Abstract
Background and Aims
Plants inhabiting arid environments tend to have leaf trichomes, but their adaptive significance remains unclear. Leaf trichomes are known to play a role in plant defence against herbivores, including gall makers. Because gall formation can increase water loss partly through increased surface area, we tested the novel hypothesis that leaf trichomes could contribute to avoiding extra water stress by impeding gall formation, which would have adaptive advantages in arid environments.
Methods
We focused on Metrosideros polymorpha, an endemic tree species in the Hawaiian Islands, whose leaves often suffer from galls formed by specialist insects, Hawaiian psyllids (Pariaconus spp.). There is large variation in the amount of leaf trichomes (0–40 % of leaf mass) in M. polymorpha. Three gall types are found on the island of Hawaii: the largest is the ‘cone’ type, followed by ‘flat’ and ‘pit’ types. We conducted laboratory experiments to quantify the extent to which gall formation is associated with leaf water relations. We also conducted a field census of 1779 individuals from 48 populations across the entire range of habitats of M. polymorpha on the island of Hawaii to evaluate associations between gall formation (presence and abundance) and the amount of leaf trichomes.
Key Results
Our laboratory experiment showed that leaf minimum conductance was significantly higher in leaves with a greater number of cone- or flat-type galls but not pit-type galls. Our field census suggested that the amount of trichomes was negatively associated with probabilities of the presence of cone- or flat-type galls but not pit-type galls, irrespective of environmental factors.
Conclusion
Our results suggest that leaf trichomes in M. polymorpha can contribute to the avoidance of extra water stress through interactions with some gall-making species, and potentially increase the fitness of plants under arid conditions.
Keywords: Leaf trichome, water limitation, plant, insect interaction, defence, gall, Hawaiian psyllid, Pariaconus, Metrosideros polymorpha
Introduction
Plants under water-stress conditions, such as arid and sun-exposed environments and during dry seasons, tend to have a greater density or amount of leaf trichomes compared with those under mesic conditions (e.g. Aronne and De Micco, 2001; Agrawal et al., 2009; Ichie et al., 2016). These patterns may be attributable to one or multiple functions of leaf trichomes, including reflecting sunlight (e.g. Ehleringer, 1984), saving water by increasing the boundary layer (e.g. Wuenscher, 1970) and promoting foliar water uptake (e.g. Benzing et al., 1976). On the other hand, defence against herbivores, which is one of the major functions of leaf trichomes for a wide range of taxa, is not typically considered to be a key function in water-stress conditions. Leaf trichomes play a role of physical and chemical resistance against insect oviposition and/or feeding (Johnson, 1975; Levin, 1973; Dalin et al., 2008), which appears to be independent of drought tolerance. However, we consider that leaf trichomes could indirectly contribute to the plant’s adaptation to arid environments through plant–herbivore interactions, especially for plants suffering from gall-makers. Gall formation is likely to increase water stress in host plants (Florentine et al., 2005; Nabity et al., 2012) because galls increase the surface area of leaves and in theory increase evaporative water loss. In line with this hypothesis, Bailey et al. (2015) reported that genes associated with drought tolerance were notably expressed in galled leaves compared with ungalled leaves. Based on these studies, we propose the novel hypothesis that leaf trichomes have adaptive significance under arid conditions by impeding gall formation, which otherwise increases water stress.
Metrosideros polymorpha, an endemic and dominant tree species in the Hawaiian Islands, shows extreme phenotypic variations across a wide range of habitat conditions: mean annual temperature (MAT) ranges from 8 to 23 °C, mean annual precipitation (MAP) from <400 to >10 000 mm year−1 and soil age (SA) from a few decades to >4 000 000 years (Kitayama and Mueller-Dombois, 1995; Cordell et al., 1998; Cornwell et al., 2007). The variation in the amount of trichomes (non-glandular) on the lower leaf surface is remarkable, ranging from 0 to 150 g m−2, and trichomes account for up to 40 % of total leaf mass (Joel et al., 1994; Tsujii et al., 2016; Amada et al., 2017). Such large phenotypic diversity is mostly genetically determined rather than a result of acclimation to habitat conditions (Tsujii et al., 2016) and is associated with three major varieties on the island of Hawaii: a glabrous variety (var. glaberrima) and two pubescent varieties (var. polymorpha and var. incana). It should be noted that there are many intermediate forms due to a lack of reproductive isolation mechanisms among the varieties (Stacy et al., 2014, 2016). Glabrous individuals are often abundant in moderately wet areas whereas pubescent individuals are more abundant in dryland, high-elevation or bog areas where plants may suffer from drought, low-temperature or anaerobic conditions (Vitousek et al., 1992; Stacy et al., 2014; Tsujii et al., 2016). While previous studies have repeatedly considered that leaf trichomes have adaptive significance in arid environments (Joel et al., 1994; Hoof et al., 2008; Tsujii et al., 2016), Amada et al. (2017) showed that the increased boundary-layer resistance due to the leaf trichomes of M. polymorpha had negligible effects on water-use efficiency, which is calculated as the ratio of assimilation rate to transpiration rate. Therefore, leaf trichomes should have ecological functions, other than boundary-layer resistance, that can contribute to higher fitness under water stress.
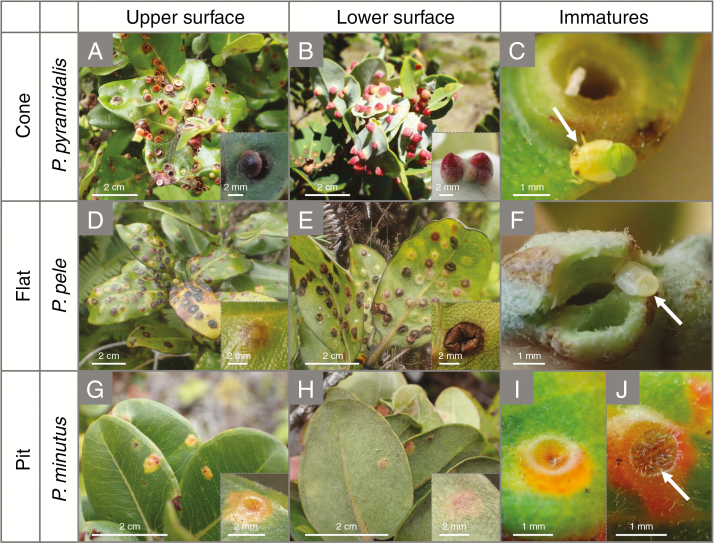
In the present study we focus on plant–insect interactions in relation to water stress. Leaves of M. polymorpha often suffer from galls of various morphological types made by endemic Hawaiian psyllids (Pariaconus spp.; Fig. 1; Nishida et al., 1980; Lee, 1981; Gruner 2004; Percy, 2017). Thirty-six Pariaconus species, which all occur only on M. polymorpha, have diversified from a single ancestor probably in response to diverse phenotypes of the host tree species across the Hawaiian islands (Percy, 2017). This M. polymorpha–Pariaconus relationship offers an ideal model system to understand how plant–insect interactions are formed and how they are related to plant adaptation under water stress. The morphology of galls differs depending on psyllid species (but not all psyllid species make galls as some species are free-living; Percy, 2017). On the island of Hawaii, three morphological types of gall are commonly observed on leaves of M. polymorpha: cone-, flat- and pit-type galls, which are induced by P. pyramidalis, P. pele and P. minutus, respectively (Fig. 1; Nishida et al., 1980; Percy, 2017). Pariaconus pyramidalis makes enclosed cone-type galls that considerably extend from the leaf lower surface, and the galls open on the leaf upper surface by a circular fissure resembling a trapdoor (Fig. 1A–C; Percy, 2017). Pariaconus pele makes enclosed flat-type galls that extend moderately from both sides of the leaves, and the galls open on the leaf’s lower surface by irregular fissures (Fig. 1D–F; Percy, 2017). Pariaconus minutus makes small open pit-type galls that occur only on the upper leaf surface, and the immature psyllids develop on the galls, which do not dehisce (Fig. 1G–J; Percy, 2017). Pariaconus pyramidalis and P. pele attack leaves from the leaf’s lower surface while P. minutus attacks from the leaf’s upper surface (Percy, 2017; leaf trichomes are located on the leaf’s lower side).
