Abstract
Introduction:
Poisoning exposure cases involving e-cigarettes have increased since 2010, coinciding with increasing rates of e-cigarette use in the United States (US). Given the increasing prevalence of e-cigarette use and ever-changing product designs, particularly the development of new products with high nicotine levels, it is important to conduct ongoing surveillance of poisoning exposure cases involving e-cigarettes. The objective of this study is to describe trends and characteristics of poisoning exposure cases involving e-cigarettes and e-liquids reported to poison control centers in the US.
Methods:
We analyzed e-cigarette exposure cases from the National Poison Data System (NPDS) during 2010–2018 by year and other characteristics.
Results:
The annual number of e-cigarette exposure cases increased greatly between 2010 and 2014, reaching a peak of 3742 in 2014, and then decreasing each year between 2015 and 2017. Between 2017 and 2018, the overall number of e-cigarette exposure cases increased by 25.0% (from 2320 to 2901). Approximately two-thirds (64.8%) of all cases were in children under age five, and 14.7% were in children aged 5–17 years or young adults aged 18–24 years. A small proportion of cases developed life-threatening symptoms (0.1%); and cases with more serious medical outcomes tended to be exposed to a higher e-liquid or nicotine quantity.
Conclusions:
Annual declines in e-cigarette exposure cases between 2015 and 2017 did not continue in 2018. The rapid changes in the occurrence of poisoning exposure cases involving e-cigarettes coupled with the development of new tobacco products and ever-evolving tobacco use landscape underscore the importance of continued surveillance of these poisoning exposure cases. Continuous monitoring of these poisoning exposure cases may inform efforts aimed at preventing e-cigarette poisoning exposures.
Keywords: Poison, exposure, electronic cigarette, e-cigarette, liquid nicotine
Introduction
Electronic cigarette (e-cigarette) use patterns are changing in the United States (US). Among youth, current e-cigarette use has increased dramatically since 2011 [1]. During 2017–2018, current e-cigarette use increased by 77.8% (from11.7% to 20.8%) among high school students and by 48.5% (from 3.3% to 4.9%) among middle school students [1,2]. More than 3.6 million US middle and high school students were current e-cigarette users in 2018 [1,2]. Among US adults, current e-cigarette use decreased between 2014 and 2017 (from 3.7% in 2014 to 3.2% in 2016 and to 2.8% in 2017) [3,4]. In 2017, 6.9 million US adults were current e-cigarette users [4].
Since e-cigarettes were first introduced to the US market in 2007 [5], e-cigarette designs have continually changed. For example, a relatively new type of e-cigarette product resembles a USB flash drive and is characterized by high nicotine concentration, no vape clouds, and easy concealment. JUUL is the most popular e-cigarette brand of this type, although there are other e-cigarettes that resemble USB flash drives [6,7]. During a relatively short 3-year period in 2015–2017, JUUL and other JUUL-like products have been increasingly used by youth and young adults, with JUUL becoming the largest retail e-cigarette brand and accounting for more than 40% of e-cigarette retail market share in the US in 2017 [8]. While some e-cigarette products sold in the US do not contain nicotine, retail sales data from US stores in 2015, excluding vape shops, tobacco specialty shops, and online sources, suggested that 99% of e-cigarette products contained nicotine with various nicotine concentration levels [9].
As e-cigarette use has become increasingly popular, the number of individuals experiencing unintended or potentially unintended effects from ingestion, inhalation, or other routes of contact with e-cigarettes and e-liquids (i.e., e-cigarette exposure cases) also increased dramatically in both the general population and the pediatric population in the US [10–14]. For example, Chatham-Stephens et al. reported that the number of such cases involving e-cigarettes and e-liquid only in the general population increased from one in September 2010 to a peak of 401 in April 2014, followed by a decline to 295 in December 2014 [10]. Wang and Rostron analyzed data on tobacco related exposure calls to US poison control centers in 2001–2016 involving children younger than five years old. They found that the number of e-cigarette related cases increased from seven in 2010 to 2558 in 2015 [13]. Govindarajan et al. estimated that the annual e-liquid exposure rate per 100,000 children younger than age six increased from 0.7 in 2012 to 10.4 in 2015, and subsequently decreased to 8.3 in 2016 [14]. To date, there are no published data showing whether the trend of decreased e-cigarette exposure cases continued into 2018.
Given the increasing prevalence of e-cigarette use and ever-changing product designs, particularly the development of new products with high nicotine levels, it is important to conduct ongoing surveillance of e-cigarette exposure cases. In this study, we assessed the overall number of e-cigarette exposure cases between 2010 and 2018 using data from the National Poison Data System (NPDS) and described trends and case characteristics. In addition, we examined e-liquid and nicotine quantity of exposures for cases with data available stratified by medical outcomes, an area that has not been addressed by previous studies using NPDS.
