Abstract
Organophosphate esters (OPEs) are a class of semi-volatile organic compounds (SVOCs) used as flame retardants, plasticizers, and anti-foaming agents. Due to stringent flammability standards in vehicles and the ability of OPEs to migrate out of end-use products, elevated concentrations of OPEs have been found in car dust samples around the world. As many residents of Southern California spend a significant amount of time in their vehicles, there is potential for increased exposure to OPEs associated with longer commute times. As approximately 70% of the University of California, Riverside’s undergraduate population commutes, the objective of this study was to use silicone wristbands to monitor personal exposure to OPEs and determine if exposure was associated with commute time in a subset of these students. Participants were asked to wear wristbands for five continuous days and complete daily surveys about the amount of time spent commuting. Data were then used to calculate a participant-specific total commute score. Components of Firemaster 550 (triphenyl phosphate, or TPHP, and isopropylated triaryl phosphate isomers) and Firemaster 600 (TPHP and tert-butylated triaryl phosphate isomers) – both widely used commercial flame retardant formulations – were strongly correlated with other OPEs detected within participant wristbands. Moreover, the concentration of tris(1,3-dichloro-2-propyl) phosphate (TDCIPP) was significantly correlated with the concentration of several Firemaster 500 components and tris(2-chloroisopropyl) phosphate (TCIPP). Finally, out of all OPEs measured, TDCIPP was significantly and positively correlated with total commute score, indicating that longer commutes are associated with increased human exposure to TDCIPP. Overall, our findings raise concerns about the potential for chronic TDCIPP exposure within vehicles and other forms of transportation, particularly within densely populated and traffic-congested areas such as Southern California.
Keywords: Organophosphate esters; Silicone wristband; Tris(1,3-dichloro-2-propyl) phosphate; Human exposure; Transportation
1. Introduction
Organophosphate esters (OPEs) are a class of semi-volatile organic compound (SVOCs) used as flame retardants, plasticizers, and anti-foaming agents (Wei et al., 2015). OPEs are used in a wide range of products including cosmetics, textiles, polyurethane foam, upholstery, electric cables, lubricants, floor waxes/polishes, construction materials, and vehicles (Marklund et al., 2003; Wei et al., 2015). As OPEs are not chemically bound to these products, OPEs have the potential to readily migrate into surrounding environmental media such as indoor air and dust. Interestingly, OPE concentrations within indoor air are significantly higher relative to outdoor air, suggesting that the built environment represents a primary source of OPEs (Carlsson et al., 1997). Indeed, OPEs are ubiquitous within indoor dust around the world, with concentrations ranging from 1 to 577 μg/g within industrialized countries such as the United States, Switzerland, Denmark, Sweden, China, and Japan (Araki et al., 2014; Bergh et al., 2011; Hartmann et al., 2004; He et al., 2015; Langer et al., 2016; Stapleton et al., 2009). The wide range of concentrations may be attributed to different country-specific flammability standards and approaches to meet these standards.
In addition to buildings and homes, dust samples collected from vehicles also have OPE concentrations ranging from 1.1 ng/g to 1100 μg/g (Abdallah and Covaci, 2014; Ali et al., 2013; Brandsma et al., 2014; Harrad et al., 2016). Interestingly, the total mass of OPEs is higher in car dust samples compared to indoor dust samples from buildings and homes (Ali et al., 2016; Brandsma et al., 2014; Brommer and Harrad, 2015; Christia et al., 2018; Zhou et al., 2017). As OPEs are commonly used as flame retardants (Wei et al., 2015), this finding is likely attributed to more stringent, vehicle-specific flammability standards. For example, Federal Motor Vehicle Safety Standard (FMVSS) No. 302 – the federal-level flammability standard for car interiors in the United States – is more rigorous than the California-specific smolder resistance test for upholstered furniture (Technical Bulletin 117–2013). Within FMVSS No. 302, the ignition source (a Bunsen burner) is placed 19 mm below interior materials tested (e.g., seat cushions, seat backs, seat belts, etc.), and these materials fail if any are burned or transmit a flame across its surface (> 102 mm/min).
While prior studies have used active samplers on lapels (Tsai and Vincent, 2001) and within backpacks (Nethery et al., 2012) to monitor individual human exposure to environmental chemicals, these samplers often require a battery-powered pump to force air through the sampling module (Bohlin et al., 2007). Biological samples such as urinary and serum biomarkers are also used to measure human exposure, but biological sample collection may be invasive and result in smaller sample sizes due to lower study participation rates (Aerts et al., 2018; Needham et al., 2005). Alternatively, passive sampling represents another method of measuring human exposure, where air-borne SVOCs diffuse into the lipophilic membrane of passive samplers (Anderson et al., 2017).
Silicone wristbands are non-invasive and have been rapidly adopted to support human exposure assessments within the United States and abroad. For example, wristbands have been used to measure (1) brominated flame retardants (BFRs) and OPEs in preschool children (Kile et al., 2016); (2) pesticides, environmental chemicals, and personal care products in agricultural and urban communities (Bergmann et al., 2017); and (3) polycyclic aromatic hydrocarbons (PAHs) in pregnant women (Dixon et al., 2018). Interestingly, PAHs detected on wristbands were more strongly associated with urinary metabolites compared to active air monitoring samplers (Dixon et al., 2018). Furthermore, when comparing measurements of OPEs on hand wipes and metabolites in pooled urine samples, OPEs measured on wristbands were more strongly associated with urinary metabolites compared to hand wipes (Hammel et al., 2016). Likewise, polybrominated diphenyl ethers (PBDEs) measured within wristbands were positively associated with serum biomarkers from the same participants (Hammel et al., 2018), and OPEs detected in wristbands were positively associated with hand wipes, brooches, and active air samples (Wang et al., 2019). Therefore, wristbands have the potential to account for both inhalation and dermal routes of exposure and represent a cost-effective and non-invasive tool to measure biologically relevant human exposure.
In Southern California (particularly within the Inland Empire), residents spend an average of 62.45 min per day commuting to and from work (Sirotnik and Aldana, 2018). As approximately 19% of commuters spend two or more hours within their vehicles per commute (Sirotnik and Aldana, 2018), this raises concerns about the potential health risks associated with spending extended periods of time in their personal vehicles over multiple days, weeks, years, and possibly decades. While elevated urinary concentrations of the primary TDCIPP metabolite (bis (1,3-dichloro-2-propyl) phosphate, or BDCIPP) were previously associated with spending more than one hour per day within vehicles (Hammel et al., 2016), to our knowledge human OPE exposure as a function of total commute time has not been evaluated to date. Therefore, the overall objective of the study was to leverage silicone wristbands to monitor personal OPE exposure within a subset of commuter vs. non-commuter undergraduate students at the University of California, Riverside (UCR). As 70% of UCR’s undergraduate population commutes to campus multiple times a week from all over Southern California, we hypothesized that OPE exposure is elevated for students who spend longer amounts of time commuting to and from UCR.
2. Materials and methods
2.1. Study design
Study participants (N = 88) were recruited in January/February 2019 from UCR. Participants were eligible for the study if they were (1) at least 18 years old; (2) willing to wear a silicone wristband continuously for five days; and (2) willing to complete five, one-minute online questionnaires. All study protocols and materials used for this study were approved by UCR’s Institutional Review Board (IRB Number: HS-18–162), and each participant provided informed consent prior to enrolling in the study.
