Abstract
BACKGROUND
Transgender individuals receiving masculinising or feminising gender-affirming hormone therapy with testosterone or estradiol respectively, are at increased risk of adverse cardiovascular outcomes, including myocardial infarction and stroke. This may be related to the effects of testosterone or estradiol therapy on body composition, fat distribution, and insulin resistance but the effect of gender-affirming hormone therapy on these cardiovascular risk factors has not been extensively examined.
AIM
To evaluate the impact of gender-affirming hormone therapy on body composition and insulin resistance in transgender individuals, to guide clinicians in minimising cardiovascular risk.
METHODS
We performed a review of the literature based on PRISMA guidelines. MEDLINE, Embase and PsycINFO databases were searched for studies examining body composition, insulin resistance or body fat distribution in transgender individuals aged over 18 years on established gender-affirming hormone therapy. Studies were selected for full-text analysis if they investigated transgender individuals on any type of gender-affirming hormone therapy and reported effects on lean mass, fat mass or insulin resistance.
RESULTS
The search strategy identified 221 studies. After exclusion of studies that did not meet inclusion criteria, 26 were included (2 cross-sectional, 21 prospective-uncontrolled and 3 prospective-controlled). Evidence in transgender men suggests that testosterone therapy increases lean mass, decreases fat mass and has no impact on insulin resistance. Evidence in transgender women suggests that feminising hormone therapy (estradiol, with or without anti-androgen agents) decreases lean mass, increases fat mass, and may worsen insulin resistance. Changes to body composition were consistent across almost all studies: Transgender men on testosterone gained lean mass and lost fat mass, and transgender women on oestrogen experienced the reverse. No study directly contradicted these trends, though several small studies of short duration reported no changes. Results for insulin resistance are less consistent and uncertain. There is a paucity of prospective controlled research, and existing prospective evidence is limited by small sample sizes, short follow up periods, and young cohorts of participants.
CONCLUSION
Further research is required to further characterise the impact of gender-affirming hormone therapy on body composition and insulin resistance in the medium-long term. Until further evidence is available, clinicians should aim to minimise risk by monitoring cardiovascular risk markers regularly in their patients and encouraging healthy lifestyle modifications.
Keywords: Transgender persons, Insulin resistance, Body composition, Gender dysphoria, Metabolic syndrome
Core tip: Evidence in transgender men suggests that testosterone therapy increases lean mass, decreases fat mass and has no impact on insulin resistance. Evidence in transgender women suggests that feminising hormone therapy (estradiol, with or without anti-androgen agents) decreases lean mass, increases fat mass, and may worsen insulin resistance. There is a paucity of prospective controlled research, and existing prospective evidence is limited by small sample sizes, short follow up periods, and young cohorts of participants. Until further evidence is available, clinicians should aim to minimise risk by monitoring cardiovascular risk markers regularly in their patients and encouraging healthy lifestyle modifications.
INTRODUCTION
Transgender healthcare is a rapidly expanding field of medicine and transgender individuals are presenting in increasing numbers for advice and assistance in transitioning[1]. Research indicates that many physicians are unsure how to optimally manage gender dysphoria and support individuals through the process of transition. Further research and education in this area is vital to meet the increasing demand for treatment[2,3].
The term transgender refers to an individual who has a gender identity which differs from their sex assigned at birth. Gender dysphoria denotes the distress that may accompany this incongruence, and the goal of therapy is to alleviate this distress[4]. The exact prevalence of transgender people and gender dysphoria is unknown and, for various reasons, including the lack of clear nomenclature, is likely to be underestimated[5]. Population-based studies have found a higher prevalence than clinical studies, ranging from 0.5% in the United States to 0.6%-1.1% in the Netherlands and Belgium[6]. The range of management options available is broad and encompasses many disciplines of medicine, including psychological, hormonal and surgical interventions. Whether or not an individual chooses to undertake any or all of these interventions is variable, and management is ideally tailored to the individual’s goals.
Gender-affirming hormone therapy (GAHT) is commonly given to align an individual’s physical characteristics to more closely match their gender identity with the goal of improving mental health and reducing dysphoria. Masculinising hormonal therapy for individuals assigned female sex at birth (transgender men) most commonly involves administration of intramuscular or transdermal testosterone in doses similar to those used for hypogonadal men. We use the term transgender in this review to also include people with gender diverse or non-binary identities. Feminising hormonal therapy for individuals assigned male sex at birth (transgender women) involves oestrogen replacement usually with oral or transdermal formulations and concurrent suppression of endogenous testosterone with androgen antagonists[7].
A number of studies and reviews have demonstrated the safety of gender-affirming hormone therapy in the short and medium-term, however there is a paucity of long term clinical safety and efficacy data[6]. Notably, two recent large cohort studies have demonstrated that transgender individuals on hormone therapy have higher rates of adverse cardiovascular events, with transmen on testosterone at increased risk of myocardial infarction, and transwomen on oestrogen at increased risk of ischaemic stroke[8,9]. Given the sexually dimorphic nature of cardiovascular disease and its associated risk factors, manipulation of sex hormones may underlie this increase in cardiovascular risk[10-12]. However, there are few reviews looking at the effects of hormone therapy on cardiovascular risk. Specifically, there is a lack of reviews into the available evidence in the effect of GAHT on insulin resistance, and its relationship to the changes in body composition brought about by hormone therapy. Both oestrogen and testosterone are capable of altering insulin sensitivity in both cisgender women and men[11,13-16], however, the impact of gender-affirming hormone therapy on insulin resistance in transgender individuals is less clear.
Studies demonstrating that transgender people on hormone therapy are at increased risk of adverse cardiovascular events highlight the importance of investigating surrogate markers while awaiting further long-term research. We aimed to investigate the existing evidence of the effects of gender-affirming hormone therapy on insulin resistance and body composition, as this may provide insight into the long-term risks of hormone therapy in transgender individuals.
