Abstract
Background:
The effect of surgeon factors on patient-reported quality of life outcomes after breast conserving therapy (BCT) is unknown and may help patients make informed care decisions.
Methods:
We performed a survey study of women age ≥67 with non-metastatic breast cancer diagnosed in 2009 treated with guideline-concordant BCT to determine the association of surgeon factors with patient-reported outcomes. The treating surgeon was identified using Medicare claims and surgeon factors identified via the AMA Physician Masterfile. The primary outcome was patient-reported cosmetic satisfaction measured by CanSORT Satisfaction with Breast Cosmetic Outcome. Secondary outcomes included BREAST-Q subdomains. All patient, treatment, and surgeon covariables were included in a saturated multivariable linear regression model with backward elimination applied until remaining variables were P<0.1.
Results:
Of 1650 women randomly selected to receive the questionnaire, 489 responded and of these, 289 underwent BCT. Median age at diagnosis was 72 years and time from diagnosis to survey was 6 years. Mean adjusted CanSORT score was higher for patients treated by surgical oncologists than patients treated by non-surgical oncologists (4.01 [95% CI 3.65–4.38] vs. 3.53 [95% CI 3.28–3.77]), P=0.006). Similarly, mean adjusted BREAST-Q Physical Well-Being (91.97 [95% CI 86.13–97.80] vs. 83.04 [95% CI 80.85–85.22], P=0.006) and Adverse Radiation Effects (95.28 [95% CI 91.25–99.31] vs. 88.90 [95% CI 86.23–91.57], P=0.004) scores were better among patients treated by surgical oncologists.
Conclusions:
Specialized surgical oncology training is associated with improved long-term patient-reported outcomes. These findings underscore the value of specialized training and may be useful to patients choosing their care team.
Introduction
Older breast cancer patients face many decisions regarding their treatment, including type of primary surgery, reconstruction (if any), and radiation therapy, including the option to forego radiation therapy in some cases.1,2 Patients rely on their physicians to discuss the advantages and drawbacks of each treatment option,3 and there is an increasing body of evidence to assist with these discussions.4–8 Previous studies have shown that the treating surgeon can have a significant impact on the type of therapy a patient receives,9–11 and that older patients are more likely than younger patients to defer to their physicians for treatment decisions.12,13
Beyond the influence of treating surgeons on patient treatment decisions, there is evidence to suggest an association between the treating surgeon and patient outcomes. For example, surgeon expertise has been shown to correlate with overall patient satisfaction with their treatment experience.14 However, little is known about how individual surgeons’ characteristics influence long-term patient-reported outcomes (PROs). Such information may be helpful for patients choosing among multiple potential providers and treatment settings and in targeting quality improvement interventions.
We recently performed a national survey study of older breast cancer patients following definitive treatment to determine the associations between local therapy type and long-term patient-reported QOL outcomes.15 Within the subgroup of patients treated with breast-conserving local therapy, where optimal cosmetic outcome is both a key goal of therapy and known to vary considerably,16 we hypothesized that the specialization of the treating surgeon contributes to patient-reported cosmetic satisfaction. To test this hypothesis, we evaluated whether surgeon factors were associated with long-term cosmetic satisfaction and other important PROs in older breast cancer survivors treated with breast-conserving therapy.
Methods
This study was approved by the Institutional Review Board of the University of Texas MD Anderson Cancer Center as well the Centers for Medicare and Medicaid Services.
Cohort Selection
We identified a cohort of potential study participants by applying a validated algorithm to national Medicare claims, which identified women age 67 years of age or older diagnosed with early stage invasive breast cancer in 2009 (N = 42,735).17,18 Of these, we included only patients who were treated with one of five guideline-concordant local therapy strategies: lumpectomy with whole breast irradiation (Lump+WBI), lumpectomy with brachytherapy (Lump+Brachy, inclusive of both intracavitary balloon-based and interstitial high dose rate procedures), lumpectomy with endocrine therapy alone (Lump alone), mastectomy without radiation (Mast alone), or mastectomy with radiation (Mast+RT). Patients had to be alive as of May 2015, possess continuous Medicare Part A and B coverage from diagnosis through 2011, and be without new diagnosis cancer following breast cancer diagnosis (N = 26,069). Study participants were selected from the remaining patients (eTable 1).
Determination of Local Therapy
We used International Classification of Diseases (ICD-9) and Common Procedural Terminology (CPT) code sets to determine receipt of surgery and/or radiation. “Lump+WBI” was defined as lumpectomy with 15 or more external beam radiation fractions and no brachytherapy. “Lump+Brachy” was defined as a lumpectomy followed by brachytherapy and no external beam radiation treatment. “Lump alone” was defined as a lumpectomy with a dispensed prescription for endocrine therapy. “Mast alone” was defined as a mastectomy and no radiotherapy in the subsequent year. “Mast+RT” was defined as a mastectomy with 15 or more external beam radiation fractions.
Sampling Strategy
Lump+WBI was the most common local treatment (N = 2,731) and therefore served as the referent group for all analyses. We determined that including 250 patients per local therapy group would result in 80% power to detect a difference of 0.31 points on a 5-point Likert scale, which was based on prior published data from the CanSORT Breast Cosmetic Outcome instrument. Estimating a 75% response rate, we sampled 330 patients per local therapy group. We first randomly sampled 330 patients who received Lump+WBI and subsequently used sampling without replacement with probability proportional to size to sample 330 patients who received each of the other four types of local therapies so that the age distribution resembled the Lump+WBI group.
