Abstract
Background
Intraventricular haemorrhage (IVH) is a major complication of preterm birth. Large haemorrhages are associated with a high risk of disability and hydrocephalus. Instability of blood pressure and cerebral blood flow are postulated as causative factors. Another mechanism may involve reperfusion damage from oxygen free radicals. Phenobarbital has been suggested as a safe treatment that stabilises blood pressure and may protect against free radicals.
Objectives
To determine the effect of postnatal administration of phenobarbital on the risk of IVH, neurodevelopmental impairment or death in preterm infants.
Search methods
We used the search strategy of the Neonatal Collaborative Review Group. The original review author (A Whitelaw) was an active trialist in this area and had personal contact with many groups in this field. He handsearched journals from 1976 (when cranial computed tomography (CT) scanning started) to October 2000; these included: Pediatrics, Journal of Pediatrics, Archives of Disease in Childhood, Pediatric Research, Developmental Medicine and Child Neurology, Acta Paediatrica, European Journal of Pediatrics, Neuropediatrics, New England Journal of Medicine, Lancet and British Medical Journal. We searched the National Library of Medicine (USA) database (via PubMed) and the Cochrane Central Register of Controlled Trials (CENTRAL, 2012, Issue 10) through to 31 October 2012. We did not limit the searches to the English language, as long as the article included an English abstract. We read identified articles in the original language or translated.
Selection criteria
We included randomised or quasi‐randomised controlled trials in which phenobarbital was given to preterm infants identified as being at risk of IVH because of gestational age below 34 weeks, birthweight below 1500 g or respiratory failure. Adequate determination of IVH by ultrasound or CT was also required.
Data collection and analysis
In addition to details of patient selection and control of bias, we extracted the details of the administration of phenobarbital. We searched for the following endpoints: IVH (with grading), posthaemorrhagic ventricular dilation or hydrocephalus, neurodevelopmental impairment and death. In addition, we searched for possible adverse effects of phenobarbitone, for example hypotension, mechanical ventilation, pneumothorax, hypercapnia and acidosis.
Main results
We included 12 controlled trials that recruited 982 infants. There was heterogeneity between trials for the outcome IVH, with three trials finding a significant decrease in IVH and one trial finding an increase in IVH in the group receiving phenobarbital. Meta‐analysis showed no difference between the phenobarbital‐treated group and the control group in either all IVH (typical risk ratio (RR) 0.91; 95% CI 0.77 to 1.08), severe IVH (typical RR 0.77; 95% CI 0.58 to 1.04), posthaemorrhagic ventricular dilation (typical RR 0.89; 95% CI 0.38 to 2.08), severe neurodevelopmental impairment (typical RR 1.44; 95% CI 0.41 to 5.04) or death before hospital discharge (typical RR 0.88; 95% CI 0.64 to 1.21). There was a consistent trend in the trials towards increased use of mechanical ventilation in the phenobarbital‐treated group, which was supported by the meta‐analysis (typical RR 1.18; 95% CI 1.06 to 1.32; typical risk difference 0.129; 95% CI 0.04 to 0.21), but there was no significant difference in pneumothorax, acidosis or hypercapnia.
Authors' conclusions
Postnatal administration of phenobarbital cannot be recommended as prophylaxis to prevent IVH in preterm infants and is associated with an increased need for mechanical ventilation.
Plain language summary
Postnatal phenobarbital for the prevention of intraventricular haemorrhage in preterm infants
Large bleeds in the centre of the brain can cause disability or death in preterm babies. Unstable blood pressure and blood flow to the brain are believed to cause intraventricular haemorrhage (IVH) (bleeding into the fluid‐filled cavities of the brain (ventricles). The drug phenobarbital is believed to stabilise blood pressure and, therefore, potentially help prevent IVH. The review of trials found that there was not enough evidence that postnatal phenobarbital is effective in preventing IVH. Furthermore, phenobarbital suppresses breathing in infants who are breathing spontaneously, causing a need for mechanical ventilation.
Background
Description of the condition
Intraventricular haemorrhage (IVH) is a major complication of preterm birth and large haemorrhages or haemorrhages associated with parenchymal brain lesions have a high rate of disability (Vohr 1989). Massive IVH may result in death from hypovolaemia and large haemorrhages may result in hydrocephalus in infants who survive (Volpe 1995). IVH in preterm infants originates, not from an artery, but from capillaries of the subependymal germinal matrix. The particular vulnerability of premature infants is thought to result from a) a subependymal germinal matrix that is rich in immature vessels poorly supported by connective tissue (Hambleton 1976; Gould 1987), b) marked fluctuations in cerebral blood flow (Perlman 1983), and c) severe respiratory problems that result in major swings in intrathoracic and venous pressure that are then transmitted to the fragile germinal matrix (Nakamura 1990). In addition, there is evidence that ischaemia followed by reperfusion plays a role in the pathogenesis and that cerebral ischaemia may result from IVH. This may take the form of periventricular haemorrhagic infarction (PHI) (Volpe 1995). PHI lesions are typically unilateral and in continuity with the margin of the lateral ventricle. The aetiology is thought to be obstruction of venous drainage by a blood clot in the germinal matrix. Interventions aimed at prevention of IVH or its consequences might be targeted at any one (or more) of the above mechanisms.
The non‐invasive diagnosis of IVH during life was first made by cerebral computed tomography (CT) but the need for transport and the ionising radiation made this method unsuitable for studies of whole populations.
Diagnosis of intraventricular haemorrhage by ultrasound
Cranial ultrasound can be carried out at the cot side and exposes the infant to no ionising radiation. This enables whole populations of infants to be safely and ethically examined. Papile's classification of IVH was originally developed for CT (Papile 1978), but was quickly implemented by ultrasonographers. Grade I haemorrhage is confined to the subependymal germinal matrix with no blood clot in the lumen. Grade II haemorrhage is a small haemorrhage within the ventricular lumen without ventricular dilation. Grade III haemorrhage is a large haemorrhage sufficient to expand the ventricle from the amount of blood. Grade IV haemorrhage is IVH plus parenchymal haemorrhagic venous infarction (Volpe 1995). Although ultrasound diagnosis of germinal matrix haemorrhage is not perfect with sensitivity of 61% and specificity 78%, the diagnosis of IVH shows high sensitivity (91%) and specificity (81%), as does diagnosis of parenchymal haemorrhage (sensitivity 82% and specificity 97%) (Hope 1988).
Timing of intraventricular haemorrhage
Approximately 80% of IVH occurs within 72 hours of birth but a considerable proportion of IVH is visible on the first scan within a few hours of birth (Levene 1982). This means that interventions to prevent IVH should ideally start before delivery and should be commenced soon after birth.
Description of the intervention
Phenobarbital is a barbiturate that acts on the gamma aminobutyric acid (GABA)A receptors in the central nervous system. Phenobarbital prolongs and potentiates the action of GABA on GABAA receptors and at higher concentrations activates the receptors directly. It is frequently used in children as an anticonvulsant.
How the intervention might work
Postnatal phenobarbital
The administration of postnatal phenobarbital to prevent IVH in low birthweight infants is based on:
the observation that phenobarbital may dampen fluctuations in systemic blood pressure in premature infants (Wimberley 1982);
evidence that treatment with phenobarbital reduces the incidence of intracranial haemorrhage in newborn beagles made hypertensive with phenylephrine (Goddard 1987);
experimental evidence that barbiturates can partially protect the brain against hypoxic‐ischaemic damage (Steen 1979);
the suggestion that the free radical scavenging capacity of phenobarbital may protect the brain after hypoxia‐ischaemia (Ment 1985).
Drug side effects
Phenobarbital and other barbiturates have pharmacological effects in high doses that could be detrimental to preterm infants. These effects include respiratory depression with consequent respiratory acidosis and need for mechanical ventilation, cardiac depression and hypotension.
Why it is important to do this review
One previous systematic review on this topic (Horbar 1992), including eight trials, concluded that postnatal phenobarbital did not reduce the frequency or severity of IVH in preterm infants. This Cochrane systematic review was undertaken in order to a) include studies after 1988 and b) include outcomes not included in the first review by Horbar 1992. This is an update of the existing review "Postnatal phenobarbital for the prevention of intraventricular haemorrhage" published in The Cochrane Library (Whitelaw 2007).
Objectives
To determine the effect of postnatal administration of phenobarbital on the risk of IVH, neurodevelopmental impairment or death, and whether significant adverse effects are associated with postnatal phenobarbital administration in preterm infants.
Methods
Criteria for considering studies for this review
Types of studies
All controlled trials, whether randomised or quasi‐randomised, in which postnatal phenobarbital was compared with control treatment of preterm infants at risk of IVH.