Fig. 1.
Representive images of galls induced by Hawaiian psyllids (Pariaconus spp.) on leaves of Metrosideros polymorpha. (A–C) Cone-type galls (large size) from the upper (A) and lower leaf surface (B), and an immature psyllid uncovered by dissection of the enclosing gall (C). (D–F) Flat-type galls (intermediate size) from the upper (D) and lower leaf surface (E), and an immature psyllid uncovered by dissection of the enclosing gall (F). (G–I) Pit-type galls (small size) from the upper (G) and lower leaf surface (H), and an immature psyllid developed in the hollow of the open gall (I, J). White arrows indicate immature forms of each psyllid (C, F, J).
Previous studies showed that the number of psyllid galls was smaller on pubescent individuals than on glabrous ones at some sites but not at other sites on the island of Hawaii without classifying the morphological types of galls (Lee, 1981; Gruner et al., 2005). Recently, Percy (2017) reported that cone- and flat-type galls were found in both glabrous and pubescent individuals, but pit-type galls were typically found in pubescent individuals. Nishida et al. (1980) found that the distributions of these psyllid species were different along elevation, at least on a south-eastern slope of Mauna Loa, implying that psyllid species may have distinct preferences for environmental conditions (Price et al., 1987; Stone and Schönrogge, 2003) such as temperature (Henson, 1958), aridity (Fernandes and Price, 1992; Price et al., 1998) or soil fertility (Blanche and Westoby, 1995). Therefore, the impact of leaf traits on the abundance of the Hawaiian psyllids needs to be examined for each species with consideration of these environmental conditions.
In order to test the above-mentioned novel hypothesis, that leaf trichomes have adaptive significance under arid conditions by impeding gall formation, first we conducted a laboratory experiment to examine whether water loss is higher in leaves with a greater number of galls. Second, across 48 populations on the island of Hawaii (1799 individuals), we examined the extent to which in situ variation in leaf trichomes is associated with the presence/absence or abundance of each type of gall, while considering other leaf traits and environmental conditions. Based on these examinations, we discuss the ecological significance of the large variation in leaf trichome amounts in M. polymorpha.
MATERIALS AND METHODS
Study species
Metrosideros polymorpha (Myrtaceae) is an endemic and dominant tree species in the Hawaiian islands. This species is distributed across a wide range of habitat conditions, as described later, and shows a large degree of phenotypic polymorphism (Stemmermann, 1983; Joel et al., 1994; Tsujii et al., 2016). While the variation in phenotypic traits is continuous (Tsujii et al., 2016), M. polymorpha on the island of Hawaii has been classified into three major varieties, namely var. polymorpha, var. glaberrima and var. incana. In this study, however, we did not discriminate between these varieties as there were many intermediate forms. Instead we measured some key traits, such as the amount of leaf trichomes, in a quantitative manner described later. In the following sections, we describe (1) experiments that quantified the extent to which gall abundance is associated with leaf water relations, and (2) field investigations that evaluated associations between galls (presence and abundance) and leaf traits across 48 study sites covering almost the full range of habitat conditions of M. polymorpha on the island of Hawaii.
Leaf physiological functions with and without galls
To evaluate the associations between galls and leaf water relations, we conducted physiological and morphological measurements on leaf samples that were collected from one site where all three types of galls were found (ML-E-700-Y; altitude 750 m, MAT 17.1 °C, MAP 5934 mm year−1; Supplementary Data Table S1, Fig. S1). To determine the size of galls, we measured thicknesses of the galled parts and the intact parts of mature leaves using a thickness gauge (7173, Mitsutoyo, Japan) (11, 28 and 7 pairs were measured for cone-, flat- and pit-type galls, respectively).
To examine whether the presence of galls is associated with foliar specific mass and water content in leaves, we compared mass per area and water content per area between the intact and galled parts of mature leaves for each gall type separately. Prior to this measurement, leaves were fully hydrated with a wet paper towel sealed in Ziploc plastic bags overnight. We punched out three 6-mm-diameter discs for galled parts and intact parts, respectively, using a hole punch. We measured the weights of these discs before and after drying in an oven (70 °C) for more than 2 d. Mass per area (g m−2) and water content per area (g m−2) were defined as dry mass and water mass per disc area, respectively (10, 10 and 8 leaves were measured for cone-, flat- and pit-type galls, respectively). Because all diameters of galls were <6 mm (i.e. the size of a leaf disc), the excised discs included intact leaf as well as galled parts; therefore, the increases in mass per area and water content per area due to gall formation should be considered as conservative estimates.
To examine whether the abundance of galls is associated with water loss in leaves, we measured leaf minimum conductance (gmin), which is defined as water vapour conductance when stomata are assumed to be closed (Duursma et al., 2019). Leaf minimum conductance was measured from the rate of water loss from a leaf in a dark condition (photosynthetic photon flux density <10 µmol m−2 s−1; Sack et al., 2003). We selected a pair of mature leaves: one with galls and another without galls for each individual (1–28 galls per leaf). We included six pairs of leaves that had no galls to quantify natural variation between pairs of leaves. After the leaves had been fully hydrated with a wet paper towel in Ziploc plastic bags overnight, we dried leaves in a dark condition at room temperature (21.0 ± 1.0 °C; mean ± s.e.) and relative humidity 73.0 ± 5.0 %, and then measured changes in the weights of leaves more than eight times at intervals of 30 min. Across the measurements, the leaf weight and drying time showed a linear relationship (R2 > 0.99), which suggested that the stomata were closed (Sack et al., 2003). After the mass measurement, leaf areas were measured from the scanned leaf images (GT-S630, Epson, Japan) using ImageJ (National Institutes of Health, USA). We calculated leaf minimum conductance per area (gmin; mmol m−2 s−1) from the rate of decrease in leaf weight per area (13, 21 and 10 paired leaves were measured for cone, flat and pit types, respectively).