MATERIALS AND METHODS
A search of the MEDLINE and EMBASE databases using the Ovid platform was conducted in March 2019. Terms defining the participant population included “Transgender”, “Gender dysphoria”, “Transsexual”, “Gender identity”, “Gender variant”, “Trans men”, “Trans women”, “Trans people”, “Two-spirit”, “FTM” and “MTF”. Terms defining the intervention included “Cross-sex hormone therapy”, “Gonadal steroid hormones”, “Estrogen replacement therapy”, “Estradiol/ Oestradiol”, “Estrogen/Oestrogen”, “Testosterone”, ‘Gonadotropin-Releasing Hormone analogues”, “Androgen antagonists”, “Antiandrogen”, “Spironolactone”, “Hormone therapy”, “Hormone treatment”, “Sex steroid”, “Progestin”, and “Androgen”. Terms used for the study outcomes included “Body Composition”, “Lean mass”, “Muscle mass”, “Fat mass”, “Adipose fat or tissue”, “Intra-abdominal fat”, “Central or abdominal obesity”, “Body fat distribution”, “Anthropometry”, “Waist or body circumference”, “Waist-hip ratio”, “Insulin resistance”, “Insulin sensitivity”, “HbA1c”, “Blood glucose”, “Insulin-Secreting Cells”, “beta cell” and “Diabetes mellitus, Type 2”. The full search strategy is included in Supplementary Tables 1 and 2. After removal of conference abstracts and duplicates, this search yielded a total of 221 studies for title and abstract screening.
Screening was conducted using the Rayyan web application[17]. Studies were selected for full-text analysis if they investigated transgender individuals on any type of gender-affirming hormone therapy and reported impacts (or lack thereof) on lean mass, fat mass or insulin resistance. Studies were excluded if they did not meet these inclusion criteria, or if they only reported fasting insulin levels, fasting plasma glucose or HbA1c in isolation as these were not taken to be sufficiently representative of insulin sensitivity.
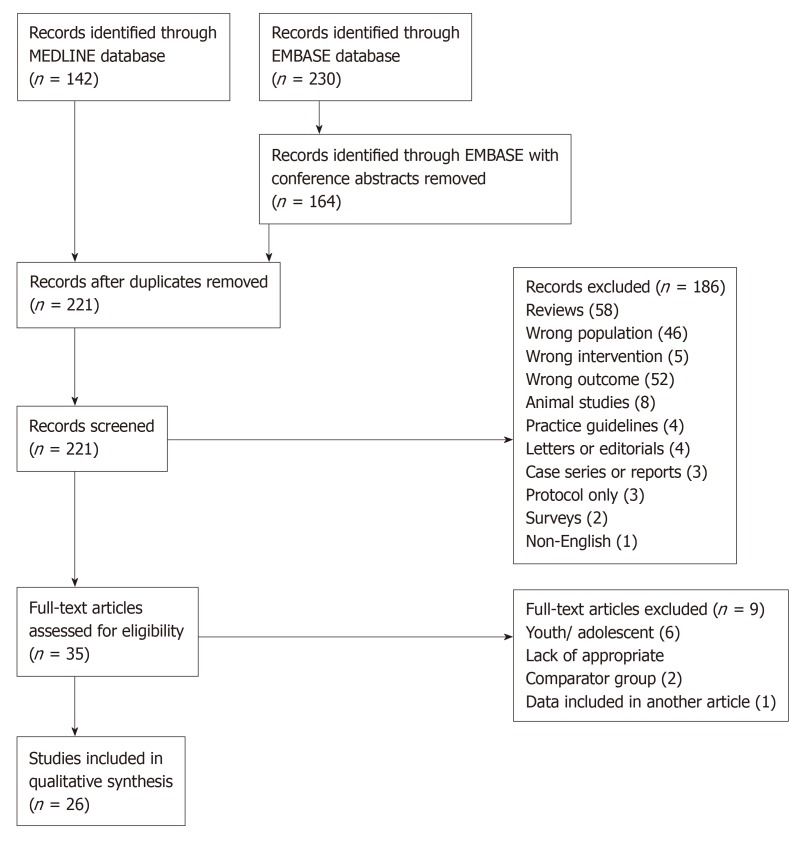
A summary of the study selection process is included (Figure 1). After exclusion (reasons listed in Figure 1), 26 studies were included in the final analysis.
Figure 1.
Study selection flowchart.
RESULTS
Overall
Of the 26 studies evaluated, 21 examined transgender men and 16 examined transgender women[18-43]. In total, these studies included 751 transgender male and 689 transgender female participants. A large majority of the research was conducted in the Netherlands and Belgium (11 studies) and other European countries (6 Italian, 4 German, 2 Spanish, 1 Norwegian). Two studies were conducted outside Europe; 1 in the United States and 1 in Brazil. Results are summarised in Table 1.
Table 1.
