Abstract
Purpose
Anaphylaxis is an immediate allergic reaction characterized by potentially life-threatening, severe, systemic manifestations. While studies have evaluated links between serious illness and posttraumatic stress disorder (PTSD), few have investigated PTSD after anaphylaxis in adults. We sought to investigate the psychosocial burden of recent anaphylaxis in Korean adults.
Methods
A total of 203 (mean age of 44 years, 120 females) patients with anaphylaxis were recruited from 15 university hospitals in Korea. Questionnaires, including the Impact of Event Scale-Revised-Korean version (IES-R-K), the Korean version of the Beck Anxiety Inventory (K-BAI), and the Korean version of the Beck Depression Inventory (K-BDI), were administered. Demographic characteristics, causes and clinical features of anaphylaxis, and serum inflammatory markers, including tryptase, platelet-activating factor, interleukin-6, tumor necrosis factor-α, and C-reactive protein, were evaluated.
Results
PTSD (IES-R-K ≥ 25) was noted in 84 (41.4%) patients with anaphylaxis. Of them, 56.0% had severe PTSD (IES-R-K ≥ 40). Additionally, 23.2% and 28.1% of the patients had anxiety (K-BAI ≥ 22) and depression (K-BDI ≥ 17), respectively. IES-R-K was significantly correlated with both K-BAI (r = 0.609, P < 0.0001) and K-BDI (r = 0.550, P < 0.0001). Among the inflammatory mediators, tryptase levels were lower in patients exhibiting PTSD; meanwhile, platelet-activating factor levels were lower in patients exhibiting anxiety and depression while recovering from anaphylaxis. In multivariate analysis, K-BAI and K-BDI were identified as major predictive variables of PTSD in patients with anaphylaxis.
Conclusions
In patients with anaphylaxis, we found a remarkably high prevalence of PTSD and associated psychological distresses, including anxiety and depression. Physicians ought to be aware of the potential for psychological distress in anaphylactic patients and to consider psychological evaluation.
Keywords: Anaphylaxis; stress disorders, post-traumatic; psychological distress; anxiety; depression
INTRODUCTION
Anaphylaxis is a rapid and potentially fatal systemic response and is characterized by sudden degranulation of mast cells and basophils initiated by an immunological or non-immunological response.1,2 It is a relatively rare allergic disease, with a known prevalence ranging from 0.05% to 2%,3 and its causes, pathophysiology, and severity can vary greatly. Moreover, there are no biomarkers with which to guarantee a diagnosis of anaphylaxis. During anaphylaxis, the release of immunological mediators, such as histamine, tryptase, prostaglandin, and leukotriene, produce symptoms ranging from urticaria and angioedema at the mildest severity to dyspnea, loss of consciousness, and shock at the greatest severity. In result, patients can experience emotional stress leading to various mental diseases.4
Posttraumatic stress disorder (PTSD) is a psychiatric disorder with a reported lifetime prevalence of 6.8% in Americans.5 Its essential features comprise exposure to a traumatic event, re-experiencing the event, avoidance of stimuli reminding an individual of the trauma, and hyperarousal.6 One study reported that people who had suffered from anaphylactic shock could develop PTSD and other psychosocial dysfunctions.7 Since the trauma experienced during an anaphylaxis event can have a direct effect on the quality of life, investigating the psychological burden placed on patients with anaphylaxis is warranted.
PTSD has been found to exhibit relationships with diseases in which immune activation plays an important role, and some have speculated that alteration of inflammatory profiles, as observed in anaphylaxis, may contribute to the development of PTSD.8,9,10,11 Various inflammatory mediators are involved in the pathogenesis of anaphylaxis.4 Tryptase, which is released from activated mast cells in severe anaphylaxis, is the most reliable biomarker of anaphylaxis.4 Tryptase has been shown to stimulate monocytes and eosinophils to produce interlukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-1β.12 Among these, IL-6 is known to augment the acute phase response in inflammatory diseases, resulting in increasing C-reactive protein (CRP) levels.13,14 Another study described increased platelet-activating factor (PAF) levels in patients with anaphylaxis.15 Interestingly, several studies have suggested that elevated levels of these inflammatory mediators are associated with PTSD.16,17,18 Accordingly, we hypothesized that assessment of these mediators in patients with anaphylaxis would reveal links between them and psychiatric disorders, such as PTSD, anxiety, and depression.
In this study, we evaluated psychological distress (PTSD, anxiety, and depression) in patients with anaphylaxis using validated versions of related questionnaires translated in Korean and investigated relationships among inflammatory markers and distress severity.