Survey Collection
The Centers for Medicare and Medicaid Services Beneficiary Contact Service mailed an informational flyer including a description of our study and an opt-out, postage-paid response card to 1,650 potential participants in September 2015. We received 397 (24%) opt-out responses. In November 2015, a study invitation letter, $10 gift card, and questionnaire were sent by mail to 1,253 potential participants and followed up with non-responders by mail and phone through January 2016.
Survey Measures
We developed a paper questionnaire to collect baseline patient information and PROs in breast cancer patients. We piloted the questionnaire in 10 breast cancer patients returning for follow up care at our institution. Cognitive interviews were conducted to obtain feedback on the length and clarity of the questionnaire.19
The survey assessed demographic characteristics including race/ethnicity, education, and income. Other personal and clinical items included current height and weight, weight at diagnosis, bra cup size, smoking history, subsequent local or distant breast cancer recurrence or mastectomy, and second cancer diagnoses.
The primary outcome, defined a priori, was patient-reported cosmetic satisfaction evaluated using the Cancer Surveillance and Outcomes Research Team (CanSORT) Satisfaction with Breast Cosmetic Outcome instrument.4 This validated tool consists of six items scored with a 5-point Likert scale. The total score is the arithmetic mean of the six items, and a higher score indicates a higher level of satisfaction. A difference in mean scores of 0.3 or greater was considered clinically relevant, as our prior data from this cohort indicated that 0.3 is the approximate magnitude of the difference in satisfaction between breast-conserving therapy and mastectomy.15
Secondary outcomes were measured using up to five independent BREAST-Q modules20 including Satisfaction with Breasts, Physical Well-Being, Adverse Effects of Radiation, Psychosocial Well-Being, and Sexual Well-Being. The BREAST-Q is a validated measure of patient satisfaction and QoL after breast cancer surgery. For each measure, the sum score is converted to an equivalent Rasch transformed score that ranges from 0–100. Higher scores indicate better outcomes. Clinically relevant differences in BREAST-Q scores have been reported for the core modules as follows: satisfaction with Breasts, 8 points; Physical Well-Being, 7 points; Psychosocial Well-Being, 10 points; and Sexual Well-Being, 10 points.21
Determination of Surgeon Factors
For each patient, we identified the treating surgeon, defined as the surgeon who performed the initial local breast surgery via Medicare claims.10 The following surgeon factors were obtained via linkage to the American Medical Association (AMA) Physician Masterfile by National Provider Identification number: board certification (yes versus no), location of training (United States versus outside of the United States), type of medical degree (MD versus DO), decade of medical school graduation (before 1980 versus after 1980), surgical specialty (surgical oncology versus general surgery/other), surgeon sex (male versus female), and Medicare breast surgery case volume (≤40 cases versus 41–100 cases versus >100 cases). Surgeon subspecialty is determined via self-report. Options for self-reporting include surgical oncology and general surgery; breast surgery is not an option. Surgical oncologists have completed additional fellowship training after general surgery residency focusing on the advanced surgical management of cancers. Case volume was defined as the number of Medicare fee-for-service lumpectomy and mastectomy cases performed by the surgeon from 2007–2010.
Statistical Analysis
Baseline patient characteristics including age, Charlson Comorbidity Index, geographic region, and receipt of axillary surgery or chemotherapy were obtained from Medicare enrollment data, Medicare claims, and self-report (see Survey Measures). Baseline patient characteristics between different local therapy groups were compared using the chi-square test or Fisher’s exact test when cell sizes were small.
Primary and secondary outcomes were analyzed using multivariable linear regression. We included all patient, surgeon, and treatment variables in a multivariable model and applied backward elimination to identify patient, treatment, and surgeon factors associated with each outcome at P<0.1. Type of local therapy was retained in the models regardless of P value. The effect of type of local therapy on PROs is reported elsewhere.15 As a sensitivity analysis, we retained all surgeon factors in the final multivariable models regardless of their significance.
In the study population, 258 surgeons treated one patient each and only 13 surgeons treated two patients each, thus we used a traditional single-level modeling approach for all outcomes and a multilevel model of patients nested within surgeons was created only for the primary outcome as an exploratory analysis. We created non-response weights to account for differential sample selection across treatment groups and age categories. Non-response weights were scaled based on the ratios of numbers of responders versus the total samples in each cell. All analyses used these weights to compensate for different probabilities of sample selection and non-response so that results were more representative of the sampled population.4 Statistical analyses were performed in SAS v9.3 with significance level of two-sided P<0.05.
Results
Patient Characteristics
A total of 489 women responded (30% response rate). In the current study, we included the 289 responders treated with one of three breast-conserving local therapies, including 108 (37%) Lump+WBI, 103 (36%) Lump+Brachy, and 78 (27%) Lump alone. Compared to non-responders, responders were more likely to be younger, white, have not received Lump alone, have not received chemotherapy, have lower Charlson Comorbidity Index, and live in the South geographic region (P<0.05 for all) (eTable 2). The median age at breast cancer diagnosis was 72 years (range, 67–87) and elapsed time from diagnosis to survey completion was about 6 years for all respondents. Other patient characteristics can be found in Table 1.
Table 1.