Methods
Data source
Data for this study are from the NPDS, maintained by the American Association of Poison Control Centers (AAPCC), an organization responsible for providing information on substance toxicity and advice on poisoning exposure management involving human and animals, collecting accurate data with up-to-date coding, responding to continuing need for public and professional education about poisoning prevention and management. Details on NPDS have been described elsewhere [15]. Briefly, NPDS is a data repository of telephone calls to poison control centers (PCCs) in the US and its territories, which are staffed with specially trained and certified health care professionals who respond to telephone calls for information on specific substances or for advice on poisoning exposure management, 24 h a day, 365 days a year, free of charge in 150 languages. During each telephone call, staff collect and document the following information in a structured database: call time, caller location, exposure site, demographic characteristics of the person who may have experienced poisoning exposure (e.g., age, gender), substance(s) or product(s) involved, route of exposure, reason for exposure (e.g., unintentional, intentional misuse or abuse, suicide or suspected suicide), clinical effect (i.e., symptoms), health care facility level of care, and medical outcome. The information from all telephone calls is uploaded to NPDS in 8.2 min on average, becoming a near-real-time national poisoning exposure surveillance system [15]. NPDS contains exposure information on more than 430,000 products, including e-cigarettes since 2010 and e-liquid since 2011 [15–17].
Case selection criteria
We identified human exposure cases involving e-cigarettes and e-liquids that occurred in the US and its territories between 1 January 2010 and 31 December 2018 using both generic and product codes for e-cigarettes and e-liquid. We excluded cases involving more than one substance other than e-cigarettes or e-liquid to separate the effects due to other substances (n = 441). We also excluded cases that were confirmed non-exposure (n = 46) or for which a clinical effect was determined to be unrelated to an e-cigarette or e-liquid (n = 594).
Study variables
Study variables included call time, caller site, age and gender of the exposed individual, substance involved in exposure, route of exposure, reason for exposure (e.g., unintentional or intentional), clinical effect (i.e., symptoms), health care facility level of care (e.g., admitted to an intensive care unit), and medical outcome. Information on the substance (i.e., e-liquid) involved in the exposure included formulation, quantity unit (documented as mg or mL), quantity, and substance certainty (exact, estimate, and maximum possible). Substance certainty indicates confidence in substance information recorded. PCC staff who answer the telephone calls obtain information from callers regarding formulation, concentration, unit, and quantity. Exact substance certainty is preferred when available, generally indicating information on the product labels. When information on substance (e.g., nicotine) concentration and volume is available, PCC staff may calculate substance quantity in mg; otherwise, they document substance volume in mL. If the exact quantity cannot be determined, the best possible estimate or maximum quantity possible is recorded. Medical outcome was classified as no effect (no signs or symptoms as a result of the exposure); minor effect, (some signs or symptoms, but minimally bothersome and self-limited without residual disability or disfigurement); moderate effect (signs or symptoms that were more pronounced, more prolonged, usually required medical attention, but not life-threatening); major effect (signs or symptoms that were life-threatening or resulted in significant residual disability or disfigurement); and death (died as a result of the exposure).
Data analysis
We computed the frequency of e-cigarette exposure cases by year, age, and other key characteristics such as medical outcome and clinical effects. For analyses on substance(i.e., e-liquid) quantity involved in the exposures, data were restricted to liquid formulation cases with exact substance quantity and known medical outcomes (including no effect, minor, moderate, and major effect, or death) to provide data with a high level of certainty (n = 289). We analyzed e-liquid and nicotine quantity data for cases with substance quantity unit of mL (n = 64) and mg (n = 18) separately. We conducted data analysis using SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA).
This study was determined as exempt by the US Food and Drug Administration (FDA) Institutional Review Board for Human Subject Protection because data were previously collected and did not contain personally identifiable information.
