Abstract
The neurotransmitter dopamine is a neuromodulator in the positive and negative regulation of brain circuits. Dopamine insufficiency or overload has been implicated in aberrant activities of neural circuits that play key roles in the pathogenesis of neurological and psychiatric diseases. Dopaminergic neurons are vulnerable to environmental insults. The neurotoxin methylmercury (MeHg) produces dopaminergic neuron damage in rodent as well as in Caenorhabditis elegans (C. elegans) models. Previous studies have demonstrated the utility of C. elegans as an alternative and complementary experimental model in dissecting out mechanism of MeHg-induced dopaminergic neurodegeneration. However, a sensitive pathological change that marks early events in neurodegeneration induced by environmental level of MeHg, is still lacking. By establishing a chronic exposure C. elegans model, for the first time, we have shown the propensity of MeHg (5 μM, 10 days) to induce bright puncta of dat-1::mCherry aggreagtes in the dendrites of cephalic (2 CEPs) dopaminergic neurons in a dose- and time-dependent manner, while these changes were not found in other dopaminergic neurons: anterior deirids (2 ADEs) and posterior deirids (2 PDEs), cholinergic neurons (2 AlYs) or glutamatergic neurons (2 PVDs). The bright puncta appear as an aggregation of mCherry proteins accumulating in dendrites. Further staining shows that the puncta were not inclusions in lysosome, or amyloid protein aggregates. In addition, features of the puncta including enlarged sphere shape (0.5 – 2 μm diameters), bright and accompanying with the shrinkage of the dendrite suggest that the puncta are likely composed of homologous mCherry molecules packaged at the dendritic site for exportation. Moreover, in the glutathione S-transferase 4 (gst-4) transcriptional reporter strain and RT-PCR assay, the expression levels of gst-4 and tubulins (tba-1 and tba-2) genes were not significantly modified under this chronic exposure paradigm, but gst-4 did show significant changes in an one day exposure paradigm. Collectively, these results suggest that CEP dopaminergic neurons are a sensitive target of MeHg, and the current exposure paradigm could be used as a model to investigate mechanism of dopaminergic neurotoxicity.
Keywords: methylmercury, C. elegans, dopamine, toxicity, puncta formation
Introduction
Mercury (Hg) is an environmental pollutant occurring in different chemical forms: elemental Hg (Hg°), inorganic Hg compounds (Hg2+), and organic Hg compounds, such as methylmercury (MeHg) (Clarkson, 2002). The main source of MeHg exposure is through seafood consumption. Brain is the most susceptible organ affected by MeHg. Neuronal death and disruption of neurotransmission are the pathogenic basis for MeHg-induced behavioral abnormity (Bjorklund et al., 2017).
The neurotransmitter dopamine is involved in fundamental regulation of brain activities. Since its discovery in 1957, knowledge on the functional diversity of dopamine in the brain has progressed substantially (Yeragani et al., 2010). In mammalian brain, the dopaminergic neurons reside primarily in nine distinct cell groups (A8-A16) from the mesencephalon to the olfactory bulb (Bjorklund and Dunnett, 2007). The dopaminergic neurons in these areas project to discrete brain regions to regulate voluntary movement, reward behaviors, and endocrine functions (Gerfen and Surmeier, 2011). For example, dopaminergic neurons in the substantia nigra (SN) project to the dorsal striatum to regulate motor activities through direct (dis-inhibitory) and indirect pathways (dis-dis-inhibitory) (Przedborski et al., 1995). The delicate and precise control of motor functions by dopamine transmission is fulfilled by the signaling of dopamine receptors (Borroto-Escuela et al., 2018). At least five dopamine receptors (D1-D5) have been discovered, and these receptors exhibit heterogeneous pharmacological properties (Beaulieu and Gainetdinov, 2011).
Dopamine insufficiency or overload is implicated in aberrant activities of neural circuits that play key roles in the pathogenesis of neurological and psychiatric diseases, such as Parkinson’s disease (PD) and depression (Borroto-Escuela et al., 2018). It has been shown that the number of dopaminergic neurons declines with age (Bogerts et al., 1983), and loss of the dopaminergic neurons in SN is a hallmark of the PD, one of the most prevalent neurological diseases in elderly people (Bogerts et al., 1983). Treatment of PD patients with the dopamine receptor agonist, L-dihydroxyphenylalanine (L-dopa), is effective in the early stages of the disease (Fahn, 2015), while in the late stage of the disease, the loss of dopaminergic neurons in SN cannot be prevented with pharmacological agents (Tarakad and Jankovic, 2017).
Dopaminergic neurons are vulnerable to numerous environmental insults (Chaudhuri et al., 2007). For example, the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) selectively targets the dopaminergic neurons in SN, and is commonly used as a mitochondrial neurotoxic model of PD (Meredith and Rademacher, 2011). A genomic and proteomic study has found that MeHg has overlapping targets with MPTP (Shao et al., 2015). Notably, gene products altered by both MeHg and MPTP are involved in dopamine signaling pathway (Shao et al., 2015). Importantly, MeHg is known to affect numerous endpoints related to brain dopamine homeostasis, inducing a concentration-dependent increase in spontaneous dopamine release in the caudate-putamen (O’Kusky et al., 1988) and in mouse striatal slices (Kalisch and Racz, 1996). In agreement to the above, intrastriatal administration of MeHg in rats led to a concentration-dependent increase in the dopamine release (Faro et al., 2000). Moreover, MeHg has been shown to cause significant changes in the morphology of cultured ventral mesencephalic neurons, such as cell shrinkage and decreased number of neurites (Gotz et al., 2002). However, the mechanisms associated with the dopaminergic neurodegeneration upon MeHg exposure have yet to be delineated.
Caenorhabditis elegans (C. elegans) has emerged as an important complementary and alternative experimental model to study the interaction between environmental and genetic factors leading to neurotoxicity. C. elegans hermaphrodite has only eight dopaminergic neurons, but they possess enzymes necessary for dopamine biosynthesis, dopamine receptors for signaling transduction, and dopamine neural circuits that regulate locomotion and egg laying (Schafer and Kenyon, 1995). MeHg-induced morphological changes in dopaminergic neurons were also investigated in C. elegans (Caito and Aschner, 2016; Vanduyn et al., 2010). MeHg led to neurodegenerative phenotypes in the dendrites of dopaminergic neurons in worms deficient in SKN-1, the homolog of mammalian nuclear factor erythroid 2-related factor2 (Nrf-2) (Vanduyn et al., 2010). Furthermore, in wild-type worms, MeHg led to puncta formation, loss of dendrites and shrinkage of soma in dopaminergic neurons (Caito and Aschner, 2016). These studies establish the utility of C. elegans in dissecting out the mechanism of dopaminergic neurodegeneration. However, a sensitive pathologic change that marks early events in neurodegeneration resulting from toxicity of environmental level of MeHg is still lacking (Caito and Aschner, 2016; Vanduyn et al., 2010). As the toxicity of MeHg in the brain exhibits different features upon acute and chronic exposure paradigms (Ekino et al., 2007), we hypothesized that a neurodegenerative phenotype in dopaminergic neurons could be observed by moving to a chronic MeHg exposure model in liquid culture. Here we tested a chronic treatment of MeHg (5 μM, 10 days) in liquid culture, and examined neuronal morphology with a dat-1::mCherry (dat-1, dopamine active transporter 1) reporter line. This led to the discovery of aberrant puncta formation in the dendrites of cephalic (CEP) dopaminergic neurons in C. elegans following MeHg exposure. This manuscript reports this novel observation, and a characterization of these puncta and associated neurotoxicological phenotypes.
