Abstract
Background:
Preclinical investigations of the effects of general anesthesia on the young brain show differences in vulnerability of males and females to anesthetic exposure at different times during development. However, the mechanism underlying this sex difference is poorly understood. Perinatal testosterone is the primary determinant of sexual differentiation and likely plays an important role in defining the period of susceptibility to anesthetic injury. We investigated whether the removal of testosterone through gonadectomy shortly after birth would improve cognitive outcomes in male rodents after early anesthesia exposure.
Methods:
Male Sprague Dawley rats underwent gonadectomy at postnatal day 2 (P2), followed by exposure to 6 hours of isoflurane at P7. A control cohort of gonad-intact male littermates was simultaneously exposed. All rats were subjected to a series of object recognition and association tasks beginning at P42. Cell death in the thalamus and hippocampus was assessed in a separate cohort.
Results:
All groups performed similarly on the Novel Object Recognition task, however the gonad-intact isoflurane group exhibited decreased performance in the more difficult tasks. This deficit was ameliorated in the gonadectomized group. Cell death was similar between both isoflurane-exposed groups, regardless of gonadectomy.
Conclusions:
The absence of testosterone does not block cell death after anesthesia in specific brain regions of interest, however does provide some neuroprotection as evidenced by the improved cognitive test performance during adulthood. These findings suggest that testosterone may be mechanistically involved in the sex-specific effects of anesthetic injury on the developing brain by extending the vulnerable period in male rats.
Keywords: Isoflurane/TO, Sex Factors, Behavior, Animal
INTRODUCTION
Developmental sex factors may play an important role in determining the cognitive outcomes after early anesthesia exposure in rodents. Male rats exposed to volatile anesthetics at postnatal day 7 (P7) display more severe forms of behavioral and cognitive impairment compared to age-matched females1. However, female rats exhibit similar cognitive deficits when exposed at an earlier postnatal age2. This suggests that a critical age-defined period of vulnerability to anesthetic neurotoxicity exists and is influenced by sex differences during brain development. The actions of sex hormones, such as testosterone in the male brain, are instrumental in the early formation of some brain structures and sexual differentiation before and shortly after birth3,4. Neonatal testosterone and its metabolites influence synapse formation, neurogenesis, cell differentiation, and expression of proteins critical to development5–7. Thus, the developmental expression of sex hormones and their role in sex-specific effects of anesthesia on the brain is an important area of investigation.
Males are at higher risk for developing certain neurological diseases and are more susceptible to early neurological injury, such as neonatal hypoxic ischemia8,9. This sex bias is observed widely in clinical diagnoses and replicated in animal models, however the mechanism of vulnerability in the male brain – or protection in the female brain – is poorly understood. Multiple factors inherent to male neurodevelopment, such as the prolonged excitatory action of GABA and increased inflammatory factors compared to females, may prime the male brain to be more sensitive to disease and injury9. These sex-specific characteristics are primarily driven by the perinatal surge in testosterone production and aromatization of testosterone into estradiol derivatives, which is largely responsible for the masculinization of the brain.
Here we investigate the behavioral and neurotoxic effects of anesthesia exposure on the developing brain of male rats after alteration of testosterone levels through perinatal gonadectomy. We hypothesize that a testosterone-mediated delay in early brain development increases the susceptibility of males to anesthetic injury and leads to the difference in cognitive outcomes between males and females on P7. Removal of the primary testosterone source causes the males to develop more similarly to females, potentially shifting the window of anesthetic vulnerability during the first postnatal week and providing protection at the time of exposure. Through the current study we aim to gain insight into the underlying sex-dependent mechanism and developmental timing of anesthetic injury in the developing brain.
