Abstract
Objective:
The cerebellum is a target of alcoholism-related brain damage in adults. Yet no study has prospectively tracked deviations from normal cerebellar growth trajectories in adolescents before and after initiating drinking.
Method:
MRI tracked developmental volume trajectories of 10 cerebellar lobule and vermis tissue constituents in 548 no-to-low drinking youth age 12-21 years at induction into this five-site, National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) study. Over the 3-4 year longitudinal examination yielding 2043 MRIs, 328 youth remained no/low drinkers while 220 initiated substantial drinking after initial neuroimaging.
Results:
Normal growth trajectories derived from no/low drinkers indicated that gray matter volumes of lobules V-VI, Crus II, VIIB, and X declined faster with age in male than female youth, whereas white matter volumes in Crus I-II and lobules VIIIA-VIIIB expanded faster in female than male youth; CSF volume expanded faster in most cerebellar regions of male than female youth. Drinkers exhibited accelerated gray matter decline in anterior lobules and vermis, accelerated vermian white matter expansion, and accelerated CSF volumes expansion of anterior lobules relative to youth who remained no/low drinkers. Analyses including both alcohol and marijuana did not support an independent role for marijuana on alcohol effects on cerebellar gray matter trajectories.
Conclusions:
Alcohol use-related cerebellar growth trajectory differences from normal involved anterior lobules and vermis of youth who initiated substantial drinking. These regions are commonly affected in alcohol-dependent adults, raising the possibility that cerebellar structures affected with youthful drinking may be vulnerable to age-alcohol interactions in later adulthood.
Keywords: adolescence, alcohol, marijuana, cerebellum, brain, development
INTRODUCTION
For nearly a century, clinical and neuropathological studies have observed vulnerability of the cerebellum to excessive alcohol consumption (1, 2). Initial findings on cell macrostructure and cellular dysmorphology were guided by detection of ataxia of gait notable in older dependent drinkers with neuropathological examination revealing a selectivity of damage to the anterior lobules (2-4). MRI studies later confirmed cerebellar volume shrinkage in Alcohol Use Disorder (AUD) in vivo with selective effect on anterior superior lobules (5-8) and regions of the corpus medullare (9). Despite this legacy of AUD-related cerebellar dysmorphology, little attention has been given to its potential insult in adolescents who initiate substantial drinking, which is now known to alter the trajectory of normal cortical development (10, 11).
Only a few in vivo neuroimaging studies have measured cerebellar volume in high-drinking compared with low-drinking adolescents, and all have been cross-sectional making it difficult to rule out the role of pre-exposure factors causing group differences (12). Youth who engaged in binge drinking exhibited volume deficits of the cerebellar hemispheres that were significant for gray matter but marginal for white matter and without sex differences; volume deficit severity was related to binge-drinking intensity measured as peak number of drinks in the 3 months before MRI acquisition (13). A cross-sectional study of adolescents and young adults with adolescent-onset AUD identified volume deficits in the total cerebella of male (n=8) but not female (n=6) youth relative to their sex-matched control groups (14). A role for family history of alcoholism was identified in late adolescents and young adults, where high-risk offspring (n=72) had larger volumes than low-risk offspring (n=59) of the corpus medullare and a cerebellar region inferior to the horizontal fissure (lobules VIIA to X and tonsil). Although history of alcohol use was accounted for statistically for the 22 high-risk participants who met diagnostic criteria for alcohol or drug abuse or dependence before their MRI, a follow-up analysis of this subgroup was not reported (15).
The recognized influence of alcohol on the cerebellum in adult AUD, together with a few small-scale studies supporting cerebellar vulnerability during growth years of adolescence, provide justification for conducting longitudinal investigation using refined measurement approaches to track potential trajectory deviations from normal regional cerebellar development (16). The first step in this endeavor requires establishment of normal growth trajectories and consideration of sexual dimorphism in maturing adolescents, with adolescence age range now hypothesized to extend into early 20s (17). Cross-sectional MRI studies (18-20) confirmed with longitudinal examination (10, 11, 21) describe cortical growth patterns by tissue type and region, indicating gray matter volume decline complemented by white matter and CSF volume increase during adolescence into young adulthood. This systematic neurorestructuring may be a reflection of gray matter pruning to accommodate environmental experience and genetic influence and white matter growth to expand connectivity for increasing potential for complex cognition. To date, the few studies of cerebellar development in adolescence (22-25) have focused on volume of the total structure and indicate nonspecific declines with age.
An initial longitudinal study measured volumetric changes in regions of the cerebellar vermis and hemispheres of 25 male and 25 female participants, age 5 to 24 years at baseline, selected quasi-randomly from the NIMH longitudinal study of normal brain development to have had at least 3 MRIs at 2-year intervals (26). Adjustment for total brain volume attenuated but did not fully remove the ubiquitous sex differences of male greater than female cerebellar volumes, notable in the superior posterior region. Sex differences in developmental trajectories of other regions may have been blurred because volumes were not analyzed by tissue type, i.e., gray matter, white matter, and cerebrospinal fluid (CSF), each of which follow different growth trajectories in the cerebral cortex (19, 21, 27-29) and may also do so in the cerebellums (30). Further, sexual dimorphism in cerebellar volume change over intervals of 1.5 to 5.6 years indicated that boys followed a quadratic function with a peak in volume at age 15.6 years, whereas girls showed steady decline over the 7 to 24.3 year age range of the 53 youth examined two or more times (31). Despite the strength of longitudinal studies, none to date have tracked developmental changes in the separate tissue types or in parcellated lobules of this complex structure (32, 33), which may develop differentially by age and sex (34) and be differentially vulnerable to environmental insult from alcohol use (35).
Herein we report a novel longitudinal analysis of structural MRI data collected at three to four annual visits on 548 youth of the National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) study (36). All participants had met study entry criteria for no-to-low drinking and drug consumption at initial MRI. By the fourth MRI, 220 had initiated drinking beyond levels permitted at study entry and 328 remained no-to-low drinkers, thus providing the basis for a prospective study on the effects of drinking on the adolescent cerebellum. Accordingly, this study had three aims: 1) to characterize normal developmental trajectories and potential sexual dimorphism of gray matter, white matter, and CSF volumes of the total cerebellum in youth who remained no-to-low drinkers for all MRI examinations; 2) to determine normal growth patterns by sex of cerebellar volumes by lobule; and 3) to localize patterns of deviations from normal by region and tissue type in youth who initiated moderate to heavy alcohol use. Additional analyses explored whether the magnitudes of cerebellar volume trajectory deviations were related to quantity or frequency of drinking, co-use of alcohol and marijuana, or motor performance.
METHODS
Participants
This longitudinal analysis included 548 participants who were no/low drinkers at baseline and had 2 or 3 additional (annual) follow-up MRIs: 80 participants had 3 MRIs and 468 had 4 MRIs, totaling 2043 MRIs. The Institutional Review Boards of the five NCANDA sites approved this study: University of California at San Diego (UCSD), SRI International, Duke University Medical Center, University of Pittsburgh (UPitt), and Oregon Health & Science University (OHSU) (36).
