Abstract
Introduction:
Understanding temporal and anatomic patterns of lung cancer recurrence could guide disease management and monitoring. However, these data are not available in population-based datasets and are not routinely recorded in clinical trials.
Methods:
We identified stage 1-3 lung cancer cases diagnosed January 1, 2000, to December 31, 2017, in the tumor registry of a National Cancer Institute-designated comprehensive cancer center. For cases with documented disease recurrence, we recorded anatomic site(s) and timing. We estimated time to recurrence using Kaplan-Meier methods. Associations between case characteristics and recurrence features were assessed using univariable and multivariable logistic regression models and Cox regression models.
Results:
1,619 stage 1-3 lung cancer cases from 1,549 patients were included in the analysis. Of these, 466 patients (30%) developed recurrent lung cancer. The most common type of first recurrence was distant disease, most commonly central nervous system (CNS) (37%). In multivariable analyses, race (P=0.02) and primary treatment modality (P<0.001) correlated with recurrent disease, while tumor histology (P=0.004), and primary treatment modality (P<0.001) were associated specifically with distant recurrence. Patient age (P=0.05) and initial TNM stage (P=0.001) correlated with timing of recurrence.
Conclusion:
In this single-center series of stage 1-3 lung cancer, recurrent disease was associated with race, histology and treatment modality, and most commonly occurred in the CNS. Modulation of clinical and radiographic disease monitoring according to recurrence risk, timing, and site may offer a means to identify future lung cancer when it remains asymptomatic and highly treatable.
Keywords: latency, metastases, monitoring, surveillance
MicroAbstract
Timing and site of lung cancer recurrence are largely unknown. We reviewed 1,619 stage 1-3 lung cancer cases, and analyzed risk, timing, and site of recurrence. We found that distant recurrence occurred earlier than local or regional recurrence, and that the central nervous system was the most common site of recurrence. These findings have implications for disease monitoring and management.
Introduction
Despite advances in screening, detection, molecular classification, and therapy, lung cancer accounts for almost one-fourth of all cancer-related deaths in the U.S., more than breast, prostate, and colorectal cancer combined.1,2 Contributing to these poor outcomes is the nature of presentation, with more than 70% diagnosed initially with metastatic disease.3 Even with aggressive, multi-modality therapy, a substantial proportion of individuals who initially present with localized or locoregional disease eventually succumb to recurrent malignancy.
Given the aging of the U.S. population and the uptake of lung cancer screening, the number of lung cancer cases eligible for and receiving definitive, potentially curative therapy is expected to grow. For this population, which achieves a clinical disease-free state, a thorough understanding of recurrence patterns is essential to optimize post-treatment monitoring. However, to date, obtaining information on patterns of lung cancer recurrence has been hampered by the lack of relevant information in population-based datasets and clinical trials. Surveillance Epidemiology and End Results (SEER), the National Cancer Database (NCDB), and the Veterans Affairs (VA) database do not capture recurrence data such as site or timing. Earlier publications that did characterize disease recurrence focused almost exclusively on stage 1 non-small cell lung cancer (NSCLC),4–6 which accounts for fewer than 20% of all cases.7
Because the nature of disease recurrence has potential relevance to patient management and monitoring, we determined the type and timing of recurrent lung cancer in a diverse population treated at a regional cancer center.
Materials and Methods
Data Extraction
This study was approved by the University of Texas Southwestern Medical Center at Dallas (UT Southwestern) Institutional Review Board (STU 042018-102). Within the UT Southwestern Tumor Registry (which is credentialed by the American College of Surgeons and the American Society of Clinical Oncology), we identified individuals with initial stage 1-3 lung cancer diagnosis between January 1, 2000, and December 31, 2017. We selected this time interval because the relevant clinical data was first consistently collected by the registry in 2000, and because the 2017 cut-off provided at least one-year follow-up for the most recently diagnosed cases. We excluded cases that (1) had incomplete disease information, or (2) never achieved a disease-free state. When necessary, we reviewed individual medical records to clarify registry data abstraction.
Recording and Definition of Variables
For each case, we recorded demographics (sex, age, race/ethnicity), primary tumor characteristics (diagnosis date, tumor-nodes-metastasis [TNM] stage, histology), and treatment modality. Race/ethnicity was categorized as Caucasian, African American, and other. Treatment was categorized as local (surgery and/or radiation therapy) or systemic (chemotherapy alone or in combination with surgery and/or radiation therapy). The UT Southwestern Tumor Registry distinguished second and third primary cases from recurrence based on histology, topography and timing in accordance with NCI SEER guidelines.8 Recurrence was categorized by tumor registrars as local, regional or distant recurrence based on the North American Association of Central Registries (NAACCR), ACoS – Commission on Cancer and SEER/NCI guidelines as outlined in the Standards for Oncology Registry Entry (STORE) Manual.9 These categories were defined as follows: local, recurrence in initial primary organ; regional, recurrence in adjacent organ or lymph nodes draining the organ; distant, recurrence in a location beyond regional. For cases with subsequent recurrence, we recorded timing, type (local, regional, distant), and anatomic site. We characterized initial site of distant recurrence as lung (if contralateral to the primary tumor), bone, central nervous system, liver, multiple, and other (distant lymph nodes, skin, peritoneum, pleura, generalized not otherwise specified [NOS], and other).
