Abstract
Introduction and hypothesis
New Zealand white rabbits are an inexpensive large-animal model. This study explored the rabbit as a model for mesh-augmented colpopexy using the intra-abdominal vagina. We hypothesized that polypropylene mesh would negatively impact rabbit vaginal smooth muscle (VSM) morphology and contractile function, similar to the nonhuman primate (NHP)—the established model for prolapse mesh evaluation.
Methods
Restorelle was implanted onto the vagina of ten rabbits via lumbar colpopexy after a hysterectomy. Ten rabbits served as sham. Twelve weeks post-implantation, the vagina was excised and VSM morphology and vaginal contractility were assessed. Outcome measures were compared using independent samples t and Mann-Whitney U tests with a Bonferroni correction, where appropriate. Results from the rabbits were compared with published NHP data.
Results
Animals had similar age, parity and BMI. VSM was 18% thinner after Restorelle implantation, P = 0.027. Vaginal contractility was 43% decreased in response to 120 mM KCl (P = 0.003), similar to the 46% reduction observed in the NHP vagina implanted with Restorelle (P = 0.027). Three meshes wrinkled in vivo, resulting in dramatic thinning of the underlying vagina in the area of the mesh causing a mesh exposure.
Conclusions
Polypropylene mesh negatively impacts VSM morphology and vaginal contractility in the rabbit, similar to the NHP, suggesting that the rabbit may serve as an alternative large-animal model. The vaginal thinning and appearance of a mesh exposure in the area of a mesh wrinkle suggest the rabbit may also serve as a model for understanding the pathophysiology of mesh exposure.
Keywords: New Zealand white rabbit, Vaginal smooth muscle, Pelvic organ prolapse, Modified abdominal sacrocolpopexy, Lumbar colpopexy, Polypropylene mesh
Introduction
Polypropylene mesh is commonly used in the surgical treatment of pelvic organ prolapse (POP) [1]. Unfortunately, mesh usage has been hampered by complications, with mesh exposure through the vaginal epithelium and pain most commonly reported [1]. The precise etiology of mesh complications is unclear. Animal models have improved our understanding of the impact of mesh on the vagina and provided insight into mechanisms of mesh complications [2–6].
The nonhuman primate (NHP), Rhesus macaque, is currently the gold standard model for investigating the impact of mesh on the vagina. NHPs are advantageous as they spontaneously develop prolapse and their pelvic anatomy and physiology are similar to those of humans [7]. Additionally, the NHP’s pelvis is large enough to accommodate implantation of a prolapse mesh of reasonable dimensions onto the vagina in a flat configuration via an abdominal sacrocolpopexy (ASC), similar to humans. However, NHPs are a limited resource and are expensive, which limits the number and length of studies that can be conducted with this model. Hence, there is an urgent need for a cheaper large-animal model that can be used to investigate mechanisms of mesh complications.
The ewe is a cheaper and more accessible animal model relative to the NHP, and previous studies have utilized the ewe to implant mesh onto the vagina via a transvaginal approach [8–10]. However, performing abdominal surgery on ewes is surgically difficult because of the large rumen, and bowel obstructions are extremely common after surgery. The New Zealand White rabbit, on the other hand, is an alternative large-animal model that is cheaper than the NHP and is not a ruminant. Additionally, the rabbit vagina is large enough (~15 cm long and 2.5–4 cm wide) to allow for the implantation of mesh in a flat configuration. It is important to note that unlike in NHPs and humans, the rabbit vagina consists of two parts—an intra-abdominal portion and an external portion. The external vagina has previously been used for mesh implantation studies [11–15]. However, meshes implanted on the external vagina cannot be placed on tension because of limited accessibility of the pelvic side wall and spine. In contrast, mesh can be implanted in a flat configuration on the intra-abdominal vagina and placed on tension by attaching it to the spine, similar to an ASC. Additionally, compared with the external vagina, the intra-abdominal vagina is more representative of POP in women because it naturally lacks lateral and apical support, common sites of support defects in women with POP. In this way the rabbit may serve as an alternative model for addressing certain questions regarding the impact of mesh on the vagina such as the impact of tensioning and loading. We describe a novel method for implanting mesh onto the internal rabbit vagina and then define the impact of mesh on rabbit vaginal smooth muscle (VSM) morphology and vaginal contractility. Additionally, the results of this study were compared to the results from previous studies in which mesh was implanted onto the NHP vagina to assess the validity of using the rabbit as an alternative model to the NHP [4–6]. As studies utilizing the NHP have found that polypropylene mesh negatively impacts VSM morphology and vaginal contractility [4–6], we hypothesize the rabbit, if an appropriate model, would behave similarly.
Materials and methods
Animals
Twenty female New Zealand white rabbits, retired breeders, ages 2 to 3 years, were utilized according to experimental protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC #16035431). Rabbits were housed in standard cages, on a 12-h alternating light/dark cycle, with water and a standard rabbit diet supplemented with hay and greens ad libitum.
Surgical procedures
Sterile samples (12 × 3 cm2) of Restorelle (Coloplast, Minneapolis, MN) were implanted onto the anterior and posterior vagina via a modified ASC. Restorelle was chosen as it is widely used in humans and in previous studies had the least negative impact on vaginal morphology and function [4–6]. Following laparotomy, the bladder and rectum were gently dissected off of the vagina. Muscles overlying the lumbar spine were divided, and two 2–0 PDS II sutures, which served as the mesh-vagina anchoring sites, were placed through the ligamentous portion of the vertebral body. Next, a complete hysterectomy was performed without excision of the ovaries, and two straps of mesh were secured to the anterior and posterior vagina with 3–0 PDS II sutures. The two straps were then anchored to the lumbar spine with the previously placed 2–0 PDS II sutures (Fig. 1, right). For sham animals, the vagina with no mesh attached was anchored to the lumbar spine (Fig. 1, left). Lastly, the abdominal muscle layer was closed with 2–0 PDS II, and the skin was closed with a continuous subcuticular stitch (2–0 Vicryl). Note the more appropriate terminology for this modified ASC is lumbar colpopexy, a term that will be used throughout this manuscript. Twelve weeks post-implantation, the vagina with and without mesh was excised and harvested for histomorphology and biomechanical analyses.
Fig. 1.