MATERIALS AND METHODS
Subjects and data collection
A total of 203 adult patients with anaphylaxis (83 males and 120 females, mean age of 44 years) participated in this study. Patients who had been diagnosed with anaphylaxis in an emergency department or in an inpatient or outpatient setting were recruited. It was confirmed that all the patients in this study met the diagnostic criteria of anaphylaxis proposed by National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network.19 For the follow-up, all patients visited an allergy outpatient clinic for 10 to 102 days after anaphylaxis, at which time blood sampling and psychiatric questionnaires were administered. No control subject was included in this study. Informed consent was obtained from each patient. This study received Institutional Review Board approval (MED-SUR-16-012).
Demographic characteristics, including age, sex, atopy, underlying diseases (allergic, psychological, or other medical diseases), prior anaphylaxis, causes of anaphylaxis, and the severity of anaphylaxis, were collected. Severe anaphylaxis was defined as the presence of hypotension, hypoxemia, collapse, loss of consciousness, or incontinence.20,21 Information on the presence of any underlying psychiatric diseases was also acquired. Atopy was defined as at least 1 positive reaction to a skin prick test for 55 common inhalant allergens (Bencard Co., Brentford, UK), in which a positive reaction was defined as a ratio of the mean diameter of an allergen wheal greater than or equal to that of a histamine wheal. Administration of a systemic steroid or epinephrine was also recorded.
Korean versions of the Impact of Event Scale (IES-R-K)-Revised and other questionnaires
The IES-R-K, which is a modified Korean version of the IES-R, was used to detect PTSD.22 It is a self-rating questionnaire consisting of 22 questions designed to evaluate PTSD features, such as hyperarousal (feeling watchful and on guard), avoidance (an effort to avoid reminders of the trauma), and intrusion (dreams about the trauma). We categorized IES-R-K scores among the patients into three groups: normal (IES-R-K ≤ 24), wherein the risk of PTSD was low; mild-to-moderate (25 < IES-R-K ≤ 39); and severe (IES-R-K ≥ 40). The original cutoff points for the categorization of IES-R-K scores were 24, 39, and 60, allowing researchers to categorize subjects into one of four groups (≤ 24 = normal, 25-39 = mild/moderate, 40-59 = severe, ≥ 60 = very severe). However, as there were only 16 patients with an IES-R-K score over 60, we integrated the severe and very severe groups to maintain balance among groups.
For anxiety and depression, the Korean version of the Beck Anxiety Inventory (K-BAI) and the Beck Depression Inventory (K-BDI) were adopted. The K-BAI and K-BDI are self-rated questionnaires designed to evaluate anxiety and depression, respectively, both of which consist of 21 questions. Each of the questions is scaled from 0 to 3. Generally, a score of 22 or greater on K-BAI is considered to reflect the presence of anxiety symptoms, while a score of 17 or more on K-BDI is indicative of the presence of depressive symptoms.23,24
Measurement of serum inflammatory markers in the recovery phase
Sera from all patients were collected at each participating hospital and sent to Ajou University Medical Center for the assessment of inflammatory markers. Serum tryptase (ThermoFisher Scientific, Uppsala, Sweden), IL-6 (R&D Systems, Minneapolis, MN, USA), TNF-α (Thermo Fisher Scientific), CRP (R&D Systems), and PAF (LifeSpan BioSciences, Seattle, WA, USA) levels were determined by enzyme-linked immunosorbent assay. All experiments were conducted in accordance with manufacturer guidelines for each product.
Statistical analysis
Statistical analyses were performed using SPSS 22.0. Student's t test or the Mann-Whitney U test was applied for the analysis of continuous variables. Discrete variables were analyzed by the χ2 test or Fischer's exact test. For comparisons among 3 groups, an analysis of variance test or Kruskal-Wallis test was adopted according to the normality of the data. The Wilcoxon signed rank test was performed to evaluate quantitative changes in variables at different time points. Simple correlation analysis was used for the determination of correlation between continuous variables. To identify factors predictive of PTSD, single and multiple logistic regression analyses were conducted. All P values less than 0.05 were considered statistically significant.
RESULTS
Clinical characteristics of the study population
The study subjects comprised 83 (40.7%) males and 120 (59.3%) females (Table 1). Atopy was present in 69 (33.9%) patients. Among 88 (43.3%) patients with other allergic diseases, allergic rhinitis (80 patients, 39.6%) was the most common comorbid allergic condition, followed by food allergy (66 patients, 32.6%), atopic dermatitis (17 patients, 8.4%), asthma (13 patients, 6.4%), and chronic urticaria (11 patients, 5.4%). Underlying psychiatric diseases were noted in 6 (2.9%) patients. Drugs were the most common cause of anaphylaxis (114 patients, 56.1%), followed by food (52 patients, 25.6%), food-dependent exercise-induced anaphylaxis (16 patients, 7.8%), and bee stings (11 patients, 5.4%). Fifty-three patients (26.1%) had prior anaphylaxis events. More than half of the patients (110 patients, 54.2%) had severe anaphylaxis. Epinephrine and systemic steroids were administered in 61 (30.0%) and 63 (31.0%) patients, respectively. The mean time interval between an anaphylaxis event and administration of the questionnaires was 33.4 ± 19.6 days (range, 10-102 days).
