Abstract
BACKGROUND
Vitamin C (VC) is a common antioxidant with cell protection potentials. However, its possible protective effect on cardiac autonomic nerves from diabetic induced insults is yet to be explored.
AIM
To investigate the effects of VC on diabetic cardiac autonomic neuropathy.
METHODS
Thirty male Wistar rats were equally grouped into control, diabetic and diabetic + VC. Type 2 diabetes was induced with fructose diet and alloxan. VC (1 g/kg) was administered for 4 wk via oral canula. Blood pressure and heart rate were measured non-invasively using tail flick blood pressure monitor. Spectral analysis of heart rate variability (HRV) was used to assess cardiac autonomic neuropathy. Blood was collected from the ocular sinus for biochemical analysis. Urethane (1 g/kg-ip) was used for anaesthesia prior to HRV and cervical dislocation to harvest hearts. Intracardiac autonomic nerve was assessed using tyrosine hydroxylase immunohistochemistry on fixed heart sections.
RESULTS
Results were analysed using ANOVA at α0.05. Unlike VC and control groups, diabetic rats showed significantly (P < 0.0001) reduced HRV, increased heart-rate and blood pressure, initial increase in cardiac tyrosine hydroxylase activities at week-2 and sparse activity at week-4 of diabetes. Furthermore, apolipoprotein B, Oxidative stress and inflammatory markers were significantly (P < 0.01) reduced in VC treated rats.
CONCLUSION
VC possesses cardio-autonomic nerve protective potential and ameliorates the symptoms of cardiac autonomic neuropathy in type 2 diabetes. The possible mechanisms via which VC exert these effects may be via downregulation of oxidative stress, inflammation and apolipoprotein B.
Keywords: Vitamin C, Oxidative stress, Heart rate variability, Tyrosine hydroxylase, Autonomic nerve, Neuropathy, Type 2 diabetes
Core tip: This study reveals that vitamin C maintained normal heart rate variability, blood pressure and sympathetic tone in type 2 diabetic rats. Thus, preventing the symptoms of cardiac autonomic neuropathy. This is done via mechanisms related to downregulation of oxidative stress, inflammation and dyslipidaemia. Vitamin C may therefore possess cardio-autonomic nerve protection potential and may be used to prevent the development of cardiac autonomic neuropathy in type 2 diabetic subjects.
INTRODUCTION
Cardiac autonomic neuropathy (CAN) is an independent predictor of cardiovascular-disease mortality and morbidity in diabetes. It is the impairment of autonomic control of the cardiovascular system in diabetic condition after the exclusion of other causes[1]. Diabetes is a metabolic disorder of multiple aetiology characterised by chronic hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects of insulin secretion, insulin action, or both[2].
CAN has been detected at the time of diagnosis of diabetes in patients irrespective of age, suggesting that CAN presentation is not limited by age or type of diabetes and can occur before diabetes is evident clinically[3]. CAN is detected in about 7% of both type 1 and type 2 diabetes at the time of initial diagnosis and it is estimated that the risk for developing CAN increases annually by approximately 2% to 6% in diabetic patients[4]. Ziegler et al[5] estimated that about 20% of asymptomatic diabetic patients have abnormal cardiovascular autonomic function; and, with increase in diabetic age, CAN progresses and worsens[6]. The prevalence of CAN varies between 1% to 90% in patients with type 1 diabetes and 20% to 73% in patients with type 2 diabetes[5,7]. This has been widely reported to be greater in type 2 diabetes (34.5%-65%) than in type 1 diabetes (25.3%-38%)[5,7,8].
In the aetiology of CAN, hyperglycaemia activates pathways such as the aldose reductase, polyol, hexosamine, protein kinase C and advanced glycation end products (AGEs) pathways all of which leads to the production of reactive oxygen and nitrogen species and consequently oxidative stress[4]. AGE is formed from non-enzymatic reactions between reducing sugars and proteins/lipids. This reaction results in the activation of inflammatory cascades and release of cytokines which may further lead to cell death or oxidative injury[9,10]. In diabetic nerves, the biochemical damage induced by AGEs results in impaired nerve blood flow, diminished neurotrophic support and hence neuronal death[11,12]. Low-density lipoproteins (LDLs), can be modified by glycation or oxidation to generate reactive oxygen and nitrogen species. Furthermore, oxidized lipids from LDL particles accumulate in the endothelial wall of arteries, monocytes then infiltrate the arterial wall and differentiate into macrophages, which accumulate oxidized lipids to form foam cells. Once formed, foam cells stimulate macrophage proliferation and attraction of T-lymphocytes which, in turn, induce smooth muscle proliferation in the arterial walls and collagen accumulation causing endothelial damage and atherosclerotic plaque formation[13].
Hence, glycation and oxidation of lipids/proteins is the basis for neuronal call damage, inflammation, endothelial dysfunction and atherosclerosis which may give rise to neuronal and cardiovascular abnormalities in diabetes. This makes reduction of oxidative stress an attractive therapeutic pathway in the prevention and/or management of CAN. Palacka et al[14] reported that type 2 diabetic patients taking an antioxidant cocktail had improved cardiovascular function. Antioxidants, like alpha lipoic acid, have been reported to improve autonomic functions[1] while chronic vitamin E administration has been shown to improve the ratio of cardiac sympathetic to parasympathetic tone in patients with type 2 diabetes[14,15]. Vitamin E and alpha lipoic acid are lipid-soluble vitamins however, vitamin C (VC) is a very cheap and readily available aqueous phase antioxidant.
VC is capable of scavenging oxygen-derived free radicals in the aqueous phase before it gets to the lipid cell membrane. Besides, it is effective in the prevention of the non-enzymatic glycosylation of proteins[16]. It has also been reported to reduce oxidative stress, improve nitric oxide beneficial bioactivity, and enhance vascular and endothelial function in high dose[17]. High doses of VC have been shown to improve blood glucose regulation, reduce serum cholesterol and triglyceride in type 2 diabetic patients[18]. However, VC level has been found to be reduced in diabetic humans and rats[19,20]. However, there is paucity of literature concerning effects of VC on cardiac autonomic neuropathy in type 2 diabetic subjects. This study, thus, hypothesises that administration of high dose of 1 g/kg·d of VC to type 2 diabetic subjects may improve cardiac autonomic function together with the risk markers of cardiac autonomic neuropathy such as dyslipidaemia, oxidative stress and inflammation. This study was therefore, carried out to investigate the effects of VC on cardiac autonomic innervation and function, oxidative stress, as well as dyslipidaemia in type 2 diabetic rats.