Statistical Analysis
We generated descriptive statistics (medians and means for continuous variables and percentages for discrete variables) for baseline demographic, tumor, and initial treatment characteristics. We categorized tumor histology as adenocarcinoma, squamous cell carcinoma, non-small cell/other, and small cell. We reported summary statistics for patient characteristics using counts and percentages for categorical variables and using medians and interquartile ranges (IQR) for continuous variables. Univariable and multivariable logistic regression models were used to assess the association between recurrence and case characteristics. Variables with a univariable P-value of ≤0.2 were entered in a backward selection algorithm to yield the parsimonious multivariable regression model. When we did not identify differences among the four histology categories, we merged them as small cell vs. other to assess statistical relevance. Time to recurrence was estimated using Kaplan-Meier methods. Median time to recurrence and 95% confidence intervals (CI) were reported. Univariable and multivariable Cox regression models were used to assess the association between time to recurrence and patient characteristics. Odds ratios (OR) with 95% CI and hazard ratios (HR) with 95% CI were reported. Two-sided P-values were reported. A P-value <0.05 was considered statistically significant. All data analysis was performed using SAS 9.4 (SAS Institute, NC).
Results
Study Cohort
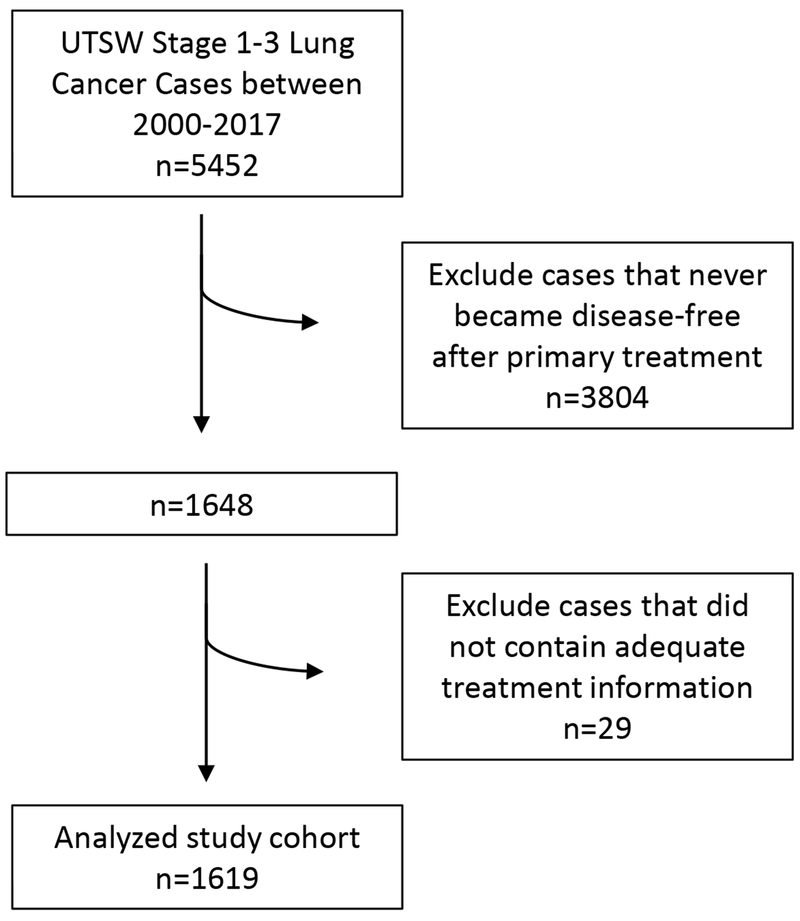
A total of 1,619 stage 1-3 lung cancer cases from 1,549 patients met inclusion for analysis (Figure 1). These included 1,481 primary lung cancer cases, 132 second primary lung cancer cases, and six third primary lung cancer cases. Within the overall cohort, approximately half were female, half age ≤65 years, and half stage 1. Additional case characteristics are listed in Table 1. A total of 130 cases underwent testing for genomic alterations, of which 11 were found to have a driver mutation.
Figure 1.
CONSORT diagram
Table 1.