Patient demographic and clinical characteristics (n=289)
| Characteristic | No. (%)a |
|---|---|
| Local therapy | |
| Lump+WBI | 108 (37.4) |
| Lump+Brachy | 103 (35.6) |
| Lump alone | 78 (27.0) |
| Age, years | |
| Median (range) | 72 (67–87) |
| 67–74 | 200 (57.5) |
| ≥75 | 89 (42.5) |
| Patient-reported race | |
| White | 250 (86.7) |
| Non-white | 39 (13.3) |
| Charlson Comorbidity Index | |
| 0 | 197 (68.5) |
| 1 | 71 (24.8) |
| 2+ | 21 (6.6) |
| Patient-reported baseline BMI | |
| Underweight / Normal | 93 (35.7) |
| Overweight | 77 (21.9) |
| Obese | 103 (34.8) |
| Unknown | 16 (7.7) |
| Patient-reported current bra cup size | |
| A-B | 89 (31.0) |
| C | 88 (31.4) |
| D+ | 81 (25.8) |
| Unknown | 31 (11.8) |
| Axillary surgery | |
| Axillary dissection/axillary surgery NOS | 94 (36.2) |
| Sentinel lymph node biopsy | 126 (45.7) |
| No axillary surgery | 69 (18.0) |
| Patient-reported endocrine therapy | |
| No | 95 (39.5) |
| Yes | 194 (60.5) |
| Chemotherapy | |
| No | 254 (84.7) |
| Yes | 35 (15.3) |
| Region | |
| Northeast | 50 (22.2) |
| Midwest | 68 (20.8) |
| South | 119 (39.3) |
| West or unknown | 51 (17.7) |
| Patient-reported education | |
| High school or less | 95 (32.9) |
| Associate degree | 97 (33.6) |
| College or higher | 82 (28.4) |
| Unknown | 15 (5.2) |
| Patient-reported current household income | |
| <$40K | 120 (44.5) |
| $40–60K | 45 (12.5) |
| >$60K | 67 (22.8) |
| Unknown | 57 (20.2) |
| Has smoked 100 cigarettes in lifetime | |
| No | 154 (53.8) |
| Yes | 119 (40.0) |
| Unknown | 16 (6.2) |
Abbreviations: Lump+WBI = lumpectomy plus whole-breast irradiation; Lump+Brachy = lumpectomy plus brachytherapy; Lump alone = lumpectomy plus endocrine therapy alone; NOS = not otherwise specified.
All percentages are weighted percentages except for local therapy; weights accounted for differential selection and response rates.
Surgeon Factors
Treating surgeon data were available for 284 out of 289 patients (98%), representing 271 unique surgeons (including 13 surgeons who had treated 2 patients each). Most were US-trained (88%), had an MD degree (93%), were male (61%), and did not have specialized surgical oncology training (86%). Surgical oncologists had higher breast surgery case volume compared to non-surgical oncologists from 2007–2010 (61.6% vs. 38.4% with >100 cases, P<0.001). Additional surgeon characteristics are listed in Table 2.
Table 2.
Treating surgeon characteristics (n=271a)
| Characteristic | No. (%)b |
|---|---|
| Board certification | |
| Yes | 244 (87.5) |
| No | 27 (12.5) |
| Location of training | |
| United States | 238 (85.7) |
| Outside of United States | 22 (11.5) |
| Unknown | 11 (2.8) |
| Type of medical degree | |
| MD | 251 (91.2) |
| DO/Unknownc | 20 (8.8) |
| Decade of medical school graduation | |
| Before 1980 | 91 (38.1) |
| 1980 and after | 169 (59.1) |
| Unknown | 11 (2.8) |
| Surgical specialty | |
| General surgery/other | 233 (86.7) |
| Surgical oncology | 38 (13.3) |
| Gender | |
| Male | 164 (64.2) |
| Female | 96 (32.9) |
| Unknown | 11 (2.8) |
| Case volumed | |
| ≤40 | 70 (29.8) |
| 41–100 | 97 (36.3) |
| >100 | 104 (33.9) |
Surgeon data were available for 284 out of 289 patients (98%), and there were 271 unique surgeons.
All percentages are weighted percentages; weights accounted for differential selection and response rates.
Due to small cell sizes, “DO” and “Unknown” categories were combined to avoid compromising provider identity in accordance with the Data User’s Agreement with Centers for Medicare and Medicaid Services.
Number of Medicare fee-for-service cases from 2007–2010
Multivariable Analysis of CanSORT Satisfaction with Breast Cosmetic Outcome and BREAST-Q Satisfaction with Breasts
In multivariable analysis adjusting for type of local therapy, CanSORT Satisfaction with Breast Cosmetic Outcome was higher among patients treated by surgical oncologists compared to non-surgical oncologists (effect size 0.48, [95% CI 0.14–0.83]; P=0.006) (Table 3). This finding was stable in an exploratory multilevel model of patients nested within surgeons (effect size 0.49, [95% CI 0.14–0.83]; P=0.006). Sensitivity analysis with all surgeon factors retained in the final model regardless of their significance showed similar results (eTable 4).
Table 3.
Multivariable linear regression models for CanSORT Satisfaction with Breast Cosmetic Outcome and BREAST-Q Satisfaction with Breasts
| CanSORT (n=282) | BREAST-Q Satisfaction with Breasts (n=273) | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate Coefficient | SE | 95% CI | P Value | Estimate Coefficient | SE | 95% CI | P Value | |
| Intercepta | 3.62 | 0.12 | 3.40 to 3.85 | <0.001 | 60.69 | 3.28 | 54.26 to 67.11 | <0.001 |
| Surgical specialty | ||||||||
| General surgery/other | Referent | |||||||
| Surgical oncology | 0.48 | 0.18 | 0.14 to 0.83 | 0.006 | ||||
| Local therapy | ||||||||
| Lump+WBI | Referent | Referent | ||||||
| Lump+Brachy | 0.35 | 0.13 | 0.10 to 0.61 | 0.007 | 7.06 | 3.25 | 0.70 to 13.42 | 0.031 |
| Lump alone | 0.56 | 0.24 | 0.09 to 1.02 | 0.02 | 8.54 | 4.84 | −0.95 to 18.03 | 0.08 |
| Charlson Comorbidity Index | ||||||||
| 0 | Referent | Referent | ||||||
| 1 | 0.02 | 0.18 | −0.34 to 0.38 | 0.91 | −0.61 | 4.06 | −8.57 to 7.34 | 0.88 |
| 2+ | −1.23 | 0.30 | −1.81 to −0.64 | <0.001 | −19.42 | 5.52 | −30.25 to −8.60 | <0.001 |
| Region | ||||||||
| South | Referent | |||||||
| Northeast | 2.42 | 5.25 | −7.86 to 12.70 | 0.65 | ||||
| Midwest | 8.89 | 4.44 | 0.18 to 17.60 | 0.046 | ||||
| West | −4.73 | 4.70 | −13.95 to 4.48 | 0.31 | ||||
Abbreviations: Lump+WBI = lumpectomy plus whole-breast irradiation; Lump+Brachy = lumpectomy plus brachytherapy; Lump alone = lumpectomy plus endocrine therapy alone; SE = standard error; CI = confidence interval.
