Abstract
Background
Despite Stebbins’ principle of the most efficient pollinator being proposed decades ago, the most important pollinators are still mainly identified using the frequency of visits to flowers. This shortcoming results in a gap between the characterization of the flower visitors of a plant species and a reliable estimation of the plant fitness consequences of the mutualistic interaction. The performance of a mutualistic visitor depends on its abundance, behaviour, effectiveness (pollen removal and deposition per unit time) and efficiency (seed set per unit time) conditioned by the temporal matching between pollinator activity and temporal patterns of maturation of the sexual functions of flowers. Although there have been recent attempts to provide a conceptual and methodological framework to characterize pollinators’ performance, few have combined all key elements of visitors and plants to provide an accurate estimation of pollinators’ performance under natural conditions.
Methods
We complement information on the flower biology and mating system of the sub-shrub Lepechinia floribunda (Lamiaceae) to provide a daily quantitative estimation of performance (effectiveness and efficiency) of the more abundant pollinators, i.e. native bumble-bees (Bombus spp.) and leafcutter bees (Megachile sp.), and the exotic honey-bee (Apis mellifera).
Key Results
Unlike honey-bees or leafcutter bees, native bumble-bees matched the daily pattern of nectar production and stigma receptivity, and showed higher effectiveness and efficiency. Despite the overabundance of honey-bees, visits occurred mainly when stigmas were not receptive, thus reducing the honey-bees’ overall performance.
Conclusions
Bumble-bees appear to be the most important pollinators and potential historical mediators of reproductive trait evolution in L. floribunda. Because the production of seeds by bumble-bees involved fewer pollen grains for plants and less investment in floral display than honey-bees, contemporary and expected changes in pollinator abundance may affect future L. floribunda floral evolution. If bumble-bees were to be further displaced by anthropogenic disturbance or by competition with honey-bees, their lower efficiency will select for a larger floral display increasing reproductive costs. This scenario may also impose selection to reduce dichogamy to match honey-bee foraging activity.
Keywords: Foraging behaviour, Lepechinia floribunda, outcrossing rate, plant–pollinator interactions, pollen deposition, pollen removal, Stebbins’ principle, visitation frequency
INTRODUCTION
The relationship between flowering plants and pollinators seldom occurs in a pairwise fashion because the majority of plant species are visited by more than one pollinator species (Ashworth et al., 2015). Thus, a central aspect is the identification of the floral visitors that exert the strongest positive effect on plant fitness (Mayer et al., 2011). Following Stebbins’ principle of the most efficient pollinator (Stebbins, 1970), these are expected to impose selection on floral traits and will be considered key agents of selection to explain phenotypic evolution (Poblete Palacios et al., 2019). However, even though the importance of a pollinator depends on its effect on plant fitness (Fenster et al., 2004), there has been a historical bias to infer the ecological and evolutionary consequences of a plant–pollinator interaction based on its frequency of occurrence (Vazquez et al., 2005). Although the frequency of visits to flowers provides a useful approximation, it is by no means a definitive assessment of pollinator performance and of its effect on plant fitness (Waser et al., 1996). Despite the fact that recent theoretical and methodological proposals argue for a more precise estimation of pollinator performance (Ne’eman et al., 2010; Freitas, 2013; Schupp et al., 2017, Minnaar et al., 2019), the identification of the pollen vectors that play a central role in plant reproduction and evolution remains a major challenge and awaits further investigation.
Pollinator performance can be defined as the absolute contribution of a given pollen vector to plant fitness and involves at least two main sequential factors: (1) abundance of pollinators and (2) pollen removal and deposition. The interaction between these two ultimately determines the role of each pollinator as a vector of gametes affecting mating and plant fitness (Herrera, 1987). The association between the abundance of pollinators and pollen removal/deposition performance is not necessarily linear, because less abundant pollinators may deposit more pollen per visit, and elicit a higher seed set than the most abundant ones (Zych, 2007; Barrios et al., 2016), and vice versa (Sahli and Conner, 2007; Medel et al., 2018). Ne’eman et al. (2010) defined pollinator performance as resulting from pollination effectiveness and efficiency. Pollination effectiveness quantifies the ability of a floral visitor to remove and deposit pollen on stigmas per visit per unit time. Pollination efficiency indicates to what extent pollen deposition contributes to female plant fitness per visit per unit time (i.e. including pollen quality). These definitions reveal that an effective floral visitor in terms of pollen deposition could not be efficient in terms of seed production (Ne’eman et al., 2010). This may occur in dichogamous species if pollinator activity is concentrated during only one phase of sexual maturation, either when anthers open or when stigmas are receptive (Zych, 2007). However, an efficient pollen vector is certainly effective.
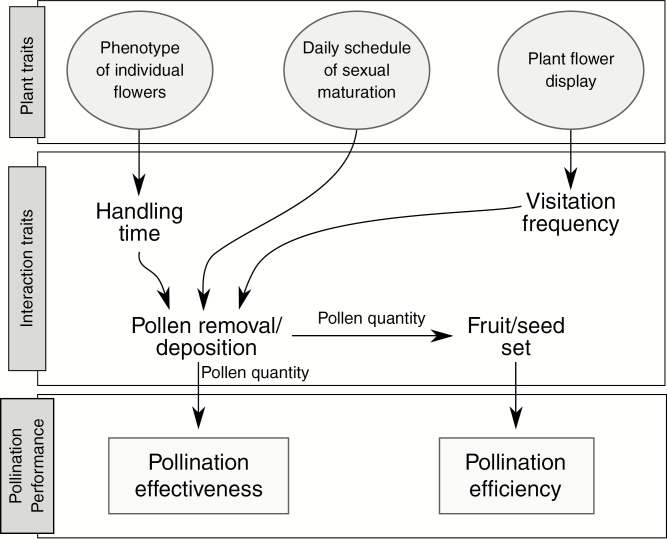
The relationship between floral traits and interaction traits accounting for the variation in pollinators’ performance in natural conditions is depicted in Fig. 1. The availability of pollinators for a focal plant species in a given patch and community context usually depends on its floral display (Proctor et al., 1996). Plants with large floral displays usually receive more visits (Harder and Johnson, 2009), and those with high visitation frequency produce more seeds. Nevertheless, these sequential events can be modulated by the flower/pollinator adjustments during each pollination event (Poblete Palacios et al., 2019). From arrival at a flower, morphological matching between a pollinator and floral architecture, together with pollinator behaviour, modulate handling time and effectiveness of both pollen removal and deposition (Barrios et al., 2016). The efficiency of a pollinator is given by its contribution to the final fitness of a plant (Fig. 1). Thus, both pollination effectiveness and efficiency translate into the final performance of each pollinator species. In addition, within a population, pollen movement among plants and the resulting outcrossing rate depend on the co-ordination between the period of pollinator activity and the timing of maturation of sexual phases among flowers (Herrera, 2000). Hence, the variation in the number of receptive flowers during the day, relative to the timing and duration of pollinator activity, should also be taken into account for a reliable estimation of pollinator performance (Albercht et al., 2012). Because daily fluctuations in environmental conditions and pollinator activity affect pollination effectiveness and efficiency (Fig. 1; Herrera, 1987, 2000), those pollinators that better match the population pattern of anther maturation and stigma receptivity will have greater performance.
Fig. 1.
Schematic representation of the relationships among floral traits and interaction traits determining pollination effectiveness, efficiency and performance.
Due to the multifactorial context of the pollination process, there is an important gap between the characterization of the floral visitor community in a given plant population and the quantitative assessment of pollinator performances. This gap limits our ability to identify the most important selective agents affecting floral evolution and manipulate efficient pollinators to warrant outcrossing in the context of population conservation and crop production.