Summary of studies investigating body composition and insulin resistance in transgender individuals
| Ref. | Country | Design | Time period | n | Average age (yr) | Number of controls | Methods | Lean mass | Fat mass | IR | WHR |
| Studies in Transgender men | |||||||||||
| Van Caenegem et al[41] | Belgium | Prospective controlled | 12 mo | 23 | 27 (9) | 23 age-matched | DXA and pQCT | ↑10.4% | ↓9.7% | ↔ | |
| Haraldsen et al[31] | Norway | Prospective controlled | 3 mo, 12 mo | 21 | 25.1 (4.8) | 45, not age-matched | DXA | ↑ | ↔ | ||
| Cupisti et al[24] | Germany | Prospective controlled | 12 mo | 29 | 29.9 (18-40) | 240 PCOS, age-matched | HOMA-IR | ↔ | |||
| Aranda et al[19] | Spain | Prospective | 6 mo, 12 mo | 20 | 27.1 (8.0) | DXA and HOMA-IR | ↑5.8% | ↓6.3% | ↔ | ↑A:G ratio | |
| Auer et al[21] | Germany | Prospective | 13 mo | 45 | 27.5 (1.3) | DXA and HOMA-IR | ↑ | ↓ | ↓ | ↔ | |
| Klaver et al[32] | The Nether-lands and Belgium | Prospective | 12 mo | 162 | 24 (18-58) | DXA | ↑10% | ↓9% | ↑ | ||
| Gava et al[30] | Italy | Prospective | 12 mo, 36 mo, 60 mo | 50 | 30.1 (6.1) | DXA and HOMA-IR | ↑ | ↔ | ↔ | ↔ | |
| Aranda et al[18] | Spain | Prospective | 6 mo, 12 mo | 12 | 27.1 (17-43) | HOMA-IR | ↔ | WC ↔ | |||
| Auer et al[20] | Belgium and Norway | Prospective | 12 mo | 20 | NR | DXA and HOMA-IR | ↑ | ↔ | ↔ | ↔ | |
| Colizzi et al[23] | Italy | Prospective | 12 mo, 24 mo | 43 | 28.8 (5.6) | HOMA-IR | ↔ | WC ↑ | |||
| Pelusi et al[37] | Italy | Prospective | 30 wk, 54 wk | 45 | 29.5 | DXA and HOMA-IR | ↑ | ↓ | ↔ | ↔ | |
| Wierckx et al[42] | Belgium | Prospective | 12 mo | 53 | 24.5 (7.0) | DXA | ↑ | ↓ | ↑ | ||
| Mueller et al[35] | Germany | Prospective | 12 mo, 24 mo | 45 | NR | DXA | ↑ | ↔ | |||
| Yahyaoui et al[43] | United States | Prospective | 12 mo, 24 mo | 47 | 25.7 (6.0) | HOMA-IR | ↓ | ||||
| Merigg-iola et al[34] | Italy | Prospective | 12 mo | 15 | 35.7 (5.0) | DXA and HOMA-IR | ↑ | ↔ | ↔ | ↑ (NS) | |
| Berra et al[22] | Italy | Prospective | 6 mo | 16 | NR | BIA and HOMA | ↑ | ↓ | ↔ | WC ↔ | |
| Elbers et al[27] | The Nether-lands | Prospective | 12 mo | 17 | 23 (16-34) | MRI and EGC | ↑ in VAT | ↔ | |||
| Elbers et al[25] | The Nether-lands | Prospective | 4 mo, 12 mo | 15 | 23 (16-34) | MRI and BIA | ↓ | ||||
| Elbers et al[26] | The Nether-lands | Prospective | 12 mo, 36 mo | 10 | 24 (16-33) | MRI | ↑ thigh muscle | ↑ in VAT | |||
| Polderman et al[38] | The Nether-lands | Prospective | 4 mo | 13 | 23.1 (18-33) | BIA and EGC | ↑ | ↑ | |||
| Van Caenegem et al[39] | Belgium | Cross-sectional | 10 yr on GAHT (3-28) | 50 | 37 (8) | 50 age-matched | DXA and pQCT | 9% more | 30% less | Larger | |
| Studies in Transgender women | |||||||||||
| Haraldsen et al[31] | Norway | Prospective controlled | 3 mo, 12 mo | 12 | 29.3 (7.8) | 77, not age-matched | DXA | ↓ | ↑ | ||
| Auer et al[21] | Germany | Prospective | 12 mo | 24 | 34.8(1.4) | DXA and HOMA-IR | ↔ | ↑ | ↑ | ↓ | |
| Klaver et al[32] | The Nether-lands and Belgium | Prospective | 12 mo | 179 | 29 (18-66) | DXA | ↓3% | ↑28% | ↓ | ||
| Fighera et al[28] | Brazil | Prospective | 31 mo | 46 | 33.7 (10.3) | DXA | ↓ | ↑ | |||
| Aranda et al[18] | Spain | Prospective | 6 mo, 12 mo | 6 | 18.8 (16-21) | HOMA-IR | ↔ | WC ↑ | |||
| Auer et al[20] | Belgium and Norway | Prospective | 12 mo | 20 | NR | DXA and HOMA-IR | ↓ | ↑ | ↑ | ↔ | |
| Gava et al[29] | Italy | Prospective | 12 mo | 40 | 31.2 (9.8) | DXA and HOMA-IR | ↓ (NS) | ↑ | ↔ | ↔ | |
| Colizzi et al[23] | Italy | Prospective | 12 mo, 24 mo | 79 | 30.2 (9.6) | HOMA-IR | ↑ | WC ↑ | |||
| Van Caenegem et al[40] | Belgium | Prospective | 12 months, 24 months | 46 | 33 (12) | 49 (not followed prospective-ly) | DXA and pQCT | ↓ | ↑ | ↓ | |
| Wierckx et al[42] | Belgium | Prospective | 12 mo | 53 | 30.3 (14.0) | DXA | ↓ | ↑ | ↓ | ||
| Mueller et al[36] | Germany | Prospective | 12 mo, 24 mo | 84 | NR | DXA | ↓ | ↑ | |||
| Yahyaoui et al[43] | United States | Prospective | 12 mo, 24 mo | 22 | 23.1 (9.4) | HOMA-IR | ↔ | ||||
| Elbers et al[27] | The Nether-lands | Prospective | 12 mo | 20 | 26 (18-36) | MRI and EGC | ↑ VAT | ↑ | |||
| Elbers et al[25] | The Nether-lands | Prospective | 4 mo, 12 mo | 17 | 26 (18-37) | MRI and BIA | ↔ | ||||
| Polderm-an et al[38] | The Nether-lands | Prospective | 4 mo | 18 | 26.5 (18-36) | BIA and EGC | ↓ (NS) | ↑ | |||
| Lapauw et al[33] | Belgium | Cross-sectional | 8 yr on GAHT (4-20) | 23 | 41 (7) | 46, age-matched | DXA, pQCT | 20% lower | 30% higher | ||
A:G ratio: Android-Gynoid ratio; BIA: Bioelectrical Impedance Analysis; DXA: Dual-energy X-ray Absorptiometry; ECG: Euglycaemic clamp; HOMA-IR: Homeostasis model assessment of Insulin Resistance; IR: Insulin resistance; MRI: Magnetic Resonance Imaging; NR: Not reported; NS: Not significant; pQCT: Peripheral Quantitative Computed Tomography: VAT: Visceral adipose tissue; WC: Waist circumference; WHR: Waist-hip ratio. Ages given as mean (SD or range).
All studies managed transgender men with testosterone and transgender women with oestrogen. With the exception of one study[31] all transgender women also received antiandrogen therapy (cyproterone acetate 50-100 mg or, in one study, goserelin acetate[36]) unless they were post-orchiectomy. The majority of studies treated transgender men with testosterone undecanoate 1000 mg IM every 12 wk (n = 12), or testosterone esters 250 mg IM every 2-3 wk (n = 6). Regimens in transgender women were more variable, though most studies used ethinyl estradiol or estradiol valerate in dosages of 100 g or 1-4 mg, respectively.