Subject demographics.
As described previously (11), participants were characterized by age, sex, self-identified ethnicity, and socioeconomic status (SES) determined as the highest level of education achieved by either parent (37) (Table 1). All participants submitted samples to a 14-panel urine toxicology screen for data exclusion if positive on the study day (11, 36).
Table 1.
National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) demographics for 548 youth at baseline and final MRI visit of subgroups defined by interim drinking
| Baseline | Longitudinal (values at final MRI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Full Group | Maintained | Transitioned to Drinking | No/low vs. Heavy |
|||||||
| No/low | No/low | Moderate | Heavy | All | Moderate | All | ||||
| Age at baseline (years) | ||||||||||
| Male | mean= | 15.61 | 17.82 | 19.18 | 19.60 | 19.42 | t= | −3.808 | −6.049 | −6.126 |
| SD= | 2.27 | 2.26 | 2.20 | 1.86 | 2.02 | p= | 0.000 | 0.000 | 0.000 | |
| N= | 272 | 159 | 50 | 63 | 113 | |||||
| Female | mean= | 15.65 | 17.83 | 19.69 | 19.77 | 19.72 | t= | −6.088 | −5.108 | −7.110 |
| SD= | 2.33 | 2.26 | 2.10 | 2.06 | 2.08 | p= | 0.000 | 0.000 | 0.000 | |
| N= | 276 | 169 | 70 | 37 | 107 | |||||
| Male+female | mean= | 15.63 | 17.82 | 19.48 | 19.66 | 19.56 | t= | −7.159 | −8.023 | −9.354 |
| SD= | 2.30 | 2.26 | 2.15 | 1.92 | 2.05 | p= | 0.000 | 0.000 | 0.000 | |
| N= | 548 | 328 | 120 | 100 | 220 | |||||
| Socioeconomic status† | mean= | 16.74 | 16.66 | 16.85 | 16.91 | 16.88 | t= | −0.710 | −0.951 | −1.030 |
| SD= | 2.49 | 2.52 | 2.58 | 2.28 | 2.45 | p= | 0.478 | 0.343 | 0.305 | |
| N= | 548 | 328 | 120 | 100 | 220 | |||||
| Body mass index (BMI) percentile | mean= | 58.59 | 59.52 | 59.20 | 51.25 | 55.86 | t= | 0.109 | 2.447 | 1.571 |
| SD= | 28.45 | 29.22 | 26.84 | 29.70 | 28.39 | p= | 0.913 | 0.016 | 0.117 | |
| N= | 548 | 326 | 120 | 100 | 220 | |||||
| Internalizing symptoms T-score | mean= | 47.58 | 47.43 | 46.58 | 46.05 | 46.35 | t= | 0.704 | 1.134 | 1.147 |
| SD= | 8.23 | 10.08 | 11.71 | 10.52 | 11.17 | p= | 0.482 | 0.259 | 0.252 | |
| N= | 548 | 324 | 119 | 95 | 214 | |||||
| Externalizing symptoms T-score | mean= | 47.35 | 46.89 | 46.38 | 44.65 | 45.62 | t= | 0.456 | 2.115 | 1.480 |
| SD= | 8.08 | 9.34 | 10.91 | 8.99 | 10.12 | p= | 0.649 | 0.036 | 0.140 | |
| N= | 548 | 324 | 119 | 95 | 214 | |||||
| Lifetime drinking days | mean= | 0.39 | 0.96 | 18.98 | 67.18 | 40.89 | t= | −10.079 | −10.226 | −11.429 |
| SD= | 1.42 | 2.65 | 19.52 | 64.72 | 51.77 | p= | 0.000 | 0.000 | 0.000 | |
| N= | 548 | 325 | 120 | 100 | 220 | |||||
| Lifetime drinks | mean= | 0.01 | 1.18 | 42.39 | 249.07 | 140.24 | t= | −9.694 | −11.777 | −10.970 |
| SD= | 0.14 | 3.98 | 42.23 | 198.57 | 173.77 | p= | 0.000 | 0.000 | 0.000 | |
| N= | 474 | 283 | 99 | 89 | 188 | |||||
| Lifetime binges | mean= | 0.00 | 0.003 | 2.27 | 26.07 | 13.09 | t= | −7.129 | −8.551 | −8.144 |
| SD= | 0.00 | 0.055 | 3.48 | 30.49 | 23.83 | p= | 0.000 | 0.000 | 0.000 | |
| N= | 548 | 325 | 120 | 100 | 220 | |||||
| Lifetime marijuana days | mean= | 28.22 | 3.66 | 29.36 | 107.17 | 64.73 | t= | −2.860 | −5.203 | −5.723 |
| SD= | 102.88 | 24.65 | 90.34 | 195.90 | 152.62 | p= | 0.005 | 0.000 | 0.000 | |
| N= | 548 | 328 | 120 | 100 | 220 | |||||
| Moderate | No/low vs. Heavy |
All | ||||||||
| Cigarette smokers†† | ||||||||||
| no, yes= | 520, 27 | 304, 24 | 87, 33 | 47, 53 | 134, 86 | χ2= | 32.229 | 108.400 | 82.861 | |
| p= | 0.00001 | 0.00001 | 0.00001 | |||||||
| Family history of alcoholism | ||||||||||
| negative, positive= | 502, 46 | 302, 26 | 111, 9 | 89, 11 | 200, 20 | χ2= | 0.0220 | 0.917 | 0.232 | |
| p= | 0.881 | 0.338 | 0.630 | |||||||
| Self-declared ethnicity | ||||||||||
| Caucasian | N= | 407 | 227 | 95 | 85 | 180 | χ2= | 9.3210 | 9.731 | 14.352 |
| African-American | N= | 74 | 58 | 8 | 8 | 16 | p= | 0.025 | 0.021 | 0.002 |
| Asian | N= | 59 | 37 | 16 | 6 | 22 | ||||
| Other | N= | 8 | 6 | 1 | 1 | 2 | ||||
| Site (scanner manufacturer) | ||||||||||
| UPitt (Siemens) | N= | 72 | 46 | 15 | 11 | 26 | χ2= | 4.361 | 6.630 | 8.618 |
| SRI (GE) | N= | 96 | 52 | 24 | 20 | 44 | p= | 0.359 | 0.157 | 0.071 |
| Duke (GE) | N= | 112 | 79 | 19 | 14 | 33 | ||||
| OHSU(Siemens) | N= | 118 | 68 | 28 | 22 | 50 | ||||
| UCSD (GE) | N= | 150 | 83 | 34 | 33 | 67 | ||||
Highest education of a parent
yes =ever smoked a cigarette
Criteria for alcohol grouping.