The present study attempts to narrow this gap using as study system Lepechinia floribunda (Benth.) Epling (Lamiaceae), that is visited by several species of native bees, bumble-bees and the cosmopolitan honey-bee Apis mellifera (Roldan and Ashworth, 2018). As native bees have a longer history of interaction with the selected plant species than honey-bees, it is expected that native bees will show greater effectiveness and/or efficiency than honey-bees. Nevertheless, if honey-bees are more abundant than native bees (Magrach et al., 2017; Valido et al., 2019), a lower effectiveness and efficiency may be compensated by a higher visitation frequency. In the present study, we (1) describe the floral biology of L. floribunda (daily patterns of both nectar secretion and stigma receptivity); (2) examine the relevance of pollinators for seed production through outcrossing rate (t) estimation; (3) assess relative abundance, visitation rate and relative visits of the whole community of floral visitors; and (4) compare pollination effectiveness (i.e. the contribution of each pollinator to pollen removal and deposition per unit time) and efficiency (i.e. female fitness per pollinator per time unit) between native and non-native pollinators.
MATERIALS AND METHODS
System and study site
Lepechinia floribunda (Lamiaceae) is a perennial sub-shrub native of the montane forests in Argentina, Bolivia and Peru commonly found in dry open habitats from 500 to 3500 m above sea level (Epling, 1938). It is a dominant species that blooms from early October to late February, producing several short bilabiate white hermaphroditic flowers per plant throughout the flowering season. Receptive flowers last 1 day and present bifid stigmas and four ovules. They are incomplete protandrous (anthers mature before stigmas), and can self-fertilize autonomously (Roldan and Ashworth, 2018). Fruits mature approx. 3 weeks after pollination (Camina et al., 2018).
The study was performed in a natural population at the Reserva Natural Los Manantiales (31°9'40.34''S, 64°21'03.67''W) in Central Argentina where 164 tagged individuals were studied during three blooming seasons (2013–2014, 2014–2015 and 2018–2019).
Floral biology: nectar production, stigma receptivity and mating system
To characterize the floral biology of L. floribunda, during the flowering season of 2014–2015, daily nectar production was calculated using 115 randomly selected floral buds from 26 plants bagged before 07.00 h. Opened flowers were harvested every hour (12–21 bagged flowers per hour) from 08.00 h to 15.00 h when nectar production dropped. Nectar volume (μL) and concentration (μg μL–1) were recorded using 1 or 5 μL micro-caps and a temperature-compensated hand refractometer (concentration range 0–32° BRIX units; American Optical 10431). These variables were used to calculate nectar sugar content (μg) following Kearns and Inouye (1993). The percentage of receptive stigmas per hour was recorded using 253 randomly selected floral buds from 52 plants (15–68 bagged flowers per census) to estimate the population stigmatic receptivity throughout the day. After a preliminary evaluation, only pollen deposition on stigmatic branches opened at 90° produced seeds; thus, only completely opened stigmas were considered receptive (Camina, 2018).
To characterize the mating system after the reproductive season of 2014–2015, we sowed 10–20 seeds for each of 15 plants in germination chambers following the protocol proposed by Ashworth et al. (2017) and, after 1 month, the first leaves were collected and freeze-dried (n = 188 seedlings). We extracted DNA and amplified 12 microsatellites specifically developed for L. floribunda (molecular techniques details and microsatellite features are provided in Supplementary Data Information S1).
We used a maximum likelihood approach to estimate the outcrossing rate by genotyping each family and the whole sample, and using a mixed mating system model of Ritland and Jain (1981) implemented in the program MLTR v.3.4 (Ritland, 2002). MLTR calculates multilocus (tm) and single-locus (ts) outcrossing rates using the Newton–Raphson iteration. These estimations range between 0 (indicating complete selfing) and 1 (indicating complete outcrossing). Outcrossing rate standard errors and 95% confidence intervals were obtained from 1000 bootstrap replicates. The outcrossing rate tuning parameter, allele frequency tuning parameter and initial population outcrossing rate were set to 0.05, 0.1 and 0.5, respectively.
Floral visitor abundance
To identify the most abundant floral visitors, we characterized the floral visitor community and its period of greatest activity, and determined the abundance of its members by recording mean visitation rate (visits per minute; Vr), relative visits (visits per observed flowers; Rv) and relative abundance (Ra; Table 1) of each floral visitor species during two consecutive reproductive seasons. Direct observations of visitors were made between 08.00 h and 17.00 h, in periods of 15 min per plant, during 10 d, accumulating a total of 17 and 28 man-hours observation in the reproductive seasons of 2013–2014 and 2014–2015, respectively.
Table 1.
Summary of metrics used to study the pollination performance in Lepechinia floribunda population.
| Measured | Definition | Abbreviation | References |
|---|---|---|---|
| Visitation rate | Total visits per species per minute of observation | Vr | Herrera (1989) |
| Relative visits | Total visits per species over the total observed flowers | Rv | Herrera (1989) |
| Visitor relative abundance | Percentage of individuals of a given species in relation to the total number of individuals observed | Ra | This study |
| Visitation frequency | The proportion of visited flowers per species per hour | Vf | Dafni et al. (2005) |
| Handling time | The time between arrival and departure from a flower by an insect | Ht | Zych et al. (2013) |
| Pollen removal | The difference in the number of pollen grains recorded between non-visited and visited flowers | Pr | This study |
| Pollen deposition | The difference in the number of pollen grains on the receptive inner blades of the stigmatic lobules between non-visited and visited flowers | Pd | This study |
| Fruit/flower | The proportion of flower setting fruits per plant after one visit | Fs | Ma et al. (2018) |
| Seed/fruit | Number of mature seeds per fruit after one visit | Ss | Ma et al. (2018) |
| Pollen exportation effectiveness | Number of pollen grains removed from anthers multiplied by the visitation frequency: removal per visit per hour | Dte | This study following Ne’eman et al. (2010 |
| Pollen deposition effectiveness | Number of pollen grains deposited in the stigma multiplied by the visitation frequency: deposition per visit per hour | Dtd | Ne’eman et al. (2010 |
| Pollination efficiency | Number of seeds produced after one visit divided by the maximum seed set potential of the flower (s), multiplied by the visitation frequency: seeds set per visit per hour | PE | Ne’eman et al. (2010 |
Based on this characterization of the floral visitor community, we carried out a series of experiments to determine pollination effectiveness and efficiency of the two most abundant visitors using individual performance measures (such as visitation frequency and pollen deposition on the stigma) and combining these individual measures following the approach proposed by Ne’eman et al. (2010 (Table 1). To define the most important flower visitors, we considered their visitation consistency throughout two reproductive seasons (see the Results and Table 2).
Table 2.