Intercept is the expected mean value of the outcome when all covariables are referent.
All analyses incorporated weights to adjust for differential sample selection and response rates.
Adjusted mean CanSORT cosmetic satisfaction score was 4.01 (95% CI 3.65–4.38) for patients treated by surgical oncologists versus 3.53 (95% CI 3.28–3.77) for patients treated by non-surgical oncologists (P=0.006) (Table 4 and Figure 1). Compared to Lump+WBI, patients undergoing Lump alone (effect size 0.56, [95% CI 0.09–1.02]; P=0.02) and Lump+Brachy (effect size 0.35, [95% CI 0.10–0.61]; P=0.007) reported higher CanSORT cosmetic satisfaction. The only patient characteristic associated with CanSORT cosmetic satisfaction was Charlson Comorbidity Index, with higher comorbidity score associated with worse CanSORT cosmetic satisfaction.
Table 4.
Adjusted mean scores for CanSORT Satisfaction with Breast Cosmetic Outcome, Breast-Q Physical Well-Being and Adverse RT Effects
| CanSORTa (n=282) | Breast-Q Physical Well-Beingb (n=275) | Breast-Q Adverse RT Effectsc (n=201) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (95% CI) | P Value | n | Mean (95% CI) | P Value | n | Mean (95% CI) | P Value | |
| Surgical oncologist | 36 | 4.01 (3.65 to 4.37) | 0.006 | 35 | 91.97 (86.13 to 97.80) | 0.006 | 26 | 95.28 (91.25 to 99.31) | 0.004 |
| Non-surgical oncologist | 246 | 3.53 (3.28 to 3.77) | 240 | 83.04 (80.85 to 85.22) | 175 | 88.90 (86.23 to 91.57) | |||
Final model for CanSORT was additionally adjusted for local therapy and Charlson Comorbidity Index.
Final model for Breast-Q Physical Well-Being was additionally adjusted for local therapy and geographic region.
Final model for Breast-Q Adverse Radiation Effects was additionally adjusted for local therapy and bra cup size.
All analyses incorporated weights to adjust for differential sample selection and response rates.
Figure 1.
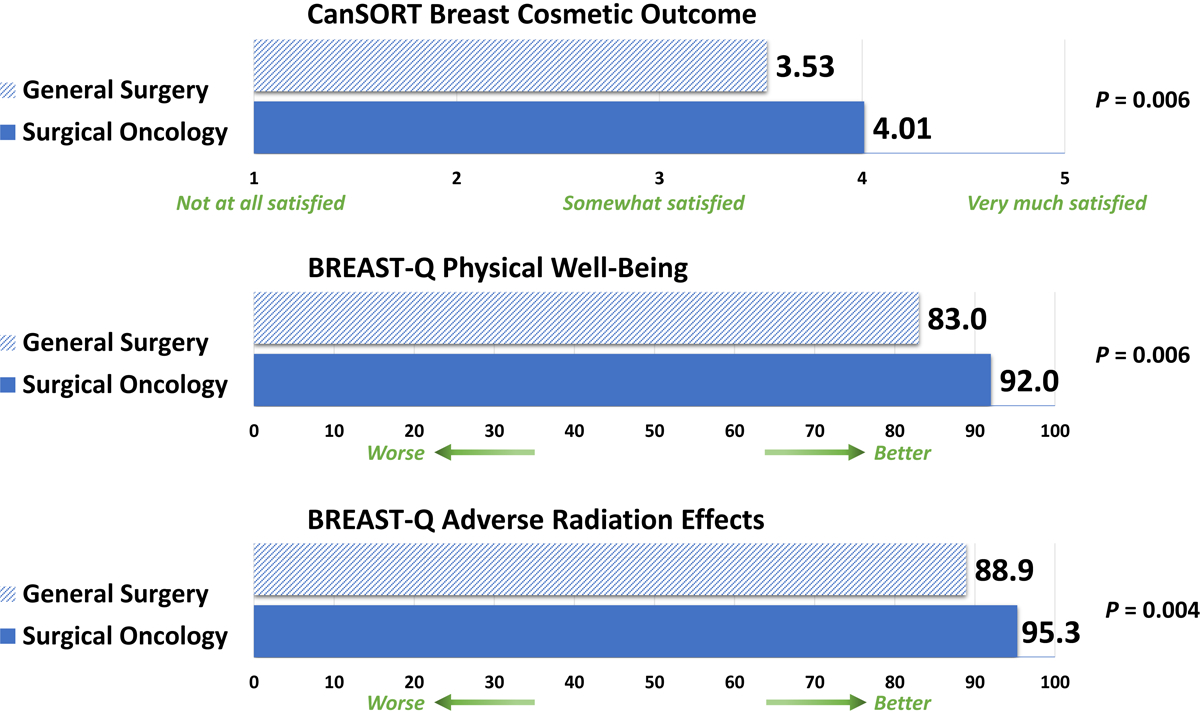
Mean adjusted patient-reported outcome scores by surgical specialization
No significant difference in BREAST-Q Satisfaction with Breasts mean score was detected among patients of surgical oncologists compared to non-surgical oncologists in unadjusted analysis (67.4 vs. 64.2, P=0.42) and no surgeon factors were retained in multivariable analysis. In multivariable analysis, Lump+WBI, higher Charlson Comorbidity Index score, and living in the South geographic region were associated with lower Satisfaction with Breasts (Table 3).