Transgender men
Controlled prospective studies: There are few controlled prospective studies (Table 1). Only 3 studies in transgender men included cisgender female control participants[24,31,41]. An initial 2007 study assessed 21 transgender men at initiation of testosterone therapy and followed them for 12 mo[31]. Comparisons were made with controls at baseline only and notably were not age-matched, with female controls almost 10 years older than the transgender male participants. This study noted no differences between groups at baseline, and that transgender men gained lean mass and lost fat mass, becoming more similar in body composition to male references over the 12-mo time period. These changes were noted as early as 3 mo after commencing hormone therapy[31].
A Belgian study of 23 transgender men compared them with 23 age-matched control women over 12 mo and found that treated transgender men gained lean mass and lost fat mass while the control women gained fat and lost lean mass and muscle strength[41]. The researchers also used peripheral quantitative computed tomography (pQCT) to investigate cross-sectional muscle area, finding significant increases in transgender men, along with increased grip strength.
To examine insulin resistance, 29 transgender men before and after commencing testosterone therapy were compared to 240 cisgender women with polycystic ovary syndrome (PCOS)[24]. Whilst the control women with PCOS and had significantly higher insulin resistance than the transgender men at baseline, there did not appear to be any induction of insulin resistance with testosterone therapy. Further, there was no correlation between serum testosterone concentrations and insulin resistance.
Prospective studies without controls: All uncontrolled prospective studies examining body composition have found that testosterone was associated with increases in lean mass (Table 1). The majority also found an associated decrease in fat mass, but despite this two imaging studies showed that testosterone was associated with an increase in visceral fat area[26,27]. The magnitude of visceral adipose tissue (VAT) gain was as high as 47% and correlated with the relative amount of weight gained during therapy. Although more recent studies have failed to report on visceral fat accumulation, several report an increase in waist-to-hip ratio. Increases in android-to-gynoid (A:G) fat ratios[19] occur, driven primarily by decreases in gynoid region fat (-14%), without increases in the android region[32].
Prospective studies reporting on insulin resistance are somewhat conflicting. Testosterone treatment was associated with increased insulin resistance measured by the hyperinsulinaemic euglycaemic clamp technique in only one study, which followed 13 people over 4 mo[38]. In contrast, no changes were seen in insulin sensitivity over 1 year in 17 healthy, nonobese young transgender men[27]. Many studies summarised in Table 1 have demonstrated no change (n = 10), or in two cases, decreased resistance. The largest prospective study of 50 transgender men followed for 5 years found that testosterone had no impact on insulin resistance[30].
Cross-sectional evidence: All of the above studies assessed transgender men newly commencing testosterone therapy. However, when comparing transgender men on established therapy (mean treatment duration 10 years) compared with age-matched cisgender female controls, lean mass remained higher and fat mass remained lower suggesting persistence of changes over time[39].
Transgender women
Prospective studies with controls: Body composition changes are summarised in Table 1. Whilst two studies made comparisons to controls at baseline[31,40], there were no studies that followed the control group over time. There were baseline differences between transgender women and control men; with transgender women having less lean mass and more fat mass than control males even before commencing any kind of hormone therapy. Over time oestrogen therapy induced a loss of lean mass and gain of fat mass in their participants, which is consistent across studies in this review.
Prospective studies without controls: As shown in Table 1, almost all prospective data in transgender women is consistent. In transgender women commencing estradiol therapy, lean mass decreases and fat mass increases. There is also a shift in body fat distribution with a decrease in waist-to-hip ratio seen in 4 studies, and rise in gynoid fat[21,32,40,42]. The largest study involving 179 transgender women, demonstrated a 42% increase in leg fat and 34% increase in gynoid fat, compared to a more modest 18% increase in android fat[32]. These changes persist over the first two years of hormone therapy[36]. Oestrogen therapy was additionally associated with an 18% increase in VAT over 12 mo, along with a 38% increase in subcutaneous fat[27].
Whether feminising hormone therapy is associated with insulin resistance is unclear. Five studies noted increases in insulin resistance and three others found no change (Table 1). Of the studies reporting increased resistance, the largest and longest-running is Colizzi et al[23], who followed 79 participants for two years. The participants, who were metabolically healthy at baseline, had an increase in Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) values of 72% in the first year of treatment and by a further 9% over the second year, suggesting that the first 12 mo is an important period. A confounding factor was the presence of participants with psychiatric comorbidities who may have been on other medications capable of altering their insulin sensitivity, however there were only 12 participants in this category. Both of the studies employing the gold-standard euglycaemic clamp method found that GAHT in transgender women decreased peripheral glucose uptake, and simultaneously noted an increase in fasting insulin of up to 50%[27,38]. This would support the notion that oestrogen therapy has negative effects on insulin sensitivity. In addition, two of the three the studies reporting no change are limited by small participant numbers (n = 22 and n = 6)[19,43].
Cross sectional evidence: One cross-sectional study compared 23 transgender women who had been on GAHT for a mean of 8 years with 46 age-matched controls[33]. Fat mass was higher and lean mass was lower than controls including a 25% decrease in lower limb muscle area measured by pQCT.
DISCUSSION
There is evidence that hormone therapy can have a significant effect on body composition within 12 mo and these changes persist long-term. The effect on insulin resistance is uncertain. Changes to body composition were consistent across almost all studies: Transgender men on testosterone gained lean mass and lost fat mass, and transgender women on oestrogen experienced the reverse. No study directly contradicted these trends, though several small studies of short duration reported no changes. These results are consistent with a recent meta-analysis investigating body composition in individuals on GAHT, which found that hormone therapy in transgender individuals affects lean and fat mass significantly, with an increase in fat mass and decrease in lean mass observed in transwomen and the reverse occurring in transmen[44]. Results for insulin resistance are less consistent and uncertain.
It would be unethical to conduct a randomised controlled trial using hormone therapy and placebo, therefore prospective cohort studies represent the highest available level of evidence. However, there is a paucity of prospective controlled data and research with a longer follow-up duration is needed. It should also be noted that most of the participants in these studies are of Caucasian ethnicity, which limits the generalisability of the findings to transgender individuals of other backgrounds.
Insulin resistance, body composition and cardiovascular risk
Insulin resistance has been shown to independently predict a variety of poor outcomes including hypertension, obesity, and dyslipidaemia, as well as cardiovascular and all-cause mortality, even in non-diabetic metabolically well individuals[45-47].
