Abstract
Brain stimulation techniques, including transcranial direct current stimulation (tDCS), were identified as promising therapeutic tools to modulate synaptic plasticity abnormalities and minimize memory and learning deficits in many neuropsychiatric diseases. Here, we revised the effect of tDCS on the modulation of neuroplasticity and cognition in several animal disease models of brain diseases affecting plasticity and cognition. Studies included in this review were searched following the terms (“transcranial direct current stimulation”) AND (mice OR mouse OR animal) and according to the PRISMA statement requirements. Overall, the studies collected suggest that tDCS was able to modulate brain plasticity due to synaptic modifications within the stimulated area. Changes in plasticity-related mechanisms were achieved through induction of long-term potentiation (LTP) and upregulation of neuroplasticity-related proteins, such as c-fos, brain-derived neurotrophic factor (BDNF), or N-methyl-D-aspartate receptors (NMDARs). Taken into account all revised studies, tDCS is a safe, easy, and noninvasive brain stimulation technique, therapeutically reliable, and with promising potential to promote cognitive enhancement and neuroplasticity. Since the use of tDCS has increased as a novel therapeutic approach in humans, animal studies are important to better understand its mechanisms as well as to help improve the stimulation protocols and their potential role in different neuropathologies.
1. Introduction
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that promotes transient polarity-dependent changes in spontaneous neuronal activity. This effect is mediated by the application of constant low-amplitude electrical currents using epicranially positioned electrodes above a specific brain region of interest [1–4]. The therapeutic use of low-amplitude electrical currents has a long historical track. Accordingly, both Greeks and Romans used electric torpedo fishes for migraine treatment, and in the 11th century, a similar therapeutic procedure was attempted to handle epilepsy [5]. In the 19th century, the application of galvanic currents was attempted to heal melancholia [6]. Over the years, the scientific community interest in brain stimulation grew, and several noninvasive brain stimulation techniques were developed such as tDCS, deep brain stimulation, or transcranial magnetic stimulation. The epicranial application of direct currents promotes a weak electric field force and produces neuronal membrane potential changes [7, 8]. These alterations occur through sodium and calcium currents [1] modulating spontaneous neuronal activity [2]. The consequent regional neuronal inhibition or excitation depends on the applied current polarity [9, 10]. So, it was overall observed that cathodal currents produce inhibitory effects, and thus hyperpolarization, whereas anodal currents increase excitability in the form of depolarization [2, 11] (Figure 1).
Figure 1.
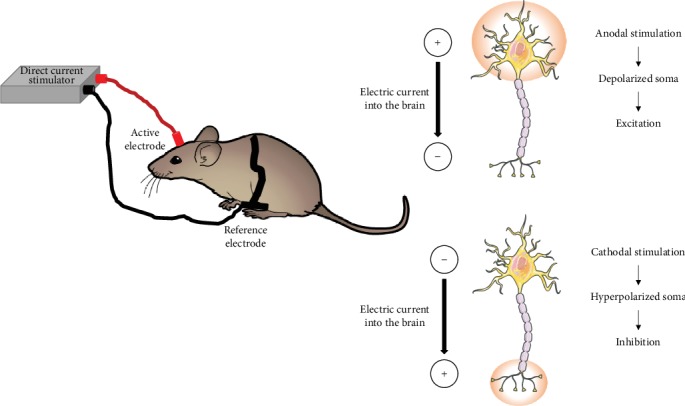
Illustration of transcranial direct current stimulation in the mice. Anodal stimulation depolarizes the neuronal membrane and enhances excitability. On the other hand, cathodal stimulation hyperpolarizes the neuronal membrane and decreases excitability.
There is nowadays an ongoing discussion regarding the factors that interfere with tDCS outcomes. The initial brain resting state of each subject [12], his/her baseline performance [13], specific individual variations in brain tissue morphology [14], or even more particular details from the experimental design or stimulation protocol used [15] influence these outcomes. In vivo and in vitro studies are consensual to demonstrate that tDCS-modulated cortical excitability depends on several stimulation parameters, such as duration and frequency of stimulation [16]; polarity, intensity, and density of the applied current [17, 18]; and electrode size and position in the scalp [18–20]. Despite that, beneficial effects of tDCS in several brain disorders, such as PD [21, 22], depression [23], stroke [24, 25], or autism [26, 27], have been documented, and there is growing evidence proposing tDCS application in multiple other disease conditions affecting cognition and neuroplasticity mechanisms.
Both preclinical and clinical studies have demonstrated therapeutic effects of tDCS. Indeed, in human studies, anodal tDCS applied intermittently in the prefrontal cortex (PFC) during slow-wave sleep period, improved recall of declarative memories (word pairs). The authors correlated these findings with enhancement of slow oscillatory electroencephalogram (EEG) activity (<3 Hz, delta (δ) waves), responsible for neuronal plasticity facilitation [28]. Also, anodal tDCS over dorsolateral prefrontal cortex (DLPFC) improved working memory in PD patients and in major depression patients by boosting cortical excitability [21, 23]. Accordingly, preclinical animal studies reported that cortical anodal tDCS improved spatial memory in both wild type (WT) [29] and the AD rat model [30]. Beneficial effects were also found during the early stage of traumatic brain injury (TBI) [31] and following a pilocarpine-induced status epilepticus in normal rats [32]. Moreover, improvements were also reported concerning short-term memory in an animal model of attention deficit hyperactivity disorder (ADHD) [33].
The molecular mechanisms underlying the tDCS-mediated cognitive improvements and neuroplasticity processes have become the focus of recent interest. Accordingly, tDCS modulation over several cognition-related plasticity genes and their signaling pathways has been studied. In this review, we provide a state of the art on the application of different protocols of tDCS in animal models highlighting its effectiveness on neuroplasticity mechanisms and, consequently, their related learning and memory processes. Since the published systematic reviews focused on human application of tDCS, here, we provide a comprehensive revision of the effect of tDCS in in vivo rodent models of normal and pathological brain functioning.
2. Methods
2.1. Data Sources and Search
Studies included in this review were identified by searching PubMed. The search was run until 31 October 2019. The search terms were (“transcranial direct current stimulation”) AND (mice OR mouse OR animal). Articles were firstly assessed based on their abstracts and titles, aiming to include studies that reported applying tDCS to cognitive impairment in animal models. Simultaneously, the following exclusion criteria were adopted to reject studies: (1) not written in English; (2) performing reviews; (3) in human subjects; (4) in vitro models; (5) employing other brain stimulation techniques (e.g., transcranial magnetic stimulation (TMS), deep brain stimulation (DBS), or transcranial alternating current stimulation (tACS)); and (6) not explicitly describing the tDCS protocol (stimulation area, number of sessions, frequency, intensity, and pattern).
2.2. Data Extraction
A data extraction sheet was developed seeking to retrieve relevant information from each study, namely, study design, sample size, animal model, whether additional therapy was performed, details of the tDCS protocol, outcome measures, and behavioral results.
2.3. Study Selection
The database search was elaborated according to the PRISMA statement requirements [34]. 404 records were found, which underwent a preliminary screening (of titles and abstracts), with 314 records being excluded because they did not meet the eligibility criteria. After the full-text analysis of each of the 90 individual articles, 44 rodent studies focusing on tDCS effects over cognition and neuroplasticity in both healthy and neuropathological animal models were selected (Figure 2).
Figure 2.
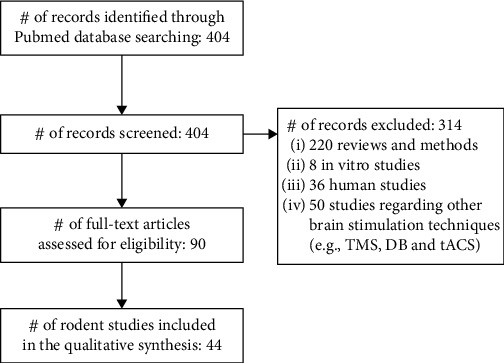
Search flow diagram (in accordance with PRISMA statement). Abbreviations: DB: deep brain stimulation; tACS: transcranial alternating current stimulation; TMS: transcranial magnetic stimulation.
3. Results
3.1. Role of Anodal tDCS in Cognition Processing in Healthy Animals
In healthy animals, studies demonstrated memory improvement in association with induction of synaptic plasticity mechanisms. In fact, tDCS to prefrontal cortex improved monkey's performance on an associative learning task by altering low-frequency oscillations and functional connectivity, both locally and between distant brain areas [35]. Regarding rodent models, data are controversial regarding fear condition. Right frontal anodal tDCS administered 24 h before behavioral task did not alter contextual and auditory learning and memory [36]. Additionally, another study described that while the anodal stimulation did not affect fear retrieval, posttraining cathodal stimulation improved fear memory retrieval [37, 38]. However, left prefrontal anodal and cathodal tDCS impaired the acquisition of both contextual and cued fear memory, which could be explained by activity modulation of deep structures such as the amygdala and hippocampus [39].
Concerning learning and memory, de Souza Custódio and colleagues [29] reported better spatial working memory performance following administration of anodal currents to the medial prefrontal cortex (mPFC). In agreement, it was described that administration of hippocampal anodal tDCS improves learning and memory in the Morris water maze and novel object recognition tests [40]. Moreover, memory performance in the passive avoidance learning task was enhanced by anodal stimulation [41]. Also, cortical cathodal stimulation together with visuospatial memory training led to cognitive improvement [42].
The revised in vivo animal model studies regarding tDCS effects in memory and cognition of healthy animals are listed below in Table 1.
Table 1.
