Abstract
Background
Preterm premature rupture of membranes (PPROM) is a leading cause of perinatal morbidity and mortality. Amnioinfusion aims to restore amniotic fluid volume by infusing a solution into the uterine cavity.
Objectives
The objective of this review was to assess the effects of amnioinfusion for PPROM on perinatal and maternal morbidity and mortality.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (2 December 2013).
Selection criteria
Randomised trials of amnioinfusion compared with no amnioinfusion in women with PPROM.
Data collection and analysis
Three review authors independently assessed trials for inclusion. Two review authors independently assessed trial quality and extracted data. Data were checked for accuracy.
Main results
We included five trials, of moderate quality, but we only analysed data from four studies (with a total of 241 participants). One trial did not contribute any data to the review.
Transcervical amnioinfusion improved fetal umbilical artery pH at delivery (mean difference 0.11; 95% confidence interval (CI) 0.08 to 0.14; one trial, 61 participants) and reduced persistent variable decelerations during labour (risk ratio (RR) 0.52; 95% CI 0.30 to 0.91; one trial, 86 participants).
Transabdominal amnioinfusion was associated with a reduction in neonatal death (RR 0.30; 95% CI 0.14 to 0.66; two trials, 94 participants), neonatal sepsis (RR 0.26; 95% CI 0.11 to 0.61; one trial, 60 participants), pulmonary hypoplasia (RR 0.22; 95% CI 0.06 to 0.88; one trial, 34 participants) and puerperal sepsis (RR 0.20; 95% CI 0.05 to 0.84; one trial, 60 participants). Women in the amnioinfusion group were also less likely to deliver within seven days of membrane rupture (RR 0.18; 95% CI 0.05 to 0.70; one trial, 34 participants). These results should be treated with circumspection as the positive findings were mainly due to one trial with unclear allocation concealment.
Authors' conclusions
These results are encouraging but are limited by the sparse data and unclear methodological robustness, therefore further evidence is required before amnioinfusion for PPROM can be recommended for routine clinical practice.
Plain language summary
Amnioinfusion for preterm premature rupture of membranes
There is some evidence to show that restoring amniotic fluid volume with saline or a similar fluid (amnioinfusion) following preterm premature rupture of the membranes (PPROM) may be beneficial for preterm babies (by preventing infection, lung damage and death) and mothers (by preventing infection of the womb after childbirth). However, current evidence is insufficient to recommend amnioinfusion for routine use in PPROM.
Preterm premature rupture of membranes is the single most identifiable cause of preterm labour. The sac (membranes) surrounding the baby and fluid in the womb (uterus) usually breaks (ruptures) during labour. If the membranes rupture before labour and preterm (before 37 weeks) the baby has an increased risk of infection. Reduced fluid around the baby also increases the chance of the umbilical cord being compressed, which can reduce the baby's supply of nutrients and oxygen. In addition, insufficient fluid in the womb may interfere with normal lung development in very small babies and can cause fetal distress, with changes in heart rate. Extra liquid can be injected through the woman's vagina (transcervical amnioinfusion) or abdomen (transabdominal amnioinfusion) into the womb, providing more liquid to surround the baby. The review of five randomised controlled trials (with data from a total of 241 participants analysed) found some evidence to show that amnioinfusion with a saline solution may improve health outcomes and be beneficial for babies and mothers following PPROM. However, the evidence is currently insufficient to recommend its routine use because of the limited number of trials and low numbers of women in the trials.
Summary of findings
for the main comparison.
| Transabdominal amnioinfusion compared with no amnioinfusion for preterm rupture of membranes (PROM) | ||||
|
Patient or population: pregnant women with PROM Settings: hospital Intervention: transabdominal amnioinfusion Comparison: no amnioinfusion | ||||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Neonatal death | RR 0.30 (0.14 ‐ 0.66) | 94 (two studies) | ⊕⊕⊕⊝ moderate | Risk of neonatal death in the amnioinfusion group was 127 per 1000 compared to 426 per 1000 in the control group. |
| Neonatal sepsis/infection | RR 0.26 (0.11 ‐ 0.61) | 60 (one study*) | ⊕⊕⊕⊝ moderate | *Sepsis defined as micro‐erythrocyte sedimentation rate > 5 mm, total leucocyte count < 5000, CRP > 6 mg/dL, platelet count < 100,000 or a positive blood culture within the first 48 hours. |
| Pulmonary hypoplasia | RR 0.22 (0.06 ‐ 0.88) | 34 (one study) | ⊕⊕⊝⊝ low | Pulmonary hypoplasia was diagnosed according to strict clinical and radiological criteria, however, this study was small and blinding to group allocation was not described and so we downgraded this evidence from moderate to low. More evidence is needed. |
| Maternal puerperal sepsis | RR 0.20 (0.05 ‐ 0.84) | 60 (one study**) | ⊕⊕⊕⊝ moderate | **Defined as fever > 38° C and a positive high vaginal swab culture. |
| GRADE Working Group grades of evidence
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate. CI: confidence interval; CRP: C‐reactive protein; RR: risk ratio | ||||
Background
Description of the condition
Preterm premature rupture of membranes (PPROM) remains the single most identifiable cause of preterm labour and a major contributor to perinatal mortality and morbidity. Oligohydramnios (reduced amniotic fluid volume) following PPROM is associated with a higher risk of chorioamnionitis, neonatal fetal infection and cord compression (Keirse 1989; Vintzileos 1985). Umbilical cord compression may cause persistent variable fetal heart rate decelerations (Gabbe 1976). Oligohydramnios is also the most important predictor of perinatal mortality in very early PPROM and adequate residual amniotic fluid plays a critical role in determining the prevalence of pulmonary hypoplasia, which is a major cause of death in these babies (Vergani 1994; Vintzileos 1985). Abnormal neurological outcomes and postural deformities in the neonate may also occur as a consequence of PPROM (Locatelli 2000).
Description of the intervention
Saline fluid or Ringers lactate/Hartmans is infused transcervically through a catheter into the uterine cavity, or transabdominally, through a narrow gauge needle. Amnioinfusion was first described as a method of preventing or relieving umbilical cord compression during labour (Miyazaki 1983). The technique has since been used prophylactically in various conditions associated with oligohydramnios, including impaired intrauterine growth and PPROM. Amnioinfusion has been shown to prolong the latency period in second trimester PPROM (Locatelli 2000; Ogunyemi 2002; Turhan 2002), improve perinatal survival (Locatelli 2006; Ogunyemi 2002) and decrease rates of pulmonary hypoplasia (Locatelli 2000). Combined with antibiotics and without, it has been used to treat and to prevent infection following premature rupture of membranes (Goodlin 1981; Monahan 1995; Ogita 1988). In a Cochrane review on amnioinfusion for suspected or potential cord compression in labour at term, amnioinfusion improved short‐term measures of neonatal outcome, reduced the use of caesarean section and reduced maternal puerperal sepsis (Hofmeyr 2012). A subcutaneously implanted amniotic fluid replacement port system has been developed for long‐term amnioinfusion in women with preterm premature rupture of the membranes (Tchirikov 2010).