All 548 participants at study entry met two sets of drinking criteria determined with the Customary Drinking and Drug use Record (CDDR) (38) described previously (11, 36) and herein. First, the initial NCANDA inclusion criteria for "no/low" drinking were as follows: maximum lifetime drinking days for male and female participants was ≤5 for 12-15.9 year olds; ≤11 for 16-16.9 year olds; ≤23 for 17-17.9 year olds; ≤51 for 18 year olds and older. The maximum allowable drinks per occasion was ≤ 3 for female participants at any age but varied by age for male participants: ≤ 3 for 12-13.9 year olds, ≤ 4 for 14-19.9 year olds, and ≤ 5 for 20 year olds and older. Second, heavy, moderate, and no/low drinkers were categorized using the modified Cahalan et al. (39) inventory, comprising quantity (average and maximum consumption) and frequency combinations to classify drinking levels based on past year patterns. The final data set comprised 328 youth who remained in the no/low double criterion group and 220 youth who transitioned from the no/low to either moderate drinkers (n=120) or heavy drinkers (n=100; Table 1). Also determined was lifetime marijuana use (in days) at the time of the final MRI.
MRI Acquisition and Analysis
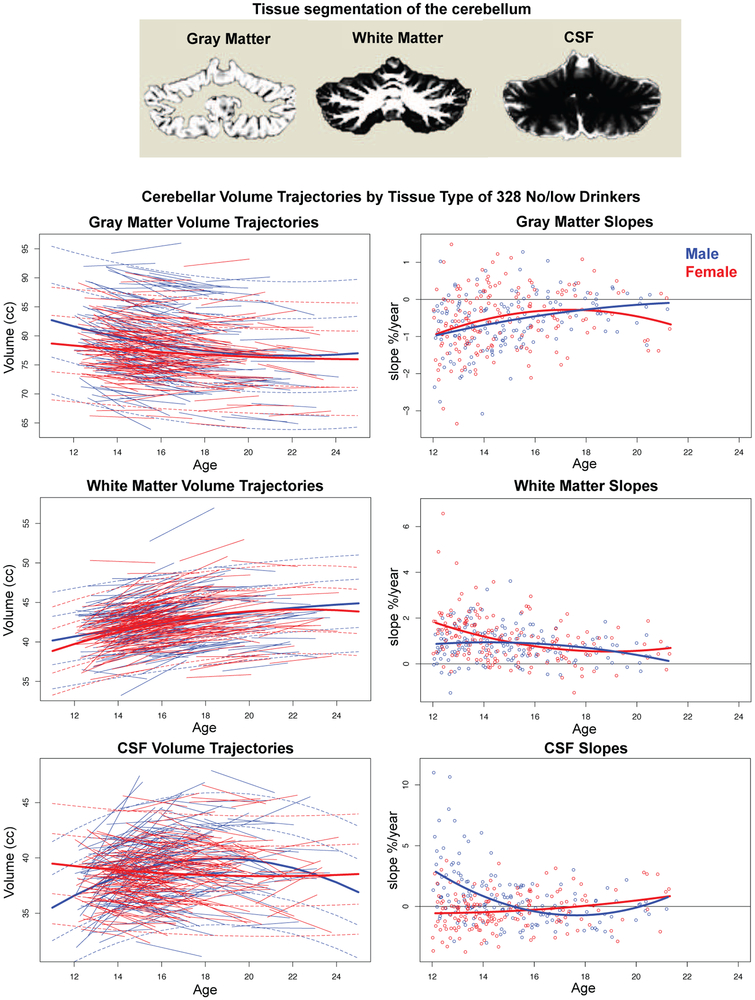
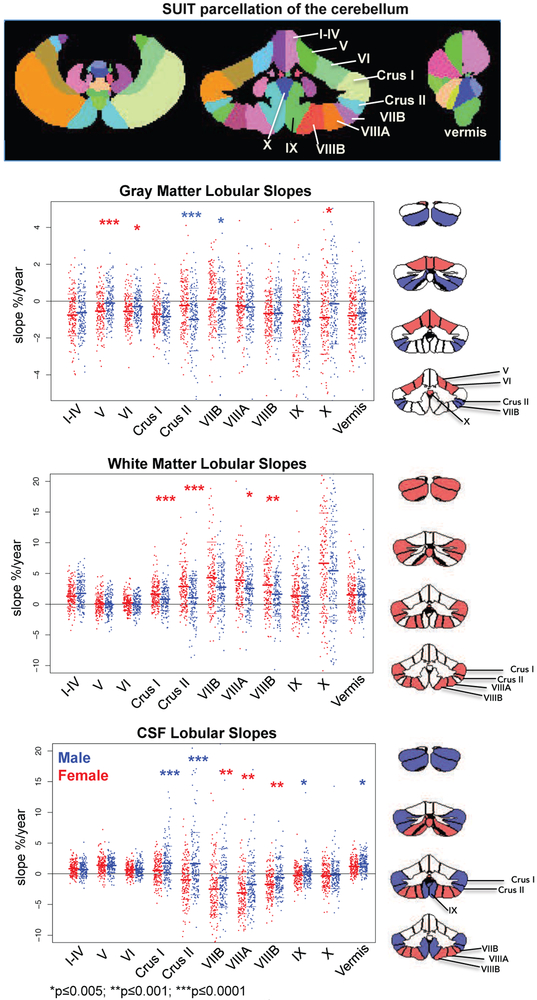
MRIs were acquired on 3T systems from two manufacturers: 3T General Electric (GE) Discovery MR750 at three sites (UCSD, SRI, and Duke) and 3T Siemens TIM TRIO scanners at two sites (UPitt and OHSU). Cerebellar tissue was segmented into gray matter, white matter, and CSF (Fig. 1); lobular quantification was accomplished with the Spatially Unbiased Infra-Tentorial (SUIT) atlas (40) (Fig. 2). Further details appear in supplemental material and described previously (11).
Figure 1.
Top: Example of tissue segmentation of a coronal slice through the cerebellum. Left set of data plots: Cerebellum volume trajectories by tissue type of the 328 no/low drinkers plotted over age at MRI. The lmer fits with +/−1 and 2 SD separately computed for boys (blue) and girls (red) are also plotted. Right set of data plots: Slope (expressed as % change per year) of each participant plotted over age at initial MRI. Although the regression fits by sex are different, overall the rate of gray matter volume loss diminishes with age, while white matter growth slows in older adolescence in both sexes. Trajectories of the CSF volumes followed quadratic function in boys and a linear function in girls; the suggestion of increasing slopes in early adulthood might herald the normal aging effect of CSF volume accrual with age-related tissue shrinkage.
Figure 2.
Top: Color-coded labels of the quantified lobules and vermis of the cerebellum. Data plots: Jitterplots of slopes (expressed as % change per year) by tissue type for each male (blue) and female (red) participant who remained as the 328 no/low drinkers. The asterisks mark sex differences in slopes meeting correction for multiple comparisons (*p≤0.005; **p≤0.001; ***p≤0.0001).
Right row of figures: The coronal cerebellar slices indicate lobules showing sex differences. For gray matter, the slopes were steeper (that is, showed faster volume declines) in female than male participants (lobules V, VI, and X) marked in red, whereas the slopes were steeper in male than female participants (Crus II and VIIB) marked in blue. For white matter, slopes indicated faster increases in Crus I, Crus II, VIIIA, and VIIIB of female than male participants, marked in red. The pattern of sexual dimorphism was complex for CSF volume changes. The slopes were steeper in Crus I, Crus II, and lobule X, marked in blue, indicating faster rates of CSF volume increases in male than female participants. By contrast, the slopes were steeper in lobules VIIB, VIIIA, and VIIIB, marked in red, indicating faster rates of CSF volume decreases in female than male participants.