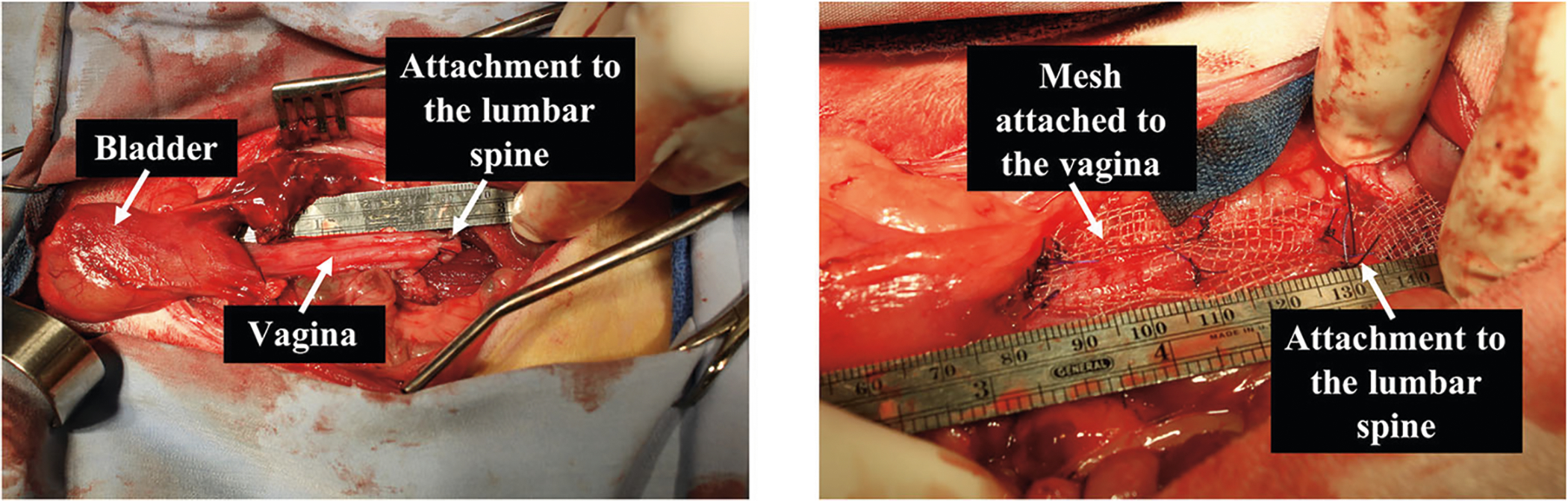
Rabbit in vivo lumbar colpopexy. Surgical image depicting a lumbar colpopexy post-hysterectomy in which the vagina is attached to the lumbar spine without (left image) and with mesh attached to the vagina (right image). Note in the right image the mesh is attached to the vagina in a flat configuration.
Histology analysis
VSM morphology was assessed using Masson’s trichrome staining. Briefly, full-thickness cross sections of the rabbit vagina with and without mesh were excised, fixed in formalin, embedded in paraffin and sectioned at 7 μm. Sections were stained with hematoxylin solution Gill no. 2 (to stain nuclei) and trichrome stain AB solution (Sigma-Aldrich, St. Louis, MO, USA) and imaged at ×100 using a Nikon Eclipse 90i imaging microscope (Melville, NY, USA). VSM thickness was measured using a custom Mathematica V11.3 (Wolfram, Champaign, IL) script, developed by Megan R. Routzong (co-author). Briefly, the smooth muscle layer was outlined by identifying the inner (i.e., the intersection where the sub-epithelium ends and the smooth muscle begins) and outer (i.e., the intersection where the smooth muscle ends and the adventitia begins) borders of the smooth muscle layer. The distance between the inner and outer border of the smooth muscle was then determined using built-in mathematical functions within Mathematica. This method was found to be within a 5% error when calculating the thickness of known geometries and manual measurements.
Vaginal contractile function analysis
The contractile function of the VSM was assessed utilizing a vaginal contractility assay as previously described [4, 5]. Briefly, strips (approximately 7 mm × 2 mm) oriented along the circumferential direction were cut from the anterior and posterior proximal vagina (2 strips per side) with and without mesh. For samples with mesh, the mesh-vagina complex was tested intact to avoid damaging the tissue or the mesh and to afford testing of the contractile function of the vagina in the presence of mesh. Strips were clamped on opposing ends, and care was taken to ensure that no mesh was placed between the clamps in the case of mesh-implanted vaginas. Contraction of the vagina was induced using three stimulants: (1) 120 mM KCl to assess function of muscle myofibers via global muscle depolarization, (2) electrical field stimulation (EFS) 20 V for 5 s at 1–64 Hz to measure nerve-mediated smooth muscle contraction and (3) 10−7 to 10−4 M phenylephrine (an α1-adreno-receptor agonist), applied non-cumulatively to measure receptor-mediated smooth muscle contraction via receptor depolarization. After each stimulant, maximum contractile force was recorded, and tissues were washed with Krebs solution prior to the application of the next stimulant. The resultant contractile forces generated in response to all three stimuli were normalized by the tissue volume. For comparison of rabbit to NHP, contractile force to EFS was also normalized by force generated in response to 120 mM KCl, both acceptable methods of normalization [4, 5, 16].
Historic nonhuman primate study design and analysis synopsis
Previously, Restorelle was implanted onto the anterior and posterior vaginal walls of eight middle-aged NHPs under nearly identical conditions—sacrocolpopexy after a hysterectomy with preservation of the ovaries [4–6]. Eight NHPs served as sham. Similar to the methods and analyses described previously, the histomorphology and thickness of the VSM were assessed after staining with Masson’s trichrome and labeling with α-smooth muscle actin, respectively. The contractile function of the vagina was also evaluated using KCl and EFS as described for the rabbits. The NHP vagina does not consistently respond to phenylephrine; therefore, the response to phenylephrine was not compared between the rabbit and NHP. Given the similar methods utilized, the impact of mesh on VSM structure and vaginal contractility between the rabbit and NHP was compared.