Effect of transcranial direct current stimulation on memory and learning of healthy animals.
| Author | Year | Animal model | Specimen; gender | N | Stimulation parameters | Main findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation electrode | Reference electrode | Anesthesia | rtDCS | ||||||||||||
| Polarity | Position | Stimulation intensity (mA) | Size (m2) | Stimulation duration (min) | Current density (A/m2) | Position | Area (cm2) | ||||||||
| Dockery et al. [42] | 2011 | NDM | Long-Evans rats; males | 41 | a-tDCS vs. c-tDCS | Frontal cortex (left or right hemisphere) | 0.2 | 0.035 | 30 | 57.1 | Back | 10.5 | N | Y (3 days) | ↑ Visuospatial working memory (c-tDCS) |
|
| |||||||||||||||
| de Souza Custódio et al. [29] | 2013 | NDM | Wistar rats; males | 23 | a-tDCS | Left mPFC | 0.4 | 0.25 | 11 | N/A | Neck | 1 | N | Y (5 days) | ↑ Spatial working memory (1 h, 4 h, and 10 h poststimulation) |
|
| |||||||||||||||
| Faraji et al. [96] | 2013 | NDM | Long-Evans rats; males | 24 | a-tDCS | Somatosensory cortex (bilateral) | 0.065 | N/A | 10 | N/A | Back of skull | N/A | N | Y | ↑ Cortical neural density ↑ Motor learning (a-tDCS applied bilaterally or into paw preferred to reaching contralateral hemisphere) |
|
| |||||||||||||||
| Podda et al. [40] | 2016 | NDM | C57BL/6 mice; males | 16 | a-tDCS vs. c-tDCS | Left parietal cortex (dorsal to hippocampal formation) | 0.35 | 0.06 | 20 | N/A | Ventral thorax | 5.2 | N | N (single session) | ↑ Spatial learning and memory (a-tDCS; benefits observable one week after) ↑ BDNF expressions in the hippocampus CREB/CBP pathway activation |
|
| |||||||||||||||
| Manteghi et al. [36] | 2017 | NDM | NMRI mice; males | 64 | a-tDCS | Right frontal cortex | 0.2 | 0.04 | 20 | N/A | Chest | 9.5 | N/A | N (single session) | ↓ Freezing time % and ↑ latency to the freezing (tDCS following 0.1 mg/kg ACPA injection) |
|
| |||||||||||||||
| Nasehi et al. [37] | 2017 | NDM | NMRI mice; males | 128 | a-tDCS vs. c-tDCS | Right frontal cortex | 0.2 | 0.04 | 20 | N/A | Ventral thorax | 9.5 | N | Y (2 sessions) | ↑ Fear memory retrieval/freezing time (a-tDCS and propranolol injection before conditioning) |
|
| |||||||||||||||
| Nasehi et al. [38] | 2017 | NDM | NMRI mice; males | 128 | a-tDCS vs. c-tDCS | Left frontal cortex | 0.2 | 0.04 | 20 | N/A | Ventral thorax | 9.5 | N | Y (2 sessions) | ↑ Contextual fear memory acquisition (a-tDCS before pre- or posttraining) |
|
| |||||||||||||||
| Abbasi et al. [39] | 2017 | NDM | NMRI mice; males | 41 | a-tDCS vs. c-tDCS | Left PFC | 0.2 | 0.04 | 20 or 30 | N/A | Chest | 9.5 | N | N (single session) | ↓ Contextual and cued fear memory |
|
| |||||||||||||||
| Martins et al. [97] | 2019 | NDM | Male Wistar rats; males | 50 | a-tDCS | Left mPFC | 0.4 | N/A | 13 | N/A | N/A | N/A | N/A | Y (5 days) | ↑ Spatial working memory ↑ GAP-43 (extinct by AMPAR antagonist PRP) |
|
| |||||||||||||||
| Yu et al. [41] | 2019 | NDM | Sprague Dawley rats; males | 224 | a-tDCS | SC dorsal to the hippocampus | 0.25 | 0.25 | 30 | N/A | Anterior chest | N/A (EEG electrode) | Y | N (single session) | ↑ Memory (passive avoidance memory retention) ↑ LTP in CA1 hippocampus (blocked by TrkB antagonist) ↑ BDNF in CA1 hippocampus |
Abbreviations: rtDCS: repetitive transcranial direct current stimulation; a-tDCS: anodal transcranial direct current stimulation; c-tDCS: cathodal transcranial direct current stimulation; SC: stereotaxic coordinates; C57BL/6: mouse strain; NMRI: Naval Medical Research Institute outbred mice; NBM: no disease model; SHR: spontaneous hypertensive rats; WKY: Wistar Kyoto rats; PFC: prefrontal cortex; mPFC: medial prefrontal cortex; ITC: inferotemporal cortex; CAI: comu ammonis 1 region in the hippocampus; PRP: perampanel; ACPA: anticitrullinated protein antibody (selective cannabinoid CBI receptor agonist); AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; Trk: tropomyosin receptor kinase receptor; CREB/CBP: cAMP response element binding protein; BDNF: brain-derived neurotrophic factor; GAP-43: growth-associated protein 43; LTP: long-term potentiation; EEG: electroencephalography; A/m2: ampere per square meter; mA: milliampere; cm2: square centimeter; mm: millimeter; h: hour; min: minute; vs.: versus; Y: yes; N: no; N/A: not available.
3.2. Beneficial Role of tDCS in Brain Diseases
Overall, reports using animal models of brain diseases described a beneficial role of tDCS in the mitigation of memory symptoms of neurologic conditions such as Alzheimer's disease (AD) or traumatic brain injury (TBI). More recent studies demonstrated that tDCS rescued AD-related cognitive symptoms, namely, spatial memory and motor skills [30, 43, 44]. The repetitive stimulation with anodal tDCS in the AD-like dementia rat model reduced the time interval animals needed to reach a food pellet and also decreased the number of errors in the attempt [43]. The same research group showed later that the abovementioned protocol rescued spatial learning and memory in a Aβ1-40-lesioned AD rat model [30]. Moreover, the impact of tDCS on cognitive performance of streptozotocin-induced diabetic rats has been evaluated. Both anodal and cathodal stimulations in the prefrontal cortex restored memory impairment [45, 46] together with restoration of LTP [45]. Other authors evaluated the potential therapeutic effects of tDCS in memory impairment in an animal model of ADHD. It was found that this neuromodulation technique was able to improve short- and long-term memory deficits in the spontaneous hypertensive rats (SHR) but not in their control, Wistar Kyoto rats [33, 47]. In addition, no changes were detected in working memory of these control rats following administration of tDCS [47].
Anodal tDCS also ameliorated behavioral and spatial memory function in the early phase after TBI when it was delivered two weeks postinjury. However, earlier stimulation only improved spatial memory [31]. In a later phase of TBI, it was possible to observe motor recovery as well as spatial memory improvement following repeated anodal tDCS [48]. A growing number of studies has been reporting promising effects of neurostimulation in models of addictive disorders, by reducing craving and maladaptive pervasive learning [49]. In fact, repeated anodal stimulation in mouse frontal cortex decreased nicotine-induced conditioned place preference and further improved working memory [50]. Same polarity currents also could prevent cocaine-induced locomotor hyperactivity and place preference conditioning [51]. In addition, it has been reported that cathodal stimulation has an anticonvulsive effect [16, 32, 52–54]. Indeed, the administration of hippocampal tDCS rescued cognitive impairment by reducing hippocampal neural death and supragranular and CA3 mossy fiber sprouting in a lithium-pilocarpine-induced status epilepticus rat [32]. Other neuroplastic effects were evidenced in the reversion of motor symptoms in PD by tDCS administration. The application of anodal currents enhanced graft survival and dopaminergic re-innervation of the surrounding striatal tissue and pronounced behavioral recovery [55].
Despite the fact that many studies reported recovery from memory deficits following tDCS stimulation, there are some opposing reports in animal models of disease affecting cognition. In a recent study from Gondard and collaborators using a triple transgenic (3xTg) mouse model of AD, it was evidenced that a neurostimulation was not able to ameliorate memory symptoms [56]. To reconcile this discrepancy, previous authors have suggested the importance of choosing an optimal current intensity in order to modulate cortical excitability since LTP alterations were dependent on current intensity [57].
The reports regarding tDCS effects in cognition and memory in animal models of brain disease are listed in Tables 2 and 3.
Table 2.
Impact of transcranial direct current stimulation on memory and learning in animal models of brain disorders.
| Author | Year | Animal model | Specimen; gender | N | Stimulation parameters | Main findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation electrode | Reference electrode | Anesthesia | rtDCS | ||||||||||||
| Polarity | Position | Stimulation intensity (mA) | Size (cm2) | Stimulation duration (min) | Current density (A/m2) | Position | Area (cm2) | ||||||||
| Kamida et al. [32] | 2011 | Lithium-pilocarpine hydrochloride (60 mg/kg) SC injection at P20-21 | Wistar rats; males | 18 | c-tDCS | Hippocampus | 0.2 | 0.035 | 30 | N/A | Back of neck | N/A | N | Y (daily for 2 weeks) | ↓ SE-induced hippocampal cell loss in CA3 region |
|
| |||||||||||||||
| Yu et al. [43] | 2014 | Scopolamine IP injection | Sprague Dawley rats; both genders | 16 | N/A | Parietal cortex (hippocampus) | 0.1 | N/A | 20 | N/A | N/A | N/A | N/A | Y (2x/day during 5 days a week, for 4 weeks) | ↓ Time and ↓ number of errors to reach food pellets Significant differences in ACh content (day 14 and day 28) ↑ Motor function |
|
| |||||||||||||||
| Pedron et al. [49] | 2014 | Nicotine IP injection (1 mh/kg 2x/day for 14 days) | Swiss mice; females | 152 | a-tDCS | Left frontal cortex | 0.2 | 0.035 | 2 × 20 | N/A | Ventral thorax | 9.5 | N | Y (5 days) | ↑ Working memory |
| ↑ Nicotine-induced place preference conditioning (3 weeks poststimulation) | |||||||||||||||
|
| |||||||||||||||
| Yu et al. [30] | 2015 | Bilateral hippocampus Aβ1-40 injection | Sprague Dawley rats; females | 36 | a-tDCS | Right frontal cortex | 0.02; 0.06; 0.1; 0.2 | 0.0314 | 20 | N/A | Ventral thorax | 10 | N | Y (10 sessions in 2 weeks) | ↑ Spatial learning performance (best with 0.1 mA and 0.2 mA stimulation) |
| ↓ GFAP expression in CA1 hippocampus region and DG (best with 0.1 mA stimulation) | |||||||||||||||
|
| |||||||||||||||
| Yoon et al. [31] | 2016 | Lateral fluid percussion method | Sprague Dawley rats; males | 36 | a-tDCS | Hippocampus | 0.2 | 0.0225 | 20 | 28.2 | Chest | 48 (corset) | Y | Y (daily for 5 days) | ↑ Perilesional area BDNF expression (tDCS 2 weeks post-TBI) ↑ Choline/creatinine ratios (tDCS 1 week post-TBI) Motor performance recovery (2 weeks of tDCS) |
|
| |||||||||||||||
| Leffa et al. [33] | 2016 | SHR | SHR rats and WKT rats; males | 48 | a-tDCS | Frontal cortex | 0.5 | 1.5 | 20 | 33.4 | Between ears | 1.5 | N | Y (8 consecutive days) | ↑ DA levels in STR in both rat strains and in the hippocampus following tDCS treatment in WKY ↑ BDNF levels in WKY rats Short-term memory improvement |
|
| |||||||||||||||
| Wu et al. [45] | 2017 | STZ-induced diabetic rats | Sprague Dawley rats; males | 130 | a-tDCS | dPFC | 0.2 | 0.0314 | 30 | N/A | Anterior chest | 0.25 | Y | Y | ↑ Spatial working memory and mPFC LTP restoring |
|
| |||||||||||||||
| Pedron et al. [50] | 2017 | Cocaine injections | Swiss mice; females | 165 | a-tDCS | Left frontal cortex | 0.2 | 0.035 | 2 × 20 | N/A | Ventral thorax | 9.5 | N | Y (5 days stimulation, twice a day; 5 h interstimulation interval) | ↓ Cocaine-induced locomotor activity No cocaine-induced place preference (5 mg/kg and 25 mg/kg) ↑ Zif268 basal expression under the electrode area, in the STR and cortex |
|
| |||||||||||||||
| Leffa et al. [55] | 2018 | ADHD | SHR and WKY rats; males | 30 | a-tDCS (bicephalic) | Frontal cortex (supraorbital area) | 0.5 | 1.5 (ECG electrode) | 20 | 33.3 | Neck | 1.5 (ECG electrode) | N/A | Y (8 days) | ↓ Inflammatory cytokines and reversion of long-term memory deficits in SHR rats |
|
| |||||||||||||||
| Bragina et al. [47] | 2018 | CCI | Mice; N/A | 40 | a-tDCS | Parietal somatosensory cortex | 0.1 | N/A | 15 | N/A | Ventral thorax | N/A | N/A | Y (daily for 4 days, over 4 weeks and 3 days interval) | Motor coordination recovery Spatial memory and learning performance improvement ↑ CBF bilaterally (regional arteriolar dilatation and hypoxia reduction) |
|
| |||||||||||||||
| Roostaei et al. [44] | 2019 | STZ-induced diabetic rats | Wistar rats; males | 64 | a-tDCS vs. c-tDCS | Left frontal cortex | 0.2 | 3.5 | 20 | N/A | Ventral thorax | 9.5 | N | Y (twice a day over 2 days) | Restoration of STZ-induced amnesia (both polarities) |
|
| |||||||||||||||
| Gondard et al. [55] | 2019 | Animal model of AD-triple transgenic (3xTg) mice | Triple transgenic (3xTg) mice; males | 27 | c-tDCS and a-tDCS | Secondary motor cortex (m2) (c-TDCS) and dorsal temporal hippocampus (a-tDCS) | 0.05 | 0.0325 | 20 | N/A | N/A | 0.0325 | N/A | Y (5 days/week for 3 weeks) | No treatment effect on memory outcome or AD neuropathological biomarkers |
Abbreviations: rtDCS: repetitive transcranial direct current stimulation; a-tDCS: anodal transcranial direct current stimulation; c-tDCS: cathodal transcranial direct current stimulation; TBI: traumatic brain injury; ADHD: attention deficit hyperactivity disorder; SHR: spontaneous hypertensive rats; WKY: Wistar Kyoto rats; CBF: cerebral blood flow; PFC: prefrontal cortex; mPFC: medial prefrontal cortex; dPFC: dorsolateral prefrontal cortex; DG: dentate gyrus; STR: striatum; M2: secondary motor cortex; ITC: inferotemporal cortex; CA3: cornu ammonis 3 region in the hippocampus, STZ: streptozotocin; AD: Alzheimer's disease; Aβ1-40: amyloid beta peptide 1–40; ACh: acetylcholine; BDNF: brain-derived neurotrophic factor; DA: dopamine; GFAP glial fibrillary acidic protein; Zif268: zinc finger transcription factor 268; LTP: long-term potentiation; IP: intraperitoneal; SC: subcutaneous; SE: status epilepticus; ECG: electrocardiography; mg: milligram; kg: kilogram; A/m2: ampere per square meter; mA: milliampere; cm2: square centimeter; mm: millimeter; h: hour; min: minute; vs.: versus; Y: yes; N: no; N/A: not available.