In 2015, increased body mass index contributed to 4.0 million deaths globally (7.1% of the total number of deaths from any cause). 39% of these deaths occurred in people with body mass index less than 30, indicating that this increased risk is not confined to people who are clinically obese, and that small changes in body fat have the potential to influence long term mortality risk[48]. The distribution of body is also important, as central or “abdominal” obesity has been shown to confer a higher risk, and an elevated waist-hip ratio correlates with adverse cardiovascular outcomes more strongly than body mass index[49]. This is significant from the perspective of gender, as male “android” obesity which is more centrally distributed is therefore more predictive of poor outcomes than female “gynoid” obesity, which involves storage of fat around the hips, thighs and buttocks[12,50].
Body fat distribution and insulin resistance are interrelated[51]. Adipose tissue has been shown to decrease sensitivity to insulin through release of multiple mediators including free fatty acids, steroid hormones and proinflammatory cytokines[51]. There is some debate about whether visceral fat in particular has a greater influence over insulin sensitivity than subcutaneous fat. Visceral adipose tissue is more metabolically active, and its proximity to portal circulation may bestow a greater impact on hepatic lipid and glucose production[12]. However, some research suggests subcutaneous fat mass is more highly correlated with insulin resistance and attribute this to its greater contribution to circulating free fatty acid levels[50]. No association between fat distribution and insulin sensitivity is evident[27], suggesting that GAHT may influence insulin resistance by mechanisms other than body fat, including direct action on tissues. More research investigating the relationships between body fat distribution and insulin resistance in transgender people on hormone therapy is needed.
Impact of testosterone therapy in transgender men
Testosterone and body composition: Testosterone therapy in transgender men is associated with an increase in lean mass and decrease in fat mass. Data suggests that changes emerge within three months. Changes seen in the early years of hormone therapy are likely to persist in the longer term, with analysis of participants on hormone therapy for 10 years demonstrating consistent changes in body composition[39].
Testosterone and visceral fat: The evidence that testosterone decreases fat mass is strong however the effects on visceral fat specifically are less clear. Whilst testosterone appears to be associated with increased VAT; there are few studies, sample sizes are small with short follow-up, and the lack of controls do not allow for the comparison with normal visceral fat accumulation with time. More recent studies reporting an increase in waist-to-hip ratio in transgender men may indicate that increases in VAT are occurring. This is supported by evidence of raised android-to-gynoid fat ratios, suggestive of changes in body fat distribution[32]. Of note, Elbers et al[26] found no changes in VAT after 12 mo, but significant increases after 3 years, indicating these changes may take time to develop. Further controlled prospective studies are required to assess not only VAT but cardiovascular outcomes and mortality.
Testosterone and insulin resistance: The effects of testosterone on insulin resistance are unclear, even in cisgender populations. In cisgender men, testosterone deficiency is associated with insulin resistance, and this improves with testosterone replacement[15,16]. In men with both hypogonadism and established diabetes, testosterone replacement does not appear to improve glycaemic control[52]. Elevated levels of testosterone in cisgender women as occurs in PCOS, are associated with increased insulin resistance. In animal models, testosterone is associated with a decrease in insulin sensitivity[11]. This review suggests that testosterone therapy has no negative effect on insulin sensitivity and may even be associated with an improvement. This raises the possibility that the impairment in insulin sensitivity in PCOS is independent of hyperandrogenism.
Impact of estradiol therapy in transgender women
Estradiol and body composition: Estradiol therapy in transgender women appears to be associated with an increase in fat mass and decrease in lean mass, although prospectively controlled data is limited. Interestingly, the studies that were controlled demonstrate these differences at baseline[31,40]. Several studies have reported significantly less weekly physical activity in transgender women compared to control men[33,39] possibly as a way to intentional to minimise muscle mass and influence body shape.
The increase in body fat associated with oestrogen therapy raises questions about the effect on cardiovascular risk. Elbers et al[27]noted that oestrogen and antiandrogen therapy was associated with a visceral fat increase of 18%, and subcutaneous fat increase of 38% over 12 mo. This represents an increase in the absolute amount of visceral fat, but a reduction in the ratio of visceral to subcutaneous fat. The implications for long-term cardiovascular health are unclear.
Estradiol and insulin resistance: Five of the eight studies reporting on insulin resistance in transwomen found that oestrogen therapy was associated with a worsening in insulin resistance measures. The remaining three studies were limited by small sample sizes. This is consistent with other high-oestrogen states such as women taking the oral contraceptive pill who have increased insulin resistance, as well as gestational diabetes mellitus during pregnancy[11]. Other research suggests that oestrogen has a protective role. Insulin sensitivity in cisgender women decreases after menopause and improves with menopausal hormone therapy[14]. Selective oestrogen receptor modulators have been shown to decrease insulin sensitivity, while Tamoxifen is associated with a 24% increase in diabetes risk in breast cancer survivors[13]. In cisgender men, decreased oestrogen production also induces insulin resistance[11]. The mechanism underlying this protective effect is largely unknown. Oestrogen has been shown to enhance peripheral insulin sensitivity via actions on skeletal muscle, liver and adipose tissue, and also has a role regulating energy intake and expenditure[13]. This review as well as other studies of high-oestrogen states may indicate that supra-physiological levels of oestrogen, in both men and women, can impair insulin sensitivity through a mechanism which is not entirely understood.
Gender-affirming hormone therapy has differing effects on body composition and insulin resistance in transgender men and women. Transgender men taking testosterone therapy have an associated gain in lean mass and decrease in fat mass. Transgender women taking oestrogen therapy have an associated decline in lean mass and gain in fat mass. The data examining the effect of hormone therapy on insulin resistance is so far inconsistent and limited. It is possible that oestrogen therapy in transgender women has a negative impact on insulin sensitivity. Both body fat and insulin resistance are known to impact cardiovascular risk and it is reasonable to conclude that gender-affirming hormone therapy may have an impact on long-term cardiovascular health.
The studies reviewed are limited by small sample sizes, young participants who may be yet to develop insulin resistance and short duration of follow-up. There is a paucity of controlled prospective research. These limitations make it difficult to draw conclusions about the long-term effects of gender-affirming hormone therapy on cardiovascular risk.