Statistical Analysis
The final unit of measure for each tissue type was its probability times the voxel volume. Dependent measures were segmented volumes of gray matter, white matter, and CSF for the whole cerebellum and for volumes of the SUIT lobular and vermis parcellations. Covariate variables were self-identified ethnicity (Asian, African-American, Caucasian, other), collection site, scanner manufacturer (GE, Siemens), and intracranial volume (ICV)
Developmental patterns derived from the no/low drinking group.
Analysis of the cerebellum as a whole and as segmented by tissue type was multi-layered, starting with a salient sex difference that all native volumes were significantly greater for male than female participants as groups. These differences were markedly attenuated but not completely eliminated, when controlling for ICV, manufacturer, site, and ethnicity using stepwise Akaike information criterion (stepAIC in R [http://www.r-project.org/]) to select variables to include in the final general linear model. Linear mixed effects modeling (lmer in R) was performed on the residual values (with the mean of all subjects added to preserve relative magnitudes) to examine the volumetric change over age.
Analysis of age-dependent trajectories of parcellated lobules by tissue type was based on slopes, which represented change in cerebellar volumes over time and comprised a series of linear changes per individual. Accordingly, for each subject the slope of 3 or 4 annual data points was computed as a function of the subject's centered age (each age – mean age) and then expressed as a percent of the first (baseline) observation. Thus, slopes were expressed as percent change/year from baseline and were computed for volumes of the total cerebellum and SUIT lobules and each tissue type. Slopes were regressed against (age+age2+ICV+maunfacture+site+ethinicity) using stepAIC to select variables to include in the final general linear model. To preserve directional information, the average slope for the whole group was added to the residuals to form the final slope metric for each subject for each SUIT lobule (also done below). Sex differences were tested with t-tests.
Testing differences between drinking groups.
Slopes of individual participants were computed for all adolescents, those who remained no/low drinkers and those who had moved from no/low consumption to the category of moderate or heavy Cahalan drinking criteria. To determine the effect of drinking, a general linear model predicted slope as a function of drinking (no/low vs. drinker) +age+age2+sex+ICV+manufacturer+site+ethinicity, using stepAIC to select variables to include in the final model. No/low vs. combined moderate plus heavy drinker slope differences were tested with t-tests. This procedure was also performed with separate drinker categories (moderate and heavy).
RESULTS
Trajectories of Normal Cerebellar Structural Growth by Sex
Total volumes by tissue type.
On average the total cerebellum comprising all gray matter, white matter, and CSF volumes was significantly larger for male than female participants (169 vs. 151 cc, t=24.023, df=1158.7, p=0 .00001) with little evidence for growth beyond mid-teen years (Supplemental Fig. 1, top). Adjustment for ICV markedly attenuated the sex difference in cerebellar total volume (Supplemental Fig. 1, bottom).
Segmenting the ICV-corrected total volume by tissue type revealed age-related sex differences in growth patterns. Gray matter volume declined faster in male than female youth with age (lmer age-by-sex Z=−5.874, p=0.0001). By contrast, white matter volume enlarged faster in female than male youth (age-by-sex Z=−3.104, p=0.0019), while CSF volume expanded faster in male than female youth over age (age-by-sex Z=8.150, p=0.0000) (Fig. 1 and Supplemental Fig. 2). The developmental trajectories were quadratic for the boys for all three tissue types but followed linear trends for gray matter and CSF in the girls (Table 2).
Table 2.
Fits of regressions of total volumes by tissue type over age in the no/low group
| Linear | Quadratic | |||||
|---|---|---|---|---|---|---|
| - | age z | age p | age z | age p | age^2 z | age^2 p |
| Male+female | ||||||
| Gray matter | −13.600 | 0.000000 | −6.530 | 0.000000 | 4.738 | 0.000002 |
| White matter | 24.370 | 0.138000 | 10.189 | 0.000000 | −6.952 | 0.000000 |
| CSF | 4.058 | 0.000050 | 5.642 | 0.000000 | −5.160 | 0.000000 |
| Total | 8.932 | 0.000000 | 7.769 | 0.000000 | −6.598 | 0.000000 |
| Male | ||||||
| Gray matter | −13.420 | 0.000000 | −7.370 | 0.000000 | 5.550 | 0.000000 |
| White matter | 15.910 | 0.000000 | 4.584 | 0.000005 | −2.468 | 0.013600 |
| CSF | 7.753 | 0.000000 | 9.710 | 0.000000 | −8.697 | 0.000000 |
| Total | 6.611 | 0.000000 | 7.241 | 0.000000 | −6.362 | 0.000000 |
| Female | ||||||
| Gray matter | −6.143 | 0.000000 | - | - | - | - |
| White matter | 18.570 | 0.000000 | 9.663 | 0.000000 | −7.172 | 0.000000 |
| CSF | −3.331 | 0.000865 | - | - | - | - |
| Total | 6.056 | 0.000000 | 3.608 | 0.000309 | −2.818 | 0.004829 |
| Age-by-sex interaction | ||||||
| Gray matter | −5.874 | 0.000000 | ||||
| White matter | −3.104 | 0.001910 | ||||
| CSF | 8.150 | 0.000000 | ||||
| Total | 1.483 | 0.138000 |
Slopes of lobular volumes by tissue type (Fig. 2, Supplemental Table 1).
Gray matter volumes declined with age in all 11 regions in male youth and in 10 regions in female youth, whose sole positive slope was for lobule VIIB. Significantly faster gray matter volume declines occurred in lobules V, VI, and X of girls relative to boys, whereas boys showed faster volume declines than girls in Crus II. Lobule VIIB trajectories were positive for the girls but negative for the boys. White matter slopes indicated positive volumetric acceleration that was significantly greater in the girls than boys in Crus I, Crus II, VIIIA, and VIIIB. CSF volume slopes of Crus I and the vermis were positive for both sexes but indicated faster increases for male than female youth. The opposite occurred for VIIB, VIIIA, and VIIIB. The CSF expansion patterns indicated positive slopes for male youth and negative slopes for female youth in Crus II and IX.
Deviations from Normal Cerebellar Development in Drinkers
All analyses seeking group differences or correlations controlled for age, sex, manufacturer, site, ethnicity, and ICV. Change in volumes (i.e., trajectory) is expressed as slope, that is, percent change per year from baseline.
Slopes of volumes by tissue type.