Table 3.
Role of transcranial direct current stimulation on neuroplasticity with a focus on animal models of neurotrauma.
| Author | Year | Animal model | Specimen; gender | N | Stimulation parameters | Main findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation electrode | Reference electrode | Anesthesia | rtDCS | ||||||||||||
| Polarity | Position | Stimulation intensity (mA) | Size (cm2) | Stimulation duration (min) | Current density (A/m2) | Position | Area (cm2) | ||||||||
| Nekhendzy et al. [97] | 2004 | Inflammatory nociception model | Sprague Dawley rats; males | 31 | c-tDCS | Frontal cortex | 2.25 | N/A | 45 | N/A | Bimastoid | N/A | Y | Y (8 days) | ↓ Nociceptive response (effects lasted up to 50 min poststimulation) |
|
| |||||||||||||||
| Taib et al. [98] | 2009 | Left hemicerebellectomy | Wistar rats; males | 9 | a-tDCS | Right or left motor cortex | 0.4 | 0.071 | 20 | 56.3 | Supraorbital region | 0.0064 | N/A | Y | ↑ T corticomuscular response amplitudes |
|
| |||||||||||||||
| Kim et al. [99] | 2010 | MCAO | Sprague Dawley rats; both genders | 61 | c-tDCS vs. a-tDCS | Left primary motor cortex (M1) | 0.1 | 0.785 | 30 | N/A | Trunk | 9 | Y | Y (2 weeks) | Neuroprotection over white matter and ischemic size ↓ (a-tDCS) |
| ↑ Motor function | |||||||||||||||
|
| |||||||||||||||
| Jiang et al. [95] | 2012 | MCAO | Sprague Dawley rats; both genders | 90 | a-tDCS and c-tDCS | Motor cortex | 0.1 | 0.785 | 30 | 1.27 | Trunk | 9 | Y | Y (daily, for 3, 7, or 14 days postlesion) | ↑ Motor function (7 and 14 days poststroke) ↑ Dendritic spine density ↓ PX1 expression (7th and 14 h day poststroke) |
|
| |||||||||||||||
| Yoon et al. [56] | 2012 | MCAO | Sprague Dawley rats; males | 30 | a-tDCS | Left primary motor cortex (M1) | 0.2 | N/A | 20 | 28.2 | Anterior chest | 48.0 | Y | Y (5 days stimulation 1 week vs. 1 day postischemia) | ↑ Motor function and Barnes maze performance (a-tDCS applied 1 week after ischemic injury) |
| ↑ MAP-2 and GAP-43 expression around the perilesional area (a-tDCS applied 1 week postischemic injury) | |||||||||||||||
|
| |||||||||||||||
| Laste et al. [100] | 2012 | CFA injection/chronic inflammation induction | Wistar rats; males | 18 | a-tDCS | Parietal cortex | 0.5 | 1.5 (ECG electrode) | 20 | 33.4 | Supraorbital area | 1.5 (ECG electrode) | N | Y (8 days) | Significant differences in nociceptive response (immediately after and 24 h poststimulation) |
|
| |||||||||||||||
| Adachi et al. [101] | 2012 | CRS | Wistar rats; males | 48 | a-tDCS | Parietal cortex | 0.5 | 1.5 (ECG electrode) | 20 | 3.3 | Supraorbital area | 1.5 (ECG electrode) | N | Y (8 days) | ↓ Nociceptive response in chronic stress condition ↓ TNF expression in the hippocampus |
|
| |||||||||||||||
| Peruzzotti-Jametti et al. [60] | 2013 | MCAO | C57BL/6 mice; males | 137 | c-tDCS vs. a-tDCS | Left parietal cortex | 0.25 | 0.0144 | 40 | 55 | Ventral thorax | 5.2 | N/A | N (single session) | ↑ Infarct volume and ↑ BBB leakage (a-tDCS ipsilesional hemisphere) |
| Cortical Glu activity ↓, functional ↑ and ischemic damage ↓ (c-tDCS ipsilesional hemisphere) | |||||||||||||||
|
| |||||||||||||||
| Notturno et al. [102] | 2014 | MCAO | Sprague Dawley rats; both genders | 53 | c-tDCS | Left motor cortex | 0.2 | 0.07 | 120 | 28.6 | Ventral thorax | 10.5 | Y | N (single session) | Ischemia volume ↓ |
|
| |||||||||||||||
| Lu et al. [103] | 2015 | MPTP injection | C57bl mice; males | 36 | a-tDCS | Left frontal cortex | 0.2 | 0.035 | 10 | 57 | Between shoulders | 9 | N/A | Y (daily for 3 weeks) | ↑ Motor coordination (until 21 days poststimulation) ↑ TH and DA expression ↓ Oxidative stress |
|
| |||||||||||||||
| Spezia Adachi et al. [104] | 2015 | Restraint stress model | Wistar rats; males | 78 | a-tDCS | Parietal cortex (midline) | 0.5 | 1.5 (ECG electrode) | 20 | N/A | Supraorbital area | 1.5 (ECG electrode) | N | Y (daily for 8 days) | ↓ Stress-induced nociceptive response ↑ Pain threshold ↓ BDNF levels (spinal cord and brainstem) in unstressed animals |
|
| |||||||||||||||
| Yoon et al. [31] | 2016 | Lateral fluid percussion method | Sprague Dawley rats; males | 36 | a-tDCS | Hippocampus | 0.2 | 0.0225 | 20 | 28.2 | Chest | 48 (corset) | Y | Y (daily for 5 days) | ↑ Perilesional area BDNF expression (tDCS 2 weeks post-TBI) |
| ↑ Choline/creatinine ratios (tDCS 1 week post-TBI) | |||||||||||||||
| Motor performance recovery (2 weeks of tDCS) | |||||||||||||||
|
| |||||||||||||||
| Leffa et al. [33] | 2016 | SHR | SHR rats and WKT rats; males | 48 | a-tDCS | Frontal cortex | 0.5 | 1.5 | 20 | 33.4 | Between ears | 1.5 | N | Y (8 consecutive days) | ↑ DA levels in STR in both rat strains and in the hippocampus following tDCS treatment in WKY ↑ BDNF levels in WKY rats |
|
| |||||||||||||||
| Liu et al. [105] | 2016 | PTI | Sprague Dawley rats; N/A | 58 | c-tDCS | Right S1FL | 2 | N/A | 20 | 20.37 | Venter | N/A | N/A | N (single session) | Ischemia expansion inhibition (c-tDCS immediately postischemia induction) ↓ NeuN expression (c-tDCS+PSS group) |
|
| |||||||||||||||
| Braun et al. [106] | 2016 | MCAO | Wistar rats; males | 41 | c-tDCS vs. a-tDCS | Left primary motor cortex (M1) | 0.5 | 0.035 | 15 | N/A | Ventral thorax | N/A | Y | Y (10-day stimulation; 5 days with 2-day interval) | Gait recovery at day 16 poststroke (both polarities) Faster recovery of limb strength (fully recovered strength at day 10 and gait at day 14 with c-tDCS) ↑ Microglia and neuroblasts in lesion ipsilateral cortex |
|
| |||||||||||||||
| Cioato et al. [107] | 2016 | Sciatic nerve chronic constriction | Wistar rats; males | 84 | a-tDCS and c-tDCS | Parietal cortex (bicephalic) | 0.5 | 1.5 (ECG electrode) | 20 | N/A | Supraorbital area | 1.5 (ECG electrode) | N | Y (8 days) | Nociceptive relieve (for up to 7 days poststimulation) Reversion of T IL-1þ levels (48 h and 7 days poststimulation) |
|
| |||||||||||||||
| Filho et al. [108] | 2016 | Partial sciatic nerve compression | Wistar rats; males | 144 | a-tDCS | Parietal cortex | 0.5 | 1.5 (ECG electrode) | 20 | N/A | Supraorbital area | 1.5 (ECG electrode) | N | Y (8 days) | ↓ BDNF expression (48 h poststimulation) Reversion of behavioral alterations (analgesic and anxiolytic) associated with neuropathic pain |
|
| |||||||||||||||
| Moreira et al. [109] | 2016 | Pain and menopause (ovariectomised animals) | Wistar rats; females | 45 | C-tDCS | Parietal cortex | 0.5 | 1.5 (ECG electrode) | 20 | N/A | Supraorbital area | 1.5 (ECG electrode) | N | Y (8 days) | ↓ Hypothalamic BDNF levels and T serum BDNF in ovariectomised animals |
|
| |||||||||||||||
| Dimov et al. [87] | 2016 | N/A | Wistar rats; males | 25 | c-tDCS | Left primary motor cortex (M1) | 0.25 | 0.0227 | 15 | N/A | Ventral thorax | N/A | N | N (single session) | Bilateral ↓ Egr-1 expression in the PAG |
| Spinal ENK immunoreactivity ↓ in the DHSC | |||||||||||||||
|
| |||||||||||||||
| Liu et al. [110] | 2017 | PTI | Sprague Dawley rats; males | 58 | c-tDCS | S1FL | 2 | N/A | 20 | N/A | 3 mm lateral to lambda | N/A | N/A | N (single session) | Prevention of ischemia injury expansion during hyperacute phase of ischemia (c-tDCS+PSS) |
|
| |||||||||||||||
| Kim & Han [111] | 2017 | Modified Tang's method [128] | Sprague Dawley rats; N/A | 31 | a-tDCS | Left motor cortex | 0.2 | 1 | 30 | 0.26 | Ventral thorax | 9 | Y | N | Early recovery of consciousness and MEP and SEP prolonged latency (tDCS applied right after TBI) ↓ Astroglial GFAP immunoreactivity |
|
| |||||||||||||||
| Winkler et al. [112] | 2017 | Striatal 6-OHDA injection | Sprague Dawley rats; females | 24 | a-tDCS and c-tDCS | Left motor cortex | N/A | 0.16 | 20 | 8 | Chest | 3 | N | Y (daily for 14 days) | Graft survival, striatal dopaminergic reinnervation and motor recovery (a-tDCS) |
|
| |||||||||||||||