Statistical analysis
Based on a power analysis, ten animals per group were needed to detect differences between groups with a power of 80% and a two-tailed significance set at P < 0.05. To determine whether the data were normally distributed, Kolmogorov-Smirnov tests were utilized. Differences in the smooth muscle thickness and contractile force within (rabbit sham vs. Restorelle) and between species (rabbit vs. nonhuman primate) were determined using independent samples t-tests in normally distributed data and Mann-Whitney U tests as a nonparametric alternative, with a Bonferroni correction where appropriate. All statistical analyses were performed utilizing SPSS 25.0 statistical software (IBM, Armonk, NY, USA).
Results
Twenty female rabbits underwent a hysterectomy with preservation of the ovaries followed by mesh implantation via lumbar colpopexy (a modified ASC) vs. sham (no mesh). One rabbit in the mesh-implanted group sustained a bowel obstruction and was excluded, leaving a final sample size of sham N = 10 and Restorelle N = 9. Animals had similar age and weight.
Gross morphology and histology analysis
At the time of tissue harvesting, vaginal tissue was well integrated within the pores of Restorelle in the mesh-implanted rabbit vagina (Fig. 2). Similar to humans and NHPs, the rabbit vagina had four layers (epithelium, subepithelium, muscularis and adventitia) (Fig. 3a) [7]. However, the rabbit vaginal epithelium is glandular, and the smooth muscle layer comprised approximately 70% of the overall thickness compared with approximately 30% of the NHP vagina (Fig. 3a and b). In six animals, meshes remained flat after implantation; however, three meshes wrinkled and were exposed through the vaginal epithelium (Fig. 4). In areas where the mesh wrinkled toward the vaginal lumen, there was an associated thinning of the underlying vagina (Fig. 5). This is in contrast to the meshes that remained flat after implantation, which displayed thinning of the muscularis without areas of extreme thinning as observed with wrinkles. Overall, the VSM layer was significantly thinner after Restorelle implantation compared with sham [1577.1 (319.4) μm vs. 1286.8 (125.0) μm, respectively (P = 0.027)].
Fig. 2.
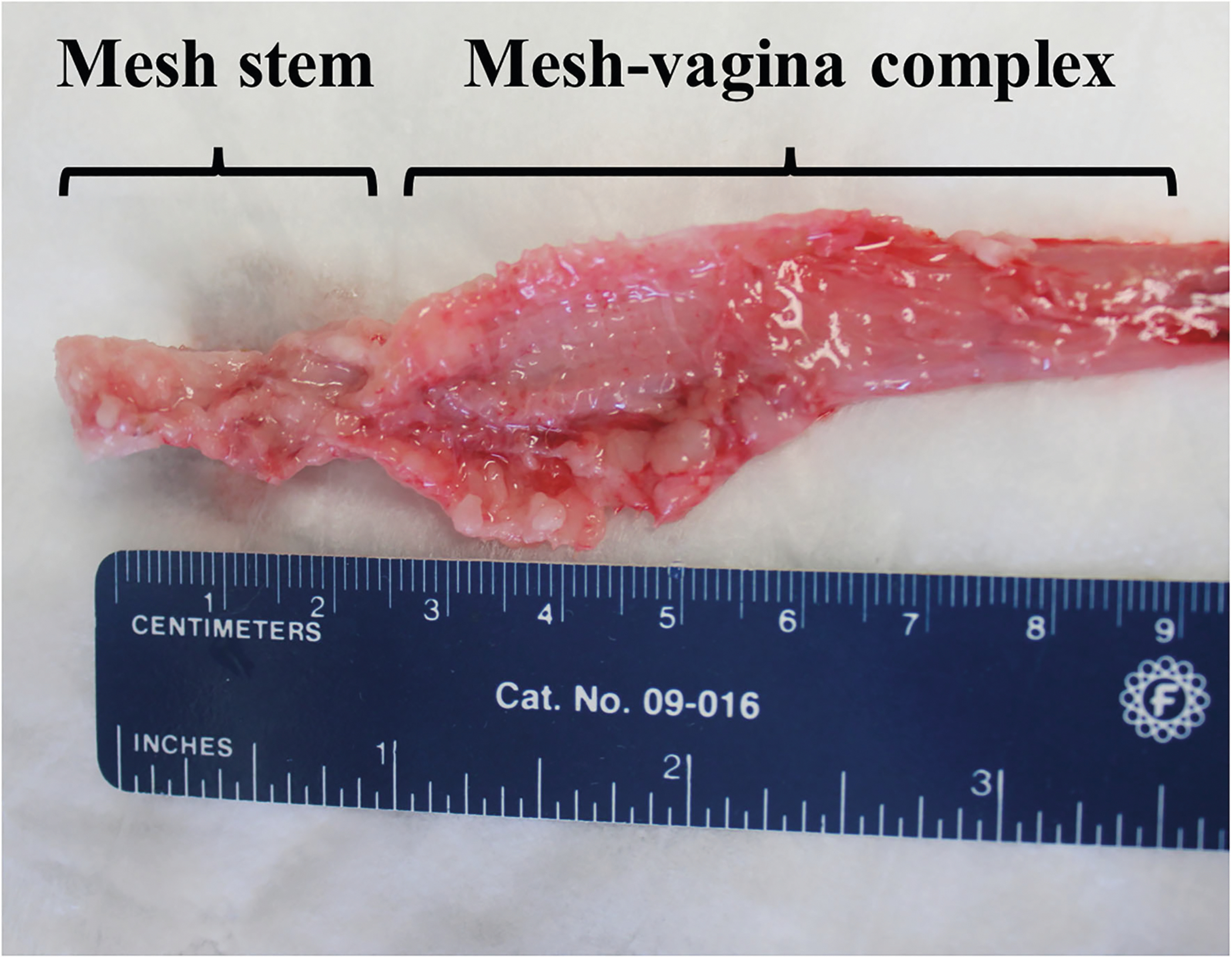
Mesh-vagina explant. Mesh-vagina complex explanted 12 weeks post lumbar colpopexy. Demonstrated in the image, vaginal tissue is incorporated between the pores of the mesh, and the mesh remained in the flat configuration in which it was implanted.
Fig. 3.