MATERIALS AND METHODS
Animals
All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee at the University of California, San Francisco. Sprague Dawley dams with P1 male-only litters were purchased from Charles River Laboratory. Pups were kept with dams in the home cage until weaning at P21, except for removal from cage at P2 and P7 for the gonadectomy procedure and anesthesia treatment. All litters were housed in the same room within the animal housing facility with a reverse 12 hr light-dark cycle. Standard chow and water were provided ad libitum. Animals in the control behavior cohort were randomly assigned to either the control group or the gonadectomy (Gx) group. Animals in the experimental behavior cohort were randomly assigned to either isoflurane (Iso), or gonadectomy plus isoflurane (Gx+Iso) group. After weaning at P21, animals were housed in groups of 3 per cage within experimental groups. Animals in the histology cohort were randomly assigned to one of four groups: control, gonadectomy, isoflurane, or gonadectomy plus isoflurane.
Gonadectomy surgery
A gonadectomy procedure was performed on the animals at P2. Anesthesia by hypothermia (cryoanesthesia)10, was induced with an ice bath immersion. The animal was then transferred to a bed of crushed ice for maintenance of hypothermic anesthesia and considered at sufficient anesthetic depth when respiration stopped and the tail pinch response was absent. A small lateral incision was made across the abdomen and both testes were removed. The muscle and skin were then sutured. A topical antibiotic was applied to the incision site. The animal was immediately removed from the ice bed and manually warmed until normal breathing resumed. The animal was subsequently transferred to a warming blanket and monitored until it regained spontaneous movement, then was returned to the dam with a control animal that was separated for a similar period of time. The incision site was checked regularly for normal healing post-surgery.
Testosterone quantification
Testosterone levels were quantified after P2 gonadectomy in rats (>P180 at time of quantification) and compared to age-matched male and female controls (n=6 per group) using an enzyme immunoassay for testosterone (EIA-1559, DRG International; Springfield, NJ). Rats were deeply anesthetized with isoflurane and 1 mL blood was collected from the left ventricle using a syringe coated with heparin. Blood was kept on ice until spun down at 14 x g for 30’ at 4 C. Plasma was collected and undiluted samples were processed according to manufacturer instructions for the ELISA kit. Optical density was read at 450 +/− 10nm using a microplate reader (FLUOstar Optima, BMG Labtech; Ortenberg, Germany) and an average absorbance was calculated for each set of samples, standards, and controls using statistical software GraphPad Prism.
Anesthesia
Animals were removed from the dams and anesthetized with isoflurane for 6 hours in a temperature-controlled incubation chamber. Isoflurane was delivered into the anesthetic chamber through a humidified gas line with 50% oxygen (0.5 L/min) and 50% air (0.5 L/min). Rats were exposed to 2 hours each of 2.0%, 1.4%, and 0.8% isoflurane in a step-down manner, for a total of 6 hours of anesthetic exposure. Temperatures were measured and recorded every 15 minutes with an infrared thermometer and the water bath temperature was adjusted as needed, with a goal pup temperature of 36.5 C. Pups were flipped onto their other side every 15 minutes. Oxygen, carbon dioxide, and isoflurane levels were continuously monitored and recorded. The rats were returned to the dams when they regained spontaneous movement and righting reflex. Reported results consist of aggregated data from two cohorts that underwent identical experimental paradigms.
Behavior
Behavioral tests were performed starting at P42 following a testing paradigm as described previously1. Briefly, testing was conducted in a dedicated behavior room within the animal facility that was dimly lit with low ambient noise. The testing context consisted of a 61 cm square base surrounded by walls that were 50 cm high. Two identical contexts were used to test two rats simultaneously for Novel Object Recognition and Object Place, and two distinct contexts were used for Object-Context. A single camera (GigE Basler) was mounted above the contexts and the video-feed was live-tracked with EthoVision XT 11.5 (Noldus, Wageningen, The Netherlands). Rats were habituated to the testing room and context for four consecutive days before testing began. Testing began with Novel Object Recognition (NOR), followed by Object-Place (OP), then Object-Context (OC). The NOR task relies on the ability of the animal to recognize a previously seen (familiar) object compared to a novel object, and consequently spend more time investigating the novel object. In OP, the animal is presented with two identical objects that have been previously seen, but one in a novel location during the test phase. This task tests the animals’ ability to associate object recognition with spatial location. In OC, the animal is presented with two different objects that have both been previously seen in different contexts during two sequential exposure phases, but one is in a novel object-context pairing during the test phase. This task tests the animals’ ability to associate object recognition with contextual cues.