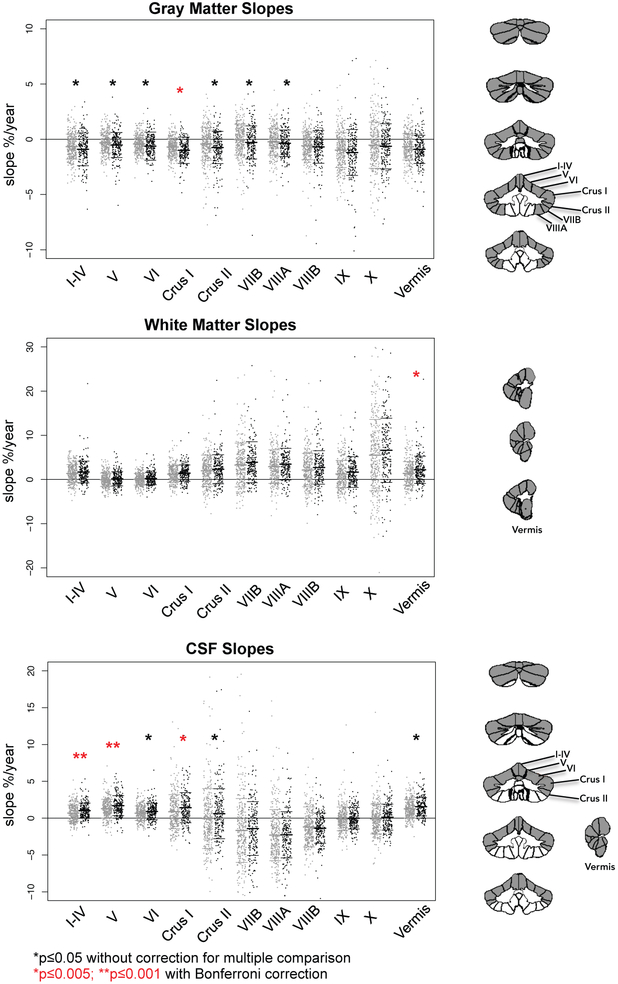
The trend across all lobules and the vermis was for the drinkers to have faster declining gray matter volumes with age than the no/low group (Supplemental Figure 2; Figs. 3-5). The gray matter slope differences were strongest in the anterior-superior lobules (I-IV, V, VI, Crus I, Crus II, VIIB with p≤0.05) with Crus I meeting the most stringent correction for multiple comparisons (Supplemental Table 2). Both the white matter and the CSF slope differences indicated that the drinkers had faster increasing volumes than the no/low group. Significant accelerations in the drinkers were present in white matter volumes of vermis and CSF volumes of lobules I-IV, V, and Crus I (Fig. 5). In no case was the group-by-sex interaction significant.
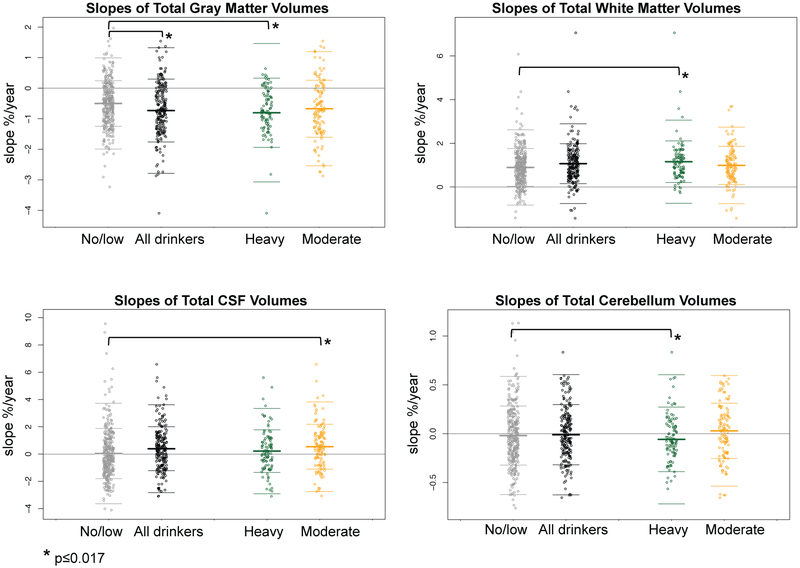
Figure 3.
Jitterplots of total cerebellar slopes (expressed as % change per year) by each tissue type for the 328 no/low drinkers (gray), all 220 youth who initiated moderate or heavy drinking (black), and the drinkers divided by amount drunk: 100 heavy drinkers (green) and 120 moderate drinkers (gold). The asterisks mark differences from the no/low drinking group in slopes meeting correction for multiple comparisons (*p≤0.017).
Figure 5.
Jitterplots of slopes (expressed as % change per year) by tissue type for the 328 no/low drinkers (gray) and all 220 youth who initiated moderate or heavy drinking (black). The red asterisks mark differences from the no/low drinking group in slopes meeting correction for multiple comparisons (*p≤0.005; **p≤0.001). The black asterisks note differences with p≤0.05 (also see Supplemental Figure 2). The cerebellar images to the right of the jitterplots display in gray the lobules showing group differences (p≤0.05) in slopes.
Exploratory correlations among and with lobular trajectories.
Examination of the degree to which cerebellar lobules had similar or dissimilar age-related developmental trajectories was examined by construction of correlational matrices for the gray matter volume slopes of the 10 cerebellar lobules and the vermis (55 pairs of correlations) separately for the no/low and drinking groups. The average within-cerebellum correlations were r=.372 for the no/low group and r=.525 for the drinking group. Although no individual pair of correlational differences between drinkers and no/lows met statistical significance criteria for comparison between two correlations, of the 55 possible pairs of correlations 53 were higher for the drinkers than the no/low drinkers (Supplemental Fig. 3). The 55 correlations for no/lows and the 55 correlations for drinkers (after r-to-z transformation) were entered as separate values into a two-group t-test and revealed that overall the correlations of the drinkers were significantly higher than those of the no/low group (t=3.736, df=107.59, p=.0003).
Simple correlations between motor performance on the Grooved Pegboard Test (dominant hand; see Supplemental Information for test description (41)) and each cerebellar gray matter slope revealed small negative correlations with slopes of lobule I-IV and the vermis that were numerically great in the total group of drinkers (IV r=−.208, p=.0022; vermis r=−.167, p=.0144) than in the controls (IV r=−.044, p=.4484; vermis r=−.031, p=.5861). The differences between correlations, however, were not significant (I-IV z=−.1.869, p=.0617; vermis z=−1.532, p=.1255).
Exploration of group differences in regional gray matter slopes in relation to family history of alcoholism identified a few modest differences, none of which would sustain correction for multiple comparisons (α=.05 for 12 comparisons, p≤.004, 2-tailed). The largest difference was observed in the drinkers, where the family history positive group had a steeper slope of lobule I-IV (i.e., faster volume decline; mean=−.913) than the family history negative group (mean=−.207; t=−2.749, p=.0127).
Differences by drinking level.
Exploratory analyses tested for differences between the no/low drinking group and separately for the moderate and heavy drinking subgroups (Table 3). For the total cerebellum the heavy drinkers had faster rates of gray matter decline and white matter increase than the no/low drinkers (p<0.015). The moderate drinkers had faster rates of CSF increase than the no/low drinkers (p=0.0065).
Table 3.