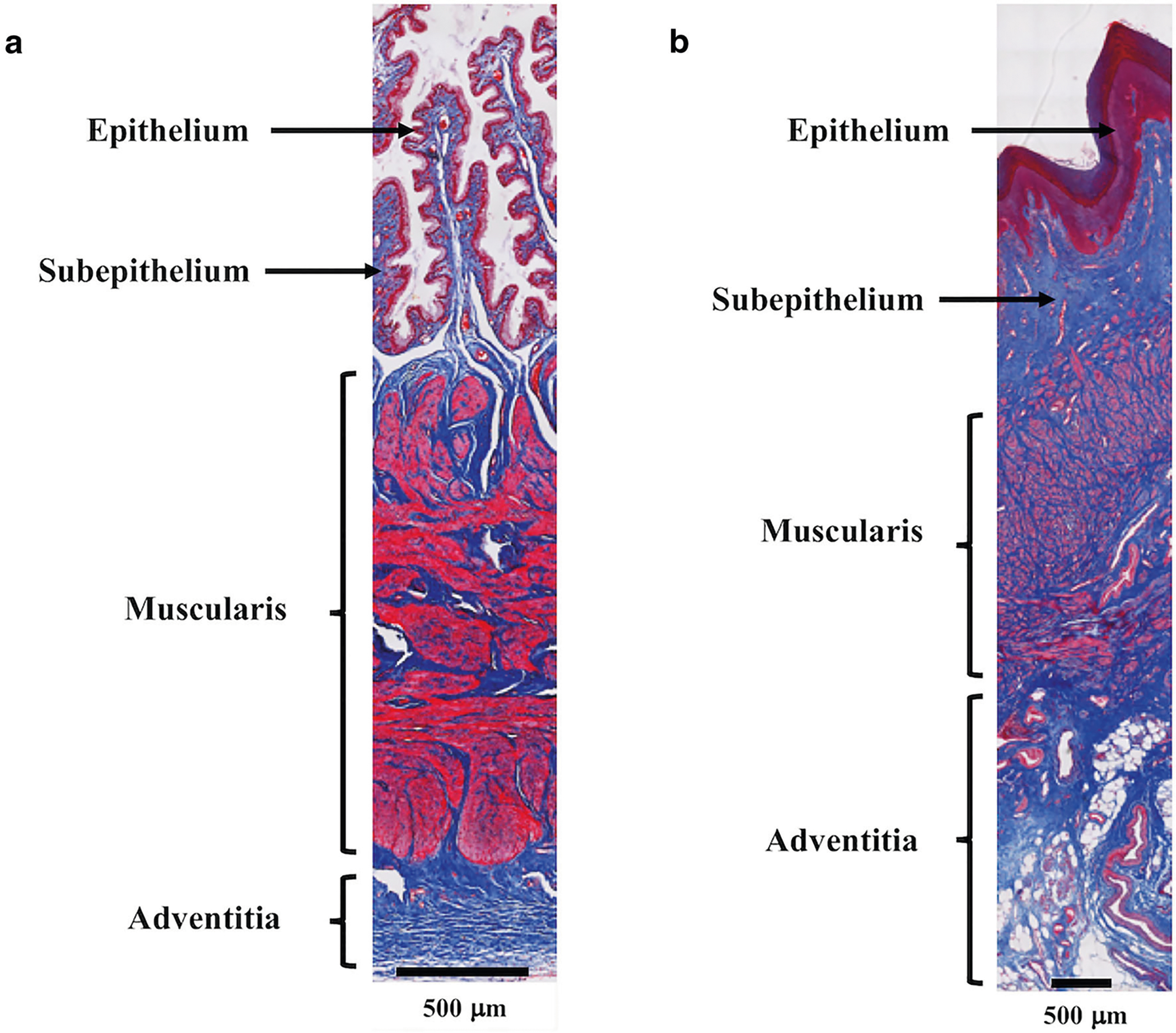
Rabbit and nonhuman primate sham vaginal cross section. Masson’s trichrome staining of the a rabbit and b nonhuman primate sham vagina demonstrating the characteristic layers of the vagina with a prominent muscularis (smooth muscle) layer. Relative to the nonhuman primate, the muscularis layer makes up a greater portion of the rabbit vagina.
Fig. 4.
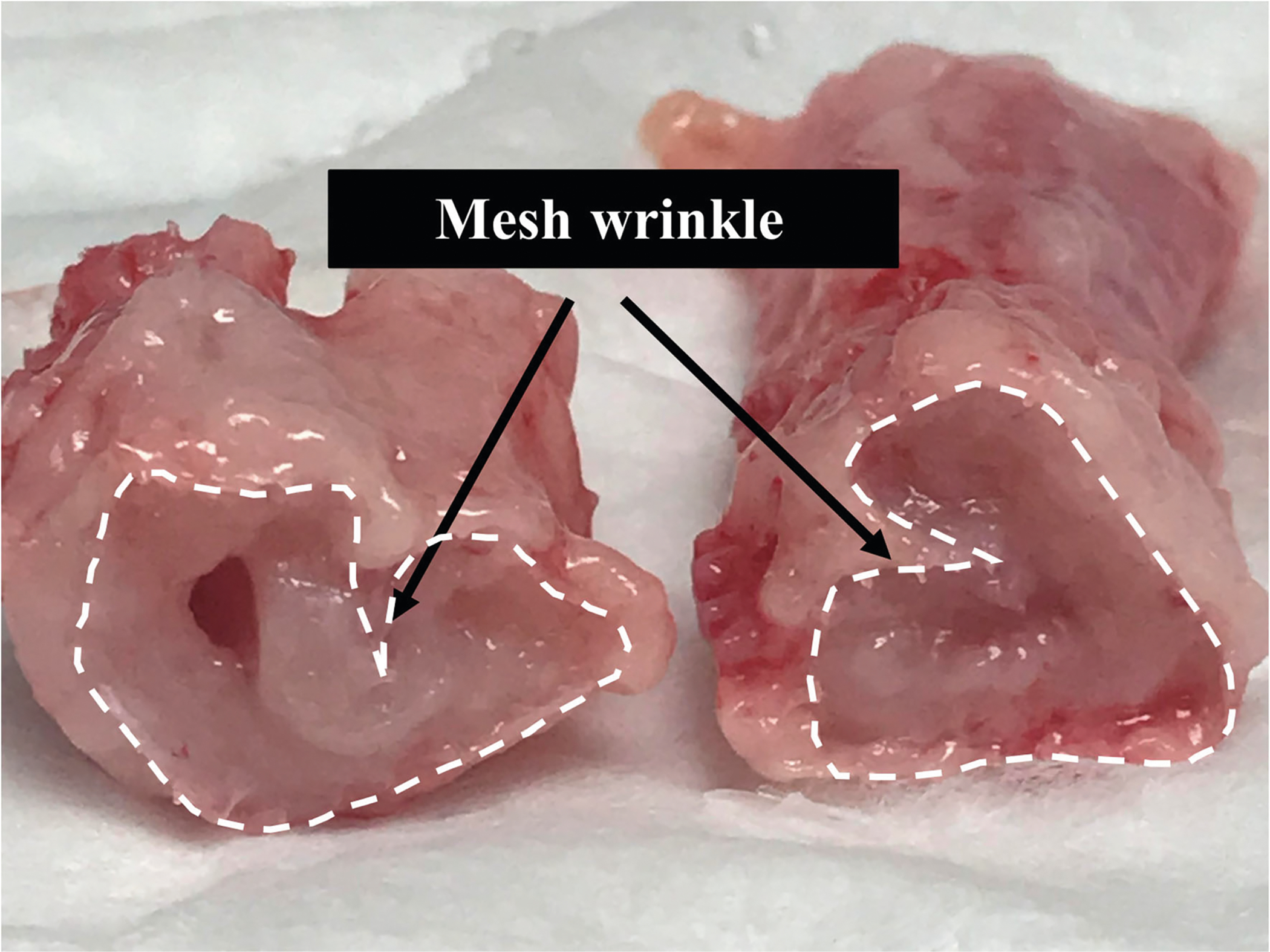
Mesh wrinkle. Cross-sectional view of the mesh-vagina complex depicting a mesh wrinkle.