Types of participants
Newborn infants (less than 24‐hours old) with a gestational age of less than 34 weeks or birthweight less than 1500 g. We included preterm infants with gestational ages 33 to 36 weeks or birthweights up to 1750 g if they were mechanically ventilated. We excluded infants with serious congenital malformations.
Types of interventions
Phenobarbitone (phenobarbital) by intravenous or intramuscular injection starting within 24 hours of birth, with or without maintenance therapy for up to seven days.
Types of outcome measures
Primary outcomes
All grades of IVH.
Severe IVH (i.e. grade III and IV IVH) (Papile 1978).
Secondary outcomes
Ventricular dilation or hydrocephalus.
Hypotension (mean arterial pressure < 30 mm Hg) during the first week.
Pneumothorax or interstitial emphysema during the first week.
Hypercapnia (> 8 kPa or 60 mm Hg) during the first week.
Acidosis (pH < 7.2) during the first week.
Mechanical ventilation (including infants who were ventilated at enrolment).
Mild neurodevelopmental impairment (developmental quotient (DQ) < 80 or motor abnormality on examination).
Severe neurodevelopmental impairment (clinical cerebral palsy or DQ below the range that can be measured).
Death before discharge from hospital.
Death at any time during the study.
Search methods for identification of studies
See the Search Strategy of the Neonatal Collaborative Review Group (neonatal.cochrane.org).
Electronic searches
We searched the National Library of Medicine (USA) database (via PubMed) and the Cochrane Central Register of Controlled Trials (CENTRAL, 2012, Issue 10) through to 31 October 2012 using the MeSH terms of newborn infant, premature infant, intracranial haemorrhage, cerebral ventricles and phenobarbital. We did not limit the searches to the English language, as long as the article included an abstract written in English. We used the search engine Google using the search term 'phenobarbital for intraventricular haemorrhage (IVH)'. We read the identified articles in the original language or translated them.
Searching other resources
The original review author (A. Whitelaw) was an active trialist in this area and had personal contact with many groups in this field. For the original review, he handsearched journals from 1976 (when cranial CT scanning started) to November 1998, which included: Pediatrics, Journal of Pediatrics, Archives of Disease in Childhood, Pediatric Research, Developmental Medicine and Child Neurology, Acta Paediatrica, European Journal of Pediatrics, Neuropediatrics, New England Journal of Medicine, Lancet and British Medical Journal.
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group (CNRG), as documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Review authors independently assessed all the potential studies identified as a result of the search strategy for inclusion.
We excluded trials without a simultaneous control group (e.g. those with historical controls). We reviewed inclusion criteria and therapeutic interventions for each trial to see how they differed between trials. We examined the outcomes in each trial to see how compatible they were between studies. We resolved any disagreement through discussion.
Data extraction and management
Review authors independently performed trial searches, assessments of methodology and extraction of data with comparison and resolution of any differences found at each stage. We entered data into Review Manager 5 software (RevMan 2011) and checked for accuracy. If information regarding any of the above was missing or unclear, we intended to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
We used the standardised review methods of the CNRG to assess the methodological quality of included studies. We assessed each identified trial for methodological quality: a) allocation concealment, b) blinding of the intervention, c) completeness of follow‐up and d) blinding of outcome ascertainment.
In addition, review authors independently assessed study quality and risk of bias using the following criteria documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Sequence generation: was the allocation sequence adequately generated?
Allocation concealment: was allocation adequately concealed?
Blinding of participants, personnel and outcome assessors for each main outcome or class of outcomes: was knowledge of the allocated intervention adequately prevented during the study?
Incomplete outcome data for each main outcome or class of outcomes: were incomplete data adequately addressed?
Selective outcome reporting: are reports of the study free of suggestion of selective outcome reporting?
Other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias? We will give particular attention to baseline imbalance in factors and to the length of follow‐up studies to identify whether any benefits claimed were robust.
We intended to request additional information and clarification of published data from the authors of individual trials. We assessed each trial for risk of bias based on the criteria listed above and marked as: 'low' risk of bias, 'unclear' risk of bias and 'high' risk of bias.
Measures of treatment effect
We analysed the results of the studies using Review Manager 5 software (RevMan 2011). We summarised data in a meta‐analysis if they were sufficiently homogeneous, both clinically and statistically.
Dichotomous data: for dichotomous data, we present results as risk ratios (RRs) with 95% confidence intervals (CIs). If there was a statistically significant reduction, we intended to report risk differences (RDs) and calculate the number needed to treat for additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH), and associated 95% CIs.
Continuous data: for continuous data, we used the mean difference (MD) if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measured the same outcome, but use different methods.
Unit of analysis issues
The unit of randomisation and the unit of analysis was the individual infant.
Dealing with missing data
We intended to contact the authors of all published studies if clarifications were required, or to provide additional information. In the case of missing data, we intended to describe the number of participants with missing data in the 'Results' section and the 'Characteristics of included studies' table. We only presented results for the available participants. We intended to discuss the implications of missing data in the discussion of the review.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity, we explored it by prespecified subgroup analysis and sensitivity analysis. We intended to grade the degree of heterogeneity as: 0% to 30% (might not be important), 31% to 50% (moderate heterogeneity), 51% to 75% (substantial heterogeneity) and 76% to 100% (considerable heterogeneity).
Data synthesis
We conducted our statistical analysis using Review Manager 5 software (RevMan 2011). We used a fixed‐effect Mantel‐Haenszel method meta‐analysis for combining data where trials were examining the same intervention, and the trials population and methods were judged to be similar.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we explored potential sources of clinical heterogeneity through the following a priori subgroup analyses.
Potential subgroups for analysis included: gestational age less than 30 weeks; infants on mechanical ventilation.
Sensitivity analysis
If sufficient data were available, we explored methodological heterogeneity through the use of sensitivity analyses. We planned to perform these through including trials of higher quality, based on the presence of any of the following: adequate sequence generation, allocation concealment and less than 10% loss to follow‐up.
Results
Description of studies
Results of the search
We identified 12 randomised or quasi‐randomised trials having a simultaneous control group, with data on 982 infants (Donn 1981; Morgan 1982; Whitelaw 1983; Bedard 1984; Porter 1985; Anwar 1986; Kuban 1986; Ruth 1988; Mas‐Munoz 1993; Sluncheva 2006; Liang 2009; Zhang 2009). One study with historical controls was not included (Hope 1982). We excluded two further studies as one was not randomised or quasi‐randomised (Chen 2008), and one did not meet the inclusion criteria for birthweight and lacked information on mechanical ventilation (Liu 2010). Sluncheva 2006 compared four groups; control, indomethacin, phenobarbital plus indomethacin, and phenobarbital plus indomethacin plus surfactant. This review used the data comparing infants who received indomethacin plus phenobarbital versus indomethacin alone.
Included studies
Participants
The infants participating were relatively similar, being preterm infants who were at risk of IVH either because of gestational age below 34 weeks, birthweight below 1500 g, respiratory distress syndrome requiring mechanical ventilation or a combination of these factors. Cranial ultrasound was carried out before trial entry in only five trials and infants who already had IVH were thereby excluded. It is very likely that some infants in the trials already had IVH before randomisation (Donn 1981; Anwar 1986; Ruth 1988; Mas‐Munoz 1993; Sluncheva 2006). Despite randomisation, three trials had unbalanced treatment groups at randomisation. Kuban's trial (Kuban 1986) had lower gestational age and birthweight in the phenobarbital group, Sluncheva's trial had greater gestational age and birthweight in the treatment group (Sluncheva 2006), and Porter's trial had lower Apgar score in the control group (Porter 1985). One trial had unequal group sizes (Liang 2009).
Variation in the intervention in included studies
Sluncheva 2006 used no loading dose of phenobarbital (infants were treated with 5 mg/kg for five days). The other 11 trials started treatment by injection of a loading dose, the dose varying between 20 mg/kg (nine trials) and 30 mg/kg (two trials). Seven of the trials divided the loading dose into two separate injections with 30‐minute, four‐hour or 12‐hour intervals. In 10 trials, maintenance therapy with phenobarbital was given for three to seven days. With the exception of Sluncheva 2006, Liang 2009 and Zhang 2009, blood levels of phenobarbital were measured in all the trials, but were not revealed to the clinicians in the two double‐blind trials (Whitelaw 1983; Kuban 1986).
Outcomes in included studies
The main outcome, IVH, was ascertained by ultrasonography in 10 trials and by CT in two trials (Liang 2009; Zhang 2009). IVH was classified in a way that made it possible to grade them as mild (grade I or II according to Papile) or severe (grade III or IV according to Papile). In Whitelaw's original paper (Whitelaw 1983), this type of grading was not used, but the scan reports by ultrasonographers blinded to treatment have been reclassified by Dr Whitelaw (who did have knowledge of treatment by this time).