Associations among galls, leaf traits and environmental conditions
During July and August 2016 and 2017, we established 48 study sites that covered almost the full range of habitat conditions of M. polymorpha on the island of Hawaii; altitude ranged from 10 to 2400 m a.s.l. (from coast to treeline), MAT from 8.9 to 23.3 °C, MAP from 470 to 6390 mm year−1, aridity index (AI) from 0.29 to 3.95 and soil age from 45 to >260 000 years (Supplementary Data Table S1, Fig. S1; Sherrod et al., 2007; Giambelluca et al., 2014). The AI was calculated as MAP/mean annual potential evapotranspiration and can be used as an index of dryness: more arid when AI is smaller (AI < 0.03, hyper-arid zone; 0.03 < AI < 0.20, arid zone; 0.20 < AI < 0.50, semi-arid zone; 0.50 < AI < 0.75, dry sub-humid zone; AI > 0.75, humid zone; UNESCO, 1979). At each site, 40 shoots were collected from the outer crown of different individuals (1779 individuals in total) to analyse the associations among galls, leaf traits and environmental conditions.
For each individual, we counted the numbers of cone-, flat- and pit-type galls on 6–14 leaves of the second-newest shoot, which is more stable and ideal for counting the number of galls than the newest shoot because psyllids typically attack the newly developing shoots. We identified each type of gall according to Percy (2017) (see Introduction section and Fig. 1). Although the number of galls is not equal to the abundance of adult psyllids, we assumed that the presence of galls reflects the presence of adult psyllids to some extent; thus we can explore distribution patterns of three psyllid species in relation to environmental conditions.
We used fully matured young leaves to measure leaf traits in order to facilitate the comparison of leaf traits among individuals or among populations. We divided leaf mass into lamina mass and trichome mass by a shaving technique (Tsujii et al., 2016). We defined leaf mass per area (LMA) associated with lamina and trichome as LMAL and LMAT, respectively, according to Tsujii et al. (2016) (i.e. LMA = LMAL + LMAT). On one half-side of a mature leaf (separated by the midrib), we shaved leaf trichomes using a rubber thimble, and punched out two 10-mm-diameter discs from both sides of the leaf. These leaf discs were dried in silica gel and used for weighing with a digital scale (0.01 mg precision). We calculated LMAT from the differences in dry weight between the discs with and without trichomes.
Statistical analyses
To evaluate the extent to which gall formation influenced leaf physiological functions, we calculated the response ratios (Rr) of thickness, mass per area, water content per area, water content per fresh mass and gmin for gall formation as follows:
| (1) |
where RrX is the ratio of a trait X with gall formation (XG) to that without galls (XI). We calculated Rr based on leaf discs except for gmin, for which whole leaves were used. We considered Rr separately for cone-, flat- and pit-type galls. As ratios have a log-normal distribution by nature (Sokal and Rohlf, 1995), Rr was natural log-transformed before all statistical analyses.
Student’s t-test was used to test the differences between galled and intact samples. One-way analysis of variance with post hoc Turkey HSD multiple comparisons was used to test the differences in traits among the three gall types. A standardized major axis (SMA) slope was used to fit bivariate relationships between the natural log-transformed Rr of gmin and the natural log-transformed number of galls per leaf area. Mean values and standard deviation of each trait in the galled and intact leaves are shown in Supplementary Data Fig. S2.
To examine whether leaf traits and environmental conditions were associated with gall formation (presence and abundance), we used a zero-altered negative-binomial generalized linear mixed model (glmmADMB package of R; Skaug et al., 2012). This model was used because many shoots had no galls and the frequency of the number of galls, except for non-galling shoots, was strongly overdispersed from the Poisson distribution (Supplementary Data Fig. S3). The zero-altered negative-binomial model is based both on the binomial distribution model for presence/absence data and on the negative binomial distribution model for count data except for zero (Martin et al., 2005; Zuur et al., 2009). Thus, we constructed two models to test the associations of environmental conditions and leaf traits with the presence or absence of galls (Model I) and with the number of galls (Model II). This is the standard analytical approach for this type of dataset (Zuur et al., 2009). In terms of the choices of leaves by psyllids, we assumed that Model I is more relevant because the presence/absence data may be related to whether or not psyllids decide to lay eggs. On the other hand, Model II may be more related to the behaviour or capacity of psyllids after they decide to lay eggs. We assumed that the associations between leaf traits and presence/absence of galls (Model I) or the number of galls (Model II) irrespective of environmental factors reflect the influences of leaf traits on gall formation. In addition, we assumed that the associations between environmental conditions and presence/absence of galls (Model I) reflect the habitat preferences of each psyllid species.
Because we intended to test whether the amount of leaf trichomes has consistent negative effects on gall formation, a linear term was assumed for this factor in the model. On the other hand, because psyllids may have a unimodal distribution across environmental gradients (e.g. elevational gradients; Nishida et al., 1980), both linear and quadratic terms were assumed for the environmental factors in the model. A significant negative quadratic term can be selected if psyllids have a unimodal distribution across the environmental gradient. We employed mean annual temperature, aridity index and soil age (as indicator of soil fertility; Vitousek et al., 1988) as explanatory variables in the models because the abundance of gall makers may be related to temperature (Henson, 1958), aridity (Fernandes and Price, 1992; Price et al., 1998) and soil fertility (Blanche and Westoby, 1995). We examined the estimated effects of LMAT, LMAL, mean annual temperature (MAT, MAT2), aridity index (AI, AI2) and soil age (SA, SA2) as fixed effects, and site as a random effect, on the numbers of each type of galls per leaf area. Although leaf traits and environmental conditions were not independent of each other on the island of Hawaii (Joel et al., 1994; Tsujii et al., 2016), we treated these parameters as independent fixed effects due to the small variance inflation factor (VIF; <1.2). Each fixed effect was standardized (zero mean and standard deviation equal to 1). Because the number of galls should increase with leaf area per shoot, total leaf area of a shoot was used as an offset term in Model II. Soil age was used as the median of soil age ranges available from Sherrod et al. (2007) for the analysis (Supplementary Data Table S1). Soil ages were natural log-transformed before standardization. Akaike’s information criterion (AIC) was used to examine which explanatory variables were important for the abundance of each type of gall (Supplementary Data Table S2).