Fig. 5.
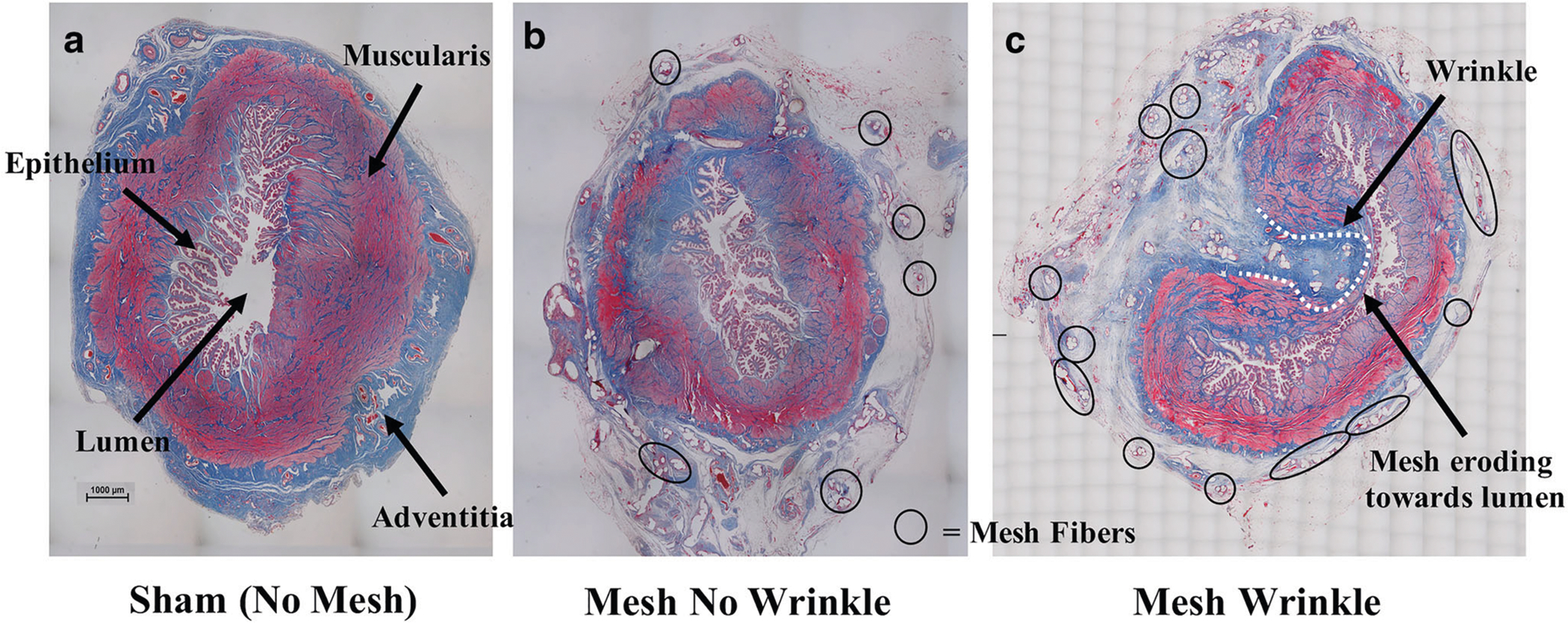
Smooth muscle thinning with mesh implantation. Cross-sectional images of the rabbit vagina stained with Masson’s trichrome staining depicting: a a vagina in which no mesh was implanted, b a mesh with no wrinkle and c a mesh wrinkle in which the mesh fibers (black circles) erode toward the lumen (dotted white line) of the vagina (characteristic of a mesh exposure). Overall, thinning of the muscularis (smooth muscle) layer was observed in the mesh-implanted rabbit vagina relative to sham. Note: For esthetics, only a few mesh fibers are outlined in b and c.
Contractile function
The contractile function of the rabbit vagina without and with mesh implanted was assessed utilizing 120 mM KCl, EFS and phenylephrine. Given that anterior and posterior strips of the vagina were evaluated, statistical analyses were performed to determine whether the contractile function of the sham anterior and posterior strips of the vagina differed. In response to all three stimuli, the resulting contractile forces were not significantly different between the sham anterior and posterior vagina (120 mM KCl P = 0.518, EFS P = 0.428 and phenylephrine P = 0.114); therefore, for the purposes of this study, the contractility data presented here represent the average contractile force of the anterior and posterior vagina.