Ten reports gave some data on mortality. Mortality data from Kuban's trial were not given in the original publication (Kuban 1986), but were subsequently supplied as a personal communication from Dr Kuban to Dr Horbar (Horbar 1992). The age‐limit for ascertainment of mortality was not stated by Morgan 1982 and Liang 2009. Sluncheva 2006 recorded mortality up to 10 days of age. Ruth 1988 provided mortality data up to 27 months of age.
Data on potential adverse effects were provided in many of the reports, for example hypotension in three, hypercapnia in five, acidosis in six and mechanical ventilation in all cases where ventilation was not a mandatory inclusion criterion. The numbers of days during which data were recorded for hypotension, hypercapnia and acidosis varied between the trials from one to seven days. The definition of acidosis varied, being less than 7.2 in three trials, less than 7.15 in two trials and need for sodium bicarbonate therapy in one trial.
See Characteristics of included studies table,
Excluded studies
We excluded one study with historical controls (Hope 1982). We excluded two further studies as one was not randomised or quasi‐randomised (Chen 2008), and one did not meet the inclusion criteria for birthweight and lacking information on mechanical ventilation (Liu 2010).
Risk of bias in included studies
Blinding of randomisation and allocation concealment
It was evident in only two of the trials that allocation concealment was achieved (Whitelaw 1983; Kuban 1986). These two trials used numbered identical vials and were double blind. Among nine other trials stated to be randomised, the method of randomisation was described only by Bedard 1984 (deck of cards), Donn 1981 (lottery) and Ruth 1988 (lottery). It was not clear how allocation concealment was achieved in any of these nine randomised trials. Morgan 1982 used alternate rather than random allocation with no attempt at allocation concealment.
Blinding of the intervention and performance bias
In the open trials by Donn 1981; Morgan 1982; Bedard 1984; Porter 1985; Anwar 1986; Ruth 1988; Mas‐Munoz 1993; Sluncheva 2006; Liang 2009 and Zhang 2009, it is likely that the medical and nursing staff knew the treatment allocation. Thus, there is the possibility that the clinical care given to the two groups could have been biased by the knowledge and beliefs of the clinical staff.
Completeness of follow‐up
In Kuban 1986, 11 out of 291 (3.8%) infants enrolled were withdrawn after randomisation.
In Ruth 1988, 10 out of 111 infants enrolled were excluded because of gestation less than 25 weeks or congenital anomaly.
In Whitelaw 1983, two of 32 (7%) infants were excluded because of congenital anomalies and these two infants were replaced in the randomisation.
None of the other trials reported any infants excluded after enrolment.
Only Ruth 1988reported long‐term follow‐up and achieved 100% ascertainment of survivors at 27 months of age.
Blinding of outcome ascertainment and detection bias
All the trials except those by Anwar 1986; Mas‐Munoz 1993; Sluncheva 2006; Liang 2009; and Zhang 2009, described the main endpoint, ultrasound or CT diagnosis of IVH, as being determined by ultrasonographers and radiologists who had no knowledge of treatment allocation. In Ruth 1988, the neurologist and psychologist assessing neurodevelopment at 27 months were blind to treatment allocation.
Effects of interventions
Prophylactic administration of phenobarbital in preterm infants at risk of developing intraventricular haemorrhage (Comparison 1)
All grades of intraventricular haemorrhage (Outcome 1.1)
There was statistical heterogeneity between the 11 trials reporting all grades of IVH (Chi2 29.07, degrees of freedom (df) = 10). The first trial published reported a reduction in IVH among the babies receiving phenobarbital (RR 0.29; 95% CI 0.11 to 0.77; RD ‐0.33; 95% CI ‐0.55 to ‐0.12) (Donn 1981). Two of the remaining 10 trials also reported a significant reduction in IVH (Liang 2009; Zhang 2009), while Kuban's trial showed a significant increase in IVH among the phenobarbital‐treated group (RR 1.83; 95% CI 1.21 to 2.75; RD 0.16; 95% CI 0.06 to 0.26), although in this trial the group receiving phenobarbital were significantly lighter and had a shorter gestation (Kuban 1986). The typical estimates from meta‐analysis provide no evidence that prophylactic phenobarbital reduces IVH (typical RR 0.91; 95% CI 0.77 to 1.08). Because of the statistical heterogeneity, these typical estimates should be interpreted with caution (Analysis 1.1).
1.1. Analysis.
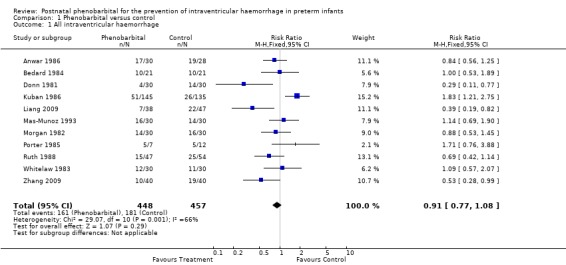
Comparison 1 Phenobarbital versus control, Outcome 1 All intraventricular haemorrhage.
Severe intraventricular haemorrhage (Outcome 1.2)
Data were available from all 12 trials on severe IVH. One trial showed a statistically significant decrease in severe IVH in the phenobarbital treated group (Zhang 2009), but the meta‐analysis provided no evidence of a significant reduction in severe IVH (typical RR 0.77; 95% CI 0.58 to 1.04) (Analysis 1.2).
1.2. Analysis.
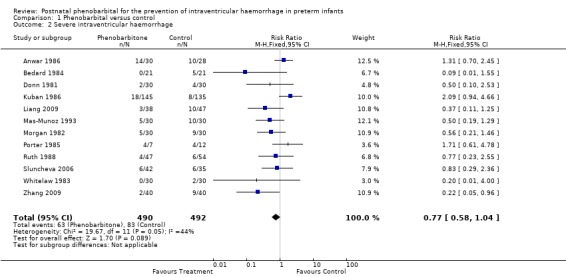
Comparison 1 Phenobarbital versus control, Outcome 2 Severe intraventricular haemorrhage.
Posthaemorrhagic ventricular dilation or hydrocephalus (Outcome 1.3)
Ventricular dilation or posthaemorrhagic hydrocephalus was reported in three trials and none of these trials reported a significant difference between the two treatment groups. The typical estimates from the meta‐analysis provided no evidence of a reduction in the risk of posthaemorrhagic ventricular dilation (typical RR 0.89; 95% CI 0.38 to 2.08, typical RD ‐0.01; 95% CI ‐0.08 to 0.06) (Analysis 1.3).
1.3. Analysis.
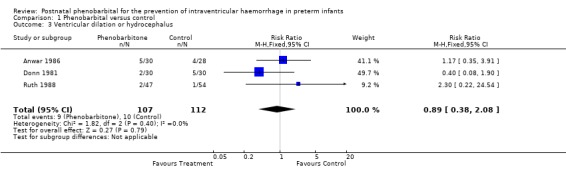
Comparison 1 Phenobarbital versus control, Outcome 3 Ventricular dilation or hydrocephalus.
Hypotension (Outcome 1.4)
Three trials reported hypotension (Donn 1981; Bedard 1984; Kuban 1986). The trial by Kuban 1986 reported a significant increase in hypotension in the infants receiving phenobarbital (RR 1.24; 95% CI 1.00 to 1.53; RD 0.12; 95% CI 0.00 to 0.23). The other two trials found no significant difference and the meta‐analysis found no significant difference in the risk of hypotension (typical RR 1.18; 95% CI 0.97 to 1.43; typical RD 0.09; 95% CI ‐0.01 to 0.19) (Analysis 1.4). Kuban's finding could have been influenced by the lower gestational age and birthweight in the group receiving phenobarbital. This would be expected to give a greater number of infants with blood pressures below 30 mm Hg as neonatal blood pressure has a positive correlation with birthweight.
1.4. Analysis.
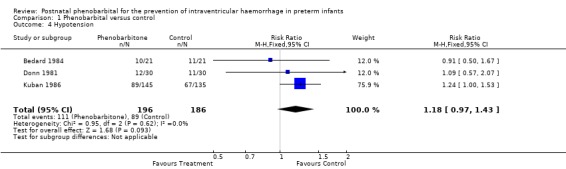
Comparison 1 Phenobarbital versus control, Outcome 4 Hypotension.
Pneumothorax/interstitial emphysema (Outcome 1.5)
Eight trials reported the number of infants with pneumothorax or interstitial emphysema. Only the trial by Kuban 1986 reported a significant increase in pneumothorax in the infants receiving phenobarbital (RR 2.11; 95% CI 1.20 to 3.70; RD 0.123; 95% CI 0.04 to 0.21). Four trials found non‐significant trends towards a reduction in pneumothorax among the infants receiving phenobarbital. The trial by Kuban 1986 had lower gestational age and birthweight in the phenobarbital‐treated group. This could have increased the risk of respiratory distress syndrome and the need for higher pressure ventilation. The meta‐analysis found no evidence of a difference in the risk of pneumothorax (typical RR 1.28; 95% CI 0.92 to 1.77; typical RD ‐0.04; 95% CI ‐0.01 to 0.10) (Analysis 1.5). There was no statistical heterogeneity.