How the intervention might work
Restoring the amniotic fluid volume cushions the fetus, thereby preventing mechanical compression of the umbilical cord and reducing fetal distress. It may prevent fetal lung hypoplasia in PPROM by preventing mechanical compression of the fetal thorax and enabling normal amniotic fluid flow into the fetal lungs (Tranquilli 2005). This effect occurs mainly in the second trimester and is considered in a separate review (Van Teeffelen 2013). Likewise, by preventing mechanical compression of the fetus, amnioinfusion may prevent postural deformities. In addition, the infused fluid may prevent intrauterine infection, possibly by the anti‐bacterial effect of saline, or a diluent effect. Improvements in fetal ductus venosus and umbilical artery flow have been demonstrated following amnioinfusion for PPROM (Hsu 2009).
Why it is important to do this review
Evidence from cohort studies suggests that replenishing the amniotic fluid volume in pregnancies complicated by PPROM is beneficial to mother and child. However, amnioinfusion is an invasive procedure and not without potential risks, thus a thorough evaluation of randomised controlled trials is required.
['Amnioinfusion for cord compression' (Hofmeyr 2012), 'Prophylactic versus therapeutic amnioinfusion' (Novikova 2012), and 'Amnioinfusion for meconium‐stained liquor' (Hofmeyr 2014), are separate reviews.]
Objectives
To assess from the best available evidence the effects of amnioinfusion for preterm premature rupture of membranes on perinatal morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing the effect of amnioinfusion for third trimester preterm premature rupture of the membranes before 37 weeks with a control group (no amnioinfusion); random allocation to treatment and control groups, with adequate allocation concealment; violations of allocated management and exclusions after allocation not sufficient to materially affect outcomes. Quasi‐randomised (alternate allocation), cross‐over and cluster trials were not considered eligible for inclusion.
Types of participants
Pregnant women with preterm premature rupture of membranes.
Types of interventions
Amnioinfusion compared with no amnioinfusion.
Types of outcome measures
Primary outcomes
Indicators of fetal condition, e.g. persistent variable decelerations, Apgar scores, cord arterial pH at birth.
Neonatal morbidity including infection, lung hypoplasia, abnormal neurological outcomes and postural deformities.
Perinatal mortality.
Secondary outcomes
Mode of delivery.
Indications for delivery.
Latency period from amnioinfusion to delivery.
Maternal morbidity, e.g. endometritis, postpartum temperature greater than 38°C.
Birthweight.
Admission to neonatal intensive or high care unit.
We considered outcomes separately for transcervical and transabdominal amnioinfusion.
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (2 December 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
monthly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
No new studies have been included for this update (2014).
For methods used in the previous version of this review, please see Hofmeyr 2011.
For methods to be used in the next update, see Appendix 1.
Selection of studies
T Lawrie (TL), AC Eke (ACE) and GJ Hofmeyr (GJH) independently assessed for inclusion all potential studies. No studies were identified from the updated search. Two studies previously in ongoing were excluded (Roberts: AMIPROM; Vergani 2007). We resolved any disagreement through discussion. We evaluated trials under consideration for methodological quality and appropriateness for inclusion according to the prespecified selection criteria, without consideration of their results.
Results
Description of studies
In total, we have included five studies; three in the transcervical amnioinfusion comparison (Gonzalez 2001; Nageotte 1985; Puertas 2007) and two in the transabdominal comparison (Singla 2010; Tranquilli 2005). One included study contributed no data (Gonzalez 2001). SeeCharacteristics of included studies. The gestational age range for recruitment to the transcervical trials was 26 to 36+ weeks and in the transabdominal trials, 24 to 34 weeks. Transcervical amnioinfusion was performed in a total of 72 women versus 75 controls (excluding unusable data from Gonzalez 2001) and transabdominal amnioinfusion was performed in 47 women versus 47 controls; thus participant numbers for this review are small.
Risk of bias in included studies
All included studies were randomised trials. SeeFigure 1 and Figure 2 for a summary of 'Risk of bias' assessments.
1.
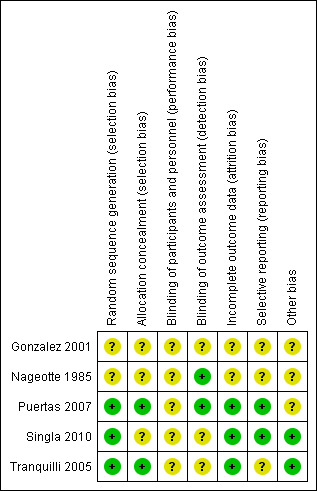
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
2.
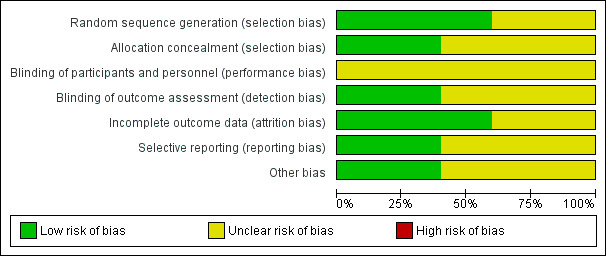
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Randomisation was by random number table in Puertas 2007 and was computer‐generated in Tranquilli 2005 and Singla 2010; the method of randomisation and group allocation was not described in Nageotte 1985 and Gonzalez 2001. Allocation concealment was described in two trials (Puertas 2007; Tranquilli 2005).
Blinding
Partial blinding was described in two trials (Nageotte 1985; Puertas 2007).
Incomplete outcome data
Incomplete outcome data were noted in the randomised clinical trial by Gonzalez et al. This is because it is an abstract only publication, hence provided insufficient detail for assessment (Gonzalez 2001). Five post‐randomisation exclusions: three for non‐vertex presentation and two for fetal distress were noted in the randomised trial by Nageotte et al (Nageotte 1985). Otherwise, outcome data were complete for Puertas 2007, Singla 2010 and Tranquilli 2005.
Selective reporting
In the randomised clinical trial by Tranquilli et al, the authors did not report the mode of delivery, Apgar scores or cord arterial pH for the babies delivered (Tranquilli 2005). No selective reporting was found in the Puertas 2007 and Singla 2010 trials. There was insufficient information to assess selective reporting in Gonzalez 2001 and Nageotte 1985.