Multivariable Analysis of BREAST-Q Physical, Psychosocial, and Sexual Well-Being and Adverse Radiation Effects
In multivariable analysis for BREAST-Q Physical Well-Being and Adverse Radiation Effects, specialization in surgical oncology was associated with improved outcomes (Table 5). The adjusted mean score for BREAST-Q Physical Well-Being was 91.97 (95% CI 86.13–97.80) for patients treated by surgical oncologists versus 83.04 (95% CI 80.85–85.22) for patients treated by non-surgical oncologists (P=0.006). Likewise, the adjusted mean score for BREAST-Q Adverse Radiation Effects was 95.28 (95% CI 91.25–99.31) for patients treated by surgical oncologists versus 88.90 (95% CI 86.23–91.57) for patients treated by non-surgical oncologists (P=0.004) (Table 4 and Figure 1). Compared to Lump+WBI, Lump+Brachy was associated with better Physical Well-Being and better Adverse Radiation Effects. Living in the South geographic region was associated with worse Physical Well-Being and D+ bra cup size was associated with worse Adverse Radiation Effects (Table 5).
Table 5.
Multivariable linear regression models for BREAST-Q Physical Well-Being and Adverse Radiation Effects
| Physical Well-Being (n=275) | Adverse Radiation Effects (n=201) | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate Coefficient | SE | 95% CI | P Value | Estimate Coefficient | SE | 95% CI | P Value | |
| Intercepta | 77.46 | 2.34 | 72.87 to 82.05 | <0.001 | 90.73 | 1.93 | 86.94 to 94.52 | <0.001 |
| Surgical specialty | ||||||||
| General surgery/other | Referent | Referent | ||||||
| Surgical oncology | 8.93 | 3.23 | 2.61 to 15.26 | 0.006 | 6.38 | 2.17 | 2.13 to 10.63 | 0.004 |
| Local therapy | ||||||||
| Lump+WBI | Referent | Referent | ||||||
| Lump+Brachy | 5.72 | 2.19 | 1.42 to 10.03 | 0.01 | 4.26 | 2.12 | 0.11 to 8.42 | 0.046 |
| Lump alone | 0.28 | 2.67 | −4.96 to 5.51 | 0.92 | . | . | . | . |
| Patient-reported current bra cup size | ||||||||
| A-B | Referent | |||||||
| C | −0.34 | 2.27 | −4.79 to 4.12 | 0.88 | ||||
| D+ | −8.49 | 3.49 | −15.33 to −1.65 | 0.02 | ||||
| Region | ||||||||
| South | Referent | |||||||
| Northeast | 8.38 | 3.06 | 2.39 to 14.39 | 0.007 | ||||
| Midwest | 2.10 | 3.59 | −4.92 to 9.13 | 0.56 | ||||
| West | 3.81 | 3.57 | −3.17 to 10.80 | 0.29 | ||||
Abbreviations: Lump+WBI = lumpectomy plus whole-breast irradiation; Lump+Brachy = lumpectomy plus brachytherapy; Lump alone = lumpectomy plus endocrine therapy alone; SE = standard error; CI = confidence interval
Intercept is the expected mean value of the outcome when all covariables are referent.
All analyses incorporated weights to adjust for differential sample selection and response rates.
In multivariable analysis for BREAST-Q Psychosocial Well-Being and Sexual Well-Being, there were no significant differences in scores by surgeon factors. Local therapy type was not predictive of Psychosocial Well-Being or Sexual Well-Being. Patient characteristics including white race, higher Charlson Comorbidity Index, lower income, and absence of axillary surgery correlated with worse Psychosocial Well-Being. D+ bra cup size and living in the South geographic region correlated with worse Sexual Well-Being (eTable 3).
