Abstract
The bi-directional links between hormones and behavior have been a rich area of research for decades. Theory on the evolution of testosterone (T) was greatly advanced by the challenge hypothesis, which presented a framework for understanding interspecific, seasonal, and social variation in T levels in males, and how they are shaped by the competing demands of parental care and male-male competition. Female competition is also widespread in nature, although it is less clear whether or how the challenge hypothesis applies to females. Here, we evaluate this issue in four parts: (1) We summarize and update prior analyses of seasonal plasticity and interspecific variation in T in females. (2) We evaluate experimental links between T and female aggression on shorter timescales, asking how T manipulations affect aggression and conversely, how social manipulations affect T levels in female mammals, birds, lizards, and fishes. (3) We examine alternative mechanisms that may link aggression to the social environment independently of T levels in circulation. (4) We present a case study, including new data analyses, in an aggressive female bird (the tree swallow, Tachycineta bicolor) to explore how variation in tissue-level processing of T may bridge the gap between circulating T and variation in behavior that is visible to natural selection. We close by connecting these multivariate levels of sex steroid signaling systems alongside different temporal scales (social, seasonal, and evolutionary) to generate broadly applicable insights into how animals respond to their social environment, regardless of whether they are male or female.
Keywords: androgen receptor, neural sensitivity, evolutionary endocrinology, 5-alpha-reductase, aromatase, amygdala, pectoralis
Introduction
“Females are generally regarded as not aggressive and testosterone is regarded as the ‘male sex hormone.’ These views are incorrect…” – Wingfield and colleagues (2000)
The sex steroid hormone testosterone (T) is thought to regulate phenotypic responses to dynamic social environments in male vertebrates. For instance, males typically produce more T during breeding stages that involve mating competition and less T during parental breeding stages (Ketterson and Nolan, 1992; Wingfield, 1984). Likewise, males may temporarily elevate T levels in response to a prospective rival or mate (Gleason et al., 2009; Wingfield, 1985). As a result, T levels may exceed what is necessary for reproduction, instead promoting behaviors that are adaptive during competition (Wingfield et al., 1990). Similar patterns may apply over evolutionary time, with higher T in species with heightened competition for territories or mates (Hirschenhauser and Oliveira, 2006; Hirschenhauser et al., 2003; Oliveira et al., 2002). Patterns of T secretion across three timescales – social, seasonal, and evolutionary – form the crux of the challenge hypothesis, which broadly suggests that variation in T secretion within and among species stems from natural selection related to trade-offs between mating effort and parental effort (Wingfield, 2012; Wingfield et al., 1990; Wingfield et al., 2001).
This framework has been largely supported in males across many taxa (Archer, 2006; Hirschenhauser and Oliveira, 2006; Hirschenhauser et al., 2003; Oliveira et al., 2002). However, we still have a relatively limited understanding of whether and how the challenge hypothesis applies to females. Our primary goal is to synthesize current data on the challenge hypothesis in females. In addition, we evaluate hypotheses that broaden the challenge hypothesis beyond its initial focus on T, asking how other hormones and tissue-level processes may modulate phenotypic responses to social competition. We begin by overviewing relationships between T and female behavior, including prior analyses of seasonal and interspecific variation in T. We then provide a novel synthesis of 80 previously published studies across vertebrate taxa to evaluate two key aspects of the challenge hypothesis: (H1) Elevated T promotes female aggression, and (H2) females respond to competition by elevating T. Next, we broaden our focus and begin to evaluate two related hypotheses: (H3) Other components of sex steroid signaling systems and (H4) non-steroidal mechanisms may influence aggression, independently of T levels in circulation, promoting the adaptive expression of aggression without necessitating elevated T per se. Finally, we describe a case study in an avian species with strong female-female competition to explore how tissue-level variation in sex steroid signaling systems may interact with seasonal patterning of T and aggression. We close by connecting these different mechanistic levels and temporal scales (social, seasonal, and evolutionary) to generate broad insights on the challenge hypothesis that are applicable not only to females but to both sexes.
Functional role of T in females
Despite its reputation as the ‘male hormone,’ T is a natural and important part of female physiology. Arguments to the contrary may stem from observations that in many species, females have lower levels of T in circulation than males, and female gonads convert more T to estrogenic metabolites, such as 17β-estradiol (E2). In addition to the costs of T incurred by males (Wingfield et al., 2001), females with high T also may experience reduced maternal care, disrupted mate choice, or delayed breeding (Clotfelter et al., 2004; Gerlach and Ketterson, 2013; McGlothlin et al., 2004; Rosvall, 2013a), meaning there are potentially fitness costs to the activational effects of T in females. Comparative studies also report correlations between female and male T levels in birds (Goymann and Wingfield, 2014; Ketterson et al., 2005; Møller et al., 2005) and fishes (Mank, 2007), suggesting that T levels in females may simply be non-functional byproducts of selection shaping T secretion in males.
However, several observations instead suggest that T is functionally relevant for females. T is critically involved in many aspects of female reproduction (Drummond, 2006). Leading up to ovulation, females experience a surge in T (Johnson and Van Tienhoven, 1980; Kobayashi et al., 1989; Rush and Blake, 1982), which can directly mediate ovulation or fertility via binding to androgen receptors (AR) (Hu et al., 2004; Rangel et al., 2006). Females can allocate T to offspring, and this maternal effect varies in relation to life history (Bentz et al., 2016; Mousseau and Fox, 1998), suggesting it may be shaped by selection. T produced from the ovary is also released into circulation, and, critically, females have all of the cellular and molecular machinery to respond to T in tissues that regulate a variety of morphological, physiological, and behavioral traits (Staub and DeBeer, 1997). Indeed, T has been linked with many adaptive female traits, including parenting, aggression, and muscle functioning (Cain and Ketterson, 2012; Cain and Ketterson, 2013; Dent et al., 2012). Like males, some of these effects may stem from T’s estrogenic metabolites acting on the brain or other target tissues (Soma et al., 2008), and the challenge hypothesis does not inherently distinguish between androgenic or estrogenic effects of T or its metabolites. We carry this perspective forward here, although we note that the activational effects of T are not necessarily identical to those of E2 (Duque-Wilckens and Trainor, 2017; Floody, 1983; French et al., 2013), a point we return to below. In sum, T is naturally produced at certain times of the female reproductive cycle, and there are many opportunities for natural selection to influence T levels via effects on organismal traits.
Seasonal and life history patterns of T secretion in females
One key component of the challenge hypothesis is that temporal patterns of circulating T are shaped by trade-offs between competition and parental care, via activational effects of T that enhance aggression and impede parental care. It is well known that, on average in many species, females provide more parental care than males (Trivers, 1972), but only recently has the adaptive function and ubiquity of female-female competition become clear. Females compete with one another in a variety of contexts across nearly all species studied to date (Clutton-Brock, 2009; Rosvall, 2011; Stockley and Bro-Jørgensen, 2011). In some cases, female competition determines access to mates, similar to how sexual selection operates in males (Eens and Pinxten, 2000; Hare and Simmons, 2019). Even when all females obtain a male, some females outcompete others for better mates, territories, nesting sites, or exclusive paternal support (Rosvall, 2011; Slagsvold and Lifjeld, 1994; Tobias et al., 2012).
Considering that this competition often occurs during breeding and pre-breeding periods when females can produce T but are not yet parenting, T could serve to promote competitive traits, like aggression, without deleterious effects on parenting. Later in the breeding season, lower T levels or less socially responsive T levels may be advantageous if elevated T also interferes with maternal care. Earlier analyses on female birds support this seasonal component of the challenge hypothesis, that T levels tend to peak during periods of territorial establishment or mate acquisition, and decline as parental demands increase (Goymann and Wingfield, 2014; Ketterson et al., 2005). We used the new resource HormoneBase (Vitousek et al., 2018) to analyze variation in female T levels in other vertebrate taxa and found similar patterns (Figure 1, see SI methods), albeit with limited taxonomic sampling in mammals and reptiles. These data show that, across vertebrates, T is elevated beyond its role in reproduction. Further, at least in species in which we have substantive data, seasonal variation in female T largely tracks expected shifts between competition and care, consistent with past work on the challenge hypothesis in males.
Figure 1.
Mean circulating T levels in female birds, fishes, mammals, and reptiles across life stages, obtained from HormoneBase (see SI methods). Prenatal care includes egg laying, incubation, and pregnancy; postnatal care includes lactation and other care of young. T significantly varied across life stages in birds and fishes. Sample sizes correspond to # of records. Error bars are SE, and letters denote significant pairwise stage differences (p<0.05), controlling for species.