Implanting Restorelle onto the rabbit vagina negatively impacted the ability of the myofibers, nerves and receptors to induce a smooth muscle contraction (Table 1). The contractile force decreased 43.3% in response to 120 mM KCl (P = 0.003), 50% following EFS (P = 0.007) and 44.9% following stimulation with phenylephrine (P = 0.012). A similar 46.2% decrease in muscle-mediated contractile function was observed with the implantation of Restorelle onto the NHP vagina, P = 0.027; however, nerve-medicated contractile function was not significantly decreased (P = 0.379), and a contractile response to phenylephrine was not consistently observed, likely because of the higher smooth muscle content in the rabbit vagina.
Table 1.
Rabbit maximum contractile force in response to stimuli
| Stimulant | Sham (N = 10) | Restorelle (N = 9) | P value |
|---|---|---|---|
| 120 mM Potassium chloride (mN/mm3) | 1.71 ± 0.52 | 0.97 ± 0.41 | 0.003a |
| Electrical field stimulation (mN/mm3) | 0.88 (1.10) | 0.44 (0.43) | 0.007b |
| Phenylephrine (mN/mm3) | 2.36 ± 0.89 | 1.30 ± 0.74 | 0.012a |
Data represented as mean ± standard deviation or median (interquartile range)
P value obtained using independent samples t-test
P value obtained using Mann-Whitney U test
Compared with the nonhuman primate, the rabbit vagina was significantly more contractile (Table 2). In response to 120 mM KCl, the rabbit vagina, without (sham) and with mesh implanted (Restorelle), was 6.6- (P < 0.001) and 6.9- (P < 0.001) fold more contractile than the NHP sham and Restorelle-implanted vagina, respectively. Similarly, the rabbit vagina without (sham) and with mesh implanted (Restorelle) was 88- and 44- fold more contractile than the NHP sham and Restorelle-implanted vagina in response to EFS, respectively (P < 0.001 for both). Normalizing the contractile force to EFS by the contractile force in response to 120 mM KCl, an alternative method of normalization, resulted in a similar decrease in smooth muscle contractility in the rabbit and NHP sham vagina (P = 0.315). The decrease in contractility between the rabbit and NHP Restorelle-implanted vagina was also similar by this method (P = 0.610).
Table 2.
Rabbit vs. nonhuman primate contractile force response to stimuli
| Stimulant | Sham | Restorelle | ||||
|---|---|---|---|---|---|---|
| Rabbit (N = 10) | NHP (N = 8) | Rabbit vs. NHP | Rabbit (N = 9) | NHP (N = 8) | Rabbit vs. NHP | |
| 120 mM Potassium chloride (mN/mm3) | 1.71 ± 0.52 | 0.26 ± 0.11 | P value < 0.001b | 0.97 ± 0.41 | 0.14 ± 0.08 | P value < 0.001b |
| Electrical field stimulation (mN/mm3) | 0.88 (1.10) | 0.01 (0.02) | P value < 0.001b | 0.44 (0.43) | 0.01 (0.02) | P value < 0.001b |
| Electrical field stimulation (g/g) | 0.46 (0.69) | 0.46 (0.44) | P = 0.315b | 0.51 (0.36) | 0.62 (0.49) | P = 0.610a |
NHP nonhuman primate
Data represented as mean ± standard deviation or median (interquartile range)
P value obtained using independent samples t-test
P value obtained using Mann-Whitney U test
Discussion
By suspending the vagina to the lumbar spine via a mesh bridge (lumbar colpopexy), we were able to (1) implant Restorelle onto the rabbit internal vagina in a flat configuration and (2) recreate the loading conditions observed during a human ASC. Interestingly, the internal vagina of the rabbit lacks lateral and apical support structures and therefore is similar to the vagina of women with advanced prolapse. The most important points of this study are that, first, implanting polypropylene mesh onto the rabbit internal vagina resulted in a significant decrease in VSM thickness and vaginal contractile function, similar to previous observations following implantation of Restorelle in the NHP. This finding is congruent with the study hypothesis and suggests that the rabbit can serve as an alternative model to the NHP. Second, significant thinning of the vagina was observed in areas where the mesh wrinkled, similar to what is seen in women with mesh exposure. In this way, the rabbit is one of the first models with potential for directly studying the mechanism(s) of mesh exposure. The third major finding was the rabbit vagina is comprised of considerably more muscle than the NHP (or human based on qualitative observation) and appears more sensitive to VSM stimulants, suggesting that it might also be a preferred model for studying the impact of vaginal implants on VSM.
VSM thickness significantly decreased with the implantation of mesh for the rabbit but not for the NHP, although the trend was similar. This is likely a result of the rabbit vagina consisting of proportionally more smooth muscle than the NHP in which the smooth muscle layer is not as predominant. Hence, changes in thickness will be more observable in the rabbit VSM than in the NHP. The relative change in the contractile force in response to EFS for the rabbit was higher than the NHP, 50% and 31%, respectively, and only the decrease in the rabbit achieved statistical significance. However, when normalizing the EFS contractile force by the force of contraction to 120 mM KCl, the observed decrease in both models was statistically similar, suggesting that the impact of mesh on nerve-mediated contractions is indeed similar for the rabbit and NHP. Likewise, the relative change in the muscle-mediated contractions was significantly decreased for both rabbit (43% decreased) and NHP (46% decreased). Together these results support that the impact of polypropylene mesh on the rabbit and NHP vagina is similar.
Vaginal smooth muscle plays an essential role in maintaining vaginal tone and the overall support of the vagina through the connection between VSM fibers and fibers of the levator ani muscles [17]. Additionally, VSM is critical to sexual function as the relaxation of VSM with sexual stimulation results in vaginal enlargement, vasocongestion, engorgement and lubrication [18]. Studies have shown that in women with POP, VSM is disorganized, the fractional area of smooth muscle is decreased, and smooth muscle apoptosis is increased [19–21]. Based on studies in the NHP and now the rabbit demonstrating the negative impact of polypropylene mesh on VSM, it is possible that surgeons are implanting mesh onto a vagina that is already compromised with regard to VSM structure and function and that mesh could be exacerbating this condition. With the abundance of VSM within the rabbit and the ability to elicit both nerve- and receptor-mediated contractions, the rabbit model provides a cost-effective model for defining the impact of mesh on VSM morphology and function.