Table 1. Demographic, clinical, and psychiatric characteristics of the patients with anaphylaxis.
| Characteristics | Values | |
|---|---|---|
| Sex (female) | 120 (59.3) | |
| Smoking history | 57 (28.2) | |
| Atopy | 69 (33.9) | |
| Allergic diseases | 88 (43.3) | |
| Asthma/AR | 13 (6.4)/80 (39.6) | |
| AD/CU/FA | 17 (8.4)/11 (5.4)/66 (32.6) | |
| Underlying psychiatric diseases | 6 (2.9) | |
| Causes of anaphylaxis | ||
| Drug | 114 (56.1) | |
| Food | 52 (25.6) | |
| FDEIA | 16 (7.8) | |
| Bee sting | 11 (5.4) | |
| Prior anaphylaxis | 53 (26.1) | |
| Severity of anaphylaxis | ||
| Nonsevere | 93 (45.8) | |
| Severe | 110 (54.2) | |
| Treatment | ||
| Epinephrine | 61 (30.0) | |
| Systemic steroids | 63 (31.0) | |
Data are shown as number (%).
AR, allergic rhinitis; AD, atopic dermatitis; CU, chronic urticarial; FA, food allergy; FDEIA, food-dependent exercise-induced anaphylaxis.
Psychological burden posed by anaphylaxis
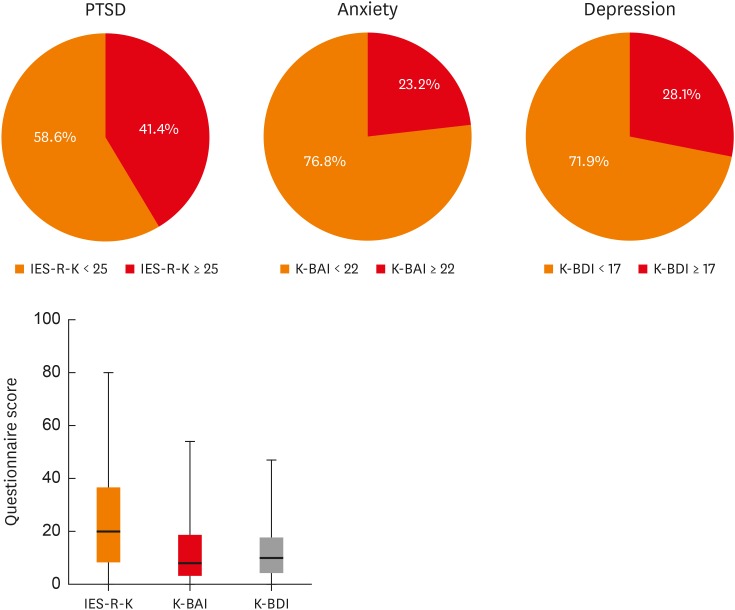
The mean IES-R-K score was 25.0 ± 20.5, and 84 (41.4%) patients had PTSD (IES-R-K ≥ 25). The mean K-BAI score was 13.1 ± 13.1, and 47 (23.2%) patients had anxiety (K-BAI ≥ 22). The average K-BDI score was 11.9 ± 9.7, and 57 (28.1%) patients had depression (K-BDI ≥ 17) (Fig. 1).
Fig. 1. Measurements of IES-R-K, K-BAI, and K-BDI and the proportion of patients who scored over the cut-off point of each questionnaire.
PTSD, posttraumatic stress disorder; IES-R-K, the Impact of Event Scale-Revised-Korean version; K-BAI, Korean version of the Beck Anxiety Inventory; K-BDI, Korean version of the Beck Depression Inventory.
Defined according to IES-R-K scores, the normal, mild-to-moderate, and severe PTSD groups comprised 119, 37, and 47 patients, respectively (Table 2). The proportion of female patients was highest in the severe PTSD group, although the difference was not statistically significant (56.3%, normal; 51.4%, mild-to-moderate; 72.3%, severe; P = 0.095). The mean ages of each group were similar (43.2 ± 14.5 year-old, normal; 46.2 ± 14.1 year-old, mild-to-moderate; 44.3 ± 14.9 year-old, severe; P = 0.409). There were 1 (0.8%) and 5 (10.7%) patients who had psychological diseases in the normal and severe groups, respectively, whereas no patient had a psychological disease in the mild-to-moderate group (P = 0.009). A history of prior anaphylaxis showed no statistical significance among the three PTSD groups (27/93 patients, 29.0%, normal; 8/29 patients, 27.6%, mild-to-moderate; 18/36 patients, 50.0%, severe; P = 0.058), nor did the severity of anaphylaxis (P = 0.484).