MATERIALS AND METHODS
Animal grouping
Thirty Wistar rats aged weighing 200-300 g were used for this study. The rats were bred in the animal house of Afe Babalola University Ado-Ekiti, Nigeria where they were exposed to a 12 h light and 12 h dark daily cycle. There were well cared for and humanely treated according to the Guide for the care and use of laboratory animals[21]. They were randomly grouped (n = 10) into control, diabetic and VC (ascorbic acid) treated. Rats in control group received standard chow and water ad libitum, while others received standard chow and 20%w/v fructose sweetened water freely for two weeks and a single dose of 150 mg/kg alloxan injection intraperitoneally to induce type 2 diabetes[22]. Animals with fasting blood glucose greater than 250 mg/dL were considered diabetic. There-after, VC group received 1 g/kg ascorbic acid (sigma) via oral cannula for four weeks after which experiments were carried out on the animals. Appropriate measures were taken to minimize pain or discomfort to the rats according to international ethical guidelines and as approved by the animal ethical committee of Afe Babalola University (ABUAD-AREC/2018/131).
Body weight measurements
Body weight of the rats was measured using an animal weighing scale.
Blood pressures and heart rate measurement
Heart rate and blood pressures were measured using CODA Kent scientific Non-Invasive Blood Pressure system. Awake rats were restrained in a small animal holder after being acclimatised to it. A tail cuff was placed on their tail which measured blood pressures and heart rate via volume pressure recording mechanism.
Cardiac autonomic function assessment
The rats were anesthetised with 1 g/kg urethane injection intraperitoneally. Urethane produces anaesthesia with minimal effects on cardiovascular and respiratory systems, preserving cardiovascular reflexes and functions[23]. Hair around the limbs and chest was shaved and electrode gel was applied on these areas. Limb electrodes were clamped on the left and right forelimb, left hind limb, while neutral electrode was placed on the right hind limb. Chest limb was placed on the chest. Electrodes were connected to EDAN cardiomaq PC ECG which was connected to computer. Five minutes heart rate variability recording was done and analysed using both “Time domain” and “Frequency domain (Fast Fourier Transform)” analysis of power spectra density. Data from the frequency domain analysis was used to plot the autonomic balance chart.
Apolipoprotein A1 and B measurements
Apolipoprotein (Apo) A1 and B were measured via immunoturbidimetric assay with kits purchased from fortress diagnostics, Antrim, United Kingdom. Each of the assay was according to manufacturers’ instruction. A volume of 250 µL phosphate buffer of pH 7.4 was added to 8 µL of undiluted serum separated from blood collected from the ocular sinus of rats. This was read against a distilled water blank at 340 nm after which 50 µL of anti-Apo-antibody was added to the sample and incubated for five minutes. The resulting antigen antibody complex was read at a wavelength of 340 nm in a spectrophotometer. The difference in absorbance was used to determine concentration from the standard curve generated from absorbance of known concentrations of Apo.
Inflammation and oxidative stress markers
Myeloperoxidase (MPO), nitric oxide (NO), super oxide dismutase (SOD), malondialdehyde (MDA), Ascorbic acid (VC) and glutathione (GSH) were assayed spectrophotometrically.
Tyrosine hydroxylase immunohistochemistry
Tyrosine hydroxylase (TH) immunohistochemistry (IHC) was done on slides prepared from excised heart of rats fixed in formalin. At weeks two and four, rats from each group were anesthetised with urethane and sacrificed by cervical dislocation. Thereafter their hearts were harvested and prepared on slides. Tyrosine hydroxylase antibody (Biolegend, United States) was used to stain for sympathetic (dopaminergic) ganglion cells on the slides. The procedure for the immuno- histochemical staining was done according to the manufacturer’s instruction. The microphotographs were scored using IHC profile on image J as positive, high positive or negative.
Statistical analysis
Data were subjected to one-way ANOVA at α0.05 to test for statistical significance. Thus, P < 0.05 was considered as statistically significant.