Other potential sources of bias
Another source of bias was noted in the randomised clinical trial by Puertas et al, where more carriers of group B streptococcal were noted in control group (14 women versus seven women) (Puertas 2007). Even though all these women with Group B streptococcal infection received prophylactic intrapartum antibiotics, the higher incidence of Group B Streptococcus in the control group was a source of bias for the trial. No other biases were reported for Singla 2010 and Tranquilli 2005. There was insufficient information to assess other sources of bias in Gonzalez 2001 and Nageotte 1985.
Effects of interventions
See: Table 1
1. Transcervical amnioinfusion
Transcervical amnioinfusion improved fetal umbilical artery pH at delivery (mean difference (MD) 0.11; 95% confidence interval (CI) 0.08 to 0.14; one trial, 61 participants; Analysis 1.6) and reduced persistent variable decelerations during labour (risk ratio (RR) 0.52; 95% CI 0.30 to 0.91; one trial, 86 participants; Analysis 1.1). No significant differences in the rates of caesarean section, low Apgar scores, neonatal death or infectious morbidity were detected.
1.6. Analysis.
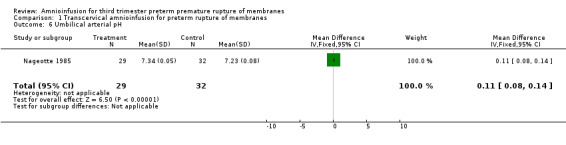
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 6 Umbilical arterial pH.
1.1. Analysis.
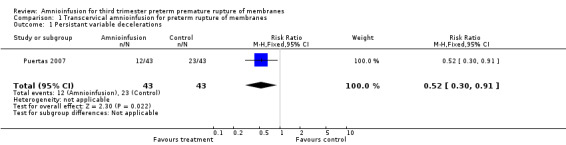
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 1 Persistant variable decelerations.
2. Transabdominal amnioinfusion
Transabdominal amnioinfusion was associated with a reduction in neonatal death (RR 0.30; 95% CI 0.14 to 0.66; two trials, 94 women; Analysis 2.7), neonatal infection/sepsis (RR 0.26; 95% CI 0.11 to 0.61; one trial, 60 participants; Analysis 2.3), and pulmonary hypoplasia (RR 0.22; 95% CI 0.06 to 0.88; one trial, 34 participants; Analysis 2.3). Women in the amnioinfusion group were also less likely to deliver within seven days of membrane rupture (RR 0.18; 95% CI 0.05 to 0.70; one trial, 34 participants; Analysis 2.4) and were less likely to experience puerperal sepsis (RR 0.20; 95% CI 0.05 to 0.84; one trial, 60 participants; Analysis 2.9). There were no significant differences between groups regarding birthweight, gestational age at delivery, admission to neonatal intensive care and neurological sequelae. Fetal distress was diagnosed less frequently in the amnioinfusion group than the control group (RR 0.27; 95% CI 0.08 to 0.88; one trial, 60 participants; Analysis 2.1), but in the absence of blinding this outcome is at risk of bias.
2.7. Analysis.
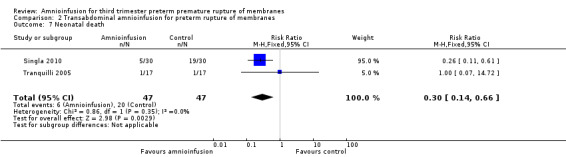
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 7 Neonatal death.
2.3. Analysis.
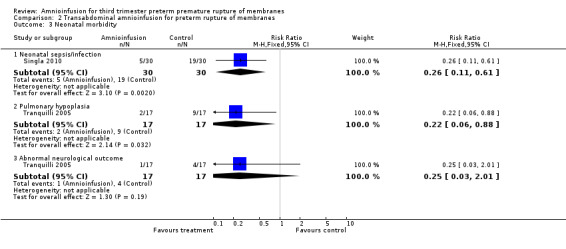
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 3 Neonatal morbidity.
2.4. Analysis.
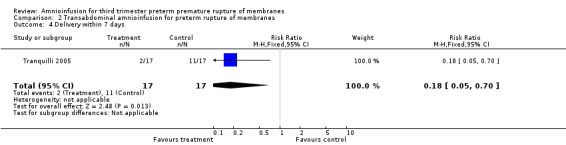
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 4 Delivery within 7 days.
2.9. Analysis.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 9 Maternal puerperal sepsis.
2.1. Analysis.
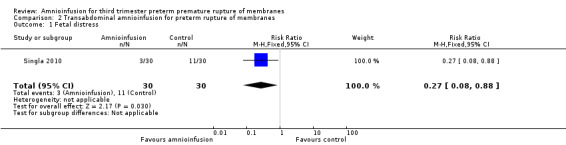
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 1 Fetal distress.
Discussion
Summary of main results
Transabdominal amnioinfusion following preterm premature rupture of the membranes (PPROM) resulted in better neonatal outcomes (decreased sepsis, infection and death) and decreased puerperal sepsis than conventional management. The data contributing to these outcomes were mainly from one trial (Singla 2010). Transcervical amnioinfusion was associated with improved fetal heart rate patterns and umbilical cord blood pH results, but improvements in substantive clinical outcomes were not statistically significant.
Overall completeness and applicability of evidence
These results are not conclusive due to the small numbers of trials and participants. In addition, the numbers are too small to detect rare potential adverse events. The lower threshold of gestational age at which amnioinfusion may be of benefit is not known.
Quality of the evidence
The evidence is of mainly moderate quality (Table 1) and limitations can largely be attributed to the small size of studies and the lack of blinding to group allocation.
Agreements and disagreements with other studies or reviews
This evidence supports the positive findings of the non‐randomised studies conducted to date.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to guide clinical practice concerning the use of amnioinfusion for preterm premature rupture of the membranes.
Implications for research.
Larger trials are needed to confirm and extend the findings of the trials reviewed here. Long‐term follow‐up of babies is desirable, especially with regard to neurological sequelae and development. Future trials and reviews may need to be stratified according to gestational age to determine whether there is a threshold for benefits and risks.