Materials and methods
C. elegans strains and maintenance
The following strain were used in this study: N2 wild type, CL2166 (dvIs19 [(pAF15)gst-4p::GFP::NLS] III), OH7193 (otIs181 [dat-1::mCherry + ttx-3::mCherry] III; him-8(e1489) IV, BZ555 (egIs1 [dat-1p::GFP] ), DA2123 (adIs2122 [lgg-1p::GFP::lgg-1 + rol-6(su1006)]), and VP596 (dvIs19 III; vsIs33 V). All strains were provided by the Caenorhabditis Genetics Center (CGC). Worms were grown on Nematode Growth Medium (NGM) agar plates carrying a lawn of E. coli OP50, and maintained at 20°C incubator (Brenner, 1974). Newly cultured worms were checked each day. Synchronization of worm cultures was achieved by bleaching of gravid hermaphrodites and glucose separation to harvest eggs. The harvested eggs were incubated at 20 °C for 18 hours. The hatch rate was checked for each strain.
MeHg exposure
Unless otherwise stated, all reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA). CH3HgCl (MeHg) was dissolved in H2O to make 40× stock solution (2, 20, 200 μM MeHg). To treat the worms, 2.5 μl MeHg were added to 100 μl NGM treatment buffer (Lehner et al., 2006) ( 3 g NaCI, 2.5 g peptone, 975 ml H2O, 1 ml cholesterol (5 mg/ml in ethanol), 1 ml nystatin, 1 ml CaCl2 1 M, 1 ml MgSO4 1 M, 25 ml KH2PO4 (pH=6)). The concentrations of MeHg used for the experiment are 0, 0.05, 0.5, and 5 μM.
Lethality and lifespan assay
For lethality assay, after treatment, worms were washed three times with M9 buffer, and then transferred to NGM plates seeded with E. coli OP50. After incubating at 20 °C for 24 hours, worms were scored as alive or dead with a stereomicroscope (stemi 2000, Zeiss, Germany). In some cases, dead worms were confirmed by touching the head region with the point of a platinum picker. For lifespan assay, after treatment with MeHg, worms were washed and transferred to NGM plates. At least 50 worms per treatment were checked each day for survival status. For worms treated with 5 μM MeHg for different time periods, they were cultured in NGM buffer in 96-well plate, and 50 μM fluorodeoxyuridine (FUDR) were added on day 4. At day 10, all worms were transferred to the FUDR-free NGM plates. These worms were transferred to new FUDR-free NGM plates twice each day during the peak egg-laying period (the first three days), and thereafter, they were transferred once a day until no live worms were observed.
RNA extraction and complementary DNA synthesis/ qPCR measurements
After treatment, at least 1,000 worms per group were pooled for mRNA samples. Total mRNA of worms per sample was extracted using TRIzol reagents as indicated by the product manual (ambion, Life Technologies, Grand Island, NY USA). mRNA samples were purified with the RNA binding column (NEB, Monarch RNA Cleanup Kit, Ipswich, MA, USA). To ensure the accuracy of qPCR data, each mRNA sample was checked with a NanoDrop 2000 spectrophotometer (Fisher, Wilmington, DE, USA). cDNA copies corresponding to each mRNA sample were synthesized using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). The mRNA levels corresponding to each gene was normalized to the relatively stable expression gene ama-1. The relative mRNA levels were determined with the 2− ΔΔCT method (Livak and Schmittgen, 2001). The predesigned probes used in the current study are tba-1 (ID: Ce02412618_gH), tba-2 (ID: Ce02420866_g1), and ama-1 (ID: Ce02462735_g1).
Dopaminergic neuron morphology
We developed a chronic treatment protocol by feeding worms with a dehydrated dead OP50 in NGM buffer as described in an early study (Ke and Aschner, 2019). We found that ~34% worms died at day 10 of MeHg treatment when fed with live bacteria, however, the death rate in dehydrated dead OP50 fed- worms was not significantly different from the control. Accordingly, worms were fed with dehydrated dead OP50 in this study. Around 100 L1 stage worms were treated in 100 μl NGM buffer in 96-well plate on a rotor at 120 rpm. To prevent evaporation, the plate was placed in a wet chamber. Starvation has tremendous impact on the biology of C. elegans (Angelo and Van Gilst, 2009). To avoid such effect, worms treated in NGM buffer were checked daily to ensure a well-feeding status. After treatment, all worms were paralyzed with 30 mM levamisole. The mCherry labeled dopaminergic neurons (strain OH7193), GFP labeled dopaminergic neurons (strain BZ555) and other stains were evaluated under 100 × objective with a fluorescence microscope (Olympus BX41, Japan). The bright, sphere-shape, enlarged puncta (thereafter named “puncta”) in dendrites of cephalic (CEP) neurons were manually counted with microscope. 15–20 worms per group on agar pad of each glass slide were scored in less than 30 minutes, in order to not compromise mCherry signal in the OH7193 strain.
Fluorescence quantification
After treatment, worms were paralyzed (30 mM levamisole) for fluorescence imaging (Leica SP8 confocal microscope, Germany). The wavelength and energy of excitation light were analogous during each scanning. Other parameters, such as magnification factor, emission gating range and scanning speed were also similar. In randomly selected 15–20 worms per sample, the mean intensity of fluorescence in the dendritic area of CEP and PVD neurons was quantified with Fiji software. To visualize lysosome, the strain DA2123 crossed with OH7193, and colocalization of GFP and mCherry was indentified with confocal microscope (Leica SP8 confocal microscope, Germany). To stain amyloid protein aggregates, the OH7193 worms dosed with MeHg were strained with the sensitive amyloid dye (X-34, sigma), according to the protocol described in an early study (Link et al., 2001).
Statistical analysis
Death rates of worms per sample were calculated and input into Graphpad 7(La Jolla, CA, USA). The LD50 for each strain was estimated by the sigmoidal dose-response model. The survival curves were generated by inputting the live status data (“1” for dead) directly into a survival datasheet in the software. Comparisons of the survival curves were made by Log-rank (Mantel-Cox) test. The average puncta numbers of CEP dendrites and soma are shown as median. Comparison of puncta numbers was made by Kruskal-Wallis test followed by Dunn’s multiple comparisons test. Other quantitative data are shown as mean ± SD. The analyses of quantitative data were made by t-test or one-way ANOVA followed by Tukey’s multiple comparisons test. Comparison of punta rate, and shrinkage rate between two groups was made by chi-square test or Fisher’s exact test, and in the case of multiple comparisons, partitions of chi-square method was employed to adjust significant level.