For each task, rats were placed into the context facing the wall and away from the objects. During the exposure phase, rats were presented with two target objects and given four minutes to investigate. The rats were removed from the context and placed back in their home cage for two minutes while the context and objects were wiped with 70% ethanol. The Object-Context task included two sequential exposure phases. Following the exposure phase, the objects were reset so that each context contained one novel target and one familiar target. The rats were then placed back into the context for the test phase. Rats were again allowed to investigate the objects for four minutes before being removed from the context.
Histology
For cell death quantification, rats were transcardially perfused 12-18 hours after an anesthesia exposure to assess neuronal death immediately induced by the anesthetic with littermate controls. Brains were dissected, post-fixed in 4% PFA at 4 C for 24 hours then incubated in 30% sucrose until sunken. Brains were frozen and sectioned at 60um thickness on a sliding microtome. Free-floating sections were collected in PBS, then every other section was mounted onto slides and allowed to dry overnight. The slides were stained with Fluorojade C (FJC, 0.001% Millipore), a fluorescent marker specific to degenerating neurons. Stained sections were imaged using a Nikon Eclipse 80i epifluorescent microscope with a 10X air objective. An intensity threshold was applied in ImageJ to isolate FJC+ pixels and quantified in specific regions of interest (ROI). The pixel area of the ROI was measured and the FJC+ pixel count was divided by the total ROI area and normalized to the control.
Statistical Analysis
GraphPad Prism 7 statistical software (GraphPad Software Inc., San Diego, CA) was used for all data analysis. Behavioral data was tested for normality using the D’Agostino-Pearson test. Normally distributed data from the recognition tasks (time spent investigating familiar vs. novel) was analyzed with the two-tailed paired t test. If the data was not normally distributed, the Wilcoxon matched-pairs rank test was used. For analysis of the discrimination index (DI), which is the difference in time investigating the novel target minus time investigating the familiar target over the total amount of time investigating either target, a one sample t test against a hypothetical mean = 0 (chance level) was used. FJC neuronal death data was analyzed with a two-way ANOVA to assess the effects of isoflurane exposure and gonadectomy.
