Abstract
Objective:
The MHADRO assesses psychosocial and medical needs, provides tailored feedback reports, and connects patients to mental health providers. This study examined the MHADRO’s effect on patient outcomes, health care utilization, and oncology provider documentation and behaviors.
Methods:
836 patients were part of a multi-site RCT and assessments were conducted at baseline, 2, 6 and 12 months.
Results:
The intervention group engaged in less emergency calls to providers. There were no differences in psychosocial outcomes at follow up assessments. Providers of patients in the intervention group were more likely to: document psychosocial symptoms and history; refer to psychosocial services; encourage support groups; seek psychological evaluations during visits. Patients who agreed to a mental health referral had decreased hospitalizations, increased mental health care interactions, and stronger ratings of counseling potential benefits. This group also reported increased psychosocial distress at all follow-up assessments.
Conclusion:
The MHADRO may increase access to mental health care, lessen utilization, and improve providers’ management of psychosocial needs, but does not appear to impact overall functioning over time.
Practice Implications:
Providers are encouraged to consider incorporating programs, like the MHADRO, into patient care as they may have the potential to impact screening and management of patients’ psychosocial needs.
Keywords: CANCER, TECHNOLOGY, PSYCHOLOGICAL DISTRESS, PSYCHOSOCIAL INTERVENTION, HEALTH CARE UTILIZATION
1. Introduction
Managing Psychological Distress in Patients with Cancer
The diagnosis and treatment of cancer can lead to an increase in psychological distress for many individuals. Changes in quality of life, anxiety, and depression are all consequences of being diagnosed with and treated for cancer [1]. An estimated 20–35% of cancer patients experience an increase in such psychosocial symptoms [2–4] and there is consensus that distress, as well as other psychosocial variables, should be screened regularly in patients with cancer [5]. Oncology care providers are expected to use this information to craft treatment interventions based on the levels of symptoms the patient endorses [5]. Such interventions may be effective in improving quality of life [6–8], decreasing levels of anxiety, depression, and overall distress [1, 9–12], and even influencing physical health outcomes such as improved immune functioning [13]. Mitchell et al. (2013) investigated the existing evidence for distress screening by reviewing 24 interventional studies, 14 of which were randomized control trials (RCT) [14]. Out of the 14 RCTs, six reported benefits to patient well-being, three showed benefits in secondary areas such as communication between clinicians/patients, and five failed to show any benefits. The authors concluded that screening for distress, as well as quality of life, is likely to benefit communication and referral for psychosocial help and can benefit patient well-being if barriers to treatment are addressed. Anderson et al. recently advised that clinicians have a vital role in mitigating the negative emotional and behavioral aspects of cancer and treating symptoms of anxiety and depression may help reduce the human cost of cancer [5].
Technology and Distress Management in Patients with Cancer
Researchers are exploring how to best to use technology for the identification and management of needs of medical patients. Erharter and colleagues [15] tested the implementation of a computer-based Quality of Life (QOL) assessment in a neuro-oncology outpatient unit. Patients with brain tumors completed a computer-based QOL questionnaire, which evaluated five main scales: physical functioning, social functioning, role functioning, emotional functioning, and cognitive functioning. Patients were also queried about cancer symptoms commonly assessed in person by nursing staff during outpatient visits. Patients were highly accepting of the computerized assessment and reported the program was efficient and easily implemented in clinical care. Loiselle, Edgar, Batist, Lu, and Lauzier tested a computer-based assessment on the quality of life of cancer patients [16]. Patients in the intervention condition received training on use of the internet to explore a list of reputable cancer web sites and how to use a CD-ROM from the Oncology Interactive Educational Series (OIES). This CD-ROM reviewed the patient’s type of cancer diagnosis and included information about symptoms, treatment options, nutrition, pain management, psychosocial care, and community support services. Female patients who received this intervention had better quality of life outcomes over time and improved patient satisfaction with cancer information and perceptions of support from their oncologist [16]. Finally, McLachlan et al. [17] conducted a large study (n=450) that allowed patients to complete a computerized assessment of quality of life, depression, physical and daily functioning, interpersonal communication, and social support. Patients received a one-page summary of their results prior to their appointment with their oncology team. A consultation nurse used the summary to build individualized care plans that addressed issues identified in the patients’ summaries. Although the authors did not find an impact on depression or quality of life, they did demonstrate that patients who had the greatest level of distress were significantly less depressed at 2 and 6 month follow-up as compared to those who were highly distressed and randomized to the control group.
Purpose of Present Study
The Mental Health Assessment and Dynamic Referral for Oncology (MHADRO) is a web-based psychosocial assessment and resource program that helps providers identify, monitor, and manage psychosocial issues in individuals who are being treated for cancer [18]. The MHADRO generates a tailored report summarizing a patient’s level of distress, anxiety, and depression, and provides feedback by comparing said scores to a large normative sample of patients with cancer. The MHADRO report also provides patients with tailored psychosocial information about cancer relevant topics and allows patients to request an electronic referral for mental health treatment, matched to their insurance and zip code. The initial development of the MHADRO system has been published elsewhere [18] and the present paper presents results of the randomized control trial (RCT) investigating provider and patient short and long-term outcomes (2, 6 and 12 months) of the MHADRO system.
Hypotheses
-
1)
Patients who were the most psychologically distressed (top 30% of distress) in the intervention group would show more positive psychosocial outcomes, greater engagement in seeking psychosocial services, and less health care utilization at 2, 6 and 12 months compared to those patients in the control group.
-
2)
Patients in the intervention group, who accepted the offer for a Dynamic Referral (DR) for mental health counseling, would have more positive psychosocial outcomes, greater engagement in seeking psychosocial services, and less health care utilization (i.e., emergency calls to the oncology care team, hospitalizations, emergency room visits) at 2, 6, and 12 months as compared to those who rejected the DR.
-
3)
Providers of the participants in the intervention group would demonstrate better communication and documentation of psychosocial history, symptoms, and resources/treatment, as compared to providers in the control group.
2. Method
2.1. Study Design
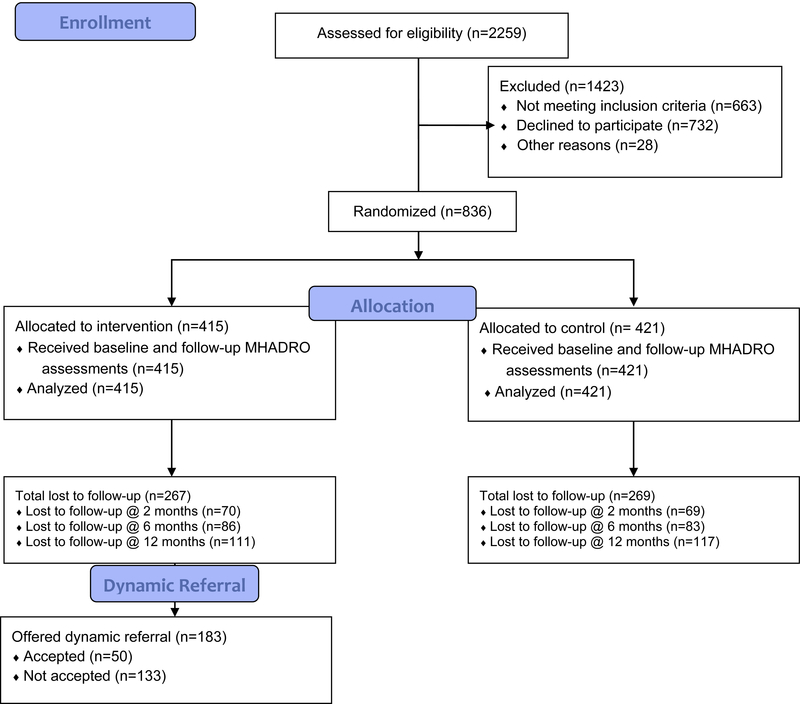
The study design is an RCT at three comprehensive cancer centers in the United States. All institutions involved received Institutional Review Board approval for this study. The full methodology of this study was published in Contemporary Clinical Trials [19]. Figure 1 is a CONSORT diagram for this study.