Results
1. Lethality and lifespan of worms exposed to MeHg
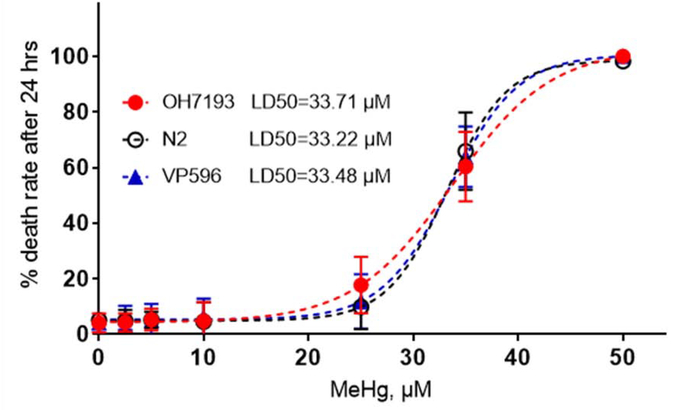
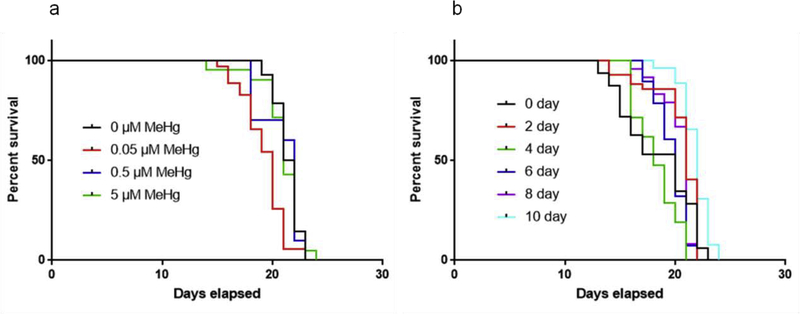
To visualize the morphology of fluorescence labeled neurons, transgenic strains OH7193 (dat-1::mCherry) and VP596 (dop-3::mCherry) were used. No statistically significant differences in the LD50 were noted among OH7193, VP596, and N2 wild type worms treated with 0–50 μM MeHg for 24 hours (Fig. 1). The lifespans were not significantly different among the OH7193 strain treated with different concentration of MeHg (Fig. 2a), or treated with 5 μM MeHg for different periods (Fig. 2b).
Fig 1.
OH7193, N2 and VP596 strains have comparable sensitivity to MeHg toxicity. 1000 worms were treated with 0–50 μM MeHg for 24 h, followed by manual counting of dead worms. Data are expressed as mean ± SD. Dose-response lethality curves and LD50 estimation were generated with a sigmoidal dose-response model with a top constraint at 100%.
Fig 2.
MeHg exposure does not affect lifespan of OH7193 strain at 0–5 μM concentration.
(a) OH7193 strain was exposed to 0–5 μM MeHg for 10 days, followed by lifespan assay.
(b) OH7193 strain was exposed to 5 μM MeHg for 0–10 days, followed by lifespan assay.
At least 50 worms per treatment were counted for lifespan assay. Comparisons of the survival curves were made using Log-rank (Mantel-Cox) test.
2. MeHg induces changes in the morphology of CEP dopaminergic neurons
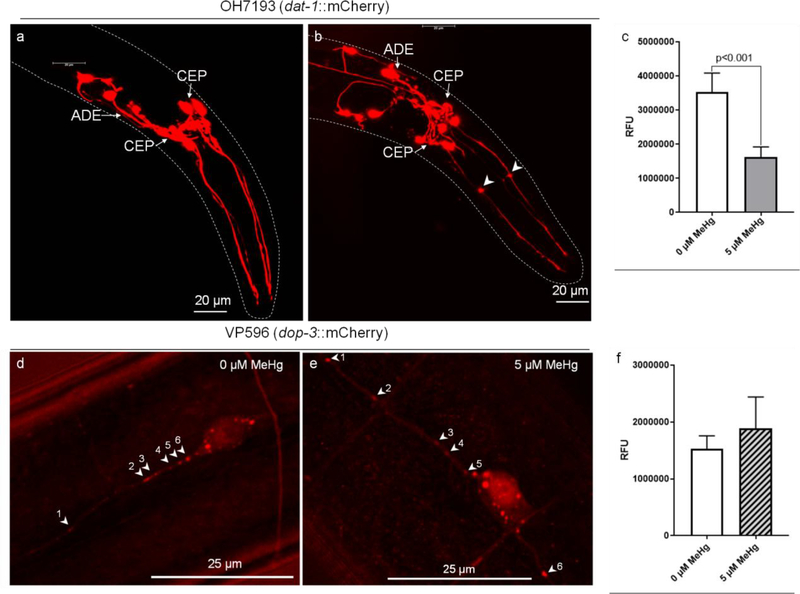
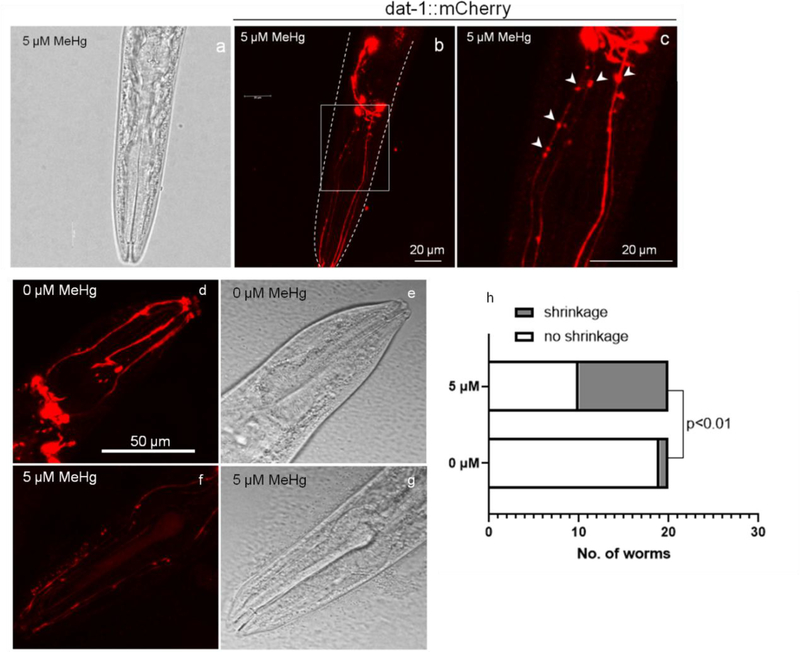
Four sets of neurons (4 dopaminergic CEPs, 2 dopaminergic anterior deirids (ADEs), 2 dopaminergic posterior deirids (PDEs) and 2 cholinergic AlYs) are labeled with mCherry in OH7193 strain, which was used to visualize the morphology of dopaminergic neurons. Interestingly, morphological changes in the dopaminergic neurons were noted in OH7193 strain following chronic exposure to 5 μM MeHg for 10 days. Specifically stationary puncta (not moving during a 0.5 hour observation) in the dendrites of CEP neurons were found in worms treated with MeHg (Fig. 3). The puncta size was variable (0.5 – 2.0 μm diameters), but all of them exhibited a sphere-like shape (Fig. 3b and 4c). In several cases, puncta formation appeared alongside shrinking dendrites, showing a beading shape (Fig. 4c). In addition, the dendritic process of CEP was thinner in exposed worms (Fig. 4), and mCherry signal was significantly lower than unexposed worms (Fig. 4). Hence, we concluded that these puncta were characterized by sphere shape, bright and accompanying with shrinkage of CEP dendrites (Fig. 3b and 4). In contrast, the puncta formation was not induced in other neurons including: 2 ADEs, 2 PDEs, cholinergic neurons (2 AIYs) or glutamatergic neurons (2 PVDs, Fig. 3).