Table 2. Demographic, clinical, and psychiatric characteristics and serum tryptase levels of the patients with anaphylaxis according to IES-R-K.
| Characteristics | IES-R-K | P value | |||
|---|---|---|---|---|---|
| Normal: IES-R-K ≤ 24 (n = 119) | Mild-to-moderate: 25 ≤ IES-R-K ≤ 39 (n = 37) | Severe: IES-R-K ≥ 40 (n = 47) | |||
| Sex (female) | 67 (56.3) | 19 (51.4) | 34 (72.3) | 0.095 | |
| Age | 43.2 ± 14.5 | 46.2 ± 14.1 | 44.3 ± 14.9 | 0.409 | |
| Psychological diseases | 1 (0.8) | 0 (0) | 5 (10.7) | 0.009 | |
| Prior anaphylaxis | 27/93 (29.0) | 8/29 (27.6) | 18/36 (50.0) | 0.058 | |
| Severity of anaphylaxis | 0.484 | ||||
| Nonsevere | 51 (42.9) | 20 (54.1) | 22 (46.8) | ||
| Severe | 68 (57.1) | 17 (45.9) | 25 (53.2) | ||
| K-BAI | 7.6 ± 8.4 | 14.5 ± 12.4 | 26.2 ± 14.0 | < 0.0001 | |
| K-BAI ≥ 22 | 9 (7.6) | 10 (27.0) | 28 (59.6) | < 0.0001 | |
| K-BDI | 8.2 ± 7.4 | 13.0 ± 8.9 | 20.3 ± 10.2 | < 0.0001 | |
| K-BDI ≥ 17 | 16 (13.4) | 13 (35.1) | 28 (59.6) | < 0.0001 | |
| Levels at the anaphylaxis | |||||
| Log (serum tryptase) | 1.19 ± 0.52/32 | 0.72 ± 0.38/12 | 0.82 ± 0.69/15 | 0.067 | |
| Levels at the recovery | |||||
| Log (serum tryptase) | 0.55 ± 0.24/77 | 0.46 ± 0.17/23 | 0.47 ± 0.30/31 | 0.017 | |
Data are shown as mean ± standard deviation or number (%).
IES-R-K, the Impact of Event Scale-Revised-Korean version; K-BAI, Korean version of the Beck Anxiety Inventory; K-BDI, Korean version of the Beck Depression Inventory.
K-BAI scores increased with more severe PTSD (7.6 ± 8.4, normal; 14.5 ± 12.4, mild-to-moderate; 26.2 ± 14.0, severe; P < 0.0001), as did K-BDI scores (8.2 ± 7.4, normal; 13.0 ± 8.9, mild-to-moderate; 20.3 ± 10.2, severe; P < 0.0001). Also increasing with more severe PTSD were the proportions of patients with anxiety (K-BAI ≥ 22) (9 patients, 7.6%, normal; 10 patients, 27.0%, mild-to-moderate; 28 patients, 59.6%, severe; P < 0.0001) and the proportions of patients with depression (K-BDI ≥ 17) (16 patients, 13.4%, normal; 13 patients, 35.1%, mild-to-moderate; 28 patients, 59.6%, severe; P < 0.0001).
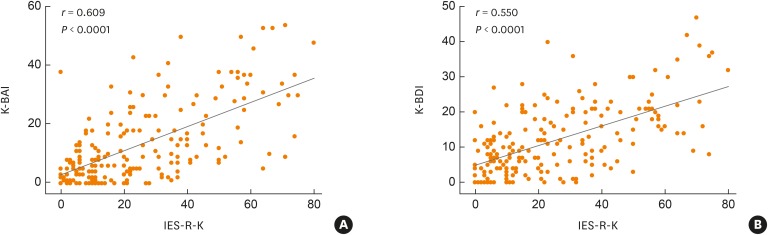
While log-transformed serum tryptase levels at the anaphylactic event were similar (1.19 ± 0.52, normal; 0.72 ± 0.38, mild-to-moderate; 0.82 ± 0.69, severe; P = 0.067), those after recovery from anaphylaxis were significantly higher in the normal group than in mild-to-moderate and severe groups (0.55 ± 0.24, normal; 0.46 ± 0.17, mild-to-moderate; 0.47 ± 0.30, severe; P = 0.017). Wilcoxon signed rank test indicated that serum tryptase levels significantly decreased during recovery, irrespective of IES-R-K score (P < 0.001). Meanwhile, IES-R-K scores in patients with anaphylaxis showed significant correlations with both K-BAI and K-BDI scores (r = 0.609, P < 0.0001 and r = 0.550, P < 0.0001, respectively) (Fig. 2).