RESULTS
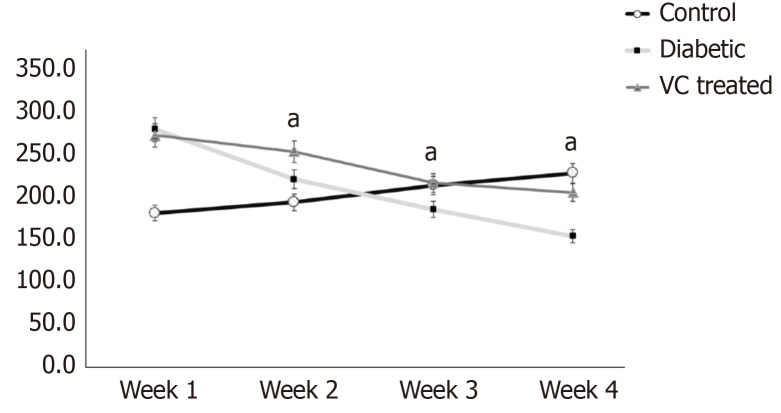
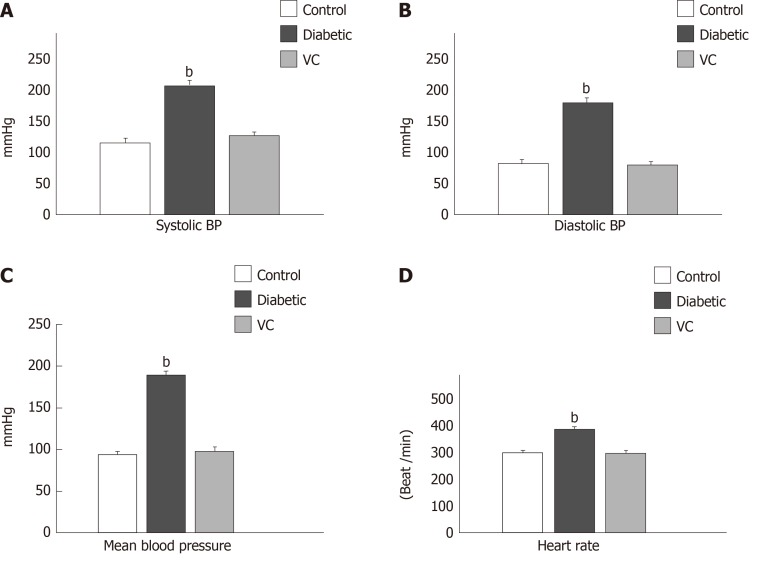
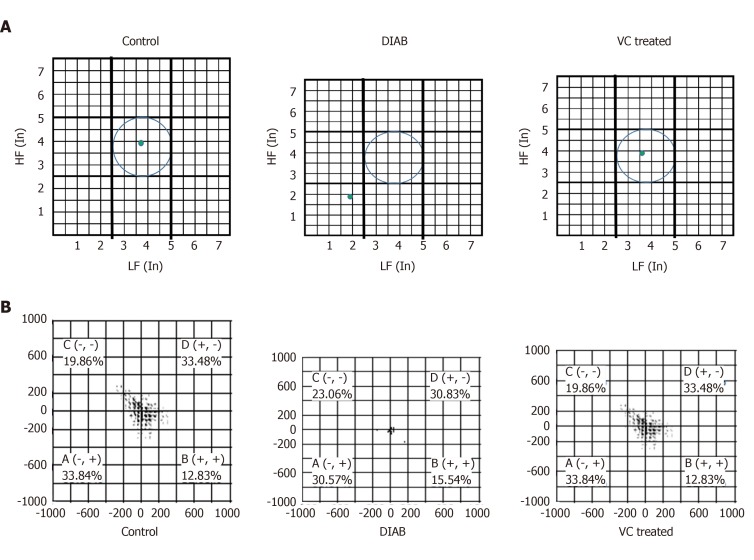
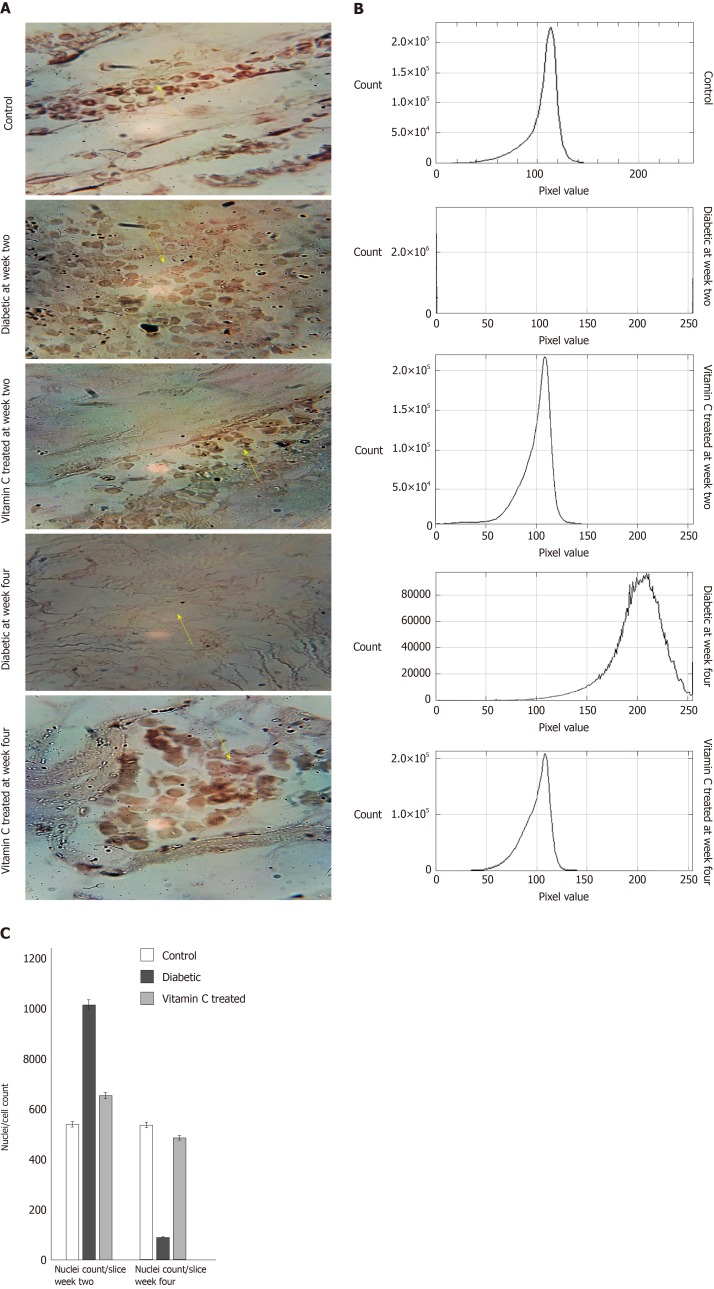
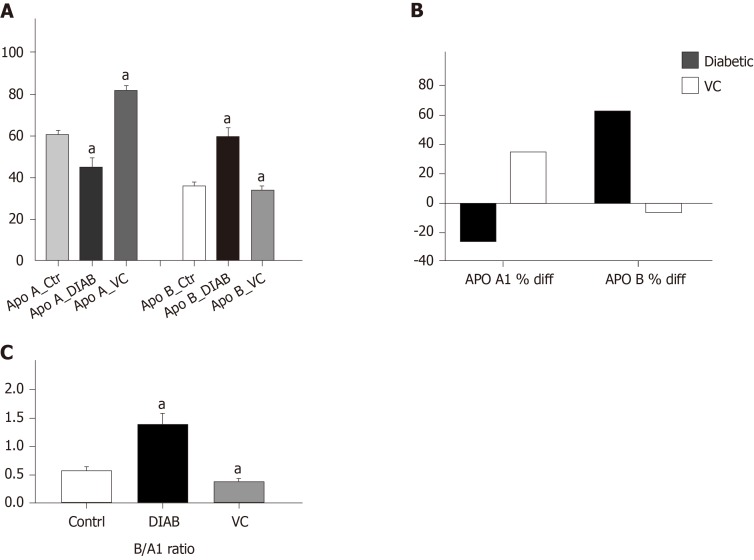
There was significant (P < 0.01) increase in the weights of the diabetic and treated rats, compared with control, at the point of diabetes induction ensuing fructose consumption (Figure 1). During the four weeks treatment period, there was significant (P < 0.01) increased and progressive weight loss in diabetic rats shown in Figure 1. Weight loss was also observed in VC treated rats; however, this was not significant compared with control. Blood pressures and heart rate were significantly (P < 0.001) increased in diabetic group, but not significantly (P > 0.05) increased in VC treated group compared with control (Figure 2). The heart rate variability (HRV) reports of control, diabetic and VC treated rats are shown in the appendices. Power spectral density analysis of HRV revealed greatly (P < 0.001) diminished high frequency (HF) and low frequency (LF) spectra in diabetic rats (Table 1). In addition, time domain variables were significantly (P < 0.01) reduced except for R-R interval which was significantly (P > 0.05) increased by diabetes (Table 1). However, VC treated rats showed no significant difference in HRV analysis compared with control (Table 1). Autonomic imbalance was observed in diabetic rats as shown in Figures 3 and 4. Also Figure 4B shows very sparse scatter points forming a tight cluster in the Poincare plot of diabetic rats. Photomicrograph of TH immunohistochemistry shows densely populated TH positive nuclei (IHC profile score: High positive) at week two of diabetes and sparse TH positive cells (IHC profile score: Negative) at week four of diabetes. However, VC treated rats showed intact TH positive nuclei (IHC profile score: Positive) (Figure 5). Biochemical analysis of oxidative and inflammatory markers in the sera of the rats are shown in Table 2. There was significant (P < 0.05) increase in MPO, NO, SOD and LPO in diabetic rats, however, these markers were not significantly different in VC treated diabetic rats compared with control. Additionally, GSH and AA was elevated (P < 0.05) by VC. Sera Apos are shown in Figure 6. Apo A1 was significantly (P < 0.05) reduced in diabetic but increased in VC treated rats, while Apo B was increased (P < 0.05) in diabetic but not in VC treated rats.