1.5. Analysis.
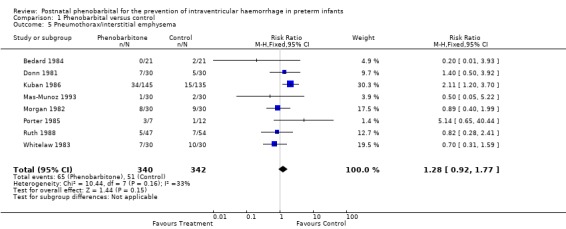
Comparison 1 Phenobarbital versus control, Outcome 5 Pneumothorax/interstitial emphysema.
Hypercapnia (Outcome 1.6)
Five trials reported the number of infants with hypercapnia. None of the trials found a significant difference and the meta‐analysis provided no evidence of a difference in the risk of hypercapnia (typical RR 1.00; 95% CI 0.73 to 1.37; typical RD 0.00; 95% CI ‐0.12 to 0.12) (Analysis 1.6).
1.6. Analysis.
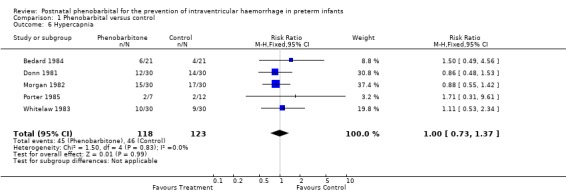
Comparison 1 Phenobarbital versus control, Outcome 6 Hypercapnia.
Acidosis (Outcome 1.7)
Six trials reported the number of infants with acidosis. None of the trials reported a significant difference and the meta‐analysis provided no evidence of a difference in the risk of acidosis (typical RR 1.16; 95% CI 0.90 to 1.51; typical RD 0.04; 95% CI ‐0.03 to 0.17) (Analysis 1.7). Because of the different definitions used for acidosis, this meta‐analysis should be treated with caution.
1.7. Analysis.
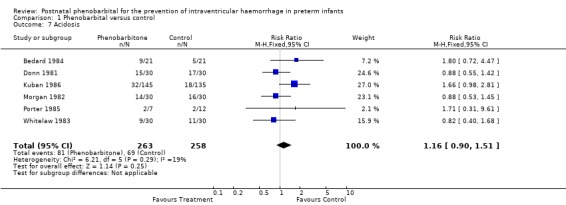
Comparison 1 Phenobarbital versus control, Outcome 7 Acidosis.
Mechanical ventilation (Outcome 1.8)
Five trials that did not require respiratory support as an obligatory entry criterion reported the number of babies who required ventilation. The trial by Ruth 1988 found a significant increase in use of mechanical ventilation in the group receiving phenobarbital (RR 1.20; 95% CI 1.01 to 1.43). Three trials found a trend towards increased use of mechanical ventilation (RR ranging from 1.09 to 1.54) with the fifth trial finding an RR of 1.00. Meta‐analysis showed a significant increase in use of mechanical ventilation in the infants receiving phenobarbital (typical RR 1.18; 95% CI 1.06 to 1.32; typical RD 0.129; 95% CI 0.05 to 0.21) (Analysis 1.8). This suggests that prophylactic phenobarbital treatment would, on average, result in one extra infant receiving mechanical ventilation for every eight preterm infants treated.
1.8. Analysis.
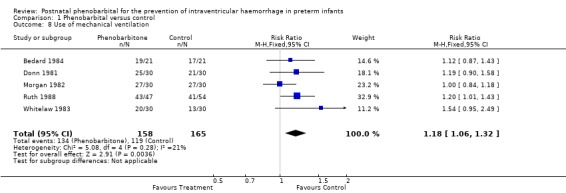
Comparison 1 Phenobarbital versus control, Outcome 8 Use of mechanical ventilation.
Neurodevelopmental impairment (Outcomes 1.9 and 1.10)
Mild neurodevelopmental impairment was reported only in Ruth 1988, and this showed no significant difference (RR 0.57; 95% CI 0.15 to 2.17; RD ‐0.05; 95% CI ‐0.16 to 0.06). Severe neurodevelopmental impairment was also reported only in Ruth 1988 and showed no significant difference (RR 1.44; 95% CI 0.41 to 5.04; RD ‐0.03; 95% CI ‐0.08 to 0.15) (Analysis 1.9; Analysis 1.10).
1.9. Analysis.
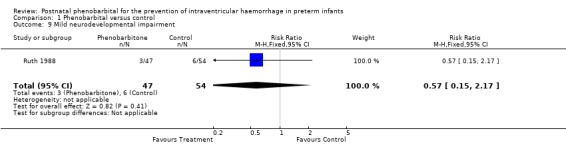
Comparison 1 Phenobarbital versus control, Outcome 9 Mild neurodevelopmental impairment.
1.10. Analysis.
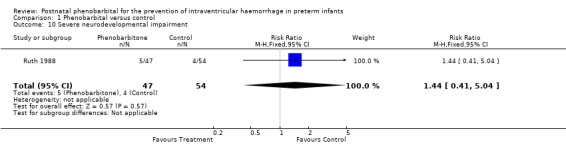
Comparison 1 Phenobarbital versus control, Outcome 10 Severe neurodevelopmental impairment.
Mortality prior to hospital discharge (Outcome 1.11)
Nine of the trials reported deaths before discharge from hospital and none reported a significant difference. The typical estimates from the meta‐analysis found no evidence of an effect on death prior to hospital discharge (typical RR 0.88; 95% CI 0.64 to 1.21; typical RD ‐0.02; 95% CI ‐0.07 to 0.03) (Analysis 1.11).
1.11. Analysis.
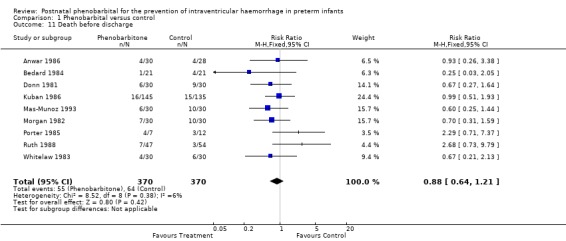
Comparison 1 Phenobarbital versus control, Outcome 11 Death before discharge.
Mortality during study period (Outcome 1.12)
Morgan 1982 and Ruth 1988 reported mortality documented after discharge from hospital while the infants were still being followed. Sluncheva 2006 reported deaths within the first 10 days of life only and Liang 2009 reported mortality without information on age at time of death. If these additional deaths are added in to give mortality during study period, none of the trials shows a significant difference and the typical estimates from the meta‐analysis provide no evidence of a difference in the risk of death during the study (typical RR 0.90; 95% CI 0.68 to 1.20) (Analysis 1.12).
1.12. Analysis.
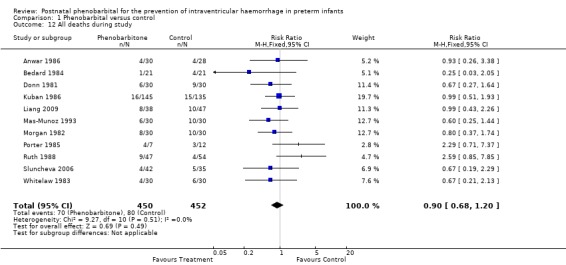
Comparison 1 Phenobarbital versus control, Outcome 12 All deaths during study.
Discussion
Horbar's systematic review of postnatal phenobarbital for preterm infants included eight trials and noted the heterogeneity between trials concerning any IVH and severe IVH (Horbar 1992). The author concluded that postnatal phenobarbital could not be recommended but the question was raised that, in specific settings, phenobarbital might be beneficial. Horbar's review did not present data on ventricular dilation, neuromotor impairment, mechanical ventilation, hypotension, pneumothorax or acidosis.
In the original review, it was possible to include one more trial than in Horbar's systematic review (Horbar 1992), and to include more data from Whitelaw's trial (Whitelaw 1983). The updated reviews in 2007 and 2012 included additional studies (one in 2007 and two in 2012). The original and subsequent updated reviews also covered ventricular dilation and neuromotor impairment, as well as possible cardiorespiratory and acid‐base side effects of the intervention. The statistical heterogeneity concerning all grades of IVH persists but no longer applied to severe IVH. This review supports Horbar's conclusion that phenobarbital does not reduce the frequency of IVH, severe IVH or death and provides new evidence that phenobarbital increases the need for mechanical ventilation. The data now available do not identify any specific setting where prophylactic phenobarbital might reduce the risk of IVH.