2.2. Wristband collection
Blue wristbands were purchased in a single size from 24HourWristbands.com (https://24hourwristbands.com/) (Houston, TX, USA) and, using previously described procedures (Hammel et al., 2016), cleaned with two, 12-h Soxhlet extractions with 1:1 ethyl acetate/hexane (v/v) and 1:1 ethyl acetate/methanol (v/v) as well as passive drying in a fume hood. Wristbands were then wrapped in pre-baked (at 450°C) aluminum foil and placed in ziplock bags. Participants were asked to wear wristbands continuously for five days, including during bathing, sleeping, and other daily activities. During the 5-d study period, weather conditions did not significantly vary, with an average outdoor temperature ranging from ~ 4.4–13.3°C (~40–56°F), average precipitation of 0.19 in, and average humidity of 60%. At the end of the study period, participants re-wrapped their wristband in either the originally provided aluminum foil or a clean (i.e., pre-baked) piece of aluminum foil, and then placed the wrapped wristband in the originally provided ziplock bag. Six solvent-rinsed wristbands were used as field blanks to account for potential OPEs present within wristbands as well as OPE exposure during shipping and handling. Field blanks were un-wrapped for 30 s at room temperature on the third floor of the Science Laboratories 1 Building at UCR, and then re-wrapped with the same aluminum foil. Participant wristbands and field blanks were stored at −20°C until overnight shipment on dry ice to Duke University (Durham, NC, USA) for extraction and analysis.
2.3. Questionnaires and calculation of commute scores
Study participants completed an initial recruitment survey. In this survey, participants were asked to provide demographic information (age, gender, ethnicity, and household income) as well as whether they use transportation to commute to campus. During the study, participants completed five short online surveys (one survey per day). Participants were asked if they had used a form of transportation on that day (personal vehicle, rideshare, vanpool, public transport, or other) and, if so, how much time they spent in each mode of transportation (< 30 min, 30–60 min, 60–180 min, or > 180 min). For this study, we did not collect personal vehicle information (year, make, and model), as prior studies have shown that OPE concentrations in car dust are not significantly correlated with these characteristics (Abdallah and Covaci, 2014; Brommer and Harrad 2015; Christia et al., 2018; Harrad et al., 2016). While we did collect information about the participant’s residence ZIP code, we were unable to rely on these data since, after completion of the study, we discovered that some study participants reported ZIP codes for permanent (e.g., family) addresses rather than addresses for current residences.
The amount of time a participant spent in a mode of transportation was used to calculate their total commute score. Each time bracket was assigned a daily commute score: < 30 min was assigned 1; 30–60 min was assigned 2; 60–180 min was assigned 3; and > 180 min was assigned 4. The daily commute scores were then summed to obtain the total commute score for each participant. For participants who did not complete all five surveys, averages were calculated based on surveys that were completed (90.9% of participants completed all five surveys) and multiplied by five (for each day of the study). For the purpose of this study, we assumed that a longer time spent in a mode of transportation was associated with a longer commute time.
2.4. Extraction and analysis of OPEs from wristbands
Wristbands were extracted and analyzed using a modified version of the method reported by Hammel et al. (2016) and Hammel et al. (2018). Each wristband (samples and field blanks) was cut into ~ 1-in fragments and the mass of each sample was recorded (~0.8 g per wristband). The wristband fragments were transferred to a clean glass centrifuge tube, spiked with isotopically-labeled compounds (Table S1), and extracted via sonication in 10 mL of a 50:50 (v:v) mixture of hexane:dichloromethane for 15 min. The extraction was repeated three times and the extracts were combined. Each sample extract was concentrated to ~ 1.0 mL using purified nitrogen gas prior to column chromatography. Extracts were purified using 8 g of deactivated, 100–200 mesh Acros Organics Florisil (Thermo Fisher Scientific, Waltham, MA, USA). An F1 fraction (40 mL hexane) and F2 fraction (40 mL ethyl acetate) were eluted and collected. The F1 and F2 extracts were combined and concentrated to 1 mL. Sample extracts were then concentrated to near dryness and reconstituted in 1 mL of hexane. Isotopically labelled recovery standards (Table S2) were spiked into each sample prior to mass spectrometry analysis.
Samples were analyzed for OPEs using a Q Exactive GC Hybrid Quadrupole-Orbitrap GC–MS/MS system (Thermo Fisher Scientific, Waltham, MA, USA) operated in full scan Electron Ionization (EI) mode. Field blanks (clean wristbands; N = 6) and lab blanks (solvent; N = 5) were processed and analyzed with each batch of wristbands for quality assurance and quality control. No significant differences were detected between field and lab blanks; therefore, field blanks were used to estimate method detection limits (MDLs). MDLs were three times the standard deviation of the field blank responses, or a value equal to ten times the signal-to-noise if the analyte was not detected in the field blanks. MDLs for all target OPEs are available in the supporting information (Table S6) and were generally at or below 1 ng/g for all target OPEs. Recoveries for all OPE surrogate standards are reported in Table S6.1, and the average across all OPE surrogate standards was 105 ± 44% (median: 100%). Analyte concentrations (in ng of analyte per g wristband) were calculated based upon standard calibration curves and all samples were recovery-corrected and blank-subtracted.
2.5. Statistical analyses
A general linear model (GLM) and Tukey’s post-hoc test (α = 0.05) was performed using SPSS Statistics 24 (IBM, Armonk, NY, USA) to identify significant differences between total commute scores and demographic data (age, gender, ethnicity, or household income). As OPE concentrations across all participant wristbands displayed a log-normal distribution, a heat map based on log10-transformed concentrations for all OPEs was generated within Morpheus (Broad Institute, Cambridge, MA, USA), and hierarchical clustering was performed using the Euclidean distance and complete linkage method. Within Prism v8.0.2 (GraphPad, San Diego, CA, USA), Spearman’s correlation coefficients (rs) were calculated to examine potential associations between OPEs. Within SPSS Statistics 24 (IBM, Armonk, NY, USA), GLM (α = 0.05) was performed to determine whether total commute scores were predictive of log10-transformed concentrations for OPEs detected on at least 62 wristbands (70% detection rate). Based on these results, statistically significant OPEs were then reanalyzed within SPSS Statistics 24 (IBM, Armonk, NY, USA) using an adjusted GLM (α = 0.05) in order to correct for age as a covariate.
3. Results and discussion
3.1. Longer commutes are associated with older study participants
Demographic data for all study participants are included within Table S3. A summary of demographic data demonstrates that, for the most part, study participants (Fig. 1) represented demographics of UCR’s entire undergraduate population (Fig. S1). While our study did not recruit participants younger than 18 years old, 89% of participants were between 18 and 21 years old (Fig. 1A) – a characteristic that was similar to the age distribution within UCR’s undergraduate population (Fig. S1). On the other hand, the gender of our study population was skewed towards a higher proportion of females compared to males (Fig. 1B) – a characteristic that was not reflective of UCR’s undergraduate population (Fig. S1) – since there were fewer male participants who had an initial interest in our study despite our diverse recruitment methods. Similar to age, the distribution of study participant ethnicity (Fig. 1C) was similar to UCR’s undergraduate population (Fig. S1), where the top four ethnicities were Hispanic, Asian, White, and Multi-Racial. The “other” ethnicity category within the undergraduate population (Fig. S1) includes ethnicities that, despite various attempts to recruit campus-wide, were not represented within our final study population (Black or African American; Native Hawaiian or Pacific Islander; or Unknown). Finally, the average household income – either reported as parental or personal income from dependent or independent students, respectively – for our study population contained a higher proportion of students within the average household income of < $30,000 and $75,001-$110,000 groups (Fig. 1D), a characteristic that was not similar to UCR’s undergraduate population (Fig. S1).