| de Souza et al. [113] | 2017 | PSNL | Swiss mice; males | N/A | a-tDCS and c-tDCS | Parietal cortex (bicephalic) | 0.5 | N/A (EEG electrode) | 5; 10; 15; 20 | N/A | Supraorbital area | N/A (EEG electrode) | N | N (single session) | Antiallodynic effect (seen 4 h poststimulation of 15 min and 20 min) |
|
| |||||||||||||||
| Leffa et al. [46] | 2018 | ADHD | SHR and WKY rats; males | 30 | a-tDCS (bicephalic) | Frontal cortex (supraorbital area) | 0.5 | 1.5 (ECG electrode) | 20 | 33.3 | Neck | 1.5 (ECG electrode) | N/A | Y (8 days) | ↓ Inflammatory cytokines and reversion of long-term memory deficits in SHR rats |
|
| |||||||||||||||
| Paciello et al. [114] | 2018 | NIHL | Wistar rats; males | 124 | a-tDCS | Temporal lobe (auditory cortex) | 0.35 | 0.0625 | 20 | 56 | Ventral thorax | 12 | N | Y (2 days) | ↑ Dendritic spines density (layer 2/3 pyramidal neurons of the auditory cortex) |
| ↑ BDNF and synaptophysin expression in auditory cortex (24 h poststimulation) | |||||||||||||||
|
| |||||||||||||||
| Fregni et al. [115] | 2018 | N/A | Wistar rats; males | 32 | N/A | N/A (bicephalic) | N/A | N/A | 20 | N/A | N/A | N/A | N/A | Y (8 days) | tDCS prior to stress exposure prevented thermal hyperalgesia |
|
| |||||||||||||||
| Lee et al. [116] | 2019 | MPTP injection | C57bl mice; male | 60 | a-tDCS | Primary motor cortex (M1) | N/A | N/A | 30 | N/A | Between shoulders | N/A | N/A | Y (daily for 5 days) | ↑ Motor coordination |
| Rescue of MTPT-induced mitochondrial dysfunction (Ç ATP and GDH and $ Drp1 levels) | |||||||||||||||
|
| |||||||||||||||
| Callai et al. [117] | 2019 | CCI-ION | Wistar rats, males | 151 | a-tDCS | Parietal cortex (bicephalic) | 0.5 | 1.5 (ECG electrode) | 20 | N/A | Supraorbital area | 1.5 (ECG electrode) | N | Y (8 days) | ↓ Mechanical hyperalgesia |
| ↓ TNF-a expression (7 days poststimulation) | |||||||||||||||
| ↓ IL-10 (7 days poststimulation) | |||||||||||||||
| ↓ LDH serum levels | |||||||||||||||
|
| |||||||||||||||
| Scarabelot et al. [118] | 2019 | CFA injection/chronic inflammation induction | Sprague Dawley rats; males | 104 | a-tDCS | Parietal cortex (bicephalic) | 0.5 | 1.5 (ECG electrode) | 20 | N/A | Supraorbital area | 1.5 (ECG electrode) | N/A | Y (8 days) | ↓ Thermal and mechanical hyperalgesia |
| ↑ IL-6 (in brainstem 24 h poststimulation) | |||||||||||||||
| ↓ IL-10 (7 days poststimulation) | |||||||||||||||
| Normalization of BDNF levels (24 h poststimulation) | |||||||||||||||
Abbreviations: rtDCS: repetitive transcranial direct current stimulation; a-tDCS: anodal transcranial direct current stimulation; c-tDCS: cathodal transcranial direct current stimulation; C57BL/6: mouse strain; SHR: spontaneous hypertensive rats; WKY: Wistar Kyoto rats; ADHD: attention deficit hyperactivity disorder; 6-OHDA: 6-hydroxydopamine; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; TBI: traumatic brain injury; PTI: photothrombic ischemia; MCAO: middle cerebral artery occlusion; PSS: peripheral sensory stimulation; NIHL: noise-induced hearing loss; CC-ION: chronic constriction of the infraorbital nerve (pain model); PSNL: partial sciatic nerve ligation (pain model); CRS: chronic restraint stress (pain model); CFA: complete Freund's adjuvant (pain model); BBB: blood–brain barrier; S1FL: forelimb region of the primary somatosensory cortex; M1: primary motor area; PAG: periaqueductal grey; DHSC: dorsal horn of the spinal cord; MEP: motor-evoked potentials; SEP: somatosensory evoked potentials; GFAP: glial fibrillary acidic protein; BDNF: brain-derived neurotrophic factor; Glu: glutamate NeuN: neuronal marker; PX1: pannexin 1; TH: thyroxine hydroxylase; Egr-1: early growth response protein 1; TNF: tumor necrosis factor; IL-1þ: interleukin 1 beta; IL-6: interleukin 6; IL-10: interleukin 10; LDH: lactate dehydrogenase enzyme; ATP: adenosine triphosphate; GDH: glutamate dehydrogenase; Drp1: dynamin-related protein; MAP-2: microtubule associated protein 2; GAP-43: growth associated protein 43; ENK: early embryo specific NK; DA: dopamine; ECG: electrocardiography; EEG: electroencephalogram; A/m2: ampere per square meter; mA: milliampere; cm2: square centimeter; min: minute; vs.: versus; Y: yes; N: no; N/A: not available.
3.3. Effect of tDCS on Cellular and Molecular Neuroplasticity Mechanisms
Neuronal network reorganization underlies neuroplasticity processes like developmental synaptogenesis, or neurogenesis and synaptic turnover later on, which ultimately contributes to optimal brain development and aging, as well as functional recovery upon trauma [58]. Interestingly, several reports using genetic engineered animals, pharmacologically induced animal models of disease, or in vitro techniques enlightened the potential of direct current stimulation (DCS) to interact with a myriad of neuroplasticity-related processes such as neuroinflammation [59, 60], neural stem cell migration [59], neurite growth [61], or neurogenesis [62]. Moreover, both human and in vivo animal studies evidenced a tDCS-induced effect on memory and learning [28, 35, 63]. However, the underlying cellular and molecular mechanisms remain to be elucidated.
3.3.1. Modulation of the Excitatory/Inhibitory Network
To date, animal experimental evidence highlighted tDCS influences on synaptic plasticity, through alterations in the functional connectivity of cognition-related areas [35] and by modulation of excitatory/inhibitory network tonus [64], which may involve both the GABAergic and glutamatergic systems. Accordingly, a study conducted with older adults remarked an anodal stimulation effect in gamma-aminobutyric acid (GABA) levels [65]. Similarly, in human healthy volunteers, an anodal tDCS effect in motor learning was correlated with a decrease in GABA levels, an outcome known to be a determinant factor in the promotion of long-term potentiation- (LTP-) dependent plasticity and therefore learning [66, 67].
Several preclinical studies probed LTP enhancement following direct current stimulation. Anodal DCS enhanced LTP in both mouse cortex [68] and rat hippocampal slices [69, 70]. Further, this neurostimulation method increased local field potential (LFPs) amplitudes in primary somatosensory cortex of rabbits [63]. Also, other works demonstrated that neurostimulation-enhanced hippocampal LTP was associated with better spatial memory performance along with an increase in brain-derived neurotrophic factor (BDNF) expression levels [40]. An opposite effect on LTP and LFPs was obtained with administration of cathodal currents. In agreement, a report from Sun et al. [71] evidenced that cathodal currents applied in mouse neocortical slices induced field excitatory postsynaptic potential depression. This type of LTD was smothered by application of an mGluR5 negative allosteric modulator [72]. These findings support a possible modulatory effect of tDCS on mGluR5-mTOR signaling [72]; these molecular pathways are recognized to disturb cognition-related synaptic plasticity.
Further evidence supporting tDCS effect on LTP-like mechanisms was recently brought to light by Stafford et al. [73]. These authors observed that a single anodal tDCS increased both the phosphorylation at the S831 of GluA1 subunit and the translocation of α-amino-3-hydroxy5-methyl-4-isoxazole propionic acid receptors (AMPARs) from cytosolic to synaptic fractions in the hippocampus. These data could be favoring learning enhancement, as this translocation has been associated with hippocampal LTP induction [72]. Accordingly, others reported a spatial working memory enhancement after anodal stimulation over left medial PFC that was lost with the administration of the AMPAR antagonist perampanel (PRP). In contrast to cathodal currents, anodal currents enhanced intracellular calcium (Ca2+) intake in cell cultures including astrocytes [74–76], a process associated with AMPAR phosphorylation and trafficking to postsynaptic density [77] and ultimately, allowing LTP facilitation, a cellular correlate of learning and memory.