Methodological considerations
There is some clinical heterogeneity between the 12 trials but the infants recruited were all similar in that they were preterm, and at risk of IVH because of their immaturity or respiratory failure or both. Although the dosages of phenobarbital varied, they all gave plasma phenobarbital concentrations in the recommended anticonvulsant range for 72 hours, the period during which IVH usually occurs. There does not appear to be a publication bias as illustrated by the funnel plot (Figure 1). The risk of bias in the included studies is summarised graphically (Figure 2; Figure 3).
1.
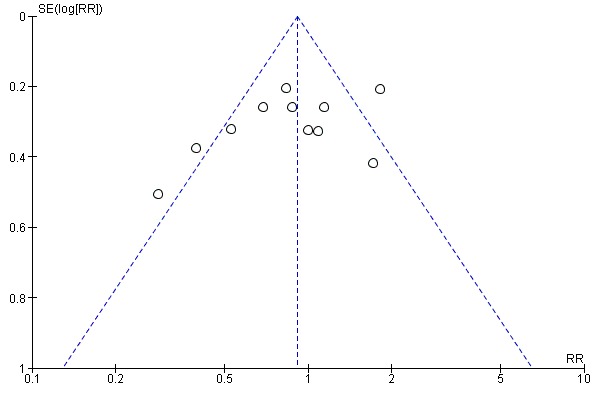
Funnel plot of comparison: 1 Phenobarbital versus control, Outcome: 1.1 All intraventricular haemorrhage.
2.
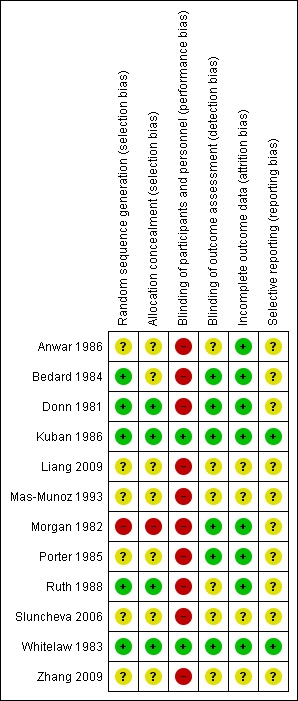
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
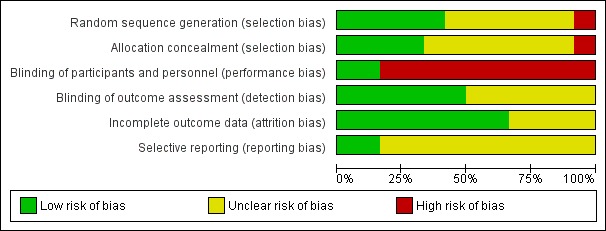
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
A cause for concern was that seven of the trials did not have a normal cranial ultrasound scan as an entry criterion. The three trials that found that postnatal phenobarbital reduced IVH were open trials that lacked a pre‐randomisation cerebral ultrasound scan (Donn 1981; Liang 2009; Zhang 2009). Some of the IVH reported could have arisen before the administration of phenobarbital. The double‐blind trial by Kuban 1986 was planned with adequate sample size; however, randomisation did not result in the two groups having similar risk factors for IVH since the group receiving phenobarbital had a significantly greater risk for IVH than did the control group at the time of randomisation. These factors in the trials by Donn 1981; Kuban 1986; Liang 2009 and Zhang 2009 could contribute to the heterogeneity found for the outcome, all grades of IVH. It is important to point out that only one of the trials showed a significant difference for severe IVH (Zhang 2009), but the meta‐analysis did not show a significant difference.
It is worth noting the relatively late timing of the initial injection of phenobarbital and the splitting of the loading dose so that it would have been well after 12 hours, in some cases, before anticonvulsant plasma concentrations of phenobarbital could have been achieved. Many IVHs have started by 12 hours of age. The difficulty in achieving therapeutic blood levels of phenobarbital before many IVHs have started was one reason for testing antenatal maternal administration of phenobarbital. Sluncheva 2006 did not use a loading dose. Prophylactic antenatal phenobarbital is the subject of a separate Cochrane systematic review by Crowther 2010, which concluded that the trials with most reliable methodology showed no evidence that the intervention was effective in reducing IVH.
Absence of therapeutic advantage
The results from the meta‐analyses of postnatal phenobarbital for preterm infants showed no significant difference between the phenobarbital‐treated group and the control group with respect to all grades of IVH, severe IVH, death, posthaemorrhagic ventricular dilation or neurodevelopmental impairment.
Potential side effects
In the current review, the only adverse effect associated with phenobarbital that reached statistical significance was mechanical ventilation, with no significant difference with respect to hypotension, acidosis, hypercapnia or pneumothorax. Increased need for mechanical ventilation is a clinically relevant adverse effect because of the associated iatrogenic risks such as tube blockage, infection, trauma to the larynx and the increased level of equipment and nursing required. Clearly, respiratory depression in spontaneously breathing infants with inadequate monitoring is potentially dangerous.
Since the original publication of this review, it has become apparent that administration of antiepileptic drugs in the newborn period may have a harmful effect on the developing brain. Phenobarbital has a proapoptotic effect in newborn rat brains (Bittigau 2002). More recently, it has been shown that neonatal rat exposure to a single dose of phenobarbital results in reduced synaptic connectivity in the striatum (Forcelli 2012).
Other approaches
Postnatal phenobarbital is not generally used in preterm infants as prophylaxis against IVH but a general decrease in IVH has been noted in developed countries since the 1980s despite an increase in survival of very immature infants. Maternal corticosteroid administration before preterm delivery has been mainly responsible for this decrease in IVH as demonstrated in a separate Cochrane review (Roberts 2006). Of the other pharmacological interventions assessed, indomethacin appeared promising, but results of a multicentre trial of indomethacin recruiting 1200 infants with birthweights below 1100 g showed that the reduction in IVH was not accompanied by an improvement in survival without disability (Schmidt 2001). Although IVH has been reduced in many centres, posthaemorrhagic hydrocephalus remains a problem without an effective treatment and requires further research into mechanisms and treatment. See Cochrane reviews on diuretic therapy (Whitelaw 2001b), repeated cerebrospinal fluid (CSF) tapping (Whitelaw 2001) and intraventricular streptokinase (Whitelaw 2001a).
Authors' conclusions
Implications for practice.
With no evidence of a reduction in intraventricular haemorrhage (IVH), neurodevelopmental impairment or death and with consistent evidence of an increase in need for mechanical ventilation, postnatal phenobarbital cannot be recommended for prophylaxis against IVH in preterm infants.
Implications for research.
There would seem to be no justification for further studies of postnatal barbiturates as prophylaxis against IVH.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2012 | New citation required but conclusions have not changed | New authorship. A repeat search on October 31, 2012 identified four more studies, of which two were eligible for inclusion in this review update. One was excluded in view of lack of randomisation, one was excluded as it failed to meet the inclusion criteria. |
| 31 October 2012 | New search has been performed | This review updates the original review "Postnatal phenobarbital for the prevention of intraventricular haemorrhage in preterm infants", published in the Cochrane Library, Issue 4, 2007 (Whitelaw 2007). |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 10 June 2008 | Amended | Converted to new review format |
| 31 May 2007 | New citation required but conclusions have not changed | Substantive amendment |
| 31 May 2007 | New search has been performed | This review updates the existing review "Postnatal phenobarbitone for the prevention of intraventricular hemorrhage in preterm infants", published in The Cochrane Library, Issue 3, 1999 (Whitelaw 1999). A repeat search 18th April 2007 identified one further eligible study. |
Acknowledgements
Thanks to Dr Yana S Kovacheva for help in translating the Sluncheva 2006 manuscript.
Thanks to Dr Xun Liu for help in translating the Liang 2009; Liu 2010; and Zhang 2009 manuscripts.