How and why these patterns of T secretion scale over evolutionary time is less certain. Møller et al. (2005) reported higher T levels in females in colonial species of birds, and Ketterson et al. (2005) found higher T in females from socially monogamous bird species. A more recent analysis included more species alongside improved phylogenetic and meta-analytical methods (Goymann and Wingfield, 2014), but results were decidedly null: maximum female T levels and seasonal plasticity in female T were unrelated to any variable studied, including mating system, coloniality, and degree of dimorphism (Goymann and Wingfield, 2014, see also Garamszegi 2014). These analyses have some limitations (Groothuis et al., 2014; Ketterson, 2014), but one implication is that the selective pressures shaping female-female competition and aggression are not related to classic indices of male-male competition and sexual selection (sensu Tobias et al., 2012). Resolving whether female T levels are shaped by the relative importance of female competition vs. care, as expected by the challenge hypothesis, will require that we re-imagine how to measure the strength of female-female competition outside of a framework derived for studying males. Echoing some of the earliest analyses on the challenge hypothesis in females (Wingfield, 1994), we urge future work that evaluates how variation in T relates to known drivers of female competition, such as competition for nesting sites, high quality males, or the direct/indirect benefits they provide. We also must systemically evaluate the organism in which this hormone acts because, ultimately, the evolutionary process depends upon how T interacts with other traits that are visible to selection, including aggression and parental care. In sum, it is not yet clear how selection generates interspecific variation in female T secretion, but it is clear that females modulate T across different breeding stages, and many species have elevated T during periods of breeding and pre-breeding social competition.
Evaluating bidirectional links between T and female aggression
Progress towards understanding how T is shaped by selection acting on females will require broad synthesis of the bidirectional links between T and female aggression, particularly on shorter time-scales where selection acts (i.e. minutes, hours, days). This relationship has been examined in several ways, including correlative studies on T and aggression and more experimental approaches in which T or social environments are manipulated. We conducted a systematic search of the literature that yielded 80 studies (n=97 cases) across mammals, birds, lizards, and fishes in which overt conspecific aggression in females was measured in relation to T (see SI methods and SI Figure S1), summarized below.
H1: Females respond to competition by increasing T
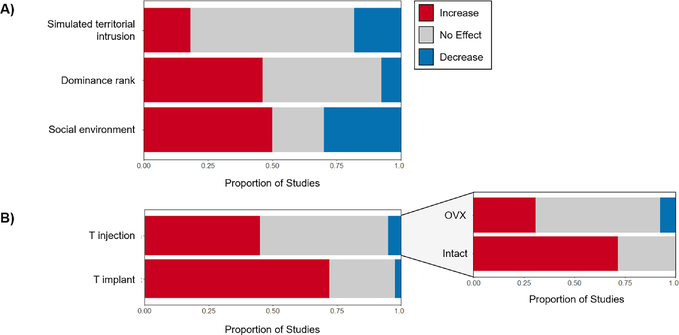
We located only 11 cases testing this hypothesis using simulated territorial intrusions (or resident-intruder tests) in which a live or stuffed decoy was presented to a focal female, aggressive response was quantified, and blood T levels were measured shortly thereafter and compared to unchallenged controls (Figure 2A; Table S1). In all cases, females responded to female intruders with robust aggression. However, we identified only 2 cases (18%) in which females exposed to territorial intrusion had higher T levels than controls (Davis and Marler, 2003; Gill et al., 2007). In all other cases, intrusions did not affect female T levels in circulation (Canoine and Gwinner, 2005; Davis and Marler, 2003; DeVries et al., 2015; Gill et al., 2007; Goymann et al., 2008; Jawor et al., 2006) or T levels were lower in challenged females compared to controls (Elekonich and Wingfield, 2000; Navara et al., 2006). Correlative studies generally mirror this same pattern, with a few reporting positive (Woodley and Moore, 1999) or negative (Cantarero et al., 2015) relationships between T and female aggression, and most reporting no such pattern (Moreno et al., 2016; Ross and French, 2011; Rosvall et al., 2012; Schwabl et al., 2005). Thus, social elevation of T is not a common female response to an acute social challenge.
Figure 2.
(A) Studies testing the effect of acute (simulated territorial intrusions) or persistent social factors (dominance rank and social environment) on female T. (B) Studies testing the effect of T on aggression (injections, implants). Injection studies are sub-divided by intact vs. ovariectomized (OVX). Color denotes proportion of studies showing that T or aggression increases (red), decreases (blue), or stays the same (no effect; grey).
The challenge hypothesis emphasizes that T levels may be more constrained during parental breeding stages, a constraint that may be stronger for females due to extended parental responsibilities. Consistent with this view, the two cases reporting increased T following a territorial intrusion occurred during pre-breeding stages (Davis and Marler, 2003; Gill et al., 2007), but these studies also repeated the challenge during later (parental) stages and found that females did not elevate T to intrusions. In addition, females in some species decrease T following social competition (Elekonich and Wingfield, 2000; Navara et al., 2006), hinting at other hormonal processes that ‘use up’ T during social competition. Existing data are too limited to statistically test the effect of breeding stage (see Table S1); however, all but one case reviewed here was conducted during various pre-breeding/breeding stages, suggesting that stage alone cannot account for these patterns.
Another possibility is that a single conspecific intrusion does not sufficiently mimic hormonal responses to real competition, a point that has been discussed in research on males (Oliveira et al., 2005; Scriba and Goymann, 2008). We therefore investigated two related analyses that may speak to the cumulative effects of repeated competitive interactions in females (Figure 2A; Table S1). First, we examined studies correlating dominance ranks with T, and we found that 46% (6/13 cases) show a positive relationship between T and female dominance (Batty et al., 1986; Beehner et al., 2005; Clarke and Faulkes, 1997; Davies et al., 2016; Desjardins et al., 2008; Renn et al., 2012). Second, looking at studies that either manipulate the social environment (e.g., by increasing breeding density) or measure natural variation in breeding density or intrusion rates, we found that 50% (5/10 cases) report higher female T levels in more competitive environments (Cantarero et al., 2015; Chapman et al., 1998; Gavasa et al., 2012; Langmore et al., 2002; Polo et al., 2010). These more persistent or naturalistic manipulations are far more consistent with the challenge hypothesis than the relatively meager support found with a single territorial intrusion (i.e. 18%), suggesting that we have work yet to do to understand how females hormonally respond to real competition in nature, echoing some emerging findings in males (Goymann et al., 2019).
H2: Elevated T promotes female aggression
The activational effects of T on female aggression have been well known for some time (see Svare, 2013; Wingfield et al., 2000; Wingfield, 1994). Our literature review found that T implants increase female aggression in 72% of cases (3¼3), while T injections increase aggression in 45% of cases (9/20; see Figure 2B; Table S2). Among the subset of studies that clearly used female-appropriate doses, most reported increased female aggression (78%; 7/9 cases). Thus, support for the hypothesis that T enhances female aggression is unlikely to be driven by supraphysiological dosing generating unnatural responses (Ketterson, 2014; Ketterson et al., 2005, see also Fusani, 2008; Goymann and Wingfield, 2014).
Our finding that T implants are more likely to increase female aggression than injections suggests that prolonged T manipulations are more behaviorally salient. Notably, only 31% of T injection studies on ovariectomized females report increased aggression (4/13 cases), whereas 71% of T injection studies on intact females report positive effects on aggression (5/7 cases). Implant experiments seem generally less influenced by this effect, with 68% (17/25) of ovariectomized vs. 78% (14/18) of intact studies reporting elevated aggression. Thus, a transient surge in T may be more likely to enhance aggression if other aspects of sex steroid signaling have primed the organism to respond. Stated another way, T does not activate aggression on its own; other co-regulated parts of the system may need to be in place before even brief elevations of T will promote aggression.
Related to this point, genetic, developmental, and physiological factors may influence the degree to which T affects aggression, via effects on other organismal and systemwide processes. For example, Sandnabba et al. (1994) found that female mice from lines bred for high aggression showed more overt aggression in response to T injections, but those from low aggression lines did not. Similarly, phylogenetic history almost surely influences the behavioral salience of T, although taxonomic biases make this challenging to quantify at this time (Table S2). Developmental effects, such as the temperature experienced embryonically, have also been linked with the effectiveness of T implants in affecting aggression in a gecko species (Crews et al., 1996; Flores and Crews, 1995). This effect was attributed to developmental differences in brain morphology and metabolic capacity impacting the behavioral salience of later T exposure. Finally, just as breeding stage may alter the effects of competition on T (see H1), stage also may alter the effects of T on behavior. Clarke et al. (1976) reported such a case in ewes, in which T implants increased aggression but only during early pregnancy – this effect was abolished during late pregnancy and attributed to increased metabolic clearance of T.