A two-way ANOVA was used to analyze the effect of age and gonadectomy on weight between gonadectomized and control male rats. Bonferroni’s multiple comparisons post-test was used to compare groups at each age point. Testosterone quantification was assessed with a one-way ANOVA and Tukey’s multiple comparisons post-test. A p value less than 0.05 was considered statistically significant for all analyses.
RESULTS
Neonatal gonadectomy and cryoanesthesia alone do not impair recognition learning and memory
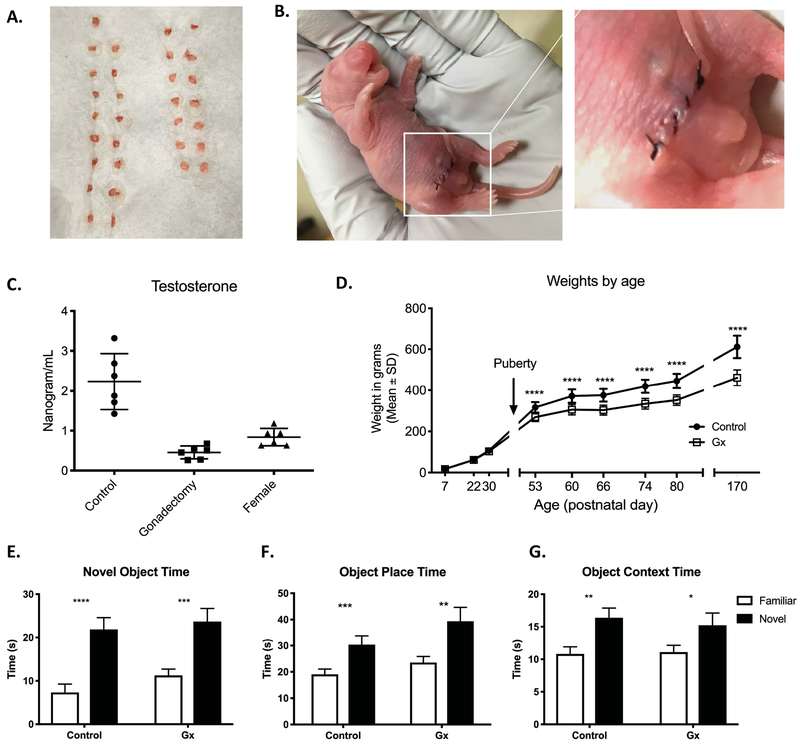
To determine whether gonadectomy and cryoanesthesia alone had an effect on recognition memory, we tested a control cohort to compare performance on specific behavioral tasks of gonadectomized rats versus gonad-intact male littermates. Rats were gonadectomized at P2 under cryoanesthesia to avoid confounding exposure to volatile anesthetic (Fig. 1a–b). The average procedure time under cryoanesthesia was 12 min, and the average time away from the dam was 33 min. Testosterone levels of gonadectomized rats were measured and compared to gonad-intact male and female litter mates during adulthood. Testosterone levels in the gonadectomized group were similar to that of females and significantly lower than gonad-intact control males (p<0.0001, One-way ANOVA, Tukey’s post-test) (Fig. 1c). Weights were measured for both groups post-weaning and showed a significant effect of time and treatment after puberty, with gonadectomized subjects weighing significantly less than control subjects (p<0.0001 age, p<0.0001 gonadectomy, p<0.0001 interaction, two-way ANOVA, Bonferroni post-test) (Fig. 1d). The divergence in weight between all rats in the two groups confirmed successful removal of testosterone-producing tissue. Both control (n=16) and gonadectomy (Gx, n=15) groups performed similarly on all object recognition tasks, spending more time with the novel target over the familiar target in the novel object (Control p<0.0001, Gx p<0.0001, paired t test), object place (Control p<0.0001 paired t test, Gx p=0.0007 Wilcoxon test), and object context tasks (Control p<0.0033 paired t test, Gx p=0.0176 Wilcoxon test) (Fig. 1e–g). Together, these results show that perinatal gonadectomy under cryoanesthesia successfully eliminates the primary source of testosterone production and does not affect performance on the object recognition tasks reported here.
Figure 1. Gonadectomy and cryoanesthesia do not impair object recognition learning and memory.
A) Testes removed during the gonadectomy procedure. B) Incision and suture site after gonadectomy procedure. C) Gonadectomized males have lower levels of circulating testosterone than controls, similar to females. D) Gx and control animals maintained similar body weights until pubertal period, after which control animals weighed significantly more. E-G) Both Control (n=16) and Gx (n=15) groups spent significantly more time with the novel target than the familiar target in the Novel Object Recognition (E), Object Place (F), and Object Context (G) tasks. Mean and SEM shown.