3.3.2. Activation of Neuroplasticity-Associated Gene Expression
Neurostimulation could have long-lasting effects in memory as data from different studies evidenced [40]. Authors have been argued that tDCS cognition modulation is associated with neuroplasticity-associated gene expression alterations [78]. One of the neuroplasticity-associated genes, known to be essential for hippocampal LTP, is BDNF [79]. Several studies elucidated the role of BDNF in memory modulation by tDCS. In fact, it was reported that anodal currents could increase BDNF expression [68], and its activation via tropomyosin receptor kinase (Trk) receptors [80], triggering NMDAR opening, and inducing a later phase LTP (L-LTP) facilitation [81]. Accordingly, Yu et al. [41] found that the administration the Trk inhibitor ANA-12 prevented the anodal tDCS-induced hippocampal CA1 LTP increase. Other studies, using the same polarity currents, revealed a link between the upregulation of BDNF and cAMP response element binding protein/CREB-binding protein (CREB/CBP) [40] involved in LTP and memory formation [82]. Also, the application of cortical anodal currents in frontal cortex was able to upregulate BDNF together with striatal dopamine [33]. The upregulation of BDNF following neurostimulation was associated with the augmentation of expression levels of immediate early genes (IEGs), such as c-fos and zif268 [69]. Moreover, Kim et al. [78] confirmed that repetitive anodal tDCS in right sensorimotor cortex of healthy rats promoted a significant increase of mRNA levels of plasticity-associated genes, namely, BDNF, cAMP response element binding protein (CREB), synapsin I, Ca2+/calmodulin-dependent protein kinase II (CaMKII), activity-regulated cytoskeleton-associated protein (Arc), and c-fos. It was also demonstrated that sensory evoked cortical responses were boosted after tDCS via alpha-1 adrenergic receptor-mediated astrocytic Ca2+/IP3 signaling, thus involving also glia and the adrenergic system [75]. Anodal tDCS actions in glia were further confirmed by Mishima et al. [76]. Using a mouse model lacking Ca2+ uptake in astrocytes, the inositol trisphosphate receptor type 2 (IP3R2) knockout (KO) mouse and also an adrenergic receptor antagonist, they confirmed decreased microglia motility along with soma enlargement in tDCS stimulated animals [76].
In poststroke recovery, it was found that anodal currents significantly increased the GAP-43 and the microtubule-associated protein 2 (MAP-2) expression around the infarct area [56]. These neuronal growth-promoting proteins are overexpressed during dendritic remodeling and axonal regrowth throughout the acute phase of stroke [83, 84]. Anodal stimulation also modulated pannexin-1 (PX1) hemichannel levels [85, 86] and, following an ischemic insult, neurostimulation decreased rat PX1 mRNA and, consequently, augmented dendritic spine density in the surrounding areas of cerebral infarction; these cellular outcomes were associated with the improvement of motor function [85]. Some authors proposed that tDCS-induced improvement of stroke/TBI symptoms might be due to increase of BDNF expression and associated with choline/creatine ratios in the perilesional cortex [31].
Overall, tDCS methodology was able to modulate molecular pathways involved in the regulation of cognition-related synaptic plasticity mechanisms (Figure 3). The revised in vivo animal studies regarding tDCS-induced effects in the cellular and molecular mechanisms of memory and learning are listed in Table 4.
Figure 3.
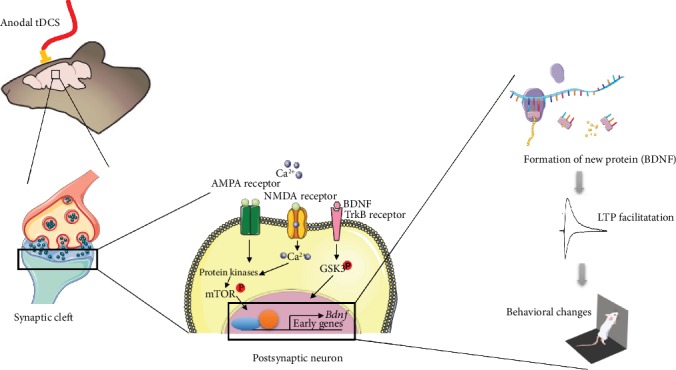
Schematic illustration of molecular mechanisms underlying the effect of anodal transcranial direct current stimulation (tDCS) on neuronal physiology. The neurostimulation in the target cortical area depolarizes neuronal membrane and glutamate released in presynaptic neuron and binds in NMDA and AMPA receptors (see book chapter Rozisky et al., 2015). Consequently, there is intracellular Ca2+ upregulation in the postsynaptic neuron, which can activate protein kinases that in turn modulate numerous neuronal signaling pathways (such as the mTOR pathway) leading to transcriptional changes. The tDCS also activates molecular cascades to promote BDNF production. As a long-term mechanism, gene transcription is modulated leading to the formation of new proteins that in turn lead to facilitation of LTP and improvement of cognition. Abbreviations: AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; BDNF: brain-derived neurotrophic factor; CBP: CREB-binding protein; CREB: cAMP response element binding protein; GSK3: glycogen synthase kinase 3; LTP: long-term potentiation; mTOR: mammalian target of rapamycin; NMDA: N-methyl-D-aspartate; TrkB: tropomyosin receptor kinase B.
Table 4.
Cellular and molecular mechanisms underlying transcranial direct current stimulation effect in the brain.
| Author | Year | Animal model | Specimen; gender | N | Stimulation parameters | Main findings | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation electrode | Reference electrode | Anesthesia | rtDCS | ||||||||||||
| Polarity | Position | Stimulation intensity (mA) | Size (cm2) | Stimulation duration (min) | Current density (A/m2) | Position | Area (cm2) | ||||||||
| Márquez-Ruiz et. [63] | 2012 | NDM | New Zealand White albino rabbits; N/A | 13 | a-tDCS and c-tDCS | Somatosensory cortex (S1) | 0.5; 1; 1.5 and 2 | 0.7857 | 10 | 3.7 | Ear | 35 | N | N (single session) | ↑ LFP in S1 (a-tDCS) and ↓ LFP S1 (c-tDCS) |
|
| |||||||||||||||
| Rohan et al. [57] | 2015 | NDM | Sprague Dawley rats; male | 34 | a-tDCS | SC dorsal to the hippocampus | 0.1 or 0.25 | 0.25 | 30 | N/A | Between shoulders | 8.04 | N | N (single session) | # LTP and PPF in the hippocampus |
|
| |||||||||||||||
| Yoon et al. [31] | 2016 | Lateral fluid percussion method | Sprague Dawley rats; males | 36 | a-tDCS | Hippocampus | 0.2 | 0.0225 | 20 | 28.2 | Chest | 48 (corset) | Y | Y (daily for 5 days) | # perilesional area BDNF expression (tDCS 2 weeks post-TBI) # choline/creatinine ratios (tDCS 1 week post-TBI) Motor performance recovery (2 weeks of tDCS) |
|
| |||||||||||||||
| Leffa et al. [33] | 2016 | SHR | SHR rats and WKT rats; males | 48 | a-tDCS | Frontal cortex | 0.5 | 1.5 | 20 | 33.4 | Between ears | 1.5 | N | Y (8 consecutive days | # DA levels in STR in both rat strains and in the hippocampus following tDCS treatment in WKY # BDNF levels in WKY rats Short-term memory improvement |
|
| |||||||||||||||
| Podda et al. [40] | 2016 | NDM | C57BL/6 mice; males | 16 | a-tDCS vs. c-tDCS | Left parietal cortex (dorsal to hippocampal formation) | 0.35 | 0.06 | 20 | N/A | Ventral thorax | 5.2 | N | N (single session) | # spatial learning and memory (a-tDCS); benefits observable one week after # BDNF expression in the hippocampus CREB/CBP pathway activation |
|
| |||||||||||||||
| Monai et al. [75] | 2016 | NDM | G7NG817 mice; N/A | 10 | a-tDCS | Primary visual cortex (VI) | 0.1 | 0.02 | 10 | N/A | Neck | N/A | N | N (single session) | Up to 50% expansion of visual evoke active area (up to 2 h poststimulation effect) tDCS-induced plasticity depends on the activity of IP, R2, and A1AR |
|
| |||||||||||||||
| Kim et al. [78] | 2017 | NDM | Sprague Dawley rats; males | 90 | a-tDCS | Right sensorimotor cortex | 0.25 | 0.071 | 20 | N/A | Right anterior chest | 0.5 | N | Y (7 days) | # BDNF, CREB, synapsin, and CaMKII mRNA expression levels (ipsilateral cortex) and c-fos (hippocampus) |
|
| |||||||||||||||
| Stafford et al. [73] | 2018 | NDM | Sprague Dawley rats, males | 16 | a-tDCS | Caudal to bregma | 0.25 | 0.25 | 30 | N/A | Ventral thorax | N/A | N | N (single session) | ↑ AMPAR translocation to the synapse in the hippocampus and ↑ phosphorylation of the S831 site on GluA1 |
|
| |||||||||||||||
| Martins et al. [97] | 2019 | NDM | Male Wistar rats; males | 50 | a-tDCS | Left mPFC | 0.4 | N/A | 13 | N/A | N/A | N/A | N/A | Y (5 days) | ↑ Spatial working memory |
| ↑ GAP-43 (extinct by AMPAR antagonist PRP) | |||||||||||||||
|
| |||||||||||||||
| Yu et al. [41] | 2019 | NDM | Sprague Dawley rats; males | 224 | a-tDCS | SC dorsal to the hippocampus | 0.25 | 0.25 | 30 | N/A | Anterior chest | N/A (EEG electrode) | Y | N (single session) | ↑ Memory (passive avoidance memory retention) |
| ↑ LTP in CA1 hippocampus (blocked by TrkB antagonist) ↑BDNF in CA1 hippocampus | |||||||||||||||
Abbreviations: rtDCS: repetitive transcranial direct current stimulation; a-tDCS: anodal transcranial direct current stimulation; c-tDCS: cathodal transcranial direct current stimulation; C57BL/6: mouse strain; SC: stereotaxic coordinates; NDM: no disease model; SHR: spontaneous hypertensive rats; WKY: Wistar Kyoto rats; PFC: prefrontal cortex; mPFC: medial prefrontal cortex; dPFC: dorsolateral prefrontal cortex; DG: dentate gyrus; STR: striatum; S1: somatosensory cortex; V1: primary visual cortex; ITC: inferotemporal cortex; CA1: cornu ammonis 1 region in the hippocampus; TBI: traumatic brain injury; PRP: perampanel; CREB: cAMP response element-binding protein (transcription factor); CREB/CBP: cAMP response element binding protein; BDNF: brain-derived neurotrophic factor; DA: dopamine; GAP-43: growth associate protein 43; CaMKII: Ca2+/calmodulin-dependent protein kinase; mRNA: messenger ribonucleic acid; IP3R2: inositol triphosphate type 2 receptor; A1AR: adenosine A2A receptor; AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GluA1: AMPA receptor subunit A1; LFP: local field potential; LTP: long-term potentiation; PPF: paired pulse facilitation; EEG: eletroencephalography; A/m2: ampere per square meter; mA: milliampere; cm2: square centimeter; mm: millimeter; h: hour; min: minute; vs.: versus; Y: yes; N: no; N/A: not available.