Foraging behaviour throughout the day by flower visitors of Lepechinia floribunda during two consecutive seasons
| 2013–2014 | 2014–2015 | Average | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flower visitor | Ra | Vr | Rv | Ra | Vr | Rv | Ra | Vr | Rv | |
| Morning (09.00–12.00 h) | Apis mellifera | 70.00 | 0.39 | 0.22 | 49.33 | 1.42 | 0.63 | 59.67 | 0.90 | 0.43 |
| Chlorostilbon lucidus | 10.00 | 0.17 | 0.10 | – | – | – | – | – | – | |
| Centris tricolor | 6.67 | 0.03 | 0.02 | 1.72 | 0.33 | 0.00 | 4.20 | 0.18 | 0.01 | |
| Bombus pauloensis | 3.33 | 0.02 | 0.01 | 20.74 | 0.69 | 0.28 | 12.04 | 0.35 | 0.14 | |
| Bombus opifex | – | – | – | 7.24 | 0.37 | 0.13 | – | – | – | |
| Megachile sp. | 6.67 | 0.02 | 0.01 | 16.2 | 0.49 | 0.27 | 11.44 | 0.25 | 0.14 | |
| Augochlora sp. | 3.33 | 0.01 | 0.01 | 1.35 | 0.26 | 0.10 | 2.34 | 0.14 | 0.05 | |
| Average | – | 0.11 | 0.06 | – | 0.53 | 0.19 | – | 0.32 | 0.13 | |
| Noon (12.00–14.30 h) | Apis mellifera | 68.42 | 0.36 | 0.25 | 33.71 | 0.31 | 0.13 | 51.07 | 0.34 | 0.19 |
| Chlorostilbon lucidus | 2.63 | 0.01 | 0.01 | – | – | – | – | – | – | |
| Bombus pauloensis | 2.63 | 0.01 | 0.01 | 14.29 | 0.41 | 0.19 | 8.46 | 0.21 | 0.10 | |
| Bombus opifex | – | – | 9.14 | 0.35 | 0.17 | – | – | – | ||
| Megachile sp. | 2.63 | 0.01 | 0.00 | 17.71 | 0.24 | 0.19 | 10.17 | 0.12 | 0.10 | |
| Augochlora sp. | 18.42 | 0.07 | 0.05 | 16.57 | 0.22 | 0.10 | 17.50 | 0.15 | 0.07 | |
| Dialictus sp. | 5.26 | 0.01 | 0.01 | 1.14 | 0.07 | 0.04 | 3.20 | 0.04 | 0.02 | |
| Centris tricolor | – | – | – | 6.29 | 0.22 | 0.00 | – | – | – | |
| Average | – | 0.08 | 0.05 | – | 0.29 | 0.13 | 0.18 | 0.09 | ||
| Afternoon (14.30–17.30 h) | Apis mellifera | 52.63 | 0.17 | 0.10 | 68.12 | 0.27 | 0.17 | 60.38 | 0.22 | 0.14 |
| Chlorostilbon lucidus | 10.53 | 0.04 | 0.02 | – | – | – | – | – | – | |
| Megachile sp. | – | – | – | 10.03 | 0.27 | 0.18 | 10.03 | 0.27 | 0.18 | |
| Bombus pauloensis | 26.32 | 0.09 | 0.05 | 11.31 | 0.48 | 0.15 | 18.82 | 0.29 | 0.10 | |
| Bombus opifex | – | – | – | 1.29 | 0.25 | 0.15 | – | – | – | |
| Dialictus sp. | 10.53 | 0.02 | 0.01 | 5.14 | 0.12 | 0.13 | 7.84 | 0.07 | 0.07 | |
| Centris tricolor | – | – | – | 4.11 | 0.2 | 0.00 | 4.11 | 0.20 | 0.00 | |
| Average | 0.08 | 0.05 | – | 0.26 | 0.11 | 0.17 | 0.08 | |||
Ra, relative abundance; Vr, visitation rate (visits min–1); Rv, relative visits (visits per flower).
The most abundant flower visitor is indicated in bold.
Following our previous characterization, subsequent estimations of pollinator performance were obtained for the honey-bee (Apis mellifera), leafcutter bees (Megachile sp.) and a set of two functionally equivalent native bumble-bee species (see below). Together, these species were responsible for 70–85 % of total visits during two consecutive years (see Table 2). Because the two Bombus species recorded (B. pauloensis and B. opifex) showed similar patterns of floral manipulation and have similar size and morphological features, in the following experiments and analyses they were combined under the same functional category (i.e. Bombus spp.).
Visitation frequency and handling time
During the flowering season of 2014–2015, visitation frequency (Vf) and handling time per visit (Ht) were recorded using a stopwatch for the three pollinator groups. Vf was calculated as the proportion of visited flowers per species per hour, and Ht as the time between arrival and departure from a flower (n = 190 observations; Table 1). Additionally, to explore the daily fluctuation in these variables, we calculated Vf and Ht every hour for each species between 09.00 h and 13.00 h, the period of highest insect activity according to previous observations.
Pollen deposition and removal
During the flowering season of 2014–2015, pollen removal (Pr) and pollen deposition (Pd) by A. mellifera, Megachile sp. and Bombus spp. after one visit were calculated using a random sample of 54 focal plants. Two to six flowers (n = 193 total flowers) per plant were chosen early in the morning and bagged before anthesis to avoid possible early visits. Once stigmas became receptive and exposed their stigmatic lobules, one or two flowers per plant were used to estimate the total number of pollen grains before a pollinator visit (n = 65; these are considered control flowers). Anthers of selected flowers were harvested with a forceps to gently collect all its pollen and this was mounted in a drop of stained glycerine jelly previously held on a slide (Baranzelli et al., 2014). The remaining bagged flowers (n = 129) were exposed to the three pollinator groups. When stigmas became receptive, one anther and the stigma of each flower were collected after one visit following the same procedure described above (one or two flowers per visitor per plant). The absolute numbers of pollen grains in the anthers and on the stigmas were counted using digital images from slides taken at ×10 magnification with an Olympus DP71 camera attached to an Olympus SZX16 stereomicroscope. ImageJ free software (National Institute of Health, USA) was used to count the total number of pollen grains per anther and on the stigmatic lobules. For each visitor, Pr was estimated for each focal plant as the difference in the number of pollen grains per anther between non-visited and visited flowers (Prunvisited flowers – Prvisited flowers). Similarly, Pd was estimated as the difference in number of pollen grains on stigmatic branches between non-visited and visited flowers (as control of flower manipulation; Table 1). These calculations were performed for every 1 h interval between 09.00 h and 13.00 h to record daily fluctuations in pollen removal and deposition by each pollinator.
Fruit set and seed set
During the flowering season of 2018–2019, fruit per flower (Fs) and seed per fruit (Ss) were calculated for 33 focal plants after a single visit of either A. mellifera or Bombus spp. Although Megachile sp. was quite good at removing pollen (see the Results), it presents very low effectiveness and visitation frequency, and was almost absent during the flowering season when efficiency was estimated; thus, it was not included in the final performance estimations. A total of 3–17 flowers per plant (n = 256 flowers) were bagged early in the morning before anthesis to avoid pollinator visits. Once stigmas became receptive, flowers were unbagged, exposed to visitation, monitored continuously and bagged again after the first visit. Four weeks later, ripe fruits were collected to obtain fruit and seed set per pollinator. Fs was estimated as the proportion of fruits per flower per plant, and Ss was calculated as the number of mature seeds per fruit (Table 1).
Pollination effectiveness and efficiency
Given that the proportion of receptive stigmas and nectar production in the population increases since flowers open in the morning until mid-day (see the Results), pollination effectiveness was calculated for every 1 h interval during pollinator activity to obtain the daily variation pattern and more accurate estimation of the response variable. Following the framework of Ne’eman et al. (2010), pollination effectiveness per time unit (Dt) for pollinators was estimated as the product of its visitation frequency (Vf) and the number of pollen grains removed from anthers (Pr) or deposited on the stigmas (Pd), as indicated below:
| (1) |
| (2) |
Pollination efficiency per time unit (PE) was calculated as the proportion of the maximum number of seeds per flower produced after one visit (as a measure of quality pollen; Ne’eman et al. 2010) multiplied by the visitation frequency. Because our studied species had a constant number of four ovules per flower, PE was estimated as indicated below:
| (3) |
Following the suggestion of Ne’eman et al. (2010), we set the upper limit of PE at 1. Hence, PE = 1 corresponds to a pollinator that visited all focal flowers during the observation period and these produced maximum seeds per fruit.
Statistical analyses
Floral biology
To compare nectar production throughout the day, linear models were fitted using time as an independent factor, and nectar volume and concentration as response variables. These analyses were implemented in the statistical software R v.3.3.2 (R Core Team, 2017), using the lm() function. Post-hoc comparisons were performed using the Tukey test with the functions glht and cld (package: multcomp; Torsten et al., 2008). To depict daily total sugar production patterns throughout the day, non-parametric cubic splines were performed using the product between nectar volume and concentration per hour as the response variable and time of the day as an independent variable. For this analysis, we used the gam function (package: mgcv; Wood, 2006) in R. Smoothing parameters were obtained by minimizing the generalized cross-validations cores (Wood, 2008), and Bayesian standard errors were obtained according to Wood (2006).