Results
Trends
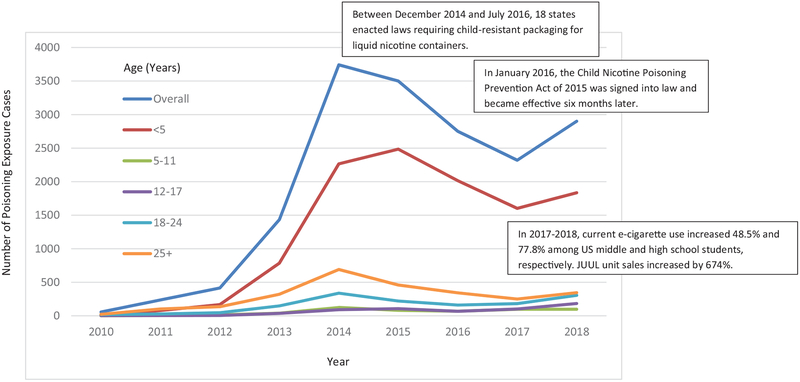
A total of 17,358 e-cigarette exposure cases were identified between 1 January 2010 and 31 December 2018. The overall annual number of e-cigarette exposure cases increased from 57 in 2010 to 2901 in 2018, a 4990% increase. The total number of cases reached a peak of 3742 in 2014 followed by annual declines in 2015–2017, and then a 25% increase in 2017–2018. We observed the largest one-year increase in absolute number of e-cigarette exposure cases among children younger than five years old in 2017–2018; the largest one-year percentage increase in e-cigarette exposure cases was among adolescents and young adults in 2017–2018. Among children younger than age five, the annual number of e-cigarette exposure cases increased from 13 in 2010 to 1835 in 2018, a 14,015.4% increase. The number of cases reached a peak of 2485 in 2015 followed by decreases in 2016 and 2017, and then a 14.5% increase from 2017 to 2018. We observed a similar trend in other age groups with the greatest one-year (2017–2018) percentage increase in e-cigarette exposure cases among adolescents aged 12–17 years (82.2%) and young adults aged 18–24 years(69.1%) (Figure 1).
Figure 1.
Trends in poisoning exposure cases involving e-cigarettes and e-liquid overall and by age, United States, 2010–2018.
Characteristics
Table 1 shows the characteristics of e-cigarette exposure cases. During 2010–2018, children younger than age five accounted for 64.8% of all e-cigarette exposure cases while children aged 5–11 years, adolescents aged 12–17 years, and young adults aged 18–24 years accounted for 14.7% of all the e-cigarette exposure cases. There were more male cases (55.5%) than female cases (44.1%). Ingestion was the most common route of exposure (77.5%), followed by dermal exposure (13.0%), inhalation or nasal exposure (10.4%), ocular exposure (7.1%), and other (0.4%) or unknown (0.2%) exposure route. Most telephone calls to PCCs concerning e-cigarette exposures originated from individuals’ residences (76.7%); 15.3% were from health care facilities; and 8.0% were from other locations. More than one quarter (27.4%) of the e-cigarette exposure cases were brought to a health care facility, where they were treated, evaluated and released; 0.8% (n = 130) of the cases were admitted to a noncritical care unit; and 0.6% (n = 99) of the cases were admitted to a critical care unit.
Table 1.
Characteristics of e-cigarette exposure cases overall and by year, United States, 2010–2018.
| Number (%) of e-cigarette exposure cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| Characteristics | Overall (n = 17,358) | (n = 57) | (n = 237) | (n = 415) | (n = 1435) | (n = 3742) | (n = 3500) | (n = 2751) | (n = 2320) | (n = 2901) |
| Age (Year) | ||||||||||
| < 5 | 11,250 (64.8) | 13 (22.8) | 79 (33.3) | 168 (40.5) | 786 (54.8) | 2266 (60.6) | 2485 (71.0) | 2015 (73.3) | 1602 (69.1) | 1835 (63.3) |
| 5–11 | 525 (3.0) | 1 (1.8) | 4 (1.7) | 14 (3.4) | 38 (2.7) | 124 (3.3) | 81 (2.3) | 68 (2.5) | 98 (4.2) | 97 (3.3) |
| 12–17 | 596 (3.4) | 2 (3.5) | 4 (1.7) | 5 (1.2) | 36 (2.5) | 91 (2.4) | 106 (3.0) | 67 (2.4) | 101 (4.4) | 184 (6.3) |