Fig. 1.
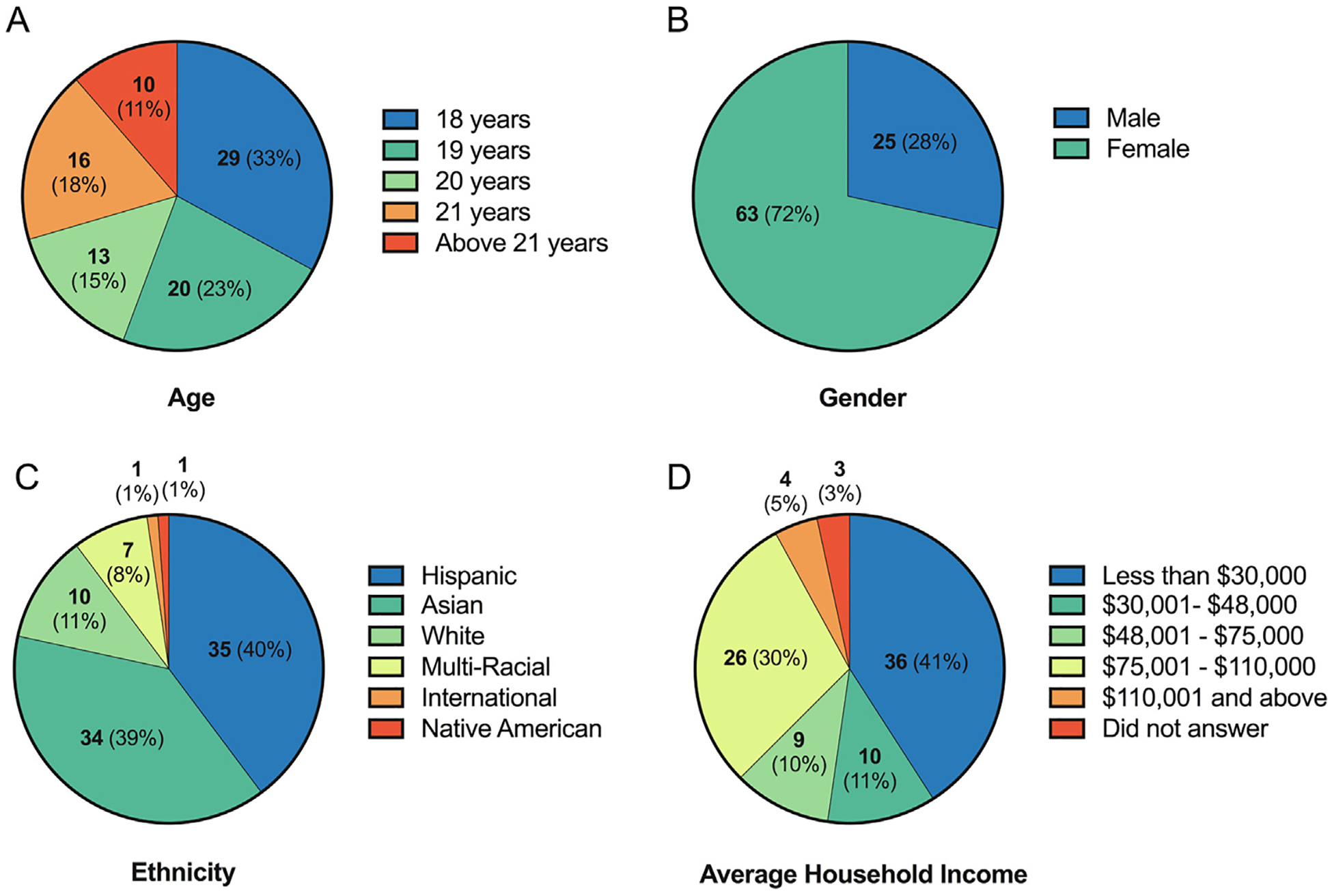
Demographics of study participants (N = 88) grouped by age (A), gender (B), ethnicity (C), or average household income (D). Bold numbers denote the number of participants within each category, and numbers within parentheses denote the percent of total study participants.
While the distribution of certain demographics was not identical to UCR’s undergraduate population, the total commute score of study participants did not vary by gender, ethnicity, nor household income (Fig. 2B, C, and D). As described within Section 2.3, total commute score was calculated based on the amount of time the participant spent in all forms of transportation – which was primarily personal vehicles – during each day of the study (Tables S4 and S5). Interestingly, total commute score was significantly (~2–3 times) higher among all age groups relative to participants that were 18 years old (Fig. 2A), a finding that was likely due to a larger number of 18-year-old freshman students that live within residential halls or are within walking distance to campus. As a result, this subset of the student population likely spends less time within personal vehicles during a typical week of the academic year.
Fig. 2.
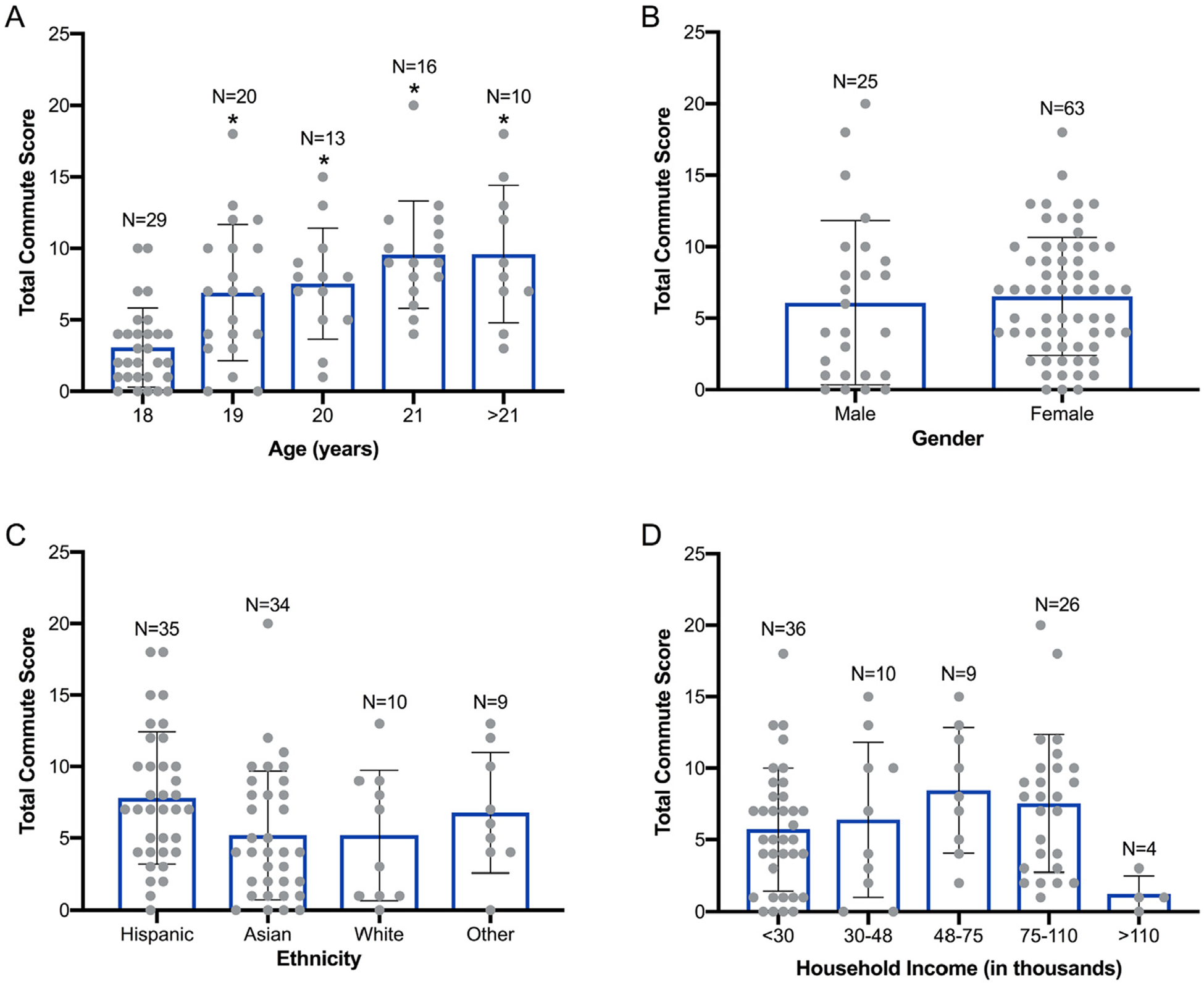
Total commute score of study participants grouped by age (A), gender (B), ethnicity (C), and household income (D). N = 88 for age, gender, and ethnicity; N = 85 for household income as a result of three participants not responding (Table S1). Asterisk (*) denotes significant difference (p < 0.05) relative to the 18-year-old age group within Panel A.