Figure 1.
MHADRO RCT CONSORT DIAGRAM
2.2. Participants
Eight hundred and thirty six cancer patients were recruited from three sites (UMass Medical Center, Worcester MA; Cooper University Hospital, Camden NJ; MD Anderson Cancer Center, Houston TX). Inclusion criteria were kept broad to be functional in a general oncology setting. Patients 18 or older with a past or current cancer diagnosis were considered for enrollment. Patients were excluded if they had any of the following: altered mental status (e.g., psychosis, delirium, and/or disorientation), hostile or agitated computer use, physical symptoms that would interfere with participation (e.g., persistent vomiting, severe pain), or factors precluding follow-up (e.g., transient residence or lack of a telephone). There was no discrimination in patient recruitment due to type, duration of illness, stage of cancer or phase of treatment. The sample size calculation for this cohort has been previously reported [19]; in brief, a sample size required to attain 80% power for a two-sided test at alpha = 0.05, assuming an exchangeable covariance structure and autocorrelation of 0.50 to 0.70, ranged from 646 to 776.
2.3. Study Procedures
Participants were approached in the exam room or an infusion chair (after the oncology provider had granted permission to approach the individual) when they arrived for routine oncology (treatment or follow-up) appointments or chemotherapy infusions. After completing a full written informed consent, the participant was randomly assigned to one of two study conditions, which are described below under Study Conditions. At 2, 6, and 12 months participants were contacted by their preferred method (phone or email) to complete a follow-up MHADRO assessment as well as other outcome measures. The research assistant administering the follow-up telephone assessments was blind to group assignment and performed the assessments from a centralized location.
2.4. Study Conditions
2.4.1. Intervention group.
Participants assigned to the intervention group (n=415) completed the full MHADRO assessment as well as were given the option of having a DR sent to a mental health provider if they met certain criteria (described below in this section). Once finished with the assignment, the oncology provider’s report was printed by the research assistant and presented to the provider. When possible, this was completed before the participant’s clinical appointment. When this was not possible, assessments were completed after the participant’s appointment and the research assistant would give the report to the provider at the next practical time before the next appointment. Once the provider had viewed the report, it was signed and placed on the patient’s electronic health record. All oncologists participating in the study were trained on how to read and interpret the provider reports; however no specific action was required in order to preserve the study’s ecological validity. That is, providers could choose to review the report with the patient or simply review the information and have it saved to the medical chart (see Appendix A for example of Provider Report).
The research assistant also printed the patient report and reviewed the sections with the patient. This patient report included information on emotional distress, relationships, concerns relating to sexuality, health management, tobacco use, alcohol use, side effects and physical symptoms, mental health referrals, and an action plan. A significant feature of the reports was the presentation of the patient’s overall psychological stress, depression, anxiety, and functional disability based on a normative database of cancer patients. Based on this database, participants scoring in the 70th percentile or higher for distress or depression were categorized in the elevated distress group, those in the 30th-70th percentile were in the average or normal group, and participants scoring less than the 30th percentile were in the low distress group (see Appendix A for example of Patient Report).
Lastly, participants in the intervention group were given the option of a DR, which was the opportunity for patients to have their personal information sent automatically, with their consent, to a mental health provider that was matched to their insurance and zip code. To be eligible, they had to meet the following criteria: 1) were not already in behavioral health treatment; 2) responded “yes” or “not sure” when asked if they felt that seeing a counselor or therapist would be beneficial; and any of the following applied: (3a) a score in the elevated range (>70th percentile) on the overall distress scale or depression subscale, OR (3b) a rating of 9 or 10 on the NCCN distress thermometer OR (3c) sexual difficulties were endorsed. This algorithm was part of the MHADRO’s programming. If patients met these criteria, participants were provided detailed information about the DR option and then asked if they were interested in having their information sent to a mental health counselor either located at the cancer center or matched to their insurance and zip code. If interested, patients could then electronically consent to transmitting their personal contact information to a designated mental health provider. DRs were either sent to a mental health specialist in the larger community or to an ‘in-house’ provider within the cancer care centers, depending on what was available and the best fit for the individual. Please note, we choose to include sexual dysfunction as a reason for DR because our group had clinically noted much sexual dysfunction in breast cancer patients and we anticipated we would have a disproportionate amount of breast cancer patients given the previous patient demographics at the treatment centers.
2.4.2. Control group.
Participants in the control condition (n=421) completed the baseline and follow-up assessments for the MHADRO. Their experience differed from that of the intervention group in that their providers did not receive a provider report, they did not receive a patient report and they were not given an option for a DR. Participants received standard care for any existing psychosocial issues.
2.5. Measures
2.5.1. Baseline assessment.
The web-based MHADRO assessment is accessed through a computer with an internet connection (e.g., PC, laptop, tablet, Ipad). The assessment covered the constructs listed in Table 1. Unless a reference is provided for a scale in Table 1, please assume the scale/survey was created by the present research team to measure the specific constructs. Full details of the constructs of the baseline assessment are published elsewhere [18, 19]; however, the primary outcome was assessed by the Behavioral Health Status (BHS). The Behavioral Health Status (BHS) scale is a global measure of mental health, which assesses subjective well-being, anxiety, and depression (see Table 1). The reliability scores of the BHS subscales for our sample ranged from 0.78–0.86, with a Cronbach’s alpha of 0.90 for the entire scale. The entire MHADRO baseline assessment was designed to take 15–20 minutes in a laboratory setting, but due to interruptions by hospital personnel (e.g. doctors or nurses), participants took an average of 28.17 (s.d. = 17.13) minutes to complete the assessment.
Table 1.