What's new
| Date | Event | Description |
|---|---|---|
| 13 January 2014 | New citation required but conclusions have not changed | Two studies previously in ongoing have been excluded (Roberts: AMIPROM; Vergani 2007). |
| 2 December 2013 | New search has been performed | Search updated. No new trials identified. Title changed from "Amnioinfusion for preterm premature rupture of membranes" to "Amnioinfusion for third trimester preterm premature rupture of membranes". See Differences between protocol and review for more details. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 3, 2016
| Date | Event | Description |
|---|---|---|
| 29 March 2011 | New citation required but conclusions have not changed | New authors helped to prepare this update. |
| 5 January 2011 | New search has been performed | Search updated, one new trial identified (Singla 2010) and included. We have also included three trials previously awaiting classification (Gonzalez 2001; Puertas 2007; Tranquilli 2005). We have also assessed the remaining trial reports from the previous search (previously in 'awaiting classification'), resulting in four excluded studies (De Santis 2003; Gowri 2004; Locatelli 2000; Mino 1999) and two ongoing studies (Roberts: AMIPROMa; Vergani 2007a). |
| 2 July 2010 | Amended | Contact details edited. |
| 3 August 2009 | Amended | Search updated. Nine reports added to Studies awaiting classification (De Santis 2003; Gonzalez 2001; Gowri 2004; Mino 1997a; Mino 1999; Roberts 2006; Puertas 2007; Tranquilli 2005; Vergani 2007a). |
| 31 October 2008 | Amended | Converted to new review format. |
Acknowledgements
Sara Roden‐Scott, Julieta Mattera and Nadia Bondarczuk for providing a translation for the Spanish trial report of Mino 1999.
Appendices
Appendix 1. Methods to be used in the next update
Data collection and analysis
Selection of studies
Two review authors will independently assess for inclusion all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult a third author.
Data extraction and management
We will design a form to extract data. For eligible studies, at least two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult a third person. We will enter data into Review Manager software (RevMan 2012) and check for accuracy.
When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and will assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see 'Sensitivity analysis'.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We will not consider cluster‐randomised trials for inclusion.
Cross‐over trials
We will not consider cross‐over trials for inclusion.
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and all participants will be analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either the Tau² is greater than zero, or there is a low P value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager software (RevMan 2012). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
Effects of transabdominal versus transcervical amnioinfusion on complications of preterm premature rupture of the fetal membranes.
Effects of transabdominal versus transcervical amnioinfusion at the various gestational ages in the third trimester.
The following outcomes will be used in subgroup analysis: persistent variable decelerations, Apgar scores, cord arterial pH at birth, neonatal morbidity including infection, lung hypoplasia, abnormal neurological outcomes and postural deformities, as well as perinatal mortality.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2012). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
Sensitivity analyses will be performed for aspects of the review that might affect the results, for example, where there is risk of bias associated with the quality of some of the included trials. Sensitivity analysis will be carried out to explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity and the effects of any assumptions made.
Data and analyses
Comparison 1. Transcervical amnioinfusion for preterm rupture of membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistant variable decelerations | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.91] |
| 2 Severe variable decelerations per hour in first stage | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐1.83, ‐0.57] |
| 3 Caesarean section | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Caesarean section overall | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.25, 1.73] |
| 3.2 Caesarean section for fetal distress | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.12, 1.55] |
| 4 Forceps/vacuum assisted delivery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Overall | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.58, 2.48] |
| 4.2 For fetal distress | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.00] |
| 5 1 minute Apgar score < 4 | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.33] |
| 6 Umbilical arterial pH | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.08, 0.14] |
| 7 Umbilical pH ≤ 7.20 | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.11] |
| 8 Neonatal morbidity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Overall | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.19, 1.34] |
| 9 Neonatal death | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.05, 5.77] |
| 10 Maternal puerperal sepsis | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.06, 2.18] |
1.2. Analysis.
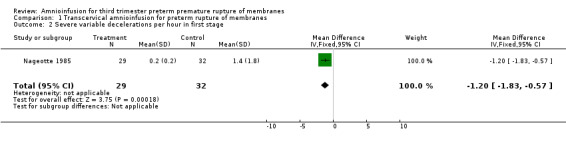
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 2 Severe variable decelerations per hour in first stage.
1.3. Analysis.
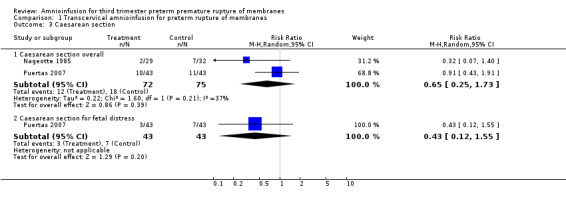
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 3 Caesarean section.
1.4. Analysis.
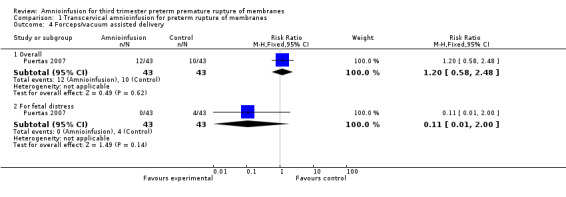
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 4 Forceps/vacuum assisted delivery.
1.5. Analysis.
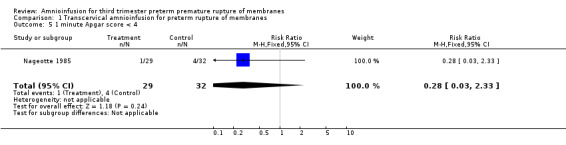
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 5 1 minute Apgar score < 4.
1.7. Analysis.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 7 Umbilical pH ≤ 7.20.
1.8. Analysis.

Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 8 Neonatal morbidity.
1.9. Analysis.
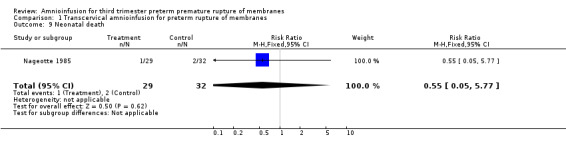
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 9 Neonatal death.
1.10. Analysis.
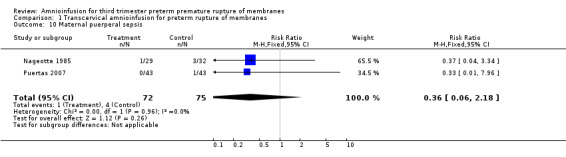
Comparison 1 Transcervical amnioinfusion for preterm rupture of membranes, Outcome 10 Maternal puerperal sepsis.