Anesthesia-induced recognition deficit is improved in gonadectomized males
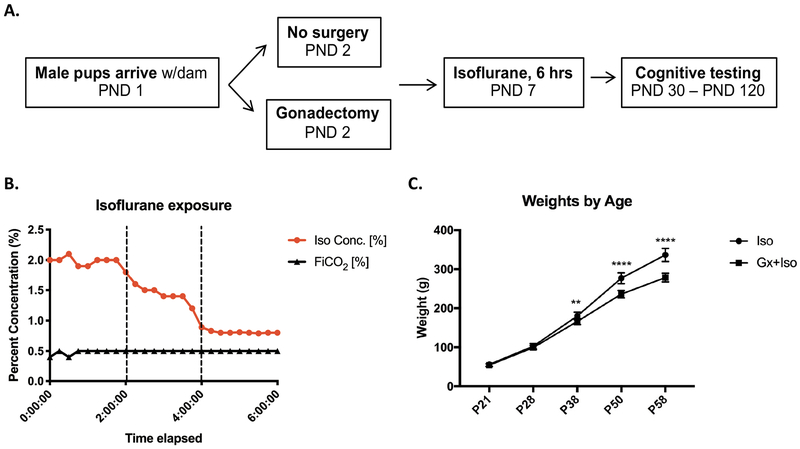
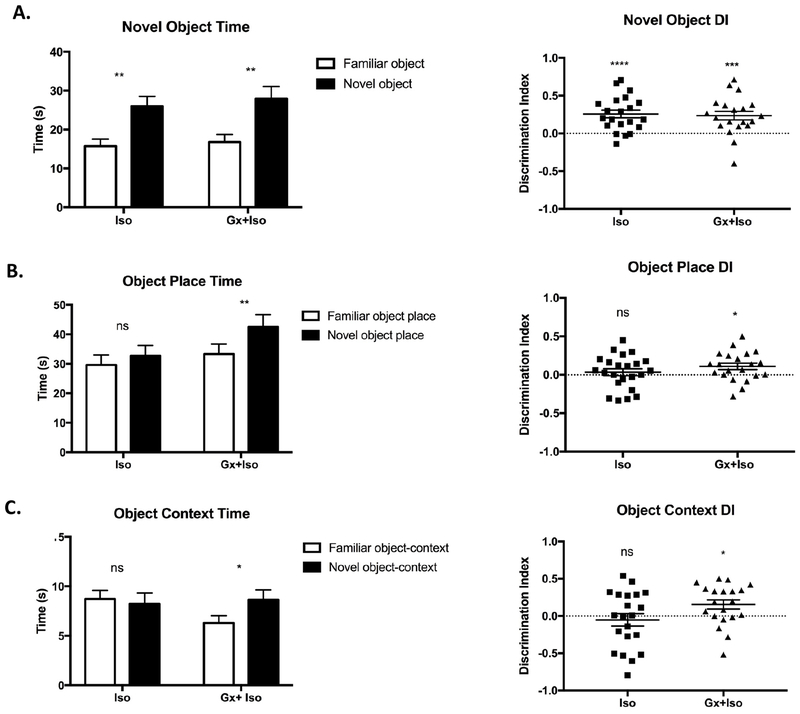
We next compared recognition memory of gonadectomized male rats to gonad-intact littermates after exposure of both groups to 6 hours of isoflurane (Fig. 2a–b). As seen with the gonadectomy control cohort, measured weights between both groups significantly diverged after puberty for all animals, indicating successful removal of the primary testosterone source (Fig. 2c). The gonadectomy plus isoflurane (Gx+Iso, n=21) and isoflurane only (Iso, n=23) groups both displayed intact novel object recognition, spending more time investigating a novel object over the familiar object (Iso p=0.0002 Wilcoxon test, Gx+Iso p=0.0028 paired t test) (Fig. 3a). The discrimination index (DI), which provides a measure of the time spent with the novel target relative to the total amount of time spent investigating either target, also reflected the ability to recognize a novel object for both groups (Iso p<0.0001, Gx+Iso p=0.0006, one sample t-test). As basic recognition memory was intact, we next moved on to more complex recognition tasks that pair object recognition with either place or context association. In the object-place task, the Iso group was not able to discriminate between a novel and familiar object-place association, as previously reported1, whereas the Gx+Iso rats spent significantly more time with the object in the novel place (Iso p=0.1679, Gx+Iso p=0.006, paired t test) (Fig. 3b). Furthermore, the same pattern was seen in the more difficult object-context recognition task; the Gx+Iso group spent more time with the novel context-object pairing during the test session, while the Iso group did not (Iso p=0.7357, Gx+Iso p=0.0405, paired t test) (Fig. 3c). The DI also showed the same learning patterns. The amount of time spent investigating the objects during the exposure phase for the tasks were not different between groups.
Figure 2. Timeline and characteristics of behavior experiment.
A) Experimental schema showing age of rats at gonadectomy, anesthetic exposure, and behavioral testing. B) Measured chamber isoflurane levels administered to the first behavioral cohort at P7. C) Gx+Iso and Iso animals maintained similar body weights until pubertal period, after which Iso animals weighed significantly more.
Figure 3. Anesthesia-induced recognition deficit is improved in gonadectomized males.