| 18–24 | 1,443 (8.3) | 17 (29.8) | 27 (11.4) | 46 (11.1) | 148 (10.3) | 337 (9.0) | 221 (6.3) | 160 (5.8) | 181 (7.8) | 306 (10.6) |
| 25+ | 2667 (15.4) | 22 (38.6) | 102 (43.0) | 135 (32.5) | 321 (22.4) | 691 (18.5) | 459 (13.1) | 342 (12.4) | 250 (10.8) | 345 (11.9) |
| Missing | 877 (5.1) | 2 (3.5) | 21 (8.9) | 47 (11.3) | 106 (7.4) | 233 (6.2) | 148 (4.2) | 99 (3.6) | 87 (3.8) | 134 (4.6) |
| Gender | ||||||||||
| Female | 7648 (44.1) | 23 (40.4) | 124 (52.3) | 201 (48.4) | 654 (45.6) | 1691 (45.2) | 1536 (43.9) | 1202 (43.7) | 984 (42.4) | 1233 (42.5) |
| Male | 9631 (55.5) | 34 (59.7) | 113 (47.7) | 213 (51.3) | 771 (53.7) | 2034 (54.4) | 1957 (55.9) | 1534 (55.8) | 1319 (56.9) | 1656 (57.1) |
| Unknown | 79 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 10 (0.7) | 17 (0.5) | 7 (0.2) | 15 (0.6) | 17 (0.7) | 12 (0.4) |
| Route of Exposurea | ||||||||||
| Ingestion | 13,456 (77.5) | 32 (56.1) | 162 (68.4) | 250 (60.2) | 1041 (72.5) | 2797 (74.8) | 2834 (81.0) | 2252 (81.9) | 1866 (80.4) | 2223 (76.6) |
| Dermal | 2258 (13.0) | 5 (8.8) | 20 (8.4) | 34 (8.2) | 162 (11.3) | 555 (14.8) | 436 (12.5) | 395 (14.4) | 285 (12.3) | 365 (12.6) |
| Inhalation/Nasal | 1807 (10.4) | 20 (35.1) | 45 (19.0) | 117 (28.2) | 195 (13.6) | 326 (8.7) | 223 (6.4) | 200 (7.3) | 246 (10.6) | 435 (15.0) |
| Ocular | 1232 (7.1) | 2 (3.5) | 22 (9.3) | 34 (8.2) | 129 (9.0) | 389 (10.4) | 261 (7.5) | 162 (5.9) | 120 (5.2) | 113 (3.9) |
| Other | 60 (0.4) | 0 (0.0) | 2 (0.8) | 2 (0.5) | 3 (0.2) | 14 (0.4) | 14 (0.4) | 7 (0.3) | 4 (0.2) | 14 (0.5) |
| Unknown | 31 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 2 (0.1) | 9 (0.2) | 5 (0.1) | 3 (0.1) | 4 (0.2) | 7 (0.2) |
| Caller Site | ||||||||||
| Health Care Facility (HCF) | 2649 (15.3) | 6 (10.5) | 22 (9.3) | 51 (12.3) | 181 (12.6) | 561 (15.0) | 623 (17.8) | 452 (16.4) | 380 (16.4) | 373 (12.9) |
| Residence | 13,319 (76.7) | 44 (77.2) | 199 (84.0) | 341 (82.2) | 1146 (79.9) | 2895 (77.4) | 2600 (74.3) | 2091 (76.0) | 1728 (74.5) | 2275 (78.4) |
| School or Workplace | 227 (1.3) | 2 (3.5) | 1 (0.4) | 3 (0.7) | 19 (1.3) | 54 (1.4) | 42 (1.2) | 23 (0.8) | 36 (1.6) | 47 (1.6) |
| Public Area or Restaurant | 72 (0.4) | 0 (0.0) | 1 (0.4) | 2 (0.5) | 4 (0.3) | 12 (0.3) | 17 (0.5) | 9 (0.3) | 12 (0.5) | 15 (0.5) |
| Unknown | 70 (0.4) | 0 (0.0 | 1 (0.4) | 2 (0.5) | 6 (0.4) | 8 (0.2) | 9 (0.3) | 11 (0.4) | 11 (0.5) | 22 (0.8) |
| Other | 1021 (5.9) | 5 (8.8) | 13 (5.5) | 16 (3.9) | 79 (5.5) | 212 (5.7) | 209 (6.0) | 165 (6.0) | 153 (6.6) | 169 (5.8) |
| Exposure Site | ||||||||||
| Health Care Facility | 11 (0.1) | 0 | 0 | 0 | 0 | 3 (0.1) | 5 (0.1) | 0 | 2 (0.1) | 1 (0.0) |
| Residence | 16,567 (95.4) | 52 (91.2) | 230 (97.1) | 399 (96.1) | 1371 (95.5) | 3581 (95.7) | 3374 (96.4) | 2644 (96.1) | 2192 (94.5) | 2724 (93.9) |
| School or Workplace | 332 (1.9) | 2 (3.5) | 1 (0.4) | 4 (1.0) | 28 (2.0) | 73 (2.0) | 56 (1.6) | 28 (1.0) | 63 (2.7) | 77 (2.7) |
| Public area or Restaurant | 121 (0.7) | 0 | 2 (0.8) | 1 (0.2) | 11 (0.8) | 23 (0.6) | 22 (0.6) | 17 (0.6) | 21 (0.9) | 24 (0.8) |
| Unknown | 132 (0.8) | 1 (1.8) | 2 (0.8) | 7 (1.7) | 10 (0.7) | 16 (0.4) | 19 (0.5) | 22 (0.8) | 18 (0.8) | 37 (1.3) |