Case characteristics.
| Characteristic | N (%) | |
|---|---|---|
| Primary Cancer | 1 | 1481 (92) |
| 2 | 132 (8) | |
| 3 | 6 (0) | |
| Gender | Male | 798 (49) |
| Female | 821 (51) | |
| Age at Dx | <= 65 | 780 (48) |
| >= 65 | 829 (52) | |
| Race | Caucasian | 1213 (75) |
| African American | 250 (15) | |
| Other | 156 (10) | |
| TNM stage | 1 | 856 (53) |
| 2 | 255 (16) | |
| 3 | 367 (23) | |
| Missing | 141 (8) | |
| Histology | Adenocarcinoma | 838 (52) |
| Squamous | 361 (22) | |
| Small cell | 88 (5) | |
| Non-small cell, other | 332 (21) | |
| Primary treatment | Local only | 980 (61) |
| Local + systemic | 639(39) | |
| Recurrence | Yes | 487 (30) |
| No | 1086 (67) | |
| Unknown | 46 (3) | |
| Type of first recurrence | No recurrence | 1086 (67) |
| Local | 108 (7) | |
| Regional | 103 (6) | |
| Distant | 273 (17) | |
| Unknown | 49 (3) | |
| Site of first distant recurrence | Bone | 31 (11) |
| CNS | 102 (37) | |
| Liver | 12 (4) | |
| Lung | 14 (5) | |
| Other | 93 (34) | |
| Multiple | 21 (8) | |
Lung Cancer Recurrence and Timing
Of the 1,619 analyzed cases, 487 (30%) had recurrent lung cancer. In univariate analysis, age at diagnosis, race, tumor stage, histology, and primary treatment modality were significantly associated with development of recurrent disease. Recurrence developed in 35% of individuals age ≤65 years, compared to 26% for age >65 years (P<0.001). African American patients had 25% rate of recurrence, compared to 31% for Caucasian, and 38% for other (P=0.02). Recurrence developed in 22% of stage 1 cases, 33% of stage 2 cases, and 40% of stage 3 cases (P<0.001). By histology, recurrence rates were 30% for non-small cell and 53% for small cell (P<0.001). Among cases receiving systemic chemotherapy, 45% had recurrence, compared to 22% for those receiving local therapy alone (P<0.001). In multivariable analysis, likelihood of recurrence remained significantly associated with receipt of chemotherapy and race (Table 2). Specifically, African American patients and those patients not administered chemotherapy had lower likelihood of disease recurrence. To determine whether competing causes of mortality accounted for lower recurrence rates among African American patients, we examined vital status of individuals not developing recurrent disease. Notably, mortality rate was lower among African American patients (29%) than among Caucasian patients (39%).
Table 2.
Univariable and multivariable analyses of disease recurrence. Recurrence (Yes/No) by all patients.
| # Recurrence | UVA | MVA with all variables | MVA after model selection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | OR (95% CI) | p | Overall p | OR (95% CI) | p | Overall p | OR (95% CI) | p | Overall p | |
| Gender | |||||||||||
| Male | 527 | 247 | Reference | 0.42 | Reference | 0.71 | not used in MVA | ||||
| Female | 559 | 240 | 0.92 (0.74 - 1.13) | 0.420 | 0.96 (0.75 - 1.21) | 0.71 | |||||
| Age | |||||||||||
| <= 65 | 495 | 268 | 1.51 (1.22 - 1.88) | <0.001 | <0.001 | 1.21 (0.95 - 1.55) | 0.13 | 0.13 | removed in model selection | ||
| >= 65 | 589 | 211 | Reference | Reference | |||||||
| Race | |||||||||||
| Caucasian | 810 | 370 | Reference | 0.02 | Reference | 0.03 | Reference | 0.02 | |||
| African American | 182 | 60 | 0.72 (0.53 - 0.99) | 0.044 | 0.67 (0.47 - 0.95) | 0.02 | 0.68 (0.49 - 0.94) | 0.020 | |||
| Other | 94 | 57 | 1.33 (0.93 - 1.89) | 0.114 | 1.28 (0.87 - 1.88) | 0.22 | 1.24 (0.86 - 1.79) | 0.240 | |||
| TNM Staging | |||||||||||
| 1 | 651 | 186 | Reference | <0.001 | Reference | 0.60 | removed in model selection | ||||
| 2 | 168 | 81 | 1.69 (1.24 - 2.30) | 0.001 | 1.10 (0.77 - 1.56) | 0.61 | |||||
| 3 | 214 | 140 | 2.29 (1.75 - 2.99) | <0.001 | 1.20 (0.85 - 1.69) | 0.31 | |||||
| Histology | |||||||||||
| Small cell | 39 | 44 | 2.67 (1.71 - 4.16) | <0.001 | <0.001 | 1.428 (0.819 - 2.490) | 0.21 | 0.21 | removed in model selection | ||
| Other | 1047 | 443 | Reference | Reference | |||||||
| Primary Treatment | |||||||||||
| Local therapy alone | 742 | 211 | 0.35 (0.28 - 0.44) | <0.001 | <0.001 | 0.42 (0.31 - 0.58) | <0.001 | <0.001 | 0.35 (0.28 - 0.44) | <0.001 | <0.001 |
| Systemic therapy included | 344 | 276 | Reference | Reference | Reference | ||||||