Fig 3.
Chronic MeHg exposure induces mCherry puncta formation in dendrites of CEP neurons. (a,b) Representative images of dopaminergic CEP neurons in worms treated without (a) or with MeHg (b). Arrowheads show the puncta in the dendrites of CEP neurons following MeHg treatment. (c) Effect of MeHg on the mCherry fluorescence level of CEP dendrites. (d, e) Representative images of glutaminergic PVD neurons in worms treated without (d) or with MeHg (e). Arrowheads show the normal mCherry aggregates in PVD neurons. (f) Effect of MeHg on the mCherry fluorescence level of PVD dendrites. The fluorescence level in dendritic areas of CEP and PVD were assessed with ImageJ software. Data are expressed as mean ± SD (one-way ANOVA, n=3).
Fig. 4.
Characterization of CEP dendrites of worms treated with MeHg. (a-c) Representative images of puncta positive dendrites of CEP neurons in worms treated with MeHg. Arrowheads show the beading-shape puncta in the dendrites of CEP. scable bar = 20 μm. (d-g) Shrinkage of CEP dendrites in worms treated with MeHg. scable bar = 50 μm. (h) Number of worms with CEP dendritic shrinkage following MeHg treatment (Fisher’s exact test).
It is known that MeHg can promote aggregation of cellular proteins (Kanda et al., 2014), and the mCherry probe has propensity to form aggregates (Shemiakina et al., 2012). Thus we proposed that the puncta are likely composed of aggregated mCherry protein. Indeed, these puncta seem much brighter than surrounding areas (Fig. 3), and morphologically distinct from those inherent to normal mCherry fluorescent protein aggregates found in the soma of CEP neurons or in PVD neurons (indicated by numbered arrows in Fig. 3d and 3e).
To verify neurons other than CEPs that were labeled with mCherry also have puncta in the dendritic area after chronic MeHg exposure, the VP596 strain was used to investigate the morphology of PVD glutamatergic neurons, which are characterized by extensive branching patterns. In the VP596 strain, mCherry expression is driven by the dopamine receptor gene promoter dop-3 (dop-3, an ortholog of human DRD2 (dopamine receptor D2)), which labels PVD glutamatergic neuron in the posterior body of the worm. Interestingly, either in untreated (Fig. 3d), or in MeHg-treated worms (Fig. 3e), mCherry aggregates appeared in the dendritic area of PVD neurons, but they were not as bright as the puncta in CEP neurons of MeHg-treated worms (Fig. 3, and Fig. 4). There was no statistical difference between the untreateded and treated groups (supplemental Fig. 2). Notably, these aggregates in PVD neurons were not sphere-like shaped, and they conformed to the dendritic shape, exhibiting various sizes, brightness and motility (marked with numbered arrows in Fig. 3d and 3e). The mCherry signal of worms with or without MeHg was not significantly different (Fig. 3f).
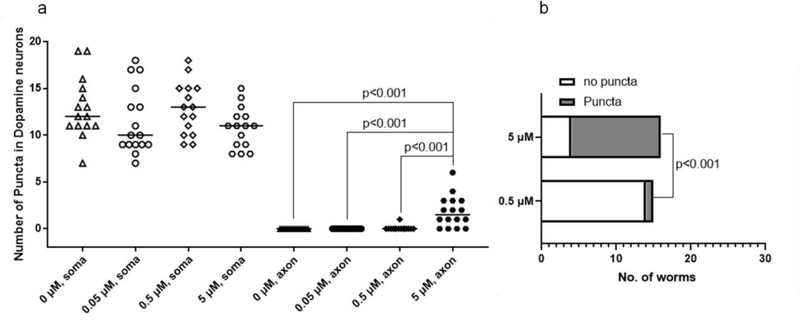
To further characterize a sublethal chronic MeHg exposure and puncta formation in CEP neurons, worms were treated with graded concentrations of MeHg (0, 0.05, 0.5, 5 μM) for 10 days. 5 μM MeHg significantly increased the puncta number in the dendrites, but not in the soma (Fig. 5a). Less than 10% of the worms showed puncta formation in the dendrites after treatment with 0.5 μM MeHg (Fig. 5b), whilst following treatment with 5 μM MeHg, more than 70% worms showed puncta in the CEP dendrites (Fig. 5b).
Fig. 5.
MeHg dose-dependently increases puncta in the dendrites of CEP neurons. (a) OH9173 strain was treated with 0–5 μM MeHg for 10 days, followed by counting the number of puncta in CEP soma and dendrites. The horizontal lines represent median values. (Kruskal-Wallis test followed by Dunn’s multiple comparisons test, n=3). (b) Reanalysis of data presented in (a) showing the number of worms with positive puncta (Fisher’s exact test).
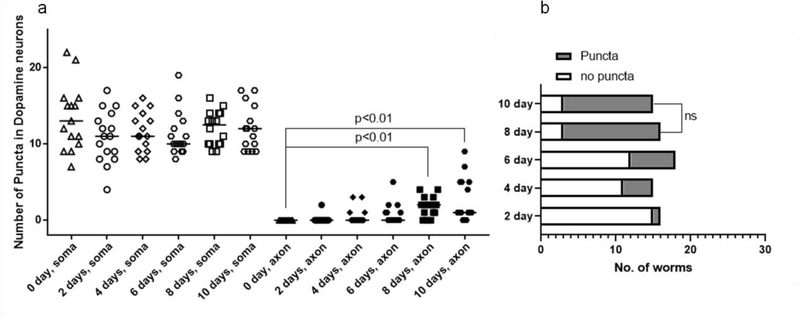
To test if the rate of puncta formation correlates with MeHg treatment periods, worms were treated with 5 μM MeHg for up to 10 days. An increase in the dendritic puncta number following MeHg treatment was noted. No alterations were observed in the soma of the CEP neurons. The puncta number significantly increased in the 4, 6, 8, and 10 - day groups as compared with the 0-day group (Fig. 6a). The percentage of worms with positive puncta in the dendrites was also significantly increased in the 4, 6, 8, 10 -day groups as compared with the 0-day group (Fig. 6b). Although the percentages in the 8-day and 10-day groups are not statistically different, they are both statistically higher than the percentages in 2-, 4- and 6-day groups (Fig. 6b).
Fig. 6.