In sum, T does increase female aggression, especially when T is present at sufficient background levels as seen with T implants and T injections into intact females (Figure 2B, SI Table S2), mirroring the findings in H1 that more prolonged, cumulative social interactions are more likely to elevate T (Figure 2A; SI Table S1; SI Figure S2). Because this behavioral effect occurs using a fraction of the T needed to elicit a similar response in males, females may in some ways be considered more sensitive to T than males. Alternatively, females may achieve these behavioral effects with lower T because T is converted to E2. E2 undoubtedly plays a role in aggression in females much like it does in males (summarized above), but it appears unlikely that E2 alone drives the findings summarized here. Critically, a subset of the cases in which T treatment increased female aggression also contained a separate E2 treatment. However, E2 elicited increased (Albert et al., 1990; Greene et al., 1978), decreased (DeBold and Miczek, 1981) or no effect on aggression (Archawaranon and Wiley, 1988; Barkley and Goldman, 1978; Compaan et al., 1993; Simon et al., 1985; Winkler and Wade, 1998), despite T increasing aggression in all of these cases. Taken together, these findings suggest that our understanding of T’s effect on behavior will be improved by consideration of the rest of the system in which this hormone operates.
Many roads lead to aggression: extensions of the challenge hypothesis beyond T in circulation
If the challenge hypothesis provides a framework for linking T and aggression over different timescales and contexts, then a natural extension is that other endocrine-molecular mechanisms should influence the strength of those patterns. Indeed, we know that T does not act in a vacuum (Adkins-Regan, 2005; Fuxjager and Schuppe, 2018). Once produced, gonadally-derived T is transported through the bloodstream to multiple target tissues, including brain, muscle, and immune cells. There, T can bind to AR, which interacts with co-factors and response elements to alter gene expression (Peterson et al., 2013; Peterson et al., 2014; van Nas et al., 2009). T can have downstream effects after 5α-reductase (5αR) converts T to dihydrotestosterone (DHT) which binds AR with higher affinity than T itself. T can also be converted to E2 via aromatase (AROM), which is present in many tissues, including the brain and ovary. E2 can bind to estrogen receptors (ER) or initiate rapid effects via secondary messenger systems (Remage-Healey et al., 2008). The effectiveness of steroids can also be modulated by liver metabolic processes (Mueller et al., 2015) or binding globulins (Deviche et al., 2001; Swett and Breuner, 2008) that essentially lower the availability of bioactive steroids. Furthermore, many components of the androgenic signaling system interact with other behaviorally active signaling molecules, including glucocorticoids, nonapeptides, catecholamines, and more, all of which can modulate T’s effect on behavior or influence aggression (Filby et al., 2010; Nelson and Trainor, 2007; Soma et al., 2008). In short, T levels in circulation barely scratch the surface of the full suite of endocrine-molecular processes that may influence variation in aggressive behavior.
Critically, natural selection does not differentiate among these diverse endocrine-molecular mechanisms. Rather, it is their consequence for organismal traits that matter in the eyes of selection: differences in behavior, morphology, and physiology that generate variation in survival and reproductive success. We therefore envision two interacting phenotypic landscapes that can guide our thinking on how trade-offs between competition and care may influence the evolution of T-aggression relationships (Figure 3). The first landscape (Figure 3, top) predicts variation in intrasexual aggression, which should be highest when competition is most intense and parental demands are low, and vice versa. Animals may shift along the phenotypic landscape with varying social or seasonal cues, and species may be restricted to certain areas of the landscape via evolutionary change. The second landscape (Figure 3, bottom) predicts variation in T secretion in an analogous way, with one key difference: selection does not assay T; it only shapes T indirectly via its consequence for the behavioral landscape and presumably other inter-related phenotypic landscapes not shown here (e.g. immune function). The original challenge hypothesis primarily dealt with individuals or species in the upper left or bottom right quadrants, and the degree to which T and aggression are bi-directionally linked. For females and other animals that experience co-occurring pressures of competition and care (upper right quadrant), alongside pleiotropic costs of T that may affect breeding and parenting, we should expect other mechanisms to support aggression (Rosvall, 2013b), thereby decoupling the aggression landscape and the T landscape to some degree. With this in mind, we envision two extensions of the challenge hypothesis that move beyond the initial focus on T in circulation (H3 and H4, below).
Figure 3.
Natural selection acts on the aggression landscape (upper panel), whereas variation in the testosterone landscape (below) is only linked with the behavioral landscape in some contexts. The basic tenets of the challenge hypothesis (e.g. H1 and H2, which bidirectionally link T and aggression) operate in the upper left quadrant of either surface. In the upper right quadrant, however, other mechanisms (e.g. H3, H4) may be more adaptive to promote aggression that co-occurs with parental care. The exact shapes of these surfaces are heuristic, and we imagine that individuals, sexes, and species move along these surfaces across social, seasonal, and evolutionary time.
H3: Other components of sex steroid signaling systems promote aggression when T is low
Recent analyses suggest that organisms can modulate tissue-level sensitivity to T independently from one tissue to the next, and independently of T levels in circulation (Hunter et al., 2018; Lipshutz et al., accepted). Consequently, it is reasonable to expect that selection might shape the specific constellation of tissues with high vs. low androgen sensitivity to adaptively suit the competing demands of social competition vs. care (Hau, 2007; Ketterson et al., 2009). By extension, aggression in females may be modified via changes in how efficiently T is transported, how rapidly it is converted to other steroids in specific tissues, and how many steroid receptors are available to receive that signal within specific tissues (Rosvall 2013b).
Indeed, there is good evidence that variation in T sensitivity, conversion, or metabolism within specific areas of the brain can predict female aggression even when circulating T levels cannot. For instance, in female dark-eyed juncos (Junco hyemalis), neural expression of AR, ERα, and AROM predicts individual variation in response to simulated territorial intrusions, whereas T levels do not (Rosvall et al., 2012). Likewise, in the white-throated sparrow (Zonotrichia albicollis), females of the more aggressive white-striped morph differ from the less aggressive tan-striped morph in ERα expression in key nodes of the social behavior network (Horton et al., 2014), and behavioral differences between morphs persist even when sex steroid levels are experimentally equalized (Maney et al., 2009; Merritt et al., 2018). Sex-role reversed species in which females are the more aggressive sex further suggest that enhanced sensitivity to T in the brain may promote aggression in the face of low T (Voigt, 2016; Voigt and Goymann, 2007). These comparative and correlative results are bolstered by experimental work linking AR, ERα, and AROM to aggression (Duque-Wilckens and Trainor, 2017), lending support to the hypothesis that female aggression relates to T and E2 processing in the brain.
Other steroids like progesterone (PROG) and dehydroepiandrosterone (DHEA) also can be converted to T or E2 in the brain to influence aggression. In Siberian hamsters (Phodopus sungorus), for example, females are especially aggressive during the non-breeding season when gonadal sex steroid secretion is low, but adrenal DHEA production is high (Rendon et al., 2015). These females also exhibit greater ERα expression in brain regions mediating aggression (Rendon et al., 2017). In addition, social challenges reduce levels of DHEA in circulation (Rendon and Demas, 2016), suggesting that DHEA may be ‘used up’ during competition, similar to the competition-induced decreases in T described above. Several other studies also point to interactions between PROG and female aggression (Davis and Marler, 2003; Goymann et al., 2008; Rubenstein and Wikelski, 2005), with some indication that the enzyme 5αR may influence aggression via its joint effects on concentrations of PROG, T, or the ratio between the two, which has been linked with aggression as well (Pibiri et al., 2006; Pinna et al., 2005). Together, these studies highlight the potential for other sex steroids and prohormones to mediate aggression at times when circulating T levels are low – a hormonal state that often applies to females.
To our knowledge, no studies have addressed whether social challenges induce local changes to the female brain in a manner that parallels elevations in circulating T predicted by the challenge hypothesis. However, evidence from males suggests that challenges may influence neurosteroidogenesis (Charlier et al., 2011), conversion of steroids into more active forms (Pradhan et al., 2010; Soma et al., 2014; Soma et al., 2008), and AR expression (Fuxjager et al., 2010) in ways that promote aggression. These mechanisms may be particularly relevant when T levels are low, including male aggression during the non-breeding season (Hau et al., 2004; Soma et al., 2014). We envision that similar mechanisms occur in females, perhaps explaining why T levels sometimes decrease following competitive interactions (see H1). Future work should explore whether these putatively adaptive responses to the social environment occur in females, and whether seasonal or evolutionary changes in these mechanisms predict female aggression, even when T levels do not.