The long-term effects of hormone therapy on body composition and insulin resistance are unknown. Significant changes in body composition occur, most detrimentally in transgender women who gain fat mass and also have an increase in insulin resistance. Further research is therefore needed and given the long-term nature of cardiovascular disease progression, large prospectively controlled cohort studies are required. In the meantime, further research into the impact of hormone therapy on surrogate markers of cardiovascular risk is encouraged, and it would be prudent for clinicians to monitor markers of cardiovascular risk in patients taking gender-affirming hormone therapy.
ARTICLE HIGHLIGHTS
Research background
Transgender individuals receiving masculinising or feminising gender-affirming hormone therapy with testosterone or estradiol respectively, are at increased risk of heart disease and stroke. Testosterone and estradiol play important roles in regulating body fat and muscle and in the general population, males who have relatively high levels of testosterone compared with females are at higher risk for heart disease. The increased risk of heart disease may be related to the effects of testosterone or estradiol therapy on fat and muscle distribution, as well as insulin resistance, a measure of diabetes and heart disease risk. The effect of gender-affirming hormone therapy on these cardiovascular risk factors has not been extensively examined.
Research motivation
Studies demonstrating that transgender people on hormone therapy are at increased risk of adverse cardiovascular events highlight the importance of investigating surrogate markers while awaiting further long-term research. Both oestrogen and testosterone are capable of altering insulin resistance in both cisgender women and men, however, the impact of gender-affirming hormone therapy on insulin resistance in transgender individuals is less clear. This review was conducted to examine the relationship between insulin resistance and its relationship to the changes in body composition brought about by hormone therapy in order to enable clinicians to proactively lower risk factors which may contribute to heart disease and diabetes.
Research objectives
We aimed to investigate the existing evidence of the effects of gender-affirming hormone therapy on insulin resistance and body composition, as this may provide insight into the long-term risks of hormone therapy in transgender individuals.
Research methods
We performed a systematic review of the literature based on PRISMA guidelines. MEDLINE, Embase and PsycINFO databases were searched for studies examining body composition, insulin resistance or body fat distribution in transgender individuals aged over 18 years on established gender-affirming hormone therapy. Studies were selected for full-text analysis if they investigated transgender individuals on any type of gender-affirming hormone therapy and reported effects on lean mass, fat mass or insulin resistance.
Research results
The search strategy identified 221 studies. After exclusion of studies that did not meet inclusion criteria, 26 were included (2 cross-sectional, 21 prospective-uncontrolled and 3 prospective-controlled). Evidence in transgender men suggests that testosterone therapy increases lean mass, decreases fat mass and has no impact on insulin resistance. Evidence in transgender women suggests that feminising hormone therapy (estradiol, with or without anti-androgen agents) decreases lean mass, increases fat mass, and may worsen insulin resistance. Changes to body composition were consistent across almost all studies: transgender men on testosterone gained lean mass and lost fat mass, and transgender women on oestrogen experienced the reverse. No study directly contradicted these trends, though several small studies of short duration reported no changes. Results for insulin resistance are less consistent and uncertain. There is a paucity of prospective controlled research, and existing prospective evidence is limited by small sample sizes, short follow up periods, and young cohorts of participants.
Research conclusions
Masculinising gender-affirming hormone therapy increases lean mass, decreases fat mass and has no impact on insulin resistance. Feminising gender-affirming hormone therapy decreases lean mass, increases fat mass, and may worsen insulin resistance. Further research is required to further characterise the impact of gender-affirming hormone therapy on body composition and insulin resistance in the medium-long term. Until further evidence is available, clinicians should aim to minimise risk by monitoring cardiovascular risk markers regularly in their patients and encouraging healthy lifestyle modifications is paramount.
Research perspectives
Clinicians need to be aware of body composition changes and potential insulin resistance changes. Proactive lowering of cardiovascular risk factors such as optimising diet and physical activity as well as managing weight, lipids, blood pressure and glucose are important. Long-term prospective controlled studies will provide further insights in the future.
Footnotes
Conflict-of-interest statement: All the authors declare that they have no competing interests.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Endocrine Society; Endocrine Society of Australia; Australian Professional Association for Trans Health; European Society of Endocrinology; Australian Medical Association.
Peer-review started: October 31, 2019
First decision: December 4, 2019
Article in press: January 19, 2020
Specialty type: Endocrinology and Metabolism
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Galva VG, Sherif Z S-Editor: Ma L L-Editor: A E-Editor: Ma YJ
Contributor Information
Cassandra Spanos, Trans Medical Research Group, Department of Medicine (Austin Health), University of Melbourne, Victoria 3084, Australia.
Ingrid Bretherton, Trans Medical Research Group, Department of Medicine (Austin Health), University of Melbourne, Victoria 3084, Australia.
Jeffrey D Zajac, Trans Medical Research Group, Department of Medicine (Austin Health), University of Melbourne, Victoria 3084, Australia.
Ada S Cheung, Trans Medical Research Group, Department of Medicine (Austin Health), University of Melbourne, Victoria 3084, Australia. adac@unimelb.edu.au.