Fig. 2. Correlations for IES-R-K with K-BAI (A) and K-BDI (B) in patients with anaphylaxis.
IES-R-K, the Impact of Event Scale-Revised-Korean version; K-BAI, Korean version of the Beck Anxiety Inventory; K-BDI, Korean version of the Beck Depression Inventory.
Differences in serum inflammatory markers according to anaphylaxis severity and IES-R-K, K-BAI, and K-BDI scores
Various serum inflammatory markers were compared in the present study (Table 3). None of the inflammatory markers, including serum tryptase, IL-6, TNF-α, CRP, and PAF, differed between the mild-to-moderate and severe anaphylaxis groups (tryptase, 3.6 ± 2.1 ng/mL vs. 3.5 ± 2.7 ng/mL, P = 0.741; IL-6, 5.3 ± 11.0 pg/mL vs. 9.3 ± 25.2 pg/mL, P = 0.223; TNF-α, 0.8 ± 4.1 pg/mL vs. 1.4 ± 7.7 pg/mL, P = 0.594; CRP, 1.1 ± 0.8 µg/mL vs. 1.2 ± 0.9 µg/mL, P = 0.636; PAF, 4.5 ± 3.2 ng/mL vs. 4.4 ± 3.2 ng/mL, P = 0.868). None of these markers, except for serum tryptase, showed significant differences when dichotomized according to an IES-R-K score < or ≥ 25 (tryptase, 3.2 ± 2.5 ng/mL vs. 3.8 ± 2.4 ng/mL, respectively, P = 0.050; serum IL-6, 8.2 ± 20.8 pg/mL vs. 6.9 ± 19.2 pg/mL, P = 0.434; serum TNF-α, 1.1 ± 4.8 pg/mL vs. 1.1 ± 7.1 pg/mL, P = 0.797; serum CRP, 1.0 ± 0.8 µg/mL vs. 1.2 ± 0.9 µg/mL, P = 0.338; serum PAF, 4.0 ± 3.0 ng/mL vs. 4.8 ± 3.3 ng/mL, P = 0.156). Only serum PAF levels exhibited significant differences between patients grouped according to K-BAI scores ≥ 22 or < 22 (3.6 ± 3.0 ng/mL vs. 4.8 ± 3.2 ng/mL, respectively, P = 0.045) and between those grouped according to K-BDI scores ≥ 17 or < 17 (PAF, 3.5 ± 2.8 ng/mL vs. 4.9 ± 3.3 ng/mL, respectively, P = 0.028).
Table 3. Differences of serum inflammatory mediators measured at the recovery phase according to the severity of anaphylaxis and the cut-off values of psychiatric questionnaires (IES-R-K, K-BAI, and K-BDI).
| Mediators | Severity of anaphylaxis | IES-R-K | K-BAI | K-BDI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonsevere (n = 67) | Severe (n = 75) | P value | ≥ 25 (n = 58) | < 25 (n = 84) | P value | ≥ 22 (n = 36) | < 22 (n = 106) | P value | ≥ 17 (n = 40) | < 17 (n = 102) | P value | |
| Tryptase (ng/mL) | 3.6 ± 2.1 | 3.5 ± 2.7 | 0.741 | 3.2 ± 2.5 | 3.8 ± 2.4 | 0.050 | 3.3 ± 2.7 | 3.6 ± 2.3 | 0.261 | 3.2 ± 2.8 | 3.7 ± 2.3 | 0.072 |
| IL-6 (pg/mL) | 5.3 ± 11.0 | 9.3 ± 25.2 | 0.223 | 8.2 ± 20.8 | 6.9 ± 19.2 | 0.434 | 4.6 ± 10.6 | 8.4 ± 22.0 | 0.460 | 5.1 ± 13.0 | 8.3 ± 21.9 | 0.105 |
| TNF-α (pg/mL) | 0.8 ± 4.1 | 1.4 ± 7.7 | 0.594 | 1.1 ± 4.8 | 1.1 ± 7.1 | 0.797 | 1.8 ± 6.0 | 0.9 ± 6.4 | 0.511 | 1.5 ± 5.7 | 1.0 ± 6.5 | 0.757 |
| CRP (µg/mL) | 1.1 ± 0.8 | 1.2 ± 0.9 | 0.636 | 1.0 ± 0.8 | 1.2 ± 0.9 | 0.338 | 1.0 ± 0.9 | 1.2 ± 0.8 | 0.265 | 0.9 ± 0.8 | 1.2 ± 0.9 | 0.069 |
| PAF (ng/mL) | 4.5 ± 3.2 | 4.4 ± 3.2 | 0.868 | 4.0 ± 3.0 | 4.8 ± 3.3 | 0.156 | 3.6 ± 3.0 | 4.8 ± 3.2 | 0.045 | 3.5 ± 2.8 | 4.9 ± 3.3 | 0.028 |
IES-R-K, the Impact of Event Scale-Revised-Korean version; K-BAI, Korean version of the Beck Anxiety Inventory; K-BDI, Korean version of the Beck Depression Inventory; IL-6, interlukin-6; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein; PAF, platelet-activating factor.