H4: Other signaling systems regulate female responses to the social environment
A related but non-mutually exclusive hypothesis is that female aggression depends less on T and more on other signaling systems, such as nonapeptides, catecholamines, or glucocorticoids (Filby et al., 2010; Nelson and Trainor, 2007). Many of these ‘alternative’ mechanisms are still regulated to some extent by T. For instance, experimental treatment with T alters neural gene expression of monoamine oxidase A (an enzyme involved in catecholamine signaling) in female dark-eyed juncos (Peterson et al., 2013). T and E2 also regulate signaling of vasopressin, oxytocin, and their taxon-specific homologs (e.g. Auger et al., 2011; Kabelik et al., 2008; Nugent et al., in press), and variation in these nonapeptides predicts differences in aggression among individuals, sexes, sex roles, seasons, and species (e.g. Aubin-Horth et al., 2007; Goodson et al., 2012; Renn et al., 2008; Schumer et al., 2011, reviewed in Kelly and Vitousek, 2017). Like T levels in circulation, these mechanisms vary flexibly in response to changing social environments (Hsu et al., 2006; Kelly and Vitousek, 2017; O’Connell and Hofmann, 2011), and these mechanisms may also influence aggression independently of T. Thus, it is clear that we should look beyond levels of T in circulation as we continue to explore how hormone-behavior relationships relate to female competition and vary over social, seasonal, and evolutionary timescales.
Testing and expanding the challenge hypothesis in females: a case study
We have begun to integrate these extensions of the challenge hypothesis in a set of studies on the North American tree swallow (Tachycineta bicolor), a bird that is ideal for studying ecologically relevant female-female competition. Tree swallows need access to a cavity in order to breed, but they cannot excavate one themselves, meaning that a female without a nesting cavity will have zero reproductive success (Winkler et al., 2011). More aggressive females are more likely to obtain these limited resources, suggesting that female aggression is adaptive during pre-breeding competition for nesting sites (Rosvall, 2008). Female floaters are abundant in this system, with some estimates that one in four females do not breed, only because they do not obtain a nesting site (Stutchbury and Robertson, 1985). Floaters frequently intrude at territories, and they can evict or even kill other females, their eggs, and/or their offspring (Leffelaar and Robertson, 1985; Lombardo, 1986; Stutchbury and Robertson, 1987).
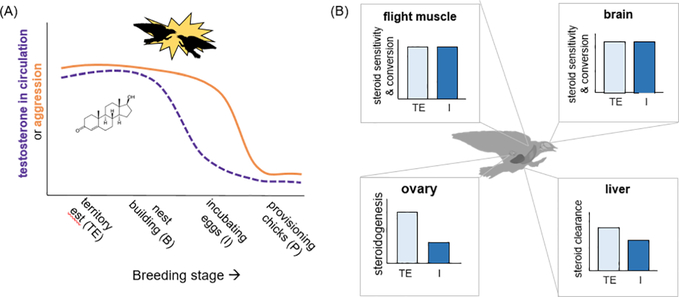
Early in the breeding season prior to egg laying, females respond to simulated territorial intrusions with robust aggression, including physical attacks, toward a female decoy placed on or near their nestbox (Bentz et al., 2019b; Rosvall, 2008). The density of nestboxes also predicts the frequency of female aggression (Bentz et al., 2013; Whittingham and Schwabl, 2002), which can escalate to displacements, acrobatic chases, and grappling in the air (pers. obs). Naturally occurring aggressive interactions may be more common during territorial establishment (George and Rosvall, 2018; Robertson et al., 1986); however, floater intrusions can persist later in the breeding season (Harris, 1979; Stutchbury and Robertson, 1987), potentially selecting for females that retain the ability to respond aggressively long after territory-establishment. Consistent with this view, female aggressiveness toward a simulated intruder remains relatively high through incubation. It is not until chicks hatch that we see a precipitous decline in female aggressiveness (Bentz et al., 2019b). Females therefore mount strong behavioral responses to social challenges, although the magnitude of these responses declines during the busy period of provisioning offspring (Figure 4a), consistent with the behavioral component of the challenge hypothesis.
Figure 4.
Mismatches between T and aggression, such as what we observed in female tree swallows (A) may be resolved by considering how T is processed elsewhere in the brain and body (B). Data not to scale. See text for details.
T plays a role in modulating aggression in this system as well. Implants with female-appropriate doses of T significantly increased aggression in incubating females (Rosvall, 2013a), consistent with H2. Females are physiologically capable of elevating T in response to a pulse of GnRH, at least during early breeding stages (George and Rosvall, 2018), suggesting they may be capable of elevating T in response to social challenges. Yet, when we challenged pre-breeding females with a simulated territorial intrusion or a more naturalistic manipulation in nestbox availability, we did not find evidence of elevated T (George et al., in prep). Therefore, evidence for bidirectional links between T and aggression in this system largely echoes the taxa-wide findings for H1 and H2, described above.
Seasonal patterns of T secretion largely follow expectations of the challenge hypothesis (Figure 4a). During early breeding stages (prior to egg laying), female tree swallows have significantly higher baseline T than they do during parental stages (George and Rosvall, 2018). Females sampled during territorial establishment (i.e. before the fertile period) also expressed high levels of ovarian 5αR mRNA, which encodes the enzyme producing the potent androgen DHT (Bentz et al., 2019a), consistent with the idea that androgen production may serve another (behavioral) purpose beyond its role in fertility. T levels dramatically decline during incubation, a period when experimental T treatment suppresses maternal care (Rosvall, 2013a). This seasonal decline in T coincides with other organismal and tissue-level changes (H3), including a roughly 2× reduction in gene expression for other ovarian steroidogenic enzymes and a roughly 0.5× decrease in steroid-metabolizing enzyme gene expression in the liver (Bentz et al., 2019a), demonstrating that patterns of T secretion co-occur with a host of other organismal and tissue-specific processes that may affect behavioral phenotypes (Figure 4b).
Notably, seasonal patterns of T secretion do not completely mirror those of female aggression (Figure 4a), despite experimental links connecting the two. During incubation, this mismatch is especially striking because baseline T levels were quite low (George and Rosvall, 2018), but average aggression levels had not significantly declined below those measured during territorial establishment or nest building (Bentz et al., 2019b). We hypothesized that tissue-level metabolism and binding of T may bridge this gap between seasonal changes in circulating T and aggression, which we address here with two previously unpublished datasets (see SI for full methods, SI Table S3). Using quantitative PCR, we measured gene expression for AR, 5αR, ERα, and AROM in n=5 females collected in each of two breeding stages (territorial establishment or incubation). First, we measured AR, 5αR, ERα, and AROM mRNA in the ventromedial telencephalon and hypothalamus, for their role in regulating aggression. Second, we quantified expression of these genes in the pectoral muscle because almost all aggressive interactions among tree swallows occur in flight, and androgen signaling in skeletal muscle has been linked with behavioral performance in other systems (Fuxjager and Schuppe, 2018). We reasoned that local steroid sensitivity and conversion may allow for continued aggression after T levels decline (i.e. during parental phases), with the prediction that incubating females would show enhanced or at least sustained sex steroid-related gene expression in both brain and muscle.
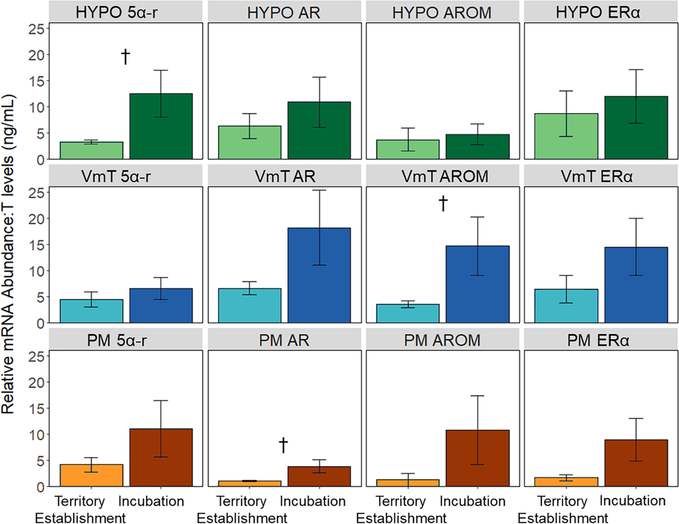
Expression of AR, 5αR, ERα, and AROM was detected in all tissues, but almost none changed in abundance as females transitioned from territory establishment to incubation (SI Figure S3). When considered in tandem with the seasonal decline in gonadal T secretion, this means that females have a higher receptor:hormone or enzyme:hormone ratio during incubation. Studies using receptor antagonists and enzyme inhibitors effectively alter this ratio and subsequent behavior (Adkins-Regan, 2005), suggesting that changes in the ratio of hormone receptor or enzyme to hormone levels themselves may be functionally relevant. Viewed this way, 5αR in the hypothalamus, AROM in the ventromedial telencephalon, and AR in the pectoral muscle all showed near-significant increases in the ratio of gene expression to T during incubation (Figure 5), potentially indicating that incubating females are more sensitive to what little T is still in circulation (H3). We are currently taking a genome-wide approach to explore how aggression-related genes in non-steroidal pathways change across the breeding season to promote aggression when T is low (Bentz et al., submitted).