4. Discussion
This systematic review collected several studies that confirm the potential effects of tDCS on neuronal activity and synaptic plasticity. Here, we documented a variable combination of stimulation protocols, stimulation areas, and healthy and disease animal models. Most of the existent literature is focused on human application of tDCS. The comprehensive revision of the effect of tDCS on rodent models of normal and pathological brain functioning does therefore provide a novel contribution to the field. Overall, the revised studies indicated that tDCS was able to modulate synaptic plasticity and, consequently, learning and memory processes [87, 88].
Memory formation and consolidation are recognized to rely on activity-dependent modifications, such as LTD and LTP [89], both dependent on the activation of calcium-dependent kinases (e.g., CaMKs), which in turn control the trafficking of NMDARs and AMPARs [90]. Despite the wide set of stimulation protocols, tDCS-induced modulation of NMDAR signaling and synaptic protein upregulation resulting in LTP and cognitive enhancement have been consistently reported in animal studies. Anodal tDCS increased AMPAR synapse translocation [73, 89] and induced spatial memory improvement by involving both CREB and BDNF expression alterations [53]. Also, an increase in hippocampal and cortical mRNA levels of c-fos, synapsin, CaMKII, and Arc was observed poststimulation [78].
Similar results highlighting tDCS effects in neuroplasticity were obtained with in vitro studies. Accordingly, Ranieri and coworkers [69] probed that anodal currents increased NMDAR-dependent LTP in hippocampal CA3-CA1 synapses [69], in part, due to production of BDNF [68]. In addition, it was demonstrated that tDCS-induced hippocampal BDNF release is dependent on histone acetylation of BDNF gene promoters [40]. Overall, the abovementioned works provide positive evidence for the effect of tDCS on cognitive function enhancement.
Although tDCS impaired the acquisition of both contextual and cued fear memory [39], there are no studies on possible cascades/proteins involved in tDCS-induced neuroplasticity alterations following fear memory changes. Nevertheless, a very recent paper demonstrated chronic repetitive TMS of the ventromedial prefrontal cortex reversed stress-induced behavior impairments with an increase of c-fos activity [91].
Cortical anodal currents have been shown to be mostly excitatory and support memory enhancement and neuroplasticity. The literature is also consistent with the notion that the stimulation over the cortical region functionally involved in a certain cognitive task increases performance in that specific task. Marshall et al. demonstrated that anodal currents over the PFC, a region involved in memory encoding, during slow wave sleep improved declarative memory [28]. However, it was described that cortical cathodal stimulation simultaneously with training task was able to increase visuospatial working memory, in spite of the fact that it was associated with decreased excitability [42]. This suggests that modulatory effects of tDCS were influenced by the polarity-dependent electrical dynamics established between the stimulated area and its related neuronal networks. In agreement, a recent report observed an inhibitory effect in motor learning tasks following anodal currents in the cerebellum; the anodal excitatory effect over the Purkinje cell activity led to an overall inhibition of downstream structures, reducing as a result the vestibulo-ocular reflex gain [90]. Similar paradoxical results have been observed in humans. Recently, Moliadze and collaborators [92] reported that tDCS-induced neural modulation depended on several parameters, namely, the age. In fact, an excitatory effect was seen in young subjects, but not in the older participants.
Nowadays, TMS, another important noninvasive brain stimulation technique, is useful for evaluating excitability in the primary motor cortex (M1) and conductivity along the cortical-spinal tract. This technique has been amply used in rehabilitation of stroke patients [93] and in neuropsychiatric disorders, namely, depression [94]. tDCS and TMS are undergoing the most active investigation and share a capacity to modulate regional cortical excitability, and both are well-tolerated by children and adults [95]. However, TMS has been already approved for clinical use and tDCS is still undergoing investigation as a plausible therapy for a range of neuropsychiatric disorders [95]. The rational, in part, for this is because data on the efficacy and safety of tDCS are sparse and employ heterogeneous stimulation protocols. Indeed, there is a paucity of strictly conducted randomized, sham controlled clinical trials, and case considerable follow-up periods, which makes it difficult to use these results to inform clinical practice concerning the putative beneficial role of tDCS. Moreover, tDCS effects seem to be clearly dependent on structure, connectivity, and function of the target brain region. Importantly, these outcomes were intrinsically correlated with GABAergic neurotransmission which raises the issue that one has to take into account that during development GABA can act as an excitatory neurotransmitter [96].
5. Conclusions
There is growing evidence that tDCS modulates brain activity and, consequently, enhances synaptic plasticity and cognitive performance. Overall, reports from laboratory animal research present tDCS as a promising noninvasive brain stimulation technique. The presented evidence is therefore consistent with human studies suggesting that this technique is useful to mitigate neurologic symptoms of several brain disorders, thus improving learning and memory. Further research is needed so that this technique can be fully translated into optimal therapeutic strategies.
Acknowledgments
This work was supported by grants POCI-01-0145-FEDER-016428 and CENTRO-01-0145-FEDER-000016 financed by Centro 2020 FEDER, COMPETE, FLAD Life Sciences Ed 2 2016, FCT/UID 4950 COMPETE, POCI-01-0145-FEDER-007440, FCT, and European Grant H2020 STIPED.
Contributor Information
Joana Gonçalves, Email: jgoncalves@fmed.uc.pt.
Miguel Castelo-Branco, Email: mcbranco@fmed.uc.pt.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Joana Gonçalves and Miguel Castelo-Branco share senior authorship.
References
- 1.Ruffini G., Wendling F., Merlet I., et al. Transcranial current brain stimulation (tCS): models and technologies. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2013;21(3):333–345. doi: 10.1109/TNSRE.2012.2200046. [DOI] [PubMed] [Google Scholar]
- 2.Nitsche M. A., Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology. 2000;527(3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner T., Valero-Cabre A., Pascual-Leone A. Noninvasive human brain stimulation. Annual Review of Biomedical Engineering. 2007;9(1):527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 4.Nitsche M., Liebetanz D., Antal A., Lang N., Tergau F., Paulus W. Modulation of cortical excitability by weak direct current stimulation-technical, safety and functional aspects. Supplements to Clinical Neurophysiology. 2003;56:255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- 5.Kellaway P. The part played by electrical fish in the early history of bioelectricity and electrotherapy. Bulletin of the History of Medicine. 1946;20(2):130–134. [PubMed] [Google Scholar]
- 6.Parent A. Giovanni Aldini: from animal electricity to human brain stimulation. The Canadian Journal of Neurological Sciences. 2004;31(4):576–584. doi: 10.1017/s0317167100003851. [DOI] [PubMed] [Google Scholar]
- 7.Bullock T., Terzuolo C. Diverse forms of activity in the somata of spontaneous and integrating ganglion cells. The Journal of Physiology. 1957;138(3):341–364. doi: 10.1113/jphysiol.1957.sp005855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poreisz C., Boros K., Antal A., Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Research Bulletin. 2007;72(4-6):208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Bindman L., Lippold O., Redfearn J. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. The Journal of Physiology. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terzuolo C. A., Bullock T. H. Measurement of imposed voltage gradient adequate to modulate neuronal firing. Proceedings of the National Academy of Sciences of the United States of America. 1956;42(9):687–694. doi: 10.1073/pnas.42.9.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitsche M. A., Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–1901. doi: 10.1212/WNL.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 12.Antal A., Terney D., Poreisz C., Paulus W. Towards unravelling task-related modulations of neuroplastic changes induced in the human motor cortex. The European Journal of Neuroscience. 2007;26(9):2687–2691. doi: 10.1111/j.1460-9568.2007.05896.x. [DOI] [PubMed] [Google Scholar]
- 13.Krause B., Cohen Kadosh R. Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Frontiers in Systems Neuroscience. 2014;8 doi: 10.3389/fnsys.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikson M., Rahman A., Datta A. Computational models of transcranial direct current stimulation. Clinical EEG and Neuroscience. 2012;43(3):176–183. doi: 10.1177/1550059412445138. [DOI] [PubMed] [Google Scholar]
- 15.Fertonani A., Miniussi C. Transcranial electrical stimulation: what we know and do not know about mechanisms. The Neuroscientist. 2017;23(2):109–123. doi: 10.1177/1073858416631966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liebetanz D., Klinker F., Hering D., et al. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47(7):1216–1224. doi: 10.1111/j.1528-1167.2006.00539.x. [DOI] [PubMed] [Google Scholar]
- 17.Liebetanz D., Koch R., Mayenfels S., König F., Paulus W., Nitsche M. A. Safety limits of cathodal transcranial direct current stimulation in rats. Clinical Neurophysiology. 2009;120(6):1161–1167. doi: 10.1016/j.clinph.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Jackson M., Truong D., Brownlow M., et al. Safety parameter considerations of anodal transcranial direct current stimulation in rats. Brain, Behavior, and Immunity. 2017;64:152–161. doi: 10.1016/j.bbi.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitsche M., Cohen L., Wassermann E., et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimulation. 2008;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Monte-Silva K., Kuo M., Liebetanz D., Paulus W., Nitsche M. A. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS) Journal of Neurophysiology. 2010;103(4):1735–1740. doi: 10.1152/jn.00924.2009. [DOI] [PubMed] [Google Scholar]
- 21.Boggio P. S., Ferrucci R., Rigonatti S. P., et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson's disease. Journal of the Neurological Sciences. 2006;249(1):31–38. doi: 10.1016/j.jns.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 22.Horiba M., Ueki Y., Nojima I., et al. Impaired motor skill acquisition using mirror visual feedback improved by transcranial direct current stimulation (tDCS) in patients with Parkinson’s disease. Frontiers in Neuroscience. 2019;13:p. 602. doi: 10.3389/fnins.2019.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fregni F., Boggio P. S., Nitsche M. A., Rigonatti S. P., Pascual-Leone A. Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depression and Anxiety. 2006;23(8):482–484. doi: 10.1002/da.20201. [DOI] [PubMed] [Google Scholar]
- 24.Fregni F., Boggio P., Mansur C., et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16(14):1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- 25.Hummel F., Celnik P., Giraux P., et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(3):490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 26.English M. C. W., Kitching E. S., Maybery M. T., Visser T. A. W. Modulating attentional biases of adults with autistic traits using transcranial direct current stimulation: a pilot study. Autism Research. 2018;11(2):385–390. doi: 10.1002/aur.1895. [DOI] [PubMed] [Google Scholar]