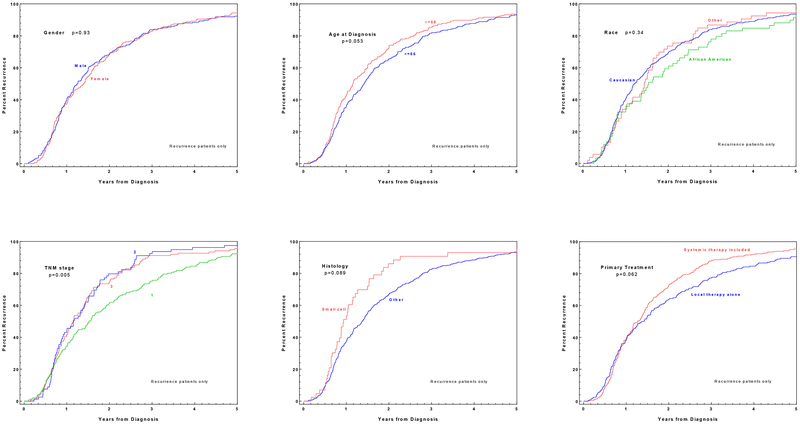
By five years after diagnosis, more than 90% of eventual recurrences had occurred (Figure 2). This time-course differed according to case characteristics. For stage 2 and 3 lung cancer, 80% of eventual recurrences had occurred by two years, whereas only 60% of stage 1 recurrences had occurred by that time-point. More than 90% of small cell recurrences had occurred by two years, compared to approximately 60% of NSCLC recurrences. Interestingly, timing of recurrence was essentially identical between cases treated with and without chemotherapy for the first 18 months, then subsequently separated. When considering all analyzed cases, race (P=0.001), TNM stage (P=0.03), histology (P=0.02), and primary treatment modality (P<0.001) significantly correlated with recurrence timing in multivariable analyses (Supplemental Table 1). Analyzing only those cases with disease recurrence, increasing age at diagnosis (P=0.05) and TNM stage (P=0.001) were significantly associated with timing of cancer recurrence (Supplemental Table 2).
Figure 2.
Time to recurrence according to case characteristics (among cases with eventual recurrence)
Type and Site of First Recurrence
The most common type of first recurrence was distant disease (56%). Among cases with recurrence, distant disease developed in 79% of small cell cases, compared to 54% of NSCLC cases. Distant disease was also more common in cases receiving systemic chemotherapy (60% of recurrences) than in cases treated with local therapy alone (51% of recurrences). In multivariable analysis, recurrence type remained significantly associated with histology (P=0.004) and primary treatment modality (P<0.001) (Table 3). The most common site of distant disease was central nervous system (37%), followed by other (34%), bone (11%), multiple (8%), lung (contralateral) (5%), and liver (4%) Because small case numbers precluded calculation of odds ratios for several case characteristics, we incorporated liver metastases into the “other” category in our multivariable analysis. In that analysis, specific site of distant recurrence was associated with histology (p=0.004), and primary treatment (p<0.001) (Table 4). Among cases with distant recurrence, 34% were brain metastases for adenocarcinoma histology, versus 16% for squamous histology. Regarding lymph node recurrence, 103 cases were categorized as regional, and six cases were categorized as distant. Supplemental Figure 1 displays the timing of disease recurrence according to type and site. We observed a near-significant trend with regional and distant recurrences developing sooner than local recurrences. Approximately 75% of brain metastatic recurrences developed within two years after diagnosis.
Table 3.
Type of first recurrence.
| # Cases | MVA after model selection |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Local | Regional | Distant | Overall p | ||||||||||
| Local | Regional | Distant | No Rec. | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||||
| Gender | |||||||||||||
| Male | 52 | 56 | 136 | 527 | not used in MVA | ||||||||
| Female | 56 | 47 | 137 | 559 | |||||||||
| Age at Dx | |||||||||||||
| <= 65 | 55 | 57 | 157 | 495 | removed in model selection | ||||||||
| >= 66 | 52 | 44 | 111 | 589 | |||||||||
| Race | |||||||||||||
| Caucasian | 83 | 83 | 202 | 810 | removed in model selection | ||||||||
| African American | 12 | 12 | 35 | 182 | |||||||||
| Other | 13 | 8 | 36 | 94 | |||||||||
| TNM stage | |||||||||||||
| 1 | 50 | 43 | 90 | 651 | removed in model selection | ||||||||
| 2 | 14 | 23 | 44 | 168 | |||||||||
| 3 | 27 | 27 | 86 | 214 | |||||||||
| Histology | |||||||||||||
| Small cell | 7 | 2 | 34 | 39 | 1.21 (0.51 - 2.836) | 0.67 | 0.33 (0.08 - 1.39) | 0.13 | 2.17 (1.31 - 3.58) | 0.003 | 0.004 | ||
| Other | 101 | 101 | 239 | 1047 | Reference | Reference | Reference | ||||||
| Primary treatment | |||||||||||||
| Local therapy alone | 53 | 50 | 108 | 742 | 0.46 (0.30 - 0.66) | <0.001 | 0.41 (0.27 - 0.62) | <0.001 | 0.34 (0.25 - 0.45) | <0.001 | <0.001 | ||
| Systemic therapy included | 55 | 53 | 165 | 344 | Reference | Reference | Reference | ||||||
Table 4.