Predictors of PTSD severity
Univariate and multivariate logistic regression analyses were performed to identify predictors for PTSD (Table 4). The variables included demographic and clinical characteristics (female sex, prior anaphylaxis, causes of anaphylaxis, and severe anaphylaxis), as well as K-BAI and K-BDI scores (K-BAI ≥ 22 and K-BDI ≥ 17). In the univariate analysis, only K-BAI and K-BDI scores were predictors for PTSD (odds ration [OR], 10.10; 95% confidence interval [CI], 4.52-22.56; P < 0.0001, K-BAI ≥ 22; OR, 6.14; 95% CI, 3.11-12.10; P < 0.001, K-BDI ≥ 17). Multivariate analysis showed a K-BAI score of 22 or more as the most significant variable (OR, 6.15; 95% CI, 2.37–15.95; P < 0.0001), followed by a K-BDI score of 17 or more (OR, 2.63; 95% CI, 1.09–6.38; P = 0.032).
Table 4. Results of the univariate and multivariate logistic regression analyses for the presence of posttraumatic stress disorder.
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Sex (female) | 1.33 | 0.75–2.35 | 0.333 | 1.07 | 0.50–2.28 | 0.865 | |
| Prior anaphylaxis | 1.63 | 0.84–3.18 | 0.152 | 1.51 | 0.71–3.22 | 0.286 | |
| Cause | |||||||
| Drug | 1.06 | 0.60–1.85 | 0.851 | 1.29 | 0.49–3.38 | 0.601 | |
| Food | 0.85 | 0.45–1.62 | 0.621 | 1.01 | 0.35–2.93 | 0.982 | |
| Severe anaphylaxis | 1.33 | 0.76–2.34 | 0.315 | 1.23 | 0.59–2.59 | 0.578 | |
| K-BAI ≥ 22 | 10.10 | 4.52–22.56 | < 0.0001 | 6.15 | 2.37–15.95 | < 0.0001 | |
| K-BDI ≥ 17 | 6.14 | 3.11–12.10 | < 0.0001 | 2.63 | 1.09–6.38 | 0.032 | |
OR, odds ratio; CI, confidence interval; K-BAI, Korean version of the Beck Anxiety Inventory; K-BDI, Korean version of the Beck Depression Inventory.
DISCUSSION
This is the first multicenter study to investigate the relationships between anaphylaxis and psychological distress in Korea. Our results indicated that patients who experienced anaphylaxis were likely to develop psychiatric disorders, such as PTSD, anxiety, and depression. Among all 203 patients with anaphylaxis in this study, 84 (41.4%) had PTSD, and the development of PTSD did not appear to depend on the severity of anaphylaxis, although patients with higher IES-R-K scores had more severe anxiety and depression. While serum tryptase levels during anaphylaxis did not differ according to PTSD status, serum PAF levels were lower in patients with anxiety and depression during recovery from anaphylaxis. Anxiety and depression were identified as predictors of PTSD in patients who had recently experienced anaphylaxis.
Generally, the diagnosis of psychiatric diseases is established by psychiatrists using the Diagnostic and Statistical Manual of Mental Disorders. However, IES-R-K is commonly used by researchers to evaluate the presence of PTSD in Korea, since not all patients with possible PTSD visit a psychiatric specialist. In a previous study, the reliability and validity of the IES-R-K were deemed satisfactory as an instrument for PTSD assessment in Korea.25
Serum tryptase is considered a reliable marker of anaphylaxis and is widely used for the diagnosis of anaphylaxis.26 In the current study, the mean serum tryptase level during anaphylaxis was 24.7 ng/mL, and serum tryptase levels during anaphylaxis above the known cutoff value of anaphylaxis (11.4 ng/mL) were only present in 44.1% of patients.27 Also, we discovered that serum tryptase levels neither during anaphylaxis nor during recovery were correlated with the severity of anaphylaxis (P = 0.827 and P = 0.973, respectively). While baseline serum tryptase levels have been proposed as a possible biomarker for risk prediction of anaphylaxis,27 their ability to reflect the severity of anaphylaxis appears to be limited.