Pollinator performance
To disentangle differences in pollination performance between the two main visitor categories, generalized linear mixed-effect models (GLMMs; Zuur et al., 2009) implemented in R were applied individually to each component of performance as described in the previous sections (Vf, Ht, Pd, Pr, Fs and Ss). Models included flower visitor (A. mellifera, Megachile sp. or Bombus spp.) as a fixed effect, and plants as a random effect within flower visitor. Significance of the fixed effects was estimated using restricted maximum-likelihood estimations and Akaike information criterion (AIC) comparisons of models. For Vf, Ht, Pd and Pr variables, models were performed using a Gaussian error distribution and the function lmer (package: lme4; Bates et al., 2015), while Fs and Ss were compared using a binomial distribution of errors [family = binomial (logit)] with the function glmer (package: lme4).
Because pollen effectiveness (Dt) was recorded every hour during pollinator activity, weighted mean effectiveness was obtained for each pollinator as where n is the period of observation following Eqns (1) and (2). The uncertainty of Dt and PE per hour was calculated by bootstrapping 1000 times. Resampling was performed with the function boot (package: boot; Canty and Riplay, 2016) in R. Comparison of Dt between pollinators for both pollen exportation (Dte) and pollen deposited on the stigma (Dtd) was performed using estimates of effectiveness for the whole period of pollinator activity (09.00–13.00 h) and for the period when >70 % of the opened flowers had their stigma receptive in the population (11.00–13.00 h; see Fig. 2). Comparisons were performed using linear models with the lm() function in R. In all cases, we implemented post-hoc multiple comparisons using Tukey range test with the functions glht and cld.
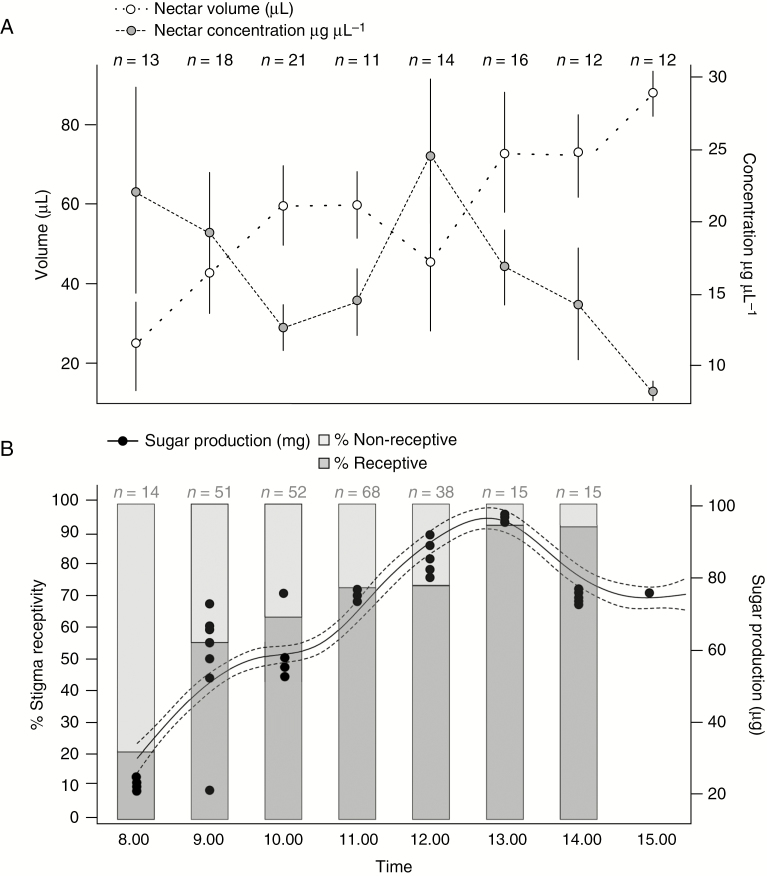
Fig. 2.
(A) Nectar volume and concentration over time in a random sample of flowers in a natural population of Lepechinia floribunda. (B) Percentage of receptive and non-receptive stigmas and sugar production (nectar volume × nectar concentration). Sample size per harvest is indicated above each hour. Non-parametric regression analysis and a cubic spline adjustment were performed to show the sugar production pattern through time. Dotted lines show ±1 Bayesian standard error interval.
Comparison of PE between A. mellifera and Bombus spp., following Eqn (3), was performed using zero-inflated negative binomial (ZINB) models to consider overdispersion and excess of zeros in the data. The ZINB model was tested as suggested by Zuur et al. (2009; see the Results), using the zeroinfl function (package: pscl; Zeileis et al., 2008).
We established the significance level at a P-value of <0.05 in all analyses. All response variables satisfied homoscedasticity and independence of error. Finally, we used the ggplot function (package: ggplot2; Wickham, 2009) to build bar plots and the lineplotCI function (package: sciplot; Murdoch, 2017) to build daily variation plots.
RESULTS
Floral biology
Early in the morning, mean (± s.d.) nectar volume was 2.4 ± 1.1 (μL), with a mean sugar concentration of 2.22 ± 0.70 (μg μL–1). Nectar volume continuously increased throughout the day, reaching 9.4 ± 1.6 μL before 15.00 h. There were significant differences in nectar volume across time (F = 3.794; P = 0.001), in particular among the first hours of the morning (08.00 h and 9.00 h), mid-day (10.00 h to 14.00 h) and afternoon (15.00 h; Fig. 2A). Sugar concentration showed a nearly decreasing trend throughout the day, with a peak around 12.00 h (2.5 ± 1.4 μg μL–1), and a minimum concentration at 15.00 h (0.8 ± 0.1 μg μL–1; Fig. 2A) but without statistically significant differences across time (F = 1.959; P = 0.073). Throughout the day, flowers produced an average of 5.9 ± 0.4 μL of nectar with 1.6 ± 0.1 μg μL–1 sugar per flower. Sugar production increased throughout the day (explained deviance = 79.7 %; P < 0.0001), with a peak at around 12.00–13.00 h (9.4 ± 0.5 mg; Fig. 2B).
The proportion of receptive stigmas followed a similar trend to volume and sugar production. The first receptive stigmas were observed early in the morning at around 08.00 h but at low frequency (20 %). It was not until 11.00 h that >70 % of the observed flowers had their stigmas open with an angle of >90º that indicated receptiveness. By 13.00 h, >80% of stigmas were receptive (Fig. 2B).
Based on a random sample of 15 maternal plant families, genetic analyses indicated that their progenies (n = 188 seedlings) showed a total of 42 alleles using 12 nuclear microsatellite loci with a range of 2–6 alleles per locus. Multilocus outcrossing rate estimation (tm) at family level ranged between 0.235 ± 0.06 and 1.000 ± 0.249, (population mean ± s.d. = 0.753 ± 0.113; details in Supplementary Data Information S1).
Flower visitor assemblage
Our censuses of floral visitors during two seasons recorded a total of nine bee species from three families (Apidae, Halictidae and Megachilidae), and one hummingbird, Chlorostilbon lucidus (Trochilidae; Table 2). Hummingbirds were observed only in one season (in 2013–2014), while other visitors appeared in both seasons (Table 2). The sweat bee Dialictus sp. appeared at noon and in the afternoon. Taking all floral visitors into account, the highest Vr and Rv were recorded in the morning (0.32 visits min–1; 0.13 visits per flower) and early afternoon (0.18 visits min–1; 0.09 visits per flower). All floral visitors were observed collecting either pollen, nectar or both. Together, bumble-bees, leafcutter bees and honey-bees represented >70 and 85 % of the observed visits in 2013–2014 and 2014–2015 seasons, respectively (Table 2). Moreover, these visitors appeared throughout the day, with the highest visitation rates per flower and/or per minute (Table 2).