Mesh exposure is one of the most common complications reported, occurring in approximately 10–20% of the meshes placed transvaginally and 10.5% of meshes placed transabdominally with rates that increase with time [1, 22–25]. Infection, host reaction to a foreign material, stress mis-matches between the mesh and the vagina, and micro-motion are all proposed mechanisms [2, 4, 6]. Clinically, mesh exposures are often observed in the areas of a mesh wrinkle and/or contraction [26, 27]. Furthermore, mesh explants removed from women with exposure demonstrate marked deformation and wrinkling associated with increased pro-MMP-9 and cytotoxic T cells relative to control tissue without mesh, indicative of tissue degradation [28, 29]. Indeed, thinning of the underlying tissue in the area of mesh wrinkles, as observed in this study, strongly suggests that mesh wrinkling leading to tissue degradation is a plausible mechanism for mesh exposure.
Together, our data establishing the rabbit model as an alternative to the NHP comprise a crucial next step toward understanding mechanisms of mesh complications, particularly those whose pathophysiology involves smooth muscle dysfunction and atrophy. The availability, size, ability to implant mesh via a lumbar colpopexy and relatively low expense of the rabbit give scientists the ability to investigate multiple research questions and to conduct long-term studies.
The strengths of this study include the use of an inexpensive large-animal model that lacks apical and lateral vaginal support similar to women with POP. Additionally, the methods utilized to assess the contractile function of the rabbit vagina are the same as those used previously for the NHP and women with prolapse, which allows for a direct comparison between species [4, 5, 30]. The NHP ASC model also served as a way to validate the rabbit lumbar colpopexy model developed in this study. This study however has some limitations which must be considered. First, the rabbit vagina is structurally different from that of humans. Specifically, the rabbit vagina has a glandular epithelium, it is primarily smooth muscle and is thinner than the human vagina (which can make the rabbit vagina more susceptible to mesh complications), and it lacks lateral and apical support (typical of women with POP but not women with normal support). Furthermore, the rabbit is a quadruped and it does not develop POP spontaneously. Due to the inherent challenges in conducting longitudinal experiments on tissue in women undergoing prolapse procedures, researchers must utilize animal models that mimic the human condition. Indeed, if the appropriate animal model is chosen based on the research question investigated, the limitations of utilizing an animal model can be reduced. Second, the contractile function of the VSM from sham and Restorelle-implanted animals was indirectly measured with the vagina or mesh-vagina complex completely intact (i.e., the VSM was not isolated from the other layers of the vagina). This was done to (1) avoid any damage that would occur when separating the smooth muscle layer from the other layers of the vagina, especially since the mesh was well integrated within the vaginal tissue, and (2) to determine how vaginal tissue functions (i.e., contracts) in the presence of mesh. In this study, the contractile function reported was normalized to the volume of the sample tested to account for any differences in the dimensions of the samples (e.g., length, width and thickness). This addresses questions about the overall contractile function of the organ as a whole, but does not provide information on the contractile phenotype of specific cells or cell layers. Lastly, stimulating rabbit vaginal tissue with 120 mM KCl did not produce the maximal contractile response (i.e., phenylephrine stimulation produced the highest response in this study), but did allow for a direct comparison to that of the NHP since 120 mM KCl was also utilized on the NHP vagina. Moreover, we performed a preliminary dose-response curve of the rabbit vagina (the smooth muscle was not isolated from the other layers of the vagina) in response to KCl and found that the dose for maximal stimulation (over 160 mM KCl) substantially exceeds physiologic ranges.
Overall, this study demonstrated that polypropylene mesh negatively impacts rabbit vaginal morphology and function, similar to what we have observed in the NHP, suggesting that the rabbit model can serve as an alternate model. Perhaps most interestingly and unexpectedly, we were able to recreate conditions consistent with mesh exposures in women. In this way, the rabbit may serve as a model for understanding the mechanism(s) of mesh exposure.
Acknowledgments
We are grateful for the financial support from the Department of Defense (DOD) (grant no. W81XWH-16-1-0133). The DOD did not provide any assistance with the study design, the collection, analysis and interpretation of data or in the writing of this report or in the decision to submit the article for publication. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award no. TL1TR001858. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Dr. Naoki Yoshimura MD, PhD (Professor of Urology, Pharmacology, and Cell Biology, University of Pittsburgh), for allowing us to use the organ bath system to collect the contractility data reported and Stacy Palcsey for her assistance with this study.
Funding This study was funded by the Department of Defense (grant no. W81XWH-16-1-0133) and the National Center for Advancing Translational Sciences of the National Institutes of Health (award no. TL1TR001858).
Footnotes
Conference Presentation American Urogynecology Society 39th Annual Scientific Meeting, Chicago, IL, October 9–13, 2018
Conflicts of interest None.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FDA. Surgical mesh for treatment of women with pelvic organ prolapse and stress urinary incontinence: FDA executive summary. 2011.