MeHg time-dependently increases puncta in the dendrites of CEP neurons. (a) OH9173 strain was treated with 5 μM MeHg for 0–10 days, followed by counting the number of puncta in CEP soma and dendrites. The horizontal lines represent median values (Kruskal-Wallis test followed by Dunn’s multiple comparisons test, n=3). (b) Reanalysis of data presented in (a) showing the number of worms with positive puncta (Chi-square test).
3. gst-4 and tubulins (tba-1 and tba-2) expression were not modified after chronic MeHg exposure
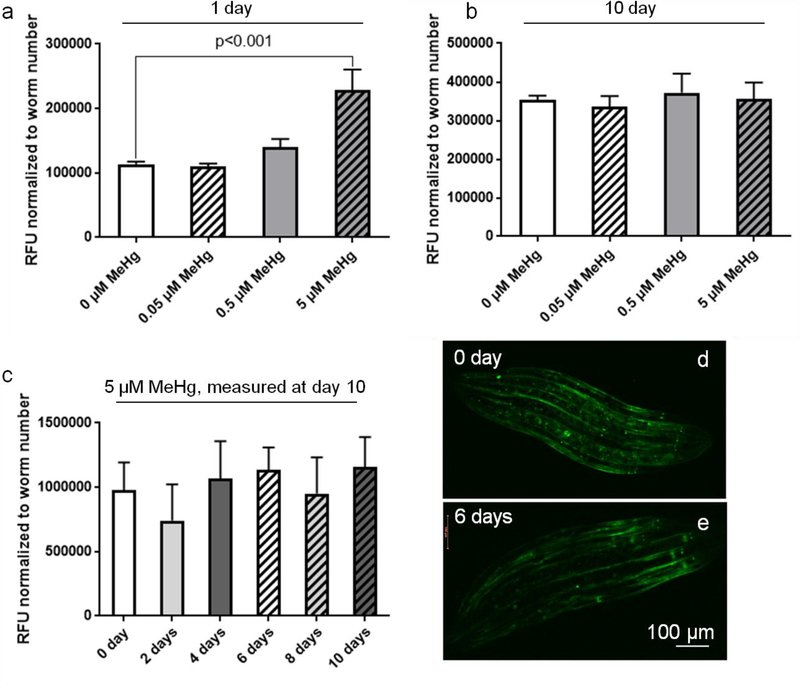
Puncta formation in response to MeHg treatment was only present following chronic MeHg exposure, which leads us to test if this event was related to MeHg-induced changes in the antioxidant status. Glutathione S-transferases (GSTs) are detoxification enzymes that have been proposed to facilitate MeHg excretion by catalyzing MeHg-GSH conjugation (Vorojeikina et al., 2017). We used a strain (CL2166) with gst-4::GFP to analyze expression levels of gst-4 after chronic MeHg exposure. It was found that MeHg induced a dose-dependent upregulation of gst-4 transcriptional levels one day after exposure, which was indicated by increased level of GFP signals (Fig. 7a). However, the levels of gst-4 expression were not changed 10 days after MeHg treatment (Fig. 7b). Worms treated with MeHg for different periods did not show significant change in gst-4 expression (Fig. 7c, 7d and 7e).
Fig. 7.
Chronic exposure to MeHg doesn’t invoke overexpression of gst-4. (a) CL2166 strain was treated with 0–5 μM MeHg for 1 day, followed by measurement of fluorescence intensity with ImageJ software. (b) CL2166 strain was treated with 0–5 μM MeHg for 10 days, followed by measurement of fluorescence intensity with ImageJ software. (c) CL2166 strain was treated with 5 μM MeHg for 0–10 days, followed by measurement of fluorescence intensity with ImageJ software. (d,e) Representative images of worms treated without MeHg (d) or with MeHg for 6 days (e). Data are expressed as mean ± SD (one-way ANOVA followed by Tukey’s post hoc test, n=3).
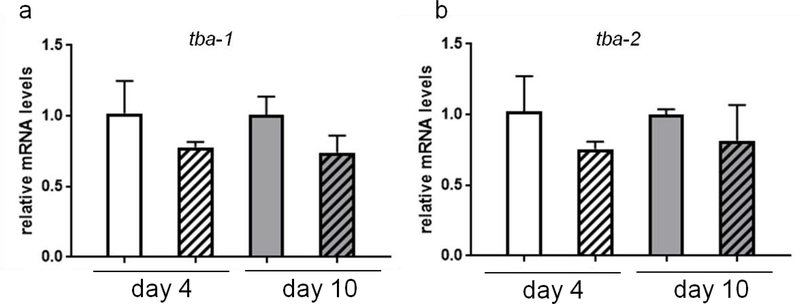
Tubulins play important roles in transporting organelles and nutrients from soma to dendritic area. It has been reported that MeHg binded to thiols in the tubulin, affecting tubulin assembly (Vogel et al., 1985), and down regulating tubulin expression (Miura et al., 1998). Here, we hypothesized that the formation of puncta in the CEP dendritic area, could be a consequence of disrupted expression of tubulins after MeHg exposure. To test this hypothesis, we analyzed the mRNA levels of two tubulin homologs (tba-1, tba-2) in C. elegans. However, treatment with 5 μM MeHg for 4 days or 10 days failed to alter the tubulin mRNA levels (Fig. 8).
Fig. 8.
Expression of tubulin genes is not associated with MeHg exposure. (a) N2 strain was treated with 5 μM MeHg for 4 or 10 days, followed by measurement of tba-1 mRNA with RT-PCR. (b) N2 strain was treated with 5 μM MeHg for 4 or 10 days, followed by measurement of tba-2 mRNA with RT-PCR. Data are expressed as mean ± SD (one-way ANOVA followed by Tukey’s post hoc test; n=3).
Discussion
Our findings, for the first time, demonstrate that exposure to a sublethal level of MeHg produces significant morphology changes in dopaminergic neurons in C. elegans. In addition, we report that puncta formation selectively appeared in CEP dopaminergic neurons, which increased with MeHg doses and treatment periods. We also found that gst-4 expression was induced in a relatively short exposure paradigm, while chronic exposure did not invoke significant changes in gst-4, tba-1, or tba-2 gene expression levels. These results indicate that CEP dopaminergic neurons are selectively sensitive to the toxicity of a sublethal dose of MeHg, and that complex mechanisms underlie MeHg’s neurotoxicity, culminating in the morphological changes in these neurons.
The integrity of the dopaminergic neurons and dopamine-mediated neurotransmission is a requisite for optimal brain function. Damage to dopaminergic neurons and circuits is implicated in several brain diseases (Borroto-Escuela et al., 2018). MeHg is an environmental neurotoxin that can target dopaminergic neurons. In fact, previous studies with C. elegans have demonstrated that MeHg is able to induce morphological changes in dopaminergic neurons (Caito and Aschner, 2016; Vanduyn et al., 2010). However, sensitive morphological biomarkers of dopaminergic neurodegeneration that precede frank MeHg neurotoxicity and neuronal cell death are lacking. Accordingly, we hypothesized that morphological changes in dopaminergic neurons might be induced by chronic treatment with a sublethal dose of MeHg.