3.2. OPE mixtures detected on study participant wristbands are likely driven by overlapping use patterns
Descriptive statistics for all OPEs measured on study participant wristbands are provided within Table S6 and summarized within Fig. 3A. Although total OPE concentrations across all participants ranged from 0.06 to 7604 ng/g, the average concentration by OPE class did not significantly vary by commute score (Fig. 3B). Spearman’s correlation coefficients were calculated for OPEs detected on at least 62 wristbands (70% detection rate) (Fig. 4). Within the correlation matrix, OPEs were ranked by -log10(p-value) derived from unadjusted GLM-based analyses of total commute scores and OPE concentrations (Fig. 4 inset; Table S7), with TDCIPP representing the only OPE that was significantly affected by total commute score.
Fig. 3.
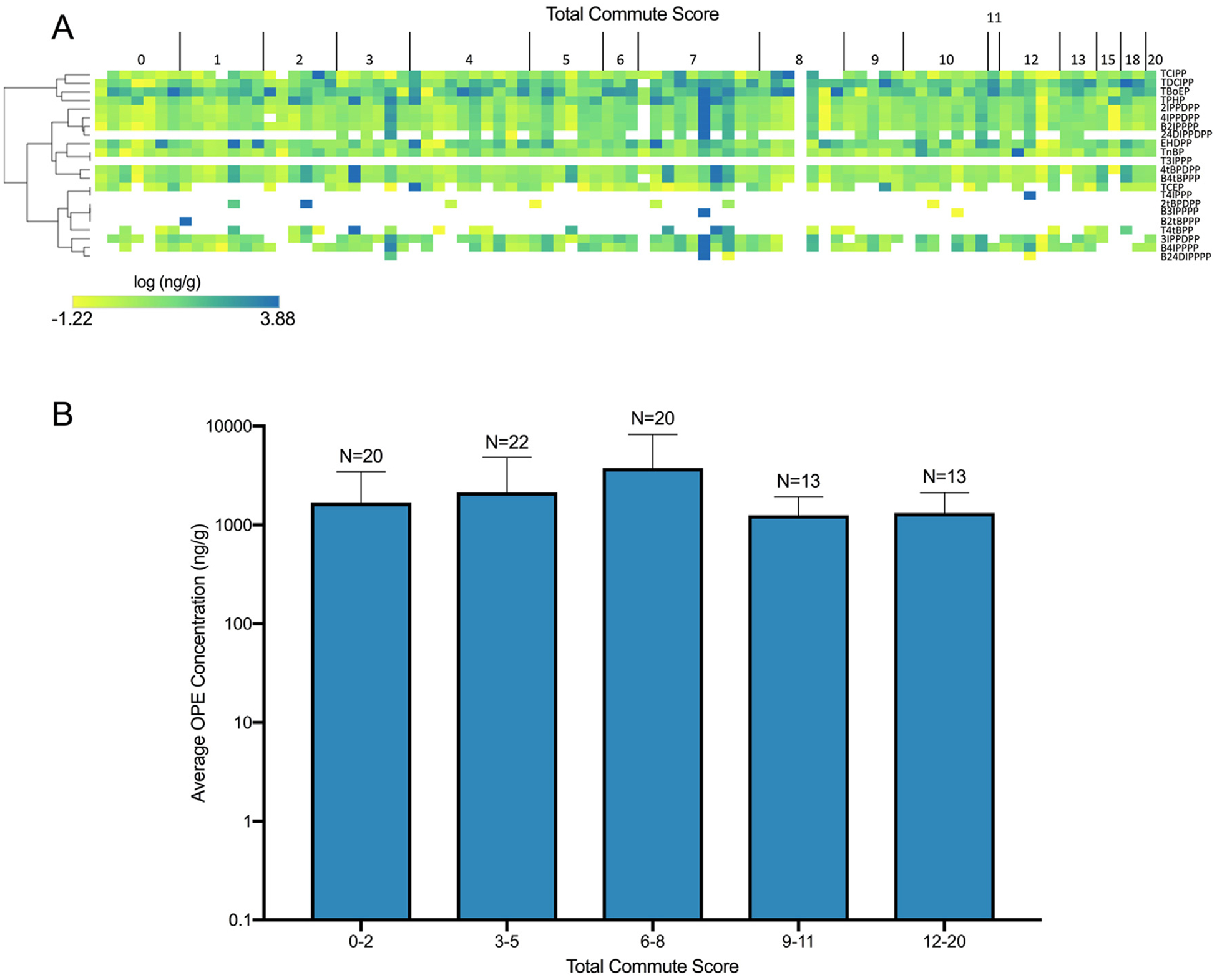
Heat map representing wristband concentrations of individual OPEs vs. total commute scores (A). OPE concentration data shown within the heat map were log10-transformed, and hierarchical clustering was performed using the Euclidean distance and complete linkage method. Average concentration (± standard deviation) of all OPEs and total commute score (B).
Fig. 4.