Data and analyses
Comparison 1. Phenobarbital versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All intraventricular haemorrhage | 11 | 905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.08] |
| 2 Severe intraventricular haemorrhage | 12 | 982 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.04] |
| 3 Ventricular dilation or hydrocephalus | 3 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.08] |
| 4 Hypotension | 3 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.97, 1.43] |
| 5 Pneumothorax/interstitial emphysema | 8 | 682 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.92, 1.77] |
| 6 Hypercapnia | 5 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| 7 Acidosis | 6 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.90, 1.51] |
| 8 Use of mechanical ventilation | 5 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.06, 1.32] |
| 9 Mild neurodevelopmental impairment | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.15, 2.17] |
| 10 Severe neurodevelopmental impairment | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.41, 5.04] |
| 11 Death before discharge | 9 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.21] |
| 12 All deaths during study | 11 | 902 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.68, 1.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anwar 1986.
| Methods | Open randomised controlled trial Blinding of randomisation: cannot determine No blinding of intervention Complete follow‐up: yes Blinding of main outcome measurement: cannot determine | |
| Participants | Preterm infants with a birthweight < 1500 g with no congenital malformations and no maternal phenobarbital administration. n = 58 | |
| Interventions | 2 loading doses of phenobarbital 10 mg/kg intravenously starting before 6 h of age and the second loading dose 12 h later, followed by a maintenance dose of 2.5 mg/kg every 12 h for 7 days. Maintenance doses were adjusted to achieve trough phenobarbital concentrations of 20‐30 mg/L | |
| Outcomes | Papile grade of IVH by ultrasound on days 1, 3 and 7; posthaemorrhagic hydrocephalus; death. It is not clear that the ultrasonographers were blind to treatment allocation | |
| Notes | Cerebral ultrasound was not carried out prior to trial entry so it was not possible to exclude babies who already had IVH before the first dose of phenobarbital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on how allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention was most likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up of all participants |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
Bedard 1984.
| Methods | Open randomised controlled trial Randomisation was by using a deck of cards but it is not clear how blinding to treatment allocation was achieved Blinding of intervention: no Blinding of main outcome measurement: yes Complete follow‐up: yes | |
| Participants | Infants < 24 h old with birthweights < 1500 g or gestation < 33 weeks were all eligible. Infants with gestational ages 33‐36 weeks or birthweight > 1500 g were eligible if they required mechanical ventilation for RDS. Another requirement was a cranial ultrasound scan showing no haemorrhage. n = 42 | |
| Interventions | 2 intravenous loading doses of phenobarbital 10 mg/kg 12 h apart, followed by maintenance doses of 2.5 mg/kg intravenously or orally every 12 h for 6 days | |
| Outcomes | Ultrasound diagnosis of grade of IVH as mild (grade I or II on Papile scale) or medium/severe (grade III or IV on Papile scale), death mechanical ventilation, pneumothorax, hypotension (< 2 SD below mean), pH < 7.2, pCO2 > 60 mm Hg, pCO2 < 25 mm Hg, bicarbonate administration (for metabolic acidosis) | |
| Notes | Of 95 potential trial participants, 42 were excluded because of IVH on the initial ultrasound scan. The control group were, on average, 1.1 weeks less mature and 220 g lighter than the phenobarbital group. No infants excluded after enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by using a deck of cards |
| Allocation concealment (selection bias) | Unclear risk | It is not clear how blinding to treatment allocation was achieved |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention was most likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was done by a paediatric radiologist unaware of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up was complete |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
Donn 1981.
| Methods | Open randomised controlled trial. Randomisation was described as by lottery but there is no description of how allocation concealment was achieved Blinding of intervention: no Complete follow‐up: yes Blinding of main outcome measurement: yes | |
| Participants | Infants with birthweights < 1500 g, admitted to the NICU within 6 h, without congenital malformations and where the mother had not received barbiturates during pregnancy. n = 60. No information on infants excluded or lost after enrolment | |
| Interventions | 2 loading doses of 10 mg/kg phenobarbital each administered intravenously 12 h apart. Maintenance dose of 2.5 mg/h every 12 h was begun 12 h after. Doses were adjusted to maintain serum concentrations in the 20‐30 μg/mL range for 7 days | |
| Outcomes | Papile grade of IVH on ultrasound, ventriculomegaly, mechanical ventilation, pneumothorax requiring drainage, hypercapnia (pCO2 > 60 mm Hg), hypotension (systolic blood pressure 10 mm Hg below expected value or impaired perfusion), bicarbonate therapy, death | |
| Notes | Cerebral ultrasound was not carried out prior to trial entry so it was not possible to exclude babies who already had IVH before the first dose of phenobarbital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation is described as by lottery |
| Allocation concealment (selection bias) | Low risk | No information provided, but it is likely the next allocation was not known in advance as a lottery system was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Most likely there was no blinding of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was done by ultrasonographers and neuroradiologists unaware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were followed‐up. The infants that died had a postmortem examination to ensure complete diagnosis of IVH |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
Kuban 1986.
| Methods | Randomised, double‐blind, controlled trial. Identical numbered ampoules were prepared by the pharmacy Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of main outcome measurement: yes | |
| Participants | Inclusion criteria were a) birthweight <1751 g, b) endotracheal intubation before 12 h, c) absence of congenital anomaly, d) no evidence of intracranial haemorrhage on ultrasound scan, e) neonatal phenobarbital level < 5 μg/mL. n = 280. Of 291 infants enrolled, 11 had to be withdrawn and were excluded from analysis. 48 infants were excluded from enrolment because IVH was already present | |
| Interventions | 2 loading doses of phenobarbital 10 mg/kg or placebo intravenously with a 30‐minute interval. 12 h later, the baby received the first of 9 maintenance doses of 2.5 mg/kg or placebo at 12‐h intervals | |
| Outcomes | Papile grade of IVH on ultrasound scan (any haemorrhage or severe grade III or IV), haemorrhage, acidosis (pH < 7.2 on day 1), pneumothorax/pulmonary interstitial emphysema, hypotension (< 30 mm Hg on day 1). Mortality data were by personal communication between Dr Kuban and Dr Horbar although age at death was not clear | |
| Notes | The randomisation did not give a similar gestational age in the 2 treatment groups. Thus 52.4% of the phenobarbital group had a gestational age < 30 weeks but this was true of only 41.5% of the control group. The authors attempted to allow for this imbalance by analysis within weight groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers used |
| Allocation concealment (selection bias) | Low risk | Insufficient information provided, but as a table of random numbers was used it is likely the next allocation was not known in advance |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical numbered ampoules were prepared by the pharmacy, participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The ultrasonographers were not aware of the treatment allocation when assessing the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants were followed‐up, the infants that died had a postmortem examination to assess for IVH. 11 out of 291 (3.8%) infants enrolled were withdrawn after randomisation |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but it appears the published report included all reported outcomes, including those that were prespecified |
Liang 2009.
| Methods | Open randomised trial. The method of randomisation and means of allocation concealment were not described. Despite randomisation, group sizes were unequal with 38 subjects in the phenobarbital group versus 47 in the control group Blinding of intervention: no Complete follow‐up: uncertain Blinding of outcome measurement: uncertain |
|
| Participants | Preterm infants with gestational age 28‐34 weeks from a single centre were included. No birthweight or need for mechanical ventilation criteria. No information given on withdrawal or loss of subjects after enrolment | |
| Interventions | Phenobarbital 20 mg/kg split in 2 doses 12 h apart, started within 6 h of birth. Followed 12 h later by a maintenance dose of 5 mg/kg/day for 5 days. Route of administration was not specified. Drug levels were not monitored. No use of a placebo | |
| Outcomes | Grade of IVH (graded 1‐4 with 3 and 4 being severe) on brain CT within 1 week of age. Mortality data were given, but age at death was unclear | |
| Notes | Randomisation resulted in unequal group sizes. The authors did not explain this. High mortality rate noted, with uncertainty about whether any subjects died prior to undergoing CT or underwent postmortem to identify IVH. No assessment of IVH prior to trial entry | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were probably not blinded for intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
Mas‐Munoz 1993.
| Methods | Open controlled trial. The method of randomisation and means of allocation concealment were not described Blinding of intervention: no Complete follow‐up: yes Blinding of outcome measurement: cannot determine | |
| Participants | Newborn infants with gestational ages 27‐34 weeks and who were ventilator dependent. n = 60. No information on infants excluded or lost after enrolment | |
| Interventions | Phenobarbital 20 mg/kg intravenously as a loading dose within 12 h of birth followed by phenobarbital 2.5 mg/kg every 12 h for the next 5 days | |
| Outcomes | Cerebral ultrasound every 48 h for 14 days, IVH graded as I/II or III/IV on the Papile scale, death. It is not clear whether the ultrasonographers were blind to treatment allocation | |
| Notes | Cerebral ultrasound was not carried out prior to trial entry so it was not possible to exclude babies who already had IVH before the first dose of phenobarbital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were most likely not blinded for intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear whether the ultrasonographers were blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on infants excluded or lost after enrolment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
Morgan 1982.