Description of the constructs assessed at baseline and at follow up from which tailored feedback reports were created
| Construct | Description |
|---|---|
| Demographic and Cancer Information | Age, sex, marital status, education level, race, ethnicity, insurance provider, cancer history (e.g., type of cancer, time since diagnosis, treatment received, number of times in remission, duration of treatment) |
| Mental Health Assessment | History of mental health diagnoses; if positive a drop down menu was presented that listed 11 common mental health diagnoses (i.e., depression, bipolar, alcohol abuse, drug abuse, anxiety, panic attacks, PTSD, ADD/ADHD, anorexia, bulimia, schizophrenia) |
| BHS *Depression (5 items) | Feelings of sadness; decreased pleasure in activities; feelings of worthlessness; hopelessness; trouble concentrating |
| BHS *Anxiety (5 items) | Worry; tension or anxiety; irritability or easily angered; keyed up or on edge; trouble concentrating |
| BHS *Functional Disability (5 items) | Time had to cut down on work and spent activities as a result of any emotional problems; physical health limitations (e.g., carrying groceries, climbing stairs); managing day-to-day life; getting along with others; work, school, or household performance |
| BHS *Subjective Well Being (1 item) | How well getting along emotionally and psychologically |
| *Behavioral Health Status | An average of the anxiety, depression, functional disability, and subjective well-being scores; this scale is psychometrically validated (30). |
| *Self-Reported Distress | National Comprehensive Cancer Network’s (NCCN) distress thermometer |
| Social Support | Help and advice from others; emotional support, comfort, and understanding; people to help with difficult time. |
| General Health Information | Documented health problems per patient report and general health status |
| *Alcohol Use | Alcohol Use Disorders Identification Test (AUDIT); frequency consuming alcoholic beverages; number of drinks typically consumed when drinking; and frequency of binge drinking (Saunders et al, 31). |
| *Tobacco Use | History of tobacco use; tobacco products used in the 30 days prior to enrollment; quit attempts; number of cigarettes per day; how many minutes after waking first cigarette (Heatherton et al, 32) |
| Total Number of Symptoms | Pain, tiredness or fatigue, nausea or vomiting, insomnia or sleep difficulties, difficulty with bowel movements, and sexual difficulties or lack of interest in sex |
| *Patient-Provider Partnership (5 items) | Treated the patient in a friendly and courteous manner, cared about the patient as a person, listened to patient, answered all questions, and had good communication with each other. |
| *Behavioral Health Recommendations | Whether or not oncologist had made specific recommendations (i.e., quit smoking, exercise daily, reduce alcohol use, go to support groups or counseling services, increase fluid intake, eat nutritious foods). Participants chose from a checklist of recommendations, indicating which recommendations had been made explicitly by their oncologist. |
| *Counseling/therapy Status | Whether or not presently in therapy |
| *Perception of Benefit of Therapy | If not in counseling, perception of potential benefit to engaging in therapy at present time |
| * Interest in DR (Intervention Group only) | Whether or not the patient is interested in having their information, in the form of a tailored report, sent to a mental health counselor, matched to their insurance and zip code, who will contact them to set up an initial therapy appointment. |
indicates construct was also assessed at 2, 6, and 12 month follow up
2.5.2. Follow-up assessment.
Follow-up assessments were similar to the baseline assessments (see Table 1). There were additional questions that assessed health care utilization (i.e., hospitalizations, emergency room visits, and acute oncology provider visits), health behavior provider recommendations (i.e., did oncology providers discuss diet, exercise, smoking or alcohol), return to work, use of groups/classes, oncology provider discussion of mental health, mental health provider treatment initiation, and psychotropic medications. All follow up assessments were conducted on the phone at 3, 6, and 12 months, by a research assistant who was blind to the patients’ assigned conditions [18].
2.5.3. Medical chart review.
Chart reviews of participants’ oncology-related medical records, who consented to having a full chart review, were conducted. Researchers were blind to group assignment during chart assessments and evaluated providers’ documented discussions involving mental health and health behaviors with the participants during the course of the previous 6 months (see Table 2). The reports generated by the MHADRO were not used as source documents to avoid artificially inflating documentation rates.
Table 2.
Chart Review Data Comparing Intervention and Control Groups
| Variable | Intervention (n=405) |
Control (n=360) |
P-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Mental Health Indicators | |||
| Depression | 149 (36.8) | 79 (21.9) | <.0001 |
| Anxiety | 63 (15.6) | 36 (10.0) | 0.0223 |
| Sleep disturbance | 52 (12.8) | 12 (3.3) | <.0001 |
| Sexual/intimacy difficulties | 7 (1.7) | 7 (1.9) | 0.8239 |
| Social support issues | 3 (0.7) | 4 (1.1) | 0.5913 |
| Marital problems | 2 (0.5) | 1 (0.3) | 0.6332 |
| Drug use/abuse | 12 (3.0) | 6 (1.7) | 0.2377 |
| Alcohol use | 12 (3.0) | 17 (4.7) | 0.2035 |
| Tobacco use | 1 (0.2) | 0 (0.0) | 0.3455 |
| PTSD | 5 (1.2) | 2 (0.6) | 0.3249 |
| Mental Health Documentation - Positive | 232 (57.3) | 120 (33.3) | 0.8518 |
| Mental Health Counseling | 106 (26.2) | 28 (7.8) | <.0001 |
| Alcohol Counseling | 15 (3.7) | 3 (0.8) | 0.0087 |
| Smoking Counseling/Treatment | 22 (5.4) | 6 (1.7) | 0.0052 |
| Attending Support Group | 163 (40.2) | 4 (1.1) | <.0001 |
| Attended Classes Offered at Hospital or Cancer Center | 22 (5.4) | 3 (0.8) | 0.0003 |
| Psychotropic Medication use During Review Period | 124 (30.6) | 125 (34.7) | 0.2236 |
| Change in Psychotropic Medication Use | |||
| Psychotropics never used | 80 (19.8) | 94 (26.1) | 0.0363 |
| Psychotropics remained stable | 26 (6.4) | 14 (3.9) | 0.1165 |
| At least 1 psychotropic changed | 22 (5.4) | 14 (3.9) | 0.3144 |
| Dose of at least 1 psychotropic changed | 282 (69.6) | 238 (66.1) | 0.2978 |
| Patient Received Materials about Mental Illness/Coping | 16 (3.9) | 3 (0.8) | 0.0053 |
| Status of Onsite Evaluation for Mental Health Issues by a MHP | |||
| No, not evaluated | 354 (87.4) | 342 (95.0) | 0.0004 |
| Yes, with negative results (the patient is not distressed) | 11 (2.7) | 5 (1.4) | |
| Yes, with positive results (the patient is distressed) | 39 (9.6) | 11 (3.1) | |
| Patient has Documented ED Visits in the Past 6 Months | 59 (14.6) | 66 (18.3) | 0.1520 |
| Patient has Documented Hospitalizations in the Past 6 Months | 65 (16.0) | 74 (20.6) | 0.1074 |
| Patient has Documented Outpatient Visits in the Past 6 Months | 382 (94.3) | 346 (96.1) | 0.2494 |
| Patient has Documented Emergency Calls in the Past 6 Months | 47 (11.6) | 63 (17.5) | 0.0231 |
Note: p-values from χ2 test and Fisher’s exact test where cell sizes are <5
2.5.4. Follow-up assessment for mental health providers.
Mental health providers involved in referrals were contacted four weeks after the initial referral was made for those patients who consented and signed the appropriate release forms. The mental health provider indicated whether they had successfully contacted the patient after the individual’s personal contact information was received. The providers were also asked whether the participant had completed the initial evaluation and the number of appointments that had occurred. Personal information regarding di agnoses or treatment/therapy content was not discussed nor recorded.
3. Results
3.1. Data Analytic Plan
We examined baseline demographic and psychometric characteristics among all 836 participants. Characteristics were compared by group assignment (MHADRO intervention vs. control) using chi-squared tests (or Fisher’s exact test when cell sizes were <5) for categorical variables and Student’s t-test for continuous variables (with a Satterthwaite adjustment when equality of variances significantly differed between groups). We then repeated these demographic and psychometric comparisons between intervention and control patients with the worst 30% of Behavioral Health Status scores (BHS score ≤3.7506) and between patients who were offered and accepted a DR to patients who were offered and rejected a DR. Chart review data for intervention and control participants was compared using chi-squared tests (or Fisher’s exact test when cell sizes were <5). As this information was only examined at one time point (i.e., at the time of chart review), only bivariate analyses were conducted with this data.