Figure 1.
Body weight of diabetic and vitamin C treated rats. Results are presented as mean ± SE, n = 10. aP < 0.001, significantly different from control. VC: Vitamin C treated.
Figure 2.
Cardiovascular parameters of diabetic rats after vitamin C treatment. A: Systolic blood pressure; B: Diastolic blood pressure; C: Mean blood pressure; D: Heart rate. Results are presented as mean ± SE, n = 10; bP < 0.01, significantly different from control.
Table 1.
Heart rate variability in type 2 diabetic rats
| HRV Indices | Control | Diabetic | Vitamin C |
| HR (bpm) | 282.2 ± 8 | 78.3 ± 17.5c | 277.8 ± 11 |
| RR Interval (ms) | 220.5 ± 14 | 872.5 ± 94d | 222.7 ± 13 |
| Max/Min RR | 3.4 ± 0.2 | 1.4 ± 0.2a | 3.5 ± 0.1 |
| SDNN (ms) | 72.1 ± 11 | 48.6 ± 1.6a | 68.2 ± 7 |
| RMSSD (ms) | 107.5 ± 11.5 | 65.1 ± 1.0b | 98 ± 13 |
| TP (ms2) | 45159.3 ± 7839 | 290.4 ± 0.5d | 44927.8 ± 7832 |
| LF (ms2) | 5434 ± 877 | 126.5 ± 4.6d | 5338.7 ± 1198 |
| HF (ms2) | 19574.1 ± 4019 | 108.4 ± 7d | 14634.1 ± 4866 |
| nLF | 5.9 ± 2.3 | 44.2 ± 2c | 5.7 ± 1 |
| nHF | 16.2 ± 3.4 | 38.5 ± 2a | 13.4 ± 3 |
Results are presented as mean ± SE, n = 10;
P < 0.05,
P < 0.01,
P < 0.001,
P < 0.0001, significantly different from control. HRV: Heart rate variability; HR: Heart rate; RR interval: Interval between two QRS wave complex; SDNN: Standard deviation of successive normal intervals; RMSSD: Root mean square of the difference between successive normal RR interval; TP: Total power; LF: Low frequency; HF: High frequency; nLF: Normalised low frequency; nHF: Normalised high frequency.
Figure 3.
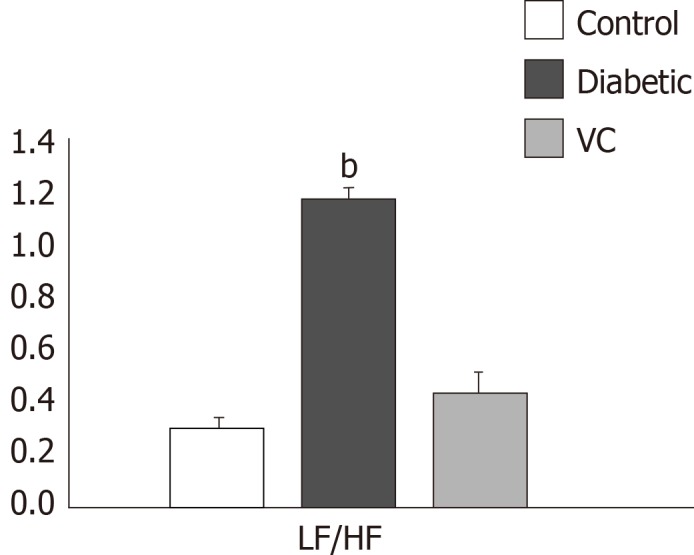
Sympathovagal balance of diabetic and vitamin C treated rats. Results are presented as mean ± SE, n = 10; bP < 0.001 significantly different from control. LF: Low frequency; HF: High frequency.
Figure 4.