- 27.Kang J., Cai E., Han J., et al. Transcranial direct current stimulation (tDCS) can modulate EEG complexity of children with autism spectrum disorder. Frontiers in Neuroscience. 2018;12:p. 201. doi: 10.3389/fnins.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall L., Mölle M., Hallschmid M., Born J. Transcranial direct current stimulation during sleep improves declarative memory. Journal of Neuroscience. 2004;24(44):9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Souza Custódio J. C., Martins C. W., Lugon M. D. M. V., Fregni F., Nakamura-Palacios E. M. Epidural direct current stimulation over the left medial prefrontal cortex facilitates spatial working memory performance in rats. Brain Stimulation. 2013;6(3):261–269. doi: 10.1016/j.brs.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Yu X., Li Y., Wen H., Zhang Y., Tian X. Intensity-dependent effects of repetitive anodal transcranial direct current stimulation on learning and memory in a rat model of Alzheimer’s disease. Neurobiology of Learning and Memory. 2015;123:168–178. doi: 10.1016/j.nlm.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Yoon K. J., Lee Y. T., Chae S. W., Park C. R., Kim D. Y. Effects of anodal transcranial direct current stimulation (tDCS) on behavioral and spatial memory during the early stage of traumatic brain injury in the rats. Journal of the Neurological Sciences. 2016;362:314–320. doi: 10.1016/j.jns.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Kamida T., Kong S., Eshima N., Abe T., Fujiki M., Kobayashi H. Transcranial direct current stimulation decreases convulsions and spatial memory deficits following pilocarpine-induced status epilepticus in immature rats. Behavioural Brain Research. 2011;217(1):99–103. doi: 10.1016/j.bbr.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 33.Leffa D. T., de Souza A., Scarabelot V. L., et al. Transcranial direct current stimulation improves short-term memory in an animal model of attention-deficit/hyperactivity disorder. European Neuropsychopharmacology. 2016;26(2):368–377. doi: 10.1016/j.euroneuro.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Moher D., Liberati A., Tetzlaff J., Altman D. G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7, article e1000097) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krause M. R., Zanos T. P., Csorba B. A., et al. Transcranial direct current stimulation facilitates associative learning and alters functional connectivity in the primate brain. Current Biology. 2017;27(20):3086–3096.e3. doi: 10.1016/j.cub.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Manteghi F., Nasehi M., Zarrindast M. Precondition of right frontal region with anodal tDCS can restore the fear memory impairment induced by ACPA in male mice. EXCLI Journal. 2017;16:1–13. doi: 10.17179/excli2016-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasehi M., Soltanpour R., Ebrahimi-Ghiri M., Zarrabian S., Zarrindast M. R. Interference effects of transcranial direct current stimulation over the right frontal cortex and adrenergic system on conditioned fear. Psychopharmacology. 2017;234(22):3407–3416. doi: 10.1007/s00213-017-4722-6. [DOI] [PubMed] [Google Scholar]
- 38.Nasehi M., Khani-Abyaneh M., Ebrahimi-Ghiri M., Zarrindast M. R. The effect of left frontal transcranial direct-current stimulation on propranolol-induced fear memory acquisition and consolidation deficits. Behavioural Brain Research. 2017;331:76–83. doi: 10.1016/j.bbr.2017.04.055. [DOI] [PubMed] [Google Scholar]
- 39.Abbasi S., Nasehi M., Lichaei H. R. S., Zarrindast M. R. Effects of left prefrontal transcranial direct current stimulation on the acquisition of contextual and cued fear memory. Iranian Journal of Basic Medical Sciences. 2017;20(6):623–630. doi: 10.22038/IJBMS.2017.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podda M., Cocco S., Mastrodonato A., et al. Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression. Scientific Reports. 2016;6:p. 22180. doi: 10.1038/srep22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu T. H., Wu Y. J., Chien M. E., Hsu K. S. Transcranial direct current stimulation induces hippocampal metaplasticity mediated by brain-derived neurotrophic factor. Neuropharmacology. 2019;144(1):358–367. doi: 10.1016/j.neuropharm.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Dockery C., Liebetanz D., Birbaumer N., Malinowska M., Wesierska M. J. Cumulative benefits of frontal transcranial direct current stimulation on visuospatial working memory training and skill learning in rats. Neurobiology of Learning and Memory. 2011;96(3):452–460. doi: 10.1016/j.nlm.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Yu S., Park S., Sim K. The effect of tDCS on cognition and neurologic recovery of rats with Alzheimer’s disease. Journal of Physical Therapy Science. 2014;26(2):247–249. doi: 10.1589/jpts.26.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang C. H., Lane H. Y., Lin C. H. Brain stimulation in Alzheimer’s disease. Frontiers in Psychiatry. 2018;9 doi: 10.3389/fpsyt.2018.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roostaei A., Vaezi G., Nasehi M., Haeri-Rohani A., Zarrindast M. R. The Involvement of D1 and D2 dopamine receptors in the restoration effect of left frontal anodal, but not cathodal, tDCS on streptozocin-induced amnesia. Archives of Iranian Medicine. 2019;22(3):144–154. [PubMed] [Google Scholar]
- 46.Wu Y., Lin C., Yeh C., et al. Repeated transcranial direct current stimulation improves cognitive dysfunction and synaptic plasticity deficit in the prefrontal cortex of streptozotocin-induced diabetic rats. Brain Stimulation. 2017;10(6):1079–1087. doi: 10.1016/j.brs.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 47.Leffa D. T., Bellaver B., Salvi A. A., et al. Transcranial direct current stimulation improves long-term memory deficits in an animal model of attention-deficit/hyperactivity disorder and modulates oxidative and inflammatory parameters. Brain Stimulation. 2018;11(4):743–751. doi: 10.1016/j.brs.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Bragina O., Lara D., Nemoto E., Shuttleworth C. W., Semyachkina-Glushkovskaya O. V., Bragin D. E. Increases in microvascular perfusion and tissue oxygenation via vasodilatation after anodal transcranial direct current stimulation in the healthy and traumatized mouse brain. Advances in Experimental Medicine and Biology. 2018;1072:27–31. doi: 10.1007/978-3-319-91287-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spagnolo P., Goldman D. Neuromodulation interventions for addictive disorders: challenges, promise and roadmap for future research. Brain. 2017;140(5):1183–1203. doi: 10.1093/brain/aww284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedron S., Monnin J., Haffen E., Sechter D., van Waes V. Repeated transcranial direct current stimulation prevents abnormal behaviors associated with abstinence from chronic nicotine consumption. Neuropsychopharmacology. 2014;39(4):981–988. doi: 10.1038/npp.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pedron S., Beverley J., Haffen E., Andrieu P., Steiner H., van Waes V. Transcranial direct current stimulation produces long-lasting attenuation of cocaine-induced behavioral responses and gene regulation in corticostriatal circuits. Addiction Biology. 2017;22(5):1267–1278. doi: 10.1111/adb.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamida T., Kong S., Eshima N., Fujiki M. Cathodal transcranial direct current stimulation affects seizures and cognition in fully amygdala- kindled rats. Neurological Research. 2013;35(6):602–607. doi: 10.1179/1743132813Y.0000000170. [DOI] [PubMed] [Google Scholar]
- 53.Zobeiri M., van Luijtelaar G. Noninvasive transcranial direct current stimulation in a genetic absence model. Epilepsy & Behavior. 2013;26(1):42–50. doi: 10.1016/j.yebeh.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 54.Dhamne S. C., Ekstein D., Zhuo Z., et al. Acute seizure suppression by transcranial direct current stimulation in rats. Annals of Clinical and Translational Neurology. 2015;2(8):843–856. doi: 10.1002/acn3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rohan J. G., Carhuatanta K. A., McInturf S. M., Miklasevich M. K., Jankord R. Modulating hippocampal plasticity with in vivo brain stimulation. The Journal of Neuroscience. 2015;35(37):12824–12832. doi: 10.1523/JNEUROSCI.2376-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gondard E., Soto-Montenegro M., Cassol A., Lozano A. M., Hamani C. Transcranial direct current stimulation does not improve memory deficits or alter pathological hallmarks in a rodent model of Alzheimer’s disease. Journal of Psychiatric Research. 2019;114:93–98. doi: 10.1016/j.jpsychires.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Yoon K. J., Oh B. M., Kim D. Y. Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Research. 2012;1452:61–72. doi: 10.1016/j.brainres.2012.02.062. [DOI] [PubMed] [Google Scholar]
- 58.Hebb D. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 59.Rueger M., Keuters M., Walberer M., et al. Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PLoS One. 2012;7(8, article e43776) doi: 10.1371/journal.pone.0043776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peruzzotti-Jametti L., Cambiaghi M., Bacigaluppi M., et al. Safety and efficacy of transcranial direct current stimulation in acute experimental ischemic stroke. Stroke. 2013;44(11):3166–3174. doi: 10.1161/STROKEAHA.113.001687. [DOI] [PubMed] [Google Scholar]
- 61.McCaig C., Sangster L., Stewart R. Neurotrophins enhance electric field-directed growth cone guidance and directed nerve branching. Developmental Dynamics. 2000;217(3):299–308. doi: 10.1002/(SICI)1097-0177(200003)217:3<299::AID-DVDY8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 62.Pikhovych A., Stolberg N. P., Jessica Flitsch L., et al. Transcranial direct current stimulation modulates neurogenesis and microglia activation in the mouse brain. Stem Cells International. 2016;2016:9. doi: 10.1155/2016/2715196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Márquez-Ruiz J., Leal-Campanario R., Sánchez-Campusano R., et al. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(17):6710–6715. doi: 10.1073/pnas.1121147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krause B., Márquez-Ruiz J., Kadosh R. C. The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Frontiers in Human Neuroscience. 2013;7(602) doi: 10.3389/fnhum.2013.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antonenko D., Schubert F., Bohm F., et al. tDCS-induced modulation of GABA levels and resting-state functional connectivity in older adults. The Journal of Neuroscience. 2017;37(15):4065–4073. doi: 10.1523/JNEUROSCI.0079-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stagg C., Nitsche M. Physiological basis of transcranial direct current stimulation. The Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 67.Stagg C. J., Bachtiar V., Amadi U., et al. Local GABA concentration is related to network-level resting functional connectivity. eLife. 2014;3(3):1–9. doi: 10.7554/eLife.01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fritsch B., Reis J., Martinowich K., et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ranieri F., Podda M., Riccardi E., et al. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. Journal of Neurophysiology. 2012;107(7):1868–1880. doi: 10.1152/jn.00319.2011. [DOI] [PubMed] [Google Scholar]