Visitation frequency and handling time
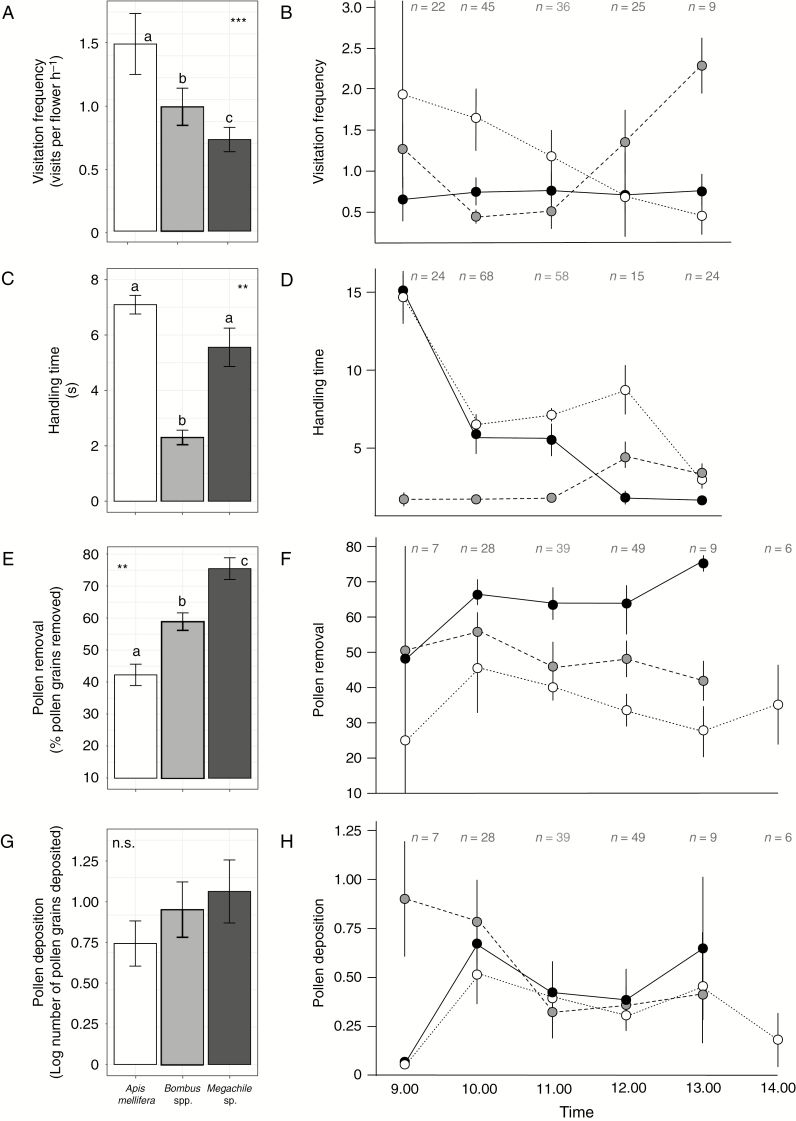
Average visitation frequency (Vf) was significantly higher for A. mellifera (1.29 ± 0.21 visits per flower h–1) than for Bombus spp. (0.83 ± 0.13 visits per flower h–1) or Megachile sp. (0.62 ± 0.08 visits per flower h–1; F = 4.67; P = 0.0013; Fig. 3A). Apis mellifera was the main visitor until 11.00 h, whereas Bombus spp. increased its frequency between 11.00 h and 13.00 h (Fig. 3B). Megachile sp. showed a low but constant Vf throughout the day. Handling time (Ht) varied between flower visitors (F = 5.85; P = 0.005). Post-hoc comparisons showed that Bombus spp. had, on average, significantly shorter handling time (2.30 ± 1.72 s per flower) than Megachile sp. (5.56 ± 3.53 s per flower) and A. mellifera (7.09 ± 3.70 s per flower); Fig. 3C). While A. mellifera and Megachile sp. showed a consistent reduction throughout the day in the handling time per flower, Bombus spp. were much more constant in this regard (Fig. 3D). Despite differences detected between pollinators, a significant plant effect on handling time per visit was also detected (ΔAIC = 3130; P = 0.007), accounting for 10 % of the total variation.
Fig. 3.
Components of pollination performance for Apis mellifera, Bombus spp. and Megachile sp. in Lepechinia floribunda. Left panels represent average values and right panels correspond to the per hour estimates. (A and B) Visitation frequency, (C and D) handling time, (E–F) pollen removal (difference in the number of pollen grains recorded between non-visited and visited flowers expressed as a percentage) and (G and H) pollen deposition (logarithm of the difference in the number of pollen grains on the receptive inner blades of the stigmatic lobules among visited flowers). Vertical lines within the left panels represent the s.e. Sample size per hour of data recording is indicated above each hour. n.s., non-significant difference; *P < 0.05; **P < 0.01.
Pollen removal and deposition
The number of pollen grains available per anther of non-visited flowers ranged between 1301 and 2860, and the number of pollen grains removed per visitor species ranged between 0 and 2529 (mean ± s.d = 968 ± 514). The estimated percentage of pollen removed per visit ranged from 0 to 94.47 %. Pollen removal (Pr) was significantly different between pollinators (F = 37.847; P < 0.001). Megachile sp. removed around 20 % more pollen per visit than Bombus sp. and 50 % more than A. mellifera (Fig. 3E). The three species showed relatively constant removal patterns throughout the day (Fig. 3F). Pollen grains deposited on the stigmas (Pd) varied between 0 and 80 grains per flower (mean ± s.d = 5.84 ± 13.09) and showed a rather constant pattern throughout the day. No significant differences were detected among visitors (F = 0.896; P = 0.350; Fig. 3G, H). Variation among plants also accounted for a significant amount of variation in Pr (ΔAIC = 9.478; P = 0.002), explaining 29 % of the variation.
Fruit set and seed set
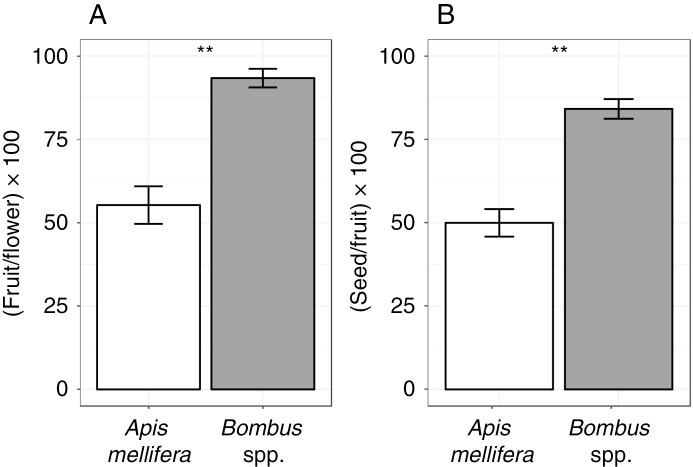
Among tagged plants, fruit per flower (Fs) varied from 33 to 100 %, and seed per fruit (Ss) from 0 to 100 %. There were significant differences in Fs and Ss between flower visitors (z = 5.891, P < 0.001). On a single visit basis, Bombus spp. had both Fs and Ss about 40 % higher than A. mellifera (Fig. 4).
Fig. 4.
(A) Percentage of mean number of fruits per flowers, and (B) seeds per flower formed after a visit of Apis mellifera or Bombus spp. in a natural population of Lepechinia floribunda. Vertical lines in each bar represent the s.e. **P < 0.001.