Autonomic imbalance was observed in diabetic rats. A: Autonomic balance diagram of diabetic rats after vitamin C treatment; B: Poincare plot of diabetic and vitamin C treated rats. DIAB: Diabetic; VC: Vitamin C; LF: Low frequency; HF: High frequency.
Figure 5.
Photomicrograph and immunohistochemistry profile of tyrosine hydroxylase antibody stained slices of cardiac tissue of diabetic rats after vitamin C treatment. A: Photomicrograph; B: Immunohistochemistry profile; C: Shows nuclei count per slice. Magnification: x 400.
Table 2.
Markers of oxidative stress and inflammation
| Markers | Control | Diabetic | VC treated |
| MPO (µmol/mL/mg protein) | 1.63 ± 0.3 | 2.3 ± 0.07a | 1.62 ± 0.31 |
| NO (M/dL) | 9.5 ± 0.3 | 20.1 ± 0.6b | 8.7 ± 1.11 |
| SOD (unit SOD/min) | 0.7 ± 0.1 | 1.4 ± 0.04b | 0.6 ± 0.11 |
| LPO (nmol/mg protein) | 8.1 ± 1.0 | 16.4 ± 1.2b | 8.6 ± 0.71 |
| GSH (mg/dL) | 11.3 ± 1.0 | 5.89 ± 0.8b | 9.2 ± 1.91 |
| AA (µg/mL) | 61 ± 10.6 | 39.2 ± 2.0b | 62.3 ± 6.21 |
Results are mean ± SE, n = 10;
No significant difference from control;
P < 0.05,
P < 0.01, significantly different from control. MPO: Myeloperoxidase; NO: Nitric oxide; SOD: Super oxide dismutase; MDA: Malondialdehyde; GSH: Glutathione; AA: Ascorbic acid.
Figure 6.
Apolipoprotein A1 and B in serum of diabetic rats after vitamin C treatment. A: Apolipoproteins B and A1; B: Percentage difference in apolipoprotein level compared with control; C: Apolipoprotein B/A1 ratio. Results are presented as mean ± SE, n = 10. aP < 0.01, significantly different from control.
DISCUSSION
There was initial significant increase in weight in diabetic and treated groups after the two weeks of free fructose feeding before alloxan injection. Feeding rats with twenty percent fructose prior to alloxan injection induces a diabetic condition that closely mimics human type 2 diabetes with obesity and insulin resistance preceding hyperglycaemia[22,24]. After alloxan injection, the diabetic rats continued to lose weight over the four weeks observational period and by the third week their weights were significantly less than control. However, the weight loss in the treated rats was controlled significantly by VC administration, such that by the third and fourth week their weight was stabilised and was the same with control. This means that VC facilitated a controlled and beneficial loss of excess weight gained during fructose feeding prior to alloxan injection. This is corroborated by the study of Molz et al[25] who reported the anti-obesogenic effect of VC on sugar intake.
The presence of hypertension, tachycardia, reduced or abnormal HRV, among others, are indication of CAN. However, this study has demonstrated the cardio autonomic protective potential of VC in the amelioration of CAN. Type 2 diabetes has been associated with the development of hypertension as shown in this study, with several mechanisms proposed to mediate the link between these two. However, normotension was achieved in diabetic rats treated with VC in this study. The blood pressure lowering potential of VC has earlier been reported[26,27]. The mechanism by which VC may exert normotensive effect may be by improving endothelial function[27], and enhancing physiological nitric oxide synthase activities as demonstrated in this study and corroborated by Huang et al[28].
Resting tachycardia was evident in awake diabetic rats in this study. According to Vinik and Ziegler[29], resting tachycardia not only reflects vagal impairment, but also reflects autonomic dysfunction and sympathetic over reactivity. Normal resting heart rate was observed in VC treated diabetic rats; thus, VC preserved normal cardiac autonomic functions. Also, administration of VC to diabetic rats in this study restored normal cardiac reflex response to Ethyl carbamate (urethane). Urethane produces anaesthesia with minimal effects on cardiovascular and respiratory systems and maintains spinal reflexes. This was corroborated by this study as there was no significant difference between the heart rate of control rats when awake and when anesthetised with 1 g/kg urethane[23]. However, bradycardia was observed in anesthetised type 2 diabetic rats thus indicating abnormal cardiac reflex response resulting from autonomic dysfunction.
VC intervention was found to restore normal HRV in diabetic Wistar rats. The Standard deviation of the normal to normal intervals between adjacent QRS complexes (SDNN) resulting from sinus node depolarisations is widely accepted as a primary measure of autonomic influence on heart rate variability while the Root Mean Square of the Differences between Successive QRS intervals (RMSSD) is an estimate of the short-term components of HRV that reflects parasympathetic regulation of the heart. Both SDNN and RMSSD were reduced significantly in diabetic rats[30] thus, indicating depression of both parasympathetic and sympathetic control of the heart. However, administration of VC to type 2 diabetic rats restored normal autonomic control of heart rate variability as SDNN and RMSSD were augmented back to levels similar to control, indicating that VC may have cardio autonomic protective effects[31]. Total power, LF and HF were significantly reduced in type 2 diabetic rats. Total power is a measure that reflects the overall autonomic activity where sympathetic activity is a primary contributor; HF represents parasympathetic (vagal) control of the heart, while LF represents mainly sympathetic modulation of heart rate. The reduction in total power, LF and HF shows that both parasympathetic and sympathetic control of the heart were at very low levels and close to being lost. General reduction in HRV has been shown to precede the clinical expression of cardiac autonomic neuropathy in both type 1 and type 2 diabetes[32]. However, in this study, VC restored normal HRV and, thus, normal autonomic control of heart in type 2 diabetic rats.