Comparison 2. Transabdominal amnioinfusion for preterm rupture of membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fetal distress | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.08, 0.88] |
| 2 Gestational age at delivery (weeks) | 2 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐2.63, 1.65] |
| 3 Neonatal morbidity | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Neonatal sepsis/infection | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.61] |
| 3.2 Pulmonary hypoplasia | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.88] |
| 3.3 Abnormal neurological outcome | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.01] |
| 4 Delivery within 7 days | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.70] |
| 5 Time to delivery (days) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐2.86, 4.00] |
| 6 Admission to neonatal intensive care unit | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.90, 1.12] |
| 7 Neonatal death | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.14, 0.66] |
| 8 Birthweight (grams) | 2 | 94 | Mean Difference (IV, Random, 95% CI) | 15.65 [‐254.02, 285.32] |
| 9 Maternal puerperal sepsis | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.84] |
2.2. Analysis.
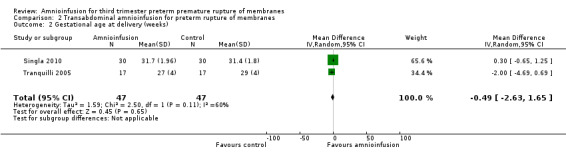
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 2 Gestational age at delivery (weeks).
2.5. Analysis.
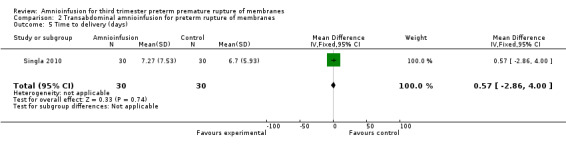
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 5 Time to delivery (days).
2.6. Analysis.

Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 6 Admission to neonatal intensive care unit.
2.8. Analysis.
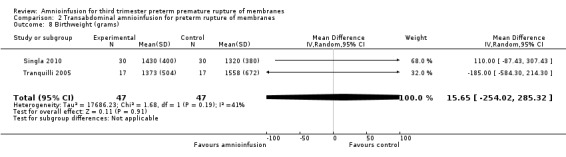
Comparison 2 Transabdominal amnioinfusion for preterm rupture of membranes, Outcome 8 Birthweight (grams).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gonzalez 2001.
| Methods | Randomised trial. Type of randomisation not specified. Abstract only. | |
| Participants | 44 pregnant women with gestational ages from 189‐258 days (24 in amnioinfusion group and 20 controls). | |
| Interventions | Amnioinfusion versus no amnioinfusion. Inclusion and exclusion criteria not specified. | |
| Outcomes | Fetal heart rate variability, mode of delivery, cord arterial pH and perinatal morbidity. | |
| Notes | This is an abstract only; we are not aware of any published final results. The abstract results are presented as percentages only without denominators and so could not be used in this review. Amnioinfusion reduced the percentage of caesarean sections done for fetal distress compared with the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only, insufficient detail provided for assessment. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only, insufficient detail provided for assessment. |
| Other bias | Unclear risk | Abstract only, insufficient detail provided for assessment. |
Nageotte 1985.
| Methods | 'Assigned in a random fashion.' | |
| Participants | Inclusion criteria: spontaneous premature rupture of membranes; gestational age 26 to 35 weeks; decreased or absent amniotic fluid; vertex presentation; no gross fetal anomalies. Exclusion criteria: premature labour on admission; vaginal bleeding; previous caesarean section and no desire for trial of labour; antepartum fetal distress; consent refused. 1 woman with twin pregnancy and 1 with previous caesarean section were allocated to the amnioinfusion group. Of 66 women allocated, 3 were excluded for a change from the vertex presentation at the time of labour, and 2 for antepartum fetal distress. Of the remainder, 29 were allocated to amnioinfusion and 32 to the control group. |
|
| Interventions | Labour commenced spontaneously, or induced for confirmed fetal pulmonary maturity or infection. All women had fetal scalp electrodes and uterine pressure catheters placed as early in labour as possible. Amnioinfusion with warmed saline at 10 mL per minute for 1 hour (repeated if a large volume of fluid was lost), then 3 mL per minute (total volume infused mean 1160, range 735‐1650 mL); compared with no amnioinfusion. Caesarean section was performed for persistent late decelerations; recurrent severe variable decelerations; recurrent or uncorrectable prolonged decelerations (no mention of fetal scalp blood sampling). |
|
| Outcomes | Amnionitis (2 of the following: fever 38 degrees celsius or more; leucocytosis > 15,000 per microlitre; foul‐smelling amniotic fluid; uterine tenderness); intrapartum fetal heart rate tracings, evaluated in a blinded manner; endometritis (postpartum fever > 38 degrees celsius twice 4 hours apart, and uterine tenderness or foul‐smelling lochia); Apgar scores; umbilical cord arterial and venous pH; neonatal morbidity and mortality. | |
| Notes | Long Beach, California, USA. March 1984 to March 1985. Of 66 women allocated, 5 (7.6%) were excluded, 3 for a change from the vertex presentation at the time of labour, and 2 for antepartum fetal distress. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Fetal heart rate tracings evaluated in a blinded manner. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 post‐randomisation exclusions: 3 for non‐vertex presentation and 2 for fetal distress. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Unclear risk | Unclear. |
Puertas 2007.
| Methods | Random allocation to 2 groups using a random number table and opaque sealed envelopes. | |
| Participants | Inclusion criteria: women with preterm premature rupture of membranes between 27 and 35 weeks of gestation; included if labour had begun spontaneously or after induction. Exclusion criteria: women with multiple gestation, presentation other than cephalic, cervical dilatation > 5 cm, cardiotocography signs compatible with non‐reassuring fetal status, meconium‐stained amniotic fluid, umbilical cord prolapse, uterine scarring, placenta praevia, premature detachment of the placenta, any vaginal bleeding of unknown cause, presence of oligohydramnios (amniotic fluid index < 5) prior to premature rupture of the membranes, maternal infection that could be transmitted to the fetus (human immunodeficiency virus and herpes simplex virus), fetal anomalies incompatible with life, or any known obstetric or maternal complication other than premature rupture of membranes. | |
| Interventions | Intrapartum transcervical amnioinfusion versus a control group that had an intrauterine pressure catheter inserted but without amnioinfusion. Physiological saline at 37 degree celsius, at a rate of 600 mL/hr during the first hour. After 1 hour, amniotic fluid index was determined, and if amniotic fluid index was greater than 15, amnioinfusion was stopped. In all other women in the study group amnioinfusion continued at a rate of 180 mL/hr until the cervix was completely dilated. Fetal heart rate and uterine activity were recorded continuously throughout labour. All women who were group B streptococcus carriers received prophylactic intrapartum antibiotics. | |
| Outcomes | Mode of delivery, PH and arterial blood concentrations, neonatal morbidity, puerperal morbidity. | |
| Notes | More group B streptococcal carriers in control group (14 women versus 7 women). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Partial blinding. Changes in fetal heart rate analysed with the Cabaniss classification by an independent investigator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data complete. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Unclear risk | More group B streptococcal carriers in control group (14 women versus 7 women). |
Singla 2010.