References
- 1.Telfer M, Tollit M, Feldman D. Transformation of health-care and legal systems for the transgender population: The need for change in Australia. J Paediatr Child Health. 2015;51:1051–1053. doi: 10.1111/jpc.12994. [DOI] [PubMed] [Google Scholar]
- 2.Davidge-Pitts C, Nippoldt TB, Danoff A, Radziejewski L, Natt N. Transgender Health in Endocrinology: Current Status of Endocrinology Fellowship Programs and Practicing Clinicians. J Clin Endocrinol Metab. 2017;102:1286–1290. doi: 10.1210/jc.2016-3007. [DOI] [PubMed] [Google Scholar]
- 3.Bretherton I, Thrower E, Grossmann M, Zajac JD, Cheung AS. Cross-sex hormone therapy in Australia: the prescription patterns of clinicians experienced in adult transgender healthcare. Intern Med J. 2019;49:182–188. doi: 10.1111/imj.14035. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Gender Dysphoria. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. 2013 [Google Scholar]
- 5.Collin L, Reisner SL, Tangpricha V, Goodman M. Prevalence of Transgender Depends on the "Case" Definition: A Systematic Review. J Sex Med. 2016;13:613–626. doi: 10.1016/j.jsxm.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.T'Sjoen G, Arcelus J, Gooren L, Klink DT, Tangpricha V. Endocrinology of Transgender Medicine. Endocr Rev. 2019;40:97–117. doi: 10.1210/er.2018-00011. [DOI] [PubMed] [Google Scholar]
- 7.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, 3rd, Spack NP, Tangpricha V, Montori VM Endocrine Society. Endocrine treatment of transsexual persons: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009;94:3132–3154. doi: 10.1210/jc.2009-0345. [DOI] [PubMed] [Google Scholar]
- 8.Getahun D, Nash R, Flanders WD, Baird TC, Becerra-Culqui TA, Cromwell L, Hunkeler E, Lash TL, Millman A, Quinn VP, Robinson B, Roblin D, Silverberg MJ, Safer J, Slovis J, Tangpricha V, Goodman M. Cross-sex Hormones and Acute Cardiovascular Events in Transgender Persons: A Cohort Study. Ann Intern Med. 2018;169:205–213. doi: 10.7326/M17-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nota NM, Wiepjes CM, de Blok CJM, Gooren LJG, Kreukels BPC, den Heijer M. Occurrence of Acute Cardiovascular Events in Transgender Individuals Receiving Hormone Therapy. Circulation. 2019;139:1461–1462. doi: 10.1161/CIRCULATIONAHA.118.038584. [DOI] [PubMed] [Google Scholar]
- 10.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95:136–147. doi: 10.1007/s00392-006-0351-5. [DOI] [PubMed] [Google Scholar]
- 11.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6 Suppl 1:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–119. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev. 2013;34:309–338. doi: 10.1210/er.2012-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8:538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 15.Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, Green K, Makdissi A, Hejna J, Chaudhuri A, Punyanitya M, Dandona P. Insulin Resistance and Inflammation in Hypogonadotropic Hypogonadism and Their Reduction After Testosterone Replacement in Men with Type 2 Diabetes. Diabetes Care. 2016;39:82–91. doi: 10.2337/dc15-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon D, Charles MA, Lahlou N, Nahoul K, Oppert JM, Gouault-Heilmann M, Lemort N, Thibult N, Joubert E, Balkau B, Eschwege E. Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-month randomized placebo-controlled trial. Diabetes Care. 2001;24:2149–2151. doi: 10.2337/diacare.24.12.2149. [DOI] [PubMed] [Google Scholar]
- 17.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aranda G, Fernández-Rebollo E, Pradas-Juni M, Hanzu FA, Kalko SG, Halperin I, Mora M. Effects of sex steroids on the pattern of methylation and expression of the promoter region of estrogen and androgen receptors in people with gender dysphoria under cross-sex hormone treatment. J Steroid Biochem Mol Biol. 2017;172:20–28. doi: 10.1016/j.jsbmb.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Aranda G, Mora M, Hanzu FA, Vera J, Ortega E, Halperin I. Effects of sex steroids on cardiovascular risk profile in transgender men under gender affirming hormone therapy. Endocrinol Diabetes Nutr. 2019;66:385–392. doi: 10.1016/j.endinu.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Auer MK, Cecil A, Roepke Y, Bultynck C, Pas C, Fuss J, Prehn C, Wang-Sattler R, Adamski J, Stalla GK, T'Sjoen G. 12-months metabolic changes among gender dysphoric individuals under cross-sex hormone treatment: a targeted metabolomics study. Sci Rep. 2016;6:37005. doi: 10.1038/srep37005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auer MK, Ebert T, Pietzner M, Defreyne J, Fuss J, Stalla GK, T'Sjoen G. Effects of Sex Hormone Treatment on the Metabolic Syndrome in Transgender Individuals: Focus on Metabolic Cytokines. J Clin Endocrinol Metab. 2018;103:790–802. doi: 10.1210/jc.2017-01559. [DOI] [PubMed] [Google Scholar]
- 22.Berra M, Armillotta F, D'Emidio L, Costantino A, Martorana G, Pelusi G, Meriggiola MC. Testosterone decreases adiponectin levels in female to male transsexuals. Asian J Androl. 2006;8:725–729. doi: 10.1111/j.1745-7262.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 23.Colizzi M, Costa R, Scaramuzzi F, Palumbo C, Tyropani M, Pace V, Quagliarella L, Brescia F, Natilla LC, Loverro G, Todarello O. Concomitant psychiatric problems and hormonal treatment induced metabolic syndrome in gender dysphoria individuals: a 2 years follow-up study. J Psychosom Res. 2015;78:399–406. doi: 10.1016/j.jpsychores.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Cupisti S, Giltay EJ, Gooren LJ, Kronawitter D, Oppelt PG, Beckmann MW, Dittrich R, Mueller A. The impact of testosterone administration to female-to-male transsexuals on insulin resistance and lipid parameters compared with women with polycystic ovary syndrome. Fertil Steril. 2010;94:2647–2653. doi: 10.1016/j.fertnstert.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 25.Elbers JM, Asscheman H, Seidell JC, Frölich M, Meinders AE, Gooren LJ. Reversal of the sex difference in serum leptin levels upon cross-sex hormone administration in transsexuals. J Clin Endocrinol Metab. 1997;82:3267–3270. doi: 10.1210/jcem.82.10.4284. [DOI] [PubMed] [Google Scholar]
- 26.Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab. 1997;82:2044–2047. doi: 10.1210/jcem.82.7.4078. [DOI] [PubMed] [Google Scholar]
- 27.Elbers JM, Giltay EJ, Teerlink T, Scheffer PG, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 2003;58:562–571. doi: 10.1046/j.1365-2265.2003.01753.x. [DOI] [PubMed] [Google Scholar]