Next, multivariable general estimating equation (GEE) models were conducted to evaluate the association between patient group and specific outcome measures. GEE models were used to adjust for the inherent correlation among the data collected at repeated visits for the same subject, where a subject identifier was used as the clustering variable in the GEE models. The variance-covariance structure was specified as exchangeable (compound symmetric). All main effects models converged within normal parameters. Data was reorganized into a longitudinal format for each subject where their original single data record was converted into four records per subject with a visit indicator. Psychometric scores, healthcare utilization, engagement in therapy, and health behavior variables were treated as the dependent variables in individual models. Each model was tested with a visit/group assignment interaction to determine if outcomes differed by group over time. We examined these outcomes in the top 30% of distressed patients and in patients who were offered a DR, as described below.
Mechanisms of action were examined in the intervention participants through their level of treatment engagement, defined as follow-up participation in any of the following treatments: receipt of counseling or therapy, attendance at a support group, or receipt of a prescription for psychotropic medication. Responses to these three questions were examined at each of the follow-up time points (2 months, 6 months, and 12 months) and participants were given a score of 0 (no treatment engagement) to 3 (all three levels of treatment engagement) based on engagement at any point during follow-up. The association between BHS score and level of treatment engagement was examined using ANOVA models. All analyses were conducted in SAS 9.3 [20].
3.2. Intervention Effect – MHADRO vs. Control Group (n=836)
3.2.1. Intervention Effect – MHADRO vs. Control Group - Chart Review (n=765)
Chart reviews were conducted on the patients in the intervention group and the control groups at the 6 months post baseline. Table 2 provides information regarding significant results regarding provider documentation and patient health care utilization.
3.2.2. Group Effects.
Group effects between intervention and control patients were compared for the entire group (n=836) and also for patients who were in the top 30% of distress (n=251 patients). Overall, there were no significant differences in distress found between the control versus intervention group (see Table 3) for the entire sample or for the sample which consisted only of the top 30% of distressed patients (see Table 4) on demographics, history of mental health conditions, healthcare utilization, or psychometric measures.
Table 3.
Differences Between Control and Intervention Group at Baseline
| Variable | Total n=836 |
Intervention n=415 |
Control n=421 |
P- value |
|---|---|---|---|---|
| n (%) | ||||
| BHS Worst 30% | 251 (30.0) | 121 (29.2) | 130 (30.9) | 0.5870 |
| Dynamic Referral Assignment | ||||
| Offered, Rejected | 133 (15.9) | 133 (32.0) | ||
| Offered, Accepted | 50 (6.0) | 50 (12.0) | - | - |
| Not Offered | 232 (27.8) | 232 (55.9) | ||
| Sex | ||||
| Male | 118(14.1) | 55 (13.2) | 63 (15.0) | 0.4774 |
| Female | 718(85.9) | 360 (86.8) | 358(85.0) | |
| Marital Status | ||||
| Married | 542 (64.8) | 271 (65.3) | 271 (64.4) | 0.4307 |
| Separated/Divorced/Widowed | 219 (26.2) | 112 (27.0) | 107 (25.4) | |
| Never Married | 75 (9.0) | 32 (7.7) | 43 (10.2) | |
| Education Level | ||||
| <High school | 43 (5.1) | 22 (5.3) | 21 (5.0) | 0.5282 |
| High school graduate | 214 (25.6) | 113 (27.2) | 101 (24.0) | |
| >High school | 579 (69.3) | 280 (67.5) | 299 (71.0) | |
| Hispanic/Latino Ethnicity | 35 (4.2) | 18 (4.3) | 17 (4.0) | 0.8289 |
| Ever seen a counselor or therapist | ||||
| Yes, currently | 68 (8.1) | 32 (7.7) | 36 (8.6) | 0.2190 |
| Yes, previously | 239(28.6) | 130 (31.3) | 109 (25.9) | |
| No | 529 (63.3) | 253 (61.0) | 276 (65.6) | |
| Depression – ever | 204 (24.4)) | 97 (23.4) | 107 (25.4) | 0.4919 |
| Bipolar/Manic Depression – ever | 16 (1.9) | 10 (2.4) | 6 (1.4) | 0.2989 |
| Alcohol abuse/dependence – ever | 18 (2.2) | 9 (2.2) | 9 (2.1) | 0.9754 |
| Drug abuse/dependence – ever | 8 (1.0) | 5 (1.2) | 3 (0.7) | 0.4648 |
| Anxiety disorder – ever | 124 (14.8) | 59 (14.2) | 65 (15.4) | 0.6190 |
| Panic attacks/disorder – ever | 56 (6.7) | 24 (5.8) | 32 (7.6) | 0.2932 |
| PTSD – ever | 39 (4.7) | 19 (4.6) | 20 (4.8) | 0.9060 |
| ADD/ADHD – ever | 16 (1.9) | 8 (1.9) | 8 (1.9) | 0.9769 |
| Anorexia – ever | 6 (0.7) | 4 (1.0) | 2 (0.5) | 0.4484 |
| Schizophrenia – ever | 2 (0.2) | 1 (0.2) | 1 (0.2) | 0.9999 |
| Ever taken med for emotional problem | ||||
| Yes, currently | 168 (20.1) | 81 (19.5) | 87 (20.7) | 0.8299 |
| Yes, previously | 92(11.0) | 48 (11.6) | 44 (10.4) | |
| No | 576 (68.9) | 286 (68.9) | 290 (68.9) | |
| Ever used tobacco on a regular basis | 376 (45.0) | 190 (45.8) | 186(44.2) | 0.5554 |
| Currently in remission | 443(53.0) | 225 (54.2) | 218(51.8) | 0.8158 |
| Type of cancer most recently diagnosis | ||||
| Breast | 410 (49.0) | 198 (47.7) | 212 (50.4) | 0.5639 |
| Lung | 44 (5.3) | 20 (4.8) | 24 (5.7) | |
| Colon/rectal | 45 (5.4) | 21 (5.1) | 24 (5.7) | |
| Endometrial | 72 (8.6) | 31 (7.5) | 41 (9.7) | |
| Prostate | 13 (1.6) | 7 (1.7) | 6 (1.4) | |
| Leukemia | 10 (1.2) | 5 (1.2) | 5 (1.2) | |
| Other | 242 (29.0) | 133 (32.0) | 109 (25.9) | |
| Received most recent diagnosis | ||||
| Today | 22 (2.6) | 12 (2.9) | 10 (2.4) | 0.6243 |
| Within past week | 5 (0.6) | 3 (0.7) | 2 (0.5) | |
| 1–4 weeks ago | 41 (4.9) | 24 (5.8) | 17 (4.0) | |
| 1–3 months ago | 119 (14.2) | 57 (13.7) | 62 (14.7) | |
| 4–6 months ago | 97 (11.6) | 42 (10.1) | 55 (13.1) | |
| >6 months ago | 552 (66.0) | 277 (66.7) | 275 (49.8) | |
| Mean +/− Standard Deviation (Range) |
||||
| Score variables (high is good) | ||||
| BHS | 4.1 +/−0.7(1.5–5.0) | 4.1 +/− 0.7(1.5–5.0) | 4.1 +/−0.7(1.7–5.0) | 0.5946 |
| Functional Disability | 4.2 +/−0.8(1.4–5.0) | 4.2 +/− 0.9(1.6–5.0) | 4.2 +/−0.8(1.4–5.0) | 0.9448 |