A) Both Iso (n=23) and Gx+Iso (n=21) were able to discriminate the novel object from a familiar object in the Novel Object Recognition task. Both groups spent more time investigating the novel object and had a discrimination index (DI) significantly greater than 0. B) The Iso group, but not the Gx+Iso group, was impaired in the Object Place recognition task. C) The Iso group, but not the Gx+Iso group, was impaired in the Object-Context recognition task. Mean and SEM shown.
Gonadectomy does not protect from isoflurane-induced cell death
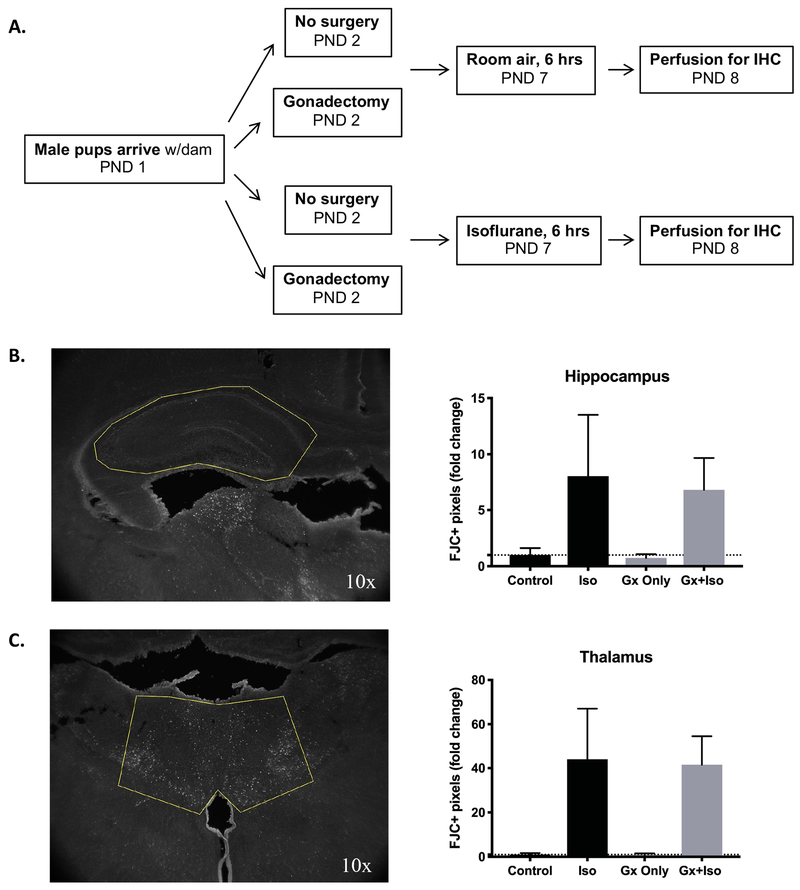
We next asked whether the gonadectomy procedure provided any neuroprotection from immediate cell death that is consistently observed with isoflurane exposure1,11,12(Fig. 4a). Fluorojade C, a fluorescent marker for degenerating neurons, showed increased rates of neuronal death in both Iso (n=7) and Gx+Iso (n=6) groups 12 hours post-anesthesia compared to their respective controls (n=5, Gx n=7) in the thalamus and hippocampus. Gonadectomy itself did not lead to any difference in cell death in the analyzed brain regions compared to controls or after isoflurane exposure at P7 (Thalamus: Iso p<0.0001, Gx p=0.7999, Interaction p=0.8236; Hippocampus: Iso p<0.0001, Gx p=0.5740, Interaction p=0.7121; Ordinary two-way ANOVA) (Fig. 4b–c).
Figure 4. Isoflurane, but not gonadectomy, alters neuronal death levels in the hippocampus and thalamus.
A) Experimental schema showing groups and ages for gonadectomy and/or isoflurane exposure before perfusion for immunohistochemical (IHC) staining with neurodegenerative cell marker Fluorojade C (FJC). Control (n=5), Gx only (n=7), Iso (n=7), Gx+Iso (n=6). B) ROI of hippocampus analyzed for FJC (left). Isoflurane increased levels of cell death in the hippocampus compared to control regardless of gonadectomy procedure (right). C) ROI of thalamus analyzed for FJC (left). Isoflurane increased levels of cell death in the thalamus compared to control regardless of gonadectomy procedure (right). Mean and SD shown.