It was evident in this study that although both parasympathetic and sympathetic regulatory activities were greatly reduced, parasympathetic regulation was much more reduced, giving rise to sympathetic dominance and disruption of normal autonomic balance between sympathetic and parasympathetic regulations. Sympathetic dominance in the diabetic rats was demonstrated by the significantly increased normalised low frequency and LF/HF ratio. In addition, autonomic balance diagram revealed a shift from the region of optimal balance to the region of both sympathetic and parasympathetic inhibition or burnout accompanied with sympathetic dominance. Also, in this study, TH positive neurons were identified in the intracardiac nerve ganglion of all groups. But, unlike VC group, the diabetic group had markedly increased density of TH positive neurons at week-2 and sparse neurons at week-4 of diabetes showing sympathetic overactivation[33,34] which progresses into autonomic burnout/low autonomic tone and denervation. Sympathetic dominance on heart rate variability is a clear indication of cardiovascular autonomic neuropathy[32,35]. Furthermore, autonomic imbalance or dysfunction impairs the ability of the autonomic nervous system to regulate the cardiovascular system[9]. VC administration not only restored both vagal and sympathetic tone to normal levels, but also re-established normal vagal dominance and sympathovagal balance, thus ameliorating CAN.
Hyperglycaemia of diabetes has been implicated as the chief cause of CAN by causing excessive non-enzymatic glycation of proteins, with marked inactivation of enzymes; tissue damage, increased lipid peroxidation and a great imbalance in the antioxidant defence systems[36,37]. Reduced VC and GSH were observed in diabetic rats. In addition, there was increased lipid peroxidation, SOD, NO and MPO in diabetic rats in this study. However, plasma VC and GSH deficiencies were ameliorated upon exogenous administration of VC. Glutathione is known to catalyse the detoxification of lipid peroxides and to regenerate oxidized VC back to its active form[38,39], while VC, in turn, spares GSH from oxidation (Interdependence). SOD is required for the dismutation of superoxide and is used as an indirect assessment of super oxide anion production. Although SOD is considered as one of the enzymatic antioxidants defence and is the first in line in scavenging super oxide anion, it depends on other enzymatic antioxidants like glutathione peroxidase/glutathione to neutralise its product of dismutation. Increased plasma superoxide generation has been reported to predict cardiac autonomic nerve function and mortality in diabetic patients[40]. In addition, overproduction of superoxide promotes the production of hydroxyl radicals which initiate lipid peroxidation process. Lipid peroxidation has been said to play an important role in the pathogenesis of diabetic complications. In this study, MDA, a product of lipid peroxidation, was increased in type 2 diabetic rats. The increased lipid peroxidation may be due to oxidative stress or hyperglycaemia and is a major cause of atherosclerosis, oxidative damage to cells and cardiovascular disease in diabetic condition. VC inhibited lipid peroxidation in type 2 diabetic rats as shown by the reduction in MDA in treated rats. VC is an excellent source of electrons; therefore, it can donate electrons to free radicals such as hydroxyl and superoxide radicals, and quench their reactivity[41] thus, ameliorating oxidative stress which is a key factor in CAN production.
Elevated levels of NO and MPO observed in diabetes are particularly deleterious to the cardiovascular system. At a moderate physiological level, NO is a signalling molecule in diverse physiological processes such as neurotransmission, blood pressure regulation, smooth muscle relaxation and immune regulation[42]. However, excess NO is cytotoxic because it acts as a pro-oxidant at high concentrations and gives rise to the formation of other reactive nitrogen species such as peroxynitirte which oxidizes lipids, DNA and activates poly ADP-ribose polymerase pathway – one of the major pathways implicated in the aetiology of CAN[43]. Also, nitric oxide has been found to modulate the catalytic activity of MPO[44]. Myeloperoxidase catalyses the production of hypochlorous acid (an oxidant) as an immune response, albeit elevated MPO is harmful as it catalyses vascular nitro tyrosine formation[45], oxidizes lipoproteins and is proatherogenic[46]. However, VC administration to the diabetic rats downregulated NO and MPO to physiological levels, thus, preserving their beneficial bioactivity.
Apo B is the structural backbone of all atherogenic lipids, while Apo A1 is the structural backbone of HDL and all good lipids. Apo B/A1 is a better marker of dyslipidaemia and cardiovascular disorders risk prediction than convention lipid measures such as LDL/HDL[47]. In addition, Apo B/A1 ratio can be used to evaluate cardiovascular disease risk in diabetic patients[48]. In this study, Apo B was increased in untreated type 2 diabetic rats but not in VC treated diabetic rats while apo A1 was increased in VC treated rats giving rise to an increased apo B/A1 ratio in diabetic rats and decreased apo B/A1 ratio in VC treated rats. Apo A1 has been found to have a strong inverse association with the development of cardiac autonomic neuropathy in diabetic patients[49]. VC has been shown to increase plasma HDL (which is mainly made up of apo A1), and also protect against its oxidation, preserving its cardioprotective properties[50]. This study also showed that VC directly correlated with Apo A1 and inversely correlated with both Apo B and Apo B/A1 ratio, which is an index of cardiovascular disease[48]. VC may, thus, have hypolipidemic effect reducing circulating atherogenic lipids and, as a result, protecting cardiovascular functions.
In conclusion, this study has demonstrated that VC may have direct and indirect effects on the amelioration of cardiac autonomic neuropathy by possibly combating oxidative stress, protecting the autonomic nerves from oxidative stress damage, preventing lipid peroxidation, promoting nerve regeneration, maintaining normal autonomic tone and balance, and ameliorating dyslipidaemia. Thus, VC may be an adjuvant therapy in the prevention or amelioration cardiac autonomic neuropathy in patients living with type 2 diabetes. However, human studies may be necessary to evaluate this.
ARTICLE HIGHLIGHTS
Research background
Complications of diabetes such as cardiac autonomic neuropathy has been attributed to increased oxidative stress secondary to hyperglycaemia. This makes reduction of oxidative stress an attractive therapeutic pathway in the prevention and/or management of cardiac autonomic neuropathy. Thus, this study hypothesises that administration of high dose of 1 g/kg·d of vitamin C (VC) to type 2 diabetic subjects may prevent or ameliorate cardiac autonomic neuropathy.
Research motivation
The motivation for this study is to increase the quality of life of diabetics, reducing the morbidity and mortality due to cardiovascular and neuronal events in them. This study proposes that high dose VC may possess neuronal and cardio protective effects thus preventing or ameliorating cardiac autonomic neuropathy in diabetic subjects.
Research objectives
The main objectives of this study were to evaluate the cardiovascular ad neuro- protective effects of VC administration in diabetics. The effects of VC on cardiovascular functions, autonomic functions, oxidative stress and lipid metabolism were investigated.