| Methods | Randomised controlled trial conducted in Delhi, India between August 2005 and 2007. | |
| Participants | 60 women with singleton pregnancies (30 in each group) between 26 and 33 + 6 weeks' gestation with amniotic fluid index less than the 5th percentile. Excluded if evidence of chorioamnionitis, placental or fetal anomalies or active labour. Diagnosis of preterm rupture of membranes was made via the litmus paper test and confirmed using ultrasound. | |
| Interventions | Transcervical amnioinfusion with normal saline repeated weekly if amniotic fluid index < 5 versus no amnioinfusion. All women received bed rest, antibiotics and steroids. | |
| Outcomes | Preterm rupture of membranes to delivery interval; neonatal outcome including birthweight, intrapartum fetal distress defined as persistent tachycardia and recurrent, late or severe decelerations; early neonatal sepsis; neonatal mortality; causes of neonatal mortality; mode of delivery; postpartum sepsis. | |
| Notes | No loss to follow‐up. Baseline characteristics were similar between groups. 5 women received amnioinfusion twice, 1 woman received it 3 times. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described: "To exclude observer bias, randomisation was performed ....using a computer generated random number table..." There was no mention of allocation concealment (e.g. sealed opaque envelopes). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No selective reporting. |
| Other bias | Low risk | None other bias was noted. |
Tranquilli 2005.
| Methods | Randomised controlled trial using opaque sealed envelopes and a computer random‐number generator. 44 women met inclusion criteria, 4 refused to participate and 6 delivered before randomisation, leaving 34 women to be randomised. | |
| Participants | Pregnant women included if: singleton pregnancies complicated by preterm premature rupture of membranes, gestational age between 24 and 33 weeks, evidence of preterm premature rupture of membrane within 24 hours of admission, oligohydramnios, absence of uterine contractions at the time of hospitalisation, no placental anomalies or major structural fetal anomalies and normal cardiotocography at the time of admission. | |
| Interventions | 17 women were allocated to amnioinfusion and 17 to no amnioinfusion. All women received bedrest and antibiotic prophylaxis and corticosteroid therapy. Prophylactic tocolytic treatment was administered if there were no clinical signs of chorioamnionitis or placental abruption. Women in the treatment group received weekly serial amnioinfusion if the amniotic fluid index fell below the 5th centile and/or a median pocket of amniotic fluid was < 2 cm. The mean volume infused was 250 mL N/saline (range 120‐350 mL), the aim was to restore the amniotic fluid index to > 10th percentile. The amniotic fluid index was measured after the procedure and it was repeated after 24 hours and at least once weekly. If amniotic fluid index was ≤ 5, the amnioinfusion was repeated weekly until 27 weeks of gestation. A non‐stress test was performed daily. Caesarean section was done for chorioamnionitis and fetal distress (defined as persistent tachycardia with reduced variability, recurrent late or severe variable decelerations). | |
| Outcomes | Birthweight; admission to neonatal intensive care unit; gestational age at delivery (weeks); pulmonary hypoplasia diagnosed on the basis of strict clinical and radiological criteria at 1 and 3 months after delivery; abnormal neurological outcomes. The preterm premature rupture of membrane‐delivery interval was also determined. | |
| Notes | Study conducted at the Salesi Mothers' and Children's Hospital, Ancona, Italy, from January‐December 2002. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were complete. |
| Selective reporting (reporting bias) | Unclear risk | Authors do not report mode of delivery, Apgar scores or cord arterial pH. |
| Other bias | Low risk | No other bias noted. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| De Santis 2003 | The quality of the randomisation process was poor. Women were by chance admitted into 1 of the 2 divisions of the department of obstetrics and gynaecology. Among the 71 women who were included in the study, 37 underwent serial amnioinfusion and 34 were used as controls. Also included in the study were women who underwent preterm rupture of membrane after amniocentesis for prenatal diagnosis. 10 patients (27.0%) in the amnioinfusion group and 8 patients (23.5%) in the control group. The study is also limited by the long period in which it was performed due to the low prevalence of this disease. The control group was selected without proper randomisation therefore bias could not be excluded during the selection process. |
| Gowri 2004 | Randomised controlled trial of amnioinfusion versus no amnioinfusion for oligohydramnios. Small numbers (9 controls versus 8 study participants), only 3 study participants who received amnioinfusion had premature rupture of membranes and these outcome data were not extractable from totals. |
| Locatelli 2000 | The study was not a randomised controlled trial. All singleton pregnancies with preterm premature rupture of membranes at < 26 weeks' gestation and lasting > 4 days between January 1991 and June 1998 were included. Consenting women with persistent (> 4 days) oligohydramnios (maximum cord‐free pocket of amniotic fluid volume less or equal to 2 cm) received serial transabdominal amnioinfusions to maintain an amniotic pocket > 2 cm. The pregnancy, neonatal, and long‐term neurological outcomes of the cases that spontaneously maintained a median amniotic fluid pocket > 2 cm (amnioinfusion‐not‐necessary group) were compared with those of women with oligohydramnios who underwent amnioinfusion but continued to have a median amniotic fluid pocket after preterm premature rupture of membranes (amniotic fluid pocket less or equal to 2 cm/persistent oligohydramnios group) and with those of women in whom oligohydramnios was alleviated by amnioinfusion for at least 48 hours (successful amnioinfusion group). The study also incorporated 18 cases that had been previously reported in Vergani 1997. |
| Mino 1999 | Study population were women with ruptured membranes at term, not preterm. |
| Roberts: AMIPROM | Excluded because even though this is a randomised controlled trial involving preterm premature rupture of membrane and amnioinfusion, the gestational ages when the preterm premature rupture of membrane occurred were in the 2nd trimester of pregnancy. There are no reported data for 3rd trimester outcomes. This was a prospective non‐blinded randomised controlled trial with randomisation stratified for pregnancies where the membranes ruptured between 16 + 0 and 19 + 6 weeks' gestation and 20 + 0 and 23 + 6 weeks' gestation to minimise the risk of random imbalance in gestational age distribution between randomised groups. This study was carried out in 4 UK hospital‐based Fetal Medicine Units (Liverpool Women’s NHS Trust, St. Mary’s Hospital Manchester, Birmingham Women’s NHS Foundation Trust, Wirral University Hospitals Trust). Participants were randomly allocated to either serial weekly trans‐abdominal amnioinfusions if the deepest pool of amniotic fluid was < 2 cm or expectant management until 37 weeks of pregnancy. Short‐term maternal, pregnancy and neonatal and long‐term outcomes for the child were studied. Long‐term respiratory morbidity was assessed using validated respiratory questionnaires at 6, 12 and 18 months of age and infant lung function test at around 12 months of age. Neurodevelopment was assessed using Bayley’s Scale of Infant Development II at corrected age of 2. |
| Vergani 2007 | Excluded because even though this is an randomised controlled trial involving preterm premature rupture of membrane and amnioinfusion, the gestational ages to be considered are preterm premature rupture of membranes that occurred in the 2nd trimester of pregnancy (< 24 weeks). The study will consider singleton pregnancies, early spontaneous preterm premature rupture of membrane < 24.3 weeks, oligohydramnios (deepest vertical pocket < 2 cm) for at least 4 days and no longer than 15 days at enrolment. The study is open and will be run through a dedicated password protected website, and with a minimal number of outcome measures. Primary outcomes include survival until discharge from the neonatal intensive care unit, while secondary outcomes include latency time from preterm premature rupture of membrane to delivery, gestational age at birth, indication for delivery, number of days of ventilatory support, serious neurologic morbidity, neonatal sepsis prevalence, need for oxygen at 36 weeks post‐conception. Statistics: 38 patients in each arm are necessary to show an increase in neonatal survival from 10% to 40%, with a power of 0.80 and alpha = 0.05. An interim analysis after recruitment of 75% of the study population will be held in order to re‐calculate sample size according to the difference between the groups in latency time as the main secondary outcome. |
Differences between protocol and review
In order to eliminate overlap between this review and a separate review (Van Teeffelen 2013), the title of this review has been changed from "Amnioinfusion for preterm premature rupture of membranes" to "Amnioinfusion for third trimester preterm premature rupture of membranes".