DISCUSSION
Our study found that perinatal gonadectomy ameliorated cognitive deficits observed in adult male rats that were exposed postnatally to volatile general anesthesia. Further, we showed that the gonadectomy procedure under cryoanesthesia alone does not affect object recognition learning and memory. These results suggest that developmental sex differences in cognitive outcomes may be driven by testosterone mediated effects on neurodevelopment. As reported previously1, the Iso group was still able to successfully distinguish a novel object from a familiar one, despite impaired performance on the later, more difficult tasks. This aligns with clinical studies that show evidence of anesthetic effects on more complex subdomains of memory or behavior rather than on measures of global intelligence.
Gonadectomy did not alter the level of cell death observed in the thalamus and hippocampus after isoflurane exposure. We have previously shown that P7 females exposed to isoflurane have similar rates of cell death compared to their male counterparts, but do not exhibit the anesthesia-associated cognitive deficit later in life1. Similar results showing that cell death does not necessarily correlate with cognitive function have been shown by other groups as well13,14. These results, in conjunction with our current study, suggest that cell death per se may not be causative of the observed behavioral outcome. Rather, it supports that early anesthesia exposure at a specific postnatal time point may disrupt critical developmental processes, such as circuitry formation or cellular differentiation, that lead to the lasting deficits. Exposure before certain circuits are developed could inhibit their formation through disrupted cell migration or altered functional input (decreased activity or death of essential cells involved in the circuit formation), which would result in permanent changes to the brain. Sexual dimorphism during brain development occurs during the early postnatal weeks in males due to circulating (although very low levels of) testosterone. The perinatal removal of the testes and lowering of testosterone in the males before anesthesia exposure likely primes the brains of the gonadectomized males to be more developmentally similar to females. While the anesthesia may have induced the same amount of cell death regardless of gonadectomy, the developmental stage of the neural circuits at the time of exposure may have been more advanced due to the hormonal alteration resulting from the surgery, affording protection from the changes in cell activity.
Rats undergo a transient testosterone surge within a few hours of birth that is instrumental in the sexual differentiation of the brain15. Since the gonadectomy procedure in this study was not performed until P2, only testosterone produced after this timepoint would have been eliminated. This suggests that the cognitive and behavioral differences in the Gx+Iso versus Iso group are not dependent on the initial testosterone surge, but rather the testosterone production closer to the time of exposure or thereafter.
Our findings further show that perinatal gonadectomy and cryoanesthesia alone do not affect performance on object recognition tasks. To our knowledge, this is the first time it has been shown that the combination of this surgical procedure and anesthetic does not in itself lead to lasting changes in recognition memory in adult rodents. We believe this may be useful for future cognitive or behavioral experiments in animals that require postnatal gonadectomy but also need to avoid using general anesthesia due to confounding neurotoxic effects.
LIMITATIONS
While our current study provides evidence that sex hormones influence anesthetic vulnerability, the mechanism of injury and precise timing of the hormonal effects remain unclear. Future studies will be necessary to determine how the elimination of testosterone provides neuroprotection and whether its removal is only necessary during or around the time of exposure to the anesthetic to produce the same lasting outcome. Further, while the use of gonadectomized male rats allows us to draw some parallels to female outcomes after anesthesia, additional experiments at different time points would be useful in determining if gonadectomy establishes the same time-sensitive window of anesthetic vulnerability seen in females.
Spatial reference memory has been shown to be affected by early anesthesia2,11, however it is also impaired by gonadectomy in males16. Therefore, we could not accurately assess the combined effects of anesthesia and gonadectomy on spatial reference memory due to confounding. While our current results show that postnatal gonadectomy rescue the effects of anesthesia exposure on recognition and associative memory, we cannot conclude whether the rescue phenotype can be extended to all domains that have previously been shown to be affected by anesthesia. Further experiments that use androgen blocking methods might be useful to separate these effects since later development would continue normally with testes intact.