| Methods | An open controlled trial using alternate allocation to phenobarbital or no injection Blinding of randomisation: no Blinding of intervention: no Complete follow‐up: yes Blinding of main outcome measurement: yes | |
| Participants | Infants with birthweights below 1250 g and infants with birthweights 1250‐1500 g who required mechanical ventilation in the first 24 h. An ultrasound scan showing absence of IVH was also a requirement. N = 60. No information on infants excluded or lost after enrolment | |
| Interventions | A loading dose of 20 mg/kg phenobarbital intramuscularly at a median time of 2 h after birth (range 1‐22 h) | |
| Outcomes | Papile grade of IVH on ultrasound, death, pneumothorax, hypercapnia (pCO2 > 8 kPa), acidosis (pH < 7.15). The age limit for death is not specified but "one cot death" occurred at home at 4 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation (quasi‐random) |
| Allocation concealment (selection bias) | High risk | Next allocation always known as alternate allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were most likely not blinded for intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An experienced observer unaware of treatment allocation assessed outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All subjects are followed up, but no information provided on postmortem diagnoses in infants that died |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
Porter 1985.
| Methods | Open randomised controlled trial. The method of randomisation was not described Blinding of randomisation: cannot determine Blinding of intervention: no Complete follow‐up: yes Blinding of main outcome measurement | |
| Participants | Newborn infants with birthweight < 1500 g with a normal cerebral ultrasound scan before 6 h of birth and receiving respiratory support. n = 19. No information on infants excluded after enrolment | |
| Interventions | A loading dose of phenobarbital 30 mg/kg intravenously within 6 h of birth, followed by a maintenance dose of 5 mg/kg per day for 72 h | |
| Outcomes | Cerebral ultrasound scans were carried out daily by sonographers who were blind to the initial treatment allocation. IVH was graded according to the Papile scale, mechanical ventilation, pneumothorax, hypercapnia (> 60 mm Hg), acidosis (pH < 7.15), death | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment allocation was most likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cerebral ultrasound scans were carried out daily by sonographers who were blind to the initial treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
Ruth 1988.
| Methods | Open randomised controlled trial. Randomisation was by "lottery" Blinding of randomisation: cannot determine Complete follow‐up: yes Blinding of outcome measurement: yes |
|
| Participants | Infants with birthweights < 1501 g and gestational age ≥ 25 weeks, < 4 h old. Infants with malformations or maternal barbiturate treatment were excluded. n = 101. 111 infants were originally enrolled but 10 were excluded (7 in the phenobarbital group and 3 in the control group) either because the gestational age was < 25 weeks or because of congenital anomaly | |
| Interventions | 2 loading doses of phenobarbital 15 mg/kg intravenously were given 4 h apart. Maintenance treatment with phenobarbital 5 mg/kg per day was started 24 h after the first dose and continued for 5 days | |
| Outcomes | Cerebral ultrasound scans were carried out on days 1, 3, 5 and 7 and then weekly; IVH was graded according to the Papile scale; neurodevelopmental assessment at 27 months of age; neonatal death; postnatal death; mechanical ventilation (total and > 7 days); pneumothorax | |
| Notes | Cerebral ultrasound was not carried out prior to trial entry so it was not possible to exclude babies who already had IVH before the first dose of phenobarbital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by lottery |
| Allocation concealment (selection bias) | Low risk | No information provided, but next allocation unlikely to have been known in advance as lottery system used for treatment allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided, but participants and personnel were most likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment both for cranial ultrasound and for neurodevelopmental outcome at 27 months |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 111 infants were originally enrolled but 10 were excluded (7 in the phenobarbital group and 3 in the control group) either because the gestational age was < 25 weeks or because of congenital anomaly. Long‐term (27 months) follow‐up reported for all survivors |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
Sluncheva 2006.
| Methods | Randomised controlled trial | |
| Participants | Infants with birthweights < 1500 g and under 32 weeks' gestation | |
| Interventions | 5 mg/kg/day dose of phenobarbital intravenously for the first 5 days | |
| Outcomes | Cerebral ultrasound scans were carried out on days 1, 3, 5 and 10; IVH was graded according to the Papile scale; neonatal death; pulmonary haemorrhage; oxygen requirement; respiratory rate; patent ductus arterious up to 10 days of age | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided, but participants and personnel were most likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on infants excluded or lost after enrolment |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
Whitelaw 1983.
| Methods | Randomised double‐blind controlled trial. The infants received numbered, identical ampoules for injection Blinding of randomisation: yes Blinding of intervention: yes Complete follow‐up: yes Blinding of outcome measurement: yes | |
| Participants | Infants < 1500 g with a normal cerebral ultrasound scan in the first 4 h. n = 60. 2 infants were excluded after randomisation because of congenital malformations and they were replaced | |
| Interventions | Phenobarbital 20 mg/kg or isotonic saline given intravenously or intramuscularly within 4 h of birth. No maintenance doses given | |
| Outcomes | IVH on cerebral ultrasound scans carried out daily for the 2 weeks and then weekly. Grading 1, 2, 3 according to Levene initially, subsequently reclassified to be compatible with Papile grading. Mechanical ventilation after injection, pneumothorax, hypercapnia (pCO2 > 8 kPa), acidosis (pH < 7.2), death before discharge from hospital | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The method of randomisation was not described in the paper, but was clarified by personal communication with Prof Whitelaw as a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | No risk of prior knowledge of next allocation as random numbers table was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The infants received numbered, identical ampoules for injection and participants and personnel were unaware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cranial ultrasound was performed and assessed by personnel unaware of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 infants were excluded after randomisation because of congenital malformations and they were replaced |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes, including those prespecified |
Zhang 2009.
| Methods | Open randomised trial. No description of randomisation method or allocation concealment. 40 infants were assigned to each group (intervention versus control) Blinding of intervention: no Complete follow‐up: uncertain Blinding of outcome measurement: uncertain |
|
| Participants | Preterm infants < 34 weeks' gestation were included. No birthweight or mechanical ventilation criteria. No information on infants excluded or lost after enrolment | |
| Interventions | Phenobarbital loading dose 2 mg/kg split in 2 doses of 10 mg/kg intravenously. Maintenance dose 12 h later, 5 mg/kg every 12 h for 5 days. Aim to give phenobarbital within 6 h of birth. No placebo used. No drug level monitoring | |
| Outcomes | IVH on CT within 3 days of birth (graded 1‐4, with 3 and 4 being severe). No assessment of IVH prior to trial entry | |
| Notes | 18 infants received the dose of phenobarbital later than 6 h, mean age at time of loading dose was 9.1 h. CT was done early (within 3 days), this may result in missing infants with late progression of IVH. In view of high rate of IVH, it is likely there was mortality too, but the authors do not give mortality data. This raises the question whether any infants died prior to having had their CT scan to assess IVH | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment allocation was most likely not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on infants excluded or lost after enrolment. In view of high rate of IVH, it is likely there was mortality too, but the authors do not give mortality data. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a judgement as we have no access to a trial protocol |
CT: computed tomography; IVH: intraventricular haemorrhage; NICU: neonatal intensive care unit; pCO2: partial pressure of carbon dioxide; RDS: respiratory distress syndrome; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2008 | Not a randomised or quasi‐randomised trial |
| Hope 1982 | Not a randomised or quasi‐randomised trial |
| Liu 2010 | Did not meet inclusion criteria for gestational age combined with birthweight (infants < 35 weeks' gestation were included). Mean birthweight in intervention group was 2165 g and in control group was 2188 g. No information available on whether these infants were ventilated or not (infants with gestation 33‐36 weeks can only be included in this review if ventilated and birthweight was < 1750 g) |
Differences between protocol and review
We have updated the methodology for judging risk of bias.
Contributions of authors
AW carried out a literature search and wrote the first draft of the protocol and the full review.
DO carried out a literature search in 2007 and updated the review and analysis.
ES carried out a literature search in 2012 and updated the review and analysis.
Sources of support
Internal sources
University of Bristol, UK.