All analyses were carried out with the R statistical package (version 3.5.0; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Leaf water relations and gall formation
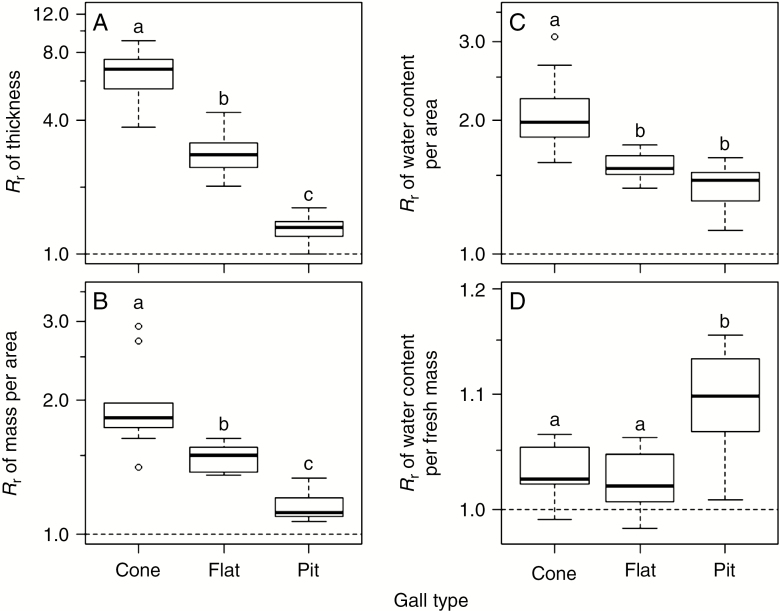
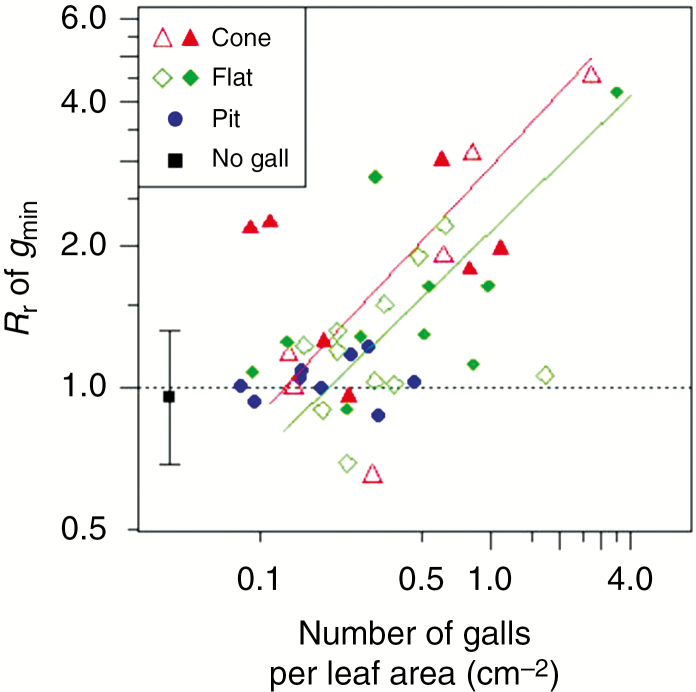
The leaf segments with galls had significantly higher thickness, mass per area, water content per area and water content per fresh mass irrespective of gall type (P < 0.05; Fig. 2A–D). These increases in thickness, mass per area and water content per area were largest in the cone type, followed by the flat and pit types (Fig. 2A–C). The increase in water content per fresh mass was larger in the pit type than in the cone and flat types (Fig. 2D). Our conservative estimates (see Materials and methods section) showed that the mean response ratio of mass per area was 197, 149 and 115 %, and the mean response ratio of water content per area was 211, 158 and 142 % for the cone-, flat- and pit-type galls, respectively (Fig. 2B, C). The response ratio of leaf minimum conductance per area (gmin) was significantly positively associated with the number of galls per leaf area in the cone or flat types (R2 = 0.31 and 0.30, respectively) but not in the pit type (Fig. 3). The slopes of regression lines (log–log scale) were 0.52 and 0.47 for the cone and flat types, respectively, suggesting that leaf minimum conductance becomes more than double compared with intact leaves when the density of these galls exceeds one per square centimetre.
Fig. 2.
Response ratio (Rr) of thickness (A), mass per area (B), water content per area (C) and water content per fresh mass (D) on a log scale. Different letters above the boxes in each panel indicate significant difference at the 5 % level (Tukey–Kramer test among the three gall types).
Fig. 3.
Response ratio (Rr) of leaf minimum conductance (gmin) plotted against the number of galls for cone, flat and pit types separately on a log–log scale. Filled symbols denote leaves with only closed galls (still immature psyllids were growing in the galls) and open symbols denote leaves with one or more opened galls (some immatures had already left). ‘No gall’ symbols represent response ratios calculated between two intact leaves (mean ± s.d.). Solid regression lines indicate significant relationship (P < 0.05).
Associations among galls, leaf traits and environmental conditions
In line with previous studies (Joel et al., 1994; Tsujii et al., 2016), pubescent individuals were dominant in dry conditions and glabrous individuals were dominant in wet conditions (Supplementary Data Fig. S4). The correlation between the amount of leaf trichomes and aridity index was negative, as expected (r = −0.42, P < 0.01). In the present study, we tested whether leaf traits and environmental conditions were associated with the presence/absence of galls (Model I) and with the abundance of galls (Model II; see Materials and methods section). Table 1 shows standardized coefficients of the best-fit model based on AIC. These coefficients reflect the relative importance of each factor for the presence or abundance of galls (Model I or Model II). Interpretation of the linear coefficient is straightforward when only a linear term is selected (higher values indicate stronger effects) but may be more complicated when a quadratic term is selected because the linear coefficient is subjected to the quadratic coefficient. Negative or positive values in the quadratic term indicate an upward or downward convex relationship within the range of each factor.
Table 1.
Standardized coefficients for the explanatory variables for presence/absence (binomial distribution model; Model I) or abundance (negative binomial distribution model; Model II) of galls.
| Model | Type | MAT2 | MAT | AI2 | AI | SA2 | SA | LMAT | LMAL |
|---|---|---|---|---|---|---|---|---|---|
| I | Cone | −2.11 | 2.96 | – | – | −0.77 | – | −0.73 | −0.44 |
| Flat | −0.56 | −0.40 | – | – | −0.24 | – | −0.26 | −0.29 | |
| Pit | −0.48 | −1.37 | −0.66 | 1.61 | – | −0.37 | – | – | |
| II | Cone | – | −0.36 | 0.29 | −1.08 | – | – | −0.11 | – |
| Flat | −0.11 | −0.17 | 0.10 | – | – | – | 0.20 | – | |
| Pit | – | −0.49 | – | −0.25 | – | – | – | 0.18 |
The best-fit model was selected based on AIC (Supplementary Data Table S2).