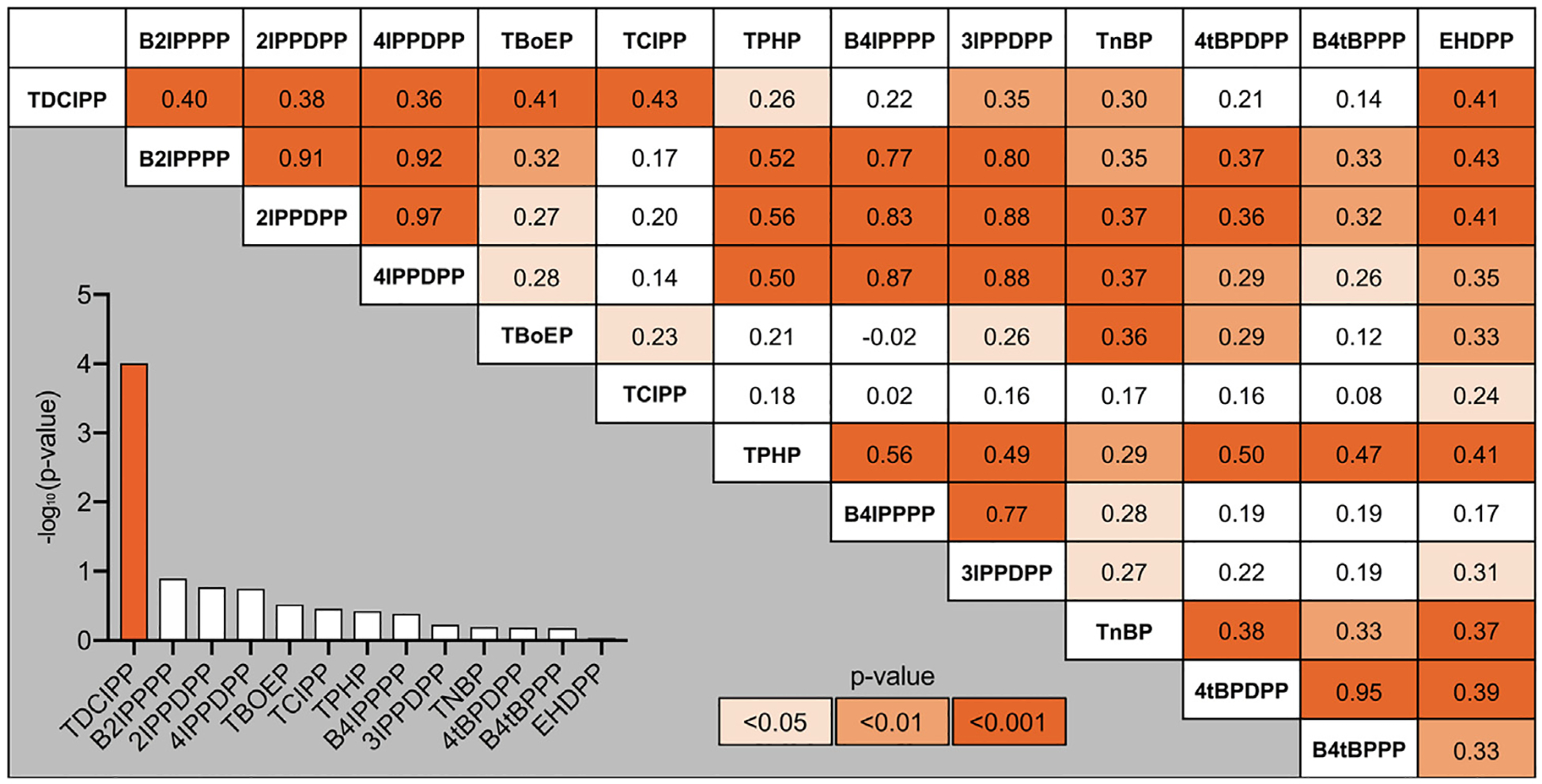
Correlation matrix showing Spearman’s correlation coefficients for OPEs with > 70% detection (at least 62 wristbands) relative to all study participants. OPEs within the matrix are ordered in descending order of −log10(p-value). Bar chart within the inset shows the −log10(p-value) derived from unadjusted GLM-based analyses of total commute scores and OPE concentrations. Darker orange denotes a lower p-value. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Not surprisingly, a large number of OPEs were positively correlated with each other, as multiple OPEs may be used within the same products as well as among different products within the same location. For example, OPEs found within Firemaster 550 (TPHP; 2-isopropylphenyl diphenyl phosphate, or 2IPPDPP; 3-isopropylphenyl diphenyl phosphate, or 3IPPDPP; 4-isopropylphenyl diphenyl phosphate, or 4IPPDPP; bis(2-isopropylphenyl) phenyl phosphate, or B2IPPPP; and bis(4-isopropylphenyl) phenyl phosphate, or B4IPPPP) (Phillips et al., 2017) – an additive flame retardant formulation introduced into automotive headliners as flame lamination and polyurethane foam – were positively correlated with each other (rs = 0.49–0.97), with isopropylated triaryl phosphate (ITP) isomers showing the strongest and most significant correlations with each other (rs = 0.77–0.97). Moreover, OPEs found within Firemaster 600 (TPHP; 4-tert-butylphenyl diphenyl phosphate, or 4tBPDPP; and bis(4-tert-butylphenyl) phenyl phosphate, or B4tBPPP) (Phillips et al., 2017) – a formulation with similar applications as Firemaster 550 – were correlated with each other (rs = 0.47–0.94), with tert-butylated triaryl phosphate (TBPP) isomers (B4tBPPP and 4tBPDPP) showing the strongest and most significant correlation with each other (rs = 0.94). Interestingly, 4tBPDPP (a component of Firemaster 600) was also positively correlated with B2IPPPP (rs = 0.37) and 2IPPDPP (rs = 0.36) (both components of Firemaster 550). Similarly, ITP and TBPP isomers within indoor dust are dominated by 2IPPDPP and 4tBPDPP, respectively (Guan et al., 2019; Phillips et al., 2018).
TDCIPP concentrations were also significantly and positively correlated with other OPEs detected on study participant wristbands (Fig. 4). In addition to Firemaster 550, TDCIPP was, until being added to California’s Proposition 65 List in 2011, one of the primary PentaBDE replacements recommended and used to meet flammability standards for upholstered furniture (i.e., California’s Technical Bulletin 117 and 117–2013) within residential and commercial environments (EPA, 2015). Therefore, a strong correlation of TDCIPP with certain Firemaster 550 components (4IPPDPP, B2IPPDPP and 21PPDPP) (rs = 0.36–0.40) suggests that TDCIPP may be applied to products located within the same environment as Firemaster 550-containing products (EPA, 2015; Stapleton et al., 2009). Finally, TDCIPP was significantly correlated with TCIPP (rs = 0.43), suggesting that, consistent with previously published studies (Stapleton et al., 2012, 2011), both OPEs are used within polyurethane foam-containing products (e.g., baby products and sofas) located within the same environment. Indeed, prior studies found that TDCIPP and TCIPP accounted for the majority of OPE mass in car dust, a finding that was likely due to co-application of both OPEs within vehicles (Brandsma et al., 2014; Brommer and Harrad, 2015; Christia et al., 2018; Zhou et al., 2017).
3.3. Longer commutes are predictive of increased TDCIPP concentrations on study participant wristbands
Out of 22 OPEs analyzed (Table S6), 13 OPEs were detected on at least 62 wristbands (70% detection rate) (Table S7). Among these 13 OPEs, TDCIPP was the only OPE that was significantly predicted by total commute score – even after adjusting for age as a covariate (Table S8) – suggesting that longer commutes are associated with increased human exposure to TDCIPP (Fig. 5). In general, OPE concentrations are higher in dust from vehicles compared to residential and commercial environments (Ali et al., 2016; Brandsma et al., 2014; Brommer and Harrad, 2015; Christia et al., 2018; Zhou et al., 2017), and TDCIPP is prevalent within dust of personal vehicles in the United Kingdom (Brommer et al., 2012). Moreover, Harrad et al. (2016) found that TDCIPP concentrations are consistently high within cars, suggesting that polyurethane foam may be a primary source of TDCIPP exposure within vehicles around the world. Indeed, TDCIPP is commonly detected within dashboard dust (Brandsma et al., 2014), suggesting that, similar to residential and commercial environments, TDCIPP readily migrates from vehicle components over time.