Discussion
In this nationally representative cohort of older breast cancer survivors treated with breast-conserving local therapy strategies, we found that long-term patient-reported cosmetic satisfaction, physical well-being, and adverse radiation effects were significantly better among patients treated by surgical oncologists compared to those treated by non-surgical oncologists. To the best of our knowledge, this is the first study to directly link surgeon specialization to long-term PROs in cancer patients. For the primary outcome of CanSORT Satisfaction with Breast Cosmetic Outcome, the adjusted effect size of surgical oncology specialization was 0.48, which is even greater than the difference in satisfaction between breast-conserving therapy and mastectomy of 0.3 found in prior data from this cohort, demonstrating the clinical relevance of these findings.15
Specialization in surgical oncology may influence PROs through a variety of mechanisms. First, surgical oncologists are more likely than general surgeons to practice within cancer centers with expertise across the diagnostic and therapeutic continuum, inclusive of breast imaging, breast pathology, medical oncology, and radiation oncology.22,23 A practice environment inclusive of other specialists focused on breast cancer is likely to synergize with surgeon expertise to promote the best patient outcomes. Aside from the multidisciplinary expertise associated with practice in a cancer center, there may also be better medical facilities and availability of support staff. Cancer centers tend to be located in metropolitan areas with easily accessible medical care, which has been shown to impact health outcomes.24
Second, surgical oncologists may be more likely to employ techniques that could improve cosmetic outcome, such as oncoplastic reconstruction or local tissue transfer with or without an accompanying plastic surgeon.25,26 Similarly, it is plausible that excision volumes may be lower among surgical oncologists compared to general surgeons. Third, patients of surgical oncologists may be less likely to undergo re-excision, as re-excision rates vary inversely with surgeon expertise27–29, and re-excision increases the likelihood of adverse cosmetic outcome.30,31
In addition, there is evidence to suggest a positive surgery case volume-outcome relationship among multiple surgical procedures and conditions.32 Within the realm of breast cancer, surgeons with higher case volume are more likely to adhere to the latest margin re-excision guidelines33 and treat patients with breast-conserving surgery.34 In our study, although surgical oncology subspecialization was associated with higher breast surgery case volume, it was surgical specialization and not case volume that was consistently associated with improved PROs in multivariable and sensitivity analyses, which suggests that additional specialty training may add value beyond what experience and case volume offer. Conversely, it is conceivable that surgeons completing competitive35 surgical oncology fellowships are selected for in terms of their prior qualifications and general surgery training, which has been shown to be related to surgical outcomes.36
Finally, surgeon-patient communication may vary between general surgeons and surgical oncologists, leading to long-term differences in PROs. For example, patients treated by surgeons specializing in breast disease are more satisfied with their surgeon-patient relationship,14 and patients who are satisfied with their surgeon-patient relationship are more likely to be satisfied with their cosmetic outcome.37,38 In a survey study of patients treated with mastectomy and reconstruction, Ho et al satisfaction with pre-operative counseling and with the surgeon-patient relationship predicted higher found that patient satisfaction with cosmetic outcome.38 Similar correlations between physician-patient relationships and PROs have been observed in prostate cancer39 and medical oncology.40 These data underscore the importance of patient-centered care, and highlight areas where surgeons – including those without specialty training in surgical oncology – may be able to impact patient outcomes outside of the operating room.
Interestingly, treatment by a surgical oncologist was also associated with improved patient-reported adverse radiation effects. This is consistent with prior studies that have associated poor post-surgery, pre-radiation cosmetic outcome with late breast fibrosis.41 As described previously, surgical oncologists are more likely than non-surgical oncologists to be working in a multidisciplinary setting with subspecialized radiation oncologists. These patients may therefore also benefit from the expertise of the treating radiation oncologist.
From a patient perspective, there is surprisingly little data to guide patients toward high quality providers. Although national movements are ongoing to promote outcome and patient satisfaction reporting, such data are not yet widely available. By demonstrating the relevance of surgeon specialization to outcomes experienced by cancer survivors after treatment, this study provides key information needed for patients to understand the implications of selecting providers with specialization in surgical oncology. For surgeons-in-training, our findings document how additional training beyond traditional residency can add value to a surgical practice by promoting optimal patient outcomes. For policymakers and professional societies, our study findings document the value of additional subspecialization within general surgery and underscore needs for ongoing medical education for general surgeons whose practice includes breast surgery. While it may be difficult for practicing surgeons who are not surgical oncologists to return for additional training, there are many opportunities for continuing medical education available at the annual meetings of the American College of Surgeons, American Society of Breast Surgeons, and the Society of Surgical Oncology. Continuing education is widely regarded as a critical component of physician practice and has been shown to impact both physician performance and health care outcomes.42
One of the limitations of the current study is that CanSORT Breast Cosmetic Outcome, but not BREAST-Q Satisfaction with Breasts was found to be significantly associated with surgical specialization. While both are validated instruments for cosmetic outcome that share similarities15, we hypothesize that more granular scoring of CanSORT Breast Cosmetic Outcome (scored on a Likert scale of 1–5, whereas BREAST-Q Satisfaction with Breasts is scored on a scale of 1–4) and overlap of physical well-being traits in CanSORT Breast Cosmetic Outcome may have led to increased ability of the CanSORT score to detect underlying differences in outcome. Finally, BREAST-Q Satisfaction with Breasts is a longer instrument (11 questions versus 6 questions) and fewer patients completed it compared to CanSORT Breast Cosmetic Outcome (282 versus 273), which suggests some respondents may have fatigued in this portion of the questionnaire.
Other study limitations include a modest response rate, which may be attributed to the older age of the study population (median age 72 at diagnosis and 78 at the time of survey) and the length of time (6 years) elapsed since diagnosis and treatment. There were significant differences in baseline characteristics between responders and non-responders, including age at diagnosis (median age 72 for responders, 75 for non-responders) and local therapy (women who received lumpectomy alone were less likely to respond compared to women who received other local treatment types) that we attempted to control for in statistical analysis with non-response weights. There were no significant differences between responders and non-responders regarding surgeon factors and therefore these differences are unlikely to have caused bias in our primary study endpoint associating surgeon factors with PROs. Our analysis did not account for relevant treatment factors that may impact cosmetic outcome such as tumor size, multifocal disease, requirement for re-excision, and radiation dose and fractionation schedule. Surgical subspecialty was provided via self-report in the AMA Physician Masterfile and breast surgery was not an option – however, it is reasonable to assume that fellowship-trained breast surgeons would self-select surgical oncology as the most appropriate specialty given the options.
In summary, older breast cancer survivors treated by surgical oncologists reported considerably better cosmetic satisfaction, physical well-being, and adverse radiation effects approximately 6 years after breast-conserving surgery. The robust association between surgeon specialization and multiple patient-reported outcome domains is highly relevant to patients seeking best care and to quality improvement efforts to optimize long-term patient outcomes.