Site of first distant metastasis.
| # Cases | MVA after model selection |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone | CNS | Lung | Multiple | Overall p | ||||||||||
| Bone | CNS | Lung | Multiple | Other | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Gender | ||||||||||||||
| Male | 18 | 42 | 6 | 11 | 59 | removed in model selection | ||||||||
| Female | 13 | 60 | 8 | 10 | 46 | |||||||||
| Age at Dx | ||||||||||||||
| <= 65 | 12 | 66 | 9 | 11 | 59 | removed in model selection | ||||||||
| >= 66 | 19 | 33 | 5 | 10 | 44 | |||||||||
| Race | ||||||||||||||
| Caucasian | 26 | 78 | 9 | 12 | 77 | Reference | Reference | Reference | 0.024 | |||||
| African American | 2 | 12 | 3 | 1 | 17 | 0.15 (0.02 - 1.20) | 0.074 | 0.71 (0.28 - 1.84) | 0.487 | 1.36 (0.31 - 6.03) | 0.683 | 0.52 (0.058 - 4.67) | 0.562 | |
| Other | 3 | 12 | 2 | 8 | 11 | 1.31 (0.29 - 5.94) | 0.728 | 1.84 (0.65 - 5.22) | 0.249 | 2.86 (0.47 - 17.38) | 0.254 | 10.73 (2.71 - 42.52) | 0.001 | |
| TNM stage | ||||||||||||||
| 1,2 | 26 | 39 | 12 | 13 | 44 | Reference | Reference | Reference | 0.001 | |||||
| 3 | 2 | 37 | 2 | 3 | 42 | 0.08 (0.02 - 0.38) | 0.001 | 0.95 (0.49 - 1.85) | 0.874 | 0.18 (0.04 - 0.87) | 0.033 | 0.20 (0.05 - 0.81) | 0.025 | |
| Histology | ||||||||||||||
| Adenocarcinoma | 20 | 43 | 7 | 8 | 49 | Reference | Reference | Reference | #### | |||||
| Squamous | 6 | 7 | 6 | 4 | 22 | 0.85 (0.27 - 2.66) | 0.776 | 0.391 (0.140 - 1.089) | 0.073 | 2.52 (0.70 - 9.07) | 0.158 | 1.86 (0.44 - 7.95) | 0.402 | |
| Small cell | 1 | 17 | 0 | 1 | 15 | 0.47 (0.050 - 4.48) | 0.511 | 1.217 (0.433 - 3.418) | 0.708 | #### | #### | 0.64 (0.06 - 7.17) | 0.721 | |
| Non-small cell, other | 4 | 35 | 1 | 8 | 19 | 1.02 (0.27 - 3.85) | 0.975 | 2.955 (1.269 - 6.876) | 0.012 | 0.72 (0.08 - 6.62) | 0.769 | 3.29 (0.74 - 14.66) | 0.118 | |
| Primary treatment | ||||||||||||||
| Local therapy alone | 18 | 30 | 9 | 12 | 39 | removed in model selection | ||||||||
| Systemic therapy included | 13 | 72 | 5 | 9 | 66 | |||||||||
Discussion
While national cancer registries provide detailed data on staging, treatment, and survival of tens of thousands of cancer cases annually, they fail to capture information on the timing, type, and site of disease recurrence. Nor do clinical trials routinely report these data. In the present study, we therefore characterized these disease factors in a diverse lung cancer population treated at a regional cancer center. In this analysis of more than 1,500 stage 1-3 lung cancer cases that achieved a disease-free state after initial therapy, approximately one-third developed recurrent lung cancer. Overall, distant metastases occurred more frequently than local or regional recurrence, with brain metastases the single most common site. Distant metastases also appeared to occur earlier than other recurrence patterns.
There are a number of potential explanations for these findings. It is possible that distant metastases had already occurred prior to local therapy, but were not detected due to inadequate initial staging, consistent with the early dissemination and parallel progression model of cancer growth.10 Clearly, subclinical microscopic disease represents a reasonable likelihood, as a 1 cm tumor nodule represents approximately 109 cancer cells and the result of 40 generational doublings.11 Thus, even with highly sensitive imaging modalities such as magnetic resonance imaging (MRI) or positron emission tomography-computed tomography (PET-CT), early tumor deposits remain undetectable. Local or regional subclinical microscopic disease may incidentally receive definitive therapy through inclusion in a resection specimen or radiotherapy port, whereas distant subclinical microscopic disease is addressed only by systemic therapy (when administered), which may account for timing differences.