These coefficients have no unit because each fixed effect was standardized (zero mean and standard deviation equal to 1).
See main text for more detail.
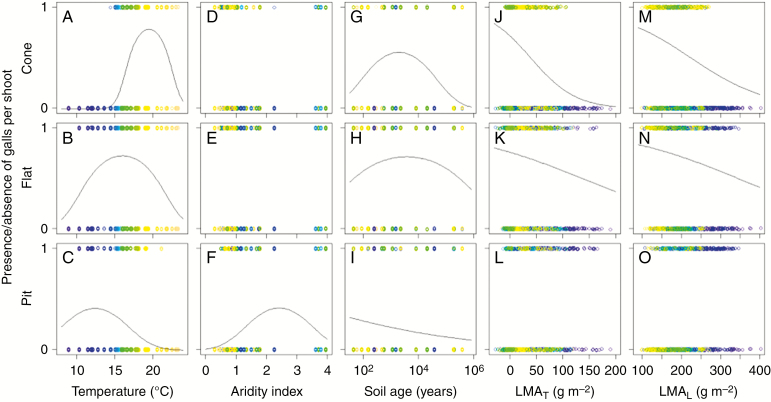
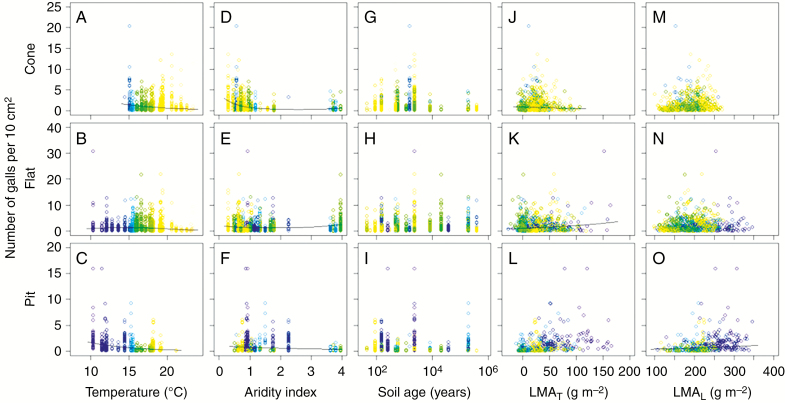
The binomial distribution model for presence/absence data (Model I) showed that the quadratic terms of temperature (MAT2) were significantly negative in each gall type, suggesting that there was a peak presence of each gall type across the temperature gradient (Table 1, Fig. 4A–C). The peak temperature for gall presence was different among the gall types; for cone, flat and pit types it was around 20, 16 and 12 °C, respectively (Fig. 4A–C). In particular, cone-type galls were rarely observed at sites below 14 °C and pit-type galls were rarely observed above 20 °C (Fig. 4A, C). The negative quadratic term for aridity index (AI2) was selected for the presence of the pit type, and the peak aridity index was 2.4 (Table 1, Fig. 4F). Similarly, the quadratic terms were negative in soil age (SA2) for the cone and flat types; the peak soil ages were roughly 2000 years for both large gall types (Table 1, Fig. 4G, H). For the pit type, soil age was negatively associated with gall presence (Table 1, Fig. 4I). In relation to leaf traits, the presence of cone and flat types was negatively associated with LMAT as well as LMAL (Table 1, Fig. 4J, K, M, N) while the presence of the pit type was not associated with these leaf traits (Table 1, Fig. 4L, O). The standardized coefficients of LMAT were larger for the cone type than the flat type (Table 1), reflecting a stronger negative association between LMAT and the presence of galls for the cone than for the flat type (Fig. 4J, K).
Fig. 4.
Presence/absence of galls plotted against environmental conditions or leaf traits. For each explanatory variable, when a significant association was found in the Model I analysis a partial regression line was calculated and drawn by fixing other explanatory variables at each mean value. Symbol colour represents the mean annual temperature at each study site.
The negative binomial distribution model for the count data (Model II) showed that the numbers of cone- and pit-type galls were negatively associated with mean annual temperature (Table 1, Fig. 5A, C). Unlike the results of the Model I analysis, negative quadratic terms were not selected in these relationships partly because zero count data (absence data) were excluded in the Model II analysis. For the flat type, the number of galls was best fitted by an upward convex curve with the peak at around 15 °C [negative quadratic term for temperature (MAT2); Fig. 5B]. Across aridity index, the numbers of cone- and flat-type galls were best fitted by a downward convex curve with the bottoms at 3.0 and 1.2, respectively [positive quadratic terms for aridity index (AI2); Fig. 5D, E]. The number of pit-type galls was weakly negatively associated with aridity index (Table 1, Fig. 5F). All types of galls were not associated with soil age (Table 1). In relation to leaf traits, the numbers of galls were negatively and positively associated with LMAT for the cone and flat types, respectively, although these associations were weak (Table 1, Fig. 5J, K). The number of pit-type galls was weakly positively associated with LMAL but not associated with LMAT (Table 1, Fig. 5L, O).
Fig. 5.
Number of galls more than zero plotted against environmental conditions or leaf traits. For each explanatory variable, when a significant association was found in the Model II analysis a partial regression line was calculated and drawn by fixing other explanatory variables at each mean value. Symbol colour represents the mean annual temperature at each study site.
Discussion
Leaf traits, physiological functions and galls
Leaf minimum conductance, which is associated with uncontrolled water loss from the plant’s surface, should be minimized, especially for plants inhabiting dry areas (Irvine et al., 1998; Medrano et al., 2002). Leaf minimum conductance increased sharply with the number of galls of the cone and flat types, while such a trend was not found for the pit type (Fig. 3). When the densities of cone- and flat-type galls exceeded one per square centimetre, leaf minimum conductance became more than double compared with intact leaves. In addition, our field census suggested that the densities of cone- and flat-type galls were greater than that of pit-type galls (Supplementary Data Fig. S3). These results suggest that formation of larger galls (i.e. cone and flat types) can lead to strong water stress in M. polymorpha, probably due to the increased surface area (Figs 1 and 2A), impaired stomata (Jiang et al., 2018) and the fissures when the psyllids mature (Fig. 1). This finding is supported by a genome study that found drought tolerance genes were significantly upregulated in galled leaves in M. polymorpha (Bailey et al., 2015). While we focused only on leaf minimum conductance, some studies focusing on other gall-makers reported that gall formation also increased stomatal conductance (Fay et al., 1996; Larson, 1998; Huang et al., 2014), whereas other studies reported the opposite trend (Larson, 1998; Florentine et al., 2005; Patankar et al., 2011; Jiang et al., 2018). Although the effect of gall formation on stomatal conductance remains inconclusive, our study clearly showed that the uncontrolled loss of leaf water was increased by gall formation in M. polymorpha.