External sources
Wellcome Trust, UK.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Anwar 1986 {published data only}
- Anwar M, Kadam S, Hiatt IM, Hegyi T. Phenobarbitone prophylaxis of intraventricular haemorrhage. Archives of Diseases in Childhood 1986;61(2):196‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bedard 1984 {published data only}
- Bedard MP, Shankaran S, Slovis TL, Pantoja A, Dayal B, Poland RL. Effect of prophylactic phenobarbital on intraventricular hemorrhage in high‐risk infants. Pediatrics 1984;73(4):435‐9. [PubMed] [Google Scholar]
Donn 1981 {published data only}
- Donn SM, Roloff DW, Goldstein GW. Prevention of intraventricular haemorrhage in preterm infants by phenobarbitone. Lancet 1981;2(8240):215‐7. [DOI] [PubMed] [Google Scholar]
Kuban 1986 {published and unpublished data}
- Kuban K, Leviton A, Brown ER, Krishnamoorthy K, Baglivo J, Sullivan KF, et al. Respiratory complications in low‐birth‐weight infants who received phenobarbital. American Journal of Diseases in Children 1987;141(9):996‐9. [DOI] [PubMed] [Google Scholar]
- Kuban KC, Leviton A, Krishnamoorthy KS, Brown ER, Teele RL, Baglivo JA, et al. Neonatal intracranial hemorrhage and phenobarbital. Pediatrics 1986;77(4):443‐50. [PubMed] [Google Scholar]
Liang 2009 {published data only}
- Liang GL, He YZ, Luo L. Phenobarbitone to prevent intraventricular hemorrhage in preterm infants ‐ an observational study (38 cases). Journal of Medical Theory and Practice 2009;22(4):449‐50. [1001‐7585(2009)04‐0449‐02] [Google Scholar]
Mas‐Munoz 1993 {published data only}
- Mas‐Munoz RL, Udaeta‐Mora E, Barrera‐Reyes RH, Rivera‐Rueda MA, Morales‐Suarez M. The effect of phenobarbital on the severity of intraventricular hemorrhage [Efecto del fenobarbital sobre la gravedad de la hemorragia intraventricular]. Boletín Médico del Hospital Infantil de México 1993;50(6):376‐82. [PubMed] [Google Scholar]
Morgan 1982 {published data only}
- Morgan ME, Massey RF, Cooke RW. Does phenobarbitone prevent periventricular hemorrhage in very low birth weight babies: a controlled trial. Pediatrics 1982;70(2):186‐9. [PubMed] [Google Scholar]
Porter 1985 {published data only}
- Porter FL, Marshall RE, Moore JA, Miller RH. Effect of phenobarbital on motor activity and intraventricular hemorrhage in preterm infants with respiratory disease weighing less than 1500 grams. American Journal of Perinatology 1985;2(2):63‐6. [DOI] [PubMed] [Google Scholar]
Ruth 1988 {published data only}
- Ruth V, Virkola K, Paetau R, Raivio KO. Early high‐dose phenobarbital treatment for prevention of hypoxic‐ischemic brain damage in very low birth weight infants. Journal of Pediatrics 1988;112(1):81‐6. [DOI] [PubMed] [Google Scholar]
Sluncheva 2006 {published data only}
- Sluncheva B, Vakrilova L, Emilova Z, Kalaĭdzhieva M, Garnizov T. Prevention of brain hemorrhage in infants with low and extremely low birth weight and infants treated with surfactants. Late observation. Akusherstvo i Ginekologiia (Sofiia) 2006;45(3):34‐8. [PubMed] [Google Scholar]
Whitelaw 1983 {published data only}
- Whitelaw A, Placzek M, Dubowitz L, Lary S, Levene M. Phenobarbitone for prevention of periventricular haemorrhage in very low birth‐weight infants. A randomised double‐blind trial. Lancet 1983;2(8360):1168‐70. [DOI] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang ZJ, Yuan J, Meng YQ, Guo JX. An observational study of phenobarbitone in preventing intraventricular hemorrhage in preterm infants. Inner Mongolian Medical Journal 2009;41(5):617‐8. [1004‐0951(2009)05‐0617‐02] [Google Scholar]
References to studies excluded from this review
Chen 2008 {published data only}
- Chen H, Wei K, Yao Y, Yang Y, Zhou C, Fang X, et al. Multicenter investigative report for the effect of prophylactic phenobarbital on intraventricular hemorrhage in premature infants in China. Journal of Clinical Pediatrics 2008;26(11):986‐93. [Google Scholar]
Hope 1982 {published data only}
- Hope PL, Stewart AL, Thorburn RJ, Whitehead MD, Reynolds EO, Lowe D. Failure of phenobarbitone to prevent intraventricular haemorrhage in small preterm infants. Lancet 1982;1(8269):444‐5. [DOI] [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu Z, Zhao Y, Chen W, Wang H. Efficacy and safety of phenobarbital in preventing intraventricular hemorrhage in premature newborns. Journal of Bengbu Medical College 2010;35(10):1030‐2. [1000‐2200(2010)10‐1030‐03] [Google Scholar]
Additional references
Bittigau 2002
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proceedings of the National Academy of Sciences USA 2002;99(23):15089‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2010
- Crowther CA, Crosby DD, Henderson‐Smart DJ. Phenobarbital prior to preterm birth for preventing neonatal periventricular haemorrhage. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD000164.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forcelli 2012
- Forcelli P, Janssen MJ, Vicini S, Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Annals of Neurology 2012;72(3):363‐72. [DOI: 10.1002/ana.23600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goddard 1987
- Goddard‐Finegold J, Armstrong DL. Reduction in incidence of periventricular intraventricular hemorrhages in hypertensive newborn beagles pretreated with phenobarbital. Pediatrics 1987;79(6):901‐6. [PubMed] [Google Scholar]
Gould 1987
- Gould SJ, Howard S. An immunohistochemical study of the germinal matrix in the late gestation human fetal brain. Neuropathology and Applied Neurobiology 1987;13(6):421‐37. [DOI] [PubMed] [Google Scholar]
Hambleton 1976
- Hambleton G, Wigglesworth JS. Origin of intraventricular haemorrhage in the preterm infant. Archives of Disease in Childhood 1976;51(9):651‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hope 1988
- Hope PL, Gould SJ, Howard S, Hamilton PA, Costello AM, Reynolds EO. Precision of ultrasound diagnosis of pathologically verified lesions in the brains of very preterm infants. Developmental Medicine and Child Neurology 1988;30(4):457‐71. [DOI] [PubMed] [Google Scholar]
Horbar 1992
- Horbar J. Prevention of periventricular‐intraventricular hemorrhage. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:562‐89. [Google Scholar]
Levene 1982
- Levene MI, Fawer CL, Lamont RF. Risk factors in the developmental of intraventricular haemorrhage in the preterm neonate. Archives of Disease in Childhood 1982;57(6):410‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ment 1985
- Ment LR, Stewart WB, Duncan CC. Beagle puppy model of intraventricular hemorrhage. Effect of superoxide dismutase on cerebral blood flow and prostaglandins. Journal of Neurosurgery 1985;62(4):563‐9. [DOI] [PubMed] [Google Scholar]
Nakamura 1990
- Nakamura Y, Okudera T, Fukuda S, Hashimoto T. Germinal matrix hemorrhage of venous origin in preterm neonates. Human Pathology 1990;21(10):1059‐62. [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [DOI] [PubMed] [Google Scholar]
Perlman 1983
- Perlman JM, McMenamin JB, Volpe JJ. Fluctuating cerebral blood‐flow velocity in respiratory‐distress syndrome. Relation to the development of intraventricular hemorrhage. New England Journal of Medicine 1983;309(4):204‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roberts 2006
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth risk of preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004454.pub2] [DOI] [PubMed] [Google Scholar]
Schmidt 2001
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long‐term effects of indomethacin prophylaxis in extremely‐low‐birth‐weight infants. New England Journal of Medicine 2001;344(26):1966‐72. [DOI] [PubMed] [Google Scholar]
Steen 1979
- Steen PA, Mitchenfelder JD. Barbiturate protection in tolerant and nontolerant hypoxic mice: comparison with hypothermic protection. Anesthesiology 1979;50(5):404‐8. [DOI] [PubMed] [Google Scholar]
Vohr 1989
- Vohr BR, Garcia‐Coll C, Mayfield S, Brann B, Shaul P, Oh W. Neurologic and developmental status related to the evolution of visual‐motor abnormalities from birth to 2 years of age in preterm infants with intraventricular hemorrhage. Journal of Pediatrics 1989;115(2):296‐302. [DOI] [PubMed] [Google Scholar]
Volpe 1995
- Volpe JJ. Neurology of the Newborn. 3rd Edition. Philadelphia: Saunders, 1995:403‐463. [Google Scholar]
Whitelaw 2001
- Whitelaw A. Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000216] [DOI] [PubMed] [Google Scholar]
Whitelaw 2001a
- Whitelaw A. Intraventricular streptokinase after intraventricular hemorrhage in newborn infants. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000498.pub2] [DOI] [PubMed] [Google Scholar]
Whitelaw 2001b
- Whitelaw A, Kennedy CR, Brion LP. Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002270] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wimberley 1982
- Wimberley PD, Lou HC, Pedersen H, Hejl M, Lassen NA, Friis‐Hansen B. Hypertensive peaks in the pathogenesis of intraventricular hemorrhage in the newborn. Abolition by phenobarbitone sedation. Acta Paediatrica Scandinavica 1982;71(4):537‐42. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Whitelaw 1999
- Whitelaw A. Postnatal phenobarbitone for the prevention of intraventricular hemorrhage in preterm infants. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001691] [DOI] [PubMed] [Google Scholar]
Whitelaw 2007
- Whitelaw A, Odd D. Postnatal phenobarbital for the prevention of intraventricular hemorrhage in preterm infants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001691.pub2] [DOI] [PubMed] [Google Scholar]