| Subjective Well-Being | 3.9 +/−0.9(1.0–5.0) | 3.8 +/− 0.9(1.0–5.0) | 3.7 +/−0.9(1.0–5.0) | 0.6587 |
| Anxiety | 3.3 +/−0.6(1.0–4.0) | 3.3 +/− 0.6(1,2–4.0) | 3.3 +/−0.6(1.0–4.0) | 0.3010 |
| Depression | 3.5 +/−0.5(1.2–4.0) | 3.5 +/− 0.5(1.2–4.0) | 3.5 +/−0.5(1.2–4.0) | 0.5363 |
| Total symptoms | 4.2 +/−0.7(1.3–5.0) | 4.2 +/− 0.7(1.3–5.0) | 4.2 +/−0.7(1.6–5.0) | 0.3577 |
| Mean +/− Standard Deviation | ||||
| Cancer team scales (1–5; high is better) | ||||
| Cares about me as a person | 4.6 +/−0.8 | 4.6 +/− 0.8 | 4.6 +/−0.7 | 0.9393 |
| Listens to what I have to say | 4.6 +/−0.8 | 4.6 +/− 0.8 | 4.6 +/−0.8 | 0.4530 |
| Answers all of my questions | 4.7 +/−0.7 | 4.7 +/− 0.8 | 4.7 +/−0.7 | 0.9963 |
| Treats me in a friendly and courteous manner | 4.7 +/−0.9 | 4.7 +/− 0.9 | 4.7 +/−0.8 | 0.8723 |
| Number of mental health diagnoses – ever | 0.6 +/−1.0 | 0.6 +/− 1.0 | 0.6 +/−1.1 | 0.4499 |
| General health rating (1–5; high is better) | 3.2 +/−0.9 | 3.1 +/− 0.9 | 3.2 +/−0.9 | 0.1412 |
| Physical health limitations in moderate activities past 2 weeks (1–3; high is worse) | 1.6 +/−0.7 | 1.6 +/− 0.7 | 1.5 +/−0.7 | 0.3021 |
| Activity level past two weeks (1–6; high is worse) | 2.0 +/−1.2 | 2.0 +/− 1.2 | 2.1 +/−1.2 | 0.2951 |
| Number of cancer treatments | 1.2 +/−0.6 | 1.2 +/− 0.6 | 1.2 +/−0.6 | 0.3737 |
Note: p-values for categorical variables from χ2 test and Fisher’s exact test where cell sizes are <5; continuous variables from Student’s t-test with Satterthwaite adjustment for unequal variances
Table 4.
Differences Between Control and Intervention Groups at Baseline for the Top 30% Distressed Patients
| Variable | Total n=251 |
Intervention n=121 |
Control n=130 |
P- value |
|---|---|---|---|---|
| n (%) | ||||
| Sex | ||||
| Male | 36 (14.3) | 15 (12.4 | 21 (16.2) | 0.3961 |
| Female | 215 (85.7) | 106 (87.6) | 109 (83.8) | |
| Marital Status | ||||
| Married | 144 (57.4) | 67 (55.4) | 77 (59.2) | 0.2242 |
| Separated/Divorced/Widowed | 80 (31.9) | 44 (36.4) | 36 (27.7) | |
| Never Married | 27 (10.8) | 10 (8.3) | 17 (13.1) | |
| Education Level | ||||
| <High school | 21 (8.4) | 11 (9.1) | 10 (7.7) | 0.2242 |
| High school graduate | 68 (27.1) | 36 (29.8) | 32 (24.6) | |
| >High school | 162 (64.5) | 74 (61.2) | 88 (66.7) | |
| Hispanic/Latino Ethnicity | 16 (6.4) | 8 (6.6) | 8 (6.2) | 0.8821 |
| Ever seen a counselor or therapist | ||||
| Yes, currently | 42 (16.7) | 21 (17.4) | 21 (16.2) | 0.7214 |
| Yes, previously | 78 (31.1) | 40 (33.1) | 38 (29.2) | |
| No | 131 (52.2) | 60 (49.6) | 71 (54.6) | |
| Depression – ever | 119 (47.4) | 56 (46.3) | 63 (48.5) | 0.7296 |
| Bipolar/Manic Depression – ever | 12 (4.8) | 5 (3.9) | 7 (5.4) | 0.4719 |
| Alcohol abuse/dependence – ever | 10 (4.0) | 5 (4.1) | 5 (3.8) | 0.9078 |
| Drug abuse/dependence – ever | 3 (1.2) | 2 (1.7) | 1 (0.8) | 0.5198 |
| Anxiety disorder – ever | 64 (25.5) | 29 (24.0) | 35 (27.0) | 0.5913 |
| Panic attacks/disorder – ever | 22 (8.8) | 9 (7.4) | 13 (10.0) | 0.4732 |
| PTSD – ever | 24 (9.6) | 13 (10.7) | 11 (8.5) | 0.5390 |
| ADD/ADHD – ever | 6 (2.4) | 2 (1.7) | 4 (3.1) | 0.4605 |
| Anorexia – ever | 4 (1.6) | 2 (1.7) | 2 (1.5) | 0.9423 |
| Schizophrenia – ever | 2 (0.8) | 1 (0.8) | 1 (0.8) | 0.9594 |
| Ever taken med for emotional problem | ||||
| Yes, currently | 94 (37.4) | 46 (38.0) | 48 (36.9) | 0.9823 |
| Yes, previously | 29 (11.6) | 14 (11.6) | 15 (11.5) | |
| No | 128 (51.0) | 61 (50.4) | 67 (51.5) | |
| Ever used tobacco on a regular basis | 126 (50.2) | 62 (51.2) | 64 (49.2) | 0.7504 |
| Currently in remission | 98 (39.0) | 45 (37.2) | 53 (40.8) | 0.9410 |
| Type of cancer most recently diagnosed | ||||
| Breast | 104 (41.4) | 45 (37.2) | 59 (45.4) | 0.3180 |
| Lung | 19 (7.6) | 7 (5.8) | 12 (9.2) | |
| Colon/rectal | 17 (6.8) | 8 (6.6) | 9 (6.9) | |
| Endometrial | 23 (9.1) | 10 (8.3) | 13 (10.0) | |
| Prostate | 5 (2.0) | 3 (2.5) | 2 (1.5) | |
| Leukemia | 2 (0.8) | 2 (1.7) | 0 (0.0) | |
| Other | 81(32.3) | 46 (38.0) | 35 (26.9) | |
| Received most recent dx | ||||
| Today | 7 (2.8) | 3 (2.5) | 4 (3.1) | 0.7482 |
| Within past week | 2 (0.8) | 2 (1.7) | 0 (0.0) | |
| 1–4 weeks ago | 17 (6.8) | 9 (7.4) | 8 (6.2) | |
| 1–3 months ago | 48 (19.1) | 22 (18.2) | 26 (20.0) | |
| 4–6 months ago | 31 (12.4) | 16 (13.2) | 15 (11.5) | |
| >6 months ago | 146 (58.2) | 69 (57.0) | 77 (59.2) | |
| Mean +/− Standard Deviation (Range) | ||||
| Score variables (high is good) | ||||
| BHS | 3.2 +/−0.4 (1.5–3.8) | 3.2 +/− 0.4 (1.5–3.8) | 3.2 +/−0.5 (1.7–3.8) | 0.8483 |
| Functional Disability | 3.2 +/−0.7 (1.4–4.7) | 3.1 +/− 0.7 (1.6–4.7) | 3.3 +/−0.6 (1.4–4.7) | 0.0843 |
| Subjective Well-Being | 2.9 +/−0.5 (1.0–4.0) | 2.9 +/− 0.5 (1.0–4.0) | 2.9 +/−0.5 (1.0–4.0) | 0.8067 |
| Anxiety | 2.7 +/−0.6 (1.0–4.0) | 2.8 +/− 0.5 (1.2–3.8) | 2.6 +/−0.6 (1.0–4.0) | 0.1119 |
| Depression | 2.9 +/−0.5 (1.2–4.0) | 2.9 +/− 0.5 (1.2–3.8) | 2.9 +/−0.5 (1.2–4.0) | 0.9893 |
| Total symptoms | 3.4 +/−0.7 (1.3–4.9) | 3.5 +/− 0.6 (1.3–4.7) | 3.4 +/−0.7 (1.6–4.9) | 0.4002 |
| Mean +/− Standard Deviation | ||||
| Cancer team scales (1–5; high is better) | ||||
| Cares about me as a person | 4.5 +/−0.8 | 4.5 +/− 0.8 | 4.5 +/−0.8 | 0.8939 |
| Listens to what I have to say | 4.5 +/−0.8 | 4.5 +/− 0.7 | 4.4 +/−0.9 | 0.1880 |
| Answers all of my questions | 4.5 +/−0.8 | 4.5 +/− 0.8 | 4.5 +/−0.8 | 0.8187 |
| Treats me in a friendly and courteous manner | 4.6 +/−0.9 | 4.6 +/− 0.9 | 4.6 +/−0.9 | 0.9685 |
| Number of mental health diagnoses – ever | 1.1 +/−1.3 | 1.0 +/− 1.2 | 1.1 +/−1.3 | 0.6157 |
| General health rating (1–5; high is better) | 2.6 +/−0.8 | 2.6 +/− 0.8 | 2.6 +/−0.9 | 0.5756 |
| Physical health limitations in moderate activities past 2 weeks (1–3; high is worse) | 2.1 +/−0.7 | 2.2 +/− 0.7 | 2.0 +/−0.7 | 0.0998 |
| Activity level past two weeks (1–6; high is worse) | 2.9 +/−1.3 | 2.9 +/− 1.3 | 3.0 +/−1.2 | 0.6341 |
| Number of cancer treatments | 1.3 +/−0.7 | 1.3 +/− 0.6 | 1.2 +/−0.7 | 0.3889 |