- 70.Kronberg G., Bridi M., Abel T., Bikson M., Parra L. C. Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimulation. 2017;10(1):51–58. doi: 10.1016/j.brs.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun Y., Lipton J., Boyle L., et al. Direct current stimulation induces mGluR5-dependent neocortical plasticity. Annals of Neurology. 2016;80(2):233–246. doi: 10.1002/ana.24708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lüscher C., Malenka R. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harbor Perspectives in Biology. 2012;4(6):p. a005710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stafford J., Brownlow M., Qualley A., Jankord R. AMPA receptor translocation and phosphorylation are induced by transcranial direct current stimulation in rats. Neurobiology of Learning and Memory. 2018;150:36–41. doi: 10.1016/j.nlm.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Perret S., Cantereau A., Audin J., Dufy B., Georgescauld D. Interplay between Ca2+ release and Ca2+ influx underlies localized hyperpolarization-induced [Ca2+]i waves in prostatic cells. Cell Calcium. 1999;25(4):297–311. doi: 10.1054/ceca.1999.0030. [DOI] [PubMed] [Google Scholar]
- 75.Monai H., Ohkura M., Tanaka M., et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nature Communications. 2016;7:p. 11100. doi: 10.1038/ncomms11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mishima T., Nagai T., Yahagi K., et al. Transcranial direct current stimulation (tDCS) induces adrenergic receptor-dependent microglial morphological changes in mice. eNeuro. 2019;6(5):1–12. doi: 10.1523/ENEURO.0204-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Opazo P., Labrecque S., Tigaret C., et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67(2):239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 78.Kim M. S., Koo H., Han S. W., et al. Repeated anodal transcranial direct current stimulation induces neural plasticity-associated gene expression in the rat cortex and hippocampus. Restorative Neurology and Neuroscience. 2017;35(2):137–146. doi: 10.3233/RNN-160689. [DOI] [PubMed] [Google Scholar]
- 79.Leal G., Bramham C., Duarte C. BDNF and hippocampal synaptic plasticity. Vitamins and Hormones. 2017;104:153–195. doi: 10.1016/bs.vh.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 80.McCaig C., Rajnicek A., Song B., Zhao M. Controlling cell behavior electrically: current views and future potential. Physiological Reviews. 2005;85(3):943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- 81.Minichiello L. TrkB signalling pathways in LTP and learning. Nature Reviews Neuroscience. 2009;10(12):850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- 82.Alberini C. M. Transcription factors in long-term memory and synaptic plasticity. Physiological Reviews. 2009;89(1):121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carmichael S. T., Archibeque I., Luke L., Nolan T., Momiy J., Li S. Growth-associated gene expression after stroke: evidence for a growth- promoting region in peri-infarct cortex. Experimental Neurology. 2005;193(2):291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Li Y., Jiang N., Powers C., Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29(9):1972–1981. doi: 10.1161/01.STR.29.9.1972. [DOI] [PubMed] [Google Scholar]
- 85.Jiang T., Xu R. X., Zhang A. W., et al. Effects of transcranial direct current stimulation on hemichannel pannexin-1 and neural plasticity in rat model of cerebral infarction. Neurosciences. 2012;226:421–426. doi: 10.1016/j.neuroscience.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 86.Bargiotas P., Monyer H., Schwaninger M. Hemichannels in cerebral ischemia. Current Molecular Medicine. 2009;9(2):186–194. doi: 10.2174/156652409787581646. [DOI] [PubMed] [Google Scholar]
- 87.Dimov L., Franciosi A., Campos A., Brunoni A. R., Pagano R. L. Top-down effect of direct current stimulation on the nociceptive response of rats. PLoS One. 2016;11(4, article e0153506) doi: 10.1371/journal.pone.0153506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rioult-Pedotti M., Friedman D., Donoghue J. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 89.Malenka R., Bear M. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 90.Lisman J., Cooper K., Sehgal M., Silva A. J. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nature Neuroscience. 2018;21(3):309–314. doi: 10.1038/s41593-018-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Legrand M., Troubat R., Brizard B., le Guisquet A. M., Belzung C., el-Hage W. Prefrontal cortex rTMS reverses behavioral impairments and differentially activates c-Fos in a mouse model of post-traumatic stress disorder. Brain Stimulation. 2019;12(1):87–95. doi: 10.1016/j.brs.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 92.Moliadze V., Lyzhko E., Schmanke T., Andreas S., Freitag C. M., Siniatchkin M. 1 mA cathodal tDCS shows excitatory effects in children and adolescents: insights from TMS evoked N100 potential. Brain Research Bulletin. 2018;140:43–51. doi: 10.1016/j.brainresbull.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 93.Fisicaro F., Lanza G., Grasso A. A., et al. Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls. Therapeutic Advances in Neurological Disorders. 2019;12:p. 1756286419878317. doi: 10.1177/1756286419878317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cantone M., Bramanti A., Lanza G., et al. Cortical plasticity in depression. ASN Neuro. 2017;9(3) doi: 10.1177/1759091417711512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palm U., Segmiller F. M., Epple A. N., et al. Transcranial direct current stimulation in children and adolescents: a comprehensive review. Journal of Neural Transmission (Vienna) 2016;123(10):1219–1234. doi: 10.1007/s00702-016-1572-z. [DOI] [PubMed] [Google Scholar]
- 96.Leinekugel X., Khalilov I., McLean H., et al. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Advances in Neurology. 1999;79:189–201. [PubMed] [Google Scholar]
- 97.Martins C. W., de Melo Rodrigues L. C., Nitsche M. A., Nakamura-Palacios E. M. AMPA receptors are involved in prefrontal direct current stimulation effects on long-term working memory and GAP-43 expression. Behavioural Brain Research. 2019;362:208–212. doi: 10.1016/j.bbr.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 98.Nekhendzy V., Fender C., Davies M., et al. The antinociceptive effect of transcranial electrostimulation with combined direct and alternating current in freely moving rats. Anesthesia and Analgesia. 2004;98(3):730–7, table of contents. doi: 10.1213/01.ane.0000096007.12845.70. [DOI] [PubMed] [Google Scholar]
- 99.Taib N. O. B., Manto M. Trains of transcranial direct current stimulation antagonize motor cortex hypoexcitability induced by acute hemicerebellectomy. Journal of Neurosurgery. 2009;111(4):796–806. doi: 10.3171/2008.2.17679. [DOI] [PubMed] [Google Scholar]
- 100.Kim S., Kim B., Ko Y., Bang M. S., Kim M. H., Han T. R. Functional and histologic changes after repeated transcranial direct current stimulation in rat stroke model. Journal of Korean Medical Science. 2010;25(10):1499–1505. doi: 10.3346/jkms.2010.25.10.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laste G., Caumo W., Adachi L., et al. After-effects of consecutive sessions of transcranial direct current stimulation (tDCS) in a rat model of chronic inflammation. Experimental Brain Research. 2012;221(1):75–83. doi: 10.1007/s00221-012-3149-x. [DOI] [PubMed] [Google Scholar]
- 102.Adachi L. N. S., Caumo W., Laste G., et al. Reversal of chronic stress-induced pain by transcranial direct current stimulation (tDCS) in an animal model. Brain Research. 2012;1489:17–26. doi: 10.1016/j.brainres.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 103.Notturno F., Pace M., Zappasodi F., Cam E., Bassetti C. L., Uncini A. Neuroprotective effect of cathodal transcranial direct current stimulation in a rat stroke model. Journal of the Neurological Sciences. 2014;342(1-2):146–151. doi: 10.1016/j.jns.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 104.Lu C., Wei Y., Hu R., Wang Y., Li K., Li X. Transcranial Direct Current Stimulation Ameliorates Behavioral Deficits and Reduces Oxidative Stress in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Mouse Model of Parkinson's Disease. Neuromodulation. 2015;18(6):442–447. doi: 10.1111/ner.12302. [DOI] [PubMed] [Google Scholar]
- 105.Adachi L. N. S., Quevedo A. S., de Souza A., et al. Exogenously induced brain activation regulates neuronal activity by top-down modulation: conceptualized model for electrical brain stimulation. Experimental Brain Research. 2015;233(5):1377–1389. doi: 10.1007/s00221-015-4212-1. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y.-H., Liao L.-D., Chan S. J., Bandla A., Thakor N. V. An integrated neuroprotective intervention for brain ischemia validated by ECoG- fPAM. 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); Aug. 2016; Orlando, FL, USA. pp. 4009–4012. [DOI] [PubMed] [Google Scholar]
- 107.Braun R., Klein R., Walter H., et al. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Experimental Neurology. 2016;279:127–136. doi: 10.1016/j.expneurol.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 108.Cioato S. G., Medeiros L. F., Filho P. R. M., et al. Long-lasting effect of transcranial direct current stimulation in the reversal of hyperalgesia and cytokine alterations induced by the neuropathic pain model. Brain Stimulation. 2016;9(2):209–217. doi: 10.1016/j.brs.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 109.Filho P. R. M., Vercelino R., Cioato S. G., et al. Transcranial direct current stimulation (tDCS) reverts behavioral alterations and brainstem BDNF level increase induced by neuropathic pain model: Long- lasting effect. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2016;64:44–51. doi: 10.1016/j.pnpbp.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 110.da Silva Moreira S. F., Medeiros L. F., de Souza A., et al. Transcranial direct current stimulation (tDCS) neuromodulatory effects on mechanical hyperalgesia and cortical BDNF levels in ovariectomized rats. Life Sciences. 2016;145:233–239. doi: 10.1016/j.lfs.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 111.Liu Y., Chan S., Pan H., et al. Integrated treatment modality of cathodal-transcranial direct current stimulation with peripheral sensory stimulation affords neuroprotection in a rat stroke model. Neurophotonics. 2017;4(4, article 045002) doi: 10.1117/1.NPh.4.4.045002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Winkler C., Reis J., Hoffmann N., et al. Anodal transcranial direct current stimulation enhances survival and integration of dopaminergic cell transplants in a rat Parkinson model. eNeuro. 2017;4(5):1–11. doi: 10.1523/ENEURO.0063-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Souza A., Martins D. F., Medeiros L. F., et al. Neurobiological mechanisms of antiallodynic effect of transcranial direct current stimulation (tDCS) in a mice model of neuropathic pain. Brain Research. 2018;1682:14–23. doi: 10.1016/j.brainres.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 114.Paciello F., Podda M. V., Rolesi R., et al. Anodal transcranial direct current stimulation affects auditory cortex plasticity in normal-hearing and noise-exposed rats. Brain Stimulation. 2018;11(5):1008–1023. doi: 10.1016/j.brs.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 115.Fregni F., Macedo I., Spezia-Adachi L., et al. Transcranial direct current stimulation (tDCS) prevents chronic stress-induced hyperalgesia in rats. Brain Stimulation. 2018;11(2):299–301. doi: 10.1016/j.brs.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 116.Lee S., Youn J., Jang W., Yang H. O. Neuroprotective effect of anodal transcranial direct current stimulation on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in mice through modulating mitochondrial dynamics. Neurochemistry International. 2019;129:p. 104491. doi: 10.1016/j.neuint.2019.104491. [DOI] [PubMed] [Google Scholar]
- 117.Callai E. M. M., Scarabelot V. L., Fernandes Medeiros L., et al. Transcranial direct current stimulation (tDCS) and trigeminal pain: a preclinical study. Oral Diseases. 2019;25(3):888–897. doi: 10.1111/odi.13038. [DOI] [PubMed] [Google Scholar]
- 118.Scarabelot V., de Oliveira C., Medeiros L., et al. Transcranial direct-current stimulation reduces nociceptive behaviour in an orofacial pain model. Journal of Oral Rehabilitation. 2019;46(1):40–50. doi: 10.1111/joor.12726. [DOI] [PubMed] [Google Scholar]


