan A, Mengoli C, et al. Comparison of cytomegalovirus (CMV) enzyme-linked immunosorbent spot and CMV quantiferon gamma interferon-releasing assays in assessing risk of CMV infection in kidney transplant recipients. J Clin Microbiol. 2013;51:2501–2507. doi: 10.1128/JCM.00563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar D, Chernenko S, Moussa G, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9:1214–1222. doi: 10.1111/j.1600-6143.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 30.Lisboa LF, Kumar D, Wilson LE, Humar A. Clinical utility of cytomegalovirus cell-mediated immunity in transplant recipients with cytomegalovirus viremia. Transplantation. 2012;93:195–200. doi: 10.1097/TP.0b013e31823c1cd4. [DOI] [PubMed] [Google Scholar]
- 31.Clari MA, Munoz-Cobo B, Solano C, et al. Performance of the QuantiFERON-cytomegalovirus (CMV) assay for detection and estimation of the magnitude and functionality of the CMV-specific gamma interferon-producing CD8(+) T-cell response in allogeneic stem cell transplant recipients. Clin Vaccine Immunol. 2012;19:791–796. doi: 10.1128/CVI.05633-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manuel O, Husain S, Kumar D, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis. 2013;56:817–824. doi: 10.1093/cid/cis993. [DOI] [PubMed] [Google Scholar]
- 33.Westall GP, Mifsud NA, Kotsimbos T. Linking CMV serostatus to episodes of CMV reactivation following lung transplantation by measuring CMV-specific CD8+ T-cell immunity. Am J Transplant. 2008;8:1749–1754. doi: 10.1111/j.1600-6143.2008.02294.x. [DOI] [PubMed] [Google Scholar]
- 34.Lochmanova A, Lochman I, Tomaskova H, et al. Quantiferon-CMV test in prediction of cytomegalovirus infection after kidney transplantation. Transplant Proc. 2010;42:3574–3577. doi: 10.1016/j.transproceed.2010.07.101. [DOI] [PubMed] [Google Scholar]
- 35.Weseslindtner L, Kerschner H, Steinacher D, et al. Prospective analysis of human cytomegalovirus DNAemia and specific CD8+ T cell responses in lung transplant recipients. Am J Transplant. 2012;12:2172–2180. doi: 10.1111/j.1600-6143.2012.04076.x. [DOI] [PubMed] [Google Scholar]
- 36.Cantisan S, Lara R, Montejo M, et al. Pretransplant interferon-γ secretion by CMV-specific CD8+ T cells informs the risk of CMV replication after transplantation. Am J Transplant. 2013;13:738–745. doi: 10.1111/ajt.12049. [DOI] [PubMed] [Google Scholar]
- 37.Hong SI, Lee YM, Park KH, Kim SH. Is the sensitivity of the QuantiFERON-TB gold in-tube test lower than that of T-SPOT.TB in patients with military tuberculosis? Clin Infect Dis. 2014;59:142. doi: 10.1093/cid/ciu248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiereghin A, Gabrielli L, Zanfi C, et al. Monitoring cytomegalovirus T-cell immunity in small bowel/multivisceral transplant recipients. Transplant Proc. 2010;42:69–73. doi: 10.1016/j.transproceed.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 39.Costa C, Astegiano S, Terlizzi ME, et al. Evaluation and significance of cytomegalovirus-specific cellular immune response in lung transplant recipients. Transplant Proc. 2011;43:1159–1161. doi: 10.1016/j.transproceed.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 40.Patel M, Stefanidou M, Long CB, et al. Dynamics of cell-mediated immune responses to cytomegalovirus in pediatric transplantation recipients. Pediatr Transplant. 2012;16:18–28. doi: 10.1111/j.1399-3046.2011.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bestard O, Lucia M, Crespo E, et al. Pretransplant immediately early-1-specific T cell responses provide protection for CMV infection after kidney transplantation. Am J Transplant. 2013;13:1793–1805. doi: 10.1111/ajt.12256. [DOI] [PubMed] [Google Scholar]
- 42.Costa C, Balloco C, Sidoti F, et al. Evaluation of CMV-specific cellular immune response by EliSPOT assay in kidney transplant patients. J Clin Virol. 2014;61:523–528. doi: 10.1016/j.jcv.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Ritta M, Costa C, Sidoti F, et al. Pre-transplant assessment of CMV-specific immune response by Elispot assay in kidney transplant recipients. New Microbiol. 2015;38:329–335. [PubMed] [Google Scholar]
- 44.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galiatsatos P, Shrier I, Lamoureux E, Szilagyi A. Meta-analysis of outcome of cytomegalovirus colitis in immunocompetent hosts. Dig Dis Sci. 2005;50:609–616. doi: 10.1007/s10620-005-2544-6. [DOI] [PubMed] [Google Scholar]
- 46.Bernard S, Germi R, Lupo J, et al. Symptomatic cytomegalovirus gastrointestinal infection with positive quantitative real-time PCR findings in apparently immunocompetent patients: a case series. Clin Microbiol Infect. 2015;21:1121. doi: 10.1016/j.cmi.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 47.Schachtner T, Stein M, Babel N, Reinke P. The loss of BKV-specific immunity from pretransplantation to posttransplantation identifies kidney transplant recipients at increased risk of BKV replication. Am J Transplant. 2015;15:2159–2169. doi: 10.1111/ajt.13252. [DOI] [PubMed] [Google Scholar]
- 48.Kim JW, Min CK, Mun YC, et al. Varicella-zoster virus-specific cell-mediated immunity and herpes zoster development in multiple myeloma patients receiving bortezomib- or thalidomide-based chemotherapy. J Clin Virol. 2015;73:64–69. doi: 10.1016/j.jcv.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Boeckh M, Boivin G. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin Microbiol Rev. 1998;11:533–554. doi: 10.1128/cmr.11.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon SM, Sung H, Kim MN, et al. Diagnostic yield of the cytomegalovirus (CMV) antigenemia assay and clinical features in solid organ transplant recipients and hematopoietic stem cell transplant recipients with CMV pneumonia. Transpl Infect Dis. 2012;14:192–197. doi: 10.1111/j.1399-3062.2011.00703.x. [DOI] [PubMed] [Google Scholar]
- 51.Cariani E, Pollara CP, Valloncini B, Perandin F, Bonfanti C, Manca N. Relationship between pp65 antigenemia levels and real-time quantitative DNA PCR for human cytomegalovirus (HCMV) management in immunocompromised patients. BMC Infect Dis. 2007;7:138. doi: 10.1186/1471-2334-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagnostic performance of pp65 and IE1 specific ELISPOT assay for prediction of significant CMV antigenemia or tissue-invasive CMV disease
Multivariable Cox proportional hazard regression model for risk factors of cytomegalovirus infection
Previous studies of CMV-specific immune monitoring using ELISPOT or Quantiferon-CMV in organ transplant patients to predict cytomegalovirus infection