The negative trends between leaf trichomes and the presence of cone- and flat-type galls but not that of pit-type galls (Table 1, Fig. 4J, K) suggest that the leaf trichomes of M. polymorpha may contribute to the impedance of cone- and flat-type gall makers but not pit-type gall makers. These different patterns could be explained by the direction of attacks of each psyllid species. The cone- and flat-type gall makers (P. pyramidalis and P. pele, respectively) typically attack from the lower surface, where leaf trichomes are present. The thickness of the leaf trichome layer ranged from 0 to 0.80 mm (Supplementary Data Fig. S5), which is often longer than the ovipositor lengths of the P. pyramidalis and P. pele (0.09 and 0.11 mm, respectively; Percy, 2017). On the other hand, as the pit-type gall-maker (P. minutus) attacks from the upper surface (Percy, 2017), the lower leaf trichomes did not hinder the attack of this species. Therefore, the leaf trichomes of M. polymorpha could effectively deter the egg deposition of P. pyramidalis and P. pele but not P. minutus. Previous studies that did not taxonomically discriminate among these psyllid species found inconsistent relationships between leaf trichomes and gall formation (Lee, 1981; Gruner et al., 2005). We speculate that such inconsistency might be partly due to different ways of egg deposition among psyllid species. On the other hand, the specific mass of the lamina (LMAL) itself was also negatively associated with the presence of cone- and flat-type galls (Table 1, Fig. 4M, N). While the leaf lamina is the tissue of photosynthesis, a large fraction of lamina mass is allocated to structural elements, including cell walls and cuticles (>40 % for evergreen woody species), which is important for leaf physical strength (Onoda et al., 2011) and resistance against herbivores and other physical stresses (Read and Stokes, 2006). Thus, it may be natural to observe negative associations of LMAL with the presence of the galls irrespective of the amount of leaf trichomes.
Contrary to the results of Model I, LMAT and LMAL had mixed associations with the number of galls in the Model II analysis, albeit rather small effects (Table 1, Fig. 5J, K, O). While trichomes are typically considered to be barriers for herbivores, some previous studies reported that some insects can utilize trichomes (e.g. Nishijima, 1960). It might be the case that some psyllid species, such as P. pele, can utilize trichomes as a foothold to oviposit and/or as a refuge to escape from environmental stresses, which may result in a weak but positive association between leaf trichomes and the abundance of galls. We also speculate that laminas with a higher LMAL may accommodate a greater number of galls, particularly smaller galls, which induce lower stress because such leaves are often more durable and long-lived (Wright et al., 2004). Moreover, while we used fully matured young leaves for leaf trait measurement, psyllid species might choose young developing leaves for oviposition. To clarify such mixed results may require more detailed observations and experiments on behaviours in relation to leaf traits, on associations between gall formation and leaf longevity, and on the timing of oviposition for each psyllid species (e.g. cafeteria experiments).
Environmental conditions and galls
The three psyllid species may differ in their habitat temperature, as suggested by the different distributions of the three gall types across temperature (Table 1, Fig. 4A–C). Because these three psyllid species have been diversified from a single ancestor on the same host tree species (Percy, 2017), they are likely to have partitioned their niches along a temperature gradient (i.e. an elevational gradient) over evolutionary timescales. This niche partitioning could be related to the size of galls; small galls (i.e. pit type) are more common in colder conditions while the intermediate (i.e. flat type) and larger galls (i.e. cone type) are more common in intermediate and warmer areas, respectively (Table 1, Fig. 4A–C). This may be in line with the temperature limitation of physiological activities known for gall-makers (Henson, 1958). Because the formation of larger galls generally requires higher energy compared with smaller galls, psyllids that form larger galls may not be adapted to low-temperature conditions. Other environmental factors, such as air pressure and UV radiation, may also be related to the elevational distribution of the three psyllid species (e.g. Hodkinson, 2005).
In the three psyllid species, only the pit-type galls showed an upward convex curve for the probability of gall presence across the aridity index (Table 1, Fig. 4F). Since pit-type galls have an open structure while the other two types have enclosed structures, the immatures of P. minutus may be more susceptible to external environmental stresses. For example, in arid conditions pit-type galls cannot protect the immature psyllids from dry air, as indicated by the particularly low abundance of pit-type galls in arid areas (AI < 0.5; Figs 4F and 5F). On the other hand, in heavy-rainfall conditions, immature psyllids seated in pit-type galls on upper leaf surfaces are often exposed to raindrops, which may increase the risk of being washed away. Such a less protected habitat for P. minutus may exclude this species from both extreme ends of the aridity gradient. In contrast, enclosed galls, i.e. cone- and flat-type galls, can protect immature psyllids from drought and physical stresses (Fernandes and Price, 1992; Stone and Schönrogge, 2003); thus, the distributions of P. pyramidalis and P. pele may be less susceptible to aridity. Other factors, such as light intensity, might also contribute to these patterns; therefore, our interpretation should be considered as a hypothesis and subject to more robust tests.
Conclusions
This study demonstrates that the formation of galls, in particular cone and flat types, greatly increases leaf water loss, possibly through the increased leaf surface area and the fissures on the leaves in M. polymorpha. Our field observations suggest that leaf trichomes can be effective in impeding colonization by psyllid species that form cone-type galls, and possibly those that form flat-type galls. These findings support our hypothesis that leaf trichomes can contribute to the avoidance of extra water stress through impeding gall formation.
It should be noted that present results do not rule out the possibility of other functions of leaf trichomes, such as saving water against evaporative demands, capturing dew, repelling dust and water, and maintaining leaf temperature (Johnson, 1975; Haworth and McElwain, 2008; Bickford, 2016; Amada et al., 2017). Moreover, it remains uncertain whether the large allocation of leaf mass to trichomes (up to 40 %) can be explained just on the basis of defence against gall formation because the thickness of the trichome layer often seems to be too great for the ovipositor lengths of psyllids (up to several-fold). That leaves with the largest amount of trichomes are common in the alpine zone, where psyllids are not so abundant, also suggests that other selective forces are involved in the adaptive development of leaf trichomes (Fig. 4). More comprehensive knowledge of these other functions is required for full understanding of the ecological significance of the large diversity of leaf trichomes in M. polymorpha.
The diversity of gall morphology and the pattern of gall abundance elucidated in our study may reflect the adaptive radiation of Pariaconus species as a consequence of coevolution with various phenotypes of M. polymorpha. Leaf trichomes of M. polymorpha must have exerted a strong selective pressure on Pariaconus species, which in turn favoured the M. polymorpha phenotype in terms of avoiding water stress from leaf surfaces. However, the associations between leaf trichomes and the abundance of galls were not strict because leaf trichomes seem to have evolved also in response to various other abiotic and biotic environmental factors on the island of Hawaii. Our study is so far the most extensive description of the relationships between leaf trichomes and the abundance of galls over the whole island of Hawaii. The knowledge obtained in this study would also be useful in unravelling the genetic basis of the history of coevolution between M. polymorpha and Pariaconus species in this unique ecosystem (Stacy et al., 2014; Izuno et al., 2016; Percy, 2017)
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following.