In this study, we found serum PAF levels to be lower in patients with anxiety and depression than in those without these conditions after an anaphylactic event. Secreted by multiple sources, including mast cells, monocytes, and macrophages, PAF is a proinflammatory phospholipid important in platelet activation, and more than 99% of serotonin, which plays an important role in mediating positive affect and mood, is found among dense platelet granules.28 Nevertheless, while there are numerous studies on the relationship between platelet activation and depression, it remains unclear whether the development of depression is attributable to increased or decreased platelet activation.29,30,31 Moreover, many factors other than PAF are thought to affect platelet activation. As the contributions of PAF to anxiety and depression in relation to platelet activation have undergone little research, further studies are warranted, employing tests of platelet aggregation or analyses of surface markers (P-selectin or activated glycoprotein IIb/IIIa) of and secreted chemicals (platelet factor 4 or β-thromboglobulin) from activated platelets.
Many psychiatric disorders are related to each other. In the present study, K-BAI and K-BDI showed incremental correlations with IES-R-K. Moreover, anxiety and depression were identified as the most reliable predictors for PTSD. Indeed, studies have shown PTSD to be accompanied by anxiety and depressive disorders after stressful events.32,33 Some have attempted to document genetic features connecting PTSD to other stress-related psychiatric disorders.34 Nevertheless, it is still unclear how psychiatric disorders exactly affect one another, and robust studies of the relationships among stress-related psychiatric disorders are needed, particularly in adults with anaphylaxis; while there are studies regarding anxiety and depression in children, their parents, or young adults with anaphylaxis, research in these areas on adult patients with anaphylaxis is scarce.35,36
There are several limitations in this study. First, clinical data on anaphylactic events were collected retrospectively. Since we planned to register the study participants when they visited for follow-up after anaphylaxis, we cannot exclude the possibility of selection bias. In addition, we were not able to compare our results with those in healthy controls. The second limitation is in relation to the timing of measurements of inflammatory markers. Except for serum tryptase, all inflammatory markers were measured only during the recovery phase of anaphylaxis, which was 102 days after the anaphylactic event at its latest. Moreover, the length of time until follow-up visit in each patient varied. As PAF has a very short half-life of 3 to 13 minutes,15 the results thereon as well as the other inflammatory markers might have differed if we had been able to measure it during the anaphylaxis. Despite these limitations, this study provides reliable information on psychological distress in patients with anaphylaxis. We prospectively evaluated the patients with questionnaires that were unified and reproducible, and the prevalence of psychiatric disorders in the anaphylaxis patients was remarkably higher than that reported in general populations.
In conclusion, we found that patients with anaphylaxis are prone to develop psychiatric disorders such as PTSD, anxiety, and depression. Accordingly, we suggest that the general management of anaphylaxis ought to include psychiatric evaluation.
ACKNOWLEDGMENTS
This research was supported by the Korean Academy of Asthma, Allergy and Clinical Immunology and partly by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2018R1A2B6006199).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.Simons FE, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8:32. doi: 10.1186/s40413-015-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. J Allergy Clin Immunol. 2002;110:341–348. doi: 10.1067/mai.2002.126811. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Allen KJ, Suaini NH, McWilliam V, Peters RL, Koplin JJ. The global incidence and prevalence of anaphylaxis in children in the general population: a systematic review. Allergy. 2019;74:1063–1080. doi: 10.1111/all.13732. [DOI] [PubMed] [Google Scholar]
- 4.Sala-Cunill A, Cardona V. Biomarkers of anaphylaxis, beyond tryptase. Curr Opin Allergy Clin Immunol. 2015;15:329–336. doi: 10.1097/ACI.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 6.Vieweg WV, Julius DA, Fernandez A, Beatty-Brooks M, Hettema JM, Pandurangi AK. Posttraumatic stress disorder: clinical features, pathophysiology, and treatment. Am J Med. 2006;119:383–390. doi: 10.1016/j.amjmed.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Chung MC, Rudd H, Wall N. Posttraumatic stress disorder following asthma attack (post-asthma attack PTSD) and psychiatric co-morbidity: the impact of alexithymia and coping. Psychiatry Res. 2012;197:246–252. doi: 10.1016/j.psychres.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Kubzansky LD, Koenen KC, Spiro A, 3rd, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry. 2007;64:109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin RD, Davidson JR. Self-reported diabetes and posttraumatic stress disorder among adults in the community. Prev Med. 2005;40:570–574. doi: 10.1016/j.ypmed.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Bedi US, Arora R. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc. 2007;99:642–649. [PMC free article] [PubMed] [Google Scholar]