- 2.Brown BN, Mani D, Nolfi AL, Liang R, Abramowitch SD, Moalli PA. Characterization of the host inflammatory response following implantation of prolapse mesh in rhesus macaque. Am J Obstet Gynecol. 2015;213(5):668e661–10. 10.1016/j.ajog.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endo M, Feola A, Sindhwani N, Manodoro S, Vlacil J, Engels AC, et al. Mesh contraction: in vivo documentation of changes in apparent surface area utilizing meshes visible on magnetic resonance imaging in the rabbit abdominal wall model. Int Urogynecol J. 2014:1–7. [DOI] [PubMed] [Google Scholar]
- 4.Feola A, Abramowitch S, Jallah Z, Stein S, Barone W, Palcsey S, et al. Deterioration in biomechanical properties of the vagina following implantation of a high-stiffness prolapse mesh. BJOG Int J Obstet Gynaecol. 2013;120(2):224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jallah Z, Liang R, Feola A, Barone W, Palcsey S, Abramowitch S, et al. The impact of prolapse mesh on vaginal smooth muscle structure and function. BJOG Int J Obstet Gynaecol. 2015. 10.1111/1471-0528.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang R, Abramowitch S, Knight K, Palcsey S, Nolfi A, Feola A, et al. Vaginal degeneration following implantation of synthetic mesh with increased stiffness. BJOG Int J Obstet Gynaecol. 2013;120(2):233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144(SUPPL 1):S146–58. [DOI] [PubMed] [Google Scholar]
- 8.Endo M, Urbankova I, Vlacil J, Sengupta S, Deprest T, Klosterhalfen B, et al. Cross-linked xenogenic collagen implantation in the sheep model for vaginal surgery. Gynecol Surg. 2015;12(2):113–22. 10.1007/s10397-015-0883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feola A, Endo M, Urbankova I, Vlacil J, Deprest T, Bettin S, et al. Host reaction to vaginally inserted collagen containing polypropylene implants in sheep. Am J Obstet Gynecol. 2015;212(4): 474.e471–8 10.1016/j.ajog.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Tayrac R, Alves A, Thérin M. Collagen-coated vs noncoated low-weight polypropylene meshes in a sheep model for vaginal surgery. A pilot study. Int Urogynecol J. 2007;18(5):513–20. 10.1007/s00192-006-0176-9. [DOI] [PubMed] [Google Scholar]
- 11.Fan X, Wang Y, Wang Y, Xu H. Comparison of polypropylene mesh and porcine-derived, cross-linked urinary bladder matrix materials implanted in the rabbit vagina and abdomen. Int Urogynecol J. 2014;25(5):683–9. 10.1007/s00192-013-2283-8. [DOI] [PubMed] [Google Scholar]
- 12.Hilger WS, Walter A, Zobitz ME, Leslie KO, Magtibay P, Cornella J. Histological and biomechanical evaluation of implanted graft materials in a rabbit vaginal and abdominal model. Am J Obstet Gynecol. 2006;195(6):1826–31. 10.1016/j.ajog.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Pierce LM, Grunlan MA, Hou Y, Baumann SS, Kuehl TJ, Muir TW. Biomechanical properties of synthetic and biologic graft materials following long-term implantation in the rabbit abdomen and vagina. Am J Obstet Gynecol. 2009;200(5):549.e541–8. 10.1016/j.ajog.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 14.Pierce LM, Rao A, Baumann SS, Glassberg JE, Kuehl TJ, Muir TW. Long-term histologic response to synthetic and biologic graft materials implanted in the vagina and abdomen of a rabbit model. Am J Obstet Gynecol. 2009;200(5):546.e541–8. 10.1016/j.ajog.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Huffaker RK, Muir TW, Rao A, Baumann SS, Kuehl TJ, Pierce LM. Histologic response of porcine collagen-coated and uncoated polypropylene grafts in a rabbit vagina model. Am J Obstet Gynecol. 2008;198(5):582.e581–7. 10.1016/j.ajog.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Skoczylas LC, Jallah Z, Sugino Y, Stein SE, Feola A, Yoshimura N, et al. Regional differences in rat vaginal smooth muscle contractility and morphology. Reproductive sciences (Thousand Oaks, Calif). 2013;20(4):382–90. 10.1177/1933719112472733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLancey JOL, Starr RA. Histology of the connection between the vagina and levator ani muscles. Implications for urinary tract function. Journal of Reproductive Medicine for the Obstetrician and Gynecologist. 1990;35(8):765–71. [PubMed] [Google Scholar]
- 18.Goldstein I, Alexander JL. Practical aspects in the management of vaginal atrophy and sexual dysfunction in perimenopausal and postmenopausal women. J Sex Med. 2005;2:154–65. 10.1111/j.1743-6109.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- 19.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(1):56–63. 10.1067/mob.2002.124843. [DOI] [PubMed] [Google Scholar]
- 20.Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA, Weber A. Morphometric properties of the posterior vaginal wall in women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(6): 1501–9. 10.1067/mob.2002.130005. [DOI] [PubMed] [Google Scholar]
- 21.Takacs P, Gualtieri M, Nassiri M, Candiotti K, Medina CA. Vaginal smooth muscle cell apoptosis is increased in women with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(11):1559–64. 10.1007/s00192-008-0690-z. [DOI] [PubMed] [Google Scholar]
- 22.Halaska M, Maxova K, Sottner O, Svabik K, Mlcoch M, Kolarik D, et al. A multicenter, randomized, prospective, controlled study comparing sacrospinous fixation and transvaginal mesh in the treatment of posthysterectomy vaginal vault prolapse. Am J Obstet Gynecol. 2012;207(4):301.e301–7. 10.1016/j.ajog.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Jacquetin B, Hinoul P, Gauld J, Fatton B, Rosenthal C, Clavé H, et al. Total transvaginal mesh (TVM) technique for treatment of pelvic organ prolapse: a 5-year prospective follow-up study. Int Urogynecol J. 2013;24(10):1679–86. 10.1007/s00192-013-2080-4. [DOI] [PubMed] [Google Scholar]
- 24.Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e231–8. 10.1016/j.ajog.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Nygaard I, Brubaker L, Zyczynski HM, Cundiff G, Richter H, Gantz M, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19): 2016–24. 10.1001/jama.2013.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feiner B, Maher C. Vaginal mesh contraction: definition, clinical presentation, and management. Obstet Gynecol. 2010;115(2 PART1):325–30. [DOI] [PubMed] [Google Scholar]
- 27.Svabik K, Martan A, Masata J, El-Haddad R, Hubka P, Pavlikova M. Ultrasound appearances after mesh implantation—evidence of mesh contraction or folding? Int Urogynecol J. 2011;22(5):529–33. 10.1007/s00192-010-1308-9. [DOI] [PubMed] [Google Scholar]
- 28.Nolfi AL, Brown BN, Liang R, Palcsey SL, Bonidie MJ, Abramowitch SD, et al. Host response to synthetic mesh in women with mesh complications. Am J Obstet Gynecol. 2016;215(2): 206.e201–8. 10.1016/j.ajog.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tennyson L, Rytel M, Palcsey S, Meyn L, Liang R, Moalli P. Characterization of the T cell response to polypropylene mesh in women with complications. Am J Obstet Gynecol. 2018. 10.1016/j.ajog.2018.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Northington GM, Basha M, Arya LA, Wein AJ, Chacko S. Contractile response of human anterior vaginal muscularis in women with and without pelvic organ prolapse. Reprod Sci. 2011;18(3): 296–303. 10.1177/1933719110392054. [DOI] [PMC free article] [PubMed] [Google Scholar]