Note: p-values for categorical variables from χ2 test; continuous variables from Student’s t-test with Satterthwaite adjustment for unequal variances
3.2.3. Intervention Effects (over time).
Analyses of data over the 3 follow-up assessments (see Appendices B–D) also demonstrated null findings as the intervention was not effective in improving psychosocial health outcomes, either overall or for the individual components of the BHS score (i.e., depression, anxiety, functional disability or subjective well-being), in the top 30% of distressed patients over time (Appendix E).
3.3. Accept DR vs. Reject DR (n=183)
3.3.1. Group Effects.
A total of 183 patients were offered a DR, with 50 patients (27%) accepting the DR. There were no differences between these patients on any of the demographic measures. However, several symptom scores differed significantly between these patients and remained significant after adjusting for multiple comparisons. These scores included BHS, functional disability, anxiety, and depression, all of which were found to be lower in those who accepted the DR suggesting worse mental health status (p<0.01 for all measures).
DR accepted patients were more likely to have seen a doctor or mental health professional regarding mental health treatment (8.7% vs 0.9%, p=0.08; 20.0% vs. 0.0%, p<0.05; 5.0% vs. 2.2%, p=0.46; at 2, 6, and 12 months, respectively). However, despite this, the longitudinal models revealed that patients who accepted the DR had worse BHS, depression, anxiety, and subjective wellbeing compared to the patients who rejected the DR across all visits (p<0.05 for all measures; data not shown).
3.3.2. Intervention Effects.
The accept DR and reject DR groups significantly varied over time on few measures, when examining models that included a group*time interaction effect. Over time, the accept DR patients had fewer hospital admissions compared to the reject DR patients (overall type 3 χ2=6.48, p=0.04). DR patients were more likely to apply for disability (overall type 3 χ2=6.25, p=0.04) and report that seeing a counselor or therapist could be beneficial for emotional problems (overall type 3 χ2=11.20, p=0.01) compared to the reject DR patients over all visits (Appendix F).
3.3.3. Mechanisms of Action Analysis.
Level of treatment engagement was examined in intervention participants (n=50). The majority of participants (n=23, 46%) did not engage in any treatment during their follow-up, with 40% (n=20), 12% (n=6), and 2% (n=1) engaging in one to three treatments (i.e., receipt of counseling or therapy; attendance at a support group; receipt of a prescription for psychotropic medication), respectively. Due to unequal cell sizes, level of treatment engagement was dichotomized into zero treatment engagement versus any treatment engagement (1–3 treatments). ANOVA models predicting BHS score by level of treatment engagement showed no difference by level of treatment engagement for two groups as mean BHS score was a non-significant 0.2 points lower in those engaging in 1–3 treatments versus those in engaging in zero treatment (p=0.1739; data not shown).
4. Discussion and Conclusion
4.1. Discussion
We evaluated our data from a number of different angles as to best appreciate the results. First, we examined the entire sample over time, regardless of intervention or control condition, and found that most patients showed overall improvements in depression, anxiety, functional disability, and subjective well-being, without the need of intervention. This suggests that without intervention patients with cancer are likely to experience a decrease in psychological difficulties. This is a contentious topic in psycho-oncology, and our results are consistent with researchers, such as James Coyne, who have argued for the normative experience of psychological distress in patients with cancer [21]. We also compared the intervention and control groups across all constructs and found that there was a lack of meaningful differences (see Table 3). We then examined the top 30% of distressed patients in both intervention and control groups and compared them across time (see Table 4). Again, patients who were randomized to the intervention group did not show improved psychosocial or physical health outcomes at the 2, 6 and 12 follow-up assessments as compared to those patients in the control group who endorsed the greatest levels of distress. We surmise that it is unlikely that a brief intervention was powerful enough to impact said outcomes of patients who were dealing such extreme stressors.
Ours is not the first study to find that psychosocial interventions do not result in positive outcomes in oncology patients [22–24]. A more intense intervention may be needed to impact such multifaceted patient outcomes such as psychological distress or quality of life. Andersen et al. [13] conducted a randomized control trail to test a detailed psychosocial intervention on female breast cancer patients. While the control group received standard care, patients in the intervention group were taught healthy coping mechanisms weekly for 4 months and then monthly for the next 8 months. Meetings were led by two clinical psychologists, and the topics consisted of introducing the patients to techniques such as progressive muscle relaxation and ways to effectively make use of their social support systems [13]. The intervention participants’ reported reduced levels of emotional distress and was related to an increase in t-cell counts. Further, Sharpe and colleagues [25] compared the effectiveness of an integrated treatment program for depression with usual treatment in an RCT that enrolled 500 cancer patients. The intervention group received a multifaceted collaborative care treatment delivered by cancer nurses and psychiatrists. Compared with patients in the usual care group, those who received the intervention reported less depression, anxiety, pain, and fatigue as well as better overall functioning, health, and quality of life. The intervention group also reported enhanced perceived quality of care for their depression at all time points.