Pollinator effectiveness and efficiency
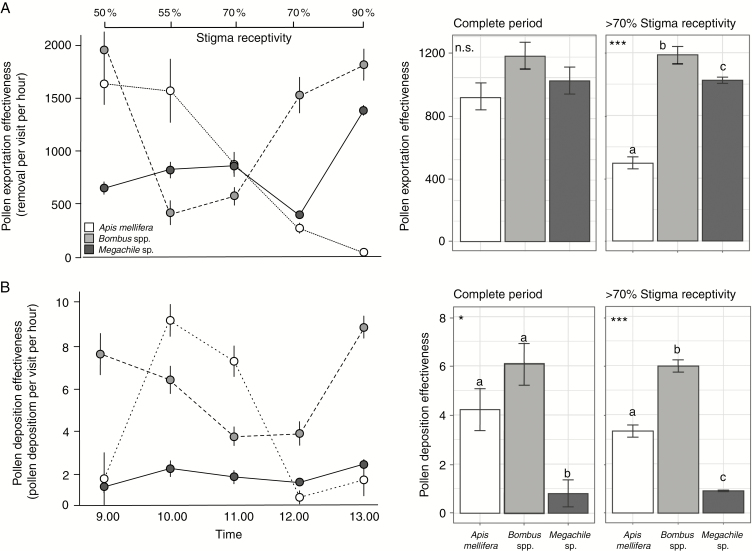
The comparison of pollination effectiveness between floral visitors (Dt) throughout the whole observation period (09.00–13.00 h) revealed similar levels of pollen removal effectiveness (Dte) across time intervals (F = 79.507; P = 0.710; Fig. 5A). In turn, significant differences in pollen deposition effectiveness (Dtd) between A. mellifera and Bombus spp. with respect to Megachile sp. were detected (F = 3.398; P = 0.043; Fig. 5A). Nevertheless, at the time of the day when >70 % of stigmas were receptive (between 11.00 h and 13.00 h), the effectiveness of Bombus spp. removing (Dte = 1186.5 ± 20) and depositing (Dtd = 6 ± 0.25) pollen grains was significantly higher than that of A. mellifera (Dte = 496 ± 12; Dtd = 3 ± 0.25) and Megachile sp. (Dte = 1024 ± 20; Dtd = 1 ± 0.03; Dte, F = 417.58, P < 0.001; Dtd, F = 156.7, P = 0.050; Fig. 5). During this period, the ratio between pollen grains removed and deposited per visit was higher for Bombus spp. than for A. mellifera and Megachile sp. Bombus spp. had its highest effectiveness early in the morning and after mid-day, A. mellifera showed a peak of high effectiveness at 10.00 h while Megachile sp. showed a low but constant pattern of effectiveness (Fig. 5).
Fig. 5.
Pollination effectiveness of Apis mellifera, Bombus spp. and Megachile sp. in Lepechinia floribunda. (A) Pollen exportation effectiveness (Dte). (B) Pollen deposition effectiveness (Dtd). Left panels show the daily variation in pollination effectiveness for each floral visitor. The s.e. was obtained after 1000 bootstrap samples for each hour. Right bar plots represent the weighted average of Dt throughout the complete observation period (08.00–13.00 h), and after >70 % of stigmas were receptive in the population (11.00–13.00 h). Vertical lines represent the s.e. after 1000 bootstrap samples. n.s., non-significant difference; *P < 0.05; ***P < 0.001.
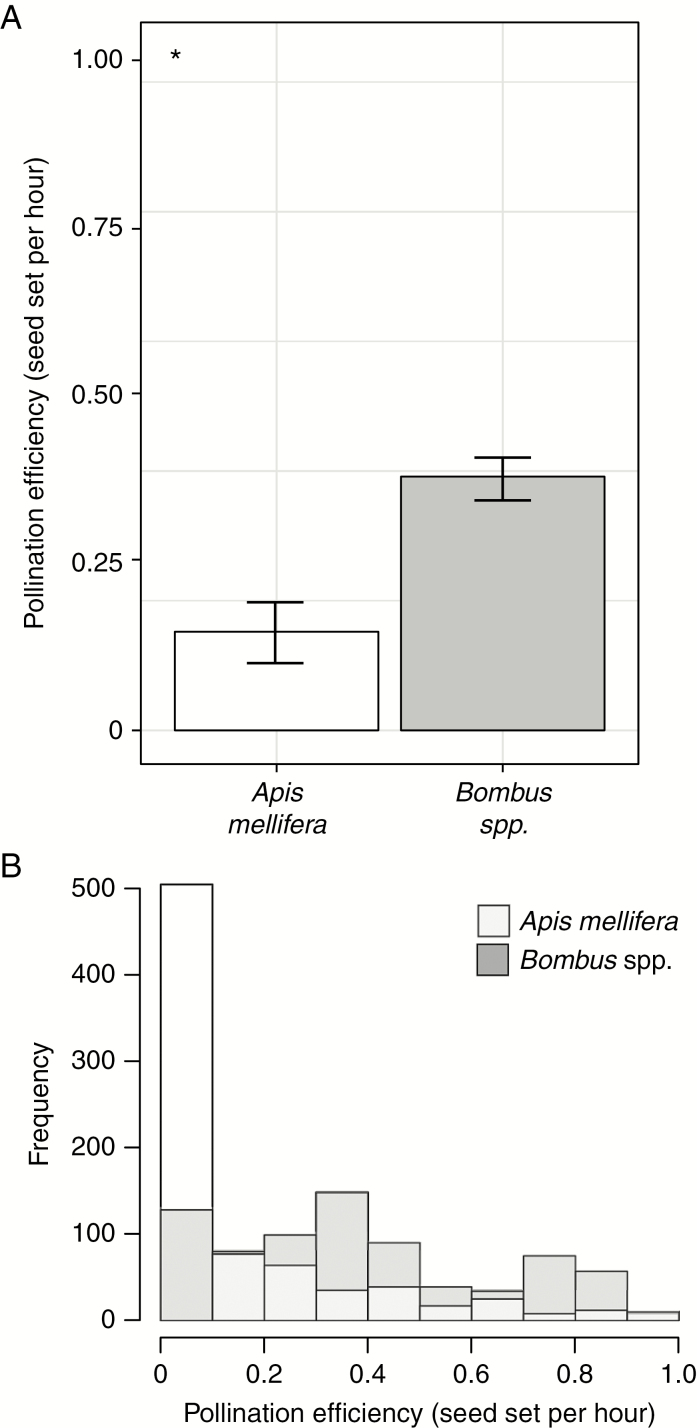
Given the low level of effectiveness and visitation frequency of Megachile sp. as well as its absence during one of the flowering seasons, it was not possible to obtain enough replicates to calculate pollination efficiency (PE) for this floral visitor. The PE of Bombus spp. per hour was significantly higher than that of A. mellifera (0.38 ± 0.01 vs. 0.14 ± 0.01) (β = –2.17, s.e. = 0.12, z = 17.59, P < 0.001; Fig. 6A). Taking into account all the flowers observed in the population, this means that the per hour contribution to maximal seed set per visited flower was 2.7 times higher for Bombus spp. despite it being a less abundant pollinator. In other words, Bombus spp. needed fewer than three visits to reach maximum seed set whereas A. mellifera needed more than six to reach that maximum. This was due to the lower probability of setting seeds after one visit by a honey-bee than by a bumble-bee. (Fig. 6B).
Fig. 6.
(A) Mean pollination efficiency of Apis mellifera and Bombus spp. Vertical lines represent the s.e. obtained after 1000 bootstrap samples. (B) Frequency distribution of pollination efficiency for each pollinator after the bootstrapping process. *P < 0.05.
DISCUSSION
We were able to disentangle pollinator performance of honey-bees (A. mellifera), bumble-bees (Bombus spp.) and leafcutter bees (Megachile sp.) through quantitative estimation of effectiveness and efficiency (sensuNe’eman et al., 2010), despite these pollinator groups belonging to the same functional guild. As expected, honey-bees were much more abundant on L. floribunda flowers than native bees. However, only one of the native visitors (Bombus spp.) was significantly more efficient than exotic bees due to its stronger overlap with daily phenological patterns of stigma receptivity and nectar production. This temporal matching accounted for the higher effectiveness and efficiency of bumble-bees over honey-bees and leafcutter bees. The results demonstrate that the higher visitation frequency of honey-bees was not enough to surpass the higher effectiveness and efficiency of the native bumble-bees. Thus, bumble-bees are probably the most important pollinators affecting the evolution of reproductive traits in L. floribunda through their positive effect on plant fitness. Under present levels of performance of honey-bees, plants would have to produce three times more flowers, or honey-bees would have to double their visitation frequency, to attain a seed production per hour as high as is attained by the native bumble-bees. This scenario may impose a strong selection favouring an increase in floral display and a reduction in dichogamy to match the foraging of activity honey-bees during the day if bumble-bees were to be displaced by anthropogenic disturbance or by competition with honey-bees. Thus, replacement of functions is likely within the pollinator assemblage of L. floribunda, but this would not be free of reproductive costs (Ashworth et al. 2015).