| Other | 195 (1.1) | 2 (3.5) | 2 (0.8) | 4 (1.0) | 15 (1.1) | 46 (1.2) | 24 (0.7) | 40 (1.5) | 24 (1.0) | 38 (1.3) |
| Level of Care at Health Care Facility | ||||||||||
| Admitted to Critical Care Unit | 99 (0.6) | 0 (0.0) | 0 (0.0) | 6 (1.5) | 8 (0.6) | 24 (0.6) | 13 (0.4) | 20 (0.7) | 15 (0.7) | 13 (0.5) |
| Admitted to Noncritical Care Unit | 130 (0.8) | 0 (0.0) | 0 (0.0) | 2 (0.5) | 9 (0.6) | 31 (0.8) | 36 (1.0) | 21 (0.8) | 13 (0.6) | 18 (0.6) |
| Admitted to Psychiatric Facility | 54 (0.3) | 0 (0.0) | 2 (0.8) | 0 (0.0) | 1 (0.1) | 11 (0.3) | 12 (0.3) | 8 (0.3) | 13 (0.6) | 7 (0.2) |
| Lost to FU or Left AMA1 | 1079 (6.2) | 4 (7.0) | 10 (4.2) | 19 (4.6) | 63 (4.3) | 229 (6.1) | 208 (5.9) | 173 (6.3) | 176 (7.6) | 198 (6.8) |
| Treated, Evaluated, and Released | 4752 (27.4) | 9 (15.8) | 38 (16.0) | 73 (17.6) | 327 (22.8) | 1033 (27.6) | 1144 (32.7) | 819 (29.8) | 647 (27.9) | 662 (22.8) |
| Refused Referral or Did Not Arrive at HCF | 679 (3.9) | 1 (1.8) | 16 (6.8) | 10 (2.4) | 41 (2.9) | 144 (3.9) | 140 (4.0) | 125 (4.5) | 78 (3.4) | 124 (4.3) |
| Not Referred to a HCF | 10,565 (60.9) | 43 (75.4) | 171 (72.2) | 305 (73.5) | 987 (68.8) | 2270 (60.7) | 1947 (55.6) | 1585 (57.6) | 1378 (59.4) | 1879 (17.8) |
| Medical Outcome | ||||||||||
| No effect | 6068 (35.0) | 12 (21.1) | 49 (20.7) | 85 (20.5) | 367 (25.6) | 1235 (33.0) | 1406 (40.2) | 1113 (40.5) | 855 (36.9) | 946 (32.6) |
| Minor effect | 3918 (22.6) | 15 (26.3) | 55 (23.2) | 105 (25.3) | 422 (29.4) | 944 (25.2) | 780 (22.3) | 563 (20.5) | 481 (20.7) | 553 (19.1) |
| Moderate effect | 578 (3.3) | 6 (10.5) | 14 (5.9) | 20 (4.8) | 47 (3.3) | 122 (3.3) | 103 (2.9) | 87 (3.2) | 77 (3.3) | 102 (5.5) |
| Major effect | 24 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 2 (0.1) | 2 (0.1) | 5 (0.1) | 7 (0.3) | 4 (0.2) | 3 (0.1) |
| Death | 2 (0.01) | 0 (0.0) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not followed or unable to follow | 6768 (39.0) | 24 (42.1) | 119 (50.2) | 203 (48.9) | 597 (41.6) | 1438 (38.4) | 1206 (34.5) | 981 (35.7) | 903 (38.9) | 1297 (44.7) |
FU: Follow-up; AMA: Against medical advice
Each case could have more than one clinical effect. Percentage total would not sum to 100%.
More than one-third (35.0%) of individuals who experienced poisoning exposures involving e-cigarettes did not have any symptoms (i.e., no effect), 22.6% developed minimally bothersome and self-limiting symptoms (i.e., minor effect), 3.3% exhibited more pronounced and prolonged symptoms (i.e., moderate effect), and 0.1% had life-threatening symptoms (i.e., major effect). There were two fatality cases in 2012 and 2014, respectively. These fatality cases have been previously reported [18,19].
Table 2 shows the most commonly reported clinical effects due to exposure to e-cigarettes or e-liquid by age group. Overall, the most commonly reported clinical effects were vomiting (n = 2297), nausea (n = 1070), and ocular irritation or pain (n = 1022). Among children younger than age five, the most commonly-reported clinical effects were vomiting (n = 1665), coughing or choking (n = 362), and drowsiness or lethargy (n = 200).
Table 2.
Top five most commonly-reported clinical effects for e-cigarette exposure cases by age, United States, 2010–2018.