Fig. 5.
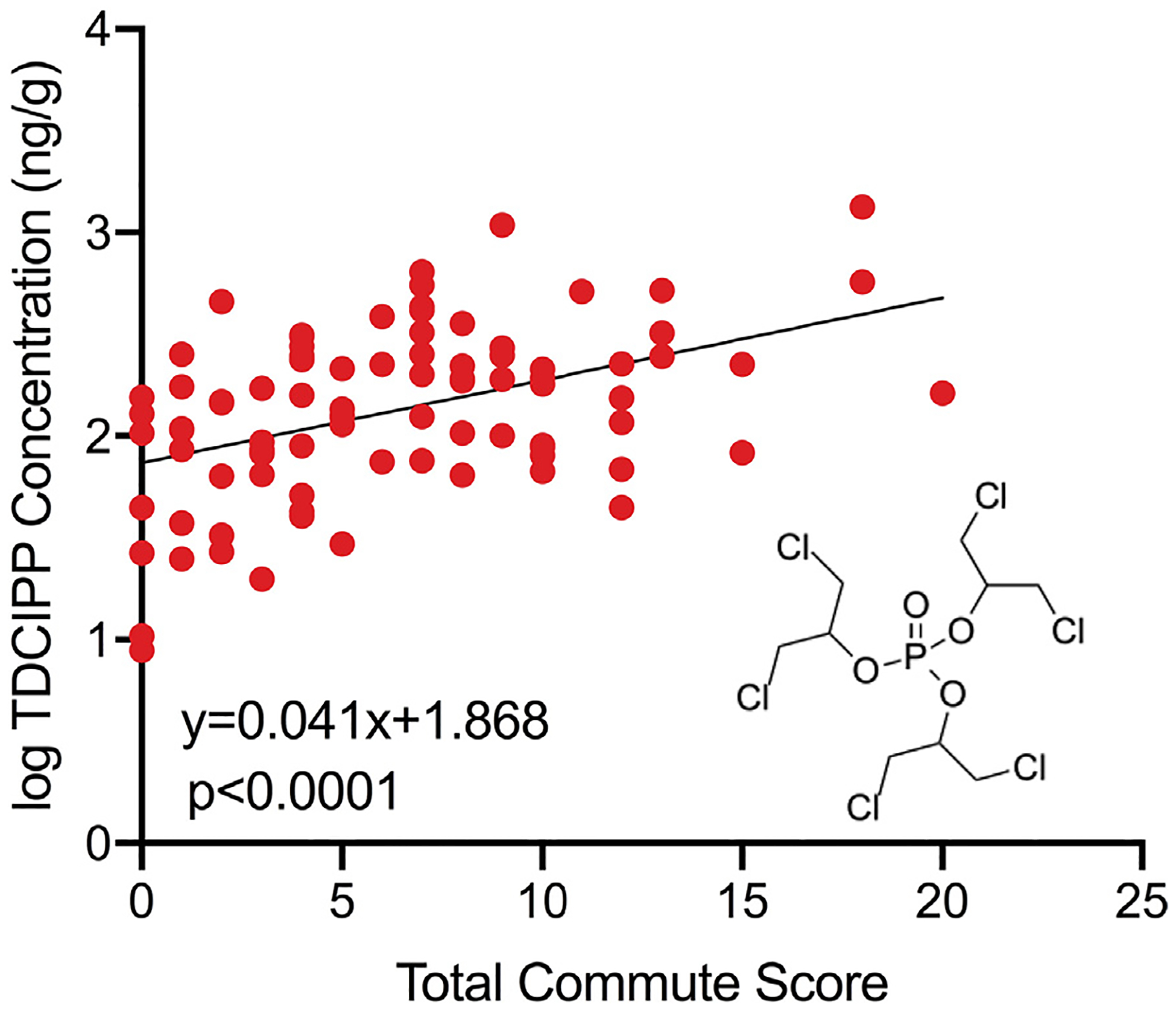
Log TDCIPP concentration as a function of total commute score. Corrected model p-value was derived from an adjusted GLM (α = 0.05) in order to correct for age as a covariate. N = 86 wristbands (out of 88 total) with detectable TDCIPP.
Following the addition of TDCIPP to California’s Proposition 65 List in 2011 and revision of Technical Bulletin 117 in 2013, the use of TDCIPP to comply with furniture-specific flammability standards has declined within California and across the United States (Cooper et al., 2016). However, as vehicles already contain numerous hazardous chemicals within California’s Proposition 65 List, there is a possibility that TDCIPP may continue to be added to permanently installed seats as well as plastic and electronics within a vehicle’s dashboard and console, respectively, within the United States in order to comply with a stringent, federal-level flammability standard (FMVSS No. 302) adopted by the () in 1971. As a result, compared to upholstered furniture within residential and commercial environments, the use and concentration of TDCIPP is likely higher in vehicle components, a recent trend that may explain why we identified a strong relationship between TDCIPP concentrations and total commute score even after adjusting for age.
4. Conclusions
In summary, our findings suggest that longer commutes are associated with increased human exposure to TDCIPP. While previous studies have detected high TDCIPP concentrations within vehicle dust samples, to our knowledge this is the first study to uncover a strong association between the amount of time spent in a vehicle and human TDCIPP exposure. Although we did not collect urine from participants within this study, silicone wristbands have previously been shown to be significantly correlated with urinary biomarkers of exposure for TDCIPP (Hammel et al., 2016), suggesting that urinary BDCIPP (the primary TDCIPP metabolite) concentrations may also be associated with longer commutes. However, since we didn’t measure TDCIPP nor other OPEs within vehicle dust, it is unknown whether vehicle dust was the primary source of TDCIPP and other OPEs detected within wristbands. Moreover, since we did not collect personal vehicle information (vehicle year, make, and model), it is unknown whether vehicle year, make, and/or model was significantly associated with TDCIPP concentrations and/or commute score.
As TDCIPP is a SVOC, the primary routes of exposure within vehicles and other forms of transportation are likely inhalation, ingestion, and transdermal permeation following dermal exposure to contaminated surfaces (Hou et al., 2016; Schreder and La Guardia, 2014; Weschler and Nazaroff, 2012). TDCIPP is known to disrupt epigenetic reprogramming, embryogenesis, behavior, liver development, and reproduction in zebrafish (Dasgupta et al., 2019, 2018, 2017; Jarema et al., 2015; Kupsco et al., 2017; Liu et al., 2016, 2013; McGee et al., 2012; Volz et al., 2016), and was added as a probable carcinogen on California’s Proposition 65 List. Moreover, TDCIPP has also been associated with abnormal pregnancy outcomes such as a decline in fertilization and a shorter gestational period (Carignan et al., 2018; Hoffman et al., 2018). Given that a large fraction of the human population within Southern California – as well as other densely populated regions across the United States – spend one or more hours commuting on a near-daily basis, our study raises concerns about the potential for chronic TDCIPP exposure within vehicles and possibly other forms of transportation. However, as many countries around the world predominately rely on public transportation rather than personal vehicles, future studies are needed to compare OPE exposure within study participants who primarily use personal vehicles vs. public transportation to commute.