Over the years, several proxies for pollinator performance have been used, but a strong bias toward using the visitation frequency still persists (Dafni et al., 2005; Willmer et al., 2017). Estimations include the percentage of open flowers with signals that pollinators had touched the stigma, the amount of pollen removal or deposition, or the handling time, among others (Ne’eman et al., 2010). However, few studies have combined any of these estimates with the final fitness consequence of the pollination event (i.e. pollination efficiency; see Barrios et al., 2016; Ma et al., 2018, Valverde et al., 2019), and even fewer studies take into account the match between daily schedule in pollen presentation and stigma receptivity as well as pollinator activity (Albrecht et al., 2012). Through a quantitative estimation of pollinator performance, the present study showed that native bumble-bees were much more efficient than exotic honey-bees and native leafcutter bees, as they contributed most of the seeds set per unit time. The better performance of bumble-bees resulted from the greater overlap between the pattern of visitation frequency and the daily variation of stigma receptivity in the population of L. floribunda. Despite the higher number of visits accomplished by honey-bees, the greater part occurred when most stigmas were still closed. Consequently, bees mostly removed pollen and were less effective in pollen deposition. On the other hand, leafcutter bees were less predictable among years, with a very low visitation frequency and effectiveness. Hence, we predict that bumble-bees are likely to be responsible for the majority of seed production in the population. Analyses revealed that neither visitation frequency nor pollen removal or deposition alone were enough to provide a reliable estimate of pollinator performance. Differences in performance between pollinators were larger when the majority of stigmas were receptive (between 11.00 h and 13.00 h) than when it was estimated throughout the entire period of pollinator activity. These results highlight the importance of a more accurate distinction among the different events during the pollination process and how each pollinator contributes to final plant fitness (Valverde et al., 2019). Thus, combining floral phenology with pollinator activity provided a more realistic estimation of pollinator performance.
Pollinator activity usually depends on climatic conditions throughout the day and availability of resources (Stone et al., 1999). A difference in the temporal pattern of visitation to flowers among flower visitors has been attributed to a distinct temperature–foraging activity ratio (Free, 1968). However, pollinators that share the same floral resource can compete in promoting displacement of foraging patterns (Valido et al., 2019). Previous studies indicate that wild bees show lower visitation rates and lower fidelity to individual plant species over time when competing with honey-bees (Isaacs and Kirk, 2010). In addition, honey-bee overabundance leads to a re-assembly of plant–pollinator interactions through increased competition with other pollinator species (Magrach et al., 2017). Although we cannot rule out the possibility that bumble-bees and honey-bees competed in our studied population, the stronger match between bumble-bee activity and the pattern of nectar production suggests that a negative effect of honey-bees on bumble-bees through competitive interactions may not explain the observed pattern. Nevertheless, we cannot rule out the possibility that the lower effectiveness of leafcutter bees resulted from competitive interactions with honey-bees and/or bumble-bees.
Parallel to the generalized decline in pollinator availability due to anthropogenic alteration of natural environments (Winfree et al., 2009), honey-bees have distributed almost all over the globe, affecting the structure and functioning of the natural pollination system ( Aizen and Harder, 2009; Ollerton et al., 2012). This change in the pollination ecology is expected to alter fitness benefits of plants, population genetic structure and future evolution of plant reproductive traits (Magrach et al., 2017). The majority of progeny (approx. 75 %) of L. floribunda resulted from outcrossing, suggesting that pollinators play a leading role in the species reproductive success. A previous study in the same population of L. floribunda showed that only one visit during the male phase of flowers strongly decreases autonomous selfing by pollen removal (Roldan and Ashworth, 2018). This finding strengthens the importance of pollinators and protandry in promoting outcrossing. Our results show that honey-bees may impose a higher cost to plant reproduction than bumble-bees, because they produced fewer seeds per visit and consume both nectar and pollen. In addition, it is likely that the longer and more frequent intra-plant visits accomplished by honey-bees can promote selfing through geitonogamy over outcrossing (Ma et al., 2018), thus reducing offspring quality through limited pollen competition (Magrach et al., 2017). However, the accumulation of pollen by honey-bees before stigmas are receptive may promote a more diverse pollen load, reducing the likelihood of geitonogamy at anthesis (Roldan and Ashworth, 2018). Thus future studies should examine the relative contribution of each pollinator to the population outcrossing rate (Valverde et al., 2019) and the costs of reproduction.
A recent study demonstrated that the more effective recent arrival of invasive Bombus terrestris to a Chilean population of Erythranthe lutea (Phrymaceae) modified selection patterns acting on floral traits (Medel et al., 2018). Our results indicate that the contribution of bumble-bees to seed production costs fewer pollen grains and less investment in the floral display for plants than honey-bee. Future changes in pollinator abundance may affect L. floribunda floral evolution (Ashworth et al., 2015). Whereas bumble-bees probably select for the maintenance of protandry, honey-bees will probably select for its reduction. If honey-bees were to increase their current abundance even more, new selective pressures are expected to act on floral display, nectar production and dichogamy. Thus monitoring selection patterns on floral traits and pollinator abundance will help to predict floral evolution in a changing world.
This comparative study provides new evidence of the factors that play a critical role when estimating pollinator performance. Our results highlight the importance of a more precise distinction among the events during the pollination process and their contribution to final plant fitness. We suggest that combining floral phenology with pollinator activity provides a more realistic model of pollinator performance. Once the best pollinator is identified, the population genetic consequences and the costs of reproduction under different pollination environments can be estimated.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of Information S1: details of molecular techniques and microsattelite features for the multilocus population outcrossing rate (t) estimation in Lepechinia floribunda.
ACKNOWLEDGEMENTS
M. T. Solano de la Cruz, G. Martinez-Martinez and R. Pérez-Ishiwara provided helpful technical assistance during the project. We thank C. Maubecin, N. Rocamundi, A. Issaly, M. Renny and M. M. Mena for providing field assistance, and the authorities of the Natural Reserve Los Manantiales. We especially thank the members of the Laboratorio de Interacciones Planta-Animal for their valuable input and suggestions on the first draft of the manuscript, and three anonymous reviewers for helpful comments to improve the final version of this manuscript. M.C.B., S.B.-V. and J.F. conceived the idea, designed the experiments and wrote the manuscript. All authors participated in data acquisition, contributed substantially to editing of the manuscript and approved the final version. The authors have no conflict of interest.
FUNDING
J.F. thanks Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT-IN210617) Universidad Nacional Autónoma de México (UNAM) for financial support. The present study was part of a sabbatical project of J.F. at Universidad Nacional de Córdoba (IMBIV-CONICET), Argentina.