Figure 5.
Ratio of mRNA abundance of androgen receptor (AR), 5α-reductase (5αR), estrogen receptor α (ERα), and aromatase (AROM) in the hypothalamus (HYPO), ventromedial telencephalon (VmT), and pectoral muscle (PM) to circulating levels of T (ng/mL) during territory establishment (n=5) and incubation (n=5). T data come from Bentz et al. 2019a; neural and muscular gene expression data from these same individuals have not been previously published. Error bars represent SE and † denotes p<0.10 without correction for multiple comparisons. For full statistical analyses, see SI Table S4.
In sum, our work on the tree swallow suggests that T mediates female aggression in some way (H2). However, differences in seasonal patterning of T and aggression, paired with the lack of support for socially-induced T elevations (H1), suggests that mechanisms beyond the scope of the original challenge hypothesis (i.e. H3, H4) may be involved in regulating female aggression at both social and seasonal timescales.
Conclusions: lessons learned from females and how to apply them towards an expanded challenge hypothesis
The challenge hypothesis has been extremely influential for several reasons, not least of which is that this framework applied the successes of early behavioral ecology and ecological determinism to understanding variation in T within and among individuals, over a lifetime, and over evolutionary time (Wingfield et al., 1990). Here we have demonstrated that many key tenets of this framework do find support in females. Females produce T outside of the fertile period, and patterns of T secretion vary within and among species. One striking pattern we revealed is that the predicted competition-induced elevation in T (H1) finds greater support when social interactions are more natural or cumulative (e.g. dominance hierarchies) as opposed to singular or acute (e.g. a simulated territorial intrusion), just as the predicted T-induced elevation in aggression (H2) also finds more support in more prolonged manipulations (implants) or naturalistic treatments (injections in intact animals). Thus, T may be more likely to affect aggression when T has already primed the animal to respond to its activational effects, and the reverse causal link is more robust when examined in contexts that may reflect the cumulative effect of many social interactions on the organism as a whole. Related to this issue, it cannot be ignored that H1 is also meeting its own challenges in males, particularly in birds in which most species largely do not elevate T in response to simulated territorial intrusions (Goymann et al., 2019). Other components of sex steroid signaling systems (H3) or other signaling systems altogether (H4) may explain variation in aggression independently of circulating T, and these alternative mechanisms may be especially salient during periods of parental care, when costs of elevated T may be high. As a consequence, females – who often bear additional parental demands beyond provisioning or defense – are particularly well suited to testing these alternative hypotheses. Our ongoing work in the tree swallow system brings together these ideas, highlighting how organismal (multi-tissue) and system-wide (AR:T ratios, metabolism, conversion) perspectives in females may extend the challenge hypothesis beyond its initial focus on T levels alone.
While the original challenge hypothesis generated a framework for asking why T levels vary within and among species, our journey through the bidirectional links between T and aggression in females highlights that today’s challenge hypothesis encompasses much more. The question is not simply whether T secretion tracks the social environment in a uniform or predictable way within and among species, but moreover, how do hormones flexibly link behavior to the social environment and how should selection shape those patterns? Freed from the expectation that T alone will provide this solution, we can more broadly explore how selection influences hormone-behavior interactions. Given the almost infinite combination of endocrine-molecular parameters that could respond to the social environment and influence aggression, it would be remarkable for T alone to hold a special place in generating social, seasonal, and evolutionary variation in aggression. Indeed, there is no gene for T and artificial selection on aggression involves changes in hundreds of genes, many of which are not repeatable across independent replications (Edwards et al., 2006; Kukekova et al., 2011). These observations, paired with mixed support for some tenets of the challenge hypothesis summarized above, highlight that the next generation of research on evolutionary endocrinology may benefit from integrating these diverse molecular and endocrine responses. Females present an opportunity to systematically identify and evaluate general principles about how animals respond to their social environment that will hopefully prompt new hypotheses in both males and females.
Supplementary Material
Highlights.
This review applies the challenge hypothesis (CH) to females
Links between T and aggression are stronger in more persistent manipulations
Other steroidal and non-steroidal mechanisms may promote aggression when T is low
Novel data from the field shows how steroid sensitivity may influence T-behavior links
Extended CH must include molecular perspectives and tissue level effects of T
Acknowledgements
Many thanks to other members of Rosvall lab, especially SE Lipshutz, SE Wolf, MJ Woodruff, and EK Dossey. The authors are supported by the National Science Foundation (IOS-1656109 to KAR; Graduate Research Fellowship to EMG), Indiana Academy of Sciences (EMG), IU Research and Teaching Preserve (EMG), and National Institutes of Health T32HD049336 (ABB and KAR). KAR also acknowledges the generous support of her dissertation improvement grant (NSF DDIG IBN-0710118), which started this journey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Adkins-Regan E, 2005. Hormones and animal social behavior. Princeton University Press, Princeton, NJ. [Google Scholar]
- Albert DJ, Jonik RH, Walsh ML, 1990. Hormone-dependent aggression in female rats: testosterone implants attenuate the decline in aggression following ovariectomy. Physiol Behav. 47, 659–64. [DOI] [PubMed] [Google Scholar]
- Archawaranon M, Wiley RH, 1988. Control of aggression and dominance in white-throated sparrows by testosterone and its metabolites. Horm Behav. 22, 497–517. [DOI] [PubMed] [Google Scholar]
- Archer J, 2006. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience and Biobehavioral Reviews. 30, 319–345. [DOI] [PubMed] [Google Scholar]
- Aubin-Horth N, Desjardins JK, Martei YM, Balshine S, Hofmann HA, 2007. Masculinized dominant females in a cooperatively breeding species. Mol Ecol. 16, 1349–1358. [DOI] [PubMed] [Google Scholar]
- Auger CJ, Coss D, Auger AP, Forbes-Lorman RM, 2011. Epigenetic control of vasopressin expression is maintained by steroid hormones in the adult male rat brain. PNAS. 108, 4242–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley MS, Goldman BD, 1978. Studies on opponent status and steroid mediation of aggression in female mice. Behav Biol. 23, 118–23. [DOI] [PubMed] [Google Scholar]
- Batty KA, Herbert J, Keverne E, Vellucci SV, 1986. Differences in Blood Levels of Androgens in Female Talapoin Monkeys Related to Their Social Status. Neuroendocrinology. 44, 347–354. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Phillips-Conroy JE, Whitten PL, 2005. Female testosterone, dominance rank, and aggression in an Ethiopian population of hybrid baboons. Am J Primatology. 67, 101–119. [DOI] [PubMed] [Google Scholar]
- Bentz AB, Becker DJ, Navara KJ, 2016. Evolutionary implications of interspecific variation in a maternal effect: a meta-analysis of yolk testosterone response to competition. Royal Society Open Science. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz AB, Dossey EK, Rosvall KA, 2019a. Tissue-specific gene regulation corresponds with seasonal plasticity in female testosterone. Gen Comp Endocrinol. 270, 26–34. [DOI] [PubMed] [Google Scholar]
- Bentz AB, Navara KJ, Siefferman L, 2013. Phenotypic plasticity in response to breeding density in tree swallows: An adaptive maternal effect? Horm Behav. 64, 729–736. [DOI] [PubMed] [Google Scholar]
- Bentz AB, Philippi KJ, Rosvall KA, 2019b. Evaluating seasonal patterns of female aggression: case study in a cavity-nesting bird with intense female-female competition. Ethology. 125, 555–564. [Google Scholar]