Somewhat surprisingly, recurrence developed less frequently in African American patients. Additionally, African American patients who did develop recurrence did so later than other patients and were significantly less likely to develop distant metastases at multiple sites. Because mortality rates among patients without recurrence were actually lower among African American patients, it seems unlikely that competing causes of death account for these favorable outcomes. These findings run counter to general observations that African American individuals with lung cancer tend to present at later stage and have worse stage-for-stage outcomes.2,12,13 Multiple factors may contribute to these long-recognized trends, including lower socioeconomic status, reduced access to care, lack of insurance, and lower likelihood of agreeing to undergo aggressive surgical resection when offered. 12–14 However, the present study examines a distinct cohort: those individuals who qualified for, underwent, and benefited from definitive therapy. Furthermore, our patient cohort—drawn from a university-based oncology clinic that provides care to an insured population—represents a socioeconomically similar population. It seems possible that some of the race-linked outcomes reported in other series reflect socioeconomic factors rather than differences in disease biology and response to therapy. Another possible explanation is differences in recurrence detection, potentially due to different clinical and radiographic follow-up patterns across races. In the present study, whether African American patients truly fare better—and, if so, reasons for this apparent advantage—requires confirmation in other populations. Alternatively, the relatively small sample size of the current study may have been underpowered to address this question formally. Unfortunately, previously conducted prospective lung cancer clinical trials may not provide sufficient case numbers to address these questions either, given the historically low representation of African American individuals and other minority populations. 12,15
Not surprisingly, higher-stage primary tumors had greater rates of recurrent disease, and these recurrences occurred sooner. Although women are known to have better prognosis than men with lung cancer,1,2,7,12 recurrence rates were similar in this study. The observation that those cases treated with systemic therapy had higher rates of recurrence seems most likely to reflect greater inherent risk leading to the decision to administer chemotherapy rather than a detrimental effect of treatment. Nevertheless, this difference persisted in multivariable analyses controlling for initial disease stage, raising the possibility that other unmeasured variables impact treatment and monitoring decisions. For instance, patients not selected for chemotherapy may have been less fit, resulting in decreased frequency and/or duration of post-treatment surveillance, which in turn could reduce detection of recurrent disease.
Currently, recommended post-treatment surveillance for stage 1-3 lung cancer is chest CT with or without intravenous contrast, initially every 6 months, then every 12 months until five years. 16–19 Based on our current findings, this duration of follow-up seems likely to capture the overwhelming majority of disease recurrences. We observed stage 1 and 2 cases having the highest recurrence in the bone, CNS and other, whereas stage 3 had the highest recurrence in CNS and other. The recommended post-treatment surveillance modality of chest CT provides adequate assessment of most sites of recurrent disease, including lung, thoracic lymph nodes, adrenal glands, much of the liver, and the thoracic spine (the most common site of bone metastases).20–21 The only common site of metastasis not captured in routine follow-up is the CNS, a noteworthy observation because brain metastases developed in approximately one-third of patients, including stage 1 and 2 cases.
Consistent with general disease patterns of disease dissemination,22–23 in the present study brain recurrence was more common in non-squamous NSCLC and in small cell lung cancer cases. In small cell lung cancer, the particularly high risk of CNS recurrence has led to recommendations for prophylactic cranial irradiation (PCI) in both limited and extensive stage disease. 24–25 Although trials of PCI for locally advanced NSCLC have not definitely demonstrated a benefit,26 given the potential for morbidity and clinical decline from symptomatic brain metastases, there may be a role for post-treatment CNS surveillance, particularly in non-squamous cases.
Limitations of this study include a single-center setting and relatively small sample size. Although we had previously incorporated our institution’s tumor registry at Parkland Health and Hospital System—the safety-net medical provider for Dallas County and a source of a large population of medically underserved patients to provide socioeconomic and racial diversity—in multiple studies, the Parkland registry has not captured recurrence data. 27–33 Staging and surveillance may not have been implemented consistently across cases. This single-center study lacks sufficient case numbers to evaluate more nuanced considerations, such as recurrence patterns within the widely heterogeneous population of stage 3 lung cancer, which ranges from single-station node-positive disease found at surgery, to non-resectable bulky disease. Additionally, very small numbers of cases with available genomic data preclude analysis of molecular alterations in recurrence patterns. Despite these concerns, because national datasets and registries fail to capture recurrence patterns, the present real-world cohort offers key insights into the growing population of lung cancer patients rendered disease-free after initial definitive therapy. Our study sample also features racial diversity and histologic variation representative of the broader U.S. lung cancer population.
Conclusion
In conclusion, the development, type, site, and timing of lung cancer recurrence vary according to patient and disease characteristics. Although lung cancer treatment varies according to factors such as histologic subtype, currently a one-size-fits all approach is applied to post-treatment surveillance. Unfortunately, only a small number of retrospective studies have examined imaging surveillance.17 The current study suggests that modulation of clinical and radiographic disease monitoring according to recurrence risk, timing, and site may offer a means to identify future lung cancer when it remains asymptomatic and highly treatable. Eventually, detailed molecular and genomic tumor characterization may also predict future recurrence risk and patterns for an individual patient.34–35 However, until that time, we encourage the collection and analysis of recurrence data in large and diverse populations—as is currently performed to inform lung cancer staging—to advance our understanding of a clinical question that will only grow in importance as the number of earlier-stage lung cancers increases.
Supplementary Material
Clinical Practice Points:
What is already known about this subject?