| Clinical effectsa (top five) | Number (% of group total) |
|---|---|
| Overall | |
| Vomiting | 2297 (25.4) |
| Nausea | 1070 (11.8) |
| Ocular irritation/pain | 1022 (11.3) |
| Red eye conjunctivitis | 494 (5.5) |
| Dizziness/vertigo | 463 (5.1) |
| < 5 Years | |
| Vomiting | 1665 (47.1) |
| Cough/choke | 362 (10.2) |
| Drowsiness/lethargy | 200 (5.7) |
| Nausea | 195 (5.5) |
| Ocular irritation/pain | 159 (4.5) |
| 5–11 years | |
| Vomiting | 58 (24.6) |
| Nausea | 38 (16.1) |
| Abdominal pain | 21 (8.9) |
| Ocular irritation/pain | 18 (7.6) |
| Dizziness/vertigo | 12 (5.1) |
| 12–17 years | |
| Nausea | 134 (20.4) |
| Vomiting | 115 (17.5) |
| Dizziness/vertigo | 63 (9.6) |
| Tachycardia | 43 (6.6) |
| Headache | 42 (6.4) |
| 18–24 years | |
| Nausea | 269 (18.7) |
| Ocular irritation/pain | 209 (14.5) |
| Vomiting | 182 (12.7) |
| Dizziness vertigo | 118 (8.2) |
| Red eye conjunctivitis | 83 (5.8) |
| 25+ years | |
| Ocular irritation/pain | 467 (18.2) |
| Red eye conjunctivitis | 348 (13.6) |
| Nausea | 223 (8.7) |
| Vomiting | 219 (8.6) |
| Dizziness/vertigo | 189 (7.4) |
One case could have more than one clinical effect.
Quantity of e-liquid and nicotine involved in exposure by medical outcome
Table 3 shows quantity of e-liquid and nicotine involved in the exposure by medical outcome for e-cigarette exposure cases. Cases with more serious medical outcomes tended to be exposed to a higher e-liquid and nicotine quantity.
Table 3.
Quantity of e-liquid and nicotine involved in exposure by medical outcome, United States, 2010–2018.
| Substance Quantity | Medical outcomea | ||
|---|---|---|---|
| No effect | Minor effect | Moderate effect | |
| Substance Quantity in mL | (n = 37) | (n = 22) | (n = 5) |
| Mean | 7.5 | 13.1 | 56.2 |
| Median | 2.0 | 3.0 | 30.0 |
| Minimum | 0.2 | 0.6 | 1.0 |
| Maximum | 60.0 | 60.0 | 200.0 |
| Substance quantity in mg | (n = 11) | (n = 7) | (n = 0) |
| Mean | 19.3 | 49.7 | — |
| Median | 12.0 | 18.0 | — |
| Minimum | 3.0 | 6.0 | — |
| Maximum | 96.0 | 240.0 | — |
Includes e-cigarette exposure cases with information on medical outcome and exact e-liquid and nicotine quantity.
Discussion
Our study provides updated information on the overall burden of e-cigarette exposure cases and description of trends and characteristics of these cases in all age groups of the general US population. For e-liquid exposure cases, the study also examines e-liquid and nicotine quantity of exposures by medical outcome, which has not been addressed in previous studies.
After several years of increase since 2010, the overall number of e-cigarette exposure cases began to decrease in 2015–2016 and continued to decrease in 2017, as did the number of e-cigarette exposure cases among young children. The decrease in e-cigarette exposure cases in recent years has been reported previously [13,14], and the decrease among young children was considered to be partially attributable to laws requiring child-resistant packaging for liquid nicotine containers and greater public awareness of risks associated with e-cigarettes [14]. The downward trend we observed coincided with two major activities. First, between December 2014 and July 2016, 18 US states enacted laws requiring child-resistant packaging for liquid nicotine containers [14,20]. Second, in January 2016, the Child Nicotine Poisoning Prevention Act of 2015 was signed into law and became effective six months later [21]. This federal law requires child-resistant packaging for liquid nicotine containers that are sold or distributed in the US. The spontaneous reporting of poisoning exposures involving e-cigarettes could have also been influenced by several other factors, such as awareness level and media coverage of e-cigarette-related poisoning and injuries.
The sharp increase in e-cigarette exposure cases between 2017 and 2018, particularly among adolescents and young adults, was unexpected. Although the reason for the increase is unclear, the 82.2% upsurge in e-cigarette exposure cases among adolescents in 2017–2018 occurred during the same time when current e-cigarette use increased 48.5% and 77.8% among US middle and high school students, respectively [1]. Additionally, the dramatic increase in e-cigarette exposure cases in 2017–2018 is consistent with a skyrocketing 674% increase in JUUL unit sales in 2017–2018 [22].
We found that approximately two-thirds of e-cigarette exposure cases occurred among children younger than age five. This finding is consistent with a previous report [10]. Unlike poisoning events involving cigarettes, more than 40% of e-cigarette poisoning events occurred among individuals aged 11–19 years and adults aged 20 years or older; in contrast, only 4.5% of cigarette-related poisoning events occurred among individuals in these age groups [10]. Although child-resistant packaging laws have been enacted to protect young children from unintentional exposure to nicotine, additional measures may be useful to prevent poisoning events involving e-cigarettes among both young children and individuals in other age groups such as education programs to address potential risk of poisoning events and proper storage and disposal. In May 2016, FDA issued a final rule to deem all products that meet the statutory definition of a tobacco product to be subject to FDA’s tobacco product authority [23]. Under the deeming rule, FDA expanded its authority to regulate the manufacture, marketing, and distribution of all tobacco products, including e-cigarettes [23]. In March 2019, FDA announced new plans aimed at preventing youth access to, and appeal of, flavored tobacco products [24]. Although these plans are aimed at preventing nicotine addiction among youth, they may also help reduce poisoning events involving e-cigarettes, particularly poisoning events associated with emerging products with a high nicotine concentration.