Group differences in slopes of volumes of cerebellar tissue type
| No/low Youth (N=328) |
Moderate Drinkers (N=120) |
No/low vs. Moderate |
|||||
|---|---|---|---|---|---|---|---|
| Slope† | Mean | SD | Mean | SD | t | df | p†† |
| Gray matter (overall) | −0.501 | 0.746 | −0.673 | 0.934 | 1.811 | 177.513 | 0.0718 |
| White matter (overall) | 0.895 | 0.863 | 0.990 | 0.876 | −1.021 | 208.942 | 0.3082 |
| CSF (overall) | 0.039 | 1.842 | 0.537 | 1.644 | −2.746 | 235.296 | 0.0065 |
| No/low Youth (N=328) |
Heavy Drinkers (N=100) |
No/low vs. Heavy |
|||||
| Mean | SD | Mean | SD | t | df | p†† | |
| Gray matter (overall) | −0.501 | 0.746 | −0.803 | 1.133 | 2.501 | 126.235 | 0.0137 |
| White matter (overall) | 0.895 | 0.863 | 1.158 | 0.952 | −2.473 | 152.008 | 0.0145 |
| CSF (overall) | 0.039 | 1.842 | 0.217 | 1.565 | −0.955 | 189.998 | 0.3407 |
| No/low Youth (N=328) |
All Drinkers (N=220) |
No/low vs. All |
|||||
| Mean | SD | Mean | SD | t | df | p†† | |
| Gray matter (overall) | −0.501 | 0.746 | −0.732 | 1.029 | 2.858 | 369.893 | 0.0045 |
| White matter (overall) | 0.895 | 0.863 | 1.067 | 0.913 | −2.203 | 451.626 | 0.0281 |
| CSF (overall) | 0.039 | 1.842 | 0.391 | 1.613 | −2.368 | 508.888 | 0.0183 |
Slopes are adjusted for ICV, age, SES, site, manufacturer
Bonferroni correction (alpha=.05, 2-tailed) for 3 tissue comparisons p ≤ 0.017
Modest nonparametric correlations emerged between the log-transformed lifetime drinking days across all participants and slopes of each tissue volume. A negative correlation with gray matter slopes (Rho=−0.098, p=0.0211) indicated steeper trajectory declines with more alcohol exposure. Positive correlations with white matter (Rho=0.111, p=0.0093) and CSF (Rho=0.116, p=0.0068) slopes indicated faster increases with greater alcohol exposure.
Marijuana and Alcohol Co-Use
Among all participants who endorsed using alcohol or marijuana (N=321), 127 (40%) youth consumed alcohol but not marijuana, 171 (53%) were co-users, and only 23 (7%) used marijuana but not alcohol. To explore the contribution of marijuana use, we used two approaches to examine the effects and interactions of lifetime marijuana use on slopes of total cerebellum volume and tissue types (Supplemental Table 3).
Firstly, to the full linear models (no/low vs. drinker and no/low vs. moderate plus heavy drinker), controlling for age, sex, manufacturer, site, ethnicity, and ICV, we added log lifetime days of marijuana use. This analysis failed to yield any significant marijuana use effects or interactions for slopes of any total cerebellum tissue type.
Secondly, using data from all 548 participants, we entered log lifetime days of marijuana use and log lifetime drinking days instead of the alcohol use categorical grouping variables into the GLMs. Using marijuana days without considering alcohol use revealed a significant effect of lifetime marijuana on the gray matter slope (Z=−1.982, p=.0480); a similar analysis of lifetime drinking days without consideration of marijuana yielded a larger effect (Z=−3.424, p=.00062). When marijuana and alcohol were entered into the same model, only the alcohol effect (Z=−2.462, p=.0141) and not a marijuana effect (Z=−0.958, p=.3384) was significant, and there was not an interaction between the two use measures (Z=0.949, p=.3432). A similar pattern of results emerged for cerebellar white matter slopes but produced no significant effects or interactions for marijuana. Further, neither lifetime drinking nor lifetime marijuana use was a significant predictor of CSF volume slopes.
DISCUSSION
In addressing the three study aims, we found that 1) the cerebellar volumes of youth who remained no/low drinkers for all MRI examinations exhibited sexual dimorphism with male youth having larger gray matter, white matter, and CSF volumes, a difference that was attenuated with adjustment for ICV; 2) normal developmental rates of tissue and lobular volumetric change differed by sex; and 3) rates of change, detected most robustly in regional gray matter and CSF volume slopes, were greater in youth who initiated substantial alcohol use than in youth who refrained from such drinking.
Patterns of Normal Cerebellar Development
In the 348 participants who remained no/low drinkers, the annual rates of change differed by tissue type and sex, effects not detectable when measuring the total, undifferentiated cerebellum. Gray matter volumes declined faster and CSF volumes increased faster in male than female youth, while white matter volume expanded at faster rates in female than male youth. Annual rates of gray matter volume declines for male youth were quadratic and on average −0.61%/year and were linear for female youth and on average −0.53%/year. By contrast, rates of white matter volume growth were 1.11%/year for female and 0.86%/year for male youth. Although CSF volumes expanded in male youth (0.71%/year), CSF volumes contracted in female youth (−0.34%/year). The gray matter findings are consistent with a previous longitudinal study (31), where boys followed a quadratic function with a peak in gray matter volume at age 15.6 years, whereas girls showed a steady decline without evidence for an inflection over the 7-24 year age range examined.
Tracking neuromaturational change in tissue constituent by lobular volume and sex is novel and extends the few existing longitudinal studies of adolescent cerebellar development. Gray matter volume declines were faster in lobules V, VI, and X of girls than boys, whereas the opposite held for Crus II and lobule VIIB; these lobules are included in the superior-posterior region and are among the last to have developed phylogenetically in evolution and ontogenetically in adolescence (26). Although white matter volume evidenced expansion in both sexes, growth rates were greater in female than male youth in Crus I and II and lobules VIIIA and VIIIB; these lobules are included in the inferior-posterior region, which were among the first to develop phylogenetically (26). Rates of CSF volume changes were variable by lobule and sex, showing greater expansion in Crus I and II, lobule IX, and the vermis of boys than girls, but greater expansion in lobules VIIIA and VIIIB of girls than boys. Division of lobule by tissue type expands depiction of age and sex influences on maturation of the cerebellum, contributing to underlying causes of its regional allometry (26). One resulting speculation suggests that If regions differ in rates of development, then it may be that untoward effects of exposure to agents such as alcohol or drugs would be magnified during active development.
Alcohol Use-related Deviations from Normal Cerebellar Development
Quantitative structural analysis of the cerebellum revealed accelerated gray matter decline and CSF expansion in the total group of youth who initiated moderate to heavy drinking. The trend across all lobules and the vermis was for the drinkers to have faster declining gray matter volumes with age than the no/low group. These alcohol-use-related cerebellar trajectory differences were located primarily in the vermis and anterior-superior lobules, which are regions commonly affected in adults with chronic alcoholism [(in vivo5, 6); (postmortem2, 3, 4)]. Remaining to be tested is whether youth who refrain from excessive alcohol consumption can show structural recovery; further, even if recovery occurs, one might question whether the structures affected with youthful drinking are selectively vulnerable to age-alcohol interactions in later adulthood (42).