Table 2 shows chart review data that suggests patients in the intervention group were more likely to have the following documented independently (i.e., not through the MHADRO report) on their medical chart: depressive and anxiety symptoms, mental health counseling history, alcohol and smoking history, support group attendance. Providers were more likely to recommend support groups and provide psycho-educational materials about cancer. Patients in the intervention group were more likely to be evaluated, during an outpatient appointment, by a psychologist. They also had less documented emergency phone calls to their oncology team and less emergency department visits and hospitalizations over the course of the study.
Those patients in the intervention group who accepted the DR versus those who rejected showed more distress and physical health difficulties at baseline and through follow-up compared to those who rejected the DR. Patients who actually accepted the DR likely did so because of greater level of difficulties, both mental and physical at baseline, and this pattern continued over time. Individuals with serious psychological issues may accept referrals for treatment as compared to those with less symptoms. Those patients who accepted the DR were more likely to begin mental health treatment at the follow-up assessments and to endorse that therapy is beneficial. This brief intervention was successful in connecting patients with mental health providers through an efficient and patient-driven approach. Another positive outcome was those who accepted the DR were less likely to be hospitalized over the course of the study. Other researchers have found similar benefits [26–28].
There are a number of limitations of this study. First, the sample of patients who accepted the DR was relatively small, limiting confidence in our findings related to the DR intervention. Second, the sample was disproportionately white women diagnosed with breast cancer and results should be interpreted with caution. Third, there was also a lack of consistency of providers receiving and reviewing the reports before seeing patients. This was simply due to the nature of a busy clinical practice. This methodological flaw likely diluted the intervention’s potential power. Fourth, the intervention would have likely been more impactful if the report would have been reviewed with the patient by the providers and not the research assistants. We designed the study so the it would not impact providers’ clinical practice and, as a result, our intervention may have been viewed as less important to the patients.
4.2. Conclusion
We used technology to efficiently assess patient psychosocial and physical functioning as well as provide real-time access for opportunities to garner psychological and social assistance. We were able to do this across three busy clinical oncology practices with minimal difficulties. Providing patients with a brief intervention, or the offer of mental health counseling that they can initiate immediately during oncology treatment visits, may impact healthcare utilization such as emergency calls to oncology care providers or even hospitalizations. However, the intervention outlined in this study was not powerful enough to alter psychological or physical outcomes over time. This is supported by researchers who have argued that a referral based on distress screening should not, in itself, alleviate distress (as suggested by Coyne and colleagues, i.e. Meijer et al., 2013) as the alleviation of distress ultimately depends on the quality of the psychosocial treatment that is delivered and received by patients, which was not in the scope of this project.
4.3. Practice Implications
The MHADRO system is effective in identifying difficulties, connecting patients with care, and improving providers’ assessing, documenting, and assisting patient’s psychosocial needs. Programs like the MHADRO may impact psychosocial documentation and health care utilization, which has the potential to affect management of patients based on their psychosocial profiles. Thus, the present results do support the effectiveness of incorporating technology into the process of psychosocial screening and referrals for patients who are receiving treatment for cancer.
Supplementary Material
Highlights:
The intervention connected patients in need with mental health services.
Patients in need of mental health services were in more distress over time.
Providers increased documenting and referring for mental health services.
Acknowledgments
Note: This grant was funded through a Small Business Technology Transfer (STTR) – Phase 2 - mechanism from the NIH/NIMH (R42 MH078432). ClinicalTrials.gov Identifier: NCT01442285
The study was approved by IRBs at the University of Massachusetts Medical School, Cooper Hospital, and MD Anderson. All patients were treated in accordance with the NIH and APA’s standards for research with human subjects and all participants went through a thorough written informed consent process prior to taking part in the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This manuscript has not been published elsewhere and is not currently under review by another journal. All authors have approved the manuscript. Intellectual property, licensing and revenue income from the POST program are shared between Polaris Health Directions and the University of Massachusetts Medical School. Dr. Boudreaux and Dr. O’Hea also consult for Polaris Health Directions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Note: The MHADRO is the name that was used for this psychosocial oncology system during the NIH grant funding period. Since ending the study the same program is now marketed by Polaris Health Directions as Polestar Oncology.
Footnotes
Compliance and Ethical Standards:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erin O’Hea, Department of Psychiatry, Corresponding author at University of Massachusetts Medical School, Worcester, MA, and Department of Psychology, Stonehill University, Easton, MA, 320 Washington Street, Shields Science Center 212, Easton, MA, USA 02357.
Aimee Kroll-Desrosiers, Department of Quantitative Health Sciences, University of Massachusetts Medical School, Worcester MA, USA.
Alexandra S. Cutillo, Medical/Clinical Psychology Doctoral Program, University of Alabama at Birmingham, Birmingham AL, USA.
Hannah R. Michalak, Yale University Alzheimer’s Disease Research Unit, One Church Street, Suite 600, New Haven CT, USA 06510.
Bruce A. Barton, Department of Quantitative Health Sciences, University of Massachusetts Medical School, Worcester MA, USA.
Tina Harralson, Polaris Health Directions, Wayne PA, USA.
Cindy Carmack, MD Anderson Cancer Center, Houston TX, USA.
Cori McMahon, MD Anderson at Cooper Cancer Center, Camden NJ, USA.
Edwin D. Boudreaux, Departments of Emergency Medicine, Psychiatry, and Quantitative Health Sciences, University of Massachusetts Medical School, Worcester MA, USA.