A study analyzed retail sales data from US stores in 2015, excluding vape shops, tobacco specialty, and online sources, suggested that 99% of e-cigarettes sold in the US in 2015 contained nicotine [9]; some emerging e-cigarette products have a high nicotine concentration [25]. However, many (63%) young current users of products with a high nicotine concentration are not aware that the products they use contain nicotine [26]. Adolescents’ use of these products poses a risk of not only nicotine addiction and potential harm to brain development [27], but also nicotine poisoning and other adverse health consequences. Findings from our study suggest that e-cigarette exposure cases with more serious medical outcomes tended to be exposed to higher quantities of e-liquid and nicotine. Although most e-cigarette exposure cases documented in NPDS did not result in serious medical outcomes, approximately one-third of the cases were treated at a health care facility and hundreds were admitted to a critical or noncritical care unit. Improved reporting of nicotine concentration and quantity to NPDS could improve our understanding of acute nicotine-induced harm and inform appropriate e-cigarette product health warnings and other regulatory actions.
Our study has some limitations. First, NPDS data is a passive surveillance system relying on spontaneous voluntary reporting of poisoning exposure cases. Therefore, the number of e-cigarette exposure cases observed in our study may not represent all the cases that occurred. Future research may estimate the magnitude of underreporting so that NPDS data could better inform regulatory activities. Second, information in NPDS is subject to reporting bias because the information is largely based on self-report, with only 14.7% of human e-cigarette exposure cases reported from health care facilities [10]. However, a unique feature of NPDS is the use of follow-up contacts to monitor case progress, obtain additional information, and determine the medical outcome of the cases. For example, follow-up was performed in nearly half (47.0%) of human exposure cases in 2017 [15]. Third, lack of denominator data (i.e., the total population exposed to e-cigarettes) for the e-cigarette exposures limited our ability to estimate prevalence or rate of poisoning exposure events involving e-cigarettes. Previous studies used US census data as a proxy of denominator to calculate e-cigarette poisoning exposure rates among young children [12,14]. These estimates are likely to be underestimated because the census data in general population do not take into account for children at risk of e-cigarette exposure (i.e., children living in households of e-cigarette users) and result in overestimation of denominator. Fourth, the small number of cases with information on substance quantity limits generalizability of substance quantity in e-cigarette exposure cases. However, we restricted our analysis of substance quantity to the data with a high level of certainty to provide the most reliable information. It is possible that substance quantity in mg is underreported due to lack of or insufficient information, such as nicotine concentration. Finally, our data may underrepresent e-cigarette exposure cases with serious medical outcomes because these serious cases may have been immediately brought to hospital emergency departments with no prior or subsequent call to PCCs.
Conclusions
Our study provides updated information on trends and characteristics of poisoning exposure cases involving e-cigarettes documented in a national surveillance system that covers the US and its territories in near-real-time, with information collected by specially trained and certified professionals who are supervised by physicians or medical toxicologists. The rapid changes in the occurrence of poisoning exposure events involving e-cigarettes coupled with the development of new tobacco products and an ever-evolving tobacco use landscape underscore the importance of continued surveillance of poisoning exposure cases. While poisoning exposure events involving e-cigarettes affected young children disproportionally, adolescents were also at a risk of poisoning exposures to e-cigarettes due to increased prevalence of e-cigarette use that results in a greater potential for poisoning exposure. It is of public health concern that thousands of poisoning events involving e-cigarettes occur each year, mostly among young children. Some of these events have serious medical outcomes. Educating the public about the potential dangers of e-cigarettes and promoting preventive measures to protect individuals, particularly young children and adolescents, from e-cigarette poisoning exposure may be useful to prevent e-cigarette-related poisoning events.
Acknowledgements
The authors would like to thank Deborah Neveleff for assistance with technical editing. The author would also like to thank Drs. Benjamin Apelberg, Robin Toblin, Bridget Ambrose, and Cindy Chang for their constructive comments.
Footnotes
Disclosure statement
The authors have no conflict of interest to report.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: This publication represents the views of the authors and does not represent FDA/CTP position or policy.