Several findings based on correlations were consistent with the possibility that deviations from normal growth trajectories were related to drinking. Firstly, albeit modest, simple correlations between greater percent changes per year of each tissue type and number of drinking days at the final MRI suggest an alcohol dose effect. Secondly, the correlations of gray matter trajectories among the lobules were higher in the moderate-to-heavy drinkers than in the no-to-low drinkers, suggesting an emergent homogeneity of interrelations among trajectories of the cerebellar lobular volumes in the drinkers. We speculate that attenuation of normal allometric heterogeneity (and possible heterochronicity) of developmental trajectories may reflect an alcohol-induced synchrony among structural developmental trajectories of the cerebellum.
Limitations
Despite the prospective nature of this study, factors in addition to alcohol consumption may have contributed to deviations from the norm. One factor was marijuana use. In our NCANDA cohort, many consumed both alcohol and marijuana, but disproportionately more youth drank alcohol only than consumed marijuana only. Including days of marijuana use in the analyses did not independently contribute to the detection of alcohol effects. Although we may not have had the power to detect specific untoward marijuana effects because marijuana use was not the primary focus of this study and so few participants used marijuana without alcohol, our results comport with a longitudinal study of 1,000 adolescent boys focused on developing marijuana use trajectories of no-to-high use (43). About a decade after initial questioning, a subset of 181 young men underwent MRI; grouping by marijuana use trajectory yielded no regional volumetric differences, leading to the conclusion that adult brain structure is not associated with adolescent marijuana use.
Further, lack of significant interactions indicating sexual dimorphism in trajectory differences does not necessarily confirm their absence; rather, they may have been below detection in our current sample because of its age range and consumption rates among other factors. In addition, reliance on self-reported alcohol and drug use, while essential without continuous monitoring, is subject to imperfect recall and guarded responses.
Conclusions
The cerebellum is aptly named the "little brain," being only 13% of the total intracranial volume. Despite its size, the cerebellum has 3.6 times the number of neurons in the cerebral cortex (44), has major circuitry relays with cortical systems (32, 33), and appears to undergo pruning and remodeling during adolescence analogous to that of the cortex (45). Taken in the context of our previous report on neurodevelopment and the toll initiation of alcohol consumption takes on the cerebral cortex (11), we conclude that adolescents who initiate drinking are vulnerable to trajectory disturbance of normal brain development affecting extensive frontal-cingulate-cerebellar systems. Continued examination of the NCANDA cohort has the potential to detect further divergence from normal trajectories with continued drinking and to localize extent of recovery with sustained abstinence.
Supplementary Material
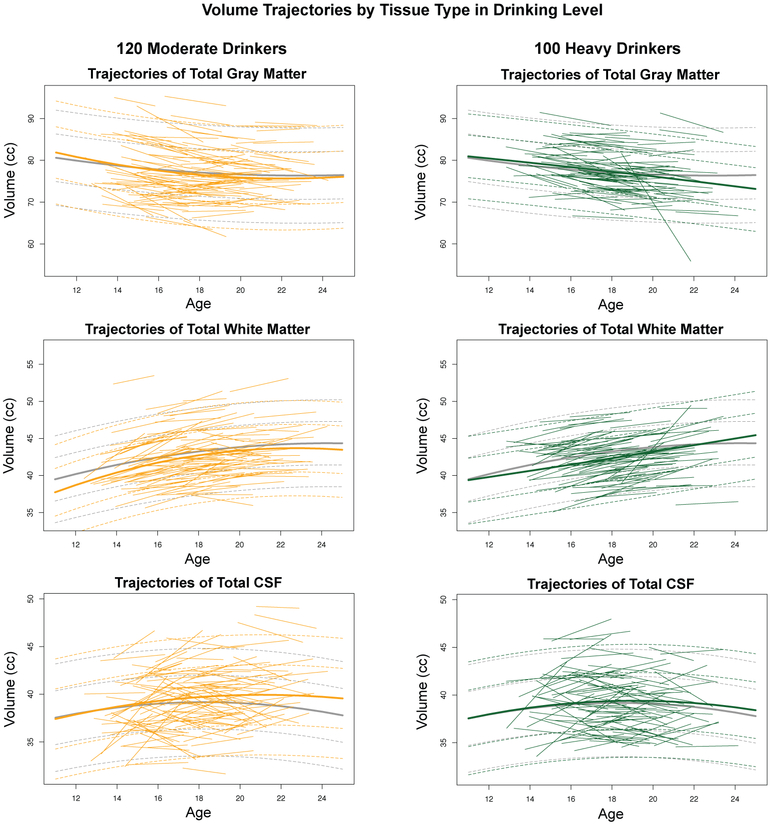
Figure 4.
Trajectories (i.e., regression lines) of individual moderate drinkers (gold) and heavy drinkers (green). The lmer fits with +/−1 and 2 SD separately computed for no/low drinkers (gray), moderate drinkers (gold), and heavy drinkers (green) are also plotted.