Research methods
Heart rate variability electrophysiology was employed to assess cardiac autonomic neuropathy in Wistar rats. Tail flick blood pressure monitor was used to assess cardiovascular functions such as blood pressure and heart rate. Apolipoproteins were measured using immunoturbidimetry, oxidative and inflammatory markers were measured spectrophotometrically while tyrosine hydroxylase immunohistochemistry was employed to study autonomic activity in the heart.
Research results
The findings of this study reveal that VC reversed cardiac autonomic neuropathy in diabetics and thus might be a useful adjuvant in the management of diabetes to improve the quality of life of diabetics. VC effects on other diabetic neuropathies should be evaluated. Also, VC as an adjuvant therapy to diabetic patients should be investigated in clinical trials.
Research conclusions
This study hypothesises that VC may ameliorate cardiac autonomic neuropathy. VC was found to reverse abnormal heart rate variability and preserve normal cardiovascular functions, protect autonomic nerves from oxidative damage and to possess lipid lowering potential in diabetic condition. The implication of this study is that VC may be used in the management of diabetic patients to prevent the progression of neuropathies and cardiovascular events in them, thereby improving their quality of life.
Research perspectives
Human studies may be needed to evaluate VC effects in diabetic patients. Interactions of VC with antidiabetic drugs in diabetic subjects should be examined. Also, further studies are required to elucidate the molecular pathway via which VC exert cardio- and neuro- protective effects in diabetic condition.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Afe Babalola University Ado-Ekiti for granting funds to support this research.
Footnotes
Institutional review board statement: The study was reviewed and approved by Afe Babalola University Institutional Review board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of Afe Babalola University with protocol number: [ABUAD-IRB/IACAU/2018/131].
Conflict-of-interest statement: There is no conflict of interest regarding this manuscript.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: September 8, 2019
First decision: September 21, 2019
Article in press: January 13, 2020
Specialty type: Endocrinology and metabolism
Country of origin: Nigeria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta B, Wan TTH S-Editor: Wang YQ L-Editor: A E-Editor: Liu MY
References
- 1.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation 2006. Switzerland: WHO Press. Available from: https://www.who.int/diabetes/publications/diagnosis_diabetes2006/en/ [Google Scholar]
- 3.Ziegler D. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev. 1994;10:339–383. doi: 10.1002/dmr.5610100403. [DOI] [PubMed] [Google Scholar]
- 4.Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Semin Neurol. 2003;23:365–372. doi: 10.1055/s-2004-817720. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler D, Dannehl K, Mühlen H, Spüler M, Gries FA. Prevalence of cardiovascular autonomic dysfunction assessed by spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses at various stages of diabetic neuropathy. Diabet Med. 1992;9:806–814. doi: 10.1111/j.1464-5491.1992.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder EB, Chambless LE, Liao D, Prineas RJ, Evans GW, Rosamond WD, Heiss G Atherosclerosis Risk in Communities (ARIC) study. Diabetes, glucose, insulin, and heart rate variability: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2005;28:668–674. doi: 10.2337/diacare.28.3.668. [DOI] [PubMed] [Google Scholar]
- 7.Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O'Brien PC, Suarez GA, Dyck PJ. Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27:2942–2947. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- 8.Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH DCCT/EDIC Research Group. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) Circulation. 2009;119:2886–2893. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig. 2013;4:4–18. doi: 10.1111/jdi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toth C, Rong LL, Yang C, Martinez J, Song F, Ramji N, Brussee V, Liu W, Durand J, Nguyen MD, Schmidt AM, Zochodne DW. Receptor for advanced glycation end products (RAGEs) and experimental diabetic neuropathy. Diabetes. 2008;57:1002–1017. doi: 10.2337/db07-0339. [DOI] [PubMed] [Google Scholar]
- 11.Wada R, Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci. 2005;1043:598–604. doi: 10.1196/annals.1338.067. [DOI] [PubMed] [Google Scholar]
- 12.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol. 2012;11:521–534. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle PJ. Diabetes mellitus and macrovascular disease: mechanisms and mediators. Am J Med. 2007;120:S12–S17. doi: 10.1016/j.amjmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Palacka P, Kucharska J, Murin J, Dostalova K, Okkelova A, Cizova M, Waczulikova I, Moricova S, Gvozdjakova A. Complementary therapy in diabetic patients with chronic complications: a pilot study. Bratisl Lek Listy. 2010;111:205–211. [PubMed] [Google Scholar]
- 15.Ziegler D, Sohr CG, Nourooz-Zadeh J. Oxidative stress and antioxidant defense in relation to the severity of diabetic polyneuropathy and cardiovascular autonomic neuropathy. Diabetes Care. 2004;27:2178–2183. doi: 10.2337/diacare.27.9.2178. [DOI] [PubMed] [Google Scholar]
- 16.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22:18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 17.McNulty PH, Robertson BJ, Tulli MA, Hess J, Harach LA, Scott S, Sinoway LI. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol (1985) 2007;102:2040–2045. doi: 10.1152/japplphysiol.00595.2006. [DOI] [PubMed] [Google Scholar]
- 18.Afkhami-Ardekani M, Shojaoddiny-Ardekani A. Effect of vitamin C on blood glucose, serum lipids & serum insulin in type 2 diabetes patients. Indian J Med Res. 2007;126:471–474. [PubMed] [Google Scholar]
- 19.Hirsch IB, Atchley DH, Tsai E, Labbé RF, Chait A. Ascorbic acid clearance in diabetic nephropathy. J Diabetes Complications. 1998;12:259–263. doi: 10.1016/s1056-8727(97)00125-6. [DOI] [PubMed] [Google Scholar]