Reference
- [1].Cullen KA, Ambrose BK, Gentzke AS, et al. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students - United States, 2011–2018. MMWR Morb Mortal Wkly Rep. 2018;67(45):1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gentzke AS, Creamer M, Cullen KA, et al. Vital signs: tobacco product use among middle and high school students - United States, 2011–2018. MMWR Morb Mortal Wkly Rep. 2019;68(6): 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bao W, Xu G, Lu J, et al. Changes in electronic cigarette use among adults in the United States, 2014–2016.JAMA. 2018; 319(19):2039–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang TW, Asman K, Gentzke AS, et al. Tobacco product use among adults - United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(44):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Food and Drug Administration, HHS. Deeming Tobacco Products To Be Subject to the Federal Food, Drug, and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act; Restrictions on the Sale and Distribution of Tobacco Products and Required Warning Statements for Tobacco Products. Final rule. Fed Regist. 2016;81(90):28973–29106. [PubMed] [Google Scholar]
- [6].Walley SC, Wilson KM, Winickoff JP, et al. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics. 2019;143(6): e20182741. [DOI] [PubMed] [Google Scholar]
- [7].JUUL. 2019. Available from: https://www.juul.com/. Accessed July 1, 2019.
- [8].Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control. 2019;28:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marynak KL, Gammon DG, Rogers T, et al. Sales of nicotine-containing electronic cigarette products: United States, 2015. Am J Public Health. 2017;107(5):702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chatham-Stephens K, Law R, Taylor E, et al. Exposure calls to U.S. poison centers involving electronic cigarettes and conventional cigarettes - September 2010–December 2014. J Med Toxicol. 2016;12(4):350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vakkalanka JP, Hardison LS Jr, Holstege CP. Epidemiological trends in electronic cigarette exposures reported to U.S. Poison Centers. Clin Toxicol. 2014;52(5):542–548. [DOI] [PubMed] [Google Scholar]
- [12].Kamboj A, Spiller HA, Casavant MJ, et al. Pediatric exposure to e-cigarettes, nicotine, and tobacco products in the United States. Pediatrics. 2016;137(6):e20160041. [DOI] [PubMed] [Google Scholar]
- [13].Wang B, Rostron B. Tobacco-related poison events involving young children in the US, 2001 – 2016. Tob Reg Sci.. 2017;3(4): 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Govindarajan P, Spiller HA, Casavant MJ, et al. E-cigarette and liquid nicotine exposures among young children. Pediatrics. 2018; 141(5):e20173361. [DOI] [PubMed] [Google Scholar]
- [15].Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th annual report. Clin Toxicol. 2018;56(12):1213–1415. [DOI] [PubMed] [Google Scholar]
- [16].Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2010 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th annual report. Clin Toxicol. 2011;49(10):910–941. [DOI] [PubMed] [Google Scholar]
- [17].Bronstein AC, Spyker DA, Cantilena LR Jr, et al. 2011 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 29th annual report. Clin Toxicol. 2012;50(10):911–1164. [DOI] [PubMed] [Google Scholar]
- [18].Mowry JB, Spyker DA, Cantilena LR Jr, et al. 2012 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th annual report. Clin Toxicol. 2013;51(10):949–1229. [DOI] [PubMed] [Google Scholar]
- [19].Mowry JB, Spyker DA, Brooks DE, et al. 2014 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd annual report. Clin Toxicol. 2015; 53(10):962–1147. [DOI] [PubMed] [Google Scholar]
- [20].Frey LT, Tilburg WC. Child-resistant packaging for e-liquid: a review of US State legislation. Am J Public Health. 2016;106(2): 266–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Congress. Child Nicotine Poisoning Prevention Act of 2015. Public Law 114–116. 2015. Available from: https://www.congress.gov/bill/114th-congress/senate-bill/142. Accessed December 31, 2018. [Google Scholar]
- [22].Ericksen A New Year for Vapor. Convenience Store Decisions. January 31, 2019. Available from: https://cstoredecisions.com/2019/01/31/new-year-for-vapor/. Accessed March 5, 2019. [Google Scholar]
- [23].FDA. Extension of certain tobacco product compliance deadlines related to the final deeming rule: guidance for industry. 2017. [Google Scholar]
- [24].FDA. Statement from FDA Commissioner Scott Gottlieb, M.D., on advancing new policies aimed at preventing youth access to, and appeal of, flavored tobacco products, including e-cigarettes and cigars. 2019. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm633291.htm. Accessed March 20, 2019, 2019.
- [25].Goniewicz ML, Boykan R, Messina CR, et al. High exposure to nicotine among adolescents who use Juul and other vape pod systems (‘pods’). Tob Control. 2018. September 7 pii: tobaccocontrol-2018–054565. [Epub ahead of print]. doi: 10.1136/tobaccocontrol-2018-054565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Willett JG, Bennett M, Hair EC, et al. Recognition, use and perceptions of JUUL among youth and young adults. Tob Control. 2019; 28:115–116. [DOI] [PubMed] [Google Scholar]
- [27].Yuan M, Cross SJ, Loughlin SE, et al. Nicotine and the adolescent brain. J Physiol.. 2015;593(16):3397–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]