Key Resource Table
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | N/A | N/A | N/A | N/A |
| Bacterial or Viral Strain | N/A | N/A | N/A | N/A |
| Biological Sample | N/A | N/A | N/A | N/A |
| Cell Line | N/A | N/A | N/A | N/A |
| Chemical Compound or Drug | N/A | N/A | N/A | N/A |
| Commercial Assay Or Kit | N/A | N/A | N/A | N/A |
| Deposited Data; Public Database | N/A | N/A | N/A | N/A |
| Genetic Reagent | N/A | N/A | N/A | N/A |
| Organism/Strain | N/A | N/A | N/A | N/A |
| Peptide, Recombinant Protein | N/A | N/A | N/A | N/A |
| Recombinant DNA | N/A | N/A | N/A | N/A |
| Sequence-Based Reagent | N/A | N/A | N/A | N/A |
| Software; Algorithm | R statistical package online | R 3.5.1 (R Core Team, 2019) | R [http://www.r-project.org/] | N/A |
| Transfected Construct | N/A | N/A | N/A | N/A |
| Other | N/A | N/A | N/A | N/A |
Acknowledgments
This work was supported by the U.S. National Institute on Alcohol Abuse and Alcoholism with co-funding from the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Child Health and Human Development [NCANDA grant numbers: AA021697 (Multiple Principal Investigators (MPI), Drs. Pfefferbaum and Pohl), AA021695 (MPI, Drs. Brown and Tapert), AA021692 (PI, Dr. Tapert), AA021696 (MPI, Drs. Colrain and Baker), AA021681 (PI, Dr. De Bellis), AA021690 (PI, Dr. Clark), AA021691 (PI, Dr. Nagel)] and NIAAA R37 AA010723 (PI, Dr. Sullivan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Lhermitte J (1935): Cortical Cerebellar Degeneration: (Section of Neurology). Proc R Soc Med. 28:379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Victor M, Adams RD, Collins GH (1989): The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition (2nd edition) Philadelphia: F.A. Davis Co. [Google Scholar]
- 3.Torvik A, Torp S (1986): The prevalence of alcoholic cerebellar atrophy: A morphometric and histological study of an autopsy material. Journal of Neurological Science. 75:43–51. [DOI] [PubMed] [Google Scholar]
- 4.Baker K, Harding A, Halliday G, Kril J, Harper C (1999): Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke's encephalopathy. Neuroscience. 91:429–438. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A (2000): Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: Relation to ataxia. Neuropsychology. 14:341–352. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan EV, Rose J, Pfefferbaum A (2006): Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex. 16:1077–1086. [DOI] [PubMed] [Google Scholar]
- 7.Sawyer KS, Oscar-Berman M, Mosher Ruiz S, Galvez DA, Makris N, Harris GJ, et al. (2016): Associations Between Cerebellar Subregional Morphometry and Alcoholism History in Men and Women. Alcohol Clin Exp Res. 40:1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan EV, Zahr NM, Saranathan M, Pohl KM, Pfefferbaum A (2019): Convergence of three parcellation approaches demonstrating cerebellar lobule volume deficits in Alcohol Use Disorder. Neuroimage Clin. 24:101974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Q, Pfefferbaum A, Podhajsky S, Pohl KM, Sullivan EV (2019): Accelerated aging and motor control deficits are related to regional deformation of central cerebellar white matter inalcohol use disorder. Addiction biology.1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, et al. (2015): Brain development in heavy-drinking adolescents. Am J Psychiatry. 172:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, et al. (2018): Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. Am J Psychiatry. 175:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson S, Malone SM, Thomas KM, Iacono WG (2015): Adolescent drinking and brain morphometry: A co-twin control analysis. Dev Cogn Neurosci. 16:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisdahl KM, Thayer R, Squeglia LM, McQueeny TM, Tapert SF (2013): Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry research. 211:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB (2005): Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 29:1590–1600. [DOI] [PubMed] [Google Scholar]
- 15.Hill SY, Lichenstein SD, Wang S, O'Brien J (2016): Volumetric Differences in Cerebellar Lobes in Individuals from Multiplex Alcohol Dependence Families and Controls: Their Relationship to Externalizing and Internalizing Disorders and Working Memory. Cerebellum. 15:744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silveri MM, Dager AD, Cohen-Gilbert JE, Sneider JT (2016): Neurobiological signatures associated with alcohol and drug use in the human adolescent brain. Neurosci Biobehav Rev. 70:244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC (2018): The age of adolescence. Lancet Child Adolesc Health. 2:223–228. [DOI] [PubMed] [Google Scholar]
- 18.Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, et al. (2016): Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex. 26:4101–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO (1994): A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 51:874–887. [DOI] [PubMed] [Google Scholar]
- 20.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nat Neurosci. 6:309–315. [DOI] [PubMed] [Google Scholar]
- 21.Raznahan A, Lerch JP, Lee N, Greenstein D, Wallace GL, Stockman M, et al. (2011): Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 72:873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taki Y, Hashizume H, Thyreau B, Sassa Y, Takeuchi H, Wu K, et al. (2013): Linear and curvilinear correlations of brain gray matter volume and density with age using voxel-based morphometry with the Akaike information criterion in 291 healthy children. Human brain mapping. 34:1857–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brain Development Cooperative G (2012): Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb Cortex. 22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernard JA, Leopold DR, Calhoun VD, Mittal VA (2015): Regional cerebellar volume and cognitive function from adolescence to late middle age. Human brain mapping. 36:1102–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace GL, Eric Schmitt J, Lenroot R, Viding E, Ordaz S, Rosenthal MA, et al. (2006): A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 47:987–993. [DOI] [PubMed] [Google Scholar]
- 26.Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN (2010): Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 49:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfefferbaum A, Zahr NM, Sassoon SA, Kwon D, Pohl KM, Sullivan EV (2018): Accelerated and Premature Aging Characterizing Regional Cortical Volume Loss in Human Immunodeficiency Virus Infection: Contributions From Alcohol, Substance Use, and Hepatitis C Coinfection. Biological psychiatry Cognitive neuroscience and neuroimaging. 3:844–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giorgio A, Watkins KE, Chadwick M, James S, Winmill L, Douaud G, et al. (2010): Longitudinal changes in grey and white matter during adolescence. Neuroimage. 49:94–103. [DOI] [PubMed] [Google Scholar]
- 29.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brouwer RM, Panizzon MS, Glahn DC, Hibar DP, Hua X, Jahanshad N, et al. (2017): Genetic influences on individual differences in longitudinal changes in global and subcortical brain volumes: Results of the ENIGMA plasticity working group. Human brain mapping. 38:4444–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Durston S (2014): Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage. 96:67–72. [DOI] [PubMed] [Google Scholar]
- 32.Kelly RM, Strick PL (2003): Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmahmann JD, Guell X, Stoodley CJ, Halko MA (2019): The Theory and Neuroscience of Cerebellar Cognition. Annu Rev Neurosci. [DOI] [PubMed] [Google Scholar]
- 34.Mankiw C, Park MTM, Reardon PK, Fish AM, Clasen LS, Greenstein D, et al. (2017): Allometric Analysis Detects Brain Size-Independent Effects of Sex and Sex Chromosome Complement on Human Cerebellar Organization. J Neurosci. 37:5221–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardenas VA, Price M, Infante MA, Moore EM, Mattson SN, Riley EP, et al. (2014): Automated cerebellar segmentation: Validation and application to detect smaller volumes in children prenatally exposed to alcohol. Neuroimage Clin. 4:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, et al. (2015): The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs. 76:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akshoomoff N, Newman E, Thompson WK, McCabe C, Bloss CS, Chang L, et al. (2014): The NIH Toolbox Cognition Battery: results from a large normative developmental sample (PING). Neuropsychology. 28:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW (1998): Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 59:427–438. [DOI] [PubMed] [Google Scholar]
- 39.Cahalan D, Cisin IH, Crossley HM (1969): American Drinking Practices: A National Study of Drinking Behavior and Attitudes. New Brunswick, NJ: Rutgers Center of Alcohol Studies. [Google Scholar]
- 40.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009): A probabilistic MR atlas of the human cerebellum. Neuroimage. 46:39–46. [DOI] [PubMed] [Google Scholar]
- 41.Klove H (1963): Clinical Neuropsychology. Med Clin North Am. 47:1647–1658. [PubMed] [Google Scholar]
- 42.Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, et al. (2018): The Role of Aging, Drug Dependence, and Hepatitis C Comorbidity in Alcoholism Cortical Compromise. JAMA psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier MH, Schriber RA, Beardslee J, Hanson J, Pardini D (2019): Associations between adolescent cannabis use frequency and adult brain structure: A prospective study of boys followed to adulthood. Drug Alcohol Depend. 202:191–199. [DOI] [PubMed] [Google Scholar]
- 44.Andersen BB, Korbo L, Pakkenberg B (1992): A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol. 326:549–560. [DOI] [PubMed] [Google Scholar]
- 45.Feinberg I, Thode HC Jr., Chugani HT, March JD (1990): Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. J Theor Biol. 142:149–161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.