LITERATURE CITED
- Aizen MA, Harder LD. 2009. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Current Biology 19: 915–918. [DOI] [PubMed] [Google Scholar]
- Albrecht M, Schmid B, Hautier Y, Müller CB. 2012. Diverse pollinator communities enhance plant reproductive success. Proceedings of the Royal Society B: Biological Sciences 279: 4845–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth L, Aguilar R, Martén-Rodríguez S, et al. . 2015. Pollination syndromes: a global pattern of convergent evolution driven by the most effective pollinator. In: Pontarotti P, ed. Evolutionary biology: biodiversification from genotype to phenotype. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Ashworth L, Camina J, Funes G. 2017. Physical seed dormancy in Lepechinia floribunda (Lamiaceae): a medicinal native shrub. Boletín de la Sociedad Argentina de Botánica 52: 689–696. [Google Scholar]
- Baranzelli MC, Sérsic AN, Cocucci AA. 2014. The search for Pleiades in trait constellations: functional integration and phenotypic selection in the complex flowers of Morrenia brachystephana (Apocynaceae). Journal of Evolutionary Biology 27: 724–736. [DOI] [PubMed] [Google Scholar]
- Barrios B, Pena SR, Salas A, Koptur S. 2016. Butterflies visit more frequently, but bees are better pollinators: the importance of mouthpart dimensions in effective pollen removal and deposition. AoB Plants 8: plw001. pii: plw001. doi: 10.1093/aobpla/plw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Camina JL, Dambolena JS, Zygadlo JA, Ashworth L. 2018. Chemical composition of essential oils of peltate glandular trichomes from leaves and flowers of Lepechinia floribunda (Lamiaceae). Boletín de la Sociedad Argentina de Botánica 53: 375–384. [Google Scholar]
- Canty A, Ripley B. 2016. boot: bootstrap R (S-Plus) functions. R package version 1.3-18. [Google Scholar]
- Dafni A, Pacini E, Nepi M. 2005. Pollen and stigma biology. In: Dafni A, Kevan PG, Husband BC, eds. Practical pollination biology. Ontario, Canada: Enviroquest Ltd, 83–142. [Google Scholar]
- Epling C. 1938. Las labiadas de la Argentina, Paraguay y Uruguay. Revista del Museo de la Plata: sección botánica. Buenos Aires: Imprenta Casa Editora Coni. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics 35: 375–403. [Google Scholar]
- Free JB. 1968. The foraging behaviour of honeybees (Apis mellifera) and bumblebees (Bombus spp.) on blackcurrant (Ribes nigrum), raspberry (Rubus idaeus) and strawberry (Fragaria × Ananassa) flowers. Journal of Applied Ecology 5: 157–168. [Google Scholar]
- Freitas L. 2013. Concepts of pollinator performance: is a simple approach necessary to achieve a standardized terminology?. Brazilian Journal of Botany 36: 3–8. [Google Scholar]
- Harder LD, Johnson SD. 2009. Darwin’s beautiful contrivances: evolutionary and functional evidence for floral adaptation. New Phytologist 183: 530–545. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 1987. Components of pollinator ‘quality’: comparative analysis of a diverse insect assemblage. Oikos 50: 79–90. [Google Scholar]
- Herrera CM. 1989. Pollinator abundance, morphology, and flower visitation rate: analysis of the ‘quantity’ component in a plant–pollinator system. Oecologia 80: 241–248. [DOI] [PubMed] [Google Scholar]
- Herrera CM. 2000. Flower-to-seedling consequences of different pollination regimes in an insect-pollinated shrub. Ecology 81: 15–29. [Google Scholar]
- Isaacs R, Kirk AK. 2010. Pollination services provided to small and large highbush blueberry fields by wild and managed bees. Journal of Applied Ecology 47: 841–849. [Google Scholar]
- Kearns CA, Inouye DW. 1993. Techniques for pollination biologists. Boulder, CO: University Press of Colorado. [Google Scholar]
- Ma Y, Yin G. Gao J, Luo YB, Bai WN. 2018. Effects of distinct pollinators on the mating system and reproductive success in Incarvillea sinensis, an annual with large floral displays. Journal of Plant Ecology 12: 137–143. [Google Scholar]
- Magrach A, González-Varo JP, Boiffier M, Vilà M, Bartomeus I. 2017. Honeybee spillover reshuffles pollinator diets and affects plant reproductive success. Nature Ecology & Evolution 1: 1299. [DOI] [PubMed] [Google Scholar]
- Mayer C, Adler L, Armbruster WS, et al. . 2011. Pollination ecology in the 21st century: key questions for future research. Journal of Pollination Ecology 3: 8–23. [Google Scholar]
- Medel R, González-Browne C, Salazar D, Ferrer P, Ehrenfeld M. 2018. The most effective pollinator principle applies to new invasive pollinators. Biology Letters 14: 20180132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnaar C, Anderson B, de Jager ML, Karron JD. 2019. Plant–pollinator interactions along the pathway to paternity. Annals of Botany 123: 225–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch M. 2017. sciplot: scientific graphing functions for factorial designs. R package version 1.1-1. [Google Scholar]
- Ne’eman G, Jürgens A, Newstrom-Lloyd L, Potts SG, Dafni A. 2010. A framework for comparing pollinator performance: effectiveness and efficiency. Biological Reviews 85: 435–451. [DOI] [PubMed] [Google Scholar]
- Ollerton J, Price V, Armbruster WS, et al. . 2012. Overplaying the role of honey bees as pollinators: a comment on Aebi and Neumann (2011). Trends in Ecology and Evolution 27: 141. [DOI] [PubMed] [Google Scholar]
- Poblete Palacios JA, Soteras F, Cocucci AA. 2019. Mechanical fit between flower and pollinators in relation to realized precision and accuracy in the hummingbird-pollinated Dolichandra cynanchoides. Biological Journal of the Linnean Society 126: 655–665. [Google Scholar]
- Proctor M, Yeo P, Lack A. 1996. The natural history of pollination. London: Harper Collins Publishers. [Google Scholar]
- R Core Team 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- Ritland K. 2002. Extensions of models for the estimation of mating systems using n independent loci. Heredity 88: 221. [DOI] [PubMed] [Google Scholar]
- Ritland K, Jain S. 1981. A model for the estimation of outcrossing rate and gene frequencies using n independent loci. Heredity 47: 35. [Google Scholar]
- Roldán JS, Ashworth L. 2018. Disentangling the role of herkogamy, dichogamy and pollinators in plant reproductive assurance. Plant Ecology & Diversity 11: 383–392. [Google Scholar]
- Sahli HF, Conner JK. 2007. Visitation, effectiveness, and efficiency of 15 genera of visitors to wild radish, Raphanus raphanistrum (Brassicaceae). American Journal of Botany 94: 203–209. [DOI] [PubMed] [Google Scholar]
- Schupp EW, Jordano P, Gómez JM. 2017. A general framework for effectiveness concepts in mutualisms. Ecology Letters 20: 577–590. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms, I: pollination mechanisms. Annual Review of Ecology and Systematics 1: 307–326. [Google Scholar]
- Stone GN, Gilbert F, Willmer P, Potts S, Semida F, Zalat S. 1999. Windows of opportunity and the temporal structuring of foraging activity in a desert solitary bee. Ecological Entomology 24: 208–221. [Google Scholar]
- Torsten H, Frank B, Westfall P. 2008. Simultaneous inference in general parametric models. Biometrical Journal 50: 346–363. [DOI] [PubMed] [Google Scholar]
- Valido A, Rodríguez-Rodríguez MC, Jordano P. 2019. Honeybees disrupt the structure and functionality of plant–pollinator networks. Scientific Reports 9: 4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde J, Perfectti F, Gómez JM. 2019. Pollination effectiveness in a generalist plant: adding the genetic component. New Phytologist 223: 354–365. [DOI] [PubMed] [Google Scholar]
- Vázquez DP, Morris WF, Jordano P. 2005. Interaction frequency as a surrogate for the total effect of animal mutualists on plants. Ecology Letters 8: 1088–1094. [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag. [Google Scholar]
- Willmer PG, Cunnold H, Ballantyne G. 2017. Insights from measuring pollen deposition: quantifying the preeminence of bees as flower visitors and effective pollinators. Arthropod-Plant Interactions 11: 411–425. [Google Scholar]
- Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. 2009. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90: 2068–2076. [DOI] [PubMed] [Google Scholar]
- Wood SN. 2006. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- Wood SN. 2008. Fast stable direct fitting and smoothness selection for generalized additive models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 70: 495–518. [Google Scholar]
- Zeileis A, Kleiber C, Jackman S. 2008. Regression models for count data in R. Journal of Statistical Software 27: 1–25. [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York: Springer Science & Business Media. [Google Scholar]
- Zych M. 2007. On flower visitors and true pollinators: the case of protandrous Heracleum sphondylium L.(Apiaceae). Plant Systematics and Evolution 263: 159–179. [Google Scholar]
- Zych M, Goldstein J, Roguz K, Stpiczyńska M. 2013. The most effective pollinator revisited: pollen dynamics in a spring-flowering herb. Arthropod-Plant Interactions 7: 315–322. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.