Contributions of authors
GJ Hofmeyr prepared the review and maintains it. For the 2011 update, G Essilfie‐Appiah assisted with the selection of studies and data extraction. T Lawrie assisted with study selection, data extraction and the text of the updated review. For the 2014 update, AC Eke assisted with the selection of studies, data extraction and the text of the updated review.
Sources of support
Internal sources
University of the Witwatersrand, South Africa.
External sources
South African Medical research Council, South Africa.
UNDP/UNFPA/WHO/World Bank (HRP), Switzerland.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Gonzalez 2001 {published data only}
- Gonzalez R, Malde J, Carrillo MP, Sancho‐Minano J, Garrote A, Munoz A, et al. The use of amnioinfusion in preterm deliveries. Preliminary results [abstract]. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):629. [Google Scholar]
Nageotte 1985 {published data only}
- Nageotte MP, Freeman RK, Garite TJ, Dorchester W. Prophylactic intrapartum amnioinfusion in patients with preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 1985;153:557‐62. [DOI] [PubMed] [Google Scholar]
Puertas 2007 {published data only}
- Puertas A, Tirado P, Perez I, Lopez MS, Montoya F, Canizares JM, et al. Transcervical intrapartum amnioinfusion for preterm premature rupture of the membranes. European Journal of Obstetrics & Gynecology and Reproductive Biology 2007;131(1):40‐4. [DOI] [PubMed] [Google Scholar]
Singla 2010 {published data only}
- Singla A, Yadav P, Vaid NB, Suneja A, Faridi MMA. Transabdominal amnioinfusion in preterm premature rupture of membranes. International Journal of Gynecology & Obstetrics 2010;108:199‐202. [DOI] [PubMed] [Google Scholar]
Tranquilli 2005 {published data only}
- Tranquilli AL, Giannubilo SR, Bezzeccheri V, Scagnoli C. Transabdominal amnioinfusion in preterm premature rupture of membranes: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112(6):759‐63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
De Santis 2003 {published data only}
- Santis M, Scavo M, Noia G, Masini L, Piersigilli F, Romagnoli C, et al. Transabdominal amnioinfusion treatment of severe oligohydramnios in preterm premature rupture of membranes at less than 26 gestational weeks. Fetal Diagnosis and Therapy 2003;18(6):412‐7. [DOI] [PubMed] [Google Scholar]
Gowri 2004 {published data only}
- Gowri R, Soundaraghavan S. Evaluation of transabdominal amnioinfusion in the antepartum management of oligohydramnios complicating preterm pregnancies. Journal of Obstetrics and Gynaecology of India 2004;54(5):460‐3. [Google Scholar]
Locatelli 2000 {published data only}
- Locatelli A, Vergani P, Pirro G, Doria V, Biffi A, Ghidini A. Role of amnioinfusion in the management of premature rupture of membranes at < 26 weeks gestation. American Journal of Obstetrics and Gynecology 2000;183:878‐82. [DOI] [PubMed] [Google Scholar]
Mino 1999 {published data only}
- Mino M, Puertas A, Carrillo M, Santiago J, Herruzo A, Miranda J. Influence of amnioinfusion on variable or prolonged decelarations: a randomised study. Acta Obstetricia et Gynecologica Scandinavica 1997;76:95. [Google Scholar]
- Mino M, Puertas A, Herruzo AJ, Miranda JA. Amnioinfusion in labor induction of term pregnancies with premature rupture of the membranes and low amniotic fluid. International Journal of Gynecology & Obstetrics 1998;61:135‐40. [DOI] [PubMed] [Google Scholar]
- Mino M, Puertas A, Miranda JA, Herruzo AJ. Amnioinfusion in term labor with low amniotic fluid due to rupture of membranes: a new indication. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;82:29‐34. [DOI] [PubMed] [Google Scholar]
- Mino M, Puertas A, Mozas J, Carrillo Badillo MP, Rodríguez Oliver A, Miranda JA. A modification of the amniotic fluid index after amnioinfusion for infants with early rupture of membranes [Modificacion del indice de liquido amniotico tras amnioinfusion en gestantes con rotura prematura de membranas a termino]. Acta Ginecologica 1997;51(1):11‐4. [Google Scholar]
- Puertas A, Mino M, Carrillo M, Mozas J, Herruzo A, Miranda J. Influence of amnioinfusion on neonatal acid‐base state: a randomized study. Acta Obstetricia et Gynecologica Scandinavica Supplement 1997;76:94. [Google Scholar]
Roberts: AMIPROM {unpublished data only}
- Roberts D, Alfirevic Z, Bricker L, Beardsmore C, Paturi P, Taylor S, et al. Amnioinfusion in preterm premature rupture of membranes (AMIPROM study). Current Controlled Trials (www.controlled‐trials.com/) (accessed 17 December 2013).