In order to establish a chronic model, we used the OH7193 (dat-1::mCherry) and VP596 (dop-3::mCherry) strains. These strains showed similar sensitivity to MeHg toxicity as the wild type, N2 strain. Chronic MeHg treatment in NGM liquid media, led to a comparable LD50 for the OH7193, VP596 and the wild-type strains (Fig. 1). Ten-day exposure to MeHg (5 μM) failed to significantly alter the worms’ life expectancy (Fig. 2). However, chronic MeHg treatment induced an increase in the large, sphere-shaped and bright puncta in the dopaminergic CEP dendrites, both dose- and time-dependently (Fig. 5, 6). These changes occurred in parallel to dendritic shrinkage (Fig. 4). Further staining shows that the puncta were not inclusions in lysosome (supplemental Fig. 3), or amyloid protein aggregates (supplemental Fig. 3). Melentijevic et al., observed that mCherry can be excreted into the extracellular space from C. elegans neurons (Melentijevic et al., 2017). They reasoned that the extracellular content of mCherry may be used to test the ability of neurons to maintain protein homeostasis (a high extracellular mCherry signal means a high potential to excrete protein aggregates) (Melentijevic et al., 2017). Accordingly, our data suggests that the puncta are likely composed of homologous mCherry molecules packaged at the dendritic site for extracellular export, which is inhibited by chronic MeHg exposure.
In C. elegans hermaphrodites, the eight dopaminergic neurons are classified into three neuronal types, namely CEPs, ADEs, and PDEs. In contrast to CEPs, we failed to find analogous changes in the dendrites of ADEs or PDEs. These changes were neither replicated in glutaminergic neurons (PVDs, Fig. 3c and 3d). The selective toxicity is probably a reflection of the unique characteristics of CEP neurons (Kang et al., 2010). The four CEP neurons have ciliated, unbranched dendrites distributed around the mouth (Kang et al., 2010). During locomotion and foraging, the activity of CEP neurons constantly turns on or off to modulate behaviors corresponding to environmental stimuli (Kindt et al., 2007). This process requires a tight coupling of energy supply and dopamine recycling (Pathak et al., 2015), both of which are targets of MeHg (Beyrouty et al., 2006; Coccini et al., 2011; Dreiem et al., 2009; Farina et al., 2011; Faro et al., 2000; Faro et al., 2002a, b; Kalisch and Racz, 1996; O’Kusky et al., 1988). In addition, CEP neurons could accumulate more Hg than ADEs and PDEs, by retrogradely absorbing or transporting MeHg through the dendritic ciliated endings in the tip of the mouth, as opposed to absorption through intestine. Evidence was accumulated on that Hg could be transported retrogradely along the neuritis (Arvidson, 1987, 1990, 1992; Arvidson and Arvidsson, 1990; Schionning, 1993). It has been previously reported that the dopaminergic neurons in the hermaphrodite worm have distinct sensitivity to 6-hydroxydopamine (6-OHDA), with the earliest and most readily detected morphological changes occurring in CEP processes (Cao et al., 2005; Nass et al., 2002). In these studies (Cao et al., 2005; Nass et al., 2002), the authors proposed that the dopamine active transporter (DAT-1), which has a relatively higher abundance in CEP neurons than other dopaminergic neurons (ADEs, PDEs) in adult stage worms (McDonald et al., 2006), was likely involved in the pathogenesis of 6-OHDA induced CEP neurodegeneration. These observations (Cao et al., 2005; Nass et al., 2002) are consistent with the results in the present model: MeHg caused selective toxicity in CEPs, while sparing other neuronal populations, such as ADEs, PDEs, AIYs and PVD neurons (Fig. 3c, 3d and supplemental Fig. 2).
In another worm strain, where dopaminergic neurons have been labeled with dat-1::GFP (BZ555), we failed to visualize puncta formations in the soma or in the dendrites (supplemental Fig. 1). The discrepancy between the OH7193 and BZ555 strains could be ascribed to the distinct biochemical properties of mCherry and GFP. The mCherry probe is prone to form aggregates in labeled cells (Shemiakina et al., 2012). In the OH7193 strain, the mCherry aggregates appeared in the soma of all eight dopaminergic neurons (unpublished data). However, these aggregates were far less frequent in CEP dendrites. The GFP (S65C) in the BZ555 strain is quenched in acidic pH (pKa~6), whereas mCherry retains fluorescence at lower pH (pKa < 4.5) (Green et al., 2008). In a previous study comparing the performance of different fluorescence proteins in mammalian cells, it was shown that monomeric RFP is more resistant to acidity and lysosomal proteases than several other GFP proteins (Katayama et al., 2008). Thus, mCherry aggregates cannot be deactivated by lysosomes, and hence are more stable than their GFP counterpart (Katayama et al., 2008).
The glutathione S-transferases (GSTs) have been proposed to play a key role in detoxifying MeHg (Vorojeikina et al., 2017). That MeHg treatment didn’t invoke gst-4 changes suggests mechanisms other than detoxification pathways might contribute to the puncta formation in CEP neurons after chronic exposure to MeHg. In this regard, the effect of reactive oxygen species (ROS) should be considered. It is known that hydrogen peroxide caused dendritic damage by oxidative stress and ATP depletion, which are important mediators of MeHg toxicity (Cambier et al., 2009; Mori et al., 2011; Roos et al., 2012). Finally, it has been reported that C. elegans adult neurons are able to extrude large membrane-surrounded vesicles (average diameter 3.8 μm) containing protein aggregates and dysfunctional organelles (Melentijevic et al., 2017), whereas the puncta sizes in CEPs of MeHg treated worm are 0.5–2.0 μm, which could be a premature form of vesicles containing protein aggregates. Accordingly, by accumulating in CEPs through retrogradely transport, MeHg might interrupt this conserved neuronal homeostatic machinery, leading to the damage of CEP dendrites.
Tubulins represent one of the major targets of MeHg cellular toxicity (Miura et al., 1984; Vogel et al., 1985). It has been previously reported that MeHg forms complexes with both polymerized or depolymerized tubulin, and increases tubulin depolymerization (Vogel et al., 1985). In mouse glioma cells, disruption of microtubules by MeHg was shown to alter tubulin synthesis via autoregulatory repression (Miura et al., 1998). However, herein, we failed to note any change in tba-1 (an ortholog of human TUBA8 (tubulin alpha 8)), or tba-2 (an ortholog of human TUBA1A (tubulin alpha 1a)) mRNA levels (Fig. 8). Given that the tubulin gene expression data in response to MeHg only reflect the global level of tba-1 and tba-2, which can not preclude neuron-specific tubulin changes in expression, a future study to compare tubulin expression at the neuron level using quantitative immunofluorescence microscopy is warranted.
In summary, by establishing a chronic exposure model, we have shown for the first time, that MeHg was able to induce bright mCherry puncta formationin the CEP dopaminergic neurons of C. elegans in a dose- and time-dependent manner. Several features of the puncta were distinguishable from normal mCherry aggregates suggest that the puncta are likely composed of homologous mCherry molecules packaged at the dendritic site for extracellular transport. Moreover, gst-4 and tubulin expression could not be invoked for the puncta formation. The identification of these alterations under a chronic exposure model may accelerate future genetic discovery on the molecular mechanism of MeHg-induced dopaminergic neurotoxicity.