Table S1: site information.
Table S2: coefficients of selected explanatory variables and AIC values in Model I and Model II for each type of gall.
Figure S1: locations of study sites on the island of Hawaii.
Figure S2: mean and standard error of thickness, mass per area, water content per area, water content per fresh mass in intact and galled parts of leaves, and Yc in intact and galled leaves.
Figure S3: frequencies of the numbers of each type of gall per shoot.
Figure S4: associations between trichome mass and environmental factors.
Figure S5: relationship between trichome thickness and trichome mass, and associations between trichome thickness and the abundance of each type of gall.
Funding
This work was partly supported by grants from the Japan Society for the Promotion of Science KAKENHI (JP17J04193 to G.A. and 15KK0255 to Y.O.).
ACKNOWLEDGEMENTS
We thank Elizabeth Stacy and Susan Cordell for advice on the selection of study sites. Elizabeth Stacy also gave kind permission to use her laboratory space in the University of Hawaii at Hilo. Michimasa Yamasaki gave advice on the statistical model. We also thank the Department of Land and Natural Resources (DOFAW) and the Hawai‘i Volcanoes National Park for permission to take plant samples, and anonymous reviewers for their constructive comments on an early draft.
LITERATURE CITED
- Agrawal AA, Fishbein M, Jetter R, et al. . 2009. Phylogenetic ecology of leaf surface traits in the milkweeds (Asclepias spp.): chemistry, ecophysiology, and insect behavior. New Phytologist 183: 848–867. [DOI] [PubMed] [Google Scholar]
- Amada G, Onoda Y, Ichie T, Kitayama K. 2017. Influence of leaf trichomes on boundary layer conductance and gas-exchange characteristics in Metrosideros polymorpha (Myrtaceae). Biotropica 49: 482–492. [Google Scholar]
- Aronne G, De Micco V. 2001. Seasonal dimorphism in the Mediterranean Cistus incanus L. subsp. incanus. Annals of Botany 87: 789–794. [Google Scholar]
- Bailey S, Percy DM, Hefer CA, Cronk QCB. 2015. The transcriptional landscape of insect galls: psyllid (Hemiptera) gall formation in Hawaiian Metrosideros polymorpha (Myrtaceae). BMC Genomics 16: 943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing DH, Henderson K, Kessel B, Sulak J. 1976. The absorptive capacities of bromeliad trichomes. American Journal of Botany 63: 1009–1014. [Google Scholar]
- Bickford CP. 2016. Ecophysiology of leaf trichomes. Functional Plant Biology 43: 807–814. [DOI] [PubMed] [Google Scholar]
- Blanche KR, Westoby M. 1995. Gall-forming insect diversity is linked to soil fertility via host-plant taxon. Ecology 76: 2334–2337. [Google Scholar]
- Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM. 1998. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: the role of phenotypic plasticity. Oecologia 113: 188–196. [DOI] [PubMed] [Google Scholar]
- Cornwell WK, Bhaskar R, Sack L, Cordell S, Lunch CK. 2007. Adjustment of structure and function of Hawaiian Metrosideros polymorpha at high vs. low precipitation. Functional Ecology 21: 1063–1071. [Google Scholar]
- Dalin P, Ågren J, Björkman C, Huttunen P, Kärkkäinen K. 2008. Leaf trichome formation and plant resistance to herbivory. In: A Schaller, ed. Induced plant resistance to herbivory. New York: Springer, 89–105. [Google Scholar]
- Duursma RA, Blackman CJ, Lopéz R, Martin-StPaul NK, Cochard H, Medlyn BE. 2019. On the minimum leaf conductance: its role in models of plant water use, and ecological and environmental controls. New Phytologist 221: 693–705. [DOI] [PubMed] [Google Scholar]
- Ehleringer J. 1984. Ecology and ecophysiology of leaf pubescence in North American desert plants. In: E Rodriguez, PL Healey, I Mehta, eds. Biology and chemistry of plant trichomes. New York: Plenum Press, 113–132. [Google Scholar]
- Fay PA, Hartnett DC, Knapp AK. 1996. Plant tolerance of gall-insect attack and gall-insect performance. Ecology 77: 521–534. [Google Scholar]
- Fernandes GW, Price PW. 1992. The adaptive significance of insect gall distribution: survivorship of species in xeric and mesic habitats. Oecologia 90: 14–20. [DOI] [PubMed] [Google Scholar]
- Florentine SK, Raman A, Dhileepan K. 2005. Effects of gall induction by Epiblema strenuana on gas exchange, nutrients, and energetics in Parthenium hysterophorus. BioControl 50: 787–801. [Google Scholar]
- Giambelluca TW, Shuai X, Barnes ML, et al. . 2014. Evapotranspiration of Hawai‘i. Final report submitted to the U.S. Army Corps of Engineers–Honolulu District, and the Commission on Water Resource Management, State of Hawai‘i. http://evapotranspiration.geography.hawaii.edu/. [Google Scholar]
- Gruner DS. 2004. Arthropods from ‘ōhi‘a lehua (Myrtaceae: Metrosideros polymorpha), with new records for the Hawaiian Islands. Bishop Museum Occasional Papers 78: 33–52. [Google Scholar]
- Gruner DS, Taylor AD, Forkner RE. 2005. The effects of foliar pubescence and nutrient enrichment on arthropod communities of Metrosideros polymorpha (Myrtaceae). Ecological Entomology 30: 428–443. [Google Scholar]
- Haworth M, McElwain J. 2008. Hot, dry, wet, cold or toxic? Revisiting the ecological significance of leaf and cuticular micromorphology. Palaeogeography, Palaeoclimatology, Palaeoecology 262: 79–90. [Google Scholar]
- Henson WR. 1958. The effects of radiation on the habitat temperatures of some poplar-inhabiting insects. Canadian Journal of Zoology 36: 463–478. [Google Scholar]
- Hodkinson ID. 2005. Terrestrial insects along elevation gradients: species and community responses to altitude. Biological Reviews 80: 489–513. [DOI] [PubMed] [Google Scholar]
- Hoof J, Sack L, Webb DT, Nilsen ET. 2008. Contrasting structure and function of pubescent and glabrous varieties of Hawaiian Metrosideros polymorpha (Myrtaceae) at high elevation. Biotropica 40: 113–118. [Google Scholar]
- Huang MY, Huang WD, Chou HM, et al. . 2014. Leaf‐derived cecidomyiid galls are sinks in Machilus thunbergii (Lauraceae) leaves. Physiologia Plantarum 152: 475–485. [DOI] [PubMed] [Google Scholar]
- Ichie T, Inoue Y, Takahashi N, Kamiya K, Kenzo T. 2016. Ecological distribution of leaf stomata and trichomes among tree species in a Malaysian lowland tropical rain forest. Journal of Plant Research 129: 625–635. [DOI] [PubMed] [Google Scholar]