References
- 1.Abernethy AP, Herndon JE II, Coan A, Staley T, Wheeler JL, Rowe K, & Lyerly HK (2010). Phase 2 Pilot Study of Pathfinders: A psychosocial intervention for cancer patients. Supportive Care in Cancer, 18(7), 893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleiker E, Pouwer F, van der Ploeg HM, Leer JH, & Ader HJ (2000). Psychological Distress Two Years After Diagnosis of Breast Cancer: Frequency and prediction. Patient and Education Counseling, 40, 209–17. [DOI] [PubMed] [Google Scholar]
- 3.Gao W, Bennett MI, Stark D, Murray S, & Higginson IJ (2010). Psychological Distress in Cancer from Survivorship to End of Life Care: Prevalence, associated factors and clinical implications. European Journal of Cancer, 46, 2036–44. [DOI] [PubMed] [Google Scholar]
- 4.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, & Piantadosi S (2001). The Prevalence of Psychological Distress by Cancer Site. Psychooncology, 10(1), 19–28. [DOI] [PubMed] [Google Scholar]
- 5.Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, Holland JC, Partridge AH, Bak K, Somerfield MR, & Rowland JH (2014). Screening, Assessment, and Care of Anxiety and Depressive Symptoms in Adults with Cancer: An American society of clinical oncology guideline adaptation. Journal of Clinical Oncology, 32(15), 1605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J, Bae SH, Jung YS, & Kim KS (2012). Quality of Life and Symptom Experience in Breast Cancer Survivors after Participating in a Psychoeducational Support Program: A pilot study. Cancer Nursing, 35(1), 34–41. [DOI] [PubMed] [Google Scholar]
- 7.Powell CB, Kneier A, Chen L, Rubin M, Kronewetter C, & Levine E (2008). A Randomized Study of the Effectiveness of a Brief Psychosocial Intervention for Women Attending a Gynecologic Cancer Clinic. Gynecologic Oncology, 111, 137–43. [DOI] [PubMed] [Google Scholar]
- 8.Rehse B & Pukrop R (2003). Effects of Psychosocial Interventions on Quality of Life in Adult Cancer Patients: Meta-analysis of 37 published controlled outcome studies. Patient Education and Counseling, 50(2), 179–86. [DOI] [PubMed] [Google Scholar]
- 9.Manos D, Sebastián J, Mateos N, & Bueno MJ (2009). Results of a Multi-componential Psychosocial Intervention Programme for Women with Early-Stage Breast Cancer in Spain: Quality of life and mental adjustment. European Journal of Cancer Care, 18(3), 295–305. [DOI] [PubMed] [Google Scholar]
- 10.McLachlan S, Allenby A, Matthews J, Wirth A, Kissane D, Bishop M, et al. (2001). Randomized Trial of Coordinated Psychosocial Interventions Based on Patient Self-Assessments Versus Standard Care to Improve the Psychosocial Functioning of Patients with Cancer. Journal of Clinical Oncology, 19(21), 4117–25. [DOI] [PubMed] [Google Scholar]
- 11.Rawl SM, Given BA, Given CW, Champion VL, Kozachik SL, Barton D, …& Williams SD (2002). Intervention to Improve Psychological Functioning for Newly Diagnosed Patients with Cancer. Oncology Nursing Forum, 29(6), 967–75. [DOI] [PubMed] [Google Scholar]
- 12.Schou I, Ekeberg O, Karesen R, & Sorensen E (2008). Psychosocial Intervention as a Component of Routine Breast Cancer Care-Who participates and does it help? Psychooncology, 17(7), 716–20. [DOI] [PubMed] [Google Scholar]
- 13.Andersen BL, Farrar WB, Golden-Kreutz D, Emery CF, Glaser R, Crespin T, & Carson W (2007). Distress Reduction from a Psychological Intervention Contributes to Improved Health for Cancer Patients. Brain, Behavior, and Immunity, 21(7), 953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell AJ (2013). Screening for Cancer-Related Distress: When is implementation successful and when is it unsuccessful? Acta Oncologica, 52(2), 216–24. [DOI] [PubMed] [Google Scholar]
- 15.Erharter A, Giesinger J, Kemmler G, Schauer-Maurer G, Stockhammer G, Muigg A, …& Holzner B (2010). Implementation of Computer-Based Quality-of-Life Monitoring in Brain Tumor Outpatients in Routine Clinical Practice. Journal of Pain and Symptom Management, 39(2), 219–29. [DOI] [PubMed] [Google Scholar]
- 16.Loiselle CG, Edgar L, Batist G, Lu J, & Lauzier S (2009). The Impact of a Multimedia Informational Intervention on Psychosocial Adjustment among Individuals with Newly Diagnosed Breast or Prostate Cancer: A feasibility study. Patient Education and Counseling, 80, 48–55. [DOI] [PubMed] [Google Scholar]
- 17.McLachlan S, Allenby A, Matthews J, Wirth A, Kissane D, Bishop M, …& Zalcberg J (2001). Randomized Trial of Coordinated Psychosocial Interventions Based on Patient Self-Assessments Versus Standard Care to Improve the Psychosocial Functioning of Patients with Cancer. Journal of Clinical Oncology, 19(21), 4117–25. [DOI] [PubMed] [Google Scholar]
- 18.Boudreaux ED, O’Hea EL, Grissom G, Lord S, Houseman J, & Grana G (2010). Initial Development of the Mental Health Assessment and Dynamic Referral for Oncology (MHADRO). Journal of Psychosocial Oncology, 29, 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Hea EL, Cutillo AS, Dietzen L, Harralson T, Grissom G, & Boudreaux ED (2013). Randomized Control Trial to Test a Computerized Psychosocial Cancer Assessment and Referral Program: Methods and research design. Contemporary Clinical Trials, 35, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SAS 9.3 (SAS Institute, Cary NC).
- 21.Coyne JC, Palmer SC, Shapiro PJ, & Thompson R (2004). Distress, Psychiatric Morbidity and Prescriptions for Psychotropic Medication in a Breast Cancer Waiting Room Sample. General Hospital Psychiatry, 26(2), 121–8. [DOI] [PubMed] [Google Scholar]
- 22.Akechi T, Taniguchi K, Suzuki S, Okamura M, Minami H, Okuyama T, & ... Uchitomi Y (2007). Multifaceted Psychosocial Intervention Program for Breast Cancer Patients after First Recurrence: Feasibility study. Psycho-Oncology, 16(6), 517–24. [DOI] [PubMed] [Google Scholar]
- 23.Cousson-Gelie F, Bruchon-Schweitzer M, Atzeni T, Houede N (2011). Evaluation of a Psychosocial Intervention on Social Support, Perceived Control, Coping Strategies, Emotional Distress and Quality of Life of Breast Cancer Patients. Psychological Reports, 108(3), 923–42. [DOI] [PubMed] [Google Scholar]
- 24.Oh PJ, & Kim SH (2010). Effects of a Brief Psychosocial Intervention in Patients with Cancer Receiving Adjuvant Therapy. Oncology Nursing Forum, 37(2), 98–104. [DOI] [PubMed] [Google Scholar]
- 25.Sharpe M, Walker J, Hansen CH, Martin P, Symeonides S, Gourley C, …& Murray G (2014). Integrated Collaborative Care for Comorbid Major Depression in Patients with Cancer (SMaRT Oncology-2): A multicentre randomized controlled effectiveness trial. The Lancet, 384(9948), 1099–1108. [DOI] [PubMed] [Google Scholar]
- 26.Rowan PJ, Davidson K, Campbell JA, Dobrez DG, & MacLean DR Depressive Symptoms Predict Medical Care Utilization in a Population-Based Sample. Psychological Medicine, 32(5), 903–8. [DOI] [PubMed] [Google Scholar]
- 27.Simon GE, VonKorff M, & Barlow W (1995). Health Care Costs of Primary Care Patients with Recognized Depression. Archives of General Psychiatry, 52(10), 850–6. [DOI] [PubMed] [Google Scholar]
- 28.Luber MP, Hollenberg JP, Williams-Russo P, DiDomenico TN, Meyers BS, Alexopoulos GS, Charlson ME (2000). Diagnosis, Treatment, Comorbidity, and Resource Utilization of Depressed Patients in a General Medical Practice. International Journal of Psychiatry Medicine, 30(1), 1–13. [DOI] [PubMed] [Google Scholar]
- 29.Coyne JC (2013). Benefits of Screening Cancer Patients for Distress still not Demonstrated. British Journal of Cancer, 108, 736–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grissom G, Lyons J, & Lutz W (2002). Standing on the Shoulders of a Giant: Development of an outcome management system based on the dose model and phase theory of psychotherapy. Psychotherapy Research, 12(4), 397–412. [Google Scholar]
- 31.Saunders JB, Aasland OG, Babor TF, de la Fuente JR & Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- 32.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, & Robinson J (1989). Measuring the Heaviness of Smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction, 84(7), 791–9.> [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.