- Bentz AB, Rusch DB, Buechlein A, Rosvall KA, submitted The neurogenomic transition from territory establishment to parenting in a territorial female songbird. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain KE, Ketterson ED, 2012. Competitive females are successful females; phenotype, mechanism, and selection in a common songbird. Behav Ecol Sociobiol. 66, 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain KE, Ketterson ED, 2013. Individual variation in testosterone and parental care in a female songbird; The dark-eyed junco (Junco hyemalis). Horm Behav. 64, 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoine V, Gwinner E, 2005. The hormonal response of female European stonechats to a territorial intrusion: The role of the male partner. Horm Behav. 47, 563–568. [DOI] [PubMed] [Google Scholar]
- Cantarero A, Laaksonen T, Jarvisto PE, Gil D, Lopez-Arrabe J, Redondo AJ, Moreno J, 2015. Nest Defence Behaviour and Testosterone Levels in Female Pied Flycatchers. Ethology. 121, 946–957. [Google Scholar]
- Chapman T, Takahisa M, Smith Hazel K, Partridge L, 1998. Interactions of mating, egg production and death rates in females of the Mediterranean fruitfly, Ceratitis capitata. Proc Biol Sci. 265, 1879–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier TD, Newman AEM, Heimovics SA, Po KWL, Saldanha CJ, Soma KK, 2011. Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol. 23, 742–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke FM, Faulkes CG, 1997. Dominance and queen succession in captive colonies of the eusocial naked mole–rat, Heterocephalus glaber. Proc Biol Sci. 264, 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke IJ, Scaramuzzi RJ, Short RV, 1976. Effects of testosterone implants in pregnant ewes on their female offspring. J Embryol Exp Morphol. 36, 87–99. [PubMed] [Google Scholar]
- Clotfelter ED, O’Neal DM, Gaudioso JM, Casto JM, Parker-Renga IM, Snajdr EA, Duffy DL, Nolan V, Ketterson ED, 2004. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm Behav. 46, 171–178. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T, 2009. Sexual selection in females. Anim Behav. 77, 3–11. [Google Scholar]
- Compaan JC, van Wattum G, de Ruiter AJ, van Oortmerssen GA, Koolhaas JM, Bohus B, 1993. Genetic differences in female house mice in aggressive response to sex steroid hormone treatment. Physiol Behav. 54, 899–902. [DOI] [PubMed] [Google Scholar]
- Crews D, Coomber P, Baldwin R, Azad N, Gonzalez-Lima F, 1996. Brain organization in a reptile lacking sex chromosomes: effects of gonadectomy and exogenous testosterone. Horm Behav. 30, 474–86. [DOI] [PubMed] [Google Scholar]
- Davies CS, Smyth KN, Greene LK, Walsh DA, Mitchell J, Clutton-Brock T, Drea CM, 2016. Exceptional endocrine profiles characterise the meerkat: sex, status, and reproductive patterns. Sci Rep. 6, 35492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ES, Marler CA, 2003. The progesterone challenge: steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus. Horm Behav. 44, 185–198. [DOI] [PubMed] [Google Scholar]
- DeBold JF, Miczek KA, 1981. Sexual dimorphism in the hormonal control of aggressive behavior of rats. Pharmacol Biochem Behav. 14 Suppl 1, 89–93. [DOI] [PubMed] [Google Scholar]
- Dent JR, Fletcher DK, McGuigan MR, 2012. Evidence for a Non-Genomic Action of Testosterone in Skeletal Muscle Which may Improve Athletic Performance: Implications for the Female Athlete. J Sports Sci Med. 11, 363–70. [PMC free article] [PubMed] [Google Scholar]
- Desjardins JK, Stiver KA, Fitzpatrick JL, Milligan N, Van Der Kraak GJ, Balshine S, 2008. Sex and status in a cooperative breeding fish: behavior and androgens. Behav Ecol Sociobiol. 62, 785–794. [Google Scholar]
- Deviche P, Breuner C, Orchinik M, 2001. Testosterone, corticosterone, and photoperiod interact to regulate plasma levels of binding globulin and free steroid hormone in Darkeyed Juncos, Junco hyemalis. Gen Comp Endocrinol. 122, 67–77. [DOI] [PubMed] [Google Scholar]
- DeVries MS, Winters CP, Jawor JM, 2015. Testosterone might not be necessary to support female aggression in incubating northern cardinals. Anim Behav. 107, 139–146. [Google Scholar]
- Drummond AE, 2006. The role of steroids in follicular growth. Reproductive Biology and Endocrinology. 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Wilckens N, Trainor BC, Behavioral neuroendocrinology of female aggression In: Nelson RJ, (Ed.), Oxford Research Encyclopedia of Neuroscience. Oxford University Press, 2017. [Google Scholar]
- Edwards AC, Rollmann SM, Morgan TJ, Mackay TFC, 2006. Quantitative Genomics of Aggressive Behavior in Drosophila melanogaster. PLOS Genetics. 2, e154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eens M, Pinxten R, 2000. Sex-role reversal in vertebrates: behavioural and endocrinological accounts. Behav Process. 51, 135–147. [DOI] [PubMed] [Google Scholar]
- Elekonich MM, Wingfield JC, 2000. Seasonality and hormonal control of territorial aggression in female song sparrows (Passeriformes: Emberizidae: Melospiza melodia). Ethology. 106, 493–510. [Google Scholar]
- Filby AL, Paull GC, Hickmore TFA, Tyler CR, 2010. Unravelling the neurophysiological basis of aggression in a fish model. BMC Genomics. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floody OR, Hormones and aggression in female mammals In: Svare BB, (Ed.), Hormones and aggressive behavior. Plenum Press, New York, NY, 1983, pp. 39–89. [Google Scholar]
- Flores DL, Crews D, 1995. Effect of hormonal manipulation on sociosexual behavior in adult female leopard geckos (Eublepharis macularius), a species with temperature-dependent sex determination. Horm Behav. 29, 458–73. [DOI] [PubMed] [Google Scholar]
- French JA, Mustoe AC, Cavanaugh J, Birnie AK, 2013. The influence of androgenic steroid hormones on female aggression in ‘atypical’ mammals. Phil Trans Royal Soc B. 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusani L, 2008. Endocrinology in field studies: Problems and solutions for the experimental design. Gen Comp Endocrinol. 157, 249–253. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA, 2010. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. PNAS. 107, 12393–12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxjager MJ, Schuppe ER, 2018. Androgenic signaling systems and their role in behavioral evolution. The Journal of Steroid Biochemistry and Molecular Biology. 184, 47–56. [DOI] [PubMed] [Google Scholar]
- Garamszegi LZ, 2014. Female peak testosterone levels in birds tell an evolutionary story: a comment on Goymann and Wingfield. Behav Ecol. 25, 700–701. [Google Scholar]
- Gavasa S, Silva AC, Gonzalez E, Molina J, Stoddard PK, 2012. Social competition masculinizes the communication signals of female electric fish. Behav Ecol Sociobiol. 66, 1057–1066. [Google Scholar]
- George EM, Bentz AB, Wolf SE, Rosvall KA, in prep Testing hormonal responses to real and simulated social challenges in a competitive female bird. [DOI] [PMC free article] [PubMed]
- George EM, Rosvall KA, 2018. Testosterone production and social environment vary with breeding stage in a competitive female songbird. Horm Behav. 103, 28–35. [DOI] [PubMed] [Google Scholar]
- Gerlach NM, Ketterson ED, 2013. Experimental elevation of testosterone lowers fitness in female dark-eyed juncos. Horm Behav. 63, 782–790. [DOI] [PubMed] [Google Scholar]
- Gill SA, Alfson ED, Hau M, 2007. Context matters: female aggression and testosterone in a year-round territorial neotropical songbird (Thryothorus leucotis). Proc Biol Sci. 274, 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA, 2009. Testosterone release and social context: When it occurs and why. Front Neuroendocrinol. 30, 460–469. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Wilson LC, Schrock SE, 2012. To flock or fight: Neurochemical signatures of divergent life histories in sparrows. PNAS. 109 Suppl 1, 10685–10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Moore IT, Oliveira RF, 2019. Challenge Hypothesis 2.0: A Fresh Look at an Established Idea. Bioscience. 69, 432–442. [Google Scholar]
- Goymann W, Wingfield JC, 2014. Male-to-female testosterone ratios, dimorphism, and life history—what does it really tell us? Behav Ecol. 25, 685–699. [Google Scholar]
- Goymann W, Wittenzellner A, Schwabl I, Makomba M, 2008. Progesterone modulates aggression in sex-role reversed female African black coucals. Proc Biol Sci. 275, 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene WA, Mogil L, Foote RH, 1978. Behavioral characteristics of freemartins administered estradiol, estrone, testosterone, and dihydrotestosterone. Horm Behav. 10, 71–84. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, de Jong B, Müller M, 2014. In search for a theory of testosterone in female birds: a comment on Goymann and Wingfield. Behav Ecol. 25, 702–704. [Google Scholar]
- Hare RM, Simmons LW, 2019. Sexual selection and its evolutionary consequences in female animals. Biological Reviews. 94, 929–956. [DOI] [PubMed] [Google Scholar]
- Harris RN, 1979. Aggression, superterritories, and reproductive success in tree swallows. Can J Zool. 57, 2072–2078. [Google Scholar]