Supplementary Material
Acknowledgments
We thank Dr. Ryan M. Johnson from the Office of Institutional Research at the University of California, Riverside for provided demographic data for UCR’s undergraduate student population.
Funding
This work was supported by a National Institutes of Health grant [R01ES027576] and USDA National Institute of Food and Agriculture Hatch Project [1009609] to D.C.V.
Footnotes
Declaration of Competing Interest
We declare that we have no conflict of interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.105499.
References
- Abdallah MA-E, Covaci A, 2014. Organophosphate flame retardants in indoor dust from Egypt: implications for human exposure. Environ. Sci. Technol 48, 4782–4789. 10.1021/es501078s. [DOI] [PubMed] [Google Scholar]
- Aerts R, Joly L, Szternfeld P, Tsilikas K, De Cremer K, Castelain P, Aerts J-M, Van Orshoven J, Somers B, Hendrickx M, Andjelkovic M, Van Nieuwenhuyse A, 2018. Silicone wristband passive samplers yield highly individualized pesticide residue exposure profiles. Environ. Sci. Technol 52, 298–307. 10.1021/acs.est.7b05039. [DOI] [PubMed] [Google Scholar]
- Ali N, Ali L, Mehdi T, Dirtu AC, Al-Shammari F, Neels H, Covaci A, 2013. Levels and profiles of organochlorines and flame retardants in car and house dust from Kuwait and Pakistan: implication for human exposure via dust ingestion. Environ. Int 55, 62–70. 10.1016/J.ENVINT.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Ali N, Eqani SAMAS, Ismail IMI, Malarvannan G, Kadi MW, Albar HMS, Rehan M, Covaci A, 2016. Brominated and organophosphate flame retardants in indoor dust of Jeddah, Kingdom of Saudi Arabia: implications for human exposure. Sci. Total Environ 569–570, 269–277. 10.1016/J.SCITOTENV.2016.06.093. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Points GL, Donald CE, Dixon HM, Scott RP, Wilson G, Tidwell LG, Hoffman PD, Herbstman JB, O’Connell SG, 2017. Preparation and performance features of wristband samplers and considerations for chemical exposure assessment. J. Expo. Sci. Environ. Epidemiol 27, 551–559. 10.1038/jes.2017.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki A, Saito I, Kanazawa A, Morimoto K, Nakayama K, Shibata E, Tanaka M, Takigawa T, Yoshimura T, Chikara H, Saijo Y, Kishi R, 2014. Phosphorus flame retardants in indoor dust and their relation to asthma and allergies of inhabitants. Indoor Air 24, 3–15. 10.1111/ina.12054. [DOI] [PubMed] [Google Scholar]
- Bergh C, Torgrip R, Emenius G, Östman C, 2011. Organophosphate and phthalate esters in air and settled dust - a multi-location indoor study. Indoor Air 21, 67–76. 10.1111/j.1600-0668.2010.00684.x. [DOI] [PubMed] [Google Scholar]
- Bergmann AJ, North PE, Vasquez L, Bello H, Del Carmen Gastañaga Ruiz M, Anderson KA, 2017. Multi-class chemical exposure in rural Peru using silicone wristbands. J. Expo. Sci. Environ. Epidemiol 27, 560–568. 10.1038/jes.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin P, Jones KC, Strandberg B, 2007. Occupational and indoor air exposure to persistent organic pollutants: a review of passive sampling techniques and needs. J. Environ. Monit 9, 501. 10.1039/b700627f. [DOI] [PubMed] [Google Scholar]
- Brandsma SH, de Boer J, van Velzen MJM, Leonards PEG, 2014. Organophosphorus flame retardants (PFRs) and plasticizers in house and car dust and the influence of electronic equipment. Chemosphere 116, 3–9. 10.1016/J.CHEMOSPHERE.2014.02.036. [DOI] [PubMed] [Google Scholar]
- Brommer S, Harrad S, 2015. Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ. Int 83, 202–207. 10.1016/J.ENVINT.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Brommer S, Harrad S, Van den Eede N, Covaci A, 2012. Concentrations of organophosphate esters and brominated flame retardants in German indoor dust samples. J. Environ. Monit 14, 2482. 10.1039/c2em30303e. [DOI] [PubMed] [Google Scholar]
- Carignan CC, Mínguez-Alarcón L, Williams PL, Meeker JD, Stapleton HM, Butt CM, Toth TL, Ford JB, Hauser R, EARTH Study Team, 2018. Paternal urinary concentrations of organophosphate flame retardant metabolites, fertility measures, and pregnancy outcomes among couples undergoing in vitro fertilization. Environ. Int 111, 232–238. 10.1016/j.envint.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson H, Nilsson U, Becker G, Östman C, 1997. Organophosphate ester flame retardants and plasticizers in the indoor environment: analytical methodology and occurrence. Environ. Sci. Technol 31, 2931–2936. 10.1021/ES970123S. [DOI] [Google Scholar]
- Christia C, Poma G, Besis A, Samara C, Covaci A, 2018. Legacy and emerging organophosphοrus flame retardants in car dust from Greece: implications for human exposure. Chemosphere 196, 231–239. 10.1016/J.CHEMOSPHERE.2017.12.132. [DOI] [PubMed] [Google Scholar]
- Cooper EM, Kroeger G, Davis K, Clark CR, Ferguson PL, Stapleton HM, 2016. Results from screening polyurethane foam based consumer products for flame retardant chemicals: assessing impacts on the change in the furniture flammability standards. Environ. Sci. Technol 50, 10653–10660. 10.1021/acs.est.6b01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Cheng V, Vliet SMF, Mitchell CA, Volz DC, 2018. Tris(1,3-dichloro-2-propyl) Phosphate Exposure During the Early-Blastula Stage Alters the Normal Trajectory of Zebrafish Embryogenesis. Environ. Sci. Technol 52, 10820–10828. 10.1021/acs.est.8b03730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Vliet SM, Kupsco A, Leet JK, Altomare D, Volz DC, 2017. Tris(1,3-dichloro-2-propyl) phosphate disrupts dorsoventral patterning in zebrafish embryos. PeerJ 5, e4156. 10.7717/peerj.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Vliet SMF, Cheng V, Mitchell CA, Kirkwood J, Vollaro A, Hur M,Mehdizadeh C, Volz DC, 2019. Complex Interplay Among Nuclear Receptor Ligands, Cytosine Methylation, and the Metabolome in Driving Tris(1,3-dichloro-2-propyl)phosphate-Induced Epiboly Defects in Zebrafish. Environ. Sci. Technol 53, 10497–10505. 10.1021/acs.est.9b04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon HM, Scott RP, Holmes D, Calero L, Kincl LD, Waters KM, Camann DE, Calafat AM, Herbstman JB, Anderson KA, 2018. Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal. Bioanal. Chem 410, 3059–3071. 10.1007/s00216-018-0992-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, U.S., 2015. Flame Retardants Used in Flexible Polyurethane Foam: An Alternatives Assessment Update. < https://www.epa.gov/sites/production/files/2015-08/documents/ffr_final.pdf >.