- Roberts D, Beardsmore C, Shaw B, Martin W, Vause S, Bricker L, et al. AMIPROM: a pilot RCT on serial transabdominal amnioinfusion versus expectant management in very early PROM. Ultrasound in Obstetrics & Gynecology 2012;40(Suppl 1):80. [Google Scholar]
- Roberts D, Vause S, Martin W, Green P, Walkinshaw S, Bricker L, et al. Amnioinfusion in very early preterm premature rupture of membranes ‐ pregnancy, neonatal and maternal outcomes in the AMIPROM randomised controlled pilot study. Ultrasound in Obstetrics & Gynecology 2013 Nov 21 [Epub ahead of print]. [DOI] [PubMed]
- Roberts D, Vause S, Martin W, Green P, Walkinshaw S, Bricker L, et al. Pregnancy outcomes in AMIPROM: a pilot RCT on serial transabdominal amnioinfusion versus expectant management in very early preterm prelabour rupture of membranes. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):10. [Google Scholar]
Vergani 2007 {published data only}
- Vergani P, Deprest J, Strobelt N, Locatelli A. Proposal for open randomized trial comparing perinatal outcome following expectant management versus amnioinfusion in PPROM < 25 weeks with persistent oligohydramnios (amnioinfusion initiative). Ultrasound in Obstetrics and Gynecology 2007;30:541. [Google Scholar]
Additional references
Gabbe 1976
- Gabbe SG, Ettinger BB, Freeman RK, Martin CB. Umbilical cord compression associated with amniotomy: laboratory observations. American Journal of Obstetrics and Gynecology 1976;126(3):353‐5. [DOI] [PubMed] [Google Scholar]
Goodlin 1981
- Goodlin RC. Intra‐amniotic antibiotic infusions. American Journal of Obstetrics and Gynecology 1981;139(8):975. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2012
- Hofmeyr GJ, Lawrie TA. Amnioinfusion for potential or suspected umbilical cord compression in labour. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD000013.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2014
- Hofmeyr GJ, Xu H, Eke AC. Amnioinfusion for meconium‐stained liquor in labour. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000014.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2009
- Hsu TY, Hsu JJ, Fu HC, Ou CY, Tsai CC, Cheng BH, et al. The changes in Doppler indices of fetal ductus venosus and umbilical artery after amnioinfusion for women with preterm premature rupture of membranes before 26 weeks' gestation. Taiwan Journal of Obstetrics and Gynecology 2009;48(3):268‐72. [DOI] [PubMed] [Google Scholar]
Keirse 1989
- Keirse MJNC, Ohlsson A, Treffers PE, Kanhai HHH. Prelabour rupture of the membranes preterm. In: Chalmers I, Enkin MW, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:666‐93. [Google Scholar]
Locatelli 2006
- Locatelli A, Ghidini A, Verderio M, Andreani M, Strobelt N, Pezzullo J, et al. Predictors of perinatal survival in a cohort of pregnancies with severe oligohydramnios due to premature rupture of membranes at < 26 weeks managed with serial infusions. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;128(1‐2):97‐102. [DOI] [PubMed] [Google Scholar]
Miyazaki 1983
- Miyazaki FS, Taylor NA. Saline amnioinfusion for relief of prolonged variable decelerations. American Journal of Obstetrics and Gynecology 1983;146:670‐8. [DOI] [PubMed] [Google Scholar]
Monahan 1995
- Monahan E, Katz VL, Cox RL. Amnioinfusion for preventing puerperal infection. A prospective study. Journal of Reproductive Medicine 1995;40(10):721‐3. [PubMed] [Google Scholar]
Novikova 2012
- Novikova N, Hofmeyr GJ, Essilfie‐Appiah G. Prophylactic versus therapeutic amnioinfusion for oligohydramnios in labour. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000176.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogita 1988
- Ogita S, Imanaka M, Matsumoto M, Oka T, Sugawa T. Transcervical amnioinfusion of antibiotics: a basic study for managing premature rupture of membranes. American Journal of Obstetrics and Gynecology 1988;158(1):23‐7. [DOI] [PubMed] [Google Scholar]
Ogunyemi 2002
- Ogunyemi D, Thompson W. A case controlled study of serial transabdominal amnioinfusions in the management of second trimester oligohydramnios due to premature rupture of membranes. European Journal of Obstetrics & Gynecology and Reproductive Biology 2002;102(2):167‐72. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Tchirikov 2010
- Tchirikov M, Steetskamp J, Hohmann M, Koelbl H. Long‐term amnioinfusion through a subcutaneously implanted amniotic fluid replacement port system for treatment of PPROM in humans. European Journal of Obstetrics & Gynecology and Reproductive Biology 2010;152:30‐3. [DOI] [PubMed] [Google Scholar]
Turhan 2002
- Turhan NO, Atacan N. Antepartum prophylactic transabdominal amnioinfusion in preterm pregnancies complicated by oligohydramnios. International Journal of Gynecology & Obstetrics 2002;76(1):15‐21. [DOI] [PubMed] [Google Scholar]
Van Teeffelen 2013
- Teeffelen S, Pajkrt E, Willekes C, Kuijk SMJ, Mol BWJ. Transabdominal amnioinfusion for improving fetal outcomes after oligohydramnios secondary to preterm prelabour rupture of membranes before 26 weeks. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD009952.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vergani 1994
- Vergani P, Ghidini A, Locatelli A, Cavallone M, Ciarla I, Cappellini A, et al. Risk factors of pulmonary hypoplasia in second trimester premature rupture of membranes. American Journal of Obstetrics and Gynecology 1994;170:1359‐64. [DOI] [PubMed] [Google Scholar]
Vergani 1997
- Vergani P, Locatelli A, Strobelt N, Mariani S, Cavallone M, Arosio P, et al. Amnioinfusion for prevention of pulmonary hypoplasia in second‐trimester rupture of membranes. American Journal of Perinatology 1997;14(6):325‐9. [DOI] [PubMed] [Google Scholar]
Vintzileos 1985
- Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Degree of oligohydramnios and pregnancy outcome in patients with premature rupture of membranes. Obstetrics & Gynecology 1985;66(2):162‐7. [PubMed] [Google Scholar]
References to other published versions of this review
Hofmeyr 1995
- Hofmeyr GJ. Amnioinfusion for preterm prelabour rupture of membranes [revised 22 April 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Database of Systematic Reviews [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Hofmeyr 1998b
- Hofmeyr GJ. Amnioinfusion for preterm rupture of membranes. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000942] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2011
- Hofmeyr GJ, Essilfie‐Appiah G, Lawrie TA. Amnioinfusion for preterm premature rupture of membranes. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000942.pub2] [DOI] [PubMed] [Google Scholar]