Supplementary Material
Highlights.
Chronic exposure to MeHg selectively induces puncta formation in CEP dopaminergic neurons.
The expression levels of gst-4 and tubulins (tba-1 and tba-2) genes are not significantly modified under chronic MeHg exposure paradigm.
Acknowledgments
This work was supported by the National Institutes of Health to MA (grant numbers, NIEHS R01ES007331, NIEHS R01ES010563 and NIEHS R01ES020852). The authors thank the Analytical Imaging Facility (AIF) at Albert Einstein College of Medicine, which is sponsored by NCI cancer center support grant P30CA013330 and Shared Instrumentation Grant (SIG) 1S10OD023591-01. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Angelo G, Van Gilst MR, 2009. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326(5955), 954–958. [DOI] [PubMed] [Google Scholar]
- Arvidson B, 1987. Retrograde axonal transport of mercury. Exp Neurol 98(1), 198–203. [DOI] [PubMed] [Google Scholar]
- Arvidson B, 1990. Accumulation of mercury in brainstem nuclei of mice after retrograde axonal transport. Acta Neurol Scand 82(4), 234–237. [DOI] [PubMed] [Google Scholar]
- Arvidson B, 1992. Accumulation of Inorganic Mercury in Lower Motoneurons of Mice. Neurotoxicology 13(1), 277–280. [PubMed] [Google Scholar]
- Arvidson B, Arvidsson J, 1990. Retrograde axonal transport of mercury in primary sensory neurons innervating the tooth pulp in the rat. Neurosci Lett 115(1), 29–32. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, 2011. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63(1), 182–217. [DOI] [PubMed] [Google Scholar]
- Beyrouty P, Stamler CJ, Liu JN, Loua KM, Kubow S, Chan HM, 2006. Effects of prenatal methylmercury exposure on brain monoamine oxidase activity and neurobehaviour of rats. Neurotoxicology and Teratology 28(2), 251–259. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB, 2007. Dopamine neuron systems in the brain: an update. Trends Neurosci 30(5), 194–202. [DOI] [PubMed] [Google Scholar]
- Bjorklund G, Dadar M, Mutter J, Aaseth J, 2017. The toxicology of mercury: Current research and emerging trends. Environ Res 159, 545–554. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Hantsch J, Herzer M, 1983. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol Psychiatry 18(9), 951–969. [PubMed] [Google Scholar]
- Borroto-Escuela DO, De la Mora MP, Manger P, Narvaez M, Beggiato S, Crespo-Ramirez M, Navarro G, Wydra K, Diaz-Cabiale Z, Rivera A, Ferraro L, Tanganelli S, Filip G, Franco R, Fuxe K, 2018. Brain Dopamine Transmission in Health and Parkinson’s Disease: Modulation of Synaptic Transmission and Plasticity Through Volume Transmission and Dopamine Heteroreceptors. Front Synaptic Neuro 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S, 1974. The genetics of Caenorhabditis elegans. Genetics 77(1), 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito SW, Aschner M, 2016. NAD+ Supplementation Attenuates Methylmercury Dopaminergic and Mitochondrial Toxicity in Caenorhabditis Elegans. Toxicol Sci 151(1), 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier S, Benard G, Mesmer-Dudons N, Gonzalez P, Rossignol R, Brethes D, Bourdineaud JP, 2009. At environmental doses, dietary methylmercury inhibits mitochondrial energy metabolism in skeletal muscles of the zebra fish (Danio rerio). Int J Biochem Cell B 41(4), 791–799. [DOI] [PubMed] [Google Scholar]
- Cao S, Gelwix CC, Caldwell KA, Caldwell GA, 2005. Torsin-mediated protection from cellular stress in the dopaminergic neurons of Caenorhabditis elegans. J Neurosci 25(15), 3801–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Bowling K, Funderburk C, Lawal H, Inamdar A, Wang Z, O’Donnell JM, 2007. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J Neurosci 27(10), 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, 2002. The three modern faces of mercury. Environ Health Perspect 110 Suppl 1, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccini T, Roda E, Castoldi AF, Poli D, Goldoni M, Vettori MV, Mutti A, Manzo L, 2011. Developmental exposure to methylmercury and 2,2 ‘,4,4 ‘,5,5 ‘-hexachlorobiphenyl (PCB153) affects cerebral dopamine D1-like and D2-like receptors of weanling and pubertal rats. Arch Toxicol 85(10), 1281–1294. [DOI] [PubMed] [Google Scholar]
- Costantini LM, Baloban M, Markwardt ML, Rizzo M, Guo F, Verkhusha VV, Snapp EL, 2015. A palette of fluorescent proteins optimized for diverse cellular environments. Nature Communications 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiem A, Shan MT, Okoniewski RJ, Sanchez-Morrissey S, Seegal RF, 2009. Methylmercury inhibits dopaminergic function in rat pup synaptosomes in an age-dependent manner. Neurotoxicology and Teratology 31(5), 312–317. [DOI] [PubMed] [Google Scholar]
- Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T, 2007. Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci 262(1–2), 131–144. [DOI] [PubMed] [Google Scholar]
- Fahn S, 2015. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disord 30(1), 4–18. [DOI] [PubMed] [Google Scholar]
- Farina M, Rocha JB, Aschner M, 2011. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci 89(15–16), 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faro LR, do Nascimento JL, San Jose JM, Alfonso M, Duran R, 2000. Intrastriatal administration of methylmercury increases in vivo dopamine release. Neurochem Res 25(2), 225–229. [DOI] [PubMed] [Google Scholar]
- Faro LRF, do Nascimento JLM, Alfonso M, Duran R, 2002a. Mechanism of action of methylmercury on in vivo striatal dopamine release - Possible involvement of dopamine transporter. Neurochem Int 40(5), 455–465. [DOI] [PubMed] [Google Scholar]
- Faro LRF, do Nascimento JLM, Alfonso M, Duran R, 2002b. Protection of methylmercury effects on the in vivo dopamine release by NMDA receptor antagonists and nitric oxide synthase inhibitors. Neuropharmacology 42(5), 612–618. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ, 2011. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34, 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz ME, Koutsilieri E, Riederer P, Ceccatelli S, Dare E, 2002. Methylmercury induces neurite degeneration in primary culture of mouse dopaminergic mesencephalic cells. J Neural Transm (Vienna) 109(5–6), 597–605. [DOI] [PubMed] [Google Scholar]
- Green RA, Audhya A, Pozniakovsky A, Dammermann A, Pemble H, Monen J, Portier N, Hyman A, Desai A, Oegema K, 2008. Expression and imaging of fluorescent proteins in the C. elegans gonad and early embryo. Methods Cell Biol 85, 179–218. [DOI] [PubMed] [Google Scholar]
- Kalisch BE, Racz WJ, 1996. The effects of methylmercury on endogenous dopamine efflux from mouse striatal slices. Toxicol Lett 89(1), 43–49. [DOI] [PubMed] [Google Scholar]