- Guan Q, Tan H, Yang L, Liu X, Fiedler H, Li X, Chen D, 2019. Isopropylated and tert-butylated triarylphosphate isomers in house dust from South China and Midwestern United States. Sci. Total Environ 686, 1113–1119. 10.1016/J.SCITOTENV.2019.06.055. [DOI] [PubMed] [Google Scholar]
- Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM, 2016. Measuring Personal Exposure to Organophosphate Flame Retardants Using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol 50, 4483–4491. 10.1021/acs.est.6b00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel SC, Phillips AL, Hoffman K, Stapleton HM, 2018. Evaluating the use of silicone wristbands to measure personal exposure to brominated flame retardants. Environ. Sci. Technol 52, 11875–11885. 10.1021/acs.est.8b03755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrad S, Brommer S, Mueller JF, 2016. Concentrations of organophosphate flame retardants in dust from cars, homes, and offices: an international comparison. Emerg. Contam 2, 66–72. 10.1016/J.EMCON.2016.05.002. [DOI] [Google Scholar]
- Hartmann PC, Bürgi D, Giger W, 2004. Organophosphate flame retardants and plasticizers in indoor air. Chemosphere 57, 781–787. 10.1016/J.CHEMOSPHERE.2004.08.051. [DOI] [PubMed] [Google Scholar]
- He C-T, Zheng J, Qiao L, Chen S-J, Yang J-Z, Yuan J-G, Yang Z-Y, Mai B-X, 2015. Occurrence of organophosphorus flame retardants in indoor dust in multiple microenvironments of southern China and implications for human exposure. Chemosphere 133, 47–52. 10.1016/j.chemosphere.2015.03.043. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Stapleton HM, Lorenzo A, Butt CM, Adair L, Herring AH, Daniels JL, 2018. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environ. Int 116, 248–254. 10.1016/J.ENVINT.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou R, Xu Y, Wang Z, 2016. Review of OPFRs in animals and humans: absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere. 10.1016/j.chemosphere.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S, 2015. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol 52, 194–209. 10.1016/j.ntt.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Scott RP, O’Connell SG, Lipscomb S, MacDonald M, McClelland M, Anderson KA, 2016. Using silicone wristbands to evaluate preschool children’s exposure to flame retardants. Environ. Res 147, 365–372. 10.1016/J.ENVRES.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsco A, Dasgupta S, Nguyen C, Volz DC, 2017. Dynamic Alterations in DNA Methylation Precede Tris(1,3-dichloro-2-propyl)phosphate-Induced Delays in Zebrafish Epiboly. Environ. Sci. Technol. Lett 4, 367–373. 10.1021/acs.estlett.7b00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S, Fredricsson M, Weschler CJ, Bekö G, Strandberg B, Remberger M,Toftum J, Clausen G, 2016. Organophosphate esters in dust samples collected from Danish homes and daycare centers. Chemosphere 154, 559–566. 10.1016/J.CHEMOSPHERE.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Liu C, Su G, Giesy JP, Letcher RJ, Li G, Agrawal I, Li J, Yu L, Wang J, Gong Z, 2016. Acute Exposure to Tris(1,3-dichloro-2-propyl) Phosphate (TDCIPP) Causes Hepatic Inflammation and Leads to Hepatotoxicity in Zebrafish. Sci. Rep 6, 19045. 10.1038/srep19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ji K, Jo A, Moon H-B, Choi K, 2013. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio). Aquat. Toxicol 134–135, 104–111. 10.1016/j.aquatox.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Marklund A, Andersson B, Haglund P, 2003. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 53, 1137–1146. 10.1016/S0045-6535(03)00666-0. [DOI] [PubMed] [Google Scholar]
- McGee SP, Cooper EM, Stapleton HM, Volz DC, 2012. Early Zebrafish embryogenesis is susceptible to developmental TDCPP exposure. Environ. Health Perspect 120, 1585–1591. 10.1289/ehp.1205316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LL, Barr DB, Calafat AM, 2005. Characterizing children’s exposures: beyond NHANES. Neurotoxicology 26, 547–553. 10.1016/J.NEURO.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Nethery E, Wheeler AJ, Fisher M, Sjödin A, Li Z, Romanoff LC, Foster W,Arbuckle TE, 2012. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. J. Expo. Sci. Environ. Epidemiol 22, 70–81. 10.1038/jes.2011.32. [DOI] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Hoffman K, Lorenzo AM, Chen A, Webster TF, Stapleton HM, 2018. Children’s residential exposure to organophosphate ester flame retardants and plasticizers: investigating exposure pathways in the TESIE study. Environ. Int 116, 176–185. 10.1016/J.ENVINT.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Hammel SC, Konstantinov A, Stapleton HM, 2017. Characterization of Individual Isopropylated and tert-Butylated Triarylphosphate (ITP and TBPP) Isomers in Several Commercial Flame Retardant Mixtures and House Dust Standard Reference Material SRM 2585. Environ. Sci. Technol 51, 13443–13449. 10.1021/acs.est.7b04179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreder ED, La Guardia MJ, 2014. Flame retardant transfers from U.S. households (dust and laundry wastewater) to the aquatic environment. Environ. Sci. Technol 10.1021/es502227h. [DOI] [PubMed] [Google Scholar]
- Sirotnik B, Aldana L, 2018. 21st Inland Empire Annual Survey Report. < https://jhbc.csusb.edu/sites/csusb_jhbc/files/AnnualFullReportSENT.pdf >.
- Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF, 2009. Detection of organophosphate flame retardants in furniture foam and U.S. House Dust. Environ. Sci. Technol 43, 7490–7495. 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, Webster TF, Blum A, 2011. Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol 45, 5323–5331. 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A, 2012. Novel and high volume use flame retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ. Sci. Technol 46, 13432–13439. 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai P-J, Vincent JH, 2001. A study of workers’ exposures to the inhalable and ‘Total’ aerosol fractions in the primary nickel production industry using mannequins to simulate personal sampling. Ann. Occup. Hyg 45, 385–394. 10.1016/S0003-4878(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Volz DC, Leet JK, Chen A, Stapleton HM, Katiyar N, Kaundal R, Yu Y, Wang Y, 2016. Tris(1,3-dichloro-2-propyl)phosphate Induces Genome-Wide Hypomethylation within Early Zebrafish Embryos. Environ. Sci. Technol 50, 10255–10263. 10.1021/acs.est.6b03656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Romanak KA, Stubbings WA, Arrandale VH, Hendryx M, Diamond ML, Salamova A, Venier M, 2019. Silicone wristbands integrate dermal and inhalation exposures to semi-volatile organic compounds (SVOCs). Environ. Int 132, 105104. 10.1016/J.ENVINT.2019.105104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G-L, Li D-Q, Zhuo M-N, Liao Y-S, Xie Z-Y, Guo T-L, Li J-J, Zhang S-Y, Liang Z-Q, 2015. Organophosphorus flame retardants and plasticizers: sources, occurrence, toxicity and human exposure. Environ. Pollut 196, 29–46. 10.1016/J.ENVPOL.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Weschler CJ, Nazaroff WW, 2012. SVOC exposure indoors: fresh look at dermal pathways. Indoor Air 22, 356–377. 10.1111/j.1600-0668.2012.00772.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Hiltscher M, Gruber D, Püttmann W, 2017. Organophosphate flame retardants (OPFRs) in indoor and outdoor air in the Rhine/Main area, Germany: comparison of concentrations and distribution profiles in different microenvironments. Environ. Sci. Pollut. Res 24, 10992–11005. 10.1007/s11356-016-6902-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