CONCLUSIONS
Our current study emphasizes the important role of sex hormones, specifically testosterone, in influencing the vulnerability of the brain to anesthetic injury during early development. Future laboratory studies will be necessary to further investigate the mechanism and timing of the critical period set by these hormones. Sex is a critical factor in postnatal anesthetic injury and outcome and should be carefully assessed in clinical trials aiming to study the effects of anesthesia on neurodevelopment.
ACKNOWLEDGEMENTS
We thank Jason Leong, Yasmine Eichbaum, and Nicole Yabut for technical assistance in work supporting this project. We thank Dr. Gregory Chinn for critical reading of the manuscript. This work was supported by National Institutes of Health Grant Award RO1GM112831 (JWS).
REFERENCES
- 1.Lee BH, Chan JT, Kraeva E, Peterson K, Sall JW. Isoflurane exposure in newborn rats induces long-term cognitive dysfunction in males but not females. Neuropharmacology. 2014;83:9–17. doi: 10.1016/j.neuropharm.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasaki Russell JM, Chinn GA, Maharjan D, Eichbaum Y, Sall JW. Female rats are more vulnerable to lasting cognitive impairment after isoflurane exposure on postnatal day 4 than 7. Br J Anaesth. 2019;122(4):490–499. doi: 10.1016/J.BJA.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filová B, Ostatníková D, Celec P, Hodosy J. The effect of testosterone on the formation of brain structures. Cells Tissues Organs. 2013;197(3):169–177. doi: 10.1159/000345567. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS, Milner TA. Understanding the Broad Influence of Sex Hormones and Sex Differences in the Brain. J Neurosci Res. 2017;95(1-2):24–39. doi: 10.1002/jnr.23809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galanopoulou AS, Moshé SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol. 2003;184(2):1003–1009. doi: 10.1016/S0014-4886(03)00387-X. [DOI] [PubMed] [Google Scholar]
- 6.Lenz KM, Nugent BM, McCarthy MM. Sexual differentiation of the rodent brain: dogma and beyond. Front Neurosci. 2012;6:26. doi: 10.3389/fnins.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowers JM, Waddell J, McCarthy MM. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ. 2010;1(1):8. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill CA, Threlkeld SW, Fitch RH. Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats. Int J Dev Neurosci. 2011;29(4):381–388. doi: 10.1016/j.ijdevneu.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy MM. Sex differences in the developing brain as a source of inherent risk. Dialogues Clin Neurosci. 2016;18(4):361–372. http://www.ncbi.nlm.nih.gov/pubmed/28179808. Accessed February 5, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libbin RM, Person P, Ayala G. Procedures for the use of neonatal rats in surgical research. J Surg Res. 1982;33(6):519–523. doi: 10.1016/0022-4804(82)90071-3. [DOI] [PubMed] [Google Scholar]
- 11.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early Exposure to Common Anesthetic Agents Causes Widespread Neurodegeneration in the Developing Rat Brain and Persistent Learning Deficits. J Neurosci. 2003;23(3):876–882. http://www.jneurosci.org/content/23/3/876.long. Accessed September 18, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stratmann G, May LDV, Sall JW, et al. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110(4):849–861. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 13.Schilling JM, Kassan A, Mandyam C, et al. Inhibition of p75 neurotrophin receptor does not rescue cognitive impairment in adulthood after isoflurane exposure in neonatal mice. Br J Anaesth. 2017;119(3):465–471. doi: 10.1093/BJA/AEW299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearn ML, Schilling JM, Jian M, et al. Inhibition of RhoA reduces propofol-mediated growth cone collapse, axonal transport impairment, loss of synaptic connectivity, and behavioural deficits. Br J Anaesth. 2018;120(4):745–760. doi: 10.1016/j.bja.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarkson J, Herbison AE. Hypothalamic control of the male neonatal testosterone surge. Philos Trans R Soc B Biol Sci. 2016;371(1688):20150115. doi: 10.1098/rstb.2015.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locklear MN, Kritzer MF. Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav. 2014;66(2):298–308. doi: 10.1016/J.YHBEH.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]