- Kanda H, Shinkai Y, Kumagai Y, 2014. S-Mercuration of cellular proteins by methylmercury and its toxicological implications. Journal of Toxicological Sciences 39(5), 687–700. [DOI] [PubMed] [Google Scholar]
- Kanda H, Toyama T, Shinohara-Kanda A, Iwamatsu A, Shinkai Y, Kaji T, Kikushima M, Kumagai Y, 2012. S-Mercuration of rat sorbitol dehydrogenase by methylmercury causes its aggregation and the release of the zinc ion from the active site. Arch Toxicol 86(11), 1693–1702. [DOI] [PubMed] [Google Scholar]
- Kang LJ, Gao JW, Schafer WR, Xie ZX, Xu XZS, 2010. C. elegans TRP Family Protein TRP-4 Is a Pore-Forming Subunit of a Native Mechanotransduction Channel. Neuron 67(3), 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama H, Yamamoto A, Mizushima N, Yoshimori T, Miyawaki A, 2008. GFP-like proteins stably accumulate in lysosomes. Cell Struct Funct 33(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Ke T, Aschner M, 2019. Bacteria affect Caenorhabditis elegans responses to MeHg toxicity. Neurotoxicology 75, 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR, 2007. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55(4), 662–676. [DOI] [PubMed] [Google Scholar]
- Lehner B, Tischler J, Fraser AG, 2006. RNAi screens in Caenorhabditis elegans in a 96-well liquid format and their application to the systematic identification of genetic interactions. Nat Protoc 1(3), 1617–1620. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Lee MG, Cowan NJ, 1985. Five mouse tubulin isotypes and their regulated expression during development. The Journal of cell biology 101(3), 852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link CD, Johnson CJ, Fonte V, Paupard MC, Hall DH, Styren S, Mathis CA, Klunk WE, 2001. Visualization of fibrillar amyloid deposits in living, transgenic Caenorhabditis elegans animals using the sensitive amyloid dye, X-34. Neurobiol Aging 22(2), 217–226. [DOI] [PubMed] [Google Scholar]
- McDonald PW, Jessen T, Field JR, Blakely RD, 2006. Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol Neurobiol 26(4–6), 593–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, Taub D, Parker JA, Neri C, Gabel CV, Hall DH, Driscoll M, 2017. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542(7641), 367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Rademacher DJ, 2011. MPTP mouse models of Parkinson’s disease: an update. J Parkinsons Dis 1(1), 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Inokawa M, Imura N, 1984. Effects of methylmercury and some metal ions on microtubule networks in mouse glioma cells and in vitro tubulin polymerization. Toxicol Appl Pharmacol 73(2), 218–231. [DOI] [PubMed] [Google Scholar]
- Miura K, Kobayashi Y, Toyoda H, Imura N, 1998. Methylmercury-induced microtubule depolymerization leads to inhibition of tubulin synthesis. J Toxicol Sci 23(5), 379–388. [DOI] [PubMed] [Google Scholar]
- Mori N, Yasutake A, Marumoto M, Hirayama K, 2011. Methylmercury inhibits electron transport chain activity and induces cytochrome c release in cerebellum mitochondria. J Toxicol Sci 36(3), 253–259. [DOI] [PubMed] [Google Scholar]
- Nass R, Hall DH, Miller DM 3rd, Blakely RD, 2002. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc Natl Acad Sci U S A 99(5), 3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kusky JR, Boyes BE, McGeer EG, 1988. Methylmercury-induced movement and postural disorders in developing rat: regional analysis of brain catecholamines and indoleamines. Brain Res 439(1–2), 138–146. [DOI] [PubMed] [Google Scholar]
- Pathak D, Shields LY, Mendelsohn BA, Haddad D, Lin W, Gerencser AA, Kim H, Brand MD, Edwards RH, Nakamura K, 2015. The role of mitochondrially derived ATP in synaptic vesicle recycling. J Biol Chem 290(37), 22325–22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patt S, Gertz HJ, Gerhard L, Cervos-Navarro J, 1991. Pathological changes in dendrites of substantia nigra neurons in Parkinson’s disease: a Golgi study. Histol Histopathol 6(3), 373–380. [PubMed] [Google Scholar]
- Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM, 1995. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience 67(3), 631–647. [DOI] [PubMed] [Google Scholar]
- Roos D, Seeger R, Puntel R, Vargas Barbosa N, 2012. Role of calcium and mitochondria in MeHg-mediated cytotoxicity. J Biomed Biotechnol 2012, 248764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Poirier MA, 2004. Protein aggregation and neurodegenerative disease. Nat Med 10(7), S10–S17. [DOI] [PubMed] [Google Scholar]
- Schafer WR, Kenyon CJ, 1995. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature 375(6526), 73–78. [DOI] [PubMed] [Google Scholar]
- Schionning JD, 1993. Retrograde Axonal-Transport of Mercury in Rat Sciatic-Nerve. Toxicol Appl Pharm 121(1), 43–49. [DOI] [PubMed] [Google Scholar]
- Shao YT, Figeys D, Ning ZB, Mailloux R, Chan HM, 2015. Methylmercury can induce Parkinson’s-like neurotoxicity similar to 1-methyl-4-phenylpyridinium: a genomic and proteomic analysis on MN9D dopaminergic neuron cells. Journal of Toxicological Sciences 40(6), 817–828. [DOI] [PubMed] [Google Scholar]
- Shemiakina II, Ermakova GV, Cranfill PJ, Baird MA, Evans RA, Souslova EA, Staroverov DB, Gorokhovatsky AY, Putintseva EV, Gorodnicheva TV, Chepurnykh TV, Strukova L, Lukyanov S, Zaraisky AG, Davidson MW, Chudakov DM, Shcherbo D, 2012. A monomeric red fluorescent protein with low cytotoxicity. Nat Commun 3, 1204. [DOI] [PubMed] [Google Scholar]
- Tarakad A, Jankovic J, 2017. Diagnosis and Management of Parkinson’s Disease. Semin Neurol 37(2), 118–126. [DOI] [PubMed] [Google Scholar]
- Toyama T, Abiko Y, Katayama Y, Kaji T, Kumagai Y, 2015. S-Mercuration of ubiquitin carboxyl-terminal hydrolase L1 through Cys152 by methylmercury causes inhibition of its catalytic activity and reduction of monoubiquitin levels in SH-SY5Y cells. Journal of Toxicological Sciences 40(6), 887–893. [DOI] [PubMed] [Google Scholar]
- Vanduyn N, Settivari R, Wong G, Nass R, 2010. SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol Sci 118(2), 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel DG, Margolis RL, Mottet NK, 1985. The effects of methyl mercury binding to microtubules. Toxicol Appl Pharmacol 80(3), 473–486. [DOI] [PubMed] [Google Scholar]
- Vorojeikina D, Broberg K, Love TM, Davidson PW, van Wijngaarden E, Rand MD, 2017. Editor’s Highlight: Glutathione S-Transferase Activity Moderates Methylmercury Toxicity During Development in Drosophila. Toxicol Sci 157(1), 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeragani VK, Tancer M, Chokka P, Baker GB, 2010. Arvid Carlsson, and the story of dopamine. Indian J Psychiatry 52(1), 87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.