A substantial proportion of individuals who initially present with localized or locoregional lung cancer eventually succumb to recurrent malignancy despite aggressive, multi-modality treatment. However, details on the site and timing of recurrence are generally not provided in national datasets or clinical trial reports.
What are the new findings?
We found that risk, timing, and site of recurrent lung cancer varied according to patient and disease characteristics, including age, race, tumor stage, histology, and primary treatment modality. The central nervous system was the most common site of recurrent disease, and distant recurrence occurred earlier than local or regional recurrence. In multivariate analysis, the site and type of recurrence was associated with histology and primary treatment modality.
How might it impact on clinical practice in the forseeable future?
Currently, post-treatment monitoring assumes a one-size-fits-all paradigm. The current study suggests that tailoring disease monitoring according to recurrence risk, timing, and site may offer a means to identify future lung cancer while it remains asymptomatic and treatable.
Acknowledgements
The authors thank Alejandra Madrigales from the UT Southwestern tumor registry for aid in collecting the cohort data, Ms. Dru Gray for assistance with manuscript preparation, and Helen Mayo, MLS, for assistance with literature searches.
Funding
Supported in part by a National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24 CA201543-01, to D.E.G.), by the Biostatistics Shared Resource of the Harold C. Simmons Comprehensive Cancer Center, which is supported in part by National Cancer Institute Cancer Center Support Grant 1P30CA142543-01, by the Cancer Prevention and Research Institute of Texas (RP150596, RP160157 to C.M.K), and, by the Mechanisms of Disease and Translational Science Graduate Track T32 Grant (GM131945 to C.M.K)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors have no conflicts of interest to report.
References:
- 1.American Cancer Society. Key Statistics for Lung Cancer. https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/key-statistics.html; 2016. Accessed 04 August 2019.
- 2.Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Lung and Bronchus Cancer. https://seer.cancer.gov/statfacts/html/lungb.html; Accessed 04 August 2019.
- 3.Walters S, Maringe C, Coleman MP, et al. , Lung cancer survival and stage diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax. 2013; 68:551–564. 10.1136/thoraxjnl-2013-203543 [DOI] [PubMed] [Google Scholar]
- 4.Maeda R, Junji Y, Ishii G, et al. Long-Term Outcome and Late Recurrence in Patients with Completely Resected Stage IA Non-small Cell Lung Cancer. Journal of Thoracic Oncology. 2010; 5(8) 1246–1250. 10.1097/JTO.0b013e3181e2f247. [DOI] [PubMed] [Google Scholar]
- 5.Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. Journal of Thoracic and Cardiovascular Surgery. 1995; 109:120–129. 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 6.Al-Kattan K, Sepsas e, Fountain SW, Townsend ER. Disease recurrence after resection for stage I lung cancer. European Journal of Cardio-thoracic Surgery. 1997; 12(3):380–384. 10.1016/S1010-7940(97)00198-X [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA: A Cancer Journal for Clinicians. 2019; 69(1): 7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 8.Dickie L, Johnson CH, Adams S, Negoita S. Lung Solid Tumor Rules 2018 (July 2019). National Cancer Institute; https://seer.cancer.gov/tools/solidtumor/Lung_STM.pdf [Google Scholar]
- 9.American College of Surgeons Commission on Cancer. Standards for Oncology Registry Entry. STORE 2018. Version 1.0 201. https://www.facs.org/-/media/files/quality-programs/cancer/ncdb/store_manual_2018.ashx
- 10.Klein CA. Parallel progression of primary tumours and metastases. Nature Reviews Cancer. 2009; 9:302–312. DOI: 10.1038/nrc2627 [DOI] [PubMed] [Google Scholar]
- 11.Rubin P, Brasacchio R, Katz A. Solitary Metasases: Illusion Versus Reality. Seminars in Radiation Oncology. 2006; 16(2):120–130. DOI: 10.1016/j.semradonc.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 12.American Cancer Society. Cancer Facts & Figures for African Americans 2019-2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/cancer-facts-and-figures-for-african-americans-2019-2021.pdf
- 13.Yang R, Cheung MC, Byrne MM, et al. Do Racial or Socioeconomic Disparities Exist in Lung Cancer Treatment? Cancer. 2010; 116(10): 2437–2447. 10.1002/cncr.24986 [DOI] [PubMed] [Google Scholar]
- 14.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950-2014: Over Six Decades of Changing Patterns and Widening Inequalities. Journal of Environmental and Public Health. 2017; 2017: Article ID 2819372, 19 pages. 10.1155/2017/2819372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duma N, Aguilera JV, Paludo J, et al. Representation of Minorities and Women in Oncology Clinical Trials: Review of the Past 14 years. Journal of Oncology Practice. 2018; 14(1):e1–e10. DOI: 10.1200/JOP.2017.025288. [DOI] [PubMed] [Google Scholar]