- 11.O'Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atiakshin D, Buchwalow I, Samoilova V, Tiemann M. Tryptase as a polyfunctional component of mast cells. Histochem Cell Biol. 2018;149:461–477. doi: 10.1007/s00418-018-1659-8. [DOI] [PubMed] [Google Scholar]
- 13.Lin RY, Trivino MR, Curry A, Pesola GR, Knight RJ, Lee HS, et al. Interleukin 6 and C-reactive protein levels in patients with acute allergic reactions: an emergency department-based study. Ann Allergy Asthma Immunol. 2001;87:412–416. doi: 10.1016/S1081-1206(10)62923-7. [DOI] [PubMed] [Google Scholar]
- 14.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 16.Baker DG, Nievergelt CM, O'Connor DT. Biomarkers of PTSD: neuropeptides and immune signaling. Neuropharmacology. 2012;62:663–673. doi: 10.1016/j.neuropharm.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-α, and IL-6 in women with PTSD. J Trauma Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- 19.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 20.Cox LS, Sanchez-Borges M, Lockey RF. World Allergy Organization systemic allergic reaction grading system: is a modification needed? J Allergy Clin Immunol Pract. 2017;5:58–62.e5. doi: 10.1016/j.jaip.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Blazowski L, Majak P, Kurzawa R, Kuna P, Jerzynska J. Food allergy endotype with high risk of severe anaphylaxis in children-Monosensitization to cashew 2S albumin Ana o 3. Allergy. 2019;74:1945–1955. doi: 10.1111/all.13810. [DOI] [PubMed] [Google Scholar]
- 22.Eun HJ, Kwon TW, Lee SM, Kim TH, Choi MR, Cho SJ. A study on reliability and validity of the Korean Version of Impact of Event Scale-Revised. J Korean Neuropsychiatr Assoc. 2005;44:303–310. [Google Scholar]
- 23.Yook SP, Kim ZS. A clinical study on the Korean version of Beck Anxiety Inventory: comparative study of patient and non-patient. Korean J Clin Psychol. 1997;16:185–197. [Google Scholar]
- 24.Rhee MK, Lee YH, Jung HY, Choi JH, Kim SH, Kim YK, et al. A standardization study of Beck Depression Inventory (II): Korean version (K-BDI): validity. Korean J Psychopathol. 1995;4:96–104. [Google Scholar]
- 25.Lim HK, Woo JM, Kim TS, Kim TH, Choi KS, Chung SK, et al. Reliability and validity of the Korean version of the Impact of Event Scale-Revised. Compr Psychiatry. 2009;50:385–390. doi: 10.1016/j.comppsych.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987;316:1622–1626. doi: 10.1056/NEJM198706253162603. [DOI] [PubMed] [Google Scholar]
- 27.Sahiner UM, Yavuz ST, Buyuktiryaki B, Cavkaytar O, Yilmaz EA, Tuncer A, et al. Serum basal tryptase may be a good marker for predicting the risk of anaphylaxis in children with food allergy. Allergy. 2014;69:265–268. doi: 10.1111/all.12317. [DOI] [PubMed] [Google Scholar]
- 28.Pivac N, Mück-Šeler D, Šagud M, Jakovljević M, Mustapić M, Mihaljević-Peleš A. Long-term sertraline treatment and peripheral biochemical markers in female depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:759–765. doi: 10.1016/S0278-5846(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 29.Ziegelstein RC, Parakh K, Sakhuja A, Bhat U. Platelet function in patients with major depression. Intern Med J. 2009;39:38–43. doi: 10.1111/j.1445-5994.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 30.Skop BP, Brown TM. Potential vascular and bleeding complications of treatment with selective serotonin reuptake inhibitors. Psychosomatics. 1996;37:12–16. doi: 10.1016/S0033-3182(96)71592-X. [DOI] [PubMed] [Google Scholar]
- 31.Uebelhack R, Franke L, Herold N, Plotkin M, Amthauer H, Felix R. Brain and platelet serotonin transporter in humans-correlation between [123I]-ADAM SPECT and serotonergic measurements in platelets. Neurosci Lett. 2006;406:153–158. doi: 10.1016/j.neulet.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Kartal D, Kiropoulos L. Effects of acculturative stress on PTSD, depressive, and anxiety symptoms among refugees resettled in Australia and Austria. Eur J Psychotraumatol. 2016;7:28711. doi: 10.3402/ejpt.v7.28711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thabet AM, Thabet SS, Vostanis P. The relationship between war trauma, PTSD, depression, and anxiety among Palestinian children in the Gaza Strip. Health Sci J. 2016;10:1. [Google Scholar]
- 34.Smoller JW. The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology. 2016;41:297–319. doi: 10.1038/npp.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbert LJ, Dahlquist LM. Perceived history of anaphylaxis and parental overprotection, autonomy, anxiety, and depression in food allergic young adults. J Clin Psychol Med Settings. 2008;15:261–269. doi: 10.1007/s10880-008-9130-y. [DOI] [PubMed] [Google Scholar]
- 36.Akeson N, Worth A, Sheikh A. The psychosocial impact of anaphylaxis on young people and their parents. Clin Exp Allergy. 2007;37:1213–1220. doi: 10.1111/j.1365-2222.2007.02758.x. [DOI] [PubMed] [Google Scholar]