- 28.Fighera TM, da Silva E, Lindenau JD, Spritzer PM. Impact of cross-sex hormone therapy on bone mineral density and body composition in transwomen. Clin Endocrinol (Oxf) 2018;88:856–862. doi: 10.1111/cen.13607. [DOI] [PubMed] [Google Scholar]
- 29.Gava G, Cerpolini S, Martelli V, Battista G, Seracchioli R, Meriggiola MC. Cyproterone acetate vs leuprolide acetate in combination with transdermal oestradiol in transwomen: a comparison of safety and effectiveness. Clin Endocrinol (Oxf) 2016;85:239–246. doi: 10.1111/cen.13050. [DOI] [PubMed] [Google Scholar]
- 30.Gava G, Mancini I, Cerpolini S, Baldassarre M, Seracchioli R, Meriggiola MC. Testosterone undecanoate and testosterone enanthate injections are both effective and safe in transmen over 5 years of administration. Clin Endocrinol (Oxf) 2018;89:878–886. doi: 10.1111/cen.13821. [DOI] [PubMed] [Google Scholar]
- 31.Haraldsen IR, Haug E, Falch J, Egeland T, Opjordsmoen S. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Horm Behav. 2007;52:334–343. doi: 10.1016/j.yhbeh.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Klaver M, de Blok CJM, Wiepjes CM, Nota NM, Dekker MJHJ, de Mutsert R, Schreiner T, Fisher AD, T'Sjoen G, den Heijer M. Changes in regional body fat, lean body mass and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol. 2018;178:163–171. doi: 10.1530/EJE-17-0496. [DOI] [PubMed] [Google Scholar]
- 33.Lapauw B, Taes Y, Simoens S, Van Caenegem E, Weyers S, Goemaere S, Toye K, Kaufman JM, T'Sjoen GG. Body composition, volumetric and areal bone parameters in male-to-female transsexual persons. Bone. 2008;43:1016–1021. doi: 10.1016/j.bone.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Meriggiola MC, Armillotta F, Costantino A, Altieri P, Saad F, Kalhorn T, Perrone AM, Ghi T, Pelusi C, Pelusi G. Effects of testosterone undecanoate administered alone or in combination with letrozole or dutasteride in female to male transsexuals. J Sex Med. 2008;5:2442–2453. doi: 10.1111/j.1743-6109.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- 35.Mueller A, Haeberle L, Zollver H, Claassen T, Kronawitter D, Oppelt PG, Cupisti S, Beckmann MW, Dittrich R. Effects of intramuscular testosterone undecanoate on body composition and bone mineral density in female-to-male transsexuals. J Sex Med. 2010;7:3190–3198. doi: 10.1111/j.1743-6109.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 36.Mueller A, Zollver H, Kronawitter D, Oppelt PG, Claassen T, Hoffmann I, Beckmann MW, Dittrich R. Body composition and bone mineral density in male-to-female transsexuals during cross-sex hormone therapy using gonadotrophin-releasing hormone agonist. Exp Clin Endocrinol Diabetes. 2011;119:95–100. doi: 10.1055/s-0030-1255074. [DOI] [PubMed] [Google Scholar]
- 37.Pelusi C, Costantino A, Martelli V, Lambertini M, Bazzocchi A, Ponti F, Battista G, Venturoli S, Meriggiola MC. Effects of three different testosterone formulations in female-to-male transsexual persons. J Sex Med. 2014;11:3002–3011. doi: 10.1111/jsm.12698. [DOI] [PubMed] [Google Scholar]
- 38.Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab. 1994;79:265–271. doi: 10.1210/jcem.79.1.8027240. [DOI] [PubMed] [Google Scholar]
- 39.Van Caenegem E, Wierckx K, Taes Y, Dedecker D, Van de Peer F, Toye K, Kaufman JM, T'Sjoen G. Bone mass, bone geometry, and body composition in female-to-male transsexual persons after long-term cross-sex hormonal therapy. J Clin Endocrinol Metab. 2012;97:2503–2511. doi: 10.1210/jc.2012-1187. [DOI] [PubMed] [Google Scholar]
- 40.Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, Kaufman JM, T'Sjoen G. Preservation of volumetric bone density and geometry in trans women during cross-sex hormonal therapy: a prospective observational study. Osteoporos Int. 2015;26:35–47. doi: 10.1007/s00198-014-2805-3. [DOI] [PubMed] [Google Scholar]
- 41.Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, Lapauw B, Kaufman JM, T'Sjoen G. Body composition, bone turnover, and bone mass in trans men during testosterone treatment: 1-year follow-up data from a prospective case-controlled study (ENIGI) Eur J Endocrinol. 2015;172:163–171. doi: 10.1530/EJE-14-0586. [DOI] [PubMed] [Google Scholar]
- 42.Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher AD, Toye K, Kaufman JM, T'Sjoen G. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: results from the European network for the investigation of gender incongruence. J Sex Med. 2014;11:1999–2011. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]
- 43.Yahyaoui R, Esteva I, Haro-Mora JJ, Almaraz MC, Morcillo S, Rojo-Martínez G, Martínez J, Gómez-Zumaquero JM, González I, Hernando V, Soriguer F. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. 2008;93:2230–2233. doi: 10.1210/jc.2007-2467. [DOI] [PubMed] [Google Scholar]
- 44.Klaver M, Dekker MJHJ, de Mutsert R, Twisk JWR, den Heijer M. Cross-sex hormone therapy in transgender persons affects total body weight, body fat and lean body mass: a meta-analysis. Andrologia. 2017:49. doi: 10.1111/and.12660. [DOI] [PubMed] [Google Scholar]
- 45.Esteghamati A, Khalilzadeh O, Abbasi M, Nakhjavani M, Novin L, Esteghamati AR. HOMA-estimated insulin resistance is associated with hypertension in Iranian diabetic and non-diabetic subjects. Clin Exp Hypertens. 2008;30:297–307. doi: 10.1080/10641960802269919. [DOI] [PubMed] [Google Scholar]
- 46.Geloneze B, Vasques AC, Stabe CF, Pareja JC, Rosado LE, Queiroz EC, Tambascia MA BRAMS Investigators. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS) Arq Bras Endocrinol Metabol. 2009;53:281–287. doi: 10.1590/s0004-27302009000200020. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Li J, Zheng S, Luo Q, Zhou C, Wang C. Fasting insulin, insulin resistance, and risk of cardiovascular or all-cause mortality in non-diabetic adults: a meta-analysis. Biosci Rep. 2017;37:BSR20170947. doi: 10.1042/BSR20170947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13–27. [Google Scholar]
- 49.Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 50.Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013;5:2019–2027. doi: 10.3390/nu5062019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gianatti EJ, Dupuis P, Hoermann R, Strauss BJ, Wentworth JM, Zajac JD, Grossmann M. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37:2098–2107. doi: 10.2337/dc13-2845. [DOI] [PubMed] [Google Scholar]