- Hau M, 2007. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays. 29, 133–144. [DOI] [PubMed] [Google Scholar]
- Hau M, Stoddard ST, Soma KK, 2004. Territorial aggression and hormones during the non-breeding season in a tropical bird. Horm Behav. 45, 40–49. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Oliveira RF, 2006. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim Behav. 71, 265–277. [Google Scholar]
- Hirschenhauser K, Winkler H, Oliveira RF, 2003. Comparative analysis of male androgen responsiveness to social environment in birds: the effects of mating system and paternal incubation. Horm Behav. 43, 508–519. [DOI] [PubMed] [Google Scholar]
- Horton BM, Hudson WH, Ortlund EA, Shirk S, Thomas JW, Young ER, Zinzow-Kramer WM, Maney DL, 2014. Estrogen receptor α polymorphism in a species with alternative behavioral phenotypes. PNAS. 111, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YY, Earley RL, Wolf LL, 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biological Reviews. 81, 33–74. [DOI] [PubMed] [Google Scholar]
- Hu Y-C, Wang P-H, Yeh S, Wang R-S, Xie C, Xu Q, Zhou X, Chao H-T, Tsai MY, Chang C, 2004. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. PNAS. 101, 11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter I, Hay CW, Esswein B, Watt K, McEwan IJ, 2018. Tissue control of androgen action: The ups and downs of androgen receptor expression. Mol Cell Endocrinol. 465, 27–35. [DOI] [PubMed] [Google Scholar]
- Jawor JM, Young R, Ketterson ED, 2006. Females competing to reproduce: Dominance matters but testosterone may not. Horm Behav. 49, 362–368. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Van Tienhoven A, 1980. Plasma Concentrations of Six Steroids and LH During the Ovulatory Cycle of the Hen, Gallus domesticus1. Biol. Reprod. 23, 386–393. [DOI] [PubMed] [Google Scholar]
- Kabelik D, Weiss SL, Moore MC, 2008. Arginine Vasotocin (AVT) Immunoreactivity Relates to Testosterone but Not Territorial Aggression in the Tree Lizard, Urosaurus ornatus. Brain Behav Evol. 72, 283–294. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Vitousek MN, 2017. Dynamic modulation of sociality and aggression: an examination of plasticity within endocrine and neuroendocrine systems. Phil Trans Royal Soc B. 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson ED, 2014. Male and female testosterone—is one sex made in the image of the other? A comment on Goymann and Wingfield. Behav Ecol. 25, 702–702. [Google Scholar]
- Ketterson ED, Atwell JW, McGlothlin JW, 2009. Phenotypic integration and independence: Hormones, performance, and response to environmental change. Integr Comp Biol. 49, 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, 1992. Hormones and life histories - an integrative approach. Am Nat. 140, S33–S62. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Sandell M, 2005. Testosterone in females: Mediator of adaptive traits, constraint on sexual dimorphism, or both? Am Nat. 166, S85–S98. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Aida K, Hanyu I, 1989. Involvement of steroid hormones in the preovulatory gonadotropin surge in female goldfish. Fish Physiology and Biochemistry. 7, 141–146. [DOI] [PubMed] [Google Scholar]
- Kukekova AV, Johnson JL, Teiling C, Li L, Oskina IN, Kharlamova AV, Gulevich RG, Padte R, Dubreuil MM, Vladimirova AV, Shepeleva DV, Shikhevich SG, Sun Q, Ponnala L, Temnykh SV, Trut LN, Acland GM, 2011. Sequence comparison of prefrontal cortical brain transcriptome from a tame and an aggressive silver fox (Vulpes vulpes). BMC Genomics. 12, 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmore NE, Cockrem JF, Candy EJ, 2002. Competition for male reproductive investment elevates testosterone levels in female dunnocks, <em>Prunella modularis</em>. Proc Biol Sci. 269, 2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffelaar D, Robertson RJ, 1985. Nest usurpation and female competition for breeding opportunities by tree swallows. Wilson Bull. 97, 221–224. [Google Scholar]
- Lipshutz SE, George EM, Bentz AB, Rosvall KA, accepted Evaluating testosterone as a phenotypic integrator: from tissues to individuals to species Mol Cell Endocrinol. [DOI] [PMC free article] [PubMed]
- Lombardo MP, 1986. A possible case of adult intraspecific killing in the tree swallow. Condor. 88, 112–112. [Google Scholar]
- Maney DL, Lange HS, Raees MQ, Reid AE, Sanford SE, 2009. Behavioral phenotypes persist after gonadal steroid manipulation in white-throated sparrows. Horm Behav. 55, 113–120. [DOI] [PubMed] [Google Scholar]
- Mank JE, 2007. The evolution of sexually selected traits and antagonistic androgen expression in actinopterygiian fishes. Am Nat. 169, 142–149. [DOI] [PubMed] [Google Scholar]
- McGlothlin JW, Neudorf DLH, Casto JM, Nolan V, Ketterson ED, 2004. Elevated testosterone reduces choosiness in female dark-eyed juncos (Junco hyemalis): evidence for a hormonal constraint on sexual selection? Proc Biol Sci. 271, 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt JR, Davis MT, Jalabert C, Libecap TJ, Williams DR, Soma KK, Maney DL, 2018. Rapid effects of estradiol on aggression depend on genotype in a species with an estrogen receptor polymorphism. Horm Behav. 98, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Garamszegi LZ, Gil D, Hurtrez-Bousses S, Eens M, 2005. Correlated evolution of male and female testosterone profiles in birds and its consequences. Behav Ecol Sociobiol. 58, 534–544. [Google Scholar]
- Moreno J, Gil D, Cantarero A, López-Arrabé J, 2016. Female aggressiveness towards female decoys decreases with mate T level in the pied flycatcher. Acta Ethologica. 19, 9–14. [Google Scholar]
- Mousseau TA, Fox CW, 1998. The adaptive significance of maternal effects. Trends Ecol Evol. 13, 403–407. [DOI] [PubMed] [Google Scholar]
- Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA, 2015. The regulation of steroid action by sulfation and desulfation. Endocrine reviews. 36, 526–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navara KJ, Siefferman LM, Hill GE, Mendonca MT, 2006. Yolk androgens vary inversely to maternal androgens in eastern bluebirds: An experimental study. Funct Ecol. 20, 449–456. [Google Scholar]
- Nelson RJ, Trainor BC, 2007. Neural mechanisms of aggression. Nat Rev Neurosci. 8, 536–546. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Stiver KA, Hofmann HA, Alonzo SH, in press Experimentally induced variation in neuroendocrine processes affects male reproductive behaviour, sperm characteristics and social interactions. Mol Ecol. 0. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA, 2011. Genes, hormones, and circuits: An integrative approach to study the evolution of social behavior. Front Neuroendocrinol. 32, 320–335. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Cameiro LA, Canario AVM, 2005. Mirror elicited aggression fails to trigger an endocrine response to a social challenge. Horm Behav. 48, 118–119. [Google Scholar]
- Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AVM, 2002. Social modulation of androgen levels in male teleost fish. Comp Biochem Physiol B. 132, 203–215. [DOI] [PubMed] [Google Scholar]
- Peterson MP, Rosvall KA, Choi J-H, Ziegenfus C, Tang H, Colbourne JK, Ketterson ED, 2013. Testosterone affects neural gene expression differently in male and female juncos: a role for hormones in mediating sexual dimorphism and conflict. PLoS ONE. 8, e61784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MP, Rosvall KA, Taylor CA, Lopez JA, Choi JH, Ziegenfus C, Tang H, Colbourne JK, Ketterson ED, 2014. Potential for sexual conflict assessed via testosterone-mediated transcriptional changes in liver and muscle of a songbird. J Exp Biol. 217, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Carboni G, Pinna G, 2006. Neurosteroids regulate mouse aggression induced by anabolic androgenic steroids. Neuroreport. 17, 1537–41. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A, 2005. Changes in brain testosterone and allopregnanolone biosynthesis elicit aggressive behavior. PNAS. 102, 2135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo V, López-Rull I, Gil D, Veiga JP, 2010. Experimental Addition of Green Plants to the Nest Increases Testosterone Levels in Female Spotless Starlings. Ethology. 116, 129–137. [Google Scholar]
- Pradhan DS, Newman AEM, Wacker DW, Wingfield JC, Schlinger BA, Soma KK, 2010. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav. 57, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel PL, Sharp PJ, Gutierrez CG, 2006. Testosterone antagonist (flutamide) blocks ovulation and preovulatory surges of progesterone, luteinizing hormone and oestradiol in laying hens. Reproduction. 131, 1109–1114. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA, 2008. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 11, 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon NM, Amez AC, Proffitt MR, Bauserman ER, Demas GE, 2017. Aggressive behaviours track transitions in seasonal phenotypes of female Siberian hamsters. Funct Ecol. 31, 1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon NM, Demas GE, 2016. Bi-directional actions of dehydroepiandrosterone and aggression in female Siberian hamsters. J Exp Zool Part A. 325, 116–121. [DOI] [PubMed] [Google Scholar]