Supplementary Material
Synopsis.
In this survey study of breast cancer survivors following breast-conserving therapy, treatment by surgeons with specialized training in surgical oncology was associated with better patient-reported cosmetic outcome, physical well-being, and adverse radiation effects compared to treatment by non-surgical oncologists.
Acknowledgements:
This work was supported by an ASTRO Comparative Effectiveness Research grant, The University of Texas MD Anderson Survivorship Institutional Research Grant, and in part by the Assessment, Intervention and Measurement (AIM) Shared Resource and Biostatistics Shared Resource through a Cancer Center Support Grant (CA016672, PI: P. Pisters, MD Anderson Cancer Center), from the National Cancer Institute, National Institutes of Health. The study was additionally supported by the Center for Radiation Oncology Research, the Duncan Family Institute, a philanthropic gift from Ann and Clarence Cazalot, and by the Department of Health and Human Services National Cancer Institute (Grant P30CA016672).
Disclosures:
Dr. Benjamin Smith is supported by the Andrew Sabin Family Fellowship, the Cancer Prevention and Research Institute of Texas (Grant RP 160674), the National Cancer Institute (R01 CA207216–01). Dr. Benjamin Smith previously received research funding from Varian Medical Systems that is unrelated to the current project. He also has an equity interest in Oncora Medical. Dr. Grace Smith is supported by the National Institute of Health (Grant K07 CA211804–01). Dr. Shaitelman receives unrelated research funding from Varian Medical Systems. Dr. DeSnyder receives unrelated research funding from Impedimed. Dr. Jagsi reports unrelated grant funding from the NIH, Doris Duke Foundation, Greenwall Foundation, and Komen Foundation; personal fees from Vizient and Amgen; and stock options in Equity Quotient. Dr. Giordano is supported by CPRIT RP160674 and Susan Komen SAC150061. Dr. Hunt is on the medical advisory board for Armada Health and Merck & Co. and receives research funding from Endomagnetics and Lumicell. The entities above had no role in the present study.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM, investigators PI. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–273. [DOI] [PubMed] [Google Scholar]
- 3.Wang SY, Kelly G, Gross C, et al. Information Needs of Older Women With Early-Stage Breast Cancer When Making Radiation Therapy Decisions. Int J Radiat Oncol Biol Phys. 2017;98(4):733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jagsi R, Li Y, Morrow M, et al. Patient-reported Quality of Life and Satisfaction With Cosmetic Outcomes After Breast Conservation and Mastectomy With and Without Reconstruction: Results of a Survey of Breast Cancer Survivors. Ann Surg. 2015;261(6):1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman KA, Shaw LK, Winch CJ, et al. Reducing Decisional Conflict and Enhancing Satisfaction with Information among Women Considering Breast Reconstruction following Mastectomy: Results from the BRECONDA Randomized Controlled Trial. Plast Reconstr Surg. 2016;138(4):592e–602e. [DOI] [PubMed] [Google Scholar]
- 6.Wong JJ, D’Alimonte L, Angus J, Paszat L, Soren B, Szumacher E. What do older patients with early breast cancer want to know while undergoing adjuvant radiotherapy? J Cancer Educ. 2011;26(2):254–261. [DOI] [PubMed] [Google Scholar]
- 7.Feldman-Stewart D, Madarnas Y, Mates M, et al. Information needs of post-menopausal women with hormone receptor positive early-stage breast cancer considering adjuvant endocrine therapy. Patient Educ Couns. 2013;93(1):114–121. [DOI] [PubMed] [Google Scholar]
- 8.Waljee JF, Rogers MA, Alderman AK. Decision aids and breast cancer: do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. 2007;25(9):1067–1073. [DOI] [PubMed] [Google Scholar]
- 9.Shahbazi S, Woods SJ. Influence of physician, patient, and health care system characteristics on the use of outpatient mastectomy. Am J Surg. 2016;211(4):802–809. [DOI] [PubMed] [Google Scholar]
- 10.Hershman DL, Buono D, McBride RB, et al. Surgeon characteristics and receipt of adjuvant radiotherapy in women with breast cancer. J Natl Cancer Inst. 2008;100(3):199–206. [DOI] [PubMed] [Google Scholar]
- 11.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA. 2009;302(14):1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277(18):1485–1492. [PubMed] [Google Scholar]
- 13.Swenson SL, Buell S, Zettler P, White M, Ruston DC, Lo B. Patient-centered communication: do patients really prefer it? J Gen Intern Med. 2004;19(11):1069–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waljee JF, Hawley S, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694–3698. [DOI] [PubMed] [Google Scholar]
- 15.Swanick CW, Lei X, Xu Y, et al. Long-term Patient-Reported Outcomes in Older Breast Cancer Survivors: A Population-Based Survey Study. Int J Radiat Oncol Biol Phys. 2018;100(4):882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munshi A, Kakkar S, Bhutani R, Jalali R, Budrukkar A, Dinshaw KA. Factors influencing cosmetic outcome in breast conservation. Clin Oncol (R Coll Radiol). 2009;21(4):285–293. [DOI] [PubMed] [Google Scholar]
- 17.Gold HT, Do HT. Evaluation of three algorithms to identify incident breast cancer in Medicare claims data. Health Serv Res. 2007;42(5):2056–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirvani SM, Jiang J, Likhacheva A, et al. Trends in Local Therapy Utilization and Cost for Early-Stage Breast Cancer in Older Women: Implications for Payment and Policy Reform. Int J Radiat Oncol Biol Phys. 2016;95(2):605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willis GB. Cognitive interviewing : a tool for improving questionnaire design. Thousand Oaks, Calif.: Sage Publications; 2005. [Google Scholar]