- 16.Postmus PE, Kerr KM. Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2017; 28(Supplement 4):1–21. DOI: 10.1093/annonc/mdx222 [DOI] [PubMed] [Google Scholar]
- 17.Colt HG, Murgu SD, Korst RJ, et al. Follow-up and Surveillance of the Patient with Lung Cancer After Curative-Intent Therapy. Diagnosis and Management of Lung Cancer, 3rd ed.: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013; 143(5), Supplement: e4375–e4545. 10.1378/chest.12-2365 [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Small Cell Lung Cancer. Version2.2019, August 5, 2019 [Google Scholar]
- 19.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer. Version2.2019, August 5, 2019 [Google Scholar]
- 20.Jazieh AM, AlSumai TS, Ali YZ, Sheblaq NR, Alkaiyat M. The pattern of bone involvement, management, and outcomes in patients with nonsmall cell lung cancer: A retrospective study. Annals of Thoracic Medicine. 2018; 13:150–155. DOI 10.4103/atm.ATM_385_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva GT, Bergmann A, Thuler LCS. Incidence, associated factors, and survival in metastatic spinal cord compression secondary to lung cancer. The Spine Journal [DOI] [PubMed] [Google Scholar]
- 22.Riihimake M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014; 86:78–84. DOI: 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
- 23.Shi AA, Digumarthy SR, Temel JS, Halpern EF, Kuester LB, Aquino SL. Does Initial Staging or Tumor Histology Better Identify Asymptomatic Brain Metastases in Patients with Non-small Cell Lung Cancer? Journal of Thoracic Oncology. 2006; 1(3): 205–210. DOI: 10.1016/S1556-0864(15)31569-0 [DOI] [PubMed] [Google Scholar]
- 24.Auperin A, Arriagada R, Pignon JP, Pechoux CL, Gregor A, Stephens RJ, et al. Prophylactic Cranial Irradiation for Patients with Small-Cell Lung Cancer in Complete Remission. New England Journal of Medicine. 1999; 341:476–484. DOI: 10.1056/NEJM199908123410703 [DOI] [PubMed] [Google Scholar]
- 25.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic Cranial Irradiation in Extensive Small-Cell Lung Cancer. New England Journal of Medicine. 2007; 357:664–672. DOI: 10.1056/NEJMoa071780 [DOI] [PubMed] [Google Scholar]
- 26.Ruysscher DD, Dingemans AMC, Praag J, et al. Prophylactic Cranial Irradiation Versus Observation in Radically Treated Stage III Non-Small-Cell Lung Cancer: A Randomized Phase III NVALT-11/DLCRG-02 Study. Journal of Clinical Oncology. 2018; 36(23):2366–2377. DOI: 10.1200/JCO.2017.77.5817 [DOI] [PubMed] [Google Scholar]
- 27.Yorio JT, Xie Y, Yan J, Gerber DE. Lung cancer diagnostic and treatment intervals in the United States: a healthcare disparity? Journal of Thoracic Oncology. 2009; 4(11):1322–1330. DOI: 10.1097/JTO.0b013e3181bbb130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasco DW, Yan J, Dowell FE, Gerber DE. Looking beyond surveillance, epidemiology, and end results: patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. Journal of Thoracic Oncology. 2010; 5(10):1529–1535. DOI: 10.1097/JTO.0b013e3181e9a00f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerber DE, Rasco DW, Le P, Yan J, Dowell JE, Xie Y. Predictors and impact of second-line chemotherapy for advanced non-small cell lung cancer in the United States: real-world considerations for maintenance therapy. Journal of Thoracic Oncology. 2011;6(2):365–371. DOI: 10.1097/JTO.0b013e3181fff142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yorio JT, Yan J, Xie Y, Gerber DE. Socioeconomic disparities in lung cancer treatment and outcomes persist within a single academic medical center. Clinical Lung Cancer. 2012; 13(6):448–457. DOI: 10.1016/j.cllc.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taiwo EO, Yorio JT, Yan J, Gerber DE. How have we diagnosed early-stage lung cancer without radiographic screening? A contemporary single-center experience. PLoS One. 2012; 7(12):e52313 DOI: 10.1371/journal.pone.0052313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutluk Cenik B, Sun H, Gerber DE. Impact of renal function on treatment options and outcomes in advanced non-small cell lung cancer. Lung Cancer. 2013; 80(3):326–332. DOI: 10.1016/j.lungcan.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn DH, Mehta N, Yorio JT, Xie Y, Yan J, Gerber DE. Influence of medical comorbidities on the presentation and outcomes of stage I-III non-small-cell lung cancer. Clinical Lung Cancer. 2013; 14(6):644–650. DOI: 10.1016/j.cllc.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito M, Miyata Y, Hirano S, et al. Synchronicity of genetic variants between primary sites and metastatic lymph nodes, and prognostic impact in nodal metastatic lung adenocarcinoma. Journal of Cancer Research and Clinical Oncology. 2019; [Epub ahead of print]. 10.1007/s00432-019-02978-0 [DOI] [PubMed] [Google Scholar]
- 35.Sim J, Kim Y, Kim H, et al. Identification of recurrence-associated microRNAs in stage I lung adenocarcinoma. Medicine. 2018; 97(25) e10996 DOI: 10.1097/MD.0000000000010996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.