- Rendon NM, Rudolph LM, Sengelaub DR, Demas GE, 2015. The agonistic adrenal: melatonin elicits female aggression via regulation of adrenal androgens. Proc Biol Sci. 282, 20152080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SCP, Aubin-Horth N, Hofmann HA, 2008. Fish and chips: functional genomics of social plasticity in an African cichlid fish. J Exp Biol. 211, 3041–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SCP, Fraser EJ, Aubin-Horth N, Trainor BC, Hofmann HA, 2012. Females of an African cichlid fish display male-typical social dominance behavior and elevated androgens in the absence of males. Horm Behav. 61, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RJ, Gibbs HL, Stutchbury BJ, 1986. Spitefulness, altruism, and the cost of aggression: evidence against superterritoriality in tree swallows. Condor. 88, 104–105. [Google Scholar]
- Ross CN, French JA, 2011. Female marmosets’ behavioral and hormonal responses to unfamiliar intruders. Am J Primatol. 73, 1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA, 2008. Sexual selection on aggressiveness in females: evidence from an experimental test with tree swallows. Anim Behav. 75, 1603–1610. [Google Scholar]
- Rosvall KA, 2011. Intrasexual competition in females: evidence for sexual selection? Behav Ecol. 22, 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA, 2013a. Life history trade-offs and behavioral sensitivity to testosterone: an experimental test when female aggression and maternal care co-occur. PLoS One. 8, e54120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA, 2013b. Proximate perspectives on the evolution of female aggression: Good for the gander, good for the goose? Phil Trans Royal Soc B. 368, 20130083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall KA, Bergeon Burns CM, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, Ketterson ED, 2012. Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proc Biol Sci. 279, 3547–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein DR, Wikelski M, 2005. Steroid hormones and aggression in female Galapagos marine iguanas. Horm Behav. 48, 329–341. [DOI] [PubMed] [Google Scholar]
- Rush ME, Blake CA, 1982. Serum Testosterone Concentrations during the 4-Day Estrous Cycle in Normal and Adrenalectomized Rats. Proceedings of the Society for Experimental Biology and Medicine. 169, 216–221. [DOI] [PubMed] [Google Scholar]
- Sandnabba NK, Lagerspetz KMJ, Jensen E, 1994. Effects of Testosterone Exposure and Fighting Experience on the Aggressive Behavior of Female and Male Mice Selectively Bred for Intermale Aggression. Horm Behav. 28, 219–231. [DOI] [PubMed] [Google Scholar]
- Schumer M, Krishnakant K, Renn SCP, 2011. Comparative gene expression profiles for highly similar aggressive phenotypes in male and female cichlid fishes (Julidochromis). J Exp Biol. 214, 3269–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H, Flinks H, Gwinner E, 2005. Testosterone, reproductive stage, and territorial behavior of male and female European stonechats Saxicola torquata. Horm Behav. 47, 503–512. [DOI] [PubMed] [Google Scholar]
- Scriba M, Goymann W, 2008. The decoy matters! Hormonal and behavioural differences in the reaction of territorial European robins towards stuffed and live decoys. Gen Comp Endocrinol. 155, 511–516. [DOI] [PubMed] [Google Scholar]
- Simon NG, Whalen RE, Tate MP, 1985. Induction of male-typical aggression by androgens but not by estrogens in adult female mice. Horm Behav. 19, 204–12. [DOI] [PubMed] [Google Scholar]
- Slagsvold T, Lifjeld JT, 1994. Polygyny in birds: the role of competition between females for male parental care. Am Nat. 143, 59–94. [Google Scholar]
- Soma KK, Rendon NM, Boonstra R, Albers HE, Demas GE, 2014. DHEA effects on brain and behavior: Insights from comparative studies of aggression. J Steroid Biochem Mol Biol. [DOI] [PubMed] [Google Scholar]
- Soma KK, Scotti MAL, Newman AEM, Charlier TD, Demas GE, 2008. Novel mechanisms for neuroendocrine regulation of aggression. Front Neuroendocrinol. 29, 476–489. [DOI] [PubMed] [Google Scholar]
- Staub NL, DeBeer M, 1997. The role of androgens in female vertebrates. Gen Comp Endocrinol. 108, 1–24. [DOI] [PubMed] [Google Scholar]
- Stockley P, Bro-Jørgensen J, 2011. Female competition and its evolutionary consequences in mammals. Biol Rev. 86, 341–366. [DOI] [PubMed] [Google Scholar]
- Stutchbury BJ, Robertson RJ, 1985. Floating Populations of Female Tree Swallows. Auk. 102, 651–654. [Google Scholar]
- Stutchbury BJ, Robertson RJ, 1987. Behavioral tactics of subadult female floaters in the tree swallow. Behav Ecol Sociobiol. 20, 413–419. [Google Scholar]
- Svare BB, 2013. Hormones and aggressive behavior. Springer Science & Business Media. [Google Scholar]
- Swett MB, Breuner CW, 2008. Interaction of testosterone, corticosterone and corticosterone binding globulin in the white-throated sparrow (Zonotrichia albicollis). Comp Biochem Physiol Part A Mol Integr Physiol. 151, 226–231. [DOI] [PubMed] [Google Scholar]
- Tobias JA, Montgomerie R, Lyon BE, 2012. The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Phil Trans Royal Soc B. 367, 2274–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL, Parental investment and sexual selection In: Cambell B, (Ed.), Sexual Selection and the Descent of Man, 1871–1971. Heinemann, London, 1972, pp. 136–179. [Google Scholar]
- van Nas A, GuhaThakurta D, Wang SS, Yehya N, Horvath S, Zhang B, Ingram-Drake L, Chaudhuri G, Schadt EE, Drake TA, Arnold AP, Lusis AJ, 2009. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology. 150, 1235–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek MN, Johnson MA, Donald JW, Francis CD, Fuxjager MJ, Goymann W, Hau M, Husak JF, Kircher BK, Knapp R, Martin LB, Miller ET, Schoenle LA, Uehling JJ, Williams TD, 2018. HormoneBase, a population-level database of steroid hormone levels across vertebrates. Scientific Data. 5, 180097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt C, 2016. Neuroendocrine correlates of sex-role reversal in barred buttonquails. Proc Biol Sci. 283, 20161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt C, Goymann W, 2007. Sex-role reversal is reflected in the brain of African black coucals (Centropus grillii). Developmental Neurobiology. 67, 1560–1573. [DOI] [PubMed] [Google Scholar]
- Whittingham LA, Schwabl H, 2002. Maternal testosterone in tree swallow eggs varies with female aggression. Anim Behav. 63, 63–67. [Google Scholar]
- Wingfield J, Jacobs J, Tramontin A, Perfito N, Meddle S, Maney D, Soma K, 2000. Toward an ecological basis of hormone–behavior interactions in reproduction of birds. Reproduction in context. 85–128. [Google Scholar]
- Wingfield JC, 1984. Environmental and endocrine control of reproduction in the song sparrow, Melospiza melodia. 1. Temporal organization of the breeding cycle. Gen Comp Endocrinol. 56, 406–416. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, 1985. Short-term changes in plasma levels of hormones during establishment and defense of a breeding territory in male song sparrows, Melospiza melodia. Horm Behav. 19, 174–187. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hormone-behavior interactions and mating systems in male and female birds In: Short RV, Balaban E, (Eds.), The differences betweeen the sexes. Cambridge University Press, Cambridge, UK, 1994, pp. 303–330. [Google Scholar]
- Wingfield JC, 2012. The challenge hypothesis: behavioral ecology to neurogenomics. J Orn. 153, 85–96. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF, 1990. The Challenge Hypothesis - theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 136, 829–846. [Google Scholar]
- Wingfield JC, Lynn SE, Soma KK, 2001. Avoiding the ‘costs’ of testosterone: Ecological bases of hormone-behavior interactions. Brain Behavior and Evolution. 57, 239–251. [DOI] [PubMed] [Google Scholar]
- Winkler DW, Hallinger KK, Ardia DR, Robertson RJ, Stutchbury BJ, Cohen RR, Tree swallow (Tachycineta bicolor), version 2.0. The Birds of North America. Cornell Lab of Ornithology, Ithaca, NY USA, 2011. [Google Scholar]
- Winkler SM, Wade J, 1998. Aromatase activity and regulation of sexual behaviors in the green anole lizard. Physiol Behav. 64, 723–31. [DOI] [PubMed] [Google Scholar]
- Woodley SK, Moore MC, 1999. Female territorial aggression and steroid hormones in mountain spiny lizards. Anim Behav. 57, 1083–1089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.