- 20.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. [DOI] [PubMed] [Google Scholar]
- 21.Cano SJ, Klassen AF, Scott A, Alderman A, Pusic AL. Interpreting clinical differences in BREAST-Q scores: minimal important difference. Plast Reconstr Surg. 2014;134(1):173e–175e. [DOI] [PubMed] [Google Scholar]
- 22.Kuerer HM, Eberlein TJ, Pollock RE, et al. Career satisfaction, practice patterns and burnout among surgical oncologists: report on the quality of life of members of the Society of Surgical Oncology. Ann Surg Oncol. 2007;14(11):3043–3053. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie WP, Rhodes RS, Biester TW. Work loads and practice patterns of general surgeons in the United States, 1995–1997: a report from the American Board of Surgery. Ann Surg. 1999;230(4):533–542; discussion 542–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly C, Hulme C, Farragher T, Clarke G. Are differences in travel time or distance to healthcare for adults in global north countries associated with an impact on health outcomes? A systematic review. BMJ Open. 2016;6(11):e013059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos G, Urban C, Edelweiss MI, et al. Long-Term Comparison of Aesthetical Outcomes After Oncoplastic Surgery and Lumpectomy in Breast Cancer Patients. Ann Surg Oncol. 2015;22(8):2500–2508. [DOI] [PubMed] [Google Scholar]
- 26.Losken A, Nahabedian MY. Oncoplastic breast surgery: past, present, and future directions in the United States. Plast Reconstr Surg. 2009;124(3):969–972. [DOI] [PubMed] [Google Scholar]
- 27.Hassani A, Griffith C, Harvey J. Size does matter: High volume breast surgeons accept smaller excision margins for wide local excision--a national survey of the surgical management of wide local excision margins in UK breast cancer patients. Breast. 2013;22(5):718–722. [DOI] [PubMed] [Google Scholar]
- 28.Hughes L, Hamm J, McGahan C, Baliski C. Surgeon Volume, Patient Age, and Tumor-Related Factors Influence the Need for Re-Excision After Breast-Conserving Surgery. Ann Surg Oncol. 2016;23(Suppl 5):656–664. [DOI] [PubMed] [Google Scholar]
- 29.DeSnyder SM, Hunt KK, Dong W, et al. American Society of Breast Surgeons’ Practice Patterns After Publication of the SSO-ASTRO-ASCO DCIS Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation. Ann Surg Oncol. 2018;25(10):2965–2974. [DOI] [PubMed] [Google Scholar]
- 30.Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of breast asymmetry after breast-conserving operation for breast cancer. J Am Coll Surg. 2008;206(2):274–280. [DOI] [PubMed] [Google Scholar]
- 31.Mook J, Klein R, Kobbermann A, et al. Volume of excision and cosmesis with routine cavity shave margins technique. Ann Surg Oncol. 2012;19(3):886–891. [DOI] [PubMed] [Google Scholar]
- 32.Morche J, Mathes T, Jacobs A, et al. Relationship between volume and outcome for congenital diaphragmatic hernia: a systematic review protocol. Syst Rev. 2018;7(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrow M, Abrahamse P, Hofer TP, et al. Trends in Reoperation After Initial Lumpectomy for Breast Cancer: Addressing Overtreatment in Surgical Management. JAMA Oncol. 2017;3(10):1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott AM, Wall DM, Waters PS, et al. Surgeon and breast unit volume-outcome relationships in breast cancer surgery and treatment. Ann Surg. 2013;258(5):808–813; discussion 813–804. [DOI] [PubMed] [Google Scholar]
- 35.Wach MM, Ruff SM, Ayabe RI, et al. An Examination of Applicants and Factors Associated with Matriculation to Complex General Surgical Oncology Fellowship Training Programs. Ann Surg Oncol. 2018;25(12):3436–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal N, Simmons KD, Epstein AJ, Morris JB, Kelz RR. Using Patient Outcomes to Evaluate General Surgery Residency Program Performance. JAMA Surg. 2016;151(2):111–119. [DOI] [PubMed] [Google Scholar]
- 37.de Blacam C, Healy C, Quinn L, et al. Is satisfaction with surgeon a determining factor in patient reported outcomes in breast reconstruction? J Plast Reconstr Aesthet Surg. 2016;69(9):1248–1253. [DOI] [PubMed] [Google Scholar]
- 38.Ho AL, Klassen AF, Cano S, Scott AM, Pusic AL. Optimizing patient-centered care in breast reconstruction: the importance of preoperative information and patient-physician communication. Plast Reconstr Surg. 2013;132(2):212e–220e. [DOI] [PubMed] [Google Scholar]
- 39.Ernstmann N, Weissbach L, Herden J, Winter N, Ansmann L. Patient-physician communication and health-related quality of life of patients with localised prostate cancer undergoing radical prostatectomy - a longitudinal multilevel analysis. BJU Int. 2017;119(3):396–405. [DOI] [PubMed] [Google Scholar]
- 40.Ong LM, Visser MR, Lammes FB, de Haes JC. Doctor-patient communication and cancer patients’ quality of life and satisfaction. Patient Educ Couns. 2000;41(2):145–156. [DOI] [PubMed] [Google Scholar]
- 41.Grossberg AJ, Lei X, Xu T, et al. Association of Transforming Growth Factor β Polymorphism C-509T With Radiation-Induced Fibrosis Among Patients With Early-Stage Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis D, O’Brien MA, Freemantle N, Wolf FM, Mazmanian P, Taylor-Vaisey A. Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes? JAMA. 1999;282(9):867–874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