- Irvine J, Perks MP, Magnani F, Grace J. 1998. The response of Pinus sylvestris to drought: stomatal control of transpiration and hydraulic conductance. Tree Physiology 18: 393–402. [DOI] [PubMed] [Google Scholar]
- Izuno A, Hatakeyama M, Nishiyama T, et al. . 2016. Genome sequencing of Metrosideros polymorpha (Myrtaceae), a dominant species in various habitats in the Hawaiian Islands with remarkable phenotypic variations. Journal of Plant Research 129: 727–736. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Veromann-Jürgenson LL, Ye J, Niinemets Ü. 2018. Oak gall wasp infections of Quercus robur leaves lead to profound modifications in foliage photosynthetic and volatile emission characteristics. Plant, Cell & Environment 41: 160–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel G, Aplet G, Vitousek PM. 1994. Leaf morphology along environmental gradients in Hawaiian Metrosideros polymorpha. Biotropica 26: 17–22. [Google Scholar]
- Johnson HB. 1975. Plant pubescence: an ecological perspective. Botanical Review 41: 233–258. [Google Scholar]
- Kitayama K, Mueller-Dombois D. 1995. Vegetation changes along gradients of long-term soil development in the Hawaiian montane rainforest zone. Vegetation 120: 1–20. [Google Scholar]
- Larson KC. 1998. The impact of two gall-forming arthropods on the photosynthetic rates of their hosts. Oecologia 115: 161–166. [DOI] [PubMed] [Google Scholar]
- Lee M. 1981. Insect damage to leaves of two varieties of Metrosideros collina subsp. polymorpha. Pacific Science 35: 89–92. [Google Scholar]
- Levin DA. 1973. The role of trichomes in plant defense. Quarterly Review of Biology 48: 3–15. [Google Scholar]
- Martin TG, Wintle BA, Rhodes JR, et al. . 2005. Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecology Letters 8: 1235–1246. [DOI] [PubMed] [Google Scholar]
- Medrano H, Escalona JM, Bota J, Gulias J, Flexas J. 2002. Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany 89: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabity PD, Hillstrom ML, Lindroth RL, DeLucia EH. 2012. Elevated CO2 interacts with herbivory to alter chlorophyll fluorescence and leaf temperature in Betula papyrifera and Populus tremuloides. Oecologia 169: 905–913. [DOI] [PubMed] [Google Scholar]
- Nishida T, Haramoto FH, Nakahara LM. 1980. Altitudinal distribution of endemic psyllids (Homoptera: Psyllidae) in the Metrosideros ecosystem. Proceedings of the Hawaiian Entomological Society 23: 255–262. [Google Scholar]
- Nishijima Y. 1960. Host plant preference of the soybean pod borer, Grapholitha glicinivorella Matsumura (Lep., Eucosmidae) 1. Oviposition site. Entomologia Experimentalis et Applicata 3: 38–47. [Google Scholar]
- Onoda Y, Westoby M, Adler PB, et al. . 2011. Global patterns of leaf mechanical properties. Ecology Letters 14: 301–312. [DOI] [PubMed] [Google Scholar]
- Patankar R, Thomas SC, Smith SM. 2011. A gall-inducing arthropod drives declines in canopy tree photosynthesis. Oecologia 167: 701–709. [DOI] [PubMed] [Google Scholar]
- Percy DM. 2017. Making the most of your host: the Metrosideros-feeding psyllids (Hemiptera, Psylloidea) of the Hawaiian Islands. ZooKeys 649: 1–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PW, Fernandes GW, Waring GL. 1987. Adaptive nature of insect galls. Environmental Entomology 16: 15–24. [Google Scholar]
- Price PW, Fernandes GW, Lara ACF, et al. . 1998. Global patterns in local number of insect galling species. Journal of Biogeography 25: 581–591. [Google Scholar]
- Read J, Stokes A. 2006. Plant biomechanics in an ecological context. American Journal of Botany 93: 1546–1565. [DOI] [PubMed] [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. 2003. The “hydrology” of leaves: coordination of structure and function in temperate woody species. Plant, Cell & Environment 26: 1343–1356. [Google Scholar]
- Sherrod DR, Sinton JM, Watkins SE, Brunt KM. 2007. Geologic map of the State of Hawaii. US Geological Survey Open-File Report 2007-1089. http://pubs.usgs.gov/of/2007/1089/.
- Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker BM. 2012. glmmADMB: generalized linear mixed models using AD Model Builder. R package version 0.7. 2.1. http://glmmadmb.r-forge.r-project.org/.
- Sokal RR, Rohlf FJ. 1995. Biometry: principles and practices of statistics in biological research, 3rd edn. New York: W. H. Freeman. [Google Scholar]
- Stacy EA, Johansen JB, Sakishima T, Price DK, Pillon Y. 2014. Incipient radiation within the dominant Hawaiian tree Metrosideros polymorpha. Heredity 113: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy EA, Johansen JB, Sakishima T, Price DK. 2016. Genetic analysis of an ephemeral intraspecific hybrid zone in the hypervariable tree, Metrosideros polymorpha, on Hawai‘i Island. Heredity 117: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmermann L. 1983. Ecological studies of Hawaiian Metrosideros in a successional context. Pacific Science 37: 361–373. [Google Scholar]
- Stone GN, Schönrogge K. 2003. The adaptive significance of insect gall morphology. Trends in Ecology and Evolution 18: 512–522. [Google Scholar]
- Tsujii Y, Onoda Y, Izuno A, Isagi Y, Kitayama K. 2016. A quantitative analysis of phenotypic variations of Metrosideros polymorpha within and across populations along environmental gradients on Mauna Loa, Hawaii. Oecologia 180: 1049–1059. [DOI] [PubMed] [Google Scholar]
- UNESCO 1979. Map of the world distribution of arid regions. Explanatory note, Man and Biosphere. Paris: UNESCO. [Google Scholar]
- Vitousek PM, Matson PA, Turner DR. 1988. Elevational and age gradients in Hawaiian montane rainforest: foliar and soil nutrients. Oecologia 77: 565–570. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Aplet G, Turner D, Lockwood JJ. 1992. The Mauna Loa environmental matrix: foliar and soil nutrients. Oecologia 89: 372–382. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. . 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Wuenscher JE. 1970. The effect of leaf hairs of Verbascum thapsus on leaf energy exchange. New Phytologist 69: 65–73. [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Zero-truncated and zero-inflated models for count data. In M Gail, K Krickeberg, J Samet, A Tsiatis, W Wong, eds. Mixed effects models and extensions in ecology with R. New York: Springer, 261–293. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.