- 20.Schaan BD, Dall'Ago P, Maeda CY, Ferlin E, Fernandes TG, Schmid H, Irigoyen MC. Relationship between cardiovascular dysfunction and hyperglycemia in streptozotocin-induced diabetes in rats. Braz J Med Biol Res. 2004;37:1895–1902. doi: 10.1590/s0100-879x2004001200016. [DOI] [PubMed] [Google Scholar]
- 21.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 22.Fabiyi-Edebor TD. Low Fructose Diet and Alloxan Induced Type II Diabetes of Low Mortality in Wister Rats. Imperial J Interdisciplinary Res. 2017;3:715–719. [Google Scholar]
- 23.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: Cardiovascular system. Experientia. 1986;42:292–297. doi: 10.1007/BF01942510. [DOI] [PubMed] [Google Scholar]
- 24.Fabiyi-Edebor TD, Fasanmade AA. Evaluation of the characteristics of diabetes induced by the administration of alloxan to fructose fed Wistar rat. Int J Pharm Sci & Res. 2019;10:881–889. [Google Scholar]
- 25.Molz P, Rael AN, Fischer MDQ, Limberger LB, Prá D, Franke SIR. Vitamin C decreases the obesogenic and hyperglycemic effect of invert sugar in prediabetic rats. Rev Nutr. 2017;30:23–32. [Google Scholar]
- 26.Vasdev S, Ford CA, Parai S, Longerich L, Gadag V. Dietary vitamin C supplementation lowers blood pressure in spontaneously hypertensive rats. Mol Cell Biochem. 2001;218:97–103. doi: 10.1023/a:1007234027421. [DOI] [PubMed] [Google Scholar]
- 27.Ettarh RR, Odigie IP, Adigun SA. Vitamin C lowers blood pressure and alters vascular responsiveness in salt-induced hypertension. Can J Physiol Pharmacol. 2002;80:1199–1202. doi: 10.1139/y02-147. [DOI] [PubMed] [Google Scholar]
- 28.Huang A, Vita JA, Venema RC, Keaney JF Jr. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J Biol Chem. 2000;275:17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 29.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Jiang YH, Jiang P, Lin HQ, Yang JL, Ma DF, Wang X, Yang CH. Analysis of Heart Rate Variability and Cardiac Autonomic Nerve Remodeling in Streptozotocin-induced Diabetic Rats. Exp Clin Endocrinol Diabetes. 2015;123:272–281. doi: 10.1055/s-0035-1547258. [DOI] [PubMed] [Google Scholar]
- 31.Manzella D, Barbieri M, Ragno E, Paolisso G. Chronic administration of pharmacologic doses of vitamin E improves the cardiac autonomic nervous system in patients with type 2 diabetes. Am J Clin Nutr. 2001;73:1052–1057. doi: 10.1093/ajcn/73.6.1052. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler D, Gries FA, Mühlen H, Rathmann W, Spüler M, Lessmann F. Prevalence and clinical correlates of cardiovascular autonomic and peripheral diabetic neuropathy in patients attending diabetes centers. The Diacan Multicenter Study Group. Diabete Metab. 1993;19:143–151. [PubMed] [Google Scholar]
- 33.Huggett RJ, Scott EM, Gilbey SG, Stoker JB, Mackintosh AF, Mary DA. Impact of type 2 diabetes mellitus on sympathetic neural mechanisms in hypertension. Circulation. 2003;108:3097–3101. doi: 10.1161/01.CIR.0000103123.66264.FE. [DOI] [PubMed] [Google Scholar]
- 34.Tran LT, Yuen VG, McNeill JH. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009;332:145–159. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- 35.Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyörälä K, Raz I, Schernthaner G, Volpe M, Wood D Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 36.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 37.Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Meister A. On the antioxidant effects of ascorbic acid and glutathione. Biochem Pharmacol. 1992;44:1905–1915. doi: 10.1016/0006-2952(92)90091-v. [DOI] [PubMed] [Google Scholar]
- 39.Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler D, Buchholz S, Sohr C, Nourooz-Zadeh J, Roden M. Oxidative stress predicts progression of peripheral and cardiac autonomic nerve dysfunction over 6 years in diabetic patients. Acta Diabetol. 2015;52:65–72. doi: 10.1007/s00592-014-0601-3. [DOI] [PubMed] [Google Scholar]
- 41.Losonczy KG, Harris TB, Havlik RJ. Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: the Established Populations for Epidemiologic Studies of the Elderly. Am J Clin Nutr. 1996;64:190–196. doi: 10.1093/ajcn/64.2.190. [DOI] [PubMed] [Google Scholar]
- 42.Bergendi L, Benes L, Duracková Z, Ferencik M. Chemistry, physiology and pathology of free radicals. Life Sci. 1999;65:1865–1874. doi: 10.1016/s0024-3205(99)00439-7. [DOI] [PubMed] [Google Scholar]
- 43.Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J, Wink DA. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem. 2004;385:1–10. doi: 10.1515/BC.2004.001. [DOI] [PubMed] [Google Scholar]
- 44.Akolkar G, da Silva Dias D, Ayyappan P, Bagchi AK, Jassal DS, Salemi VMC, Irigoyen MC, De Angelis K, Singal PK. Vitamin C mitigates oxidative/nitrosative stress and inflammation in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2017;313:H795–H809. doi: 10.1152/ajpheart.00253.2017. [DOI] [PubMed] [Google Scholar]
- 45.Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, Ma W, Tousson A, White CR, Bullard DC, Brennan ML, Lusis AJ, Moore KP, Freeman BA. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Podrez EA, Abu-Soud HM, Hazen SL. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic Biol Med. 2000;28:1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 47.Lind L. Apolipoprotein B/A1 and risk of cardiovascular disease. Lancet. 2008;372:185–186. doi: 10.1016/S0140-6736(08)61050-8. [DOI] [PubMed] [Google Scholar]
- 48.Lee B, Pratumvinit B, Thongtang N. The role of apoB measurement in Type 2 diabetic patients. Clinical Lipidology. 2015;10:137–144. [Google Scholar]
- 49.Chung JO, Park SY, Han JH, Cho DH, Chung DJ, Chung MY. Serum apolipoprotein A-1 concentrations and the prevalence of cardiovascular autonomic neuropathy in individuals with type 2 diabetes. J Diabetes Complications. 2018;32:357–361. doi: 10.1016/j.jdiacomp.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Ankit BS, Mathur G, Agrawal RP, Mathur KC. Stronger relationship of serum apolipoprotein A-1 and B with diabetic retinopathy than traditional lipids. Indian J Endocrinol Metab. 2017;21:102–105. doi: 10